Dear Editor,
Nanoparticle albumin bound (nab)–paclitaxel is a member of the taxane agents that has been proven efficient and widely applied in breast cancer as well as other kind of cancers. The taxane-induced macular edema has been recognized as an uncommon side effect which might regress after the drug withdraw. Here we presented our case as a rarely observed macular edema secondary to nab–paclitaxel therapy as follows. The consent from the patient was achieved and the research was approved by the Ethics Committee of Xijing Hospital (KY20202009-C-1).
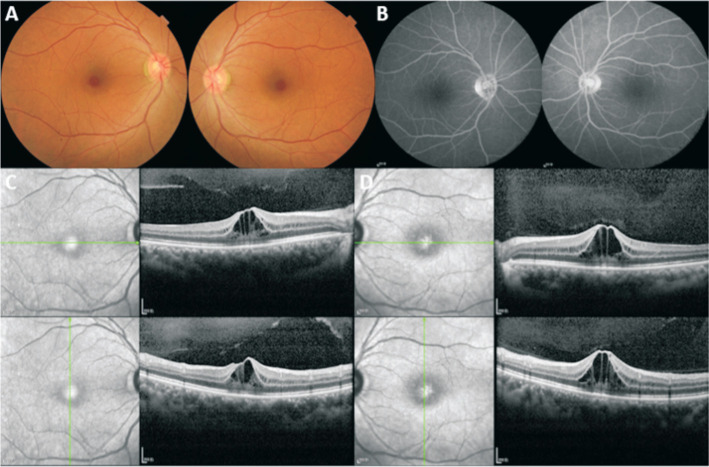
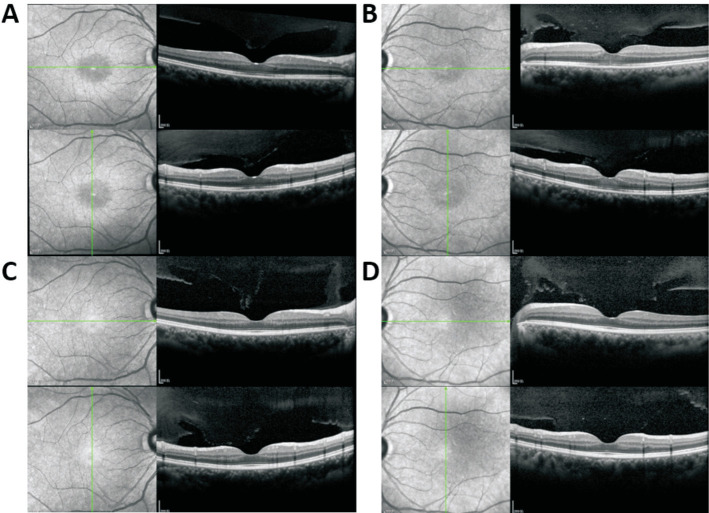
A 54-year-old woman presented to our department of ophthalmology with blurred vision in both eyes for one week. She had been diagnosed with left invasive breast cancer (ER-, PR+, Her-2++, and Ki67 40%) and started to be treated with chemotherapy of 11 times neoadjuvant nab-paclitaxel chemotherapy of EC (epirubicin 80 mg and cyclophosphamide of 0.8 g) before operation and the anti-cancer drug was withdrawn. There was a negative history of X-linked retinoschisis in her family. The external ocular examination and ocular motility of her both eyes were normal. The best corrected visual acuity tested as 20/40 oculus dexter (OD) and 30/50 oculus sinister (OS); the intraocular pressure was normal; the anterior segment of both eyes was quiescent. On fundus examination, the loss of foveal reflex and thickened fovea were observed in both eyes (Figure 1A) with no evidence of any fluorescein leakage shown on fluorescein angiography in both eyes (Figure 1B). Optical coherence tomography (OCT) revealed intraretinal cystoid spaces and dome-shaped configuration of fovea in both eyes with partial posterior vitreous detachment confirming bilateral cystoid macular edema (CME; Figure 1C and 1D) with the central foveal thickness (CFT) of 414 µm OD and 510 µm OS. Based on the patient's medication history and ocular manifestations, the final diagnosis was drug-induced macular edema secondary to nab-paclitaxel therapy. After thorough consultation and obtaining the necessary information, 0.1% sodium hyaluronate eye drops and 1% brinzolamide were administered three times daily for a duration of three months. Two weeks later, OCT images indicated that the macular edema had gradually subsided (Figure 2A and 2B) with the CFT of 174 µm OD and 185 µm OS and the edema had returned to normal in 3mo (Figure 2C and 2D) with the CFT of 190 µm OD and 179 µm OS. Over the course of one year of follow-up, her visual acuity was restored and maintained at 20/20 OD and 20/20 OS.
Figure 1. Baseline multimodal imaging.
A: Fundoscopy showed the cystoid edema in the fovea retina in both eyes; B: Fluorescein angiography showed no leakage in central macular in both eyes; C, D: Ocular coherence tomography showed cystic spaces in the retina and the increasing thickness of the central retina with the CFT of 414 µm OD (C) and 510 µm OS (D). CFT: Central foveola thickness; OS: Oculus sinister; OD: Oculus dexter.
Figure 2. Ocular coherence tomography follow-up with treatment.
A, B: After the withdraw of anti-cancer drug and being treated with 0.1% sodium hyaluronate eye drops and 1% brinzolamide, the macular edema subsided eventually with the CFT of 174 µm OD (A) and 185 µm OS (B) 2wk later. C, D: The structure of the retina turned back to normal in 3mo with the CFT of 190 µm OD and 179 µm OS. CFT: Central foveola thickness; OS: Oculus sinister; OD: Oculus dexter.
CME refers to the accumulation of extracellular fluid in the macular region, characterized by the formation of cystoid spaces in the outer plexiform layer. This condition eventually leads to blurred and distorted vision[1]. The causes for macular edema are various, ranging from retinal vascular diseases, ocular inflammation diseases, postoperations, tumor, and others[2]. Among these, diabetic retinopathy and retinal vein occlusion are the most prevalent conditions. Additionally, drug-induced macular edema by the application of topical epinephrine-like antiglaucoma drops[3]–[4], tamoxifen[5] has garnered clinical attention, indicating a potential toxic effect on the retina during drug administration. Paclitaxel and its modified form nab-paclitaxel are a staring anticancer agent and are widely used in the treatment of malignant diseases such as breast cancers, ovarian cancer and so on due to their unique mechanisms. So far, unwanted side effects of this drug have been researched in depth. Allergic reactions, fever, nausea-vomiting, loss of appetite, peripheral neuropathies, hair loss and changes in the blood picture can be frequently observed in chemotherapy patients treated with paclitaxel while vision loss and macular edema induced by the drug are comparatively rare[6]. Here we present a rare case of bilateral macular edema induced by the application of nab-paclitaxel.
Ocular or visual disturbances are fairly rare compared with other side effects induced by nab-paclitaxel, occurring in 13% of all patients treated with paclitaxel in randomized clinical trials and about 1% of all patients experience severe cases[7]. According to a cross-sectional OCT study in patients undergoing taxane-based therapy, only 0.5% of patients with macular edema are secondary to the application of the taxanes[8]. The occurrences of macular edema induced by the taxanes are often bilateral and are supposed to be reversible after drug withdrawal in most circumstances. The mechanisms are still not clear, while the toxicity of the taxnes to retinal pigment epithelial cells (RPE) and Müller cells may account for them[9]. Müller cells are responsible for maintaining the permeability gradient of the neurosensory retina, and their dysfunction may lead to the accumulation of intracellular fluid. RPE cells constitute the outermost layer of the retina, positioned between photoreceptor cells and choroid. The main functions of RPE include transporting glucose, fatty acids and other nutrients to the retina, maintaining ion balance of photoreceptor cells[10] and facilitating water transportation. The selective function of transportation and the tight junction of RPE are mainly related to claudins proteins[11]. Due to the highly active physiological processes in the retina and the high level of cell metabolism, the processes have a massive demand for glucose and oxygen resulting in the production of large amounts of water. Part of this water is transported from the anterior retina transported via Müller cells while the other is transported to choroid via RPE cells. The possible mechanisms underlying macular edema may include the effusion in Müller cells[9],[12], the fluid retention caused by the increased capillary filtration[13]–[14] and the dysfunction of RPE caused by the loss of microtubule function, which then leads to the destruction of blood-retinal barrier. RPE cells are arranged compactly, resulting in a comparatively high resistance in the paracellular pathway, and water transport is mainly discharged through transcellular pathway[15]. Moreover, according to the results of fluorescein angiography, the absence of leakage of the vessels reveals a distinct difference between macular edema caused by the chemotherapy and other common causes[9].
To relieve the symptoms, various therapies were applied. It has been recommended that 2% dozoamide, a drug that belongs to the agent of carbonic anhydrase inhibitors (CAI), may help resolve the condition of patients with macular edema induced by taxanes in the early stage[16]. In the physiological structure of retina, transmembrane carbonic anhydrase XIV is distributed on the top and outside of the base of RPE cells and end foot and non-end foot membranes on Müller cells, which regulates the transport of ions and CO2 between capillaries, RPE cells and Müller cell, maintaining pH homeostasis and thus maintaining the normal optical response of retina[17]–[18]. It is also speculated that when the paclitaxel induced CME occurs, the distribution of transmembrane carbonic anhydrase-XIV is unbalanced. The number of it on the lateral side of the base is more than that in the top in RPE[19], which provides favorable conditions for the treatment of CAI. CAI inhibits the basal lateral HCO3− transport more effectively, thus restoring the polarity of RPE cells and promoting the transport of cell fluid between retina and choroid. Furthermore, CAI promotes tighter adherence of RPE to neuroepithelium and enables the absorption of subretinal fluid, so that the symptoms of CME will be alleviated[20].
Interestingly, despite being considered first-line treatments for CME, steroid and anti-vascular endothelial growth factor (VEGF) drugs were not the optimal choice. While steroid injections have proven effective, they merely provide temporary relief rather than a resolution to the issue[21]. Anti-VEGF intravitreal injection is confirmed of little effect in the treatment of the drug-induced macular edema. This implies that VEGF should not be blamed for this kind of macular edema predominantly[16], which reveals the different mechanisms of taxanes-induced macular edema from diabetic macular edema and retinal vein occlusion-macular edema.
Most studies on macular edema primarily focus on cases related to diabetes, retinal vein occlusion, and cataract surgery while macular edema induced by nab-paclitaxel therapy is comparatively uncommon. Our patient stands out as a rare case in the Asian population diagnosed with this condition as a secondary effect of neoadjuvant nab-paclitaxel chemotherapy for breast cancer. This occurrence affirms that nab-paclitaxel therapy has the potential to trigger the uncommon side effect of macular edema, similarly to other taxane agents. The clinical trials on application of the taxanes demonstrated that there was no significant difference in the therapeutic effect among diverse races[22]. However, the side effects and toxicity may differ in Asian and Caucasian populations. Statistics have shown that over 30% of Asians but less than 5% of Caucasians experienced grade ≥3 neutropenia after the adjuvant docetaxel/cyclophosphamide therapy[23]. In another research, researchers observed that the Japanese were more sensitive to the toxicity of docetaxel and suspected that the difference in toxicity profile might due to unknown genetic factors and differences in unconjugated docetaxel concentration or baseline leukocyte count. Yet the mechanisms of different susceptibility to docetaxel toxicity in different populations have not been elucidated and have not been reported in the application of nab-paclitaxel therapy. Whether the occurrence of the side effect of macular edema has any relation to the differences in various races is still unknown.
In a nutshell, most publications related to macular edema refer to macular edema secondary to diabetes, retinal vein occlusion and surgery for cataracts. Macular edema induced by nab-paclitaxel therapy is fairly rare. After a long period of applying the taxane drugs clinically in chemotherapies for different cancers, the side effects, including neuropathies, and many other symptoms which occur more frequently are getting increasingly attention while the ocular problems are always overlooked. Most patients with tumors are the elderly who may have already suffered from the decline of visual acuity so that the similar symptoms caused by taxane-induced macular edema may fail to draw their attention. Clinical doctors should be alert for the possibility of development of macular edema in the patients receiving chemotherapies with the taxanes, regardless of the specific use of it, and it is necessary to consider baseline ophthalmological evaluation and seek professional evaluations in ophthalmology department. Meanwhile, closely follow up of the patients is also a necessity for medical practitioners, in case of the progression or persistence of the symptoms.
Footnotes
Authors' contributions: Dou GR, Zhu JT, and Sun DJ performed the examination of the patient. Zhou ZY and Ye YT were major contributors in writing the manuscript. Dou GR and Wang YS reviewed the manuscript. All authors read and approved the final manuscript.
Foundations: Supported by National Natural Science Foundation of China (No.81970814; No.82371071); Youth Science and Technology Nova Program of Shaanxi Province (No.2016KJXX-19); Xijing Supportive Grant (No.XJZT19ML19).
Conflicts of Interest: Zhou ZY, None; Ye YT, None; Zhu JT, None; Sun DJ, None; Wang YS, None; Dou GR, None.
REFERENCES
- 1.Bunch KL, Abdelrahman AA, Caldwell RB, Caldwell RW. Novel therapeutics for diabetic retinopathy and diabetic macular edema: a pathophysiologic perspective. Front Physiol. 2022;13:831616. doi: 10.3389/fphys.2022.831616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haydinger CD, Ferreira LB, Williams KA, Smith JR. Mechanisms of macular edema. Front Med (Lausanne) 2023;10:1128811. doi: 10.3389/fmed.2023.1128811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu ST, Ponugoti A, Deaner JD, Vajzovic L. Update on retinal drug toxicities. Curr Ophthalmol Rep. 2021;9(4):168–177. doi: 10.1007/s40135-021-00277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kvopka M, Smith JR, Koczwara B, Lake SR. Bilateral intermediate uveitis following treatment with paclitaxel in a patient with invasive ductal carcinoma of the breast. Int J Retina Vitreous. 2022;8(1):63. doi: 10.1186/s40942-022-00415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Ma J, Zhong Y. Ocular toxicity induced by tamoxifen: an overview. Zhonghua Yan Ke Za Zhi. 2021;57(3):232–236. doi: 10.3760/cma.j.cn112142-20200324-00221. [DOI] [PubMed] [Google Scholar]
- 6.Tapia Quijada HE, Quijada Fumero E, Mesa Lugo FI, Serrano García M, Betancor Caro N. Nepafenac for cystoid macular oedema secondary to paclitaxel. Arch Soc Esp Oftalmol (Engl Ed) 2021;96(8):434–437. doi: 10.1016/j.oftale.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O'Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 8.Kaya M, Atas F, Gulsum Guc Z, Oztop I, Durak I, Saatci AO. A cross-sectional optical coherence tomography study in patients on taxane-based therapy and a case report with the literature review. Cutan Ocul Toxicol. 2020;39(3):287–293. doi: 10.1080/15569527.2020.1790592. [DOI] [PubMed] [Google Scholar]
- 9.Sodhi M, Yeung SN, Maberley D, Mikelberg F, Etminan M. Risk of ocular adverse events with taxane-based chemotherapy. JAMA Ophthalmol. 2022;140(9):880–884. doi: 10.1001/jamaophthalmol.2022.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignatova I, Frolov R, Nymark S. The retinal pigment epithelium displays electrical excitability and lateral signal spreading. BMC Biol. 2023;21(1):84. doi: 10.1186/s12915-023-01559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirwin SJ, Kanaly ST, Hansen CR, Cairns BJ, Ren MN, Edelman JL. Retinal gene expression and visually evoked behavior in diabetic long Evans rats. Invest Ophthalmol Vis Sci. 2011;52(10):7654–7663. doi: 10.1167/iovs.10-6609. [DOI] [PubMed] [Google Scholar]
- 12.Nakao S, Ikeda Y, Emi Y, Ishibashi T. Possibility of Müller cell dysfunction as the pathogenesis of paclitaxel maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2016;47(1):81–84. doi: 10.3928/23258160-20151214-14. [DOI] [PubMed] [Google Scholar]
- 13.Telander DG, Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22(3):151–153. doi: 10.1080/08820530701457373. [DOI] [PubMed] [Google Scholar]
- 14.Yamane H, Itagaki T, Kajitani K, Koura Y, Kawabuchi Y, Ohara M. Cystoid macular edema following treatment with nanoparticle albumin-bound paclitaxel and atezolizumab for metastatic breast cancer. Case Rep Oncol. 2023;16(1):1121–1128. doi: 10.1159/000533999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu FF, Xu T, Peng SM, Adelman RA, Rizzolo LJ. Claudins regulate gene and protein expression of the retinal pigment epithelium independent of their association with tight junctions. Exp Eye Res. 2020;198:108157. doi: 10.1016/j.exer.2020.108157. [DOI] [PubMed] [Google Scholar]
- 16.Otsubo M, Kinouchi R, Kamiya T, Yoshida A. Regression of taxane-related cystoid macular edema after topical dorzolamide treatment: two case reports. J Med Case Rep. 2021;15(1):355. doi: 10.1186/s13256-021-02954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dvoriashyna M, Foss AJE, Gaffney EA, Repetto R. Fluid and solute transport across the retinal pigment epithelium: a theoretical model. J R Soc Interface. 2020;17(163):20190735. doi: 10.1098/rsif.2019.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagelhus EA, Mathiisen TM, Bateman AC, Haug FM, Ottersen OP, Grubb JH, Waheed A, Sly WS. Carbonic anhydrase XIV is enriched in specific membrane domains of retinal pigment epithelium, Muller cells, and astrocytes. Proc Natl Acad Sci U S A. 2005;102(22):8030–8035. doi: 10.1073/pnas.0503021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda Y, Yoshida N, Notomi S, Murakami Y, Hisatomi T, Enaida H, Ishibashi T. Therapeutic effect of prolonged treatment with topical dorzolamide for cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2013;97(9):1187–1191. doi: 10.1136/bjophthalmol-2012-303005. [DOI] [PubMed] [Google Scholar]
- 20.Marmor MF, Maack T. Enhancement of retinal adhesion and subretinal fluid resorption by acetazolamide. Invest Ophthalmol Vis Sci. 1982;23(1):121–124. [PubMed] [Google Scholar]
- 21.Burgos-Blasco B, Hernandez-Ruiz S, Lopez-Guajardo L, Donate-Lopez J. Dexamethasone intravitreal implant in cystoid macular edema secondary to paclitaxel therapy. Am J Ophthalmol Case Rep. 2020;18:100653. doi: 10.1016/j.ajoc.2020.100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker FE. Paclitaxel (TAXOL): side effects and patient education issues. Semin Oncol Nurs. 1993;9(4 Suppl 2):6–10. doi: 10.1016/s0749-2081(16)30036-5. [DOI] [PubMed] [Google Scholar]
- 23.Chow LWC, Biganzoli L, Leo AD, Kuroi K, Han HS, Patel J, Huang CS, Lu YS, Zhu L, Chow CYC, Loo WTY, Glück S, Toi M. Toxicity profile differences of adjuvant docetaxel/cyclophosphamide (TC) between Asian and Caucasian breast cancer patients. Asia Pac J Clin Oncol. 2017;13(6):372–378. doi: 10.1111/ajco.12682. [DOI] [PubMed] [Google Scholar]