Abstract
One of the most prevalent disorders that cause blindness worldwide is cataract, and its essence is the visual disorder caused by the opacity of the lens. The significant degree of variation in cataracts and the fact that a variety of factors can impact a patient's lens transparency make it especially crucial to investigate the pathogenesis of cataracts at the molecular level. It has been found that more than 60 genes are linked to the formation of cataracts, and the construction of a transgenic mouse model of cataract similar to the selection of human lens clouding due to a variety of causes has become an important means of studying the pathogenesis of cataract. Therefore, the research on the application of transgenic mice to the molecular pathogenesis of cataracts will be the main topic of this review of the literature.
Keywords: transgenic mice, cataract, lens
INTRODUCTION
Transgenic animals are biologically engineered to integrate exogenous genes into the genome of the recipient animal, and the alien genes multiply during cell division and have the potential to be permanently passed down to the progeny, enabling the animal to express a certain trait or show a particular appearance[1]–[2]. Genomic analysis data showed that the mouse and human genomes were highly homologous, with a genetic similarity of 95%. The developmental process and physiological and biochemical reactions are basically the same, and the metabolic changes to the environment and drugs are also very similar. Therefore, mice were mostly selected for scientific research in animal models related to human diseases. Cataract is currently the most common blinding disease in the world[3]. Its essence is visual impairment caused by lens opacification.
Thus, by means of comprehensive investigation and clarification of certain molecular pathways involved in the development and incidence of cataracts, to avoid, postpone, or even reverse lens clouding, it is possible to identify the etiology and take early intervention measures, which is an important theoretical basis and practical significance to maximize the protection of the patient's visual function, improve the quality of life, and reduce the burden on the patients and society. Further comprehensive investigations on the molecular etiology of cataracts are necessary because of the multitude of elements that might impact a patient's lens transparency and the great degree of heterogeneity displayed by cataracts. Transgenic mice have been developed as an animal model of cataract disease, which makes it easier to obtain cataract-symptomatic material that is difficult to access in the patient's eye and to quickly replicate the necessary number of samples for research[4]. This offers a dependable method and instrument for researchers to investigate the molecular pathogenesis of cataract disease. Consequently, the review of the most recent studies on the application of transgenic mice in cataract research will be the focus of this literature.
MATERIALS AND METHODS
An extensive literature search and desk review was conducted by searching databases [Google Scholar, PubMed, and The Mouse Genome Database (MGD)], using combinations of keywords to search in databases, using a combination of the following: [(“cataract” or “lens opacity”) and (“cataract” or “mice”) and (“knock in”, “knockout”, or “transgenic mice”)]. English full-text studies from 1987 to 2023 were included to answer the review question on the application of transgenic mice in the molecular pathogenesis of cataract.
RESULTS
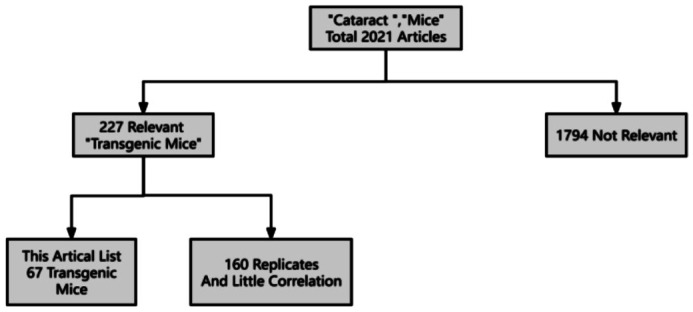
The search results were 2021 articles published in English between 1987 and 2024, and three independent assessors thoroughly reviewed all abstracts. Only 227 of the abstracts were relevant to the topic. Full English versions of these reports were downloaded and included to draw conclusions. The same approach is depicted in the flow chart (Figure 1). After repeated screening, sorting out the titles and abstracts, and deleting 160 articles that were repetitive or had little relevance, we finally obtained 67 articles closely related to the molecular pathogenesis of cataract and transgenic mice. The literature search results were shown in Tables 1[5]–[42] and 2[43]–[97].
Figure 1. Methodology for selecting articles.
Table 1. Transgenic mice in congenital cataract.
| Gene | Gene action | Mice model | Eye phenotype | References |
| Crygc | Crystallins constitute the major proteins of vertebrate eye lens and maintain the transparency and refractive index of the lens. | Crygc -/- | 1/3 of newborn mice were cured of cataract symptoms after injecting sgRNA and Cas9 directly into fertilized eggs of heterozygotes. | [5] |
| Crybb2 | Maintain the transparency and refractive index of the lens. | βB2-W151C KI mice | At 2 to 3 months of age, lens opacity rapidly progressed to complete cataracts. Additionally, multilayered LECs plaques developed beneath the lens anterior capsule in homozygous mice at 3 months of age, and severe fibrosis was observed in the whole lens capsule at 9 months of age. | [6] |
| Mip | Mip's dual roles in cell adhesion and water transport work in concert to determine the lens's biomechanical characteristics and refractive index gradient, which include its precise focus and reflection control. | Mip +/- | The lenses of heterozygous mice (Mip+/-) were clear and did not develop clouding until 24 weeks of age, Mip+/- mice have significantly reduced lens regulation, or focusing capacity. | [7] |
| AQP5 | Aquaporin 5 plays a role in the generation of saliva, tears and pulmonary secretions. | AQP5 -/- | The lens of AQP5-KO mice showed mild opacity at approximately six months of age. | [8] |
| Lim 2 | It acts as a receptor for calmodulin, and may play an important role in both lens development and cataractogenesis. | Lim2 Gt/Gt | Homozygous Lim2 gene-trap lenses (Lim2Gt/Gt) consistently developed faint, central pulverulent cataracts. | [9] |
| Nectin-3 | This gene plays a role in ocular development involving the ciliary body. Mutations in this gene are believed to result in congenital ocular defects. | Nectin-3 KO mice | Nectin-3 KO mouse lenses exhibited small, cleft-like separations between LECs, which were about to differentiate into fibroblasts, and adjacent central cortical as well as peripheral cortical fibroblasts. | [10] |
| NrCAM | This ankyrin-binding protein is involved in neuron-neuron adhesion and promotes directional signaling during axonal cone growth. | NrCAM KO mice | Cataractogenesis results from disorganization of lens fibers, disintegration of cells, and accumulation of cellular debris in NrCAM KO mice. | [11] |
| JAM-C | A mutation in an intron of this gene is associated with hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts | JAMC -/- | JAM-C KO mice exhibit nuclear cataracts, abnormal lens morphology, and defective degradation of lens fiber cell nuclei and organelles. | [12]–[15] |
| Mfn2 | This gene encodes a mitochondrial membrane protein that participates in mitochondrial fusion and contributes to the maintenance and operation of the mitochondrial network. | Mfn2 CKO mice | The lenses of Mfn2 CKO mice were smaller after birth, with slight opacities and central cortical vacuoles. The size of lens opacities and cortical vacuoles became more severe with increasing age. | [16] |
| Rest | This gene was initially identified as a transcriptional repressor that represses neuronal genes in non-neuronal tissues. | Rest KO mice | 8wk after birth, it was discovered that the rest of the KO mice's lenses clouded severely and had a decreased number of LECs, along with vacuoles forming in them. | [17] |
| c-MAF | This protein plays a role in the regulation of several cellular processes, including embryonic lens fiber cell development, increased T-cell susceptibility to apoptosis, and chondrocyte terminal differentiation. | c-MafΔTAM KO mice | In the tamoxifen-induced c-Maf KO mice,after the injection of tamoxifen, the adult c-Maf mice successfully deleted the c-MafΔTAM protein and presented severe cataracts. The adult c-Maf ΔTAM mice demonstrated abnormal lens structure and impaired differentiation of lens fiber cells. | [18] |
| Hsf4 | HSF4 stimulates the production of genes encoding γ-crystallin and is linked to lens development and differentiation. | Hsf4 -/- | 15d postnatal, Hsf4-/- mice had sparse lens fibers and downregulated multiple cataract-related genes in the lens, including Crygs and lens-specific Bfsp1 and Bfsp2. | [19] |
| Six5 | The protein encoded by this gene is a homeodomain-containing transcription factor that appears to function in the regulation of organogenesis. | Six5 +/- | Six5+/- mice were shown to acquire nuclear cataracts at 3wk, which progressively extended to the lens cortex by 8wk. | [20] |
| TGF-β | This gene encodes a secreted ligand of the TGF-β superfamily of proteins. Ligands of this family bind various TGF-β receptors leading to recruitment and activation of SMAD family transcription factors that regulate gene expression. | TGF-β CKO mice | About 50% of CKO mice develop monocular or binocular cataracts at the age of 6mo. At the same time, the development of cataracts observed in CKO mice after chronic retinal detachment is consistent with the observations in patients with retinal detachment. | [21]–[24] |
| Efna5 | Ephrin-A5, a member of the ephrin gene family, prevents axon bundling in cocultures of cortical neurons with astrocytes, a model of late stage nervous system development and differentiation. | Efna5 KO mice | 87% of mice lacking Ephrin-a5 had cataracts and that the lens fiber cells of these mice had a round or irregular cross-section, in contrast to the hexagonal cells found in mice of the wild type. | [25] |
| Gpx-1 | The protein encoded by this gene belongs to the glutathione peroxidase family, members of which catalyze the reduction of organic hydroperoxides and hydrogen peroxide (H2O2) by glutathione, and thereby protect cells against oxidative damage. | Gpx-1 KO mice | Gpx-1 null mice acquire lamellar cataracts very early after birth, which progress to mature cataracts at about 15 months of age. | [26] |
| HTRA1 | This gene encodes a member of the trypsin family of serine proteases. This protein is a secreted enzyme that is proposed to regulate the availability of IGFs by cleaving IGF-binding proteins. It has also been suggested to be a regulator of cell growth. | HTRA1 KO mice | LECs proliferated abnormally and arranged erratically in transgenic mice with loss of HTRA1. | [27] |
| P16INK4a | P16INK4a, also known as p16, is a cyclin-dependent kinase inhibitor that inhibits CDK4/6 to prevent the activation of E2F transcription factors, hence negatively regulating normal cell growth. | INK4a -/- | INK4a-/- mice were found to have unilateral or bilateral cataracts (65 out of 69), and ectopic expression of p16INK4a was found to induce gamma F-crystallin. | [28] |
| Nbn | Mutations in this gene are associated with Nijmegen breakage syndrome, an autosomal recessive chromosomal instability syndrome characterized by microcephaly, growth retardation, immunodeficiency, and cancer predisposition. | Nbn KO mice | Rly-onset cataract development in Nbn-deficient mice is accompanied by partial denucleation of fiber cells and structural disruption of the lens epithelium and fiber cells. | [29] |
| Smad4 | This gene encodes a member of the Smad family of signal transduction proteins. Smad proteins are phosphorylated and activated by transmembrane serine-threonine receptor kinases in response to TGF-β signaling. | Smad4 CKO mice | In the mice's cornea caused cataracts with corneal dysplasia, iridocorneal angle closure, and corneal adhesions. In the trabecular meshwork region of Smad4 KO mouse eyes, it was found that E- and N-cadherin expression was significantly lower. | [30] |
| Lgr4/gpr48 | The protein encoded by this gene is a G-protein coupled receptor that binds R-spondins and activates the Wnt signaling pathway. | Gpr48 -/- | Gpr48-/- mice exhibit an imbalance in iris myogenesis and its intraocular extracellular matrix homeostasis during the early stages of postnatal eye development, while 6-month-old Gpr48-/- mice with glaucoma and a thinning inner nuclear layer are found to have lost ganglion cells. | [31] |
| Tdrd7 | The protein encoded by this gene belongs to the Tudor family of proteins. This protein contains conserved Tudor domains and LOTUS domains. It is a component of RNA granules, which function in RNA processing. | Tdrd7 KO mice | Tdrd7 KO mice have heterozygous cataracts, glaucoma, and spermatogenic stoppage. | [32] |
| TRPM3 | The product of this gene belongs to the family of TRP channels. TRP channels are cation-selective channels important for cellular calcium signaling and homeostasis. | Trpm3+/-, Trpm3-M/- | Trpm3-M/M- mutants developed severe progressive anterior cone-like cataracts with microphthalmia. In contrast, anterior cone cataracts with lenses consistent with the Trpm3+/- phenotype form in heterozygous Trpm3-I/M and hemizygous Trpm3-M/- mutants. | [33] |
| FYCO1 | The gene encodes a Rab7 adapter protein that is implicated in the microtubule transport of autophagosomes. | fyco1 −/− | fyco1−/− mice exhibit initial signs of lens opacity at 4wk, develop into mild cataracts at around 8wk, and mature into cataracts at 16wk. | [34]–[36] |
| HIV-1 | HIV-1 are at risk of several intraocular diseases, the most common complication being retinal microvascular lesions, which cause retinal hemorrhage and infarction of the nerve fiber layer, forming cotton wool spots, and may also present with lens opacity. | HIV transgenic mice | In HIV transgenic mice, cataracts originated from the nucleus of the lens, subsequently evolving into cortical cataracts accompanied by uveitis. Simultaneously, LECs and fibrocytes expressed the HIV gpl20 protein, along with enhanced apoptosis of LECs and fibrocytes. | [37] |
| LAT1, TAT1 | Enables dihydrolipoyllysine-residue acetyltransferase activity. Involved in acetyl-CoA biosynthetic process from pyruvate. Located in mitochondrion. | LAT1 KO mice, TAT1 KO mice | The deletion of LAT2 results in a higher incidence of cataracts in mice, especially in aged female mice, and a synergistic effect is observed in the absence of TAT1. | [38] |
| Agps | This gene is a member of the FAD-binding oxidoreductase/transferase type 4 family. It encodes a protein that catalyzes the second step of ether lipid biosynthesis in which acyl-DHAP is converted to alkyl-DHAP by the addition of a long chain alcohol and the removal of a long-chain acid anion. | Agps KO mice | Agps KO mice presented with mild bilateral nuclear cataracts, with the lens exhibiting abnormality at 21d after birth, characterized by swelling of epithelial cells in the equatorial zone. Severe cataracts developed rapidly at 28d, accompanied by severe destruction of lens fibroblasts at 4mo. | [39] |
| MiRNA | This gene plays a well-recognized role in post-transcriptional gene regulation and are involved in lens development and pathogenesis. | miR-26 TKO | miR-26TKO develop postnatal cataracts as early as 4–6wk of age. | [40] |
| Chmp4b | This gene encodes a member of the chromatin-modifying protein/CHMP protein family. The protein is part of the ESCRT complex III (ESCRT-III), which functions in the sorting of endocytosed cell-surface receptors into multivesicular endosomes. | Chmp4b-CKO | Severe lens degeneration in Chmp4b-CKO mice leads to an immune cell response in the eye, and conditional deletion of Chmp4b leads to stasis of lens growth and differentiation. | [41] |
| S100A4 | The protein encoded by this gene is a member of the S100 family of proteins containing 2 EF-hand calcium-binding motifs. S100 proteins are localized in the cytoplasm and/or nucleus of a wide range of cells, and involved in the regulation of a number of cellular processes such as cell cycle progression and differentiation. | S100A4 -/- | S100A4-/- KO mice develop delayed cortical cataracts. | [42] |
Gene function searches were obtained from National Center for Biotechnology Information (NCBI). Crybb2 appears in both tables, Tables 1 and 2, because the transgenic mice with this gene have different types of cataracts in different studies, so they are listed separately. KO: Knockout; CKO: Conditional knockout; Crygs: γs-crystallin; Bfsp1, Bfsp2: Beaded filament proteins 1 and 2; TGF-β: Transforming growth factor-beta; IGF: Insulin-like growth factor; TRP: Transient receptor potential; DHAP: Dihydroxyacetonephosphate; CHMP: Charged multivesicular body protein; ESCRT: Endosomal sorting complex required for transport; LEC: Lens epithelial cell.
Table 2. The application of transgenic mice in acquired cataract.
| Gene | Gene action | Mice model | Eye phenotype | References |
| Vimentin | Vimentin is a type III intermediate filament protein that mostly undergoes mitotic phosphorylation. | VIM SA/SA | In vimentin KI mice in pure animals (VIMSA/SA). Disordered VIMSA/SA lens fiber membranes that resembled senile cataracts were seen under electron microscopy, along with a decrease in the number of LECs. | [43] |
| TTase | This is a crucial enzyme that maintains the lens's redox balance and guards against oxidative damage. | TTase KO mice | Cataracts in TTase KO mice appeared as early as 4 months of age and were most noticeable at 9mo. | [44] |
| Bin3 | BAR domain proteins have been implicated in endocytosis, intracellular transport, and a diverse set of other processes. | Bin3 -/- | Homozygous loss of Bin3 causes cataracts and an increased susceptibility to lymphomas during aging. The cataract phenotype was marked by multiple morphologic defects in lens fibers, including the development of vacuoles in cortical fibers and a near total loss of F-actin in lens fiber cells but not epithelial cells. | [46] |
| Clock | The protein encoded by this gene plays a central role in the regulation of circadian rhythms. | Clock -/- | Clock-/- mice live 15% less time on average than wild type mice. Additionally, compared to wild-type mice, wild-type mice showed a greater prevalence of cataracts and skin irritation. | [47] |
| Cryaa | Mammalian lens crystallins are divided into α and β γ families. They hold them in large soluble aggregates. Post-translational modifications decrease the ability to chaperone. | Cryaa -/- | Cryaa-/- gene-deficient mice's lens nuclei appeared hazy and that the cloudiness increased with age. The existence of dense inclusion bodies was demonstrated by the results of transmission electron microscopy and light microscopy in the lens fiber cells of Cryaa mutant mice. | [48] |
| Crybb2 | Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. | Crybb2 KO mice | In Crybb2 KO mice, the lens's diameter dramatically shrank with age, cataracts developed just a few months after birth, and the condition progressively got worse. | [45],[49] |
| Cryab | Alpha-A is preferentially restricted to the lens and alpha-B is expressed widely in many tissues and organs. Elevated expression of alpha-B crystallin occurs in many neurological diseases; a missense mutation cosegregated in a family with a desmin-related myopathy. Alternative splicing results in multiple transcript variants. | Cryab-R120G KI mice | Cryab-R120G KI animals show increased molecular mass, light scattering, protein aggregation, and insolubility in crystallins isolated from their lenses, and these mice develop cataracts at an early age. | [50]–[51] |
| Bfsp2 | More than 99% of the vertebrate ocular lens is comprised of terminally differentiated lens fiber cells. Two lens-specific intermediate filament-like proteins, the protein product of this gene (phakinin), and filensin, are expressed only after fiber cell differentiation has begun. | Bfsp2 KO mice | In mutant mice are slowed down after 5 weeks of age when CP49 and filensin are expressed in the lens epithelium. CP49 and filenin were also found to be necessary indicators of the differentiation of lens epithelial and fibroblast in mice. | [52] |
| Gja3 | The protein encoded by this gene is a connexin and is a component of lens fiber gap junctions. Defects in this gene are a cause of zonular pulverulent cataract type 3. | Gja3 KO mice | Gja3-deficient heterozygous mice develop nuclear cataracts | [53] |
| Gja8 | This gene encodes a transmembrane connexin protein that is necessary for lens growth and maturation of lens fiber cells. Mutations in this gene have been associated with zonular pulverulent cataracts, nuclear progressive cataracts, and cataract-microcornea syndrome. | Gja3 KO mice | In mice lacking the Gja8 gene was discovered. | [54] |
| Dnase2b | Enables endodeoxyribonuclease activity. Predicted to be involved in apoptotic DNA fragmentation. Located in cytoplasm and extracellular region. Is expressed in ganglia; medulla oblongata basal plate mantle layer; and ventral grey horn. Used to study cataract. | Dnase2b KO mice | Nuclear cataracts were also present in the lens of this mouse. | [55] |
| Epha2 | This gene belongs to the ephrin receptor subfamily of the protein-tyrosine kinase family. EPH and EPH-related receptors have been implicated in mediating developmental events, particularly in the nervous system. Mutations in this gene are the cause of certain genetically-related cataract disorders. | Epha2+/+, Epha2+/-, and Epha2-/- | Epha2-/- mice developed severe cortical cataract by 38wk and Epha2+/- mice exhibited mild cortical cataract up to 64 weeks of age. Progression of cataract in Epha2-/- and Epha2+/- female mice on C57BL/6J and mixed background, respectively, was slower than in matched male mice. | [56] |
| Sparc | This gene encodes a cysteine-rich acidic matrix-associated protein. The encoded protein is required for the collagen in bone to become calcified but is also involved in extracellular matrix synthesis and promotion of changes to cell shape. | Sparc KO mice | Cortical cataracts were discovered in Sparc KO mice at a time of one to two months, and further problems, including iris coloration and anterior chamber displacement, were noted at four months | [57] |
| Lctl | This gene encodes a member of family 1 glycosidases. Glycosidases are enzymes that hydrolyze glycosidic bonds and are classified into families based on primary amino acid sequence. | Lctl KO mice | When Lctl KO mice were young, their lens appeared normal on the outside but had aberrant regulation. As they grew older, they developed cortical cataracts. | [58] |
| Aldhla1, Aldh3a1 | It is thought to promote resistance to UV and 4-hydroxy-2-nonenal-induced oxidative damage in the cornea. | Aldh3a1-/-/Aldh1a1-/- | The Aldh1a1/Aldh3a1 and Aldh3a1-null mice develop cataracts in the anterior and posterior subcapsular regions as well as punctate opacities in the cortex by 1 month of age. The Aldh1a1-null mice also develop cataracts later in life (6–9 months of age). | [59] |
| GCLc | Glutamate-cysteine ligase, also known as gamma-glutamylcysteine synthetase is the first rate-limiting enzyme of glutathione synthesis. Mutations at this locus have been associated with hemolytic anemia due to deficiency of gamma-glutamylcysteine synthetase and susceptibility to myocardial infarction. | HET-LEGS KO mice | HET-LEGS KO mice developed nuclear opacity at 4 months of age and severe nuclear cataracts at 9 months of age. | [60] |
| HO-1 | The Nrf-2/HO-1/CO axis protects LECs from oxidant and endoplasmic reticulum stress damage. | TgHO-1 G143H transgenic FVB/N mice | Nuclear cataracts were discovered to develop in transgenic FVB/N mice overexpressing the negative dominant mutant HO-1 G143H at 4mo, 5mo earlier than in control mice. | [61] |
| hSVCT2 | hSVCT2 is a sodium-dependent vitamin C transporter | hSVCT2 overexpressing transgenic mice | The vitamin C content in the lenses of hSVCT2 overexpressing transgenic mice rises, particularly in the oxidative form, dehydroascorbic acid, leading to the development of cataracts at 12 months of age. | [62]–[63] |
| Nrf2 | This gene encodes a transcription factor which is a member of a small family of bZIP proteins. Many of these genes encode proteins involved in response to injury and inflammation which includes the production of free radicals. | Nrf2 -/- | Nrf2-/- mice exhibited notable turbidity between the ages of 11 and 15mo, giving rise to advanced cortical, posterior subcapsular, anterior subcapsular, and nuclear cataracts. | [64] |
| Sod2 | SOD exhibits a critical function in safeguarding cells against oxidative stress by catalyzing the disintegration of superoxide anions into hydrogen peroxide. | Sod2 +/- | The increment of age in Sod2+/- and WT mice, the levels of CML and pentoside in the skin collagen escalate, accompanied by an increase in cataract formation. | [65] |
| xCT | The cystine/glutamate antiporter (system xc-) is tasked with mediating the intake of extracellular cystine, while exchanging intracellular glutamate. | xCT KO mice | 3-month-old wild-type mice and xCT KO mice lenses exhibited an anterior localized cataract. The frequency of this cataract significantly increased in the KO mice compared to the wild-type mice. | [66]–[77] |
| ER | ER, which belongs to the nuclear receptor superfamily, is a ligand-dependent transcription factor. At present, two ER subtypes, namely, ERα and ERβ, are known to exist. | ER∆3 transgenic mice | In ER∆3 transgenic mice, cortical cataracts spontaneously form in ER∆3 females after puberty and progress with age. The cataracts originate from the equatorial region of the lens, where epithelial cells differentiate into elongated fibroblast-like cells. | [78] |
| Yap1 | This gene encodes a downstream nuclear effector of the Hippo signaling pathway which is involved in development, growth, repair, and homeostasis. This gene is known to play a role in the development and progression of multiple cancers as a transcriptional regulator of this signaling pathway and may function as a potential target for cancer treatment. | Yap1 +/- | Heterozygous inactivation of Yap1 in mice gives rise to cataracts with LEC phenotypic flaws in adulthood. Despite the normal early development of the eye, including the lens, most Yap1 heterozygotes develop cataracts within the first 6mo after birth. | [79] |
| GPX1 | The protein encoded by this gene belongs to the glutathione peroxidase family, members of which catalyze the reduction of organic hydroperoxides and hydrogen peroxide (H2O2) by glutathione, and thereby protect cells against oxidative damage. | GPX1 -/- | At the age of nine months, GPX1-/- mice began to present abnormal optical aberration and loss of transparency. At the age of 12mo, compared with CAT and WT, the GPX1 lens showed significant turbidity and abnormal optical aberration; these aberrations gradually increased with age and developed into cataracts at 24 months of age. | [80] |
| Sep15 | It is identified as a resident of the endoplasmic reticulum and is differentially regulated by both adaptive and acute endoplasmic reticulum stress | Sep15 KO mice | The Sep15 KO mice presented with evident nuclear cataracts in the early stages. | [81]–[84] |
| Dcoh | The HNF1 transcriptional activator family's Dcoh bifunctional protein, also known as the dimerizing co-factor for HNF1, works with HNF1 to stabilize the dimeric HNF1 complex. Tetrahydrobiopterin is catalyzed by aromatic amino acid hydroxylase, which has Dcoh as a co-factor. | Dcoh KO mice | The majority of affected mice develop cataracts by 24wk postnatal, Dcoh KO animals exhibit a minor reduction in HNF1 activity and a degree of glucose tolerance in comparison to diabetic mice, in the development of cataracts | [85] |
| Galk1 | Galactokinase is a major enzyme for the metabolism of galactose and its deficiency causes congenital cataracts during infancy and presenile cataracts in the adult population. | Galk1 KO mice | The mice lacking the gene Galk1 had impaired metabolism of galactose and accumulated galactose and galactic in their bodies. Mice lacking the Galk1 gene do not acquire cataracts following a diet high in galactose. | [86] |
| PLD | Enables phospholipase D activity. Involved in several processes, including gastrulation involving germ band extension; phototransduction; and positive regulation of Golgi vesicle fusion to target membrane. Located in cytoplasmic vesicle. | PLD2 overexpressing transgenic mice | In the presence of transgenic aldose reductase overexpression, the lens osmotic pressure of transgenic mice overexpressing PLD2 increases. These double transgenic mice develop more severe congenital cataracts and are prone to diabetic cataracts. | [87] |
| Grx2 | Enables disulfide oxidoreductase activity; glutathione peroxidase activity; and glutathione transferase activity. Involved in cellular response to oxidative stress and glutathione metabolic process. Located in cytosol and mitochondrion. | Grx2 KO mice | Grx2 KO mice were shown to have greater lens nuclear density, increased turbidity, and an earlier onset of lens turbidity at week 1 following UVB irradiation than WT mice. | [88] |
| Atm | The protein encoded by this gene belongs to the PI3/PI4-kinase family. This protein is an important cell cycle checkpoint kinase that phosphorylates. This protein and the closely related kinase ATR are thought to be master controllers of cell cycle checkpoint signaling pathways that are required for cell response to DNA damage and for genome stability. | Atm -/- | Pure mice with the Atm gene deleted have been shown to exhibit cloudiness in their lenses when exposed to various X-rays. | [89] |
| Eaf2 | Enables transcription elongation regulator activity. Involved in positive regulation of transcription by RNA polymerase II and regulation of transcription elongation from RNA polymerase II promoter. Part of transcription elongation factor complex. | Eaf2 KO mice | Eaf2 KO can reduce UV-induced apoptosis in crystalline lenses and mitigate the formation of cataracts. | [90] |
| SMP30 | The protein encoded by this gene is a highly conserved, calcium-binding protein, that is preferentially expressed in the liver and kidney. | SMP30 KO VC(-), SMP30 KO VC(+) | In the SMP30 KO VC(-) group, UVR-B-induced lens opacity is more extensive than in the SMP30 KO VC(+) group or in the WT group. | [91] |
| HPV16-E6 | Human papillomavirus type 16 | K14E6 mice | K14E6 mice is 100%, presenting as tissue loss, cortical liquefaction, and an increase in the number of hyperproliferative nuclear cells with mesenchymal-like characteristics in the lens. | [92]–[94] |
| Smad3 | The SMAD3 protein functions in the TGF-β signaling pathway, and transmits signals from the cell surface to the nucleus, regulating gene activity and cell proliferation. | Smad3 -/- | Compared with WT mice, the expression of the myofibroblast marker EMT marker α-SMA in Smad3-KO mice is significantly decreased. The proportion of apoptotic nuclei in the Smad3-/- lens is significantly higher than that in the Smad3+/+. | [95]–[96] |
| Grx2 | Enables disulfide oxidoreductase activity; glutathione peroxidase activity; and glutathione transferase activity. Involved in cellular response to oxidative stress and glutathione metabolic process. Located in cytosol and mitochondrion. | Grx2 KO mice | The Grx2 KI group showed lower expression of ILK and EMT markers than the wild-type group, whereas the Grx2 KO group showed higher expression of these markers. | [97] |
Gene function searches were obtained from National Center for Biotechnology Information (NCBI). Grx2 appears twice in the table, because Grx2 KO mice had different types of cataract in these two studies, so they are listed separately. Crybb2 appears in both Tables 1 and 2, because the transgenic mice with this gene have different types of cataracts in different studies, so they are listed separately. KI: Knock-in; KO: Knockout; WT: Wild-type; TGF-β: Transforming growth factor-beta; Nrf-2: Nuclear factor erythroid 2-like-2; HO-1: Heme oxygenase-1; CO: Carbon monoxide; LEC: Lens epithelial cell; hSVCT2: Na(+)/L-ascorbic acid transporter 2; bZIP: Basic leucine zipper; SOD: Superoxide dismutase; ER: Estrogen receptor; HNF1: Hepatocyte nuclear factor 1.
Construction Methods of Transgenic Mice
In the past, the majority of gene research involved inserting particular exogenous genes into prokaryotic or eukaryotic cells. However, since a single cell cannot accurately represent the function of a gene in a living organism, the problem was precisely resolved by creating an experimental model of transgenic mice. The process of introducing an exogenous gene into an animal's genome and analyzing it along with the transgenic animal's phenotypic to determine the gene's function is known as transgenics. Due to the short gestation period and inexpensive and easy reproduction of mice[98], as well as the fact that they can be easily bred to produce inbred variants, all of these contribute to obtaining more valid research results. In addition, the combination of the already developed technological means of genetic engineering modification could allow the establishment of transgenic mice with less time and effort than in other mammals.
Microinjection is the earliest method used for the preparation of transgenic animals, in which an exogenous gene fragment is introduced directly into prokaryotic-stage embryos or cultured cells using micromanipulation techniques, thus integrating the exogenous gene into the chromosome of the host[99]. The benefits of this approach include no requirement for a vector, ease of use, high gene integration efficiency, transgene capacity of up to 100 KB, and a shorter test duration. The drawbacks include the more challenging technical process, the more erratic integration site, the possibility of multi-copy insertion forming a continuous body, and the restriction of the insertion site, which may result in positional effects like gene silencing and changed gene expression levels, which may cause mice to die. As preparation methods have continued to advance, other approaches have progressively surfaced. These include sperm carrier[100], retrovirus infection[101], embryonic stem cell-mediated approach[102], somatic cell nuclear transplantation[103], and electroplating[104], all of which share issues with microinjection.
The advent of genome-targeted editing technologies has effectively addressed the problem of correct gene expression. The most popular genome-targeting editing technique is the clustered regularly interspaced short palindromic repeats nuclease system, also known as CRISPR/Cas9 or clustered regularly interspaced short palindromic repeats/Cas9. It is derived from the immune systems of bacteria and archaea and is mainly composed of the specific nucleic acid endonuclease Cas9 and the guide RNA, also known as single guide RNA, or sgRNA[105]. The nuclease Cas9 is directed to cleave the target sequences by specific sgRNAs that are artificially engineered to integrate exogenous target genes. These sgRNAs identify target sequences in the genome based on the complementary base pairing principle. Once the target DNA double strand is broken, homologous recombination and non-homologous end joining can be used to target genome editing in eukaryotic cells. Target gene expression may be accurately determined with this RNA-DNA interaction-based technique; however, the design and construction of sgRNA present significant challenges as well as extensive and expensive development times. Targeted genome editing is commonly achieved by the use of transcription activator-like effector nucleases (TALENs)[106], zinc finger nucleases (ZFNs)[107], gene targeting technology[108], RNA interference technology[109], and other techniques. Although these methods can realize high-precision gene expression, they also suffer from the problems of high technological difficulty and high cost.
Application of Transgenic Mice to the Molecular Pathogenesis of Cataract
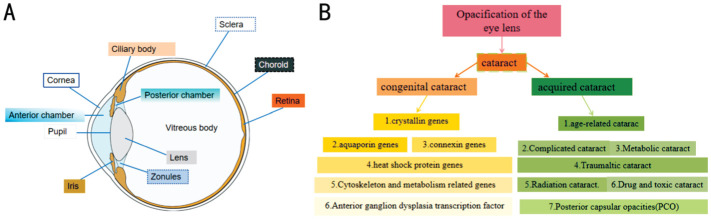
Cataracts are categorized into congenital cataract and acquired cataract (Figure 2), depending on the time of onset. Congenital cataract etiology is largely influenced by genetics,and genes closely associated with it include crystallin genes, connexin genes, aquaporin genes, heat shock protein genes, transcription factors associated with anterior segment dysplasia, and genes related to the cytoskeleton and metabolism[110].
Figure 2. Eye structure and cataract classification.
A: Eye structure; B: Cataract classification.
There are seven forms of acquired cataracts[111], which are defined as the clouding of the lens resulting from systemic or localized ophthalmopathy, nutritional and metabolic problems, toxicity, degeneration, and trauma after birth. 1) The most prevalent type of cataracts, known as senile cataract or age-related cataract, can alternatively be characterized as nuclear, cortical, or posterior capsule subcapsular cataracts based on where the lens clouding occurs. 2) Complicated cataract (caused by additional eye conditions). 3) Traumaltic cataract. 4) Metabolic cataract 5) Radiation cataract. 6) Drug and toxic cataract. 7) Posterior capsular opacities (PCO).
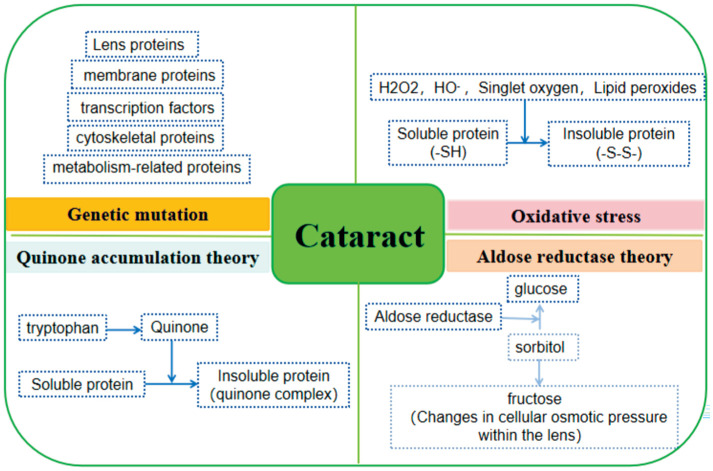
Currently, oxidative stress[112], quinone buildup[113], aberrant sorbitol metabolism[114], and genetic mutations[115] were the four main areas of interest for research on the pathogenesis of cataracts (Figure 3). Surprisingly, research on the molecular pathogenesis of cataracts can uncover the probable cause of the condition as well as its primary molecular targets. It can also examine the function and interactions of genes, proteins, metabolites, and the aforementioned pathogenic factors in the development of cataracts. Consequently, it will offer individual treatment, early diagnosis, and prevention, which holds great significance for the prevention and treatment of cataracts and the progress of ophthalmology. The subsequent discussion focused on the molecular mechanisms underlying both congenital and acquired cataracts in transgenic mice.
Figure 3. Common pathogenesis of cataract.
-SH: Mercapto group; -S-S-: Disulfide linkage.
Transgenic Mice in Congenital Cataract
Crystallin genes
Crystallin coding genes
Crygc
By utilizing CRISPR-Cas9 technology and choosing mice carrying a dominant mutation in the Crystallin, Gamma C (Crygc) gene and discovering that their lens proteins would denature and become foggy, Jinsong Li's lab was able to successfully treat the genetic condition that causes cataracts in mice. After targeting the sgRNA of the mutated Crygc gene, researchers found that 1/3 of newborn mice were cured of cataract symptoms after injecting sgRNA and Cas9 directly into fertilized eggs of heterozygotes. Passing the repaired Crygc gene to the next generation through germ cells resulted in cured mice, suggesting that the cataract genetic disease can be eradicated[5].
βB2-crystallin
One important structural protein that is essential to the development and upkeep of lens transparency is βB2-crystallin (Crybb2). Thus far, approximately 40 mutations in the Crybb2 gene, which codes for congenital cataracts, have been connected to the condition. Previous research has demonstrated that mutations in βb2-crystallin cause structural anomalies in the protein, which in turn cause the protein to become unstable and create precipitates that eventually cause cataract formation. Following the identification of a missense mutation in βB2-W151C in a patient with congenital membranous cataracts, Xiao et al[6] generated βB2-W151C knock-in (KI) mice, which underwent progressive bilateral congenital cataract development. Within two to three months of birth, the clouding of the lens had rapidly progressed into complete cataracts. Additionally, the entire lens capsule exhibited significant fibrosis at nine months of maturity, and at three months of age, purebred mice developed multilayered lens epithelial cell (LECs) plaques behind the anterior lens capsule. The rapid development of congenital cataract is eventually facilitated by endoplasmic reticulum stress (ERS), lysosomal pathways, apoptosis, and fibrosis. Congenital cataracts may benefit from treatment that inhibits the endoplasmic reticulum and lysosomal tissue proteases.
Membrane protein coding genes
Mip
Aquaporin-0 (AQP0) is another name for Mip, which is the primary intrinsic protein of the lens fiber cell plasma membrane. Mip's dual roles in cell adhesion and water transport work in concert to determine the lens's biomechanical characteristics and refractive index gradient, which include its precise focus and reflection control.It was discovered that the lenses of heterozygous mice (Mip+/-) were clear and did not develop clouding until 24 weeks of age, in contrast to the Mip-/- knockout (KO) animals, which developed polymorphic clouding of the lens from three weeks of age. Furthermore, compared to wild-type (WT) mice, Mip+/- mice have significantly reduced lens regulation, or focusing capacity. These findings demonstrated that heterozygous deletion of AQP0 was sufficient to induce cataractogenesis in mice and Mip proteins were required for lens control (focusing ability)[7].
AQP5
LECs and fiber cells both express aquaporin 5 (AQP5), a significant membrane water channel. It was discovered that at about six months of age, mice lacking AQP5 showed a little opacity in their lens[8]. Furthermore, in comparison to WT mice, this mouse exhibited higher lens turbidity and up-regulated expression of the waveform protein vimentin. It was also determined that AQP5 might use miR-124-3p.1 to control vimentin expression and preserve lens transparency.
Lim2
Lens fiber cell plasma membranes encode the second most prevalent integrin, which is called Lim2. It was discovered that lenses with abnormal refractive properties and central pulverulent opacities are produced by Lim2 (Lim2Gt/Gt) functionally impaired animals. Partially resembling those associated with a homozygous missense mutation (F105V) in human LIM218 were the lens opacities in Lim2Gt/Gt mice. Concluding that Lim2 is essential to determining the crystalline lens's accurate internal refractive characteristics[9].
Nectin-3
Nectin-3 is a key gene involved in the Nection protein-mediated cell-cell adhesion mechanism in eye development. The ciliary body and lens of mouse embryos express the nectin-3 protein, which was discovered as a result of chromosome 3 translocation[10]. Compared to control mouse lenses, Nectin-3 KO mouse lenses exhibited small, cleft-like separations between lens epithelial cells (fibroblast precursor cells), which were about to differentiate into fibroblasts, and adjacent central cortical as well as peripheral cortical fibroblasts.
Other plasma membrane related genes
NrCAM
Member of the L1 subgroup's immunoglobulin superfamily, NGCAM-related cell adhesion molecule (NrCAM) interacts with anchor proteins on cells. When fibroblasts produce primarily histological disorganization at later stages of embryonic development, including abnormalities of the cytoskeleton and gap junctional connectivity of gap junction proteins, it was discovered that cataractogenesis results from disorganization of lens fibers, disintegration of cells, and accumulation of cellular debris in NrCAM KO mice[11]. Furthermore, anchor protein b mutant mice's lenses matched NrCAM KO mice's phenotype. These findings demonstrated that anchor proteins b and NrCAM were necessary for the preservation of connections between lens fiber cells.
JAM-C
The immunoglobulin junctional adhesion molecule-C (JAM-C) is a crucial member of the JAMs family, which plays an important role in cell adhesion at junctions. Localized at the cell-cell junction, JAM-C, as a membrane protein, has been found to be associated with inflammation[12], cell polarity[13], and tumor metastasis[14]. The research indicated that JAM-C KO mice exhibit nuclear cataracts, abnormal lens morphology, and defective degradation of lens fiber cell nuclei and organelles[15]. Compared to WT control mice, the expression levels of genes related to ERS and unfolded protein response (UPR), including binding immunoglobulin protein (BiP), the mediator C/EBP homologous protein (CHOP) and F tribbles pseudokinase 3 (TRIB3), were significantly upregulated in the JAMC-/- lens, suggesting that lens developmental abnormalities are accompanied by the activation of the UPR. Additionally, an increase in cell death was also identified in the JAMC-/- lens. The presence of congenital nuclear cataract caused by JAMC deficiency is accompanied by the failure of lens fiber cell nucleus and organelle degradation, as well as lens structure disruption and UPR activation, suggesting that JAM-C is essential for the maintenance of normal lens development, and UPR activation is involved in the formation of cataracts in JAMC-deficient individuals.
Mfn2
Genetic mitochondrial mutations can lead to mitochondrial dysfunction or random oxidative damage. Cumulative mitochondrial damage is an important factor in age-related diseases such as cataracts and macular degeneration. The study found that the lenses of mitofusin-2 (Mfn2) conditional knockout (CKO) mice were smaller after birth, with slight opacities and central cortical vacuoles. The size of lens opacities and cortical vacuoles became more severe with increasing age[16]. Conditional Mfn2 deficiency affects the proliferation, apoptosis, and mitochondrial ultrastructure of LECs. It is concluded that the normal development and transparency of the lens depend on the normal function of the Mfn2 gene.
Transcription factors
Rest
As a negative regulator of neuron-specific genes in non-neuronal cells, the transcriptional repressor RE1 was found to suppress the transcription factor REST. Eight weeks after birth, it was discovered that the rest of the KO mice's lenses clouded severely and had a decreased number of LECs, along with vacuoles forming in them. Additionally, Notch signaling-related genes (also known as rest target genes) had an upregulation in expression following birth, but lens fiber-regulating proteins like Prox1 and c-Maf had a downregulation. Moreover, it was found that multiple genes linked to the Notch signaling pathway, lens differentiation, and lens function are expressed differently in lens precursor cells by rest loss[17]. These changes ultimately contribute to the development of cataracts.
c-MAF
The transcription factor c-MAF is a member of the large MAF family, which has a transactivation domain and a basic leucine zipper (bZIP) domain. c-MAF plays an important role in lens formation, T-lymphocyte differentiation, hypertrophic chondrocyte differentiation, and kidney development in mouse embryos. The research revealed that in the tamoxifen-induced c-Maf KO mice, after the injection of tamoxifen, the adult c-Maf mice successfully deleted the c-MafΔTAM protein and presented severe cataracts[18]. Moreover, the adult c-MafΔTAM mice demonstrated abnormal lens structure and impaired differentiation of lens fiber cells. Eventually, c-Maffl/fl and c-MafΔTAM C57BL/6J mice can be utilized as valuable animal models for investigating the function of c-MAF throughout various developmental stages and as a pathological model for cataracts.
Hsf4
Heat shock transcription factor 4 (HSF4) stimulates the production of genes encoding γ-crystallin and is linked to lens development and differentiation[19] and it revealed that 15d postnatal, Hsf4-/- mice had sparse lens fibers and downregulated multiple cataract-related genes in the lens, including γs-crystallin (Crygs) and lens-specific beaded filament proteins 1 and 2 (Bfsp1 and Bfsp2). Ultimately, it was determined that HSF4 can influence cataract development in three different ways: by suppressing Crygs, by decreasing the expression of lens beaded filaments, and by removing the post-translational modification of αA-crystallin.
Six5
Six5, also known as sine oculis-related homeobox5, is a transcription factor that is expressed in the mouse lens and multiple other tissues. Six5+/- mice were shown to acquire nuclear cataracts at 3wk, which progressively extended to the lens cortex by 8wk[20]. Additionally, it was determined that as Six5 levels decreased, lens clouding incidence and severity rose.
TGF-β
Transforming growth factor-beta (TGF-β) is a kind of multi-functional cytokine that can regulate cell growth, differentiation, and extracellular matrix synthesis[21]–[23]. The study showed that TGF-β-deficient mice start to develop visible cataracts in the early stage after birth, and the development of cataracts gradually became more serious with age[24]. About 50% of CKO mice develop monocular or binocular cataracts at the age of 6mo. At the same time, the development of cataracts observed in CKO mice after chronic retinal detachment is consistent with the observations in patients with retinal detachment.
Enzyme genes
Ephrin-a5
The ephrin receptor tyrosine kinase, which is found on the cell membrane and is crucial for lens growth and differentiation, is ligated by ephrin-a5 (Efna5). It was discovered that 87% of mice lacking Ephrin-a5 had cataracts and that the lens fiber cells of these mice had a round or irregular cross-section, in contrast to the hexagonal cells found in WT mice. This implies that the formation and maintenance of lenses are significantly regulated by Eph receptors and their ligands[25].
Gpx-1
Proteins in the Gpx-1 family are encoded by the glutathione peroxidase 1 (Gpx-1) gene. Gpx-1 members lower H2O2 to shield cells from oxidative damage by using glutathione. It was discovered that the activity of Gpx-1 was reduced in Gpx-1 defective animals compared to normal mice, leaving the lens susceptible to the cytotoxic effects of H2O2. Gpx-1 null mice acquire lamellar cataracts very early after birth, which progress to mature cataracts at about 15 months of age. This is because lipid peroxides are the substrate of Gpx-1, and the knockdown of Gpx-1 limits the breakdown of lipid peroxides, which causes peroxide buildup. This implies that the lens nucleus's antioxidant defense depends in large part on Gpx-1[26].
HTRA1
The enzyme HtrA Serine Peptidase 1 (HTRA1) is a serine protease that inhibits the TGF-β signaling pathway, which is crucial for lens growth. The development of lens fiber cell terminals is thought to require the TGF-β signaling pathway. Furthermore, by interfering with lens formation, TGF-β can lead to pathological alterations in subcapsular clouding of the lens. Research revealedthat posterior subcapsular congenital cataract (PSC) lens tissue exhibited an active TGF-β/Smad signaling pathway and that HTRA1 was dramatically downregulated in the lens capsule of PSC patients as compared to normal controls[27]. LECs proliferated abnormally and arranged erratically in transgenic mice with loss of HTRA1, activating the TGF-β/Smad signaling cascade and dramatically upregulating p-Smad2/3, which ultimately led to PSC.
Cell cycle genes
P16INK4a (p16)
P16INK4a, also known as p16, is a cyclin-dependent kinase inhibitor that inhibits CDK4/6 to prevent the activation of E2F transcription factors, hence negatively regulating normal cell growth. INK4a-/- mice were found to have unilateral or bilateral cataracts (65 out of 69), and ectopic expression of p16INK4a was found to induce gamma F-crystallin. These results implied that the INK4a locus was important for the development of the mouse eye and could offer fresh perspectives on the genetics of the pathogenesis of cataracts in humans[28].
Nbn
Nibrin, often known as NBN, is a part of the MRE11-RAD50-NBN (MRN complex), which is crucial for maintaining chromosomal integrity and responding to DNA damage. Studies have revealed that early-onset cataract development in Nbn-deficient mice is accompanied by partial denucleation of fiber cells and structural disruption of the lens epithelium and fiber cells. Furthermore, several lens protein genes exhibit transcriptional dysregulation in Nbn-deficient animals. These features imply that Nbn participates in the development of lens fiber cell terminals and cataractogenesis[29].
Genes involved in lens development
Smad4
Recombinant mothers against decapentaplegic homolog4 (Smad4) is a member of the SMAD family, located in the nucleus and cytoplasm, and is a component of the heterotrimeric SMAD2/SMAD3-SMAD4 complex, as well as a necessary component of the transforming growth factor-mediated signaling pathway. It was shown that a specific KO of Smad4 in the mice's cornea caused cataracts with corneal dysplasia, iridocorneal angle closure, and corneal adhesions. In the trabecular meshwork region of Smad4 KO mouse eyes, it was found that E- and N-cadherin expression was significantly lower. This suggested that Smad4 was important for eye development and regulates the epithelial-mesenchymal transition, which is one of the possible pathogenetic mechanisms for the abnormalities seen in Peters[30].
Lgr4/gpr48
The G protein-coupled receptor 48 (GPr48/LGR4) belongs to a family of receptors that are involved in the creation of structures in the anterior portion of the eye and are primarily present on cell membranes. Both anterior segment dysplasia (ASD) and cataracts are brought on by disruption of Gpr48. Gpr48-/- mice exhibit an imbalance in iris myogenesis and its intraocular extracellular matrix homeostasis during the early stages of postnatal eye development, while 6-month-old Gpr48-/- mice with glaucoma and a thinning inner nuclear layer are found to have lost ganglion cells[31].
Tdrd7
The RNA-binding protein Tudor domain containing7 (Tdrd7) is expressed in the lens's fiber cells and is located in the structural domain of Tudor. It was discovered that Tdrd7 KO mice have heterozygous cataracts, glaucoma, and spermatogenic stoppage[32]. The ability of Tdrd7 and certain lens mRNAs to form immunoprecipitations is crucial for the post-transcriptional regulation of mRNAs.
Ion channel-related genes
TRPM3
Transient receptor potential cation channel subfamily M member 3 (TRPM3) functions in a range of mammalian cell types as a multimodal calcium sensor. Through comparison of the ocular phenotypes of germline KO mice functionally deficient in TRPM3 (TRPM3-null) and germline KI mice carrying the human cataract-associated TRPM3 isoleucine-methionine mutation (p.I65M, TRPM3 mutant). The study discovered that the purebred Trpm3-M/M mutants developed severe progressive anterior cone-like cataracts with microphthalmia and were linked to the accumulation of Ca2+ phosphate-like deposits, αii-spectrin cleavage products, α-SMA+ve cells, collagen progenitor fibers, and CD68+ve cells[33]. In contrast, anterior cone cataracts with lenses consistent with the Trpm3+/- phenotype form in heterozygous Trpm3-I/M and hemizygous Trpm3-M/- mutants. These cataracts similarly have a delayed onset and slow advancement. Additionally, it is determined that whereas Trpm3 dysfunction causes progressive lens degeneration and calcification along with pro-fibrotic (α-SMA+ve) and immunological (CD68+ve) cellular responses, Trpm3 insufficiency affects lens development but not transparency.
Other Genes
FYCO1
The FYCO1 protein is encoded by the FYVE and coiled-coil domain containing 1 (FYCO1) gene[34]–[35]. As an autophagy adaptor, the FYCO1 protein is involved in the process of kinesin movement along microtubules and the targeted transport of autophagosomes to the plus end of microtubules. Currently, it has been shown that FYCO1 gene mutations can cause autosomal recessive congenital cataracts in humans, which is related to its autophagy function[36]. Studies have found that fyco1-/- mice exhibit initial signs of lens opacity at 4wk, develop into mild cataracts at around 8wk, and mature into cataracts at 16wk. It is concluded that the loss of FYCO1 function led to a decrease in autophagic flux, impaired organelle clearance, and the occurrence of cataracts.
HIV-1
Patients infected with human immunodeficiency virus-1 (HIV-1) are at risk of several intraocular diseases, the most common complication being retinal microvascular lesions, which cause retinal hemorrhage and infarction of the nerve fiber layer, forming cotton wool spots, and may also present with lens opacity. The study observed that in HIV transgenic mice, cataracts originated from the nucleus of the lens, subsequently evolving into cortical cataracts accompanied by uveitis[37]. Simultaneously, LECs and fibrocytes expressed the HIV gpl20 protein, along with enhanced apoptosis of LECs and fibrocytes. This mouse model offers a valuable means for elucidating the pathogenesis of congenital cataracts associated with viral infections that occur during fetal development.
LAT1 and TAT1
L-tryptophan and other large neutral aromatic amino acids may be transported into the lens via the ciliary epithelium with the help of at least two transporters, namely L-type amino acid transporter 2 (LAT2) and TAT1 (testis anion transporter 1). Research indicated that the neutral amino acid transporter LAT2 (Slc7a8) and the single transporter TAT1 (Slc16a10) were expressed in the mouse ciliary epithelium, and LAT2 was also present in the LECs[38]. The deletion of LAT2 results in a higher incidence of cataracts in mice, especially in aged female mice, and a synergistic effect is observed in the absence of TAT1. It is concluded that the defect of the amino acid transporter LAT2 was related to the formation of cataracts, which may be exacerbated by the defect of TAT1.
Agps
The study revealed that alkylglycerone phosphate synthase (Agps) KO mice presented with mild bilateral nuclear cataracts, with the lens exhibiting abnormality at 21d after birth, characterized by swelling of epithelial cells in the equatorial zone[39]. Severe cataracts developed rapidly at 28d, accompanied by severe destruction of lens fibroblasts at 4mo. The ultimate conclusion is that appropriate AGPS function is essential for mouse embryonic survival and the normal development of the lens, testicles, and humerus.
miRNA
MicroRNAs (miRNAs) play a well-recognized role in post-transcriptional gene regulation and are involved in lens development and pathogenesis. The lens expresses transcripts of the miRNA processing enzyme DICER, and some miRNAs are highly enriched in the lens, exhibiting spatial and temporal specificity. Study has revealed that mice lacking all three copies of miR-26 (miR-26TKO) develop postnatal cataracts as early as 4–6 weeks of age[40]. It was concluded that the deletion of miR-26 would result in changes in the lens transcriptome and drive the formation of cataracts.
Chmp4b
Charged multivesicular body protein 4B (CHMP4B) is a core component of the endosomal sorting complexes required for transport III (ESCRT-III) machinery, and can promote various membrane remodeling and shearing processes in eukaryotes. Mutations in the human CHMP4B gene are the basis of rare inherited early-onset lens opacities or cataracts. Study has found that severe lens degeneration in Chmp4b-CKO mice leads to an immune cell response in the eye, and conditional deletion of Chmp4b leads to stasis of lens growth and differentiation[41].
S100A4
S100 calcium binding protein A4 (S100A4) is a member of the multifunctional calcium-binding protein S100 family and is involved in a variety of physiological and pathological processes. Study has found that S100A4 expression was strongly induced in the differentiated lens fibrocyte, and S100A4-/- KO mice develop delayed cortical cataracts. The conclusion was drawn that S100A4 reasonably inhibits the expression of retinal genes during lens differentiation through a mechanism involving histone methylation changes[42].
Application of Transgenic Mice in Acquired Cataract
Age-related cataract
Vimentin
Vimentin is a type III intermediate filament protein (IF) that mostly undergoes mitotic phosphorylation. It was discovered that microphthalmia and cataracts were present in vimentin KI mice in pure animals (VIMSA/SA)[43]. Disordered VIMSA/SA lens fiber membranes that resembled senile cataracts were seen under electron microscopy, along with a decrease in the number of LECs.
TTase
Thioltransferase (TTase) is a crucial enzyme that maintains the lens's redox balance and guards against oxidative damage. Both TTase KO and WT mice developed cataracts more frequently as they grew older, and the majority of these cataracts were nuclear in nature. Age-related changes in the glutathione (GSH) content of the lenses were observed in both genotypes of mice: cataracts in TTase KO mice appeared as early as 4 months of age and were most noticeable at 9mo; in WT mice, cataracts appeared as early as 9mo and were most noticeable at 12 months of age. Additionally, it was discovered that TTase deletion sped up the age-related cataract formation in mice, which was connected to PSSG accumulation in the lens. Furthermore, by desulfating the produced PSSG, TTase was discovered to be essential in avoiding the formation of age-related cataract[44].
Bin3
Bridging integrator3 (BIN3) is responsible for encoding a widely expressed and evolutionarily conserved member of the guanosine triphosphatase binding (GTPase-binding) proteins and BAR superfamily, which includes pch/f-bar, i-bar articulating proteins, and BAR. These proteins are engaged in vesicular transport and signaling together. Research has demonstrated that pure deletions of the Bin3 gene were more common in diseases including lymphoma and cataracts in older people[46]. Bin3 may be related to lens development and aging because of the several morphological problems in its lens fibers, which include the creation of vacuoles in cortical fibers and the complete lack of F-actin in lens fiber cells[46].
Clock
The circadian system, which comprises transcription-related factors including the circadian clock proteins BMAL1 and CLOCK, is essential for the regulation of physiological processes. According to the study, Clock-/- mice live 15% less time on average than WT mice. Additionally, compared to WT mice, WT mice showed a greater prevalence of cataracts and skin irritation. The results indicate that the Clock gene is critical for appropriate physiological control, aging of the lens and skin, and has a significant impact on the organism's aging[47].
Cryaa
The primary structural protein I of the lens, αA crystallin, is made up of a tetramer comprising Cryaa and Cryab subunits. The research revealed that the Cryaa-/- gene-deficient mice's lens nuclei appeared hazy and that the cloudiness increased with age. The existence of dense inclusion bodies was demonstrated by the results of transmission electron microscopy and light microscopy in the lens fiber cells of Cryaa mutant mice. Based on the results, Cryaa is essential for maintaining lens transparency, likely due to specific Cryab or other proteins binding to small heat shock proteins[48].
Crybb2
It was discovered that in Crybb2 KO mice, the lens's diameter dramatically shrank with age, cataracts developed just a few months after birth, and the condition progressively got worse[45],[49]. The lens's resistance to oxidative stress and thermal stability also declined during this period. According to the study, Crybb2 may attach to other lens proteins, enhancing the lens's ability to withstand heat and function as an antioxidant.
Cryab-R120G
The lens's heterodimeric complex of αb crystallin and α crystallin is a tiny heat shock protein. Whereas amyloidogenic fibrils are more organized, crystallins can form amorphous aggregates in cataracts. A mutation in αb crystallin (Cryab-R120G) that causes arginine 120 to glycine (R120G) and loss of protein chaperone action results in the aggregation of high-molecular-weight crystallins in vitro. This has been linked to cataracts and myopathy. Cryab-R120G KI animals showed increased molecular mass, light scattering, protein aggregation, and insolubility in crystallins isolated from their lenses, and these mice develop cataracts at an early age. Furthermore, the employment of this model to investigate age-related cataracts is supported by the findings of two-dimensional infrared (2DIR)[50]–[51], which verified the existence of amyloid-like secondary structures in the cataracts of Cryab-R120G KI mice.
Bfsp2
The filamentous cytoskeletal structures known as beaded fibers (BFs) are formed by differentiated lens fiber cells. Both highly distinct members of the big intermediate filament (IF) family of proteins, CP49 (bfsp2) and filensin (Bfsp1) are necessary for the formation of BFs. According to research, lens epithelial growth and mitotic activity in mutant mice are slowed down after 5 weeks of age when CP49 and filensin are expressed in the lens epithelium[52]. CP49 and filenin were also found to be necessary indicators of the differentiation of lens epithelial and fibroblast in mice.
Gja3
The early phases of lens development and lens fiber differentiation were found to be unaffected by the gap junction protein-alpha 3 (Gja3) deficiency; nevertheless, Gja3-deficient heterozygous mice develop nuclear cataracts, the reason for which is currently believed to be connected to the hydrolysis of lens proteins[53].
Gja8
Lens development and maturation are significantly impacted by the gap junction protein-alpha 8 (Gja8), which is mostly expressed in lens fiber cells. The development of nuclear cataract and microphthalmia in mice lacking the Gja8 gene was discovered[54].
Dnase2b
Particularly expressed in eye and liver tissue, the nucleic acid-degrading enzyme deoxyribonuclease II beta (Dnase2b) is strongly abundant in the lens. The accumulation of undigested DNA in the fiber cells was discovered to be caused by the incapacity of Dnase2b gene-deficient animals to break down DNA during lens cell development. Nuclear cataracts were also present in the lens of this mouse[55].
Epha2
The abundant vertebrate lens membrane protein known as the Epha2 (or Eph receptor A2) receptor is controlled both geographically and temporally. It can be produced in differentiated fibroblasts and LECs, but in the later phases of fibroblast differentiation, it is broken down. A non-homozygous coding variant (p.R721Q) in the TK structural domain is one of at least 12 frequent single nucleotide variants in EPHA2[56] (mainly non-coding variants) that have been linked to age-related nuclear, cortical, and posterior subcapsular cataract susceptibility. Cortical cataract development was observed in a mouse model carrying a missense mutation (c.2162G>A, rs116506614) in exon 13 of EPHA2. Additionally, its lens phenotype will differ, exhibiting modest nuclear clouding with translucent patches caused by lens cell disintegration, lens rupture, and severe progressive cataract formation. Finally, it is determined that dysregulated expression of several cytoskeleton-related proteins and abnormal lens cell patterns result from mutations in the structural domain of EPHA2 tyrosine kinase.
Sparc
Osteoadhesin BM40, also known as secreted protein acidic and rich in cysteine (SPARC), is a glycoprotein that secretes calcium ions and is found in or near the basement membrane. It interacts with several components of the extracellular matrix, including collagen IV. Cortical cataracts were discovered in Sparc KO mice at a time of one to two months, and further problems, including iris coloration and anterior chamber displacement, were noted at four months[57].
Lctl
A member of the Klotho protein family, lactase-like (Lctl) is highly expressed in the lens, particularly in the equatorial epithelium. When Lctl KO mice were young, their lens appeared normal on the outside but had aberrant regulation. As they grew older, they developed cortical cataracts. Furthermore, in a mouse model of cilia/basal body-related auditory defects, the structural disruption of the lens in these mice is accompanied by a complete lack of transcription of Clic5, a protein whose transcription is crucial for the mechanisms governing the growth and assembly of lens fibroblasts. These findings imply that Lctl is necessary for the particular expression of the Clic5 gene in the lens[58].
Aldhla1 and Aldh3a1
Members of the same family of aldehyde dehydrogenases, aldehyde dehydrogenase 1 family member A1 (aldehyde dehydrogenase1 A1, Aldh1al) and aldehyde dehydrogenase 3A1 (aldehyde dehydrogenase3 A1, Aldh3a1), are found throughout the cytoplasm. While the Aldh3a1 gene is solely expressed in the mouse cornea, Aldh1al has minimal enzymatic activity in the cornea and lens. The results showed that Aldh1a1/Aldh3a1 double KO and Aldh3a1 KO mice developed cataracts at 1 month of age, with punctate clouding occurring in the cortical region and anterior and posterior subcapsular membrane regions. Six to nine months postnatally, Aldh1a1 KO mice also developed cataracts. The Aldh3a1 enzyme in the cornea and the Aldh1a1 enzyme in the lens has been demonstrated to prevent the production of cataracts through both enzymatic (detoxification) and non-enzymatic (light filtering) forms[59].
GCLc
Nuclear cataract, which is the most common type of age-related cataract, is considered to be related to the loss of GSH and the formation of disulfides, both of which are key factors in oxidative stress and the occurrence of nuclear cataract[60]. Glutamate cysteine ligase, catalytic (GCLc), which has all the activities of glutamate cysteine ligase (GCL), can reduce reactive oxygen species (ROS) and resist oxidative stress. The glutathione level in the lens is genetically reduced by disrupting the catalytic subunit of GClc. The study found that lens GSH KO mice (HET-LEGSKO) developed nuclear opacity at 4 months of age and severe nuclear cataracts at 9 months of age[60]. The conclusion is that LEGSKO mice specifically inhibit the synthesis of GSH in the lens while maintaining the integrity of all other metabolic functions, resulting in biochemical, biological, and cataract changes similar to those of age-related human nuclear cataracts. This mouse is expected to be a useful tool for the development of drugs that can restore the antioxidant reserve of the lens and prevent the distal effects of oxidative stress caused by glutathione deficiency.
HO-1
The nuclear factor erythroid 2-like-2 (Nrf-2)/heme oxygenase-1 (HO-1)/carbon monoxide (CO) axis protects LECs from oxidant and ERS damage. HO-1 and its byproduct CO, as well as biliverdin, exert cytoprotective effects through antioxidant, anti-apoptotic, and anti-inflammatory pathways. Nuclear cataracts were discovered to develop in transgenic FVB/N mice overexpressing the negative dominant mutant HO-1G143H at 4mo, 5mo earlier than in control mice. This mouse's lens also revealed reduced glutathione and protein sulfhydryl levels, as well as an accumulation of malondialdehyde and protein carbonyl[61].
hSVCT2
Na(+)/L-ascorbic acid transporter 2 (hSVCT2) is a sodium-dependent vitamin C transporter[62]. Despite the association between increased vitamin C intake and cataract prevention, there is also evidence suggesting the harm of excessive vitamin C intake. Studies reveal that the vitamin C content in the lenses of hSVCT2 overexpressing transgenic mice rises, particularly in the oxidative form, dehydroascorbic acid, leading to the development of cataracts at 12 months of age. Furthermore, the advanced glycation end products (AGEs) levels of hSVCT2 lenses escalate with advancing age[63].
Nrf2
Nrf2, also known as the nuclear factor erythroid 2-related factor 2 (Nfe2l2), is a transcription factor with distinct characteristics. It serves as a crucial cellular defense mechanism against oxidative stress. The genes activated by Nrf2 boast a remarkable cytoprotective effect, getting involved in various pathways like glutathione synthesis, detoxification, ROS elimination, and drug excretion. The research discovered that Nrf2-/- mice exhibited notable turbidity between the ages of 11 and 15mo, giving rise to advanced cortical, posterior subcapsular, anterior subcapsular, and nuclear cataracts. The conclusion drawn is that as we aged and age-related cataracts emerge, the decreased activity of NRF2 results in a reduction of downstream antioxidant effects. Consequently, NRF2 has captured people's attention as a therapeutic target for both the treatment and prevention of cataracts[64].
Sod2
Superoxide dismutase (SOD) exhibits a critical function in safeguarding cells against oxidative stress by catalyzing the disintegration of superoxide anions into hydrogen peroxide. The research indicated that with the increment of age in Sod2+/- and WT mice, the levels of CML and pentoside in the skin collagen escalate, accompanied by an increase in cataract formation[65]. Simultaneously, throughout the entire lifespan of Sod2+/- mice, the elevation in DNA oxidative damage levels gives rise to a higher incidence of cancer.
xCT
The cystine/glutamate antiporter (system xc-) is tasked with mediating the intake of extracellular cystine, while exchanging intracellular glutamate. It is a member of the heteromeric amino acid transporter family, composed of two subunits: a functional light chain known as xCT, which is responsible for the transport of amino acids, and a heavy chain subunit called 4F2hc, which anchors the transporter in the plasma membrane. xCT is expressed in various tissues of the eye, including the cornea, ciliary body, and lens (in rats, as well as the retina[66]–[77]). Simultaneously, xCT has been demonstrated to induce a decrease in GSH depletion, suggesting the role of xCT in sustaining the GSH levels within these cells. Study has revealed that the glutathione levels in the lens epithelium of gene KO mice were significantly reduced at 3mo compared to WT mice[77]. Across all age groups, the cysteine levels in WT and KO mice remained comparable, whereas the cysteine levels in the KO mice at 3, 9, and 12mo were significantly higher compared to WT mice. The conclusion drawn is that the absence of xCT leads to the consumption of glutathione in the epithelium and the oxidative shift of the cysteine/cystine ratio in the aqueous humor. These oxidative alterations may contribute to the expedited development of anterior cataracts in gene-KO mice.
ER
The estrogen receptor (ER), which belongs to the nuclear receptor superfamily, is a ligand-dependent transcription factor. At present, two ER subtypes, namely, ERα and ERβ, are known to exist. Studyon human and rodent models have demonstrated that estrogen can prevent age-related cataracts. Mice express ER∆3, a dominant negative form that inhibits the function of ERα[78]. In ER∆3 transgenic mice, cortical cataracts spontaneously form in ER∆3 females after puberty and progress with age. The cataracts originate from the equatorial region of the lens, where epithelial cells differentiate into elongated fibroblast-like cells. Ovariectomy in females before sexual maturity can prevent the formation of cataracts, but not after sexual maturity. Both male and female ER∆3 mice develop cataracts after neonatal treatment with the potent estrogen diethylstilbestrol (DES). The incidences of both spontaneous and DES-induced cataracts in ER∆3 mice are 100%, while these cataracts are absent in WT mice. These data imply that inhibiting the action of estrogen leads to the formation of cortical cataracts, as estrogen is necessary to activate the ER∆3 repressor. The evidence of DES-induced cataracts in both male and female ER∆3 mice suggests that estrogen plays an important role in the lens physiology of both sexes. The ER∆3 mouse serves as a powerful model for assessing the role of estrogen in maintaining lens transparency.
Yap1
Yes1 associated transcriptional regulator (Yap1) codes for an evolutionarily conserved transcriptional co-activator, which serves as a downstream effector of the Hippo signaling pathway that regulates tissue size and cell growth, and this gene is involved in lens development. Study revealed that heterozygous inactivation of Yap1 in mice gave rise to cataracts with LECs phenotypic flaws in adulthood[79]. Despite the normal early development of the eye, including the lens, most Yap1 heterozygotes develop cataracts within the first 6mo after birth. Prior to the onset of cataracts, there exist various morphological abnormalities in the LECs, such as decreased cell density and aberrant cell connections. The low LECs density is in line with the reduced LECs proliferation. It is concluded that the homozygosity of the Yap1 gene was crucial for the sustained proliferation of LECs and the sufficient crip1 expression needed to maintain a normally sized lens.
GPX1
Glutathione peroxidase 1 (GPX1) and catalase are expressed in lens epithelial cells and cortical fibroblasts, which can detoxify H2O2 to reduce oxidative stress, which is the main cause of cataracts. Study showed that at the age of nine months, GPX1-/- mice began to present abnormal optical aberration and loss of transparency[80]. At the age of 12mo, compared with CAT and WT, the GPX1 lens showed significant turbidity and abnormal optical aberration; these aberrations gradually increased with age and developed into cataracts at 24 months of age. Compared with the similar areas of age-matched CAT-/- and WT lenses, the GJC resistance of differentiated and mature fibroblasts in the GPX1-/- lens also significantly increased at 12 months of age.
Sep15
A15 kDa selenoprotein, Sep15, is extensively expressed in numerous tissues, with a heightened expression in the prostate, brain, kidney, liver, and testes[81]–[84]. Additionally, it is identified as a resident of the endoplasmic reticulum[85] and is differentially regulated by both adaptive and acute ERS[86]. The Sep15 KO mice presented with evident nuclear cataracts in the early stages[84]. It was determined that the protective effect of Sep15 on tm-induced human lens epithelial (HLE) cell apoptosis is accomplished through the inhibition of oxidative stress rather than the regulation of tm-induced ERS. In the context of acute stress, the role of Sep15 becomes critical.
Metabolic cataract
Dcoh
The HNF1 transcriptional activator family's Dcoh bifunctional protein, also known as the dimerizing co-factor for HNF1, works with HNF1 to stabilize the dimeric HNF1 complex[85]. Tetrahydrobiopterin is catalyzed by aromatic amino acid hydroxylase, which has Dcoh as a co-factor. It has been discovered that mice lacking the dcoh gene experience hyperphenylalaninemia and cataract development. The age at which cataracts form varies greatly, though; the majority of affected mice develop cataracts by 24wk postnatal, with indicating that genes associated with glucose metabolism may play a rolethe first cataracts being discovered at 12d postnatal. Dcoh KO animals exhibit a minor reduction in HNF1 activity and a degree of glucose tolerance in comparison to diabetic mice, in the development of cataracts.
Galk1
The enzyme galactokinase 1 (Galk1) is crucial to the metabolism of galactose. Galk1 uses ATP to catalyze the change of D-galactose to D-galactose 1-phosphate in lactose metabolism. It was discovered that mice lacking the gene Galk1 had impaired metabolism of galactose and accumulated galactose and galactic in their bodies. Mice lacking the Galkl gene do not acquire cataracts following a diet high in galactose. Conversely, cataracts occur in Galk1-deficient mice that have the human aldose reductase gene inserted[86].
PLD
Phospholipase D (PLD), a highly regulated enzyme, can generate the second messenger phosphatidic acid and is involved in signal transduction, membrane transport, and cytoskeletal reorganization. Study revealed that in the presence of transgenic aldose reductase overexpression, the lens osmotic pressure of transgenic mice overexpressing PLD2 increases[87]. These double transgenic mice develop more severe congenital cataracts and are prone to diabetic cataracts. It is concluded that the increase in PLD2 activity in the lens in the hyperglycemic state may impair its osmotic regulatory mechanism and reduce its ability to cope with osmotic stress caused by sorbitol accumulation.
Radiation cataract
Grx2
The most frequent cause of oxidative damage to the lens is prolonged sun radiation, particularly blue ultraviolet (UVA, 320–400 nm) and UVB (280–320 nm). The lens has antioxidant-reducing repair systems, one of which is the glutaraldehyde-reducing protein (Grx) system. Grx2 is mostly expressed in mitochondria, where it aids in the preservation of the mitochondria and oxidoreductase repair. Oxidative damage to the mitochondria is caused by an increase in ROS following UVB exposure. What ultimately happens in the LECs is determined by the protective effects of both oxidative stress and Grx2. Grx2 KO mice were shown to have greater lens nuclear density, increased turbidity, and an earlier onset of lens turbidity at week 1 following UVB irradiation than WT mice[88].
Atm
Ataxia-telangiectasia mutated gene (Atm) widely expressed in embryonic tissues, the ataxia telangiectasia mutant homolog is a multifunctional serine protein kinase primarily found in the nucleus. Pure mice with the Atm gene deleted have been shown to exhibit cloudiness in their lenses when exposed to various X-rays[89].
Eaf2
Lens growth and maturity are significantly influenced by Ell-associated factor 2 (Eaf2). Eaf2 knockdown was demonstrated to decrease UV-induced lens cell death and ameliorate cataract formation[90]. Eaf2 was also shown to activate caspase-3 and caspase-9, upregulate the expression of the pro-apoptotic protein Bax, and downregulate the expression of the anti-apoptotic protein bcl-2. These findings demonstrated that Eaf2 was first involved in apoptosis, which in turn led to the formation and development of cataracts.
SMP30
Aging marker protein-30 (SMP30) KO mice, due to genetic disruption of the glucose oxidase (GNL) gene, are unable to synthesize vitamin C (VC). In the SMP30 KO VC (-) group, UVR-B-induced lens opacity is more extensive than in the SMP30 KO VC (+) group or in the WT group. The VClevel in the SMP30 KO VC (-) group lenses decreased to one-third that of the SMP30 KO VC (+) group or the WT group[91]. These findings indicated that VC deficiency increased the susceptibility of lenses to UVR-B-induced oxidative stress in mice.
Posterior capsular opacities
HPV16-E6
Surgery is the most efficacious method for cataract extraction, yet the common complication after the operation is secondary cataract, namely, PCO. This is attributed to the fibrotic growth of residual LECs left in the capsular bag after the surgery[92]. Epithelial-mesenchymal transition (EMT) is a significant process in the development of human cataracts and PCO[93]. In these processes, cells with a fibroblast-like morphology migrate to the posterior capsule of the lens and accumulate in large quantities. Study has revealed that the incidence of cataracts in both eyes of K14E6 mice was 100%, presenting as tissue loss, cortical liquefaction, and an increase in the number of hyperproliferative nuclear cells with mesenchymal-like characteristics in the lens[94]. A conclusion has been drawn that the HPV16-E6 oncogene induces EMT in transgenic mice with cataracts. The molecular mechanism may involve the TGF-β and Wnt/β-catenin pathway, suggesting that the K14E6 transgenic mouse can serve as an effective model for the study or treatment of EMT and PCO[94].
Smad3
TGF-β is of great significance in the progression of PCO, and inhibiting the TGF-β pathway could be a novel treatment for PCO. Smad3 is an integral part of the TGF-β/Smad3 signaling pathway. In HLE-B3 type line, Smad3 overexpression can activate the TGF-β2 signaling pathway and increase the secretion of extracellular matrix components[95]. The research indicated that the deletion of Smad3 partially suppressed the TGF-β signaling pathway in the trauma-induced lens epithelial EMT. Compared with WT mice, the expression of the myofibroblast marker EMT marker α-SMA in Smad3-KO mice is significantly decreased. The proportion of apoptotic nuclei in the Smad3-/- lens is significantly higher than that in the Smad3+/+. It can be concluded that Smad3 KO interferes with the activation of the TGF-β signal, thereby inhibiting the occurrence of PCO[96].
Grx2
The mitochondrial glutathione-dependent oxidoreductase known as glutathione2 (Grx2) is necessary to preserve cellular viability and lens redox equilibrium. Clouding of the posterior capsule membrane is linked to the EMT in LECs caused by oxidative stress. Study has shown that, the posterior capsule membranes of lenses from Grx2 KO animal models showed a considerable upregulation of integrin-linked kinase (ILK)[97]. Additionally, the Grx2 KI group showed lower expression of ILK and EMT markers than the WT group, whereas the Grx2 KO group showed higher expression of these markers. Ultimately, it was determined that Grx2 modulated the ILK/Akt/GSK-3β axis to shield LECs against EMT caused by oxidative stress.
Concluding Remarks
The mechanisms of lens development have become better known with the advent of molecular biology techniques like gene editing and the creation of transgenic mouse models. We have improved our understanding of the molecular etiology of cataract by thoroughly examining these altered genes. Using a transgenic mouse cataract animal model, which can swiftly replicate large numbers of samples and get tissue that is not usually available from cataract patients, researchers can reliably investigate the molecular pathogenesis of cataract disease. Nevertheless, the pathophysiology of cataract remains poorly understood, and the location of its harmful genes is only partially known. Simultaneously, a large number of unidentified genes remain undiscovered. Consequently, in order to expand the opportunities for the study of cataract illness, efforts should be undertaken to produce more transgenic mice in the future. In the future, the pathophysiology of cataract will become more fully understood, opening up new therapeutic options, as more and more cataract pathogenic genes are found, cloned, and significant functional proteins expressed in the lens are found and investigated.
Footnotes
Authors' contributions: Zhang Y analyzed and wrote the manuscript; Hu SS selected the study; Chen XY and Hu YZ extracted the data. Zhang X and Zheng SF assessed the quality of study; Hu SS read and criticized the manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.82271070); the Heilongjiang Provincial Undergraduate Colleges and Universities Central to Support the Reform and Development of Local Colleges and Universities (No.2020YQ08); the Natural Science Foundation Project of Heilongjiang Province (Key Project/Outstanding Youth/Joint Guidance, No.LH2021H112); Doctoral Research Fund of Mudanjiang Medical University Affiliated Hongqi Hospital (No.2024-HQBS-03).
Conflicts of Interest: Zhang Y, None; Chen XY, None; Hu YZ, None; Zhang X, None; Zheng SF, None; Hu SS, None.
REFERENCES
- 1.Houdebine LM. Use of transgenic animals to improve human health and animal production. Reprod Domest Anim. 2005;40(4):269–281. doi: 10.1111/j.1439-0531.2005.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiels A, Hejtmancik JF. Molecular genetics of cataract. Prog Mol Biol Transl Sci. 2015;134:203–218. doi: 10.1016/bs.pmbts.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98–103. doi: 10.1097/ICU.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 4.Wang FX, Ma J, Han F, Guo XJ, Meng L, Sun YF, Jin C, Duan HJ, Li H, Peng Y. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep. 2016;6:19396. doi: 10.1038/srep19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu YX, Zhou H, Fan XY, et al. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Res. 2015;25(1):67–79. doi: 10.1038/cr.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao W, Yang WM, Zhang XY, Deng XQ, Chen XY. Endoplasmic reticulum stress and the lysosomal pathway play crucial roles in the progression of βB2-crystallin mutation-induced congenital cataracts in mice. Invest Ophthalmol Vis Sci. 2023;64(4):34. doi: 10.1167/iovs.64.4.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett TM, Zhou YF, Shiels A. Lens transcriptome profile during cataract development in Mip-null mice. Biochem Biophys Res Commun. 2016;478(2):988–993. doi: 10.1016/j.bbrc.2016.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang SZ, Di GH, Hu SH, Liu YN, Dai YH, Chen P. AQP5 regulates vimentin expression via miR-124-3p.1 to protect lens transparency. Exp Eye Res. 2021;205:108485. doi: 10.1016/j.exer.2021.108485. [DOI] [PubMed] [Google Scholar]
- 9.Shiels A, King JM, MacKay DS, Bassnett S. Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2. Invest Ophthalmol Vis Sci. 2007;48(2):500–508. doi: 10.1167/iovs.06-0947. [DOI] [PubMed] [Google Scholar]
- 10.Lachke SA, Higgins AW, Inagaki M, et al. The cell adhesion gene PVRL3 is associated with congenital ocular defects. Hum Genet. 2012;131(2):235–250. doi: 10.1007/s00439-011-1064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moré MI, Kirsch FP, Rathjen FG. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J Cell Biol. 2001;154(1):187–196. doi: 10.1083/jcb.200104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer HF, Daub K, Braun G, et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27(6):1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 13.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431(7006):320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 14.Li XD, Yin AP, Zhang WJ, Zhao F, Lv J, Lv J, Sun JP. Jam3 promotes migration and suppresses apoptosis of renal carcinoma cell lines. Int J Mol Med. 2018;42(5):2923–2929. doi: 10.3892/ijmm.2018.3854. [DOI] [PubMed] [Google Scholar]
- 15.Li JN, Tan XH, Sun QH, Li XR, Chen RY, Luo LX. Deficiency of jamc leads to congenital nuclear cataract and activates the unfolded protein response in mouse lenses. Invest Ophthalmol Vis Sci. 2022;63(10):1. doi: 10.1167/iovs.63.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Wu X, Wu D, et al. Embryonic surface ectoderm-specific mitofusin 2 conditional knockout induces congenital cataracts in mice. Sci Rep. 2018;8(1):1522. doi: 10.1038/s41598-018-19849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki H, Ogino H, Tomita H, Hara A, Kunisada T. Disruption of rest leads to the early onset of cataracts with the aberrant terminal differentiation of lens fiber cells. PLoS One. 2016;11(9):e0163042. doi: 10.1371/journal.pone.0163042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujino M, Tagami A, Ojima M, Mizuno S, Abdellatif AM, Kuno A, Takahashi S. C-MAF deletion in adult C57BL/6J mice induces cataract formation and abnormal differentiation of lens fiber cells. Exp Anim. 2020;69(2):242–249. doi: 10.1538/expanim.19-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi XH, Cui B, Wang ZG, Weng L, Xu ZP, Ma JJ, Xu GT, Kong XY, Hu LD. Removal of Hsf4 leads to cataract development in mice through down-regulation of γS-crystallin and Bfsp expression. BMC Mol Biol. 2009;10(1):10. doi: 10.1186/1471-2199-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klesert TR, Cho DH, Clark JI, Maylie J, Adelman J, Snider L, Yuen EC, Soriano P, Tapscott SJ. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25(1):105–109. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- 21.Bassols A, Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263(6):3039–3045. [PubMed] [Google Scholar]
- 22.Kulkarni A, Thyagarajan T, Letterio J. Function of cytokines within the TGF-β superfamily as determined from transgenic and gene knockout studies in mice. Curr Mol Med. 2002;2(3):303–327. doi: 10.2174/1566524024605699. [DOI] [PubMed] [Google Scholar]
- 23.Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36(5):373–384. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- 24.Honjo Y, Nagineni CN, Larsson J, Nandula SR, Hooks JJ, Chan CC, Karlsson S, Kulkarni AB. Neuron-specific TGF-beta signaling deficiency results in retinal detachment and cataracts in mice. Biochem Biophys Res Commun. 2007;352(2):418–422. doi: 10.1016/j.bbrc.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murugan S, Cheng C. Roles of Eph-ephrin signaling in the eye lens cataractogenesis, biomechanics, and homeostasis. Front Cell Dev Biol. 2022;10:852236. doi: 10.3389/fcell.2022.852236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy VN, Giblin FJ, Lin LR, et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Invest Ophthalmol Vis Sci. 2001;42(13):3247–3255. [PubMed] [Google Scholar]
- 27.Lin X, Yang T, Liu X, Fan F, Zhou X, Li H, Luo Y. TGF-β/Smad signalling activation by HTRA1 regulates the function of human lens epithelial cells and its mechanism in posterior subcapsular congenital cataract. Int J Mol Sci. 2022;23(22):14431. doi: 10.3390/ijms232214431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheong C, Sung YH, Lee J, Choi YS, Song J, Kee C, Lee HW. Role of INK4a locus in normal eye development and cataract genesis. Mech Ageing Dev. 2006;127(7):633–638. doi: 10.1016/j.mad.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Yang YG, Frappart PO, Frappart L, Wang ZQ, Tong WM. A novel function of DNA repair molecule Nbs1 in terminal differentiation of the lens fibre cells and cataractogenesis. DNA Repair. 2006;5(8):885–893. doi: 10.1016/j.dnarep.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Qin Y, Zhao FK, et al. Anterior segment dysgenesis correlation with epithelial-mesenchymal transition in Smad4 knockout mice. Int J Ophthalmol. 2016;9(7):943–947. doi: 10.18240/ijo.2016.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng JS, Luo J, Cheng XH, et al. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci U S A. 2008;105(16):6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu CF, Li HY, Liu XY, et al Q. TDRD7 participates in lens development and spermiogenesis by mediating autophagosome maturation. Autophagy. 2021;17(11):3848–3864. doi: 10.1080/15548627.2021.1894058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou YF, Bennett TM, Shiels A. Mutation of the TRPM3 cation channel underlies progressive cataract development and lens calcification associated with pro-fibrotic and immune cell responses. FASEB J. 2021;35(2):e21288. doi: 10.1096/fj.202002037R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan SY, Ali M, Kabir F, et al. The role of FYCO1-dependent autophagy in lens fiber cell differentiation. Autophagy. 2022;18(9):2198–2215. doi: 10.1080/15548627.2022.2025570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjørkøy G, Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188(2):253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng XF, Wang YL, Gong YK, Li FX, Guo YJ, Hu SC, Liu JP, Pan LF. Structural basis of FYCO1 and MAP1LC3A interaction reveals a novel binding mode for Atg8-family proteins. Autophagy. 2016;12(8):1330–1339. doi: 10.1080/15548627.2016.1185590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mozes MM, Bryant JL, Franks R, Chan CC, Kopp JB. Congenital nuclear cataracts and uveitis in HIV-transgenic mice. Eye (Lond) 2002;16(2):177–184. doi: 10.1038/sj.eye.6700101. [DOI] [PubMed] [Google Scholar]
- 38.Knöpfel EB, Vilches C, Camargo SMR, et al. Dysfunctional LAT2 amino acid transporter is associated with cataract in mouse and humans. Front Physiol. 2019;10:688. doi: 10.3389/fphys.2019.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liegel RP, Ronchetti A, Sidjanin DJ. Alkylglycerone phosphate synthase (AGPS) deficient mice: models for rhizomelic chondrodysplasia punctate type 3 (RCDP3) malformation syndrome. Mol Genet Metab Rep. 2014;1:299–311. doi: 10.1016/j.ymgmr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upreti A, Hoang TV, Li MH, et al. MiR-26 deficiency causes alterations in lens transcriptome and results in adult-onset cataract. Invest Ophthalmol Vis Sci. 2024;65(4):42. doi: 10.1167/iovs.65.4.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou YF, Bennett TM, Shiels A. A charged multivesicular body protein (CHMP4B) is required for lens growth and differentiation. Differentiation. 2019;109:16–27. doi: 10.1016/j.diff.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maddala R, Gao JY, Mathias RT, et al. Absence of S100A4 in the mouse lens induces an aberrant retina-specific differentiation program and cataract. Sci Rep. 2021;11(1):2203. doi: 10.1038/s41598-021-81611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuyama M, Tanaka H, Inoko A, et al. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J Biol Chem. 2013;288(50):35626–35635. doi: 10.1074/jbc.M113.514737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Yan H, Lou MF. Does oxidative stress play any role in diabetic cataract formation? Re-evaluation using a thioltransferase gene knockout mouse model. Exp Eye Res. 2017;161:36–42. doi: 10.1016/j.exer.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Gao Q, Sun LL, Xiang FF, Gao L, Jia Y, Zhang JR, Tao HB, Zhang JJ, Li WJ. Crybb2 deficiency impairs fertility in female mice. Biochem Biophys Res Commun. 2014;453(1):37–42. doi: 10.1016/j.bbrc.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 46.Ramalingam A, Duhadaway JB, Sutanto-Ward E, et al. Bin3 deletion causes cataracts and increased susceptibility to lymphoma during aging. Cancer Res. 2008;68(6):1683–1690. doi: 10.1158/0008-5472.CAN-07-6072. [DOI] [PubMed] [Google Scholar]
- 47.Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging. 2010;2(12):936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma XY, Jiao XD, Ma ZW, Hejtmancik JF. Polymorphism rs7278468 is associated with age-related cataract through decreasing transcriptional activity of the CRYAA promoter. Sci Rep. 2016;6:23206. doi: 10.1038/srep23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Li J, Huang CG, Xue L, Peng YJ, Fu Q, Gao L, Zhang JR, Li WJ. Targeted knockout of the mouse betaB2-crystallin gene (Crybb2) induces age-related cataract. Invest Ophthalmol Vis Sci. 2008;49(12):5476–5483. doi: 10.1167/iovs.08-2179. [DOI] [PubMed] [Google Scholar]
- 50.Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2020;27(4):217–222. doi: 10.1080/13506129.2020.1835263. [DOI] [PubMed] [Google Scholar]
- 51.Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem. 2004;279(5):3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- 52.Song SH, Landsbury A, Dahm R, Liu YZ, Zhang QJ, Quinlan RA. Functions of the intermediate filament cytoskeleton in the eye lens. J Clin Invest. 2009;119(7):1837–1848. doi: 10.1172/JCI38277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su DM, Hu SS, Guan LN, Wu XZ, Shi CG, Yang XB, Ma X. Down-regulation of GJA3 is associated with lens epithelial cell apoptosis and age-related cataract. Biochem Biophys Res Commun. 2017;484(1):159–164. doi: 10.1016/j.bbrc.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 54.Ceroni F, Aguilera-Garcia D, Chassaing N, et al. New GJA8 variants and phenotypes highlight its critical role in a broad spectrum of eye anomalies. Hum Genet. 2019;138(8-9):1027–1042. doi: 10.1007/s00439-018-1875-2. [DOI] [PubMed] [Google Scholar]
- 55.Nishimoto S, Kawane K, Watanabe-Fukunaga R, et al. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature. 2003;424:1071–1074. doi: 10.1038/nature01895. [DOI] [PubMed] [Google Scholar]
- 56.Dave A, Craig JE, Alamein M, et al. Genotype, age, genetic background, and sex influence Epha2-related cataract development in mice. Invest Ophthalmol Vis Sci. 2021;62(12):3. doi: 10.1167/iovs.62.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mansergh FC, Wride MA, Walker VE, Adams S, Hunter SM, Evans MJ. Gene expression changes during cataract progression in Sparc null mice: differential regulation of mouse globins in the lens. Mol Vis. 2004;10:490–511. [PubMed] [Google Scholar]
- 58.Fan JG, Lerner J, Wyatt MK, Cai P, Peterson K, Dong LJ, Wistow G. The klotho-related protein KLPH (lctl) has preferred expression in lens and is essential for expression of clic5 and normal lens suture formation. Exp Eye Res. 2018;169:111–121. doi: 10.1016/j.exer.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lassen N, Bateman JB, Estey T, et al. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1 (-/-)/Aldh1a1 (-/-) knock-out mice. J Biol Chem. 2007;282(35):25668–25676. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan XJ, Liu XQ, Hao SY, Wang BL, Robinson ML, Monnier VM. The LEGSKO mouse: a mouse model of age-related nuclear cataract based on genetic suppression of lens glutathione synthesis. PLoS One. 2012;7(11):e50832. doi: 10.1371/journal.pone.0050832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, Ye Z, Yin YJ, Ma TJ, Zhang Q, Shang K, Chen WQ, Li ZH. Cataract formation in transgenic HO-1 G143H mutant mice: involvement of oxidative stress and endoplasmic reticulum stress. Biochem Biophys Res Commun. 2021;537:43–49. doi: 10.1016/j.bbrc.2020.12.071. [DOI] [PubMed] [Google Scholar]
- 62.Fan XJ, Sell DR, Hao CL, et al. Vitamin C is a source of oxoaldehyde and glycative stress in age-related cataract and neurodegenerative diseases. Aging Cell. 2020;19(7):e13176. doi: 10.1111/acel.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan XJ, Reneker LW, Obrenovich ME, Strauch C, Cheng RZ, Jarvis SM, Ortwerth BJ, Monnier VM. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci U S A. 2006;103(45):16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu XF, Hao JL, Xie T, Malik TH, Lu CB, Liu C, Shu C, Lu CW, Zhou DD. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. 2017;16(5):934–942. doi: 10.1111/acel.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 66.Maruo T, Kanemaki N, Onda K, Sato R, Ichihara N, Ochiai H. Canine amino acid transport system Xc (-): cDNA sequence, distribution and cystine transport activity in lens epithelial cells. J Vet Med Sci. 2014;76(4):523–530. doi: 10.1292/jvms.13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dun Y, Mysona B, van Ells T, Amarnath L, Ola MS, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res. 2006;324(2):189–202. doi: 10.1007/s00441-005-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42(1):47–54. [PubMed] [Google Scholar]
- 69.Lall MM, Ferrell J, Nagar S, Fleisher LN, McGahan MC. Iron regulates L-cystine uptake and glutathione levels in lens epithelial and retinal pigment epithelial cells by its effect on cytosolic aconitase. Invest Ophthalmol Vis Sci. 2008;49(1):310–319. doi: 10.1167/iovs.07-1041. [DOI] [PubMed] [Google Scholar]
- 70.Hu RG, Lim J, Donaldson PJ, Kalloniatis M. Characterization of the cystine/glutamate transporter in the outer plexiform layer of the vertebrate retina. Eur J Neurosci. 2008;28(8):1491–1502. doi: 10.1111/j.1460-9568.2008.06435.x. [DOI] [PubMed] [Google Scholar]
- 71.Langford MP, Redmond P, Chanis R, Misra RP, Redens TB. Glutamate, excitatory amino acid transporters, Xc⁻ antiporter, glutamine synthetase, and gamma-glutamyltranspeptidase in human corneal epithelium. Curr Eye Res. 2010;35(3):202–211. doi: 10.3109/02713680903461489. [DOI] [PubMed] [Google Scholar]
- 72.Langford MP, Redens TB, Liang CP, Kavanaugh AS, Texada DE. EAAT and Xc⁻ exchanger inhibition depletes glutathione in the transformed human lens epithelial cell line SRA 01/04. Curr Eye Res. 2016;41(3):357–366. doi: 10.3109/02713683.2015.1017651. [DOI] [PubMed] [Google Scholar]
- 73.Lim JC, Lam L, Li B, Donaldson PJ. Molecular identification and cellular localization of a potential transport system involved in cystine/cysteine uptake in human lenses. Exp Eye Res. 2013;116:219–226. doi: 10.1016/j.exer.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Lim J, Lam YC, Kistler J, Donaldson PJ. Molecular characterization of the cystine/glutamate exchanger and the excitatory amino acid transporters in the rat lens. Invest Ophthalmol Vis Sci. 2005;46(8):2869–2877. doi: 10.1167/iovs.05-0156. [DOI] [PubMed] [Google Scholar]
- 75.Li B, Umapathy A, Tran LU, Donaldson PJ, Lim JC. Molecular identification and cellular localisation of GSH synthesis, uptake, efflux and degradation pathways in the rat ciliary body. Histochem Cell Biol. 2013;139(4):559–571. doi: 10.1007/s00418-012-1049-6. [DOI] [PubMed] [Google Scholar]
- 76.Li B, Lee MS, Lee RSY, Donaldson PJ, Lim JC. Characterization of glutathione uptake, synthesis, and efflux pathways in the epithelium and endothelium of the rat cornea. Cornea. 2012;31(11):1304–1312. doi: 10.1097/ICO.0b013e31823f76bd. [DOI] [PubMed] [Google Scholar]
- 77.Martis RM, Li B, Donaldson PJ, Lim JCH. Early onset of age-related cataracts in cystine/glutamate antiporter knockout mice. Invest Ophthalmol Vis Sci. 2021;62(7):23. doi: 10.1167/iovs.62.7.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis VL, Chan CC, Schoen TJ, Couse JF, Chader GJ, Korach KS. An estrogen receptor repressor induces cataract formation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99(14):9427–9432. doi: 10.1073/pnas.132247999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu QX, Zhang YN, Kasetti RB, Gaddipati S, Cvm NK, Borchman D, Li QT. Heterozygous loss of Yap1 in mice causes progressive cataracts. Invest Ophthalmol Vis Sci. 2020;61(12):21. doi: 10.1167/iovs.61.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varadaraj K, Gao JY, Mathias RT, Kumari SS. GPX1 knockout, not catalase knockout, causes accelerated abnormal optical aberrations and cataract in the aging lens. Mol Vis. 2022;28:11–20. [PMC free article] [PubMed] [Google Scholar]
- 81.Gladyshev VN, Jeang KT, Wootton JC, Hatfield DL. A new human selenium-containing protein. Purification, characterization, and cDNA sequence. J Biol Chem. 1998;273(15):8910–8915. doi: 10.1074/jbc.273.15.8910. [DOI] [PubMed] [Google Scholar]
- 82.Kumaraswamy E, Malykh A, Korotkov KV, et al. Structure-expression relationships of the 15-kDa selenoprotein gene. Possible role of the protein in cancer etiology. J Biol Chem. 2000;275(45):35540–35547. doi: 10.1074/jbc.M004014200. [DOI] [PubMed] [Google Scholar]
- 83.Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN. Association between the 15-kDa selenoprotein and UDP-glucose: glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2001;276(18):15330–15336. doi: 10.1074/jbc.M009861200. [DOI] [PubMed] [Google Scholar]
- 84.Yin N, Zheng XX, Zhou J, Liu HM, Huang KX. Knockdown of 15-kDa selenoprotein (Sep15) increases hLE cells' susceptibility to tunicamycin-induced apoptosis. JBIC J Biol Inorg Chem. 2015;20(8):1307–1317. doi: 10.1007/s00775-015-1309-8. [DOI] [PubMed] [Google Scholar]
- 85.Bayle JH, Randazzo F, Johnen G, Kaufman S, Nagy A, Rossant J, Crabtree GR. Hyperphenylalaninemia and impaired glucose tolerance in mice lacking the bifunctional DCoH gene. J Biol Chem. 2002;277(32):28884–28891. doi: 10.1074/jbc.M201983200. [DOI] [PubMed] [Google Scholar]
- 86.Ai Y, Zheng Z, O'Brien-Jenkins A, et al. A mouse model of galactose-induced cataracts. Hum Mol Genet. 2000;9(12):1821–1827. doi: 10.1093/hmg/9.12.1821. [DOI] [PubMed] [Google Scholar]
- 87.Huang P, Jiang ZR, Teng SZ, Wong YC, Frohman MA, Chung SK, Chung SSM. Synergism between phospholipase D2 and sorbitol accumulation in diabetic cataract formation through modulation of Na, K-ATPase activity and osmotic stress. Exp Eye Res. 2006;83(4):939–948. doi: 10.1016/j.exer.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Guo Y, Guo CJ, Zhang J, Ning XN, Yan H. The protective mechanism of Grx2 in ultraviolet-B (UVB)-induced cataract formation. Biochem Biophys Res Commun. 2022;613:107–112. doi: 10.1016/j.bbrc.2022.04.056. [DOI] [PubMed] [Google Scholar]
- 89.Worgul BV, Smilenov L, Brenner DJ, Junk A, Zhou W, Hall EJ. Atm heterozygous mice are more sensitive to radiation-induced cataracts than are their wild-type counterparts. Proc Natl Acad Sci U S A. 2002;99(15):9836–9839. doi: 10.1073/pnas.162349699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang YH, Fu RR, Zhao JY, Wu D, Qiao G, Li RX, Zhang JS. Effects of ELL-associated factor 2 on ultraviolet radiation-induced cataract formation in mice. Mol Med Rep. 2015;12(5):6605–6611. doi: 10.3892/mmr.2015.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishikawa Y, Hashizume K, Kishimoto S, et al. Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp Eye Res. 2012;94(1):85–89. doi: 10.1016/j.exer.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002;74(3):337–347. doi: 10.1006/exer.2001.1153. [DOI] [PubMed] [Google Scholar]
- 93.Eldred JA, Dawes LJ, Wormstone IM. The lens as a model for fibrotic disease. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1301–1319. doi: 10.1098/rstb.2010.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodríguez-Uribe G, Serafín-Higuera N, Damian-Morales G, et al. HPV16-E6 oncoprotein activates TGF-β and Wnt/β-catenin pathways in the epithelium-mesenchymal transition of cataracts in a transgenic mouse model. Biomed Res Int. 2018;2018:2847873. doi: 10.1155/2018/2847873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H, Yuan XY, Li J, Tang X. Implication of Smad2 and Smad3 in transforming growth factor-β-induced posterior capsular opacification of human lens epithelial cells. Curr Eye Res. 2015;40(4):386–397. doi: 10.3109/02713683.2014.925932. [DOI] [PubMed] [Google Scholar]
- 96.Meng FL, Li J, Yang X, Yuan XY, Tang X. Role of Smad3 signaling in the epithelial-mesenchymal transition of the lens epithelium following injury. Int J Mol Med. 2018;42(2):851–860. doi: 10.3892/ijmm.2018.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X, Chen Y, Li CS, Li JK, Zhang SQ, Liang C, Deng Q, Guo ZX, Guo CJ, Yan H. Glutaredoxin 2 protects lens epithelial cells from epithelial-mesenchymal transition by suppressing mitochondrial oxidative stress-related upregulation of integrin-linked kinase. Exp Eye Res. 2023;234:109609. doi: 10.1016/j.exer.2023.109609. [DOI] [PubMed] [Google Scholar]
- 98.Houdebine LM. Transgenic animal models in biomedical research. Methods Mol Biol. 2007;360:163–202. doi: 10.1385/1-59745-165-7:163. [DOI] [PubMed] [Google Scholar]
- 99.Abou Nader N, Zamberlam G, Boyer A. Transgenic mouse models to study the development and maintenance of the adrenal cortex. Int J Mol Sci. 2022;23(22):14388. doi: 10.3390/ijms232214388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moisyadi S, Kaminski JM, Yanagimachi R. Use of intracytoplasmic sperm injection (ICSI) to generate transgenic animals. Comp Immunol Microbiol Infect Dis. 2009;32(2):47–60. doi: 10.1016/j.cimid.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Souyri M, Koehne CF, O'Donnell PV, Aldrich TH, Furth ME, Fleissner E. Biological effects of a murine retrovirus carrying an activated N-ras gene of human origin. Virology. 1987;158(1):69–78. doi: 10.1016/0042-6822(87)90239-x. [DOI] [PubMed] [Google Scholar]
- 102.Dehghan Z, Darya G, Mehdinejadiani S, Derakhshanfar A. Comparison of two methods of sperm- and testis-mediated gene transfer in production of transgenic animals: a systematic review. Anim Genet. 2024;55(3):328–343. doi: 10.1111/age.13404. [DOI] [PubMed] [Google Scholar]
- 103.Malin K, Witkowska-Piłaszewicz O, Papis K. The many problems of somatic cell nuclear transfer in reproductive cloning of mammals. Theriogenology. 2022;189:246–254. doi: 10.1016/j.theriogenology.2022.06.030. [DOI] [PubMed] [Google Scholar]
- 104.Nakano K, Shimizu Y, Arai T, Kaneko T, Okamura T. The versatile electric condition in mouse embryos for genome editing using a three-step square-wave pulse electroporator. Exp Anim. 2022;71(2):214–223. doi: 10.1538/expanim.21-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamanaka Y. CRISPR/Cas9 genome editing as a strategy to study the tumor microenvironment in transgenic mice. Methods Mol Biol. 2016;1458:261–271. doi: 10.1007/978-1-4939-3801-8_19. [DOI] [PubMed] [Google Scholar]
- 106.Bhardwaj A, Nain V. TALENs-an indispensable tool in the era of CRISPR: a mini review. J Genet Eng Biotechnol. 2021;19(1):125. doi: 10.1186/s43141-021-00225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheah SS, Behringer RR. Gene-targeting strategies. Methods Mol Biol. 2000;136:455–463. doi: 10.1385/1-59259-065-9:455. [DOI] [PubMed] [Google Scholar]
- 109.Hu LF, Li YX, Wang JZ, Zhao YT, Wang YM. Controlling CRISPR-Cas9 by guide RNA engineering. Wiley Interdiscip Rev RNA. 2023;14(1):e1731. doi: 10.1002/wrna.1731. [DOI] [PubMed] [Google Scholar]
- 110.Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Mol Vis. 2010;16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- 111.Thompson J, Lakhani N. Cataracts. Prim Care. 2015;42(3):409–423. doi: 10.1016/j.pop.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 112.Zhao WJ, Yan YB. Increasing susceptibility to oxidative stress by cataract-causing crystallin mutations. Int J Biol Macromol. 2018;108:665–673. doi: 10.1016/j.ijbiomac.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 113.Sadik NAH, El-Boghdady NA, Omar NN, Al-Hamid HA. Esculetin and idebenone ameliorate galactose-induced cataract in a rat model. J Food Biochem. 2020;44(7):e13230. doi: 10.1111/jfbc.13230. [DOI] [PubMed] [Google Scholar]
- 114.Lee FK, Chung SK, Chung SS. Aberrant mRNA splicing causes sorbitol dehydrogenase deficiency in C57BL/LiA mice. Genomics. 1997;46(1):86–92. doi: 10.1006/geno.1997.4988. [DOI] [PubMed] [Google Scholar]
- 115.Pichi F, Lembo A, Serafino M, Nucci P. Genetics of congenital cataract. Dev Ophthalmol. 2016;57:1–14. doi: 10.1159/000442495. [DOI] [PubMed] [Google Scholar]