Abstract
Viruses have evolved numerous mechanisms to avoid host immune reactions. Here we report a mechanism by which Parapoxvirus ovis (PPVO) interferes with antigen presentation. PPVO (orf virus) causes orf, an acute skin disease of sheep and goats worldwide. Importantly, PPVO can repeatedly infect its host in spite of a vigorous inflammatory and host immune response to the infection. We demonstrate in a mouse system that PPVO induces apoptosis in a significant number of antigen-presenting cells after intraperitoneal injection using the CD95 pathway, thus preventing a primary T-cell response. We also show that PPVO induces a compensatory activation of the immune system. Our results may help to explain the phenomenon that natural PPVO infections in sheep occur repeatedly even after short intervals. They also suggest that the combination of immunosuppressive and immunostimulatory mechanisms is an effective survival strategy that might be used in other viruses as well.
The immune system is a highly complex surveillance network that has evolved to overcome danger to and destruction of the host (20). Viruses, however, have developed strategies to evade the host's immune response (19). Poxviruses modulate the immune response in infected hosts by (i) inhibiting the synthesis and release of cytokines (interleukin-1 [IL-1]) from infected cells; (ii) interfering with the interaction between a cytokine and its receptor (poxviruses encode soluble cytokine receptors for tumor necrosis factor alpha [TNF-α] and TNF-β, IL-1, and importantly, gamma interferon [IFN-γ]); and (iii) synthesizing virus-encoded cytokines like epidermal growth factor and transforming growth factor α which antagonize the effects of host cytokines mediating the antiviral process (25). Previously, two novel mechanisms of immune escape by poxviruses have been described. Both mechanisms work by inhibition of apoptosis in infected cells. The first mechanism is the direct inhibition of caspases by crmA of cowpox virus (31). A different mechanism is used by Molluscum contagiosum virus, which inhibits apoptosis by means of viral proteins that interfere with the CD95 signaling pathway (known as FLIPs) (1, 10, 29). The complex nature of poxviruses, their replication in the cytoplasm (24), and evidence of immune escape have made poxviruses attractive vectors for gene therapy and vector vaccines (18, 26, 28). However, it has been reported recently that vaccinia virus, an orthopoxvirus, was able to reduce the function of antigen-presenting cells (APC), which is detrimental to the use of vaccinia virus vectors for generating an antitumor or anti-infection immunity (16).
Parapoxvirus ovis (PPVO or orf virus), an epitheliotropic DNA virus, is a member of the Poxviridae family, Parapoxvirus genus, which also includes closely related bovine parapoxviruses and a deer parapoxvirus (27). PPVO causes orf, an acute skin disease of sheep and goats worldwide (8). PPVO can repeatedly infect its host in spite of a vigorous inflammatory and host immune response to the infection (5–7, 13, 23). The failure of vaccination strategies has been described previously (2). In this report, we describe the induction of apoptosis in APC as a mechanism of immune escape used by PPVO. We also demonstrate that the reduced antigen presentation does not result in a general immunosuppression that would have fatal consequences for the host. Instead, PPVO induces a compensatory systemic activation of the immune system that follows the suppression of the immune response. Our results explain the phenomenon that natural PPVO infections in sheep occur repeatedly even after short intervals. They also suggest that the combination of suppressive and stimulatory mechanisms might be an effective viral survival strategy.
MATERIALS AND METHODS
Virus.
PPVO, strain D1701, was propagated in bovine kidney cells (MDBK line). Cells were cultured in Eagle's minimal essential medium with Earle's salt, supplemented with 1% penicillin-streptomycin (10,000 U of penicillin and 10 mg of streptomycin/ml in physiological saline), 1% l-glutamine (200 mM), 1% nonessential amino acids, and 10% (vol/vol) heat-inactivated fetal calf serum. All reagents were from Life Technologies (Gaithersburg, Md.). When the cells were 80 to 90% confluent, they were infected with D1701 and incubated at 37°C and 5% CO2 for 7 days. Material was harvested when approximately 80 to 90% of the cells showed a cytopathic effect. Debris was removed by centrifugation. The supernatant was decanted and centrifuged (Beckman XL-90) for 6 h at 20,000 rpm (4°C). Subsequently, the pellet was resuspended and titrated in a plaque assay on MDBK cells. The supernatant was used as a mock control. For some experiments, the virus was inactivated using binary ethylenimine or purified through a sucrose gradient.
FACS analysis of cell populations after administration of PPVO.
Female 7- to 8-week-old BALB/cJ mice (Bomholdtgard, Ry, Denmark) and male 8- to 10-week-old MRL/MpJ and MRL/MpJ-Faslpr mice (Jackson Laboratory, Bar Harbor, Maine) were used for the experiments. The mice were divided into five treatment groups using 12 mice per group (only treatments 1 to 3 and a limit of six animals per group applied to the MRL/MpJ and MRL/MpJ-Faslpr mice): (i) placebo (nonpyrogenic phosphate-buffered saline [PBS]; Seromed, Berlin, Germany) as a negative control, (ii) supernatant from the virus purification process as another negative control (mock), (iii) active PPVO, strain D1701 (5 × 105 50% tissue culture infective dose [TCID50]), (iv) inactivated PPVO with a similar titer, and (v) complete Freund's adjuvant (CFA; Sigma Chemical Co., St. Louis, Mo.) as an adjuvant control. Treatment was performed intraperitoneally in a volume of 0.2 ml. Six and 12 h after treatment, six mice from each treatment group were sacrificed, peritoneal cells were harvested by peritoneal lavage, and gastric/epigastric/mesenterial and axillary lymph nodes were collected in prechilled PBS. The MRL/MpJ and MRL/MpJ-Faslpr mice were analyzed 6 h after treatment. Subsequently, cells were stained and measured by flow cytometry (Becton Dickinson, San Jose, Calif.). Peritoneal cells were washed twice with PBS for 10 min at 1,500 rpm in a Heraeus Minifuge RF and 4°C and resuspended in fluorescence-activated cell sorter (FACS)-Flow buffer at 106 cells/ml. Lymph nodes were homogenized in cold PBS, and the cell suspension was washed and treated as described above. The cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide for 15 min (PharMingen, Inc., San Diego, Calif.) to differentiate between apoptotic and necrotic cells. After the adequate amount of FACS-Flow buffer was added, cells were analyzed by flow cytometry. The samples were analyzed in doublets and the total number and three cell populations (monocytes/macrophages, granulocytes, and lymphocytes) were analyzed in doublets counting 15,000 cells per measurement. The identity of the cells was tested with anti-CD11b (clone M1/70, phycoerythrin [PE]), antiI-ad/I-Ed (clone 2G9, FITC), anti-CD8a (clone 53–6.7, PE), anti-CD4 (L3T4, clone H129.19, PE), anti-CD69 (clone H1.2F3, FITC), and anti-CG45R/B220 (clone RA3–6B2, PE) (all PharMingen).
Analysis of CD95/CD95L mRNA expression by quantitative reverse transcriptase PCR.
BALB/cJ mice as described above were used for the experiments. The mice were randomized and divided into three treatment groups (23/group): (i) mock, (ii) PPVO (5 × 105 TCID50), and (iii) CFA. The mice were treated intraperitoneally with an administration volume of 0.2 ml. At 6, 12, 24, and 48 h after treatment, six mice of each group (five mice at 48 h) were sacrificed. mRNA was prepared from peritoneal cells, liver, spleen, and gastric/epigastric/mesenterial lymph nodes as recommended by the supplier (Dynabeads mRNA DIRECT Kit; Dynal, Oslo, Norway). Contaminating DNA was removed by DNase I digestion (amplification grade; Life Technologies), and cDNA was synthesized (SUPERSCRIPT, Life Technologies). Expression of CD95 and CD95 ligand (CD95L) mRNA was quantified by real-time PCR (LightCycler; Roche, Mannheim, Germany). The SYBR green I format was used. Three housekeeping genes were measured as an internal standard, and the real expression was determined by establishing the ratio between the results from genes of interest (CD95 and CD95L) and the following housekeeping genes: hypoxanthine guanine phosphoribosyltransferase (HPRT), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin. The specificity of the PCR products was verified by melting curve analysis. The following primer sequences were used: β-actin (sense, 5′-ACCCTGAAGTACCCCATTGAA-3′; antisense, 5′-GGAGAGCATAGCCCTCGTAGA-3′), HPRT (sense, 5′-CCGGCTTCCTCCTCAGA-3′; antisense, 5′-CTTTTATGTCCCCCGTTGACT-3′), GAPDH (sense, 5′-CAGCCTCGTCCCGTAGACA-3′; antisense, 5′-GTTTGGCTCCACCCTTCAAGT-3′), CD95L (sense, 5′-GGCTCCTCCAGGGTCAGTTT-3′; antisense, 5′-GGGGTTGGCTATTTGCTTTTC-3′), and CD95 (sense, 5′-CTGTGGATCTGGGCTGCT-3′; antisense, 5′-TCGCAGTAGAAGTCTGGTTTG-3′). Two microliters of cDNA template was amplified in a reaction volume of 20 μl containing 10 pmol of the appropriate primer pair and 2 μl of SYBR green I. The following cycle numbers were used: 45 (β-actin, CD95) and 40 (GAPDH, HPRT, CD95L). Expression in cells from untreated animals was determined and set as 1. Relative expression in the treatment groups was calculated at each time point.
Blocking of the CD95/CD95L pathway and immunostimulation in herpesvirus-challenged mice.
BALB/cJ-mice were randomized and divided into four treatment groups (10/group): (i) placebo (nonpyrogenic PBS; Seromed); (ii) PPVO, 5 × 105 TCID50; (iii) PPVO, 5 × 105 TCID50 + 200 μg of anti-mouse-Fas ligand (anti-CD95L, NA/LE; PharMingen); and (iv) 200 μg of anti-CD95L. The mice were treated with a volume of 0.2 ml of PPVO or placebo intraperitoneally. Sixteen hours after this treatment, the antibody was administered in a volume of 0.2 ml as described above, and the mice were subsequently infected with a human herpesvirus (herpes simplex virus type 1, strain WAL-Ki, 106 PFU) intranasally.
Statistics.
Wilcoxon's t test was used to evaluate the significance of the results. The mortality rate of the animals was analyzed using log rank analysis (STATISTICA; StatSoft, Tulsa, Okla.).
RESULTS
PPVO induces cellular changes at the contact site.
BALB/cJ mice were injected intraperitoneally with the virus. Six and 12 h after administration of the virus, only low numbers of monocytes/macrophages could be detected by flow cytometry in the peritoneal lavage from PPVO-treated mice (Fig. 1A), whereas significantly more monocytes were counted in the peritoneal lavage of mice treated with a placebo (Fig. 1B, panel 1) or treated with virus-free supernatant from infected cultures (mock) (Fig. 1B, panel 2). No differences could be found among these controls, indicating that soluble cellular proteins or contents of the medium were not responsible for the low number of monocytes/macrophages. No cellular changes were observed in lymph nodes and spleens of these mice, indicating that the response is a local rather than a systemic phenomenon (not shown). Looking at other cell types, we found that the number of infiltrating lymphocytes is decreased dramatically after 12 h in these mice in comparison to the placebo (not shown). In addition, 6 h after application, a significant number of granulocytes invaded the abdominal cavity of PPV-treated animals (Fig. 1B, panels 3 and 4) as well as that of CFA-treated animals (Fig. 1B, panel 5), but the number of viable granulocytes returned to levels that were only marginally and insignificantly different from that in placebo- or mock-treated animals after 12 h.
FIG. 1.
PPVO leads to disappearance of monocytes/macrophages in peritoneal lavages 6 h after intraperitoneal injection (A). The number of cells relative to the placebo (100%) is indicated. In contrast to placebo-treated (B, panel 1) or mock-treated (B, panel 2) animals, monocytes/macrophages (R3) disappear from the application site in PPVO-treated animals (B, panels 3 [active PPVO] and 4 [inactivated PPVO]). A significant amount of granulocytes (R2) accumulates 6 h after application at the site of application of PPVO and in CFA- (B, panel 5) but not in mock- or PBS-treated animals. No changes are observed in lymphocytes (R1) at this time. The data that are shown are typical examples for six animals per group and time point. ∗, P < 0.05; ∗∗, P < 0.001.
PPVO reduces antigen presentation and prevents primary T-cell priming.
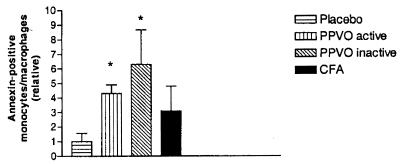
Annexin V labeling of the peritoneal cells showed evidence of early apoptosis in monocytes/macrophages 6 h after administration (Table 1) at the site of injection but not in lymph nodes, spleen, or liver. This was confirmed using a caspase-3 assay (PharMingen, not shown). Apoptosis was not found in cell types other than monocytes/macrophages at that time. In these cells, apoptosis was induced four- to sixfold by both active and inactivated virus, indicating that infection or replication of the virus is not a prerequisite for induction of apoptosis (Fig. 2). In a later experiment, we wanted to answer two other questions. (i) Are the apoptotic monocytes/macrophages indeed professional APC? (ii) Do other APC compensate for the loss and prime naive T cells? We therefore tested the activating properties in a primary T-cell response assay against ovalbumin (OVA) in vivo. The results are shown in Fig. 3. As expected, CFA was an excellent adjuvant for the primary anti-OVA delayed-type hypersensitivity response. However, PPVO was extremely insufficient as an adjuvant. The significantly weaker reaction in PPVO-treated mice than in placebo-treated mice implicates an active process resulting in loss of function for functional APC.
TABLE 1.
Apoptosis caused by different regimensa
| Cell type tested | % Apoptosis caused by
|
||
|---|---|---|---|
| Placebo | PPVO | CFA | |
| Monocytes | 3.3 ± 2.1 | 19.3 ± 6.1b | 6.7 ± 9.2 |
| Lymphocytes | 1.0 ± 0.8 | 0.6 ± 0.2 | 0.4 ± 0.2 |
| Granulocytes | 1.8 ± 1.9 | 0.4 ± 0.2 | 4.6 ± 7.8 |
Apoptosis of monocytes/macrophages is a specific early event that occurs 6 h after administration of PPVO but not of CFA or placebo. The cells were stained with annexin V antibody and analyzed by flow cytometry. The amount of apoptotic cells is indicated as the percentage of each population that was detectable.
P < 0.05 (by the Wilcoxon test) in comparison to the placebo. Other data differ insignificantly from data for the placebo control (PBS).
FIG. 2.
The induction of apoptosis does not depend on infection or viral replication. Administration of either active or inactivated PPVO leads to a four- to sixfold increase of the number of apoptotic cells 6 h after administration at the site of injection (six mice per group and time point). The total number of annexin V-positive monocytes/macrophages in mock-treated animals was set at 1. ∗, P < 0.05.
FIG. 3.
Priming of naive T cells is absent in PPVO-treated mice. BALB/cJ mice (eight per group) were injected subcutaneously with PBS, PPVO, or CFA, each mixed with 10 μg of OVA/20 μl at the base of the tail to provoke hypersensitivity. Seven days later, OVA was injected intradermally into the right hind foot. After another 48 h, the specific foot swelling was measured. To correct for the different individual sizes of feet, the diameter from the unchallenged left hind foot was taken and subtracted from the right measurement. n.s., not statistically significant.
The CD95 pathway is involved in PPVO-induced apoptosis.
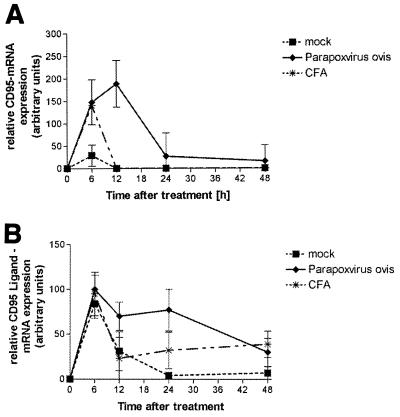
In a subsequent experiment we investigated whether the CD95 pathway is involved in this apoptosis. Results from an expression analysis using the LightCycler system (Roche) are depicted in Fig. 4A and B. CD95 mRNA expression was significantly induced by PPVO at 6 and 12 h after injection. We also detected increased CD95L expression, but it was not significantly different from that in the controls. In order to test whether the CD95/CD95L pathway plays the dominant role in PPVO-mediated apoptosis, we used CD95 (Fas) knockout mice (MRL/MpJ-Faslpr). We could find only a gradual reduction in the population of monocytes/macrophages in the Fas knockout mice (Fig. 5). Although this indicates a change in the cellular pattern, the change was not significant and the number of these cells did not change at later time points (not shown). In contrast, we could observe a significant loss of these cells in PPVO-treated wild-type mice.
FIG. 4.
CD95 mRNA expression is induced in peritoneal lavage cells of PPVO-treated (P < 0.01) and CFA-treated (P < 0.01) animals and marginally in placebo-treated animals 6 h after administration. In contrast, after 12 h, CD95 mRNA expression is detectable only in animals treated with PPVO (P < 0.0001) (A). CD95L mRNA expression is induced in peritoneal cells in all treatment groups. However, mRNA expression declines to nearly undetectable levels after 24 h in placebo-treated animals but remains high in PPVO-treated animals and detectable in CFA-treated animals (B).
FIG. 5.
The CD95/CD95L pathway plays the dominant role in the PPVO-mediated apoptosis of monocytes/macrophages. The mice (six/group) were treated with placebo, mock supernatant, or PPVO and were analyzed 6 h later. In MRL/MpJ-Faslpr mice, we found a reduction in the population of monocytes/macrophages of about 25% after PPVO treatment, whereas the number of monocytes/macrophages was reduced 68.8% in wild-type mice in comparison to the placebo control (P < 0.05).
A systemic stimulation of the immune system is a subsequent event that follows the loss of APC but is not related to the local inhibition of APC function.
We used a monoclonal antibody to block CD95L, thus inhibiting the CD95 signaling pathway. Mice were treated with PPVO, PPVO plus anti-mouse CD95L, and the relevant controls. Sixteen hours after this treatment, the animals were infected intranasally with herpes simplex virus type 1, strain WAL-Ki, which causes high mortality in mice. The results are depicted in Fig. 6. Eighty percent of the mice that were pretreated with PPVO survived for at least 12 days after the lethal challenge with the herpesvirus. In contrast, only 20% of the mice that were pretreated with placebo survived. The administration of a CD95L antibody did modulate the clinical outcome of the infection as well as leading to a higher survival rate in these mice (40%). This was not statistically significant. However, when pretreated with PPVO, 80% of these animals survived (P < 0.05).
FIG. 6.
The local suppression of the immune response is systemically compensated. BALB/cJ mice were treated with PPVO or placebo. After 16 h, anti-CD95L antibodies were administered and the mice were infected with a lethal dose of herpesvirus. Eighty percent of the placebo-pretreated mice died after 12 days. In contrast, PPVO pretreatment led to significantly prolonged survival (n = 10; P < 0.05), indicating a systemic stimulatory effect that occurs about 1 day after PPVO exposure. Blocking the CD95/CD95L pathway of apoptosis did modulate the course of herpes and led to a higher survival rate in these mice (not statistically significant). However, when pretreated with PPVO, 80% of the animals survived (P < 0.05).
DISCUSSION
PPVO can repeatedly infect sheep and goats in spite of a vigorous inflammatory and host immune response to the infection (5–8, 13, 23). In addition, vaccination strategies against PPVO have failed (2) or do not protect vaccinated animals from reinfection (8). We wanted to investigate the mechanism(s) that is responsible for PPVO escape from the immune system. An infection model in laboratory animals has not previously been described. The induction of a Th1-type immune response following a natural infection in sheep (5, 6), which was confirmed in our studies in mice (data not shown), makes it difficult if not impossible to establish a model of infection in the immunocompetent mouse. The purpose of our study was to investigate the immune response to PPVO in a well-characterized and immunocompetent system. In our experiments, PPVO induced a dramatic change of the cellular pattern in peritoneal lavage cells after intraperitoneal injection into mice of either active or inactivated virus. In an in vitro system, the presence of PPVO prevented mitogen-driven T-cell activation (unpublished data). We focused on cells that play a role in the generation of an antiviral immune response, such as APC and lymphocytes. We could demonstrate that PPVO induced apoptosis in the population of monocytes that serves as a source for APC. This was not related to viral infection or replication, since both living and inactivated virus caused apoptosis in these cells. We have also shown in different strains of mice that the CD95/CD95L pathway of apoptosis is induced by PPVO and that apoptosis is restricted to APC at the site of exposure to the virus. The causative viral protein is likely to be a structural component since, like apoptosis, CD95 expression was induced with both active and inactivated virus. Cellular components from infected cells are incorporated into the virus during morphogenesis (11, 30) but do not play a role in this process, since animals treated with virus-free supernatants from infected cultures (mock) did not show any evidence of CD95 expression or apoptosis. On the other hand, virus that was highly purified through a sucrose gradient induced CD95 expression (data not shown). Mutational analyses are in progress in order to identify the gene product(s) that causes the CD95 induction. Apoptosis was an immediate event and could already be detected at the site of application at 6 h after administration of virus. In contrast, CD95 mRNA expression peaked 12 h after administration of PPVO, at which time no macrophages could be detected. This indicates that apoptotic mechanisms different from the CD95/CD95L pathway might also contribute to the loss of APC and that the induction of CD95 expression is not restricted to monocytes/macrophages. We also made the observation that CD95 expression is enhanced in lymphocytes after administration of PPVO (not shown).
Intensive cellular changes at the site of infection also parallel the clinical picture and the clearance of the virus in natural infections with PPVO (12–15). In sheep, the early response is characterized by an influx of neutrophils followed after several days by the accumulation of CD4+ T cells, CD8+ T cells, and B cells adjacent to and underlying PPVO-infected epidermal cells (12–15). An increase of IL-1, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) levels followed by rapid decline has been described in natural PPVO infections at very early time points (5). These cytokines are involved in the recruitment of granulocytes to the site of infection as part of the host inflammatory response to this infection. The priming of neutrophils by PPVs is known and has been described elsewhere (4). The use of GM-CSF to enhance the concentration of APC in vivo has been described recently (17), and it is very likely that the increased number of granulocytes might be the source of GM-CSF for the recruitment of new APC that restore the initial loss. Indeed, accumulation of dendritic cells at the site of viral entry has been described in natural infections (12). Neutrophilic granulocytes play an important role in the inflammatory response which may result in tissue damage. However, a granulocyte colony-stimulating factor secreted by granulocytes has also been proven to be an anti-inflammatory immunomodulator (9). We did not look for anti-inflammatory granulocyte colony-stimulating factor in our experiments. However, induction of IFN-α and -β, IL-2, and TNF-α has been described following a natural infection with PPVO (3, 5, 6) and could be confirmed in our studies monitoring induction of expression of the mRNA of these cytokines (not shown).
Our observations also indicate that the PPVO-induced loss of APC is a local event that occurs at the site of application. We did not observe any cellular changes in a pool of lymph nodes or in spleens of treated mice up to 24 h after administration. In contrast, 2 to 3 days after injection of inactivated PPVO, the number of lymphocytes was found to be significantly increased (22), an increase confirmed in our studies (not shown). PPVO has been reported to have stimulatory effects on the immune system (21, 22). This cascade of immunological events would be an excellent strategy for the virus to survive. Locally, PPVO prevents the host's immune response against viral proteins and therefore generates the possibility of replicating and reinfecting the host at any time. Later and systemically, the virus protects the host from any fatal consequences of the suppression of the immune system. We confirmed that PPVO stimulates the immune system and could demonstrate that the immunostimulating properties are not related to the CD95-mediated apoptosis of APC.
Altogether, in this report we show evidence for an immune escape mechanism of a poxvirus by induction of apoptosis in APC. The combination of suppressive and stimulatory mechanisms seems to be a very successful viral survival strategy. Since this is the second poxvirus that was demonstrated to harm APC function, our results suggest that other viruses may modulate the immune system through this mechanism. These results might contribute to an explanation of the phenomenon that natural PPVO infections in sheep occur repeatedly even after short intervals. They also suggest that a careful selection of viral vectors should be made if these vectors are to be used to transduce APC for immunotherapy.
ACKNOWLEDGMENTS
We thank Holger Dethlefsen, Uwe Reimann, Diana Guntermann, Holger Spiecker, and Petra Koch for excellent technical assistance.
REFERENCES
- 1.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buddle B B, Dellers R W, Schurig G G. Contagious ecthyma virus-vaccination failures. Am J Vet Res. 1984;45:263–266. [PubMed] [Google Scholar]
- 3.Buettner M, Czerny C-P, Lehner K-H, Wertz K. Interferon induction in peripheral blood mononuclear leukocytes of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet Immunol Immunopathol. 1995;46:237–260. doi: 10.1016/0165-2427(94)05357-x. [DOI] [PubMed] [Google Scholar]
- 4.Foerster R, Wolf G, Mayr A. Highly attenuated poxviruses induce functional priming of neutrophils in vitro. Arch Virol. 1994;136:219–226. doi: 10.1007/BF01538831. [DOI] [PubMed] [Google Scholar]
- 5.Haig D M, Deane D L, Percival A, Myatt N, Thomson J, Inglis L, Rothel J, Seow H F, Wood P, Miller H R P, Reid H W. The cytokine response of afferent lymph following orf virus reinfection of sheep. Vet Dermatol. 1996;7:11–20. doi: 10.1111/j.1365-3164.1996.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 6.Haig D, McInnes C, Deane D, Lear A, Myatt N, Reid H, Rothel J, Seow H F, Wood P, Lyttle D, Mercer A. Cytokines and their inhibitors in orf virus infection. Vet Immunol Immunopathol. 1996;54:261–267. doi: 10.1016/s0165-2427(96)05687-5. [DOI] [PubMed] [Google Scholar]
- 7.Haig D, McInnes C J, Deane D, Reid H W, Mercer A A. The immune and inflammatory reponse to orf virus. Comp Immunol Microbiol Infect Dis. 1997;20:197–204. doi: 10.1016/s0147-9571(96)00045-8. [DOI] [PubMed] [Google Scholar]
- 8.Haig D M, Mercer A A. Ovine diseases. Orf. Vet Res. 1998;29:311–326. [PubMed] [Google Scholar]
- 9.Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol. 1998;5:221–225. doi: 10.1097/00062752-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Vincenz C, Buller M, Dixit V M. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;15:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 11.Ichihashi Y, Matsumoto S, Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971;46:507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson D M, Hutchinson G, Onwuka S K, Reid H W. Changes in the MHC class II dendritic cell population of ovine skin in response to orf virus infection. Vet Dermatol. 1991;2:1–9. doi: 10.1111/j.1365-3164.1991.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 13.Jenkinson D M, Hutchinson G, Reid H W. The B and T cell responses to orf virus infection of ovine skin. Vet Dermatol. 1992;3:57–64. [Google Scholar]
- 14.Jenkinson D M, McEwan P E, Onwuka S K, Moss V A, Elder H Y, Hutchinson G, Reid H W. The polymorphonuclear and mast cell responsees in ovine skin infected with orf virus. Vet Dermatol. 1990;1:71–79. doi: 10.1111/j.1365-3164.1990.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson D M, McEwan P E, Onwuka S K, Moss V A, Elder H Y, Hutchinson G, Reid H W. The pathological changes and polymorphonuclear and mast cell responses in the skin of specific pathogen free lambs following primary and secondary challenge with orf virus. Vet Dermatol. 1990;1:139–145. doi: 10.1111/j.1365-3164.1990.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenne L, Hauser C, Arrighi J F, Saurat J H, Huegin A W. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7:1575–1583. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- 17.Kass E, Parker J, Schlom J, Greiner J W. Comparative studies of the effects of recombinant GM-CSF and GM-CSF administered via a poxvirus to enhance the concentration of antigen-presenting cells in regional lymph nodes. Cytokine. 2000;12:960–971. doi: 10.1006/cyto.2000.0684. [DOI] [PubMed] [Google Scholar]
- 18.Katz E, Moss B. Immunogenicity of recombinant vaccinia viruses that display the HIV type 1 envelope glycoprotein on the surface of infectious virions. AIDS Res Hum Retrovir. 1997;13:1497–1500. doi: 10.1089/aid.1997.13.1497. [DOI] [PubMed] [Google Scholar]
- 19.Marrack P, Kappler J. Subversion of the immune system by pathogens. Cell. 1994;76:323–332. doi: 10.1016/0092-8674(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 20.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 21.Mayr A, Buettner M, Wolf G, Meyer H, Czerny C. Experimenteller Nachweis paraspezifischer Wirkungen von gereinigten und inaktivierten Pockenviren. Zentbl Vetmed Reihe B. 1989;36:81–99. [PubMed] [Google Scholar]
- 22.Mayr A, Buettner M. Paraspezifisches Immunsystem. Veterinarmedizin. 1992;7:31–33. [Google Scholar]
- 23.McKeever D J, Jenkinson D M, Hutchinson G, Reid H W. Studies on the pathogenesis of orf virus infection in sheep. J Comp Pathol. 1988;99:317–328. doi: 10.1016/0021-9975(88)90052-7. [DOI] [PubMed] [Google Scholar]
- 24.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2637–2671. [Google Scholar]
- 25.Pickup D J. Poxviral modifiers of cytokine responses to infection. Infect Agents Dis. 1994;3:116–127. [PubMed] [Google Scholar]
- 26.Robinson A J, Lyttle D J. Parapoxviruses: their biology and potential as recombinant vaccines. In: Binns M, Smith G, editors. Recombinant poxviruses. Boca Raton, Fla: CRC Press; 1992. pp. 306–317. [Google Scholar]
- 27.Robinson A J, Mercer A A. Parapoxvirus of red deer: evidence for its inclusion as a new member in the genus parapoxvirus. Virology. 1995;208:812–815. doi: 10.1006/viro.1995.1217. [DOI] [PubMed] [Google Scholar]
- 28.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 29.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schröter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler C E, Cawley E P. The microscopic appearance of ecthyma contagiosum (orf) in sheep, rabbit and man. Am J Pathol. 1956;32:535–545. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]