Abstract
Lipids are crucial for human health and reproduction and include diverse fatty acids (FAs), notably polyunsaturated FAs (PUFAs) and short-chain FAs (SCFAs) that are known for their health benefits. Bioactivities of PUFAs, including ω-6 and ω-3 FAs as well as SCFAs, have been widely studied in various tissues and diseases. Epigenetic regulation has been suggested as a significant mechanism affecting the progression of various diseases, including cancers and metabolic and inflammatory diseases. Epigenetics encompasses the reversible modulation of gene expression without altering the DNA sequence itself, mediated by mechanisms such as DNA methylation, histone acetylation, and chromatin remodeling. Bioactive FAs have been demonstrated to regulate gene expression via epigenetic modifications that are potentially important for modulating metabolic control and disease risk. This review paper discusses the evidence in support of bioactive FAs, including ω-6 and ω-3 FAs and SCFAs, eliciting various disease prevention via epigenetic regulation including methylation or acetylation.
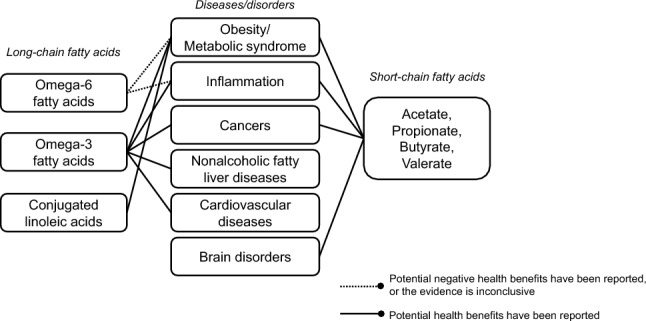
Graphical abstract
Keywords: Disease, Epigenetics, Polyunsaturated fatty acid, Regulation, Short-chain fatty acid
Introduction
Human lipids are required to maintain health. Lipids that are solid at room temperature are fats, and liquids are oils. Lipids are a diverse group of chemical compounds; among them, triglycerides are the most common type of lipid found in the body and in foods. Each triglyceride molecule consists of glycerol and three attached fatty acids (FAs). FAs vary in the number, arrangement, and configuration of double bonds along the hydrocarbon chain (Lehninger Principles of Biochemistry). The carbon atoms in unsaturated FAs contain less hydrogen than those of saturated FAs because of some unfilled bonds or double bonds. Monounsaturated FAs (MUFAs) contain at least 12 carbon atoms with single double bonds, whereas polyunsaturated FAs (PUFAs) have two or more double bonds (de Roos et al., 2009; Karapanagiotidis et al., 2007). Omega-6 (ω-6) and omega-3 (ω-3) are two major classes of PUFAs, and they are considered essential FAs that indicate that FAs should be obtained from foods since they cannot be synthesized or produced by humans.
Short-chain FAs (SCFAs) contain fewer than six carbon atoms, while medium-chain FAs are 6–12 carbons long, and long-chain FAs contain 14 or more carbon atoms. SCFAs are microbial metabolites that are formed in the large intestine by the fermentation of soluble fibers and proteins, and are excreted through feces (Morrison and Preston, 2016; Rios-Covian et al., 2016). SCFAs exhibit several health benefits. For example, SCFAs can be absorbed and transported to the liver where they suppress cholesterol synthesis, contributing to decreased serum cholesterol levels (Hara et al. 1999). Indeed, a meta-analysis by Brown et al. revealed that a diet rich in soluble fiber elicits small but significant reductions in serum cholesterol levels, particularly in the form most related to atherosclerosis and coronary heart disease (Brown et al., 1999). Since SCFAs are the products from microbial fermentation that can be absorbed in the large intestine (Bergman, 1990), to achieve health beneficial effect, daily consumption of foods rich in soluble fiber is important.
FAs can modify epigenetic landmarks, including histone acetylation, deacetylation, and methylation, thereby modulating the expression of genes involved in several pathways. These pathways are associated with lipid metabolism, insulin sensitivity, and cancer, and there have been many studies on epigenetic regulation by functional FAs (Arents et al., 1991; Goldberg et al., 2007; Li and Li, 2006). The purpose of this review paper is to summarize evidence of the crucial role of bioactive FAs including ω-6 and ω-3 FAs and SCFAs in various diseases progression via epigenetic regulation.
Epigenetic modifications
Epigenetic regulation has been suggested as a significant mechanism affecting the progression of various diseases, including cancers and metabolic and inflammatory diseases. Epigenetics refers to the phenomenon in which gene expression changes reversibly through DNA methylation, histone acetylation, and chromatin folding without changing the DNA sequence (Lillycrop and Burdge, 2015; Weinhold, 2006).
DNA methylation is a process mediated by DNA methyltransferase (DNMT) enzymes, involving the covalent addition of a methyl group to the C-5 position of cytosine nucleotides. This leads to the production of 5-methylcytosine (Jin et al., 2011; Shimizu et al., 2019) that is a methylated form of the DNA base cytosine that regulates gene transcription.
Histone modification involves the covalent attachment of functional groups to amino acids, mostly on the N-terminal histone tails, and includes acetylation, methylation, and phosphorylation (Bowman and Poirier, 2015). Histone acetyltransferases (HATs) constitute a diverse group of enzymes responsible for attaching acetyl groups to the ε-amino group of lysine residues on both histone and non-histone proteins. Histone deacetylases (HDAC) catalyze the removal of acetyl groups (Berndsen and Denu 2008; Gong and Miller, 2013; Hodawadekar and Marmorstein, 2007; Marmorstein and Zhou, 2014; Wang et al., 2014). Acetylation leads to the transformation of condensed chromatin into a more relaxed structure, making it more accessible and facilitating gene expression, whereas deacetylation causes chromatin compaction, making genes less accessible for transcription (Berndsen and Denu, 2008; Liu and Xu, 2004).
After being first defined by Waddington (1942), the term ‘epigenetics’ has been increasingly used and has been actively studied to investigate its significant role in tumor development and metastasis (Lee and Kim, 2022). Numerous studies have demonstrated that changes in histone acetylation can lead to cancer. Overexpression and increased activity of HDACs have been demonstrated to cause tumor development and metastasis by regulating histone acetylation and expression of oncogenes, including p300 and CREBP-binding protein (CBP) (Cohen et al., 2011; Sugiura et al. 2021). Additionally, HDAC1 catalyzes the deacetylation of H3K27 at the STAT1 promoter, leading to an immunosuppressive environment that promotes the progression of glioma stem-like cells (Sugiura et al., 2021). HDAC Administration has approved HDAC inhibitors for cancer treatment drug by the US FDA (Jones et al., 2016). Beyond research on cancer, studies reveal that epigenetic modifications like DNA methylation and histone acetylation influence the expression of genes associated with obesity and type 2 diabetes including INS, GLUT4, ADIPOq, FTO, and C/EBPβ (Kuroda et al., 2009; Toperoff et al., 2012; Yokomori et al., 1999; Zheng et al., 2011; Bouchard et al. 2012). Obesity and high-fat diets can induce epigenetic modifications within adipose tissue, leading to increased expression of genes encoding inflammatory cytokines and chemokines (Jung and Kang, 2021), indicating that inflammation-induced metabolic diseases can be epigenetically regulated in overweight or obese individuals.
In addition to DNA methylation and histone acetylation/deacetylation, the gut microbiota is critical for epigenetic regulation. In the folate metabolic pathway, folate is metabolized to tetrahydrofolate, 5-methyltetrahydrofolate (5-MTHF), and 5-formyltetrahydrofolate. The most effective and stable form, 5-MTHF, plays a crucial role in methylation (Liu et al., 2022) because it serves as a methyl donor by converting homocysteine to methionine in the presence of methionine synthase (MS) and vitamin B12 as a cofactor (Shane, 2008). Homocysteine and S-adenosylhomocysteine (SAH) are important products of SAM methylation by DNA methyltransferases. SAM-subtracted DNA methylation is predominantly directed towards CpG dinucleotides, where cytosine is converted to 5-methylcytosin. These CpGs tend to occur on islands that are abundant in the promoter regions of genes that are regulated in their expression by methylation (Muskiet and Kemperman, 2006). Hence, folate deficiency (and other vitamins working as co-factors) or abnormal metabolism caused by the gut microbiota is associated with several abnormal conditions, including reductions in DNA methylation, and this can promote tumor cell proliferation and growth in the intestinal mucosa (Zheng and Cantley, 2019; Rossi et al., 2011). Diet or dietary modifications can cause dramatic effects on the composition of the gut microbiota, and this act as critical regulators of health and disease (Licciardi et al., 2011), therefore, the role of SCFAs, metabolites of the gut microbiota, and bioactive FAs is important.
Epigenetic regulation of long-chain FAs in diseases progression
Omega-6 FAs
Linoleic acid (LA) is an essential FA and an important component of human diet. As the major ω-6 FAs, LA is found in various plant oils (e.g., corn oil, soybean oil, palm oil) and butter fat (Mercola and D'Adamo, 2023). Arachidonic acid (AA), a derivative of LA, is found in breast milk; therefore, it is added to infant formulas to mimic breast milk (Brenna et al., 2007). In the first chapter, ω-6 FAs LA, and AA are discussed in various diseases progression in terms of epigenetic regulation.
Obesity and metabolic disorders
LA intake has a negative effect on obesity prevention. In a cross-sectional study using young women, ω-6 PUFA intake positively associated with truncal fat, body mass index (BMI), and waist circumference (Hermsdorff et al., 2013). Some studies have demonstrated that higher ω-6 PUFA may increase the risk of obesity via epigenetic changes particularly in later generations. For example, Massiera et al. found that diet greater in LA and low in ω-3 FA (α-linolenic acid), and this are similar to the diet common in the US (Ailhaud et al., 2006; Massiera et al., 2010), change gene expressions, and this lead to increase in obesity prevalence. In their study, exposure to Western diet for four generations caused up to two-fold changes in the expression of 22 genes associated with increased adiposity, angiogenesis, and inflammation, as well as an accumulative increase in fat mass over generations (Massiera et al. 2010). AA causes gene-specific DNA methylation. Paternal or maternal exposure to AA induces cumulative weight gain in offspring generations without an increase in liver fat and with decreased hepatic Scd1 promoter methylation (de la Rocha et al., 2022). This study also found that AA exposure across generations was potentially beneficial to the innate immune system in mice (de la Rocha et al., 2022).
Inflammation and cancers
LA intake likely exhibits a negative relationship, as previously mentioned. LA has also been demonstrated to increase the risk of colon cancer (Zock and Katan, 1998). An LA diet increases the risk of colon cancer through cytochrome P450 (CYP) monooxygenase that converts LA to epoxy octadecenoic acids that are known to act as potent promoters of colon tumorigenesis (Zhang et al., 2023). Chronic intake of diet high in ω-6 PUFA activates FXR expression via CpG demethylation thereby increasing the genes whose products are involved in the regulation of bile acid homoeostasis. Meanwhile, the chronic intake of ω-6 PUFA diet caused decreased adenomatous polyposis Coli (APC) expression through CpG hypermethylation, leading to COX-2-mediated inflammation and thus together contributing to exacerbate colon cancer (Romagnolo et al., 2019). In a cross-sectional study using young women, the authors found that ω-6 PUFA intake positively related with truncal fat, and women with greater truncal adiposity had lower methylation levels of TNFα promoter in peripheral white blood cells and higher plasma TNFα concentrations (Hermsdorff et al., 2013). Linear regression model in this study suggest that TNFα promoter methylation correlated with ω-6 PUFA intake (Hermsdorff et al., 2013).
The pro-inflammatory property of AA are well known. AA induces DNA hypermethylation in human and mouse cultured cells that was observed in PPAR-α and sirtuin 1 as important mediators of FA metabolism and β-oxidation (Silva-Martinez et al., 2016). The AA-induced DNA methylation profiles were similar to those described for palmitic acid, atherosclerosis, diabetes, obesity, and autism (Silva-Martinez et al., 2016). Collectively, ω-6 PUFA including LA and AA could exacerbate the proinflammatory state and further inflammation-related diseases including cancers or metabolic diseases. Epigenetic mechanisms regulated by ω-6 PUFA in various diseases states are summarized in Table 1.
Table 1.
Epigenetic mechanism regulated by ω-6 fatty acids in various disease states
| Fatty acids | Diseases/states | Epigenetic mechanism | References |
|---|---|---|---|
| Linoleic acid (LA) | Inflammatory states, cancers | ↑ FXR expression via CpG demethylation | Romagnolo et al. (2019) |
| ↓ APC expression via CpG hypermethylation | Romagnolo et al. (2019) | ||
| ↓ TNFα promoter methylation | Hermsdorff et al. (2013) | ||
| Arachidonic acid (AA) | Weight gain | ↓ hepatic Scd1 promoter methylation | de la Rocha et al. (2022) |
| Inflammation | ↑ PPAR-α and sirtuin 1 hypermethylation | Silva-Martinez et al. (2016) |
Omega-3 FAs
-3 PUFA has long been actively studied to have a number of health benefits including protection against cardiovascular diseases (Dyerberg et al., 1975, 1978), anti-inflammatory activity (Smith and Guentzel, 2010), anti-obesity activity (Makhoul et al., 2011), improvement of endothelial function (Tousoulis et al., 2014), reduction of blood pressure (Cicero et al., 2009), and anti-cancer activity in skin and oral (Nikolakopoulou et al., 2013). However, the epigenetic mechanisms underlying these processes have not been clearly defined.
Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have unique biological effects compared to other FAs, indicating that their unique physical and biochemical properties are likely attributable to their longer chin length and higher number and position of double bonds (Deckelbaum et al., 2006; Seo et al., 2005). Female Wistar rats were fed either a control diet or an experimental diet containing EPA, DHA, and alpha-linolenic acid (ALA) for two months, mated, and continued on their diets during pregnancy. On gestation days–18–20, the rat placenta and fetal liver were isolated, and increased global DNA methylation, decreased H2A and H2B acetylation, and decreased HAT activity were detected in the placenta and fetal liver. In parallel, DNA-binding ability of PPAR-α was decreased in fetal liver, indicating that ω-3 PUFA including DHA, EPA, and ALA modify DNA methylation and HAT activity and acetylation thereby preventing the development of fatty liver and placenta (Ramaiyan and Talahalli, 2018). Another ω-3 PUFA docosapentaenoic acid (DPA) is a long-chain FA and found in fish, red meat and milk of ruminant animals in our diet. Although the health benefits of DPA have not been extensively studied, evidence exists regarding its effects on liver health. In the second chapter, ω-3 FAs ALA, EPA, DHA, and DPA are discussed in various diseases progression in terms of epigenetic regulation.
Inflammatory state
Higher ω-3 PUFA is associated with lower methylation at IL6 promoter, thereby reducing IL-6 gene expression (Ma et al., 2016). Indeed, Ma et al. found that higher methylation of IL6 promoter cg01770232 was associated with higher IL-6 plasma concentrations (p = 0.03) and greater IL-6 gene expression (p = 0.0005). Higher circulating total ω-3 PUFA was associated with lower cg01770232 methylation (p = 0.007) and lower IL-6 concentration (p = 0.02) that was analyzed using the data in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study and the Encyclopedia of DNA Elements (ENCODE) consortium (Ma et al., 2016). In PREDIMED study, Arpon et al. demonstrated the effect of the Mediterranean diet on DNA methylation. In this study, two diets 1) MedDiet with extra virgin olive oil and 2) MedDiet with nuts) were compared to a low-fat control diet during five-year follow-up. They found that the MedDiet with nuts favored hypermethylation of cg01081346 in CPT1B/CHKB-CPT 1 B genes, and the MedDiet + EVOO induced hypomethylation of CG17071192 in GNAS/GNASAS genes GNAS/GNASAS. Both diets are associated with intermediate metabolism and improved expression of genes involved in diabetes and inflammatory pathways (Arpon et al., 2017). This indicates that diet rich in ω-3 PUFA contribute to the regulation of methylation thereby contributing to prevent inflammation and related diseases progression. Anti-inflammatory effects of ω-3 PUFA via epigenetic regulation have been widely studied in a number of diseases. Rats fed with higher ω-3 PUFA diet exhibited lower tumor incidence compared to non-treated group, and this was associated to DNA methylation (Huang et al., 2016).
Cancers
Two of the most studied epigenetic modifications, DNA methylation and histone acetylation, drive and maintain the cancer phenotype. While DNA methyltransferase (DNMT) dysregulation promotes localized hypermethylation of CpG-rich regions, activated histone deacetylases (HDAC) deacetylate histone tails. These modifications cause the chromatin conformation to close, suppressing the transcription and silencing of tumor suppressor genes (Ceccarelli et al., 2020). The anticancer properties of EPA are mediated through the inhibition of HDAC1 and DNMTa, 3A, and 3 B expression, thereby promoting the expression of tumor suppressor genes in hepatocarcinoma cells (Ceccarelli et al., 2020).
Combined treatment with DHA and butyrate significantly decreased the methylation of pro-apoptotic Bcl2l11, Cideb, Dapk1, Ltbr, and Tnfrsf25 genes in HCT-116 colon cancer cells compared to untreated controls, suggesting that apoptosis induction by DHA and butyrate is partly mediated through changes in the methylation state of apoptosis-related genes (Cho et al., 2014). The anti-CRC effects of EPA and DHA via DNA methylation have been demonstrated in vitro (Moradi Sarabi et al., 2019). ALA also regulates cancer-related epigenetic modifiers by targeting key signaling pathways related to transcriptional regulation, miRNA involvement, stem cell pluripotency, and cellular senescence in cancer epigenetics (Ulhe et al., 2023).
Obesity and metabolic disorders
ω-3 PUFAs have anti-obesity effect by reducing triacylglycerol (TG) concentration (Dunbar, 2014 – Tousoulis et al., 2014), and higher EPA and DHA is associated with attenuation in TG and C-reactive protein (Makhoul et al., 2011). Tremblay et al. investigated the effects of n-3 PUFA supplementation in overweight and obese subjects on DNA methylation-mediated biological pathways (Tremblay et al., 2017). Genome-wide DNA methylation analysis indicates that following 6-month ω-3 PUFA supplementation differentially methylated 308CpG sites. Epigenetic modifications can influence pathways involved in inflammatory and immune responses, lipid metabolism, type 2 diabetes, and cardiovascular diseases (Tremblay et al., 2017). Shen et al. has studied of the effects of ω-3 PUFA on leptin promoter and thereby regulating leptin gene transcription in obesity. Higher ω-3 PUFA diet induced methylation of CpG island and the binding of methyl-CpG binding domain protein 2 (MBD2) and DNA methyltrnasferases (DNMTs) at leptin promoter. This was accompanied by hypoacetylation of H3 and H4 and increased binding of histone deacetylases (HDACs) 1, 2, and 6 to the leptin promoter (Shen et al., 2014). This study suggests that obesity inhibits enzymes that catalyze DNA methylation and histone modification, contributing to the maintenance of leptin levels within the normal range, thereby preventing or treating obesity (Shen et al., 2014). In vitro evidence further support that ω-3 PUFA attenuates lipid accumulation via HAT inhibition in 3T3-L1 adipocytes (Chung et al., 2020). CBP/p300 causes acetylation of sterol regulatory element-binding protein (SRECP-1c), a transcription factor involved in lipid accumulation, thereby promoting the expression of FA synthase, glycerol-3-phosphoate acyltransferase, etc. (Wang et al., 2015; Yang et al., 2001). Chung et al. demonstrated that treatment with EPA and DHA significantly inhibited in vitro HAT activity, thereby regulating lipid metabolism-associated gene expression. Similarly, in a study using goat mammary epithelial cells, DHA affected lipid metabolism via genome-wide H3K9ac changes, thereby regulating the PDK4-AMPK-SREBP1 signaling axis, suggesting that DHA regulates mammary cell functions through epigenetic changes (Wu et al., 2023).
Nonalcoholic fatty liver diseases
A high dietary intake of sucrose/fructose, cholesterol and saturated fat is associated with fatty liver in humans (Abdelmalek et al., 2010; Tiniakos et al., 2010), and high fat high calories diet caused nonalcoholic fatty liver diseases (NAFLD) via DNA methylation and histone modifications (Zaiou et al., 2021), On the other hand, ω-3 PUFAs may contribute to the protection of NAFLD development. Although DHA was more effective in reducing hepatic FAS levels than EPA, it was most effective in increasing hepatic DHA levels and preventing the progression of steatosis by reducing FAS and fibrosis markers (Hong et al., 2019).
Kaur et al. (2011) demonstrated that DPA treatment (equivalent to EPA and DHA) significantly reduced SREBP-1c, 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A reductase (HMG-CoA reductase), Acetyl Coenzyme A Carboxylase (ACC-1), and FA Synthase (FASn) levels in rat liver cells, suggesting the possibility of DPA preventing fatty liver. However, the epigenetic regulation of DPA has not yet been studied; thus, further studies are warranted.
Cardiovascular diseases
EPA and DHA decrease the risk of cardiovascular disease (CVD) (Mozaffarian et al., 2005; Willett, 2007). A number of studies have demonstrated that ω-3-mediated CVD protective effect was also mediated via epigenetic modifications. In a study by Trembly et al., researchers investigated the effects of ω-3 fatty acid supplementation on overweight and obese individuals. They compared genome-wide DNA methylation profiles of blood leukocytes before and after a 6-week intervention with 3 g of ω-3 FAs daily (Tremblay et al., 2017). The study also revealed correlations between changes in DNA methylation profiles of blood leukocytes, particularly at CpG sites within AKT3, ATF1, HDAC4, and IGFBP5 genes, and variations in plasma triglyceride, glucose, and cholesterol levels following ω-3 fatty acid supplementation (Trembly et al., 2017). This suggests a potential insight into the metabolic pathways following ω-3 PUFA consumption in patients with CVD. Epigenetic mechanisms regulated by ω-3 PUFA in various diseases states are summarized in Table 2.
Table 2.
Epigenetic mechanism regulated by ω-3 fatty acids in various disease states
| Fatty acids | Diseases/states | Epigenetic mechanism | References |
|---|---|---|---|
| EPA/DHA/ALA | Fatty liver | ↑ global DNA methylation | Ramaiyan and Talahalli (2018) |
| ↓ H2A and H2B and acetylation | Ramaiyan and Talahalli (2018) | ||
| ↓ IL-6 promoter methylation | Ma et al. (2016) | ||
| EPA | Cancers | Inhibition of HDAC1 and DNMT | Ceccarelli et al. (2020) |
| DHA&Butyrate | Cancers | ↓ methylation of apoptosis-related genes | Cho et al. (2014) |
| EPA&DHA | Cancer | DNA methylation | Moradi Sarabi et al. (2019) |
| ALA | Cancers | MicroRNA involvement | Ulhe et al. (2023) |
| EPA&DHA | Obesity and metabolic disorders |
Methylation of leptin promoter, hypoacetylation of H3 and H4, increased HDACs binding |
Shen et al. (2014) |
| EPA/DHA | Obesity and metabolic disorders | Inhibition of HAT activity | Chung et al. (2020) |
ω-6 to ω -3 ratio
Essential lipids play important roles in human health. Consume adequate amounts of PUFAs is important, and more importantly, sources of food which contain favorable ratios of ω-3 PUFA (ALA) to ω-6 PUFA (LA). Increased ratio of ω-6 to ω-3 is associated with increased chronic inflammatory diseases including NAFLD, CVD, obesity, and inflammatory bowel diseases, rheumatoid arthritis, and Alzheimer’s disease (Patterson et al., 2012).
While increased levels of ω-6 PUFA and ω-6/ω-3 PUFA ratio were recently demonstrated in the cancerous tissues of colorectal cancer patients (Serini et al., 2016), decreased ω-6 PUFA and ω-6/ω-3 PUFA ratio provide different results. In the study conducted by Isaac et al., female rats fed a control or different diets containing various ω-6/ω-3 ratio or protein levels for 30 days prior to mating and during pregnancy (Isaac et al., 2018). A low ω-6/ω-3 ratio (~ 1.7) with protein deficiency in the maternal diet group exhibited higher levels of H3K9Ac in neurons, H3K4Me2 in astrocytes, and LIF mRNA levels than those in the control diet group. This emphasizes the importance of dietary ω-3 availability for the brain, even under a protein-deficient condition, inducing histone modifications and increasing LIF gene transcription, involved in neural cell differentiation and reactivity (Isaac et al., 2018).
Conjugated linoleic acid
Conjugated LAs (CLAs) are positional and configurational isomers of LA (n-6) in which two double bonds are conjugated (Ma et al., 1999). CLAs mainly consist of two isomers: cis-9,trans-11 (c9,t11)-CLA and trans-10,cis-12 (t10,c12)-CLA. Various health benefits of CLAs have been reported, including anti-cancer, anti-atherosclerotic, and anti-diabetic properties, fat mass reduction, bone mass improvement, and immune-modulating functions (Dilzer and Park, 2012; Park and Pariza, 2007). CLAs are naturally found in dairy and ruminant fats (Kim et al., 2010; Rule et al., 2002).
Obesity
The anti-obesity effects of CLA have been previously studied (Woo et al., 2016). Chaplin et al. studied if CLA-mediated lipid metabolism is regulated via epigenetic modulation, including DNA methylation, in adult mice (Chaplin et al., 2017). In their experiment, mice fed a high-fat diet (43% energy from fat) supplemented with 6 mg CLA/day or CLA + calcium (12 g/kg calcium) were compared to dietary control groups. Chaplin et al. found that gene expression and methylation degree of CpG sites in promoter sequences of genes such as adiponectin (Adipoq), stearoyl-CoA desaturase (Scd1), and FA synthase (Fasn) were altered by CLA + calcium, indicating a healthier metabolic profile (Chaplin et al., 2017). Further studies are warranted to demonstrate the role of CLA in various diseases via epigenetic modifications.
Epigenetic regulation of SCFAs in diseases progression
SCFAs containing fewer than six carbons are produced through the fermentation of dietary fiber, carbohydrates, peptides, and glycoprotein precursors (Frank et al., 2007). The human gut produces three main short-chain fatty acids (SCFAs): acetate (C2), propionate (C3), and butyrate (C4), and these all been have found to reduce allergic (Roberfroid et al., 2010; Sandin et al., 2009; Thompson-Chagoyan et al., 2011) or inflammatory bowel disease (D'Argenio and Mazzacca, 1999; Huda-Faujan et al., 2010) patients compared to healthy controls. These results suggest that major SCFAs can be used as markers of disease risk. SCFAs are potential histone deacetylase 3 (HDAC3) inhibitors (Li et al. 2018; Shakespear et al., 2011; Silva et al. 2018). Similarly, valproic acid (VPA), an eight-carbon SCFA, has been used to treat epilepsy for the last 50 years (Blaheta and Cinatl, 2002). The mechanism beyond this is not clearly understood, but it has been demonstrated to enhance the level of the inhibitory neurotransmitter gamma-aminobutyric acid in the brain (Johannessen, 2000; Rosenberg, 2007). In recent years, VPA has HDAC HDAC-inhibitory activity, resulting in differentiation, cell death, and apoptosis in malignant cells (Gottlicher et al. 2001; Kramer et al. 2003; Phiel et al. 2001).
SCFA concentrations vary in the gut and circulation. Estimates for concentrations of major SCFA acetate, propionate and butyrate range from 70–140 mM in the proximal colon, 20–70 mM in the distal colon (Correa-Oliveira et al., 2016) to as low as 0.16–25.05 μM in circulation. The ratio of acetate:propionate:butyrate in the gut is about 60:20:20, though the relative concentrations of propionate and butyrate are far lower in the circulation, with butyrate circulating at ~ 0.16 μM and propionate circulating at ~ 0.62 μΜ compared to ~ 25 μM for acetate (Parada Venegas et al., 2019). Among SCFA, sodium butyrate has been the most widely studied as an HDAC inhibitor; however, the mechanism by which SCFA inhibit HDAC is not fully understood (Stein and Riber, 2023). An in vitro study demonstrated that sodium butyrate increased histone acetylation by inhibiting HDAC activity in rat hepatoma and HeLa cells (Boffa et al., 1978; Sealy and Chalkley, 1978). Since then, it has been suggested that SCFA acts as a GPCR activator (He et al. 2020). These actions affect a wide range of cellular processes including proliferation, differentiation, chemotaxis, gene expression, epigenetic remodeling, cytokine production, and apoptosis (Correa-Oliveira et al., 2016).
Many studies have demonstrated the clinical health benefits of SCFAs that are naturally produced in the human gut, non-toxic, and well tolerated at very high concentrations (Parada Venegas et al., 2019). In randomized controlled or clinical trials using SCFAs, SCFAs treatment effects were approved in inflammatory bowel and gastrointestinal disorders (Demehri et al., 2016; Hustoft et al., 2017; Mazzawi et al. 2019), diabetes and obesity (Canfora et al. 2017; van der Beek et al. 2018; Zhao et al. 2018), cancer (Hague et al. 1995), prematurity (Underwood et al., 2009), neurological disease (Erny et al. 2015), and psychosocial stress (Burokas et al., 2017). The preventive effects of SCFA on epigenetic regulation have been demonstrated in various disease models. In the third chapter, SCFA acetate, propionate, butyrate, and valerate (C5) are discussed in various disease progressions in terms of epigenetic regulation.
Inflammatory states
The anti-inflammatory effects and the redirection of the innate immune response regulated by SCFAs have been widely studied. SCFA, including butyrate and propionate, exert their effects through toll-like receptor responses in vitro, thereby regulating NF-κB and TNF-α that are similar to the small-molecule HDAC inhibitor, trichostatin A (TSA) (Lin et al., 2015). An in vitro study also found that butyrate attenuated the production of proinflammatory molecules, such as NO, IL-6, and IL-12, in bone marrow-derived macrophages and macrophages from the lamina propria of the colon. Similarly, oral administration of butyrate to mice decreased the levels of these proinflammatory molecules in colon lamina propria macrophages that likely occurred through the inhibition of HDACs (Chang et al., 2014).
Butyrate also promotes the production of IL-22, an anti-inflammatory cytokine, by G-protein receptor 41-mediated signaling and HDAC inhibition that were observed in mice fed butyrate in drinking water, as well as in CD4 + T cells and innate lymphoid cells. The mechanism beyond it was attributed to butyrate-mediated histone modification leads to increase the binding of hypoxia-inducible factor 1α (HIF1α) to the hypoxia response element (HRE) of the IL22 promoter. As a part of this effect, butyrate increases H3K9 acetylation and suppresses H3K9 trimethylation at the HRE site of the IL22 promoter (Stein and Riber—Yang et al., 2020). Likely, butyrate-mediated anti-inflammatory activity through downregulation of TNF-α and STAT1, and upregulation of anti-inflammatory IL-19 that are attributed to via H3K9 acetylation modulation (Patnala et al., 2017).
SCFA reduced production of LPS-induced production of proinflammatory ILs and TNF-α in murine macrophage cells (Liu et al., 2012; Vinolo et al., 2012). As an HDAC inhibitor, butyrate increases histone H3K9ac at the promoters of Il2, Nos2, and Il12b in macrophages; these effects were not observed with propionate or acetate (Chang et al. 2014).
One evidence indicates that sodium acetate alleviated arsenic-induced sexual dysfunction as well as biochemical and histological alterations (Besong et al., 2023), and this were accompanied acetate-driven downregulation of HDAC activity, and similarly, acetate also reduced arsenic-mediated male reproductive toxicity via suppression of HDAC and uric acid-driven NFκB-Inos-NO-involved responses (Besong et al., 2023).
Valerate has been studied as a potential inhibitor of autoimmune and inflammatory diseases (Luu et al., 2019). They found that valerate that possesses HDAC inhibitory activity, contributes to the suppression of IL-17A expression, leading to the amelioration of autoimmune inflammation in the central nervous system. Taken together, butyrate likely plays a crucial role in suppressing inflammation via various mechanisms, and acetate, propionate, and valerate exert anti-inflammatory effects via HDAC inhibition.
Cancers
HDAC removes acetyl groups from amino acids on histones and is known to be a critical target for cancer cells via the induction of apoptosis and/or cell cycle arrest (Akimova et al., 2012). Therefore, HDAC inhibition has been recognized as a preventive strategy against inflammation-related diseases, including cancer. As a natural HDAC inhibitor, SCFA also play a role in the expression of various anti-inflammatory genes within immune cells (Kim, 2018; Park et al. 2015), mimicking the action of valproic acid, such as inhibition of tight junction degradation and translocation of NFκB (Wang et al., 2011).
Direct evidence of the anticarcinogenic effects of SCFAs has been demonstrated in various experimental models, including humans. Propionate plays a crucial role in reducing cell proliferation, inducing apoptosis, and suppressing tumor progression that is attributed to significant activation of FFAR2 in colorectal adenocarcinoma (Bindels et al., 2013, 2012). Butyrate and valerate also can cause increased production of certain effector molecules including CD25, IFN-γ, and TNF-α among CAR T-cells and cytotoxic T cells (CTLs), leading to significant increases in anti-tumor reactivity and thereby improving therapeutic outcomes in cancer patients (Luu et al. 2021). Taken together, SCFAs are likely to exert their anti-carcinogenic effects through their anti-inflammatory activity. Hence, further studies are warranted to investigate the role of SCFA-mediated epigenetic regulation in cancer prevention.
Obesity and metabolic disorders
Several studies have demonstrated the protective effects of SCFAs against obesity and diabetes via epigenetic modulation; however, the mechanisms underlying these effects vary. Rumberger et al. investigated that both butyrate and propionate stimulated lipolysis in mouse adipocytes, mimicking the action of the HDAC inhibitor trichostatin A (Rumberger et al., 2014). In a streptozotocin-mediated type 2 diabetes rat model, intraperitoneal injection of sodium butyrate (i.p. injection) significantly reduced plasma glucose levels, insulin resistance, and liver steatosis that were mediated by HDAC inhibition and histone H3 hyperacetylation in the liver (Khan and Jena, 2016). Similarly, sodium butyrate (oral administration) significantly decreased obesity, improved glucose tolerance, and reduced fat deposition in the skeletal muscle of mice fed a high-fat diet, likely through sodium butyrate-mediated HDAC inhibition. In this study, H3K9 acetylation was increased at the promoters of Adipor1 and Adipor2 that encode adiponectin receptors, and Ucp2 (uncoupling protein 2) and Ucp3, thereby increasing mitochondrial thermogenesis and β-oxidation in HFD-fed mice with sodium butyrate administration (Hong et al., 2016). Another line of evidence indicates that sodium butyrate decreases glucose level and Hba1c, favoring insulin sensitivity, thereby reducing the risk of diabetes development in juvenile diabetic rats (Khan and Jena, 2014), and similarly in a high-fat diet-induced obese mouse model (Henagan et al., 2015).
Acetate-containing foods include dairy products, dried pasta, bread, liquid eggs, salt substitutes, coffee, processed meat, smoked/frozen fish, ethanol, and vinegar (Israel et al., 1994; Lim et al., 2016). Sodium acetate supplementation significantly suppresses HFD-induced weight gain compared to that in HFD control mice (Kondo et al., 2009; Lu et al., 2016). The prevention of weight gain was attributed to the action of acetate through binding to the G-protein-coupled receptors GPR43 (FFAR2) and GPR41 (FFAR3) that are characterized by increases in adipose tissue and reductions in the colon. These factors contribute to increased TG hydrolysis and FFA oxidation (through UCP-2 and CPT-1 gene expression) in the adipose tissue and liver (Kondo et al. 2009; Lu et al., 2016). However, long-term oral supplementation with acetate affects body weight management and remains controversial owing to the lack and inconsistency of data (Hernandez et al., 2019).
In a study conducted by Remely et al., methylation of the promoter region of FFAR3 was analyzed and compared in patients with obesity and type 2 diabetes and their controls (lean control group) with four-month intervention. They found decreased butyryl-CoA:acetate CoA transferase gene and significantly lower methylation of the FFAR3 promoter in obese and diabetic patients, and this was increased during the intervention, indicating a significant correlation between FFAR3 methylation and BMI, as well as LINE-3, a marker of global methylation (Remely et al., 2014).
Brain diseases
A cross-sectional study of elderly individuals found that brain amyloid deposition and endothelial dysfunction positively correlated with blood acetate levels and negatively correlated with butyrate levels (Marizzoni et al., 2020). In a study conducted by Xiang, the authors investigated the fecal microbiome and levels of SCFAs in the serum and brain of a rat model of chronic unpredictable mild stress (CUMS) and found significantly decreased butyrate in the brains of CUMS rats (Li et al., 2021). This suggests that SCFAs are detected not only in the gut but also in blood circulation, playing a role in brain function, development, and inflammation. Stein and Riber demonstrated that oral sodium butyrate decreased beta-amyloid levels and improved cognitive memory in a mouse model of early Alzheimer’s disease (Stein and Riber, 2023).
Sodium butyrate-mediated preventive effects against brain diseases are likely attributable to its ability to modulate histone acetylation. In primary cortical astrocyte cultures, sodium butyrate increases brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) transcription, accompanied by an increase in histone H3 acetylation at the GDNF promoter (Wu et al., 2008). In another study, sodium butyrate partially prevented apoptotic cell death caused by the mitochondrial toxin 1-methyl-4-phenylpyridinium, likely due to a significant increase in histone H3 acetylation in human neuroblastoma-derived and rat mesencephalon-derived cell lines (Kidd and Schneider, 2010). Additionally, in a study using a presenilin-1 and presenilin-2 conditional double-knockout mouse model, butyrate significantly increased neurogenesis in the subgranular zone of the dentate gyrus, restored contextual memory, and reversed dysregulated histone acetylation in the hippocampus and cortex (Cao et al., 2018). Several pieces of evidence have been found in a study on Parkinson’s disease. In a mouse model of Parkinson’s disease, butyrate prevented the DNA damage caused by α-synuclein, possibly by upregulating DNA repair genes, and in cell lines it rescued the decrease in histone H3 acetylation that was mediated by α-synuclein (Paiva et al., 2017). Additionally, sodium butyrate (i.p.) attenuated motor deficits, increased dopamine levels in the striatum, lowered oxidative stress, and increased striatal global histone H3 acetylation levels in a rat model of 6-hydroxydopamine-induced experimental Parkinson’s disease (Sharma et al., 2015). The epigenetic mechanisms regulated by SCFAs in various disease states are summarized in Table 3.
Table 3.
Epigenetic mechanism regulated by SCFA in various disease states
| Fatty acids | Diseases/states | Epigenetic mechanism | References |
|---|---|---|---|
| Acetate | Inflammatory states, diabetes, obesity |
HDAC inhibition, GPR43/GPR41 activation |
Parada Venegas et al. (2019), Canfora et al. (2017), Zhao et al. (2018), and Besong et al. (2023) |
| Propionate | Inflammatory states, cancers | HDAC inhibition, FFAR2 activation | Parada Venegas et al. (2019), Bindels et al. (2012, 2013) |
| Obesity | Histone acetylation | Rumberger et al. (2014) | |
| Butyrate | Inflammatory states, cancers | ↑Histone acetylation, HDAC inhibition | Chang et al. (2014) |
| Inflammatory states |
↑ H3K9 acetylation ↓H3K9 trimethylation at IL22 promoter |
Yang et al. (2020), Pantnala et al. (2017), and Chang et al. (2014) | |
| Obesity and metabolic disorders | HDAC inhibition, H3 hyperacetylation | Rumberger et al. (2014) and Khan and Jena (2016 | |
| Obesity and metabolic disorders | ↑ H3K9 acetylation at the promoter of Adipor1 and Adipor2 | Hong et al. (2016) | |
| Brain diseases | ↑ histone H3 acetylation at GDNF promoter | Wu et al. (2008), Kidd and Schneider (2010), and Sharma et al. (2015) | |
| Valerate | Inflammatory states | HDAC inhibition | Luu et al. (2019) |
In conclusion, the sensitivity of epigenetic regulation to environmental factors, such as diet, highlights its dynamic and potentially reversible nature (Lillycrop and Burdge, 2015; Weinhold, 2006). Growing evidence suggests that alterations in epigenetic modifications, including increased DNA methylation and histone acetylation triggered by various factors, contribute to the progression of certain diseases, and there is an increasing interest in foods that can alleviate these effects.
The field of food research finds epigenetics particularly attractive due to its potential to elucidate how dietary components, such as FAs, can induce reversible modifications in gene expression. This review paper summarizes the evidence that FAs affect epigenetic processes during the progression of various diseases. A number of studies have demonstrated that ω-3 PUFA majorly regulated DNA methylation as well as histone modifications, leading to various disease progression whereas the studies from ω-6 PUFA were inconclusive. Alterations in dietary fat consumption can induce epigenetic regulation of specific genes. SFA also regulate epigenetic changes in transcription and metabolism that indirectly or directly alleviate several diseases. This review highlights the importance of integrating dietary factor (fat)-microbial metabolism-epigenetics to modulate cell and tissue physiology and disease susceptibility. It would be worthwhile to conduct additional studies on the effects of epigenetic modifications induced by various functional/bioactive FA on gene function in various tissues.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [No. 2019R1F1A1053604].
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 51: 1961-1971 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailhaud G, Massiera F, Weoill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Progress in Lipid Research. 45: 203-236 (2006) [DOI] [PubMed] [Google Scholar]
- Akimova T, Beier UH, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases and T-cell immune responses. Blood. 119: 2443-245 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arents G, Burlingame RW, Wang BC, Love WE, Moudrianakis EN. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proceedings of the National Academy of Sciences of the United States of America. 88: 10148-10152 (1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpon A, Milagro FI, Razquin C, Corella D, Estruch R, Fito M, Marti A, Martinez-Gonzalez MA, Ros E, Salas-Salvado J, Riezu-Boj JI, Martinez JA. Impact of consuming extra-virgin olive oil or nuts within a Mediterranean diet on DNA methylation in peripheral white blood cells within the PREDIMED-Navarra randomized controlled trial: a role for dietary lipids. Nutrients. 10: 15 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 70: 567-590 (1990). [DOI] [PubMed] [Google Scholar]
- Berndsen CE, Denu JM. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Current Opinion in Structural Biology. 18: 682-689 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besong EE, Akhigbe TM, Obimma JN, Obembe OO, Akhigbe RE. Acetate abates arsenic-induced male reproductive toxicity by suppressing HDAC and uric acid-driven oxido-inflammatory NFkB/iNOS/NO response in rats. Biological Trace Element Research. 1-16 (2023) [DOI] [PubMed] [Google Scholar]
- Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends in Pharmacological Sciences. 34: 226-232 (2013) [DOI] [PubMed] [Google Scholar]
- Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O, Muccioli GG, Sonveaux P, Cani PD, Delzenne NM. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. British Journal of Cancer. 107: 1337-1344 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaheta RA, Cinatl J, Jr. Anti-tumor mechanisms of valproate: a novel role for an old drug. Medicinal Research Reviews. 22: 492-511 (2002) [DOI] [PubMed] [Google Scholar]
- Boffa LC, Vidali G, Mann RS, Allfrey VG. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. Journal of Biological Chemistry. 253: 3364-3366 (1978) [PubMed] [Google Scholar]
- Bouchard L, Hivert MF, Guay SP, St-Pierre J, Perron P, Brisson D. Placental adiponectin gene DNA methylation levels are associated with mothers' blood glucose concentration. Diabetes. 61: 1272-1280 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman GD, Poirier MG. Post-translational modifications of histones that influence nucleosome dynamics. Chemical Reviews. 115: 2274-2295 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. The American Journal of Clinical Nutrition. 85: 1457-1464 (2007) [DOI] [PubMed] [Google Scholar]
- Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. The American Journal of Clinical Nutrition. 69: 30-42 (1999) [DOI] [PubMed] [Google Scholar]
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological Psychiatry. 82: 472-487 (2017) [DOI] [PubMed] [Google Scholar]
- Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, Lenaerts K, Dejong CHC, Blaak EE. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Scientific Reports. 7: 2360 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T, Zhou X, Zheng X, Cui Y, Tsien JZ, Li C, Wang H. Histone Deacetylase Inhibitor Alleviates the Neurodegenerative Phenotypes and Histone Dysregulation in Presenilins-Deficient Mice. Frontiers in Aging Neuroscience. 15: 137 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli V, Ronchetti S, Marchetti MC, Calvitti M, Riccardi C, Grignani F, Vecchini A. Molecular mechanisms underlying eicosapentaenoic acid inhibition of HDAC1 and DNMT expression and activity in carcinoma cells. Biochimica et Biophysica Acta-Gene Regulatory Mechanisms. 1863: 194481 (2020) [DOI] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America. 111: 2247-2252 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin A, Palou A, Serra F. Methylation analysis in fatty-acid-related genes reveals their plasticity associated with conjugated linoleic acid and calcium supplementation in adult mice. European Journal of Nutrition. 56: 879-891 (2017). [DOI] [PubMed] [Google Scholar]
- Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Experimental Biology and Medicine (Maywood, N. J.). 239: 302-310 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero AF, Ertek S, Borghi C. Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Current Vascular Pharmacology. 7: 330-337 (2009) [DOI] [PubMed] [Google Scholar]
- Cohen I, Poreba E, Kamieniarz K, Schneider R. Histone modifiers in cancer: friends or foes? Genes & Cancer. 2: 631-647 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Oliveira R, achi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clinical & Translational Immunology. 5: e73 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Han J, Chung MY. Hypolipidemic effect of omega-3 fatty acids in 3T3-L1 preadipocytes via inhibition of histone acetyltransferase activity. Journal of the Korean Society of Food Science and Nutrition. 49: 1319-1327 (2020) [Google Scholar]
- D'Argenio G, Mazzacca G. Short-chain fatty acid in the human colon. Relation to inflammatory bowel diseases and colon cancer. Advances in Experimental Medicine and Biology. 472: 149-158 (1999) [DOI] [PubMed] [Google Scholar]
- de la Rocha C, Rodriguez-Rios D, Ramirez-Chavez E, Molina-Torres J, de Jesus Flores-Sierra J, Orozco-Castellanos LM, Galvan-Chia JP, Sanchez AV, Zaina S, Lund G. Cumulative metabolic and epigenetic effects of paternal and/or maternal supplementation with arachidonic acid across three consecutive generations in mice. Cells. 11: 1057 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. British Journal of Pharmacology. 158: 413-428 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. The American Journal of Clinical Nutrition. 83(6 Suppl): 1520S-1525S (2006) [DOI] [PubMed] [Google Scholar]
- Demehri FR, Frykman PK, Cheng Z, Ruan C, Wester T, Nordenskjold A, Kawaguchi A, Hui TT, Granstrom AL, Funari V, Teitelbaum DH, Group HCR. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. Journal of Pediatric Surgery. 51: 81-86 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilzer A, Park Y. Implication of conjugated linoleic acid (CLA) in human health. Critical Reviews in Food Science and Nutrition. 52: 488-513 (2012) [DOI] [PubMed] [Google Scholar]
- Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma lipids in Greenland Eskimos. The American Journal of Clinical Nutrition. 28: 958-966 (1975) [DOI] [PubMed] [Google Scholar]
- Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 2(8081): 117-119 (1978) [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience. 18: 965–977 (2015) [DOI] [PMC free article] [PubMed]
- Fernando W, Martins IJ, Morici M, Bharadwaj P, Rainey-Smith SR, Lim WLF, Martins RN. Sodium butyrate reduces brain amyloid-beta levels and improves cognitive memory performance in an Alzheimer's disease transgenic mouse model at an early disease stage. Journal of Alzheimer's Disease. 74: 91-99 (2020) [DOI] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 104: 13780-13785 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 128: 635-638 (2007) [DOI] [PubMed] [Google Scholar]
- Gong F, Miller KM. Mammalian DNA repair: HATs and HDACs make their mark through histone acetylation. Mutation Research. 750: 23-30 (2013) [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20: 6969-6978 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague A, Elder DJ, Hicks DJ, Paraskeva C. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. International Journal of Cancer. 60: 400-406 (1995) [DOI] [PubMed] [Google Scholar]
- Hara H, Haga S, Aoyama Y, Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. The Journal of Nutrition. 129: 942-948 (1999) [DOI] [PubMed] [Google Scholar]
- He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, Zhao Y, Bai L, Hao X, Li X, Zhang S, Zhu L. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. International Journal of Molecular Sciences. 21: 6356 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henagan TM, Stefanska B, Fang Z, Navard AM, Ye J, Lenard NR, Devarshi PP. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. British Journal of Pharmacology. 172: 2782-2798 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsdorff HH, Mansego ML, Campion J, Milagro FI, Zulet MA, Martinez JA. TNF-alpha promoter methylation in peripheral white blood cells: relationship with circulating TNFalpha, truncal fat and n-6 PUFA intake in young women. Cytokine. 64: 265-271 (2013) [DOI] [PubMed] [Google Scholar]
- Hernandez MAG, Canfora EE, Jocken JWE, Blaak EE. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 11: 1943 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodawadekar SC, Marmorstein R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene. 26: 5528-5540 (2007) [DOI] [PubMed] [Google Scholar]
- Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, Cai D, Zhao R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 7: 56071-56082 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L, Zahradka P, Cordero-Monroy L, Wright B, Taylor CG. Dietary docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) operate by different mechanisms to modulate hepatic steatosis and hyperinsulemia in fa/fa Zucker rats. Nutrients. 11: 917 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Wen J, Chen G, Ge M, Gao Y, Ye X, Liu C, Cai C. Omega-3 polyunsaturated fatty acids inhibited tumor growth via preventing the decrease of genomic DNA methylation in colorectal cancer rats. Nutrition and Cancer. 68: 113-119 (2016) [DOI] [PubMed] [Google Scholar]
- Huda-Faujan N, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, Loong YY. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. The Open Biochemistry Journal. 4: 53-58 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustoft TN, Hausken T, Ystad SO, Valeur J, Brokstad K, Hatlebakk JG, Lied GA. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterology & Motility. 29: e12969 (2017) [DOI] [PubMed] [Google Scholar]
- Isaac AR, da Silva EAN, de Matos RJB, Augusto RL, Moreno GMM, Mendonca IP, de Souza RF, Cabral-Filho PE, Rodrigues CG, Goncalves-Pimentel C, Rodrigues MCA, da Silveira Andrade-da-Costa BL. Low omega-6/omega-3 ratio in a maternal protein-deficient diet promotes histone-3 changes in progeny neural cells and favors leukemia inhibitory factor genetranscription. The Journal of Nutritional Biochemistry. 55: 229-242 (2018) [DOI] [PubMed] [Google Scholar]
- Israel Y, Orrego H, Carmichael FJ. Acetate-mediated effects of ethanol. Alcohol, Clinical and Experimental Research. 18: 144-148 (1994) [DOI] [PubMed] [Google Scholar]
- Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes & Cancer. 2: 607-617 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochemistry International. 37: 103-110 (2000) [DOI] [PubMed] [Google Scholar]
- Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nature Reviews Genetics. 17: 630-641 (2016) [DOI] [PubMed] [Google Scholar]
- Jung BC, Kang S. Epigenetic regulation of inflammatory factors in adipose tissue. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 1866: 159019 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanagiotidis IT, Bell MV, Little DC, Yakupitiyage A. Replacement of dietary fish oils by alpha-linolenic acid-rich oils lowers omega 3 content in tilapia flesh. Lipids. 42: 547-559 (2007) [DOI] [PubMed] [Google Scholar]
- Kaur G, Sinclair AJ, Cameron-Smith D, Barr DP, Molero-Navajas JC, Konstantopoulos N. Docosapentaenoic acid (22:5n-3) down-regulates the expression of genes involved in fat synthesis in liver cells. Prostaglandins, Leukotrienes and Essential Fatty Acids. 85: 155-161 (2011) [DOI] [PubMed] [Google Scholar]
- Khan S, Jena G. Sodium butyrate reduces insulin-resistance, fat accumulation and dyslipidemia in type-2 diabetic rat: a comparative study with metformin. Chemico-Biological Interactions. 254: 124-134 (2016) [DOI] [PubMed] [Google Scholar]
- Khan S, Jena GB. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: study in juvenile diabetic rat. Chemico-Biological Interactions. 213: 1-12 (2014) [DOI] [PubMed] [Google Scholar]
- Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Research. 1354: 172-178 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH. Microbiota or short-chain fatty acids: which regulates diabetes? Cellular and Molecular Immunology. 15: 88-91 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Pan JH, Park HG, Yoon HG, Kwon OJ, Kim TW, Shin DH, Kim YJ. Functional comparison of esterified and free forms of conjugated linoleic acid in high-fat-diet-induced obese C57BL/6J mice. Journal of Agricultural and Food Chemistry. 58: 11441-11447 (2010) [DOI] [PubMed] [Google Scholar]
- Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. Journal of Agricultural and Food Chemistry. 57: 5982-5986 (2009) [DOI] [PubMed] [Google Scholar]
- Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, Gottlicher M. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 22: 3411-3420 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, Mullen Y, Pfeifer GP, Ferreri K. Insulin gene expression is regulated by DNA methylation. PLoS One. 4: e6953 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Kim MY. Cancer epigenetics: Past, present and future. Seminars in Cancer Biology. 83: 4-14 (2022) [DOI] [PubMed] [Google Scholar]
- Li H, Xiang Y, Zhu Z, Wang W, Jiang Z, Zhao M, Cheng S, Pan F, Liu D, Ho RCM, Ho CSH. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. Journal of Neuroinflammation. 18: 254 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, van Esch B, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. European journal of pharmacology. 831: 52-59 (2018) [DOI] [PubMed] [Google Scholar]
- Li RW, Li C. Butyrate induces profound changes in gene expression related to multiple signal pathways in bovine kidney epithelial cells. BMC Genomics. 7: 234 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi PV, Ververis K, Karagiannis TC. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy. 2011: 869647 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Burdge GC. Maternal diet as a modifier of offspring epigenetics. Journal of Developmental Origins of Health and Disease. 6: 88-95 (2015) [DOI] [PubMed] [Google Scholar]
- Lim J, Henry CJ, Haldar S. Vinegar as a functional ingredient to improve postprandial glycemic control-human intervention findings and molecular mechanisms. Molecular Nutrition & Food Research. 60: 1837-1849 (2016) [DOI] [PubMed] [Google Scholar]
- Lin MY, de Zoete MR, van Putten JP, Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Frontiers in Immunology. 6: 554 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xu D. Inhibition of histone deacetylases. Methods in Molecular Biology. 287: 87-97 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liao C, Wu L, Tang J, Chen J, Lei C, Zheng L, Zhang C, Liu YY, Xavier J, Dai L. Ecological dynamics of the gut microbiome in response to dietary fiber. ISME J. 16: 2040-2055 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li L, Min J, Wang J, Wu H, Zeng Y, Chen S, Chu Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cellular immunology. 277: 66-73 (2012) [DOI] [PubMed] [Google Scholar]
- Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Scientific Reports. 6: 37589 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, Lamp B, Nist A, Stiewe T, Shaul Y, Adhikary T, Zaiss MM, Lauth M, Steinhoff U, Visekruna A. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nature Communications. 10: 760 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, Klein M, Wempe A, Leister H, Raifer H, Picard F, Muhammad K, Ohl K, Romero R, Fischer F, Bauer CA, Huber M, Gress TM, Lauth M, Danhof S, Bopp T, Nerreter T, Mulder IE, Steinhoff U, Hudecek M, Visekruna A. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nature Communications. 12: 4077 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DW, Wierzbicki AA, Field CJ, Clandinin MT. Conjugated linoleic acid in canadian dairy and beef products. Journal of Agricultural and Food Chemistry. 47: 1956-1960 (1999) [DOI] [PubMed] [Google Scholar]
- Ma Y, Smith CE, Lai CQ, Irvin MR, Parnell LD, Lee YC, Pham LD, Aslibekyan S, Claas SA, Tsai MY, Borecki IB, Kabagambe EK, Ordovas JM, Absher DM, Arnett DK. The effects of omega-3 polyunsaturated fatty acids and genetic variants on methylation levels of the interleukin-6 gene promoter. Molecular Nutrition & Food Research. 60: 410-419 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, O'Brien D, Hopkins SE, Stephensen CB, Stanhope KL, Havel PJ, Boyer B. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. European Journal of Clinical Nutrition. 65: 808-817 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, Mombelli E, Mazzelli M, Luongo D, Naviglio D, Coppola L, Salvatore M, Frisoni GB. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer's disease. Journal of Alzheimer's Disease. 78: 683-697 (2020) [DOI] [PubMed] [Google Scholar]
- Marmorstein R, Zhou MM. Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harbor Perspectives in Biology. 6: a018762 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, Mohsen-Kanson T, Amri EZ, Ailhaud G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. Journal of Lipid Research. 51: 2352-2361 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzawi T, Hausken T, Hov JR, Valeur J, Sangnes DA, El-Salhy M, Gilja OH, Hatlebakk JG, Lied GA. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scandinavian Journal of Gastroenterology. 54: 690-699 (2019) [DOI] [PubMed] [Google Scholar]
- Mercola J, D'Adamo CR. Linoleic acid: a narrative review of the effects of increased intake in the standard American diet and associations with chronic disease. Nutrients. 15: 3129 (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi Sarabi M, Mohammadrezaei Khorramabadi R, Zare Z, Eftekhar E. Polyunsaturated fatty acids and DNA methylation in colorectal cancer. World Journal of Clinical Cases. 7: 4172-4185 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 7: 189-200 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 111: 157-164 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskiet FA, Kemperman RF. Folate and long-chain polyunsaturated fatty acids in psychiatric disease. The Journal of Nutritional Biochemistry. 17: 717-727 (2006) [DOI] [PubMed] [Google Scholar]
- Nikolakopoulou Z, Nteliopoulos G, Michael-Titus AT, Parkinson EK. Omega-3 polyunsaturated fatty acids selectively inhibit growth in neoplastic oral keratinocytes by differentially activating ERK1/2. Carcinogenesis. 34: 2716-2725 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva I, Pinho R, Pavlou MA, Hennion M, Wales P, Schutz AL, Rajput A, Szego EM, Kerimoglu C, Gerhardt E, Rego AC, Fischer A, Bonn S, Outeiro TF. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Human Molecular Genetics. 26: 2231-2246 (2017) [DOI] [PubMed] [Google Scholar]
- Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology. 10: 277 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunology. 8: 80-93 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Pariza MW. Mechanisms of body fat modulation by conjugated linoleic acid (CLA). Food Research International. 40: 311-323 (2007) [Google Scholar]
- Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Molecular Neurobiology. 54: 6391-6411 (2017) [DOI] [PubMed] [Google Scholar]
- Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. Journal of Nutrition and Metabolism. 2012: 539426 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. Journal of Biological Chemistry. 276: 36734-36741 (2001) [DOI] [PubMed] [Google Scholar]
- Ramaiyan B, Talahalli RR. Dietary unsaturated fatty acids modulate maternal dyslipidemia-induced DNA methylation and histone acetylation in placenta and fetal liver in rats. Lipids. 53: 581-588 (2018) [DOI] [PubMed] [Google Scholar]
- Remely M, Aumueller E, Merold C, Dworzak S, Hippe B, Zanner J, Pointner A, Brath H, Haslberger AG. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 537: 85-92 (2014) [DOI] [PubMed] [Google Scholar]
- Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Frontiers in Microbiology. 7: 185 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust. A prebiotic effects: metabolic and health benefits. British Journal of Nutrition 104:1–63 (2010) [DOI] [PubMed]
- Romagnolo DF, Donovan MG, Doetschman TC, Selmin OI. n-6 Linoleic acid induces epigenetics alterations associated with colonic inflammation and cancer. Nutrients. 11: 171 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cellular and Molecular Life Sciences. 64: 2090-2103 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 3: 118-134 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule DC, Broughton KS, Shellito SM, Maiorano G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken. Journal of Animal Science. 80: 1202-1211 (2002) [DOI] [PubMed] [Google Scholar]
- Rumberger JM, Arch JR, Green A. Butyrate and other short-chain fatty acids increase the rate of lipolysis in 3T3-L1 adipocytes. PeerJ. 2: e611 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin A, Braback L, Norin E, Bjorksten B. Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatrica. 98: 823-827 (2009) [DOI] [PubMed] [Google Scholar]
- Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 14: 115-121 (1978) [DOI] [PubMed] [Google Scholar]
- Seo T, Blaner WS, Deckelbaum RJ. Omega-3 fatty acids: molecular approaches to optimal biological outcomes. Current Opinion in Lipidology. 16: 11-18 (2005) [DOI] [PubMed] [Google Scholar]
- Serini S, Ottes Vasconcelos R, Fasano E, Calviello G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opinion on Therapeutic Targets. 20: 843-858. (2016) [DOI] [PubMed] [Google Scholar]
- Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends in Immunology. 32: 335-343 (2011) [DOI] [PubMed] [Google Scholar]
- Shane B. Folate and vitamin B12 metabolism: overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food and Nutrition Bulletin. 29(2 Suppl): S5-16; discussion S17-19 (2008) [DOI] [PubMed] [Google Scholar]
- Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: Modulation of histone deacetylase activity. Behavioural Brain Research. 291: 306-314 (2015) [DOI] [PubMed] [Google Scholar]
- Shen W, Wang C, Xia L, Fan C, Dong H, Deckelbaum RJ, Qi K. Epigenetic modification of the leptin promoter in diet-induced obese mice and the effects of N-3 polyunsaturated fatty acids. Scientific Reports. 4: 5282 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Masujima Y, Ushiroda C, Mizushima R, Taira S, Ohue-Kitano R, Kimura I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Scientific Reports. 9: 16574 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Martinez GA, Rodriguez-Rios D, Alvarado-Caudillo Y, Vaquero A, Esteller M, Carmona FJ, Moran S, Nielsen FC, Wickstrom-Lindholm M, Wrobel K, Wrobel K, Barbosa-Sabanero G, Zaina S, Lund G. Arachidonic and oleic acid exert distinct effects on the DNA methylome. Epigenetics. 11: 321-334 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LG, Ferguson BS, Avila AS, Faciola AP. Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells. Journal of Animal Science. 96: 5244-5252 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KL, Guentzel JL. Mercury concentrations and omega-3 fatty acids in fish and shrimp: Preferential consumption for maximum health benefits. Marine Pollution Bulletin. 60: 1615-1618 (2010) [DOI] [PubMed] [Google Scholar]
- Stein RA, Riber L. Epigenetic effects of short-chain fatty acids from the large intestine on host cells. Microlife. 4: uqad032 (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Sato H, Kanesaka M, Imamura Y, Sakamoto S, Ichikawa T, Kaneda A. Epigenetic modifications in prostate cancer. International Journal of Urology. 28: 140-149 (2021) [DOI] [PubMed] [Google Scholar]
- Thompson-Chagoyan OC, Fallani M, Maldonado J, Vieites JM, Khanna S, Edwards C, Dore J, Gil A. Faecal microbiota and short-chain fatty acid levels in faeces from infants with cow's milk protein allergy. International Archives of Allergy and Immunology. 156: 325-332 (2011) [DOI] [PubMed] [Google Scholar]
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annual Review of Pathology: Mechanisms of Disease. 5: 145-171 (2010) [DOI] [PubMed] [Google Scholar]
- Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, Wainstein J, Friedlander Y, Levy-Lahad E, Glaser B, Hellman A. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Human Molecular Genetics. 21: 371-383 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, Maniatis K, Gouliopoulos N, Miliou A, Paraskevopoulos T, Stefanadis C. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis. 232: 10-16 (2014) [DOI] [PubMed] [Google Scholar]
- Tremblay BL, Guenard F, Rudkowska I, Lemieux S, Couture P, Vohl MC. Epigenetic changes in blood leukocytes following an omega-3 fatty acid supplementation. Clinical Epigenetics. 9: 43 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulhe A, Sharma N, Mahajan A, Patil R, Hegde M, Bhalerao S, Mali A. Decoding the therapeutic landscape of alpha-linolenic acid: a network pharmacology and bioinformatics investigation against cancer-related epigenetic modifiers. Journal of Biomolecular Structure and Dynamics. 1-26 (2023) [DOI] [PubMed] [Google Scholar]
- Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, Tancredi DJ, Bevins CL, Sherman MP. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. Journal of Pediatric Gastroenterology and Nutrition. 48: 216-225 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek CM, Canfora EE, Kip AM, Gorissen SHM, Olde Damink SWM, van Eijk HM, Holst JJ, Blaak EE, Dejong CHC, Lenaerts K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism. 87: 25-35 (2018) [DOI] [PubMed] [Google Scholar]
- Vinolo MA, Rodrigues HG, Festuccia WT, Crisma AR, Alves VS, Martins AR, Amaral CL, Fiamoncini J, Hirabara SM, Sato FT, Fock RA, Malheiros G, dos Santos MF, Curi R Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. American Journal of Physiology-Endocrinology and Metabolism. 303: E272-282 (2012) [DOI] [PubMed] [Google Scholar]
- Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nature Reviews Molecular Cell Biology. 16: 678-689 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. Journal of Cerebral Blood Flow & Metabolism. 31: 52-57 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Qin G, Zhao TC. HDAC4: mechanism of regulation and biological functions. Epigenomics. 6: 139-150 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold B. Epigenetics: the science of change. Environmental Health Perspectives. 114: A160-167 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. Journal of Cardiovascular Medicine (Hagerstown). 8 Suppl 1: S42-45 (2007) [DOI] [PubMed] [Google Scholar]
- Woo H, Chung MY, Kim J, Kong D, Min J, Choi HD, Choi IW, Kim IH, Noh SK, Kim BH. Conjugated linoleic triacylglycerols exhibit superior lymphatic absorption than free conjugate linoleic acids and have antiobesity properties. Journal of Medicinal Food. 19: 486-494 (2016) [DOI] [PubMed] [Google Scholar]
- Wu J, Luo J, He Q, Xia Y, Tian H, Zhu L, Li C, Loor JJ. Docosahexaenoic acid alters lipid metabolism processes via H3K9ac epigenetic modification in dairy goat. Journal of Agricultural and Food Chemistry. 71: 8527-8539 (2023) [DOI] [PubMed] [Google Scholar]
- Yang J, Goldstein JL, Hammer RE, Moon YA, Brown MS, Horton JD. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proceedings of the National Academy of Sciences of the United States of America. 98: 13607-13612 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori N, Tawata M, Onaya T. DNA demethylation during the differentiation of 3T3-L1 cells affects the expression of the mouse GLUT4 gene. Diabetes. 48: 685-690 (1999) [DOI] [PubMed] [Google Scholar]
- Zaiou M, Amrani R, Rihn B, Hajri T. Dietary patterns influence target gene expression through emerging epigenetic mechanisms in nonalcoholic fatty liver disease. Biomedicines. 9: 1256 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang J, Duval C, Edin ML, Williams A, Lei L, Tu M, Pourmand E, Song R, Graves JP, DeGraff LM, Wong JJ, Wang Y, Sun Q, Sanidad KZ, Wong S, Han Y, Zhang Z, Lee KSS, Park Y, Xiao H, Liu Z, Decker EA, Cui W, Zeldin DC, Zhang G. CYP eicosanoid pathway mediates colon cancer-promoting effects of dietary linoleic acid. FASEB J. 37: e23009 (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 359: 1151-1156 (2018) [DOI] [PubMed] [Google Scholar]
- Zheng S, Rollet M, Pan YX. Maternal protein restriction during pregnancy induces CCAAT/enhancer-binding protein (C/EBPbeta) expression through the regulation of histone modification at its promoter region in female offspring rat skeletal muscle. Epigenetics. 6: 161-170 (2011) [DOI] [PubMed] [Google Scholar]
- Zheng Y, Cantley LC. Toward a better understanding of folate metabolism in health and disease. Journal of Experimental Medicine. 216: 253-266 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zock PL, Katan MB. Linoleic acid intake and cancer risk: a review and meta-analysis. The American Journal of Clinical Nutrition. 68: 142-153 (1998) [DOI] [PubMed] [Google Scholar]


