Abstract
Phytoplankton respond to physical and hydrographic forcing on time and space scales up to and including those relevant to climate change. Quantifying changes in phytoplankton communities over these scales is essential for predicting ocean food resources, occurrences of harmful algal blooms, and carbon and other elemental cycles, among other predictions. However, one of the best tools for quantifying phytoplankton communities across relevant time and space scales, ocean color sensors, is constrained by its own spectral capabilities and availability of adequately vetted and relevant optical models. To address this later shortcoming, greater than fifty strains of phytoplankton, from a range of taxonomic lineages, geographic locations, and time in culture, alone and in mixtures, were grown to exponential and/or stationary phase for determination of hyperspectral UV-VIS absorption coefficients, multi-angle and multi-spectral backscatter coefficients, volume scattering functions, particle size distributions, pigment content, and fluorescence. The aim of this publication is to share these measurements to expedite their utilization in the development of new optical models for the next generation of ocean color satellites.
Subject terms: Marine biology, Microbial biooceanography
Background & Summary
The Earth’s ocean ecosystems house a myriad of physical, chemical, and biological processes that create adaptive and resilient ecological communities of organisms in the sea. These ecosystems are an integral part of the planet’s biogeochemical cycles (e.g., carbon, nitrogen, phosphorus, silica, iron, etc.), which, in turn, are coupled to and influenced by the planet’s climate; the ocean’s biological carbon pump is one such cycle1. Of central importance to the biological carbon pump is phytoplankton functional type distribution and its response to changes in the physico-chemical environment, particularly those variables associated with climate change (e.g., ocean warming, increased stratification/nutrient depletion, and CO2 enrichment). For example, Lomas et al.2 showed that in the Sargasso Sea rapid warming during the decade of the 2010’s led to decreased net primary production, but the impacts on carbon export were mitigated by rapid changes in the phytoplankton community to favor small cyanobacteria and trophic processes that maintained carbon export rates. New generations of ocean ecosystem-biogeochemical models are integrating bio-optical data, such as what is presented in Neeley et al.3, to improve the representation of optically active components, including phytoplankton, which, in turn, will advance the representation of biogeochemical cycling4,5. The representation of phytoplankton communities in these models is key to linking ecosystem model outputs to ocean color data products in a changing ocean.
The need for state-of-the-art phytoplankton community composition (PCC) algorithms that derive some level of phytoplankton taxonomic biodiversity is critical to our understanding of how climate change is and will continue to impact oceanic food webs and carbon export. A variety of satellite algorithms to derive PCC or phytoplankton functional types have been developed over the last 10+ years. Most of these algorithms, as summarized in Mouw et al.6, derive relative abundances of phytoplankton size classes or particle size distributions from inherent optical properties (i.e., particle absorption or scattering properties), or total chlorophyll a (Chla) derived from satellite algorithms. Size class information although useful for some applications, such as quantification of carbon export7, provides limited information about phytoplankton biodiversity, which can have important impacts on ocean biogeochemistry and the food web8,9. A limited number of algorithms use reflectance to either derive the dominant taxonomic group10 or the biodiversity of multiple taxonomic groups in the surface ocean11. An assumption of many of these algorithms is that Chla is a reasonable proxy for phytoplankton biomass, conversion to other biomass metrics, such as carbon, is possible but requires additional derivative procedures (e.g.12). The merging of optical characteristics, Chla and diagnostic pigment can generate a powerful tool to develop and validate algorithms for deriving phytoplankton size classes13,14.
The hyperspectral Ocean Color Instrument (OCI) on NASA’s Plankton, Aerosol, Cloud, ocean Ecosystem (PACE) mission will provide more opportunities to derive PCC at a higher level of taxonomic resolution. However, the PACE mission requires advanced algorithms to be developed for the hyperspectral ocean color data that will be generated. From the controlled laboratory conditions described in this manuscript, we have developed an optical fingerprint library for 50+ globally relevant phytoplankton taxa that may be used to develop these advanced algorithms for the determination of PCC. The advantage of this library is the inclusion of all optical properties, not just accessory pigments and Chla, which may be used to distinguish challenging taxonomic groups that look similar from multispectral ocean color data. We provide some examples of how the data could be used, but this does not even scratch the surface of its potential applicability. An in-depth analysis of the pigment ratios and recommendations for use are fully described in Neeley et al.3.
Methods
Culture strains, culture methods, and experimental treatments
The strains chosen for this study (Supplemental Table 1) were cultivated at the National Center for Marine Algae and Microbiota (NCMA) at the Bigelow Laboratory for Ocean Sciences and are from regions where they are known to occur currently (e.g., diatoms from the North Atlantic, cyanobacteria from the oligotrophic gyres). Details of culture methods can be found in Neeley et al.3 and are only described briefly here. Cultures were grown axenically on a 14:10 L:D cycle at ~80 µmol photons m−2 s−1 for the warmer growth temperatures (>14 °C) and ~50 µmol photons m−2 s−1 at the cooler growth temperatures (<10 °C). All strains were grown using L1 medium15, except Prochlorococcus marinus that was grown in Pro99 medium16. While every practical attempt was made in this study to simulate the natural environmental conditions, we acknowledge that these are data from culture experiments and optical properties may differ from the same species in the natural environment. Direct quantification of taxonomically-resolved bio-optical properties under field conditions remains an area of additional research. In vivo Chla fluorescence was measured to track biomass and determine the timing for use for experimental measurements. For in vivo Chla fluorescence measurements, subsamples taken at the same time daily were analyzed on a calibrated TD-700 fluorometer17. Daily instrument response was tracked with a commercial solid fluorescence standard. Each stock culture was acclimated for approximately 10 divisions (~3 divisions/dilution cycle) before being scaled to 20 L and used for experimental conditions outlined below. Immediately prior to use in each experiment, cultures were diluted to a cell count that approximates their abundance in natural samples using Class A volumetric glassware. Unless otherwise noted, seawater from either the local Damariscotta River (Bigelow Laboratory, Maine; salinity of 32-33) or Sargasso Sea water were filtered to <0.2 µm for use as dilution water to produce the natural abundance cultures.
A range of experimental conditions were used to assess changes in cellular carbon, pigments and optical properties. For most of the strains, measurements were made at two growth stages: mid-exponential (Exp) and stationary (Stat) growth phase, but for some strains, measurements were made during only one growth phase. As phytoplankton populations in the field are rarely unialgal, a range of mixed cultures (mc) were made to provide data that can be used in the future to assess resolution of various algorithms.. The potential for changes in pigment composition in response to in vitro culture adaption over time (Evo) was assessed by studying multiple strains of the same species (Amphidinium carterae, Ditylum brightwellii, and Heterosigma akashiwo) isolated on different historical dates, but from similar geographic areas. Synechococcus sp., Thalassiosira oceanica, and Ostreococcus lucimarinus were examined for their response in pigment composition, cellular carbon, and optical properties to ‘climate change scenarios (CC)’ of elevated temperature, irradiance and decreased pH. All the various experiments conducted, and for which data are available, are noted in Supplemental Table 1 for the appropriate strains.
Discrete measurements
HPLC pigments
Samples for pigment analysis were filtered onto glass fiber filters (~125 mm Hg), placed in aluminum foil pouches, flash frozen in liquid nitrogen before transfer to a −80 °C freezer. Phytoplankton pigment concentrations (Table 1, listed in order by retention time) were determined using well established high performance liquid chromatography methods18,19 and as described in detail in Neeley et al.3.
Table 1.
Chromatographic and optical properties of phytoplankton pigments measured in this study in order of retention time in units of minutes (min) and absorption in units of nanometers (nm). Parentheses indicate a shoulder. The table was adapted from Neeley et al.13.
| Pigment | Abbreviation | Retention time (min) | Absorption maxima (nm) |
|---|---|---|---|
| Chlorophyll c3 | Chlc3 | 3.7 | 456, 588, (626) |
| Chlorophyll c2 | Chlc1c2 | 5.9 | 446, 584, 634 |
| Chlorophyll c1 | 6.2 | 442, 580, 632 | |
| Chlorophyllide a | Chlidea | 6.3 | (380), 434, 620, 666 |
| Pheophorbide a | Phidea | 8.1 | 410, 508, 538, 610, 666 |
| Peridinin | Peri | 10.0 | 476 |
| 19′ Butanoyloxyfucoxanthin | But | 13.6 | 448, 468 |
| Fucoxanthin | Fuco | 14.0 | 452 |
| Loroxanthin-like | Loro | 14.9 | (422), 446, 474 |
| Neoxanthin | Neo | 15.1 | 412, 436, 464 |
| Prasinoxanthin | Pras | 15.3 | 458 |
| Violaxanthin | Viola | 15.6 | 416, 440, 468 |
| 19′ Hexanoyloxyfucoxanthin | Hex | 15.9 | 446, 468 |
| Diadinoxanthin | Diad | 17.2 | (424), 446, 474 |
| Alloxanthin | Allo | 18.7 | (428), 450, 480 |
| Diatoxanthin | Diato | 19.5 | (428), 450, 478 |
| Zeaxanthin | Zea | 20.3 | (428), 450, 476 |
| Lutein | Lut | 20.6 | (422), 444, 472 |
| Gyroxanthin diester | Gyro | 23.4 | (424), 444, 470 |
| Divinyl Chlorophyll b | DVChlb | 25.6 | 478, 606, 656 |
| Monovinyl Chlorophyll b | Chlb | 25.7 | 468, 602, 650 |
| Divinyl Chlorophyll a | DVChla | 28.1 | (390), 440, 624, 666 |
| Monovinyl Chlorophyll a | Chla | 28.3 | (388), 432, 618, 666 |
| Pheophytin a | Phytina | 30.2 | 408, 506, 536, 608, 666 |
| β,ε-carotene | Caro | 31.2 | (422), 444, 472 |
| β,β-carotene | Caro | 31.3 | (428), 452, 476 |
Cellular Carbon to total Chla (POC:Chla) ratios were computed using the mean POC and Chla concentrations from each growth phase or treatment. The errors associated with the separate analytical measurements of HPLC pigments and POC were propagated to POC:Chla using standard error propagation theory20. The standard deviation of each average ratio was approximated using the equation:
| 1 |
where σR is the standard deviation of the ratio, μX and μY are the average values of the pigment and carbon, respectively, CVX is the coefficient of variation of the pigment and CVY is the coefficient of variation of the carbon measurement.
Particle and dissolved absorption
Replicate filter pads, two or three from each dilution, were collected for particle absorbance measurements by concentrating particles by vacuum filtration (~125 mmHg) onto either 25 mm Whatman GF/F filters or 25 mm GF75 filters (used for smaller cell sizes) using a glass filter cup and stem. Samples were placed in HistoPrepTM tissue capsules, flash frozen in liquid nitrogen and transferred to −80 °C for storage until analysis. Measurements of filter pad particle optical density (ODfp) were performed using a Cary 4000 UV-Visible scanning spectrophotometer equipped with a 15 cm integrating sphere (Labsphere DRA-CA-900) following the protocol of Stramski et al.21 and further described in Neeley and Mannino22. Filters were moistened with 0.2 µm filtered, low dissolved organic matter seawater. The sample filter was placed on a plexiglass holder and jaw mount inside the integrating sphere chamber and measured at 0 and 90 degrees. Scans were performed between 290–850 nm with a 2 nm Slit Band Width (SBW), 0.2 nm data interval, reduced slit height and 120 nm per minute scan speed. Depigmentation of the filters was performed using the method of Kishino et al.23 and further described in Neeley and Mannino22, and analyzed as for the pigmented samples. The diameter of the filtered biomass was measured using calipers for computation of the pathlength. Blank filter scans were subtracted from the raw ODfp spectra prior to ap computation. Air scans were measured throughout the day to monitor instrument drift.
Dissolved organic carbon
Dissolved organic carbon (DOC) samples were collected from the filtrate of the particulate absorption samples in 40 mL amber glass vials (pre-cleaned from the manufacturer and combusted @450 °C for 6 h) and stored frozen (−20 °C) until analysis. For analysis, DOC samples were thawed and sonicated for 20 minutes in an ultrasonic bath. A Shimadzu TOC-L or TOC-V using the high temperature combustion catalytic oxidation method equipped with a total nitrogen unit was used to measure DOC concentrations24,25. The carbon standard potassium hydrogen phthalate (KHP) was used to generate calibration curves before each sample batch was analyzed on the instrument. Due to the broad range of DOC values, two five-point calibration curves were performed on approximately 2 mg/L and 4 mg/L KHP standards. Ultrapure water blanks (ultraviolet oxidized Milli-Q) were measured every three samples in the analysis queue to assess the instrument carbon blank, and the average water blank was subtracted from all sample values on a given analytical run. Several check standards of single concentration KHP were interspersed among samples in an analytical batch. Deep seawater consensus reference material (CRM; Rosenstiel School Hansell Organic Biogeochemistry Lab) was analyzed several times throughout each instrument sample batch to verify measurement accuracy. The carbon CRM materials measured with these samples were within reported values (CRM lot 10–17; 43.18 ± 1.07 uM C). The average concentration of carbon measured in the ultrapure water blanks throughout these analyses was 5.4 ± 1.39 uM C. Typical average percent coefficient between the measured and actual KHP was −1.4 ± 2.6%.
Particulate organic carbon and nitrogen
Samples for particulate organic carbon (POC) and nitrogen (PON) analysis, 50–100 mL depending upon the abundance of cells, were analyzed as described in Lomas et al.26. Briefly, samples were filtered onto 25 mm precombusted (450 °C, 5 h) Ahlstrom glass fiber filters and rinsed with 0.2 µm-filtered dilution seawater. Procedural blanks were created by rinsing a pre-combusted filter with an equal volume of filtered dilution seawater to account for any DOC adsorption27. All samples and blanks were frozen at −20 °C until analysis. Samples and blanks were dried at 60 °C, acid-fumed in a desiccator for 24 h, re-dried at 60 °C, and then analyzed on a Costech ECS 4010 CNS analyzer using acetanilide as a standard. Individual sample mass was corrected for the blank and converted to molar concentrations by dividing by the volume filtered.
Cell counts and biovolume
Samples for cell counts were collected at the time of sampling. Larger eukaryotic species were fixed with Alkaline Lugol’s solution (5% v/v) while cyanobacteria and Ostreococcus were fixed in freshly filtered (0.2 µm) paraformaldehyde (0.5% v/v). Lugol’s fixed samples were analyzed using a hemocytometer, counting at least 200 cells28. Cyanobacteria and Ostreococcus were counted by flow cytometry, using the volume analyzed method29. Biovolumes of larger cells were estimated from manual quantification of microscopy images, assuming a cylinder for diatoms where cell height in girdle view was assumed to be equal to the diameter, while the diameter was assumed to be equal to the height when in valve view. For all other cells, the volume was assumed to be that of an ovoid if the major and minor axes were different or a sphere when the axes were the same. Biovolumes of samples analyzed by flow cytometry were estimated by calibrated forward angle light scatter to beads and phytoplankton cultures of known diameters and assuming a spherical shape30. Direct quantification of detrital material was not conducted during sample analysis. Detrital particles and empty frustules in cultures of larger cells observed by microscopy were not common. A best qualitative assessment suggested that bias in organic biomass due to detrital particles was much less than 5%. For cultures analyzed by flow cytometry, particle events outside the cell population gate, after correcting for the sheath fluid blank, was ~5% and when converted to an estimate of biomass bias it was ~0.5% of cell biomass.
Flow-through optics
Setup of flow-through optics
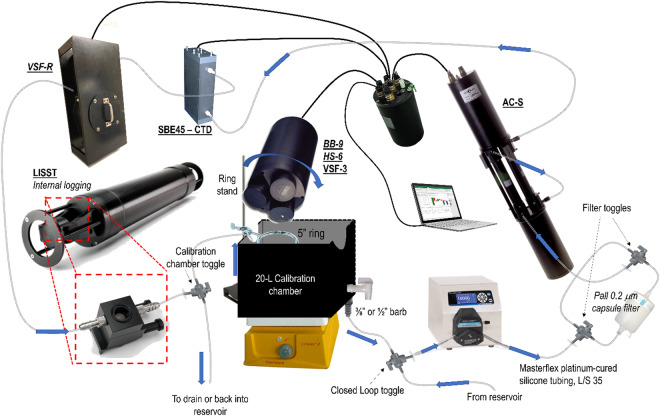
A benchtop flow-through apparatus was constructed to measure the inherent optical properties (IOPs) of the diluted phytoplankton cultures (Table 2). While not all measurements were made on all phytoplankton strains, a set of core measurements from a WETLabs AC-S (hyperspectral absorption and attenuation meter on particulate samples), at least one backscattering instrument, and the LISST-100x were performed in duplicate for all strains. Upon homogenizing diluted cultures on a stir plate for a minimum of 10 minutes at 150 rpm, samples were pumped directly from the 25-L Nalgene carboys into a closed loop benchtop flowthrough system controlled by a Masterflex Easy-Load peristaltic pump in conjunction with Masterflex platinum-cured silicone tubing, L/S 35 (Fig. 1). Water flow was dynamically directed to the instruments through a series of 3/8” barbed three-way valves, terminating in a 20-L capacity black acrylic calibration chamber31. For some experiments, a larger volume chamber (black matte interior comparable to smaller chamber) was used to accommodate the larger HS-6 scattering instrument. When the terminal calibration chamber reached volume capacity with the contents of the Nalgene carboy, a return flow to the in-line system was initiated by switching a three-way toggle valve so that sample intake would be redirected from the carboy to the calibration chamber, closing the loop of the system. With the water looping through the in-line system, the optical instrumentation was gently tapped, and/or oscillatory pressure applied to the tubing to remove bubbles from the system. If necessary, flow rates were elevated on the peristaltic pump (up to 1.8 L min−1) to forcibly remove bubbles. Any volume lost to dislodging bubbles and removing air from the in-line system was subsequently replaced by toggling the three-way valve back to the carboy to demand more sample water.
Table 2.
IOPs sampled by each specified instrument, over a defined range of wavelengths.
| Measurement | Instrument | Wavelengths (nm) | Measurement Angles | Sampling Event |
|---|---|---|---|---|
| Particulate absorption, ap | WETLabs AC-S | 400–730 (hyperspectral) | N/A | 1–5 |
| Dissolved absorption, ag | WETLabs AC-S | 400–730 (hyperspectral) | N/A | 1–5 |
| Particulate beam attenuation, cp | WETLabs AC-S | 400–730 (hyperspectral) | N/A | 1–5 |
| Dissolved beam attenuation, cg | WETLabs AC-S | 400–730 (hyperspectral) | N/A | 1–5 |
| Particulate backscatter, bbp | HOBI Hydroscat-6 (HS-6) | 375, 440, 488, 550, 620,700 | 141° | 2–5 |
| Particulate backscatter, bbp | WETLabs VSF-3 | 440,532,660 | 104°, 130°, 151° | 1–5 |
| Particulate backscatter, bbp | WETLabs VSF-R | 650 | 104°, 130°, 151° | 3–4 |
| Particulate backscatter, bbp | WETLabs BB-9 | 409, 441, 488, 508, 526, 594, 652, 679, 717 | 124° | |
| Particle Size Distribution, N(D) | Sequoia LISST-100x | N/A | N/A | 1–5 |
| Temperature/Salinity | SBE45 MicroTSG | N/A | N/A | 1–5 |
Also included are details on bbp/VSF measurement angles and sampling event in which specific instruments were used.
Fig. 1.
Schematic of in-line sampling apparatus for inherent optical properties of phytoplankton cultures.
Once system equilibrium was achieved, the flow rate on the peristaltic pump was reduced to 0.5 L min−1, and optical measurements were made for a duration of at least 2 minutes to enable statistical exclusion of data anomalies/noise in post-processing. The larger, standalone scattering instrument (BB-9, HS-6, and VSF-3) measurements were performed sequentially in the 20-L calibration chamber, with the optical windows of the instruments submerged, and measuring at a distance of 18–20 cm above the bottom of the chamber32. In some experiments, a smaller, closed, in-line acrylic chamber was alternatively/additionally utilized for the backscattering measurements (using VSF-R sensor only; 650 nm), in order to reduce volume and time demands, and enable the characterization of a larger number of phytoplankton strains. After measuring the whole sample, a series of in-line filter toggles enabled the same water to be redirected to a 0.2 µm polyethersulfone cartridge filter prior to being re-introduced to the instrumentation, in order to assess the dissolved fraction of the IOPs. A separate dedicated 20-L black calibration chamber was used for measuring dissolved backscatter. The system was allowed to cycle through the filtration for at least 10 minutes at a rate of 1.0 L min−1, after the dedicated calibration chamber was filled prior to recording any measurements. Upon termination of sampling, all optical instrumentation was thoroughly cleaned on a daily basis with 70% ethanol and rinsed with Ultra-pure water (Milli-Q UV oxidized) and dried with Opti-WipesTM. The tubing was flushed with at least 27-L of ultrapure water upon termination of sampling.
Pure water laboratory measurements and corrections
Pure water measurements were performed for all instruments daily. Ultrapure water (27-L) was collected daily and allowed to rest overnight to mitigate the impact of micro-bubbles on the measurement of inherent optical properties of the water. Following the same protocols as with sample water, the ultrapure water was introduced to the in-line IOP system through an in-line 0.2 µm cartridge filter by peristaltic pump, filling the 20-L acrylic calibration chamber before toggling the valve to loop the water through all instruments. For the AC-S, continuous measurements at discretized wavelengths were monitored over time, until all measurements > 450 nm were stable at ± 0.003 m−1 (inherent instrument noise). Measurements were compared with prior readings to ensure temporal stability over the course of sequential experiments. In addition to the ultrapure water blank measurements, dark counts on the backscatter instruments were collected by covering the optical windows with black vinyl electrical tape (3 M Scotch Super 33 + ) and submerging them in room temperature acclimated calibration chamber. A background scatter file for the LISST was collected in the zscat chamber, following procedures outlined in the LISST-100x User Manual.
Data Processing Procedures
Absorption and attenuation
All raw binary data were pre-processed with WET Labs Archive Processing program (WAP), which applies instrument specific calibration coefficients provided by the manufacturer, and temporally merges the IOP data with other data streams (e.g., temperature and salinity), outputting readable ascii files. Outlined below are the additional processing steps taken to apply daily ultrapure water calibrations, corrections for temperature, salinity, and the incomplete recovery of scattered light across the absorption tube pathlength. First, the median values from the ultrapure water calibration data were subtracted from the same-day data measurements of the non-fractionated and filtered ( < 0.2 µm) water samples. Temperature and absorption/attenuation salinity correction coefficients (ΨT, ΨSa/ΨSc respectively) provided from Sullivan et al.33 were then applied to the corresponding measured/reference temperature (Tm/Tr) and measured salinity (Sm), and subtracted from the measured absorption (am) and beam attenuation (cm):
| 2 |
| 3 |
Next, using beam attenuation values at a reference wavelength (λ ref = 715 nm) with negligible influence from absorption, a scatter correction for the a-tube was applied from Zaneveld et al.34 to yield total, scatter-corrected absorption (asc):
| 4 |
Finally, the particulate absorption (ap) and beam attenuation (cp) were derived by subtracting the median of the filtered absorption (ag) and beam attenuation (cg) data from the corresponding non-fractionated absorption (asc) and beam attenuation (ct) data. Particulate total scatter (bp) was computed as the difference between cp and ap:
| 5 |
| 6 |
| 7 |
Median values of the measurement time series were reported to mitigate the impact of anomalous bubbles or inherent instrument noise. Values were interpolated to a consistent 2.5 nm sampling interval using a piecewise cubic Hermite interpolating polynomial function in MATLAB.
Backscatter and VSF
All raw binary data from the Wetlabs instruments (BB-9, VSF-3/VSF-R) were pre-processed with the WAP program to output readable ascii files, while HS-6 data were imported directly from the instrument software. As the first step in processing, the Wetlabs instruments angle and wavelength-specific scaling factors (SF(θ,λ)) and dark offsets (DO(θ,λ)) from the manufacturer were subtracted from raw counts (V):
| 8 |
Next, a correction factor was applied to compensate for absorption across the pathlength between the light source and the detector on the backscatter instruments. The scatter-corrected absorption coefficient (asc) from the AC-S was used to correct for absorption across the pathlength:
| 9 |
In these experiments, instead of computationally correcting for the volume scattering function of seawater35 as is common for in situ measurements where one cannot isolate the dissolved component, the values of β(θ,λ) obtained from the dissolved (<0.2 µm) measurements were subtracted from the unfiltered measurements to obtain the volume scattering function of the particle field only.
| 10 |
For the BB-9 and HS-6 measurements, the particulate backscattering coefficient b(λ)bp was estimated using a sampling angle-dependent (124° for BB-9, 141° for HS-6) volume scattering function conversion coefficient, or chi factor, χ36.
| 11 |
For VSF-3 and VSF-R measurements, each spectral measurement was collected at three different angles (θ = 104 °, 130 °, 151°), and the particulate backscattering coefficient at each wavelength was obtained by integrating β(θ,λ) in the backwards direction. First, the corrected βp values were multiplied by 2πsinθ to convert to a polar steradian area. Then, a third order polynomial was fit to the three angular data points and a fourth datum of π radians = 0 (sin (π radians) = 0). Finally, an integration under the curve from π/2 to π radians yielded the b(λ)bp coefficient. The median values of the b(λ)bp time series were extracted in order to mitigate the impact of anomalous bubbles or inherent instrument noise.
Particle size and biovolume (LISST-100X)
A laser in situ scattering and transmissometer (LISST-100X Type-C, Sequoia Scientific, Inc.) was used to measure in situ particle size distribution and concentration. Detailed information of the LISST-100X operation is given in Agrawal and Pottsmith37. Clean filtered (<0.2 µm) water calibrations were performed daily. The scattering intensities measured by the LISST were post-processed using the manufacturer software, using a standard spherical inversion technique to obtain total particle volume concentrations (PVC) within 32 logarithmically spaced size classes from 2.5–500 μm (d). The particle number concentration, N(D), was calculated as:
| 12 |
To obtain an area size distribution, A(D), the number of particles in each bin is multiplied by the average area of a particle in that bin, respectively.
| 13 |
Particle absorption from the spectrophotometer
Absorption coefficients were computed using the method of Stramski et al.21. First, the optical density of suspended matter (ODs) was computed using Eq. 14.
| 14 |
Next, total particle (ap) and de-pigmented particle absorption (ad) were computed using Eq. 15:
| 15 |
where V is the filtration volume and A is the area of the particle load on the filter. The absorption coefficient of phytoplankton, aph(λ), was computed as the difference between ap(λ) and ad(λ). Values are reported as the mean ± standard deviation of replicate measurements.
For a subset of the samples, pigment extraction was not 100% efficient, as evidenced by some pigment peaks remaining in the spectra, compromising the direct calculation of ad(λ). Therefore, smooth fits were performed to estimate ad(λ) on that subset of samples using the following three equations:
| 16 |
| 17 |
| 18 |
where S defined the spectral shape of the curves, and λ0 was the reference wavelength at 400 nm. Equations 16 and 18 follow the exponential form in Roesler et al.38, while Eq. 17 follows the power law function from Twardowski et al.39. The initial values for the spectral shape in all equations were 0.012 and 0.014. Equation 18 includes a null correction, k, which was the average of the spectrum between 700–850 nm. For each sample, average values for S, determined via linear least-squares regression, were computed over the ranges 380–530 nm and 380–600 nm. The fit with the highest correlation coefficient and lowest root mean square error was retained and used.
Data Records
All optical data for these 50+ phytoplankton strains are publicly available in Microsoft Excel® XLXS format uploaded to Dryad40. The file is a multi-tab file with different data sources (e.g., particulate absorption from AC-S) on different tabs within the workbook. Each tab repeats the relevant metadata for each species to facilitate reference back to metadata. When possible, replicate analyses were conducted for each sample type. Here, in this paper, we report the mean values of the replicate samples. For all the sample data, “NA” indicate no data or not defined. All datasets are distributed under a CC0 1.0 Universal Public Domain Dedication license.
Technical Validation
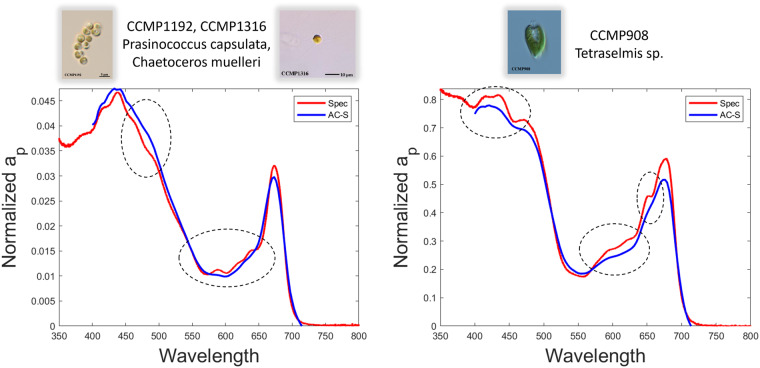
The data presented in this phytoplankton optical fingerprint library were all collected in a consistent manner across all strains. All cultures were grown under standardized conditions and diluted to cell concentrations that approximated in situ cell concentrations based upon published literature. All diluted cultures were homogenized and analyzed for a similar duration using well-vetted methods referenced above in the Methods section. Measurements of absorption were performed using a benchtop spectrophotometer via the filter pad method and an AC-S meter. Although both methods allow us to derive the absorption of particles, in this case pure phytoplankton monocultures, using the filter pad method, the absorption of de-pigmented materials can also be derived and used to compute the absorption by phytoplankton pigments alone. Additionally, the benchtop spectrophotometer provided higher resolution data (2 nm Slit Band Width, 0.2 nm data interval) compared to the AC-S data (14–18 nm Band Width, 3–5 nm interval). The advantage of the higher resolution attained with the filter pad method is that peak resolution is retained whereas some peaks in the AC-S data may be smoothed over owing to the lower spectral resolution (Fig. 2). Additionally, the spectral range of the spectrophotometric measurements reach further into the UV spectrum than the AC-S measurements (down to 290 nm in this study). The wider spectral range allows for the estimation of pigment and cellular absorption as well as the absorption by mycosporine-like amino acids (MAAs) that occur below 400 nm.
Fig. 2.
Comparison of particle absorption (ap) spectra derived from different instruments. Spectra were collected using a spectrophotometer (blue line) and an AC-S (red line) for a) mixed culture of Prasinococcus capsulata and Chaetoceros muelleri and b) unialgal culture of Tetraselmis sp.. Circled locations emphasize absorption peaks that are not resolved with the AC-S due to lower spectral resolution.
In Neeley et al.3, the pigment ratios derived from these experiments were compared to literature values for similar species. A literature review revealed a wide range of pigment ratios between species and within taxonomic groups. As observed in this study, and described in Neeley et al.3, interstrain differences within a species can occur, likely due to differences in the environmental conditions from which they were isolated. The pigment ratios determined from this study largely fell within the range of literature values (Fig. 3) for diatoms32,41–48 and peridinin-containing dinoflagellates32,41,49,50. In contrast, the strains of E. huxleyi diverged somewhat from literature values (Fig. 3c), potentially owing to the aforementioned differences in the oceanic regions from which they were isolated41,44,51,52.
Fig. 3.
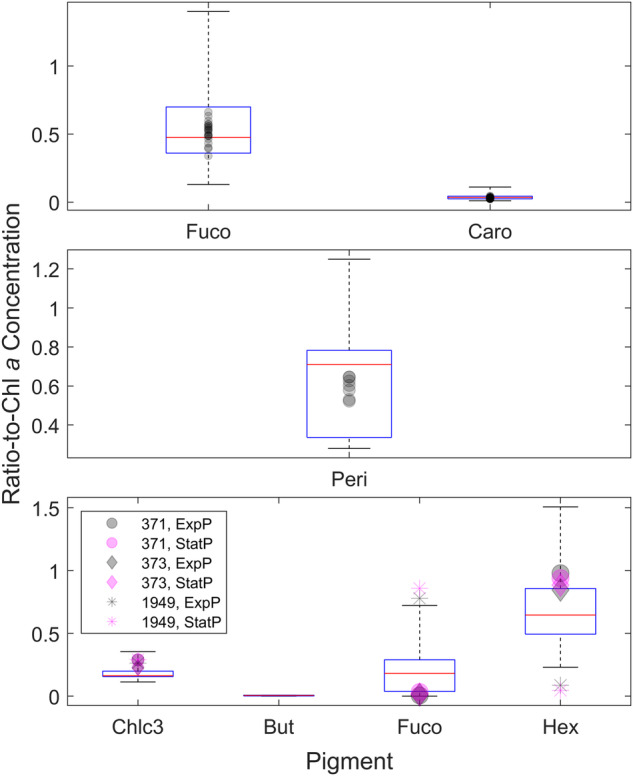
Box-whisker plots of pigment ratios normalized to Chla for different taxonomic groupings. (a) dinoflagellates (b) diatoms and (c) E. huxleyi. Literature data are presented as the box-whisker plot and are overlain with the ratios determined in this study (scatter plots).
Chlorophyll-specific absorption coefficients (a*ph) at five wavelengths determined from this study were compared to existing literature values from other culture studies32,41,53–55 Mean and standard deviation of a*ph(λ) for all species measurements within the major taxonomic groups were computed for this study and from the literature values. These values for four major taxonomic groups were reported at five wavelengths (443, 490, 500, 555 and 676 nm, Table 3). Generally, a*ph(λ) values determined during this study fell within range of those from the literature. Values for Prasinophytes from the literature were more variable but fell within range of those determined from this study,
Table 3.
Mean and standard deviation (Sd) of a*ph (λ;m−1) at five wavelengths from this study and from the literature values (Vaillancourt et al.32; Clementson and Wojtasiewicz40; Sathyendranath et al.52; Ciotti et al.53; Stuart et al.54).
| Wavelength (nm) | Haptophytes | Diatoms | Dinoflagellates | Prasinophytes | ||||
|---|---|---|---|---|---|---|---|---|
| PLOPS N = 19 | Literature N = 6 | PLOPS N = 21 | Literature N = 18 | PLOPS N = 10 | Literature N = 8 | PLOPS N = 9 | Literature N = 9 | |
| Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | Mean ± Sd | |
| a*ph(443) | 0.030 ± 0.012 | 0.033 ± 0.020 | 0.020 ± 0.009 | 0.028 ± 0.010 | 0.017 ± 0.003 | 0.022 ± 0.006 | 0.026 ± 0.015 | 0.053 ± 0.050 |
| a*ph(490) | 0.022 ± 0.010 | 0.016 ± 0.007 | 0.013 ± 0.005 | 0.020 ± 0.008 | 0.014 ± 0.002 | 0.018 ± 0.004 | 0.020 ± 0.009 | 0.038 ± 0.037 |
| a*ph(550) | 0.006 ± 0.002 | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.009 ± 0.004 | 0.007 ± 0.002 | 0.009 ± 0.002 | 0.004 ± 0.002 | 0.008 ± 0.007 |
| a*ph(555) | 0.006 ± 0.002 | 0.004 ± 0.001 | 0.005 ± 0.001 | 0.008 ± 0.004 | 0.006 ± 0.002 | 0.008 ± 0.002 | 0.004 ± 0.002 | 0.008 ± 0.008 |
| a*ph(676) | 0.016 ± 0.005 | 0.015 ± 0.008 | 0.013 ± 0.005 | 0.017 ± 0.006 | 0.013 ± 0.002 | 0.013 ± 0.003 | 0.016 ± 0.005 | 0.030 ± 0.030 |
N is the number of data points used to compute the average values.
Particulate backscatter coefficients (bbp) from this study were averaged by taxonomic group and compared with literature values (Fig. 4). These comparisons necessitate normalization since bbp varies widely with cell density and biomass. Vaillancourt et al.32 measured scattering properties of 29 phytoplankton species, including bbp at 4 wavelengths (440, 470, 510 and 620 nm), with a HS-6 instrument, similar to one of the instruments used in our study and reported bbp normalized to chlorophyll-a concentration (bbp*(λ)), as well as bbp normalized to POC and cell abundances. With the exception of the Haptophyte group, the bbp*(λ) values from the two studies matched quite well (Table 4). We examined Haptophyte strains with and without liths (Supplemental Table 1), whereas Vaillancourt et al.32 examined three strains (I. galbana/CCMP1323; Chrysomulina polylepis/CCMP1757; Pavlova sp./CCMP616), only one of which was characterized in this study. Backscattering measurements of I. galbana from our study were performed only in flow-through mode, but those bbp*(λ) values are comparable to measurements reported by Vaillancourt et al.32. bbp*(λ) values for Rhaphidophytes and cyanophytes in our study compared most closely to values in Vaillancourt et al.32. Both studies examined the same species of Raphidophytes, Heterosigma akashiwo, and the same strain (CCPM452) plus several other strains in our study. Vaillancourt et al.32 examined one Cyanophyte, Synechococcus elongatus, while this study interrogated two different Synechococcus species and Prochlorococcus marinus. Other studies investigating scattering properties of phytoplankton cultures have quantified bbp(λ), but normalized values were either not provided or not included in tabulated format to allow for intercomparisons56,57.
Fig. 4.
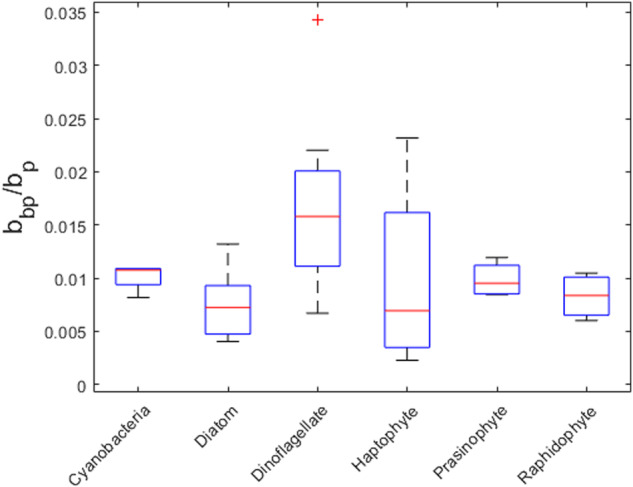
Backscatter ratio box-whisker plots for species examined in this study grouped by broad functional type.
Table 4.
Comparison of spectral particulate backscatter coefficient normalized to chlorophyll-a concentration (bbp*) from this study with literature values listed by taxonomic group.
| Taxon | bbp*(440) (m2 mg Chl-a−1) 1 | bbp*(440) (m2 mg Chl-a−1) HS6 2 | bbp*(440) (m2 mg Chl-a−1) VSF-3 2 | bbp*(441) (m2 mg Chl-a−1) BB9 2 | bbp*(510) (m2 mg Chl-a−1) 1 | bbp*(508) (m2 mg Chl-a−1) BB9 2 | bbp*(620) (m2 mg Chl-a−1) 1 | bbp*(620) (m2 mg Chl-a−1) HS6 2 |
|---|---|---|---|---|---|---|---|---|
| Chlorarachniophyte (Sd) | 1.14E-03 (1.29E-03) | 5.4E-04 (na) | 1.3E-03 (6.6E-04) | na (na) | 1.00E-03 (1.11E-03) | na (na) | 8.66E-04 (1.01E-03) | 7.0E-04 (na) |
| Cryptophyte (Sd) | 1.56E-03 (2.05E-03) | 9.3E-05 (6.8E-05) | 1.8E-04 (5.9E-05) | 2.2E-04 (8.7E-05) | 1.15E-03 (1.42E-03) | 2.0E-04 (2.8E-05) | 9.90E-04 (1.11E-03) | 1.1E-04 (1.6E-05) |
| Cyanophyte (Sd) | 9.65E-04 (na) | 7.1E-04 (4.0E-04) | 2.1E-03 (3.7E-03) | 1.2E-03 (5.7E-04) | 7.16E-04 (na) | 9.9E-04 (4.3E-04) | 5.18E-04 (na) | 5.4E-04 (3.4E-04) |
| Diatom (Sd) | 7.74E-04 (4.35E-04) | 1.5E-03 (3.0E-03) | 2.0E-03 (4.2E-03) | 1.9E-03 (3.8E-03) | 5.30E-04 (2.79E-04) | 1.6E-03 (3.3E-03) | 4.06E-04 (2.06E-04) | 1.0E-03 (2.3E-03) |
| Dinoflagellate (Sd) | 1.65E-03 (1.8E-03) | 5.8E-04 (5.4E-04) | 7.7E-04 (5.7E-04) | 8.2E-04 (5.8E-04) | 1.25E-03 (1.5E-03) | 7.9E-04 (5.2E-04) | 1.27E-03 (1.64E-03) | 4.5E-04 (3.6E-04) |
| Haptophyte (Sd) | 6.54E-04 (1.48E-04) | 7.8E-03 (1.4E-02) | 6.7E-03 (1.4E-02) | 1.7E-02 (2.1E-02) | 4.82E-04 (4.08E-05) | 1.1E-02 (1.4E-02) | 4.15E-04 (3.79E-05) | 5.3E-03 (9.1E-03) |
| Pelagophyte (Sd) | 2.38E-03 (2.92E-03) | na (na) | 9.1E-03 (3.4E-03) | na (na) | 1.61E-03 (1.86E-03) | na (na) | 1.38E-03 (1.63E-03) | na (na) |
| Prasinophyte (Sd) | 1.57E-03 (1.00E-03) | 8.9E-04 (7.1E-04) | 7.0E-04 (8.6E-04) | 2.6E-03 (3.5E-03) | 1.43E-03 (9.07E-04) | 5.9E-03 (1.0E-02) | 1.11E-03 (7.95E-04) | 5.7E-04 (2.8E-04) |
| Raphidophyte (Sd) | 3.13E-04 (na) | 1.9E-04 (2.6E-04) | 1.6E-04 (2.3E-04) | 1.8E-04 (2.3E-04) | 2.39E-04 (na) | 1.5E-04 (1.9E-04) | 2.06E-04 (na) | 1.1E-04 (1.4E-04) |
1Vaillancourt et al.32 and personal communication; 2This study.
Usage Notes
The compiled dataset represents a wide diversity of strains, collected from around the world’s oceans, and highlights the challenges of describing an ‘average diatom or dinoflagellate or cyanobacteria’ (Fig. 5). Rather than focus on this challenge, our diverse dataset can allow users to ‘regionalize’ their analysis by choosing a subset of strains that may be more representative of the focal region, as it is becoming increasingly well recognized that ‘global parameter sets’ perform poorly when downscaled to specific regions (e.g., coastal zone vs. oceanic zone). As an example of the usage of this dataset, the two species of Synechococcus sp. (CCMP1334) and Synechococcus bacillaris (CCMP1333) can be distinguished by the differential expression of the pigments phycoerythrin (PE) and phycocyanin (PC). The differential expression of the phycobilins is determined by the quality of light field in the water column58. PE is commonly expressed by species found in clear waters from which Synechococcus sp. (CCMP1334) was isolated and where blue wavelengths of light are not strongly absorbed. T. erythraeum also expresses both PE and PC. A small PE peak can be observed in the absorption spectrum of CCMP1985, which was isolated from the North Atlantic59,60. In contrast, PC is expressed in species found in more turbid, coastal waters, from which S. bacillaris was isolated, where blue light is more strongly absorbed. Microcystis aeruginosa (CCMP3462) also shows a PC absorption peak and was isolated in the turbid waters of Lake Erie. Furthermore, the wide diversity of parameters measured for each algal strain expands its utility when attempting to validate remotely sensed PCC models against direct field measurements that are not always uniformly measured.
Fig. 5.
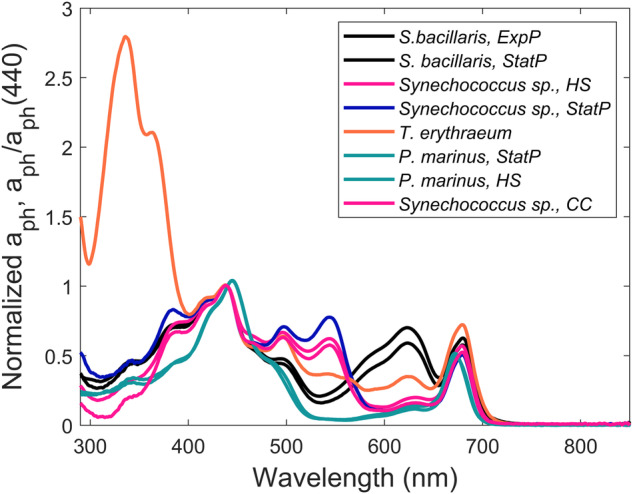
Phytoplankton absorption, aph(λ) normalized to aph(440), of cyanobacterial species measured in this study. ExpP = Exponential phase. StatP = Stationary phase. CC = Climate change. HS = HyperSAS.
Supplementary information
Acknowledgements
We acknowledge project funding support from the NASA Ocean Biology and Biogeochemistry Program (NNX17AB90G) and U.S. National Science Foundation (DEB-1349350 and −1905393) support for the National Center for Marine Algae and Microbiota that provided the cultures used in this study. We appreciate the contributions of Joan Blanchette in maintaining the stock cultures and support during the collection of samples and Grace Kim for participation in the collection and analysis of samples.
Author contributions
M.W.L. and A.M. conceived the study. M.W.L., A.M., A.R.N., and R.V. contributed to the design of the study, collection and analysis of samples, interpretation of data, and writing and editing of the manuscript.
Code availability
No custom code was generated as part of this publication or required for use of the data.
Competing interests
The authors declare no competing financial interests. The views expressed are purely those of the writers and may not under any circumstances be regarded as stating an official position of NASA or any other institution.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-024-03001-z.
References
- 1.Volk, T. & Hoffert, M. I. in The carbon cycle and atmospheric CO2: natural variations Archean to Present. (eds E. T. Sundquist & W. S. Broecker) 99-110 (AGU, 1985).
- 2.Lomas, M. W., Bates, N. R., Johnson, R. J., Steinberg, D. K. & Tanioka, T. Adaptive carbon export response to warming in the Sargasso Sea. Nat Comms13, 1211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neeley, A. R., Lomas, M. W., Mannino, A., Thomas, C. & Vandermeulen, R. Impact of growth phase, pigment adaptation, and climate change conditions on the cellular pigment and carbon content of fifty-one phytoplankton isolates. J. Phycol.58, 669–690 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Dutkiewicz, S. et al. Capturing optically important constituents and properties in a marine biogeochemical and ecosystem model. Biogeosciences12, 4447–4481 (2015). [Google Scholar]
- 5.Jones, E. M. et al. Use of remote-sensing reflectance to constrain a data assimilating marine biogeochemical model of the Great Barrier Reef. Biogeosciences13, 6441–6469 (2016). [Google Scholar]
- 6.Mouw, C. B. et al. A consumer’s guide to satellite remote sensing of multiple phytoplankton groups in the global ocean. Front Mar Sci4, 19pp (2017). [Google Scholar]
- 7.Siegel, D. A., DeVries, T., Cetinić, I. & Bisson, K. M. Quantifying the ocean’s biological pump and Its carbon cycle impacts on global scales. Ann Rev Mar Sci15, 329–356 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Gittings, J. A., Raitsos, D. E., Brewin, R. J. W. & Hoteit, I. Links between phenology of large phytoplankton and fisheries in the Northern and Central Red Sea. Remote Sensing13, 231 (2021). [Google Scholar]
- 9.Le Quere, C. et al. Ecosystem dynamics based on plankton functional types for global biogeochemistry models. Glob. Change Biol.11, 2016–2040 (2005). [Google Scholar]
- 10.Alvain, S., Moulin, C., Dandonneau, Y. & Loisel, H. Seasonal distribution and succession of dominant phytoplankton groups in the global ocean: A satellite view. Global Biogeochemical Cycles22 (2008).
- 11.Palacios, S. L. et al. Remote sensing of phytoplankton functional types in the coastal ocean from the HyspIRI Preparatory Flight Campaign. Remote Sens. Environ.167, 269–280 (2015). [Google Scholar]
- 12.Chase, A. P. et al. Plankton imagery data inform satellite-based estimates of diatom carbon. Geophys Res Lett49, e2022GL098076 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uitz, J., Claustre, H., Morel, A. & Hooker, S. B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J Geophys Res Oceans111, C08005, 10.01029/02005JC003207 (2006). 080. [Google Scholar]
- 14.Vidussi, F., Claustre, H., Manca, B. B., Luchetta, A. & Marty, J.-C. Phytoplankton pigment distribution in relation to upper thermocline circulation in the eastern Mediterranean Sea during winter. J Geophys Res106(19), 939–919,956 (2001). [Google Scholar]
- 15.Guillard, R. & Hargraves, P. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia32, 234–236 (1993). [Google Scholar]
- 16.Moore, L. R. et al. Culturing the marine cyanobacterium Prochlorococcus. Limnol. Oceanogr. Meth.5, 353–362 (2007). [Google Scholar]
- 17.Yentsch, C. & Menzel, D. W. A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep Sea Research10, 221–231 (1963). [Google Scholar]
- 18.Van Heukelem, L. & Thomas, C. S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A910, 31–49 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Hooker, S. B. et al. The second SeaWiFS HPLC analysis round-robin experiment (SeaHARRE-2). NASA Tech. Memo212785, 124 (2005). [Google Scholar]
- 20.Holmes, D. T. & Buhr, K. A. Error propagation in calculated ratios. Clin Biochem40, 728–734 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Stramski, D., Reynolds, R. A., Kaczmarek, S., Uitz, J. & Zheng, G. Correction of pathlength amplification in the filter-pad technique for measurements of particulate absorption coefficient in the visible spectral region. Applied Optics54, 6763–6782 (2015). [DOI] [PubMed] [Google Scholar]
- 22.IOCCG Protocol Series. Inherent Optical Property Measurements and Protocols: Absorption Coefficient. (Dartmouth, NS, Canada, 2018).
- 23.Kishino, M., Takahashi, M., Okami, N. & Ichimura, S. Estimation of the spectral absorption coefficients of phytoplankton in the sea. Bull. Mar. Sci.37, 634–642 (1985). [Google Scholar]
- 24.Mannino, A., Russ, M. E. & Hooker, S. B. Algorithm development and validation for satellite-derived distributions of DOC and CDOM in the U.S. Middle Atlantic Bight. J Geophys Res Oceans113, C07051 (2008). [Google Scholar]
- 25.Mannino, A. et al. Dissolved organic carbon fluxes in the Middle Atlantic Bight: An integrated approach based on satellite data and ocean model products. J Geophys Res Biogeosciences121, 312–336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomas, M. W., Baer, S. E., Acton, S. & Krause, J. W. Pumped up by the cold: elemental quotas and stoichiometry of cold-water diatoms. Front Mar Sci6, 10.3389/fmars.2019.00286 (2019).
- 27.IOCCG Protocol Series. Particulate organic matter sampling and measurement protocols: consensus towards future ocean color missions, (IOCCG, Dartmouth, NS, Canada, 2021).
- 28.Guillard, R. R. L. in Handbook of Phycological methods: culture methods and growth measurements (ed J. R. Stein) 70-85 (Cambridge University Press, 1973).
- 29.Lomas, M. W. et al. Two decades and counting: 24-years of sustained open ocean biogeochemical measurements in the Sargasso Sea. Deep Sea Research II93, 16–32 (2013). [Google Scholar]
- 30.Casey, J. R., Aucan, J., Goldberg, S. R. & Lomas, M. W. Changes in partitioning of carbon amongst photosynthetic pico- and nano-plankton groups in the Sargasso Sea in response to changes in the North Atlantic Oscillation. Deep Sea Research II93, 58–70 (2013). [Google Scholar]
- 31.Cetinić, I., Toro-Farmer, G., Ragan, M., Oberg, C. & Jones, B. H. Calibration procedure for Slocum glider deployed optical instruments. Optics Express17, 15420 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Vaillancourt, R. D., Brown, C. W., Guillard, R. R. L. & Balch, W. M. Light backscattering properties of marine phytoplankton: relationships to cell size, chemical composition and taxonomy. J. Plankton Res.26, 191–212 (2004). [Google Scholar]
- 33.Sullivan, J. M. et al. Hyperspectral temperature and salt dependencies of absorption by water and heavy water in the 400-750 nm spectral range. Appl. Optics45, 5294–5309 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Zaneveld, J. R. V., Kitchen, J. C. & Bricaud, A., Moore, C. in Proceedings SPIE Vol. Ocean Optics XI (ed G.D. Gilbert) 187-200 (1992).
- 35.Zhang, X., Hu, L. & He, M.-X. Scattering by pure seawater: Effect of salinity. Optics Express17, 5698–5710 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Sullivan, J. M. & Twardowski, M. S. Angular shape of the oceanicparticulate volume scattering function in the backward direction. Appl. Optics48, 6811–6819 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Agrawal, Y. C. & Pottsmith, C. Instruments for particle size and settling velocity observations in sediment transport. Mar Geol168, 89–114 (2000). [Google Scholar]
- 38.Roesler, C. S., Perry, M. J. & Carder, K. L. Modeling in situ phytoplankton absorption from total absorption spectra in productive inland marine waters. Limnol. Oceanogr.34, 1510–1523 (1989). [Google Scholar]
- 39.Twardowski, M. S., Boss, E., Sullivan, J. M. & Donaghay, P. L. Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar. Chem.89, 69–88 (2004). [Google Scholar]
- 40.Lomas, M. W. et al. Phytoplankton optical fingerprint libraries for development of phytoplankton ocean color satellite products. Dryad, 10.5061/dryad.rbnzs7hfg (2023). [DOI] [PMC free article] [PubMed]
- 41.Clementson, L. A. & Wojtasiewicz, B. Dataset on the absorption characteristics of extracted phytoplankton pigments. Data in brief24, 103875 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, H., Jing, H., Wong, T. H. & Chen, B. Co‐occurrence of phycocyanin‐and phycoerythrin‐rich S ynechococcus in subtropical estuarine and coastal waters of H ong K ong. Environmental Microbiology Reports6, 90–99 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Sakshaug, E., Johnsen, G., Andresen, K. & Vernet, M. Modeling of light-dependent algal photosynthesis and growth: experiments with the Barents sea diatoms Thalassiosira nordenskioldii and Chaetoceros furcellatus. Deep-Sea Res. Part A Oceangr. Res. Pap.38, 415–430 (1991). [Google Scholar]
- 44.Schlüter, L., Møhlenberg, F., Havskum, H. & Larsen, S. The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas: testing the influence of light and nutrients on pigment/chlorophyll a ratios. Mar. Ecol. Prog. Ser.192, 49–63 (2000). [Google Scholar]
- 45.Brotas, V. & Plante-Cuny, M.-R. The use of HPLC pigment analysis to study microphytobenthos communities. Acta Oecol.24, S109–S115 (2003). [Google Scholar]
- 46.Henriksen, P., Riemann, B., Kaas, H., Sørensen, H. M. & Sørensen, H. L. Effects of nutrient-limitation and irradiance on marine phytoplankton pigments. J. Plankton Res.24, 835–858 (2002). [Google Scholar]
- 47.Schanke, N. L. Photoprotective response of the sea ice diatom Fragilariopsis cylindrus to ultraviolet-B radiation under elevated temperature and light exposure. (College of Charleston, 2015).
- 48.Yao, P., Yu, Z., Deng, C., Liu, S. & Zhen, Y. Classification of marine diatoms using pigment ratio suites. Chin. J. Oceanol. Limnol.29, 1075 (2011). [Google Scholar]
- 49.Liu, S. et al. HPLC pigment profiles of 31 harmful algal bloom species isolated from the coastal sea areas of China. J. Ocean Univ. China13, 941–950 (2014). [Google Scholar]
- 50.Rodriguez, F. et al. Photoacclimation in phytoplankton: implications for biomass estimates, pigment functionality and chemotaxonomy. Mar. Biol.148, 963–971 (2006). [Google Scholar]
- 51.Zapata, M. et al. Photosynthetic pigments in 37 species (65 strains) of Haptophyta: implications for oceanography and chemotaxonomy. Mar. Ecol. Prog. Ser.270, 83–102 (2004). [Google Scholar]
- 52.Seoane, S., Laza, A. & Orive, E. Monitoring phytoplankton assemblages in estuarine waters: The application of pigment analysis and microscopy to size-fractionated samples. Estuar. Coast. Shelf Sci.67, 343–354 (2006). [Google Scholar]
- 53.Sathyendranath, S., Lazzara, L. & Prieur, L. Variations in the spectral values of specific absorption of phytoplankton. Limnol. Oceanogr.32, 403–415 (1987). [Google Scholar]
- 54.Ciotti, A. M., Lewis, M. R. & Cullen, J. J. Assessment of the relationships between dominant cell size in natural phytoplankton communities and the spectral shape of the absorption coefficient. Limnol. Oceanogr.47, 404–417 (2002). [Google Scholar]
- 55.Stuart, V. et al. Bio-optical characteristics of diatom and prymnesiophyte populations in the Labrador Sea. Mar. Ecol. Prog. Ser.201, 91–106 (2000). [Google Scholar]
- 56.Poulin, C., Antoine, D. & Huot, Y. Diurnal variations of the optical properties of phytoplankton in a laboratory experiment and their implication for using inherent optical properties to measure biomass. Optics Express26, 711–729 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Whitmire, A., Pegau, W., Karp-Boss, L., Boss, E. & Cowles, T. J. Spectral backscattering properties of marine phytoplankton cultures. Optics Express18, 15073–15093 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Haverkamp, T. H. et al. Colorful microdiversity of Synechococcus strains (picocyanobacteria) isolated from the Baltic Sea. ISME Jounal3, 397–408 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez, I. B. & Ho, T.-Y. Interactive effects of spectral quality and trace metal availability on the growth of Trichodesmium and Symbiodinium. PLoS One, 0188777 (2017). [DOI] [PMC free article] [PubMed]
- 60.Subramaniam, A., Carpenter, E. J., Karentz, D. & Falkowski, P. G. Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol. Oceanogr.44, 608–617 (1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lomas, M. W. et al. Phytoplankton optical fingerprint libraries for development of phytoplankton ocean color satellite products. Dryad, 10.5061/dryad.rbnzs7hfg (2023). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
No custom code was generated as part of this publication or required for use of the data.