Abstract
Objective
To assess the performance of known survival predictors and evaluate their stratification capability in patients with amyotrophic lateral sclerosis (ALS).
Patients and Methods
We analyzed demographic and clinical variables collected at the Mayo Clinic, Florida ALS center during the first clinical visit of 1442 (100%) patients with ALS.
Results
Our cohort had a median (interquartile range [IQR]) age at diagnosis of 64.8 (57-72) years; 1350 (92%) were non-Hispanic White; and 771 (53.5%) were male. The median (IQR) diagnostic delay was 10.1 (6-18) months, body mass index was 25.4 (23-49), and forced vital capacity was 72% (52%-87%). Approximately 12% of patients tested carried a pathologic C9orf72 hexanucleotide repeat expansion. Median (IQR) ALS functional rating scale-revised score was 35 (29-40) and ALS cognitive behavioral screen score was 15 (12-17). The median (IQR) survival after diagnosis was 17.2 (9-31) months, and survival from symptom onset was 30 (20-48) months. We found that older age decreased forced vital capacity, and fast-progressing ALS functional rating scale-revised scores significantly (P<.0001) influence survival curves and associated hazard risk.
Conclusion
Although results obtained from our cohort are consistent with other reports (eg, men with spinal onset experience a longer survival than women with bulbar onset), they remind us of the complexity of the disease’s natural history and the limited prognostic power of the most common clinical predictors.
Amyotrophic lateral sclerosis (ALS) is a fatal and clinically heterogeneous neurodegenerative disorder defined by upper and lower motor neuron degeneration. It is the most common adult-onset motor neuron disease1,2 with an estimated prevalence of 9.9/100,000 in the United States.3 It has been suggested that from 1990 to 2017, the estimated national incidence and prevalence of motor neuron diseases have increased by 86.8% and 50.4%, respectively.4 However, this increase in prevalence is not supported by direct and more careful measures, and it is unclear whether motor neuron diseases have become more common5 or clinical diagnosis has improved.6 Of note, considerable geographic variation in prevalence has been reported across the United States3,4 and worldwide.7,8 The cause of the variation is not clear. Some clusters of autosomal dominant familial forms of ALS may be explained by a founder effect,9,10 but other clusters, including genetically unexplained sporadic ALS, are still puzzling. Many environmental factors may be associated with ALS,11 but it is still unknown whether regional exposure to certain toxic entities contributes to the variation in prevalence observed. As such, center-based studies are important to understand better how time- and location-associated risk factors influence disease incidence and prevalence12,13 by generating information that may help uncover critical contributors to ALS pathogenesis, develop personalized patient management approaches, and reduce misdiagnosis and delayed therapeutic interventions.14,15 Here, we report the clinical history of patients with ALS seen at Mayo Clinic in Jacksonville, Florida, from October 2003 to October 2019 and evaluate the survival estimate performance and stratification capability of known disease predictors using data retrieved from our large clinical ALS cohort.
Patients and Methods
Study Participants
A total of 1442 patients with ALS aged 21 years or older at the time of diagnosis were included in our study (Mayo Clinic Institutional Review Board protocol # 07-005711 and 1246-03). Patients were seen at the Mayo Clinic, Florida ALS center from October 1, 2003, to October 12, 2019, and were diagnosed with possible, probable, or definite ALS using the revised El Escorial criteria.16 Patients diagnosed with primary lateral sclerosis were excluded from the analysis.
Data Collection and Clinical Predictor Variables
All variables included in this study were collected at the time of the initial ALS clinic visit (first visit). Notably, all patients scheduled in the ALS clinic had already been given an ALS diagnosis, so this visit may not represent their first health care consultation for ALS symptoms. We retrieved clinically available information for sex, age, self-reported ethnoracial group, site of disease onset, body mass index (BMI) (calculated as the weight in kilograms divided by the height in meters squared), forced vital capacity (FVC) percentage, ALS functional rating scale-revised (ALSFRS-R) score, ALS cognitive behavioral screen (ALS-CBS) score, time to diagnosis (diagnostic delay), and presence of a pathologic C9orf72 hexanucleotide repeat expansion. Patients without a confirmed date of death were excluded from the study. The date of ALS onset was determined based on patients’ self-report of initial muscle weakness. Diagnostic delay was recorded as the time between muscle weakness onset and ALS diagnosis. We calculated survival using the date of diagnosis and date of death (in months). The date of death was confirmed by querying publicly available databases. For patients who received invasive ventilatory support (n=43), the date of death was replaced by the date of tracheostomy or laryngectomy.
We placed the following variables into range categories: age (<50, 50-59, 60-69, 70-79, and ≥80 years), BMI (<18.5, 18.5-24.9, 25.0-29.9, and ≥30.0), and FVC percentage of predicted lung capacity (<50, 50-79, and ≥80). We divided the ALSFRS-R categories into 4 groups (<33, 33-39, 40-43, and ≥44).17 Progression rate (ΔFS) was calculated using the equation, ΔFS= (48- ALSFRS-R at “time of diagnosis”)/ duration, where time of diagnosis is recorded as the first visit, and duration is recorded as diagnostic delay (months),18 and then compared with a modified arbitrary range cutoff (<0.5, 0.5-0.9, 1.0-2.0, and >2.0).18,19 For the CBS assessment, patients scoring less than 15 were classified as having possible cognitive impairment, although patients scoring between 15 and 20 were classified as having preserved cognitive abilities.20,21
Statistical Analyses
Statistical analyses was performed using RStudio, version 4.2.2 (Posit software) and GraphPad Prism 9, version 9.4.0 (GraphPad software) with a significance level set at 5%. A Fisher exact test was used to compare case distribution by sex and site of onset. To evaluate group effects, we used analysis of variance and the Tukey test for parametric quantitative variables and Kruskal-Wallis and Dunn tests for non-parametric quantitative variables. Results are expressed in median and respective interquartile range (IQR). Uniform manifold approximation and projection were generated for data visualization. Pearson and Spearman r correlation coefficients were used for correlation analysis of predictors, defaulting to the exclusion of missing data. Kaplan-Meyer curves and log-rank tests were generated for survival analysis, defaulting exclusion of missing data. A univariate Cox regression analysis evaluated individual factors related to patient survival (hazard ratio), and a multivariate Cox regression analysis identified independent prognostic factors accounting for covariates in clinical outcomes. Patients were categorized as slow progressors (ΔFS <1) or fast progressors (ΔFS ≥1). P values of .05 or lower were considered statistically significant. To avoid biased outputs,22,23 no data imputation methodology was used. We performed a comprehensive medical records chart review to fill in missing data.
Results
Demographic and Clinical Characteristics
Our study cohort consisted of 1442 patients with ALS who were deceased (n=1399) or underwent tracheostomy (n=43) between October 1, 2003, and October 12, 2019, representing a prepandemic cohort. Missing clinical data were as follows: 28% for BMI, 36% for FVC, 66% for ALS-CBS, and 44% for ALSFRS-R. The study cohort was predominantly non-Hispanic White (92%), with Black, Hispanic, and other ethnoracial groups representing 6%, 2%, and 0.4% of the cohort, respectively (Table 1). There was a slight male predominance (53.5%), and men were about 2 years younger (median age) at date of diagnosis, first visit, and death when compared with women (Table 1). Patients with bulbar onset were older than those with spinal onset (66.4 years vs 63.6 years; P<.0001). The male-to-female ratio was nearly 2:1 in patients diagnosed before 50 years old and 1:1 in patients diagnosed after 70 years old. Bulbar onset disease comprised approximately 30% of our total cohort and was more frequent in women. The spinal to bulbar onset ratio was 1.7:1 in women vs 3.2:1 in men. Genetic testing as part of a research program was offered after 2008 (N=1249) and was accepted by 644 patients (52%), with 76 (11.8%) found to carry a pathologic C9orf72 hexanucleotide repeat expansion (C9 carriers). C9 carriers were younger than noncarriers at the time of first visit (median [IQR] age, 59.4 [55-66] vs 64.2 [56-71]) with increased cognitive impairment (median=13.5; P=.014), lower ALSFRS-R score (median=33, P=.030), and a shorter disease duration (median=27.3, P=.035) when compared with the subset of the cohort of noncarriers.
Table 1.
Demographics and Clinical Characteristics
| Category | No. (%) | Age at first visit, median (IQR), y | Age at disease onset, median (IQR), y | Median age at death, median (IQR), y |
|---|---|---|---|---|
| Overall | 1442 (100.0) | 64.8 (57-72) | 63.4 (56-70) | 66.6 (59-73) |
| Sex/site of onset | ||||
| Female | ||||
| Bulbar | 252 (17.5) | 66.7 (61-74) | 65.6 (60-73) | 68.9 (63-75) |
| Spinal | 419 (29.1) | 64.4 (56-72) | 63.2 (55-70) | 66.0 (58-70) |
| Total | 671 (46.5) | 65.6 (58-72) | 64.5 (57-71) | 67.8 (60-74) |
| Male | ||||
| Bulbar | 183 (12.7) | 65.8 (59-72) | 65.1 (57-71) | 67.8 (61-73) |
| Spinal | 588 (40.8) | 62.9 (54-71) | 61.5 (53-69) | 64.8 (57-69) |
| Total | 771 (53.5) | 63.7 (55-71) | 62.3 (54-70) | 65.6 (57-73) |
| Ethnoracial | ||||
| Non-Hispanic White | 1,327 (92.0) | 65.1 (57-72) | 63.2 (55-70) | 66.3 (58-73) |
| African American | 80 (5.5) | 59.8 (51-68) | 67.6 (62-74) | 71.8 (66-80) |
| Hispanic | 28 (1.9) | 61.8 (59-67) | 70.9 (63-80) | 73.4 (65-82) |
| Asian | 5 (0.3) | 64.2 (54-72) | 67.0 (59-73) | 68.4 (62-74) |
| Other | 2 (0.1) | 66.7 (49-84) | 70.8 (68-74) | 70.8 (68-74) |
Abbreviation: IQR, interquartile range.
Across our cohort, the median (IQR) diagnostic delay was 10 (6-18) months. Patients with bulbar onset were diagnosed on average 2.7 months earlier than patients with spinal onset disease (8.9 months vs 11.6 months; P<.0001). Patients were first seen at the clinic at a median (IQR) of 12.5 (8.0-22.0) months after initial onset, and for 838 (58%) patients, the first visit occurred within 12 months of symptom onset. During the course of the disease, 351 patients (24.3%) were recorded to use noninvasive ventilation, 43 (3.0%) received invasive ventilation, and 204 (14.1%) had undergone gastrostomy tube placement. Of 931 patients with recorded FVC at first visit, 576 (61.9%) already presented different levels of impaired ventilatory function (FVC <80%). Of patients with a recorded BMI, 557 (53.9%) were overweight (BMI ≥25), with 198 (19.1%) within the range of morbid obesity (BMI ≥30) (Table 2).
Table 2.
Survival Based on Clinical Characteristics at First Visit (N=1442)
| Category | No. (%) | Survival from diagnosis, median (95% CI), mo | P value |
|---|---|---|---|
| Sex | 5.7e-3 | ||
| Female | 671 (46.5) | 18.33 (16.97-20.03) | |
| Male | 771 (53.5) | 16.27 (15.23-17.27) | |
| Ethnoracial | 4.1e-2 | ||
| Non-Hispanic White | 1327 (93.6) | 17.0 (8.9-30.8) | |
| African American | 80 (5.5) | 21.3 (10.4-35.0) | |
| Hispanic | 28 (1.9) | 12.6 (10.1-21.9) | |
| Other | 7 (0.4) | 15.7 (5.1-51.3) | |
| Age range, y | <1e-4 | ||
| <50 | 178 (12.3) | 30.84 (26.17-35.67) | |
| 50-59 | 309 (21.4) | 22.70 (19.87-25.13) | |
| 60-69 | 511 (35.4) | 16.77 (15.67-18.50) | |
| 70-79 | 350 (24.3) | 12.53 (10.93-13.33) | |
| ≥80 | 94 (6.5) | 8.42 (6.83-12.57) | |
| Site of onset/sex | <1e-4 | ||
| Bulbar | |||
| Female | 252 (17.5) | 15.64 (13.33-17.03) | |
| Male | 183 (12.7) | 16.30 (13.60-18.43) | |
| Total | 435 (30.2) | 15.87 (14.43-17.03) | |
| Spinal | |||
| Female | 419 (29.1) | 16.83 (15.50-18.50) | |
| Male | 588 (40.8) | 19.75 (17.37-22.17) | |
| Total | 1,007 (69.8) | 18.00 (16.93-19.77) | |
| Diagnostic delay, mo | <1e-4 | ||
| <6.0 | 335 (23.2) | 15.97 (14.40-16.97) | |
| 6.0-11.9 | 503 (34.9) | 16.77 (15.37-17.70) | |
| 12.0-18.0 | 245 (17.0) | 17.03 (15.53-19.67) | |
| >18.0 | 359 (24.9) | 22.03 (19.33-25.20) | |
| Disease duration, mo | <1e-4 | ||
| <12 | 122 (8.5) | 3.89 (3.43-4.30) | |
| 12-24 | 392 (27.2) | 10.05 (9.27-10.50) | |
| 24-60 | 682 (47.3) | 22.37 (21.17-23.70) | |
| >60 | 246 (17.1) | 56.05 (53.17-59.03) | |
| BMI | 2.4e-3 | ||
| <18.5 | 54 (5.2) | 10.17 (5.43-14.00) | |
| 18.5-24.9 | 423 (40.9) | 16.47 (14.40-17.60) | |
| 25.0-29.9 | 359 (34.7) | 17.77 (15.90-17.60) | |
| ≥30.0 | 198 (19.2) | 17.82 (16.13-21.60) | |
| Missing | 408 (28.3) | 18.73 (17.23-21.50) | |
| FVC% | <1e-4 | ||
| >80 | 355 (38.1) | 22.43 (20.10-24.30) | |
| 50-80 | 392 (42.1) | 14.64 (13.10-16.47) | |
| <50 | 184 (19.8) | 11.14 (8.53-13.40) | |
| Missing | 511 (35.4) | 18.43 (16.73-20.03) | |
| C9orf72 status | 7.3e-3 | ||
| Carriers | 76 (11.8) | 16.95 (12.47-20.57) | |
| Noncarriers | 568 (88.2) | 19.89 (18.00-21.97) | |
| Missing | 798 (55.3) | 15.97 (14.77-16.93) | |
| ALS-CBS | 2.3e-3 | ||
| >15 | 264 (53.2) | 18.50 (16.93-21.83) | |
| ≤15 | 232 (46.8) | 15.97 (13.10-18.13) | |
| Missing | 946 (65.6) | 17.23 (16.13-18.43) | |
| ALSFRS-R | 4e-4 | ||
| >44 | 71 (5.5) | 23.85 (19.60-33.87) | |
| 40-44 | 135 (15.4) | 23.23 (19.67-27.43) | |
| 33-39 | 299 (37.9) | 18.13 (16.83-20.37) | |
| <33 | 299 (41.9) | 15.23 (12.97-17.40) | |
| Missing | 638 (44.2) | 16.2 (15.13-17.27) | |
| ΔFS | <1e-4 | ||
| <0.5 | 145 (18.0) | 28.4 (22.53-33.83) | |
| 0.5-0.9 | 207 (25.8) | 21.6 (19.23-24.40) | |
| 1.0-1.9 | 231 (28.7) | 16.93 (15.23-19.30) | |
| ≥2.0 | 221 (27.5) | 13.1 (11.53-15.67) | |
| Missing | 638 (44.2) | 16.2 (15.13-17.27) |
Abbreviations: ALS-CBS, Amyotrophic Lateral Sclerosis Cognitive Behavioral Screen scores; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised; BMI, body mass index; C9orf72, hexanucleotide repeat expansion mutation; ΔFS, progression rate; FVC%, forced vital capacity percentage.
In our cohort, 496 patients (34.4%) underwent cognitive screening at the time of their first visit. Of these patients, 232 (46.8%) scored within the impaired range (ALS-CBS score ≤15). The ALSFRS-R scores reported that most patients had some degree of functional impairment (score <44), with nearly 42% scoring below 33 (Table 2).24
Survival Analyses
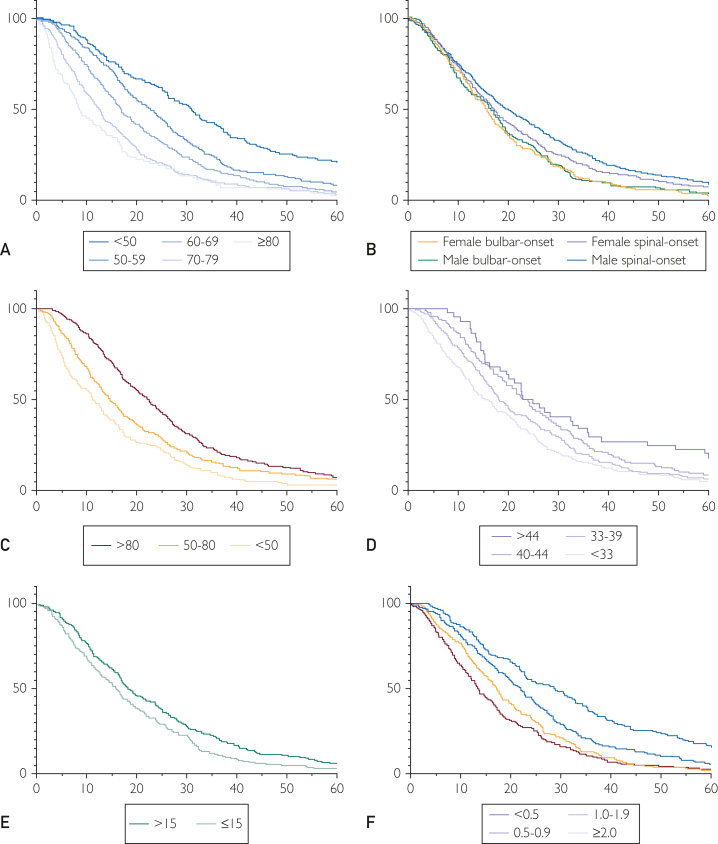
Median (IQR) survival from diagnosis was 17 (9-31) months and disease duration was 30 (20-48) months. Survival from diagnosis was used to examine the effects of clinical characteristics on survival. Survival curves (Figure) reported significant differences in stratified predictors (Table 2). Median survival decreased with increasing age at diagnosis, with a median survival of 30.8 months in younger patients less than 50 years old at diagnosis vs 8.4 months in patients 80 years or older at diagnosis (P<.0001). Median survival was also shorter in women with bulbar onset (P<.0001) and longer in men with spinal onset (P<.0001). A FVC of less than 50% was associated with 11.3 months shorter survival (P<.0001) when compared with higher FVC. Median survival in the African American subcohort reported a similar curve pattern with a longer median survival of about 4.3 years. However, the size of the cohort is insufficient for a robust comparison. Increasing diagnostic delay and BMI were associated with longer survival. A low ALS-CBS score (≤15) was associated with decreased survival by 2.5 months. A smaller ΔFS (<1.0) represents a slow disease progression history and holds lower hazard risk (1.0-1.43), correlating with a longer median survival rate (r=−0.16; P<.0001) observed in 44% of the cohort. Patients with a family history of ALS represented 5.3% of the total cohort and they had a 3-month shorter median survival (17 months; IQR, 10-25; P=.0073) (Figure and Tables 2 and 3). The FVC and age at first visit were the only predictors with visible clustering patterns in uniform manifold approximation and projection analysis (Supplemental Figure 1, available online at http://www.mcpiqojournal.org). The ALSFRS-R and FVC were significantly correlated (r=0.45; P<.0001). Nearly all predictors were significantly correlated with survival, with the strongest being age at first visit and FVC (Supplemental Figure 2, available online at http://www.mcpiqojournal.org). In the C9 carriers, survival was about 3 months shorter (median= 17.0 months, P=.026) when compared with the subset of the cohort of noncarriers.
Figure.
Kaplan-Meier survival curves. Probability of survival of (A) age at first visit (P<1e-4); (B) site of onset (P<1e-4); (C) forced vital capacity (P<1e-4); (D) Functional rating scale-revised (P=4e-4); (E) diagnostic delay—months (P<1e-4); and (F) progression rate (P<1e-4). ΔFS indicates progression rate.
Table 3.
Survival Hazard Risk of Predictors at First Visit (N=1442)
| Category | No. | Univariate model |
Multivariate model |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | Pe | ||||||
| Sex | |||||||||
| Male | 771 |  |
1.00 |  |
1.00 | ||||
| Female | 671 | 1.16 (1.05-1.29) | 4.4e-3 | 1.085 (0.86-1.36) | ns | ||||
| Age at first visit, y | |||||||||
| <50 | 178 |  |
1.00 |  |
1.00 | ||||
| 50-59 | 309 | 1.49 (1.23-1.80) | <1e-4 | 1.29 (0.83-2.06) | ns | ||||
| 60-69 | 511 | 1.87 (1.57-2.22) | <1e-4 | 1.89 (1.26-2.94) | 3.1e-3 | ||||
| 70-79 | 350 | 2.52 (2.10-3.03) | <1e-4 | 3.12 (2.00-4.99) | <1e-4 | ||||
| ≥80 | 94 | 2.85 (2.21-3.66) | <1e-4 | 1.71 (0.88-3.21) | ns | ||||
| Site of onset | |||||||||
| Spinal | 1007 |  |
1.00 |  |
1.00 | ||||
| Bulbar | 435 | 1.28 (1.15-1.44) | <1e-4 | 1.043 (0.82-1.34) | Ns | ||||
| C9orf72 status | |||||||||
| Noncarriers | 568 |  |
1.00 |  |
1.00 | ||||
| Carriers | 76 | 1.38 (1.08-1.75) | 8.9e-3 | 1.271 (0.90-1.77) | Ns | ||||
| Diagnostic delay, mo | |||||||||
| <6.0 | 335 | 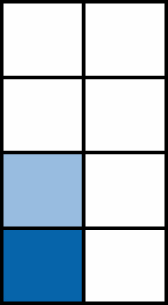 |
1.00 | ||||||
| 6.0-11.9 | 503 | 0.88 (0.76-1.01) | ns | ||||||
| 12.0-18.0 | 245 | 0.84 (0.71-0.99) | 3.7e-2 | ||||||
| >18.0 | 359 | 0.64 (0.55-0.75) | <1e-4 | ||||||
| BMI | |||||||||
| <18.5 | 54 | 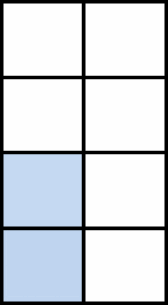 |
1.31 (0.98-1.73) | ns |  |
0.72 (0.41-1.19) | Ns | ||
| 18.5-24.9 | 423 | 1.00 | 1.00 | ||||||
| 25.0-29.9 | 359 | 0.85 (0.73-0.97) | 2.1e-2 | 0.72 (0.55-0.93) | 1.2e-2 | ||||
| ≥30.0 | 198 | 0.82 (0.69-0.97) | 2.4e-2 | 0.76 (0.56-1.05) | Ns | ||||
| FVC% | |||||||||
| >80 | 355 |  |
1.00 |  |
1.00 | ||||
| 50-80 | 392 | 1.34 (1.16-1.54) | <1e-4 | 1.40 (1.09-1.80) | 7.7e-3 | ||||
| <50 | 184 | 1.81 (1.51-2.16) | <1e-4 | 1.31 (0.91-1.88) | Ns | ||||
| ALS-CBS | |||||||||
| >15 | 264 |  |
1.00 |  |
1.00 | ||||
| ≤15 | 232 | 1.33 (1.11-1.59) | 1.7e-3 | 1.34 (1.07-1.69) | 1.4e-2 | ||||
| ALSFRS-R | |||||||||
| ≥44 | 44 |  |
1.00 | ||||||
| 40-43 | 124 | 1.24 (0.89-1.77) | ns | ||||||
| 33-39 | 299 | 1.49 (1.10-2.08) | 1.4e-2 | ||||||
| <33 | 337 | 1.74 (1.28-2.41) | 6e-4 | ||||||
| ΔFS | |||||||||
| <0.5 | 145 |  |
1.00 |  |
1.00 | ||||
| 0.5-0.9 | 207 | 1.43 (1.16-1.78) | 1.1e-3 | 1.09 (0.77-1.56) | ns | ||||
| 1.0-1.9 | 231 | 1.84 (1.49-2.28) | <1e-4 | 1.70 (1.20-2.44) | 3.4e-3 | ||||
| ≥2.0 | 221 | 2.14 (1.73-2.65) | <1e-4 | 1.63 (1.12-2.39) | 1.1e-2 | ||||
Abbreviations: ALS-CBS, amyotrophic lateral sclerosis cognitive behavioral screen scores; ALSFRS-R, amyotrophic lateral sclerosis functional rating scale-revised; BMI, body mass index; C9orf72, hexanucleotide repeat expansion; ΔFS, progression rate; FVC%, forced vital capacity percentage; HR, hazard ratio; ns, not significant.
![]()
Hazard Risk Analyses
Hazard risk was analyzed with Cox regression first using univariate methodology followed by multivariate methodology (Table 3). The multivariate model was constructed by removing diagnostic delay and ALSFRS-R because these variables were accounted for in ΔFS, which would represent a biased analysis if combined in the model. ΔFS has been suggested to better predict survival compared with ALSFRS-R and diagnostic delay.17,18
Overall, survival hazard risk regression univariate models reported significantly increased risks at first visit with increased age, female sex, bulbar onset, decreased FVC, lower ALS-CBS and ALSFRS-R scores, and faster disease progression. The highest hazard ratios were observed in older patients (2.52 for 70-79 years and 2.85 for ≥80 years) and faster disease progression (1.84 for ΔFS 1.0-1.9 and 2.14 for ΔFS ≥2.0). Of note, hazard risk was also increased 1.34-fold and 1.81-fold in patients with an FVC of 50% to 80% and less than 50%, respectively. Longer diagnostic delay was found to decrease hazard risk, a finding that is most likely showing the association between slower disease progression and longer diagnostic delay (P<.0001) (Table 3).
Five risk predictors remained significant after multivariate analysis and may be considered independent risk factors for ALS: age at first visit, BMI, FVC percentage, ALS-CBS score, and ΔFS. Patients with age at first visit of 60 to 69 or 70 to 79 years had a 1.89-fold (P=.0031) or 3.12-fold (P<.0001) increased risk, respectively, although those with BMI of 25 to 30 had a 0.72-fold decreased risk (P=.0123). Patients with FVC of 50% to 80% had a 1.40-fold increased risk (P=.0077), and those with ALS-CBS scores less than 15 had a 1.34-fold increased risk (P=.014). Patients with ΔFS of 1.0 to 2.0 had a 1.7-fold increased risk (P=.0034), and those with ΔFS of 2.0 or faster had a 1.6-fold increased risk (P=.0107) (Table 3).
Discussion
Our study included patients with ALS, primarily from northeast Florida and southern Georgia, who were evaluated at a single tertiary care center, which is one of the largest cohorts to date, comparable to the Midwest study from 2021.13 Our study mirrors previous observations from other single and regional centers and population-based studies. The median survival from diagnosis in our cohort was 17 months, which is consistent with other contemporary large US and European series,12,13 despite ethnoracial, intracontinental and intercontinental genetic variability.25,26 In our series, only 2.9% of patients were alive 10 years after diagnosis, although long survivors have been reported to represent up to 20% in some US series, and between 5%-10% in European series.8,27 The reason for differences in the frequency of long-term survivors among series is unclear. Still, they could be explained by a variety of contributing factors such as differences in diagnostic accuracy, sample size, and regional or single-center patient populations. Other studies have indicated that age at onset, disease duration, and survival rates do not vary significantly among ethnoracial groups.13 Although our data hinted at distinctions within the African American subset, the cohort’s size might not accurately support this observation. Genetic testing including C9orf72 carriership was conducted on a research basis, stretching back to 2008, identified a prevalence of 6% of the whole and 11.8% of the tested ALS cases, comparable with previous reports of a prevalence of 5-10%.28 As genetic panels advance and enhance our understanding of ALS pathogenesis, it is important to integrate clinical data with findings from genetically characterized groups.29 Older age at first visit, lower FVC percentage, and lower ALS-CBS scores emerged as the strongest independent factors of increased risk. Consistent with other series, bulbar onset disease reported a higher prevalence in women and portended a 3-month shorter median survival than spinal onset. Nevertheless, after adjustment with other factors, neither bulbar onset nor sex had independent prognostic significance for survival. This observation could be explained by the older age at diagnosis in female and bulbar onset patients, suggesting that the survival difference may be explained by advanced age. This finding is similar to those of the large Georgia single-center series by Traxinger et al12 but differ from a population-based report by Yamakawa et al.13 These discrepancies can be attributed to variations in defining the region of onset across different series or to differences in the quality of care for patients not evaluated at an ALS center.
Previous studies have also shown that BMI of 30.0 to 35.0 is a protective factor and that BMI less than 18.5 increases survival hazard.30,31 The relationship between BMI and ALS disease progression is still not fully understood. The BMI may reflect regional cohort biases,13 or perhaps a one-time BMI measure at first visit does not capture preceding weight changes.
Diagnostic delay continues to be problematic for patients with ALS, even for a well-resourced population with easy access to tertiary medical centers.15 We observed a median delay of 10.2 months between the reported onset of muscle weakness and diagnosis. This is similar to other United States and international series published over the past few decades.12, 13, 14,32, 33, 34, 35 From a national epidemiologic perspective, our results suggest that although an estimated 30,000 patients in the United States have ALS,3 another 10,000 are awaiting an ALS diagnosis. All health care system factors, such as misdiagnosis, delay in seeing a neurologist, and unnecessary procedures, influence the time to diagnosis, and patients experiencing a slower disease progression are usually diagnosed later.
As our findings and other studies suggest, clinical predictors, individually and collectively, have limited usefulness in creating individual prognostication models.27,35 Models combining clinical and molecular biomarkers have greater potential to improve prognostic accuracy.35,36 Recently, a study using a transcriptome-based disease stratification system identified correlations with age of symptom onset, age of death, and site of onset.37 Although these results support a molecular subtype-related clinical heterogeneity, more studies are needed to understand how data may be used in the clinical setting and for patient stratification and efficacy monitoring in ALS clinical trials.38
The ALSFRS-R is a widely used patient-reported outcome measure in medical settings and clinical trials.39, 40, 41 Our study confirms that using only 1 ALSFRS-R measure (at first visit) has some prognostic value; however, using the rate of change of ALSFRS-R scores to define slow and fast progressors may improve prognostication.42,43 When controlling for other factors, ΔFS contributes significantly to our model of hazard risk, suggesting that ΔFS at first visit reliably improves survival prediction compared with a single ALSFRS-R assessment. The rate of neurodegeneration or accumulation of disability measured by the ALSFRS-R score declines throughout the course of the disease as FVC declines.44,45 In fact, we observed a correlation between these 2 predictors (Supplemental Figure 2); however, FVC displayed a better role in population stratification compared with all other predictors, including ALSFRS-R (Supplemental Figure 1). Therefore, the combination of FVC and ΔFS may improve survival and risk estimates, particularly if also associated with age and ALS-CBS score at first visit. Currently, these measures and molecular markers in use cannot strongly support disease diagnosis, prognosis, or drug efficacy monitoring. Evolving new antisense nucleotide therapies have reported this.38 The importance of early diagnosis and intervention, as well as the monitoring of disease progression and drug efficacy, cannot be overstated. Timely identification and management of ALS can significantly impact clinical outcomes and enhance patients’ quality of life. Furthermore, ongoing monitoring is crucial for assessing disease progression and the effectiveness of interventions, particularly in clinical trials.
Limitations
This study is not representative of global ethnoracial and socioeconomic diversity, as patients in our cohort were predominantly non-Hispanic Whites. The large number of missing data represents a limitation in the analysis. The data from the 408 patient with a complete data set is shown in the Supplemental Table, there are no large differences between the 2 data sets. As this was a center-based study, ascertainment biases are present. Data were collected during routine clinical care at the first visit. Clinical referrals imply a selection bias that may limit data generalization to regional populations, particularly due to a large number of slow-progressing patients (eg, our male spinal onset group). Still, detailed center-based studies are highly relevant and clinically important, as they provide insights into the epidemiology of ALS and advise on using specific prognostic factors not only for individuals but also at the population level.8,35
Conclusion
Our population-based research affirms established prognostic factors in ALS, reinforcing prior findings. Surprisingly, clinical outcomes in ALS appear consistent across diverse geographical groups, emphasizing the enduring importance of epidemiologic research. Notably, ALS clinical outcomes are intricately linked to various demographic and clinical factors. Younger men, especially those with spinal onset, experience slower disease progression. These findings recapitulate recurring patterns in ALS natural progression and underscoring the challenge of accurate disease diagnosis and risk prediction in the absence of reliable clinical and molecular biomarkers.
Potential Competing Interests
Dr Shah reports research grants from Healey Center and the Muscular Dystrophy Association. Dr Belzil reports research grants from NIH, US Department of Defense, LiveLikeLou Foundation, Gerstner Family Foundation, Mayo Clinic Center for Individualized Medicine, and ALS Association. Dr Oskarsson serves as a consultant for Columbia University/Tsumura Inc, Biogen, MediciNova, uniQure, and Mitsubishi; reports research grants from Columbia University/Tsumura Inc, Biogen, MediciNova, Cytokinetics, Mitsubishi, Calico, Ashwatta, Sanofi, AZTherapies, Orion, and TARGET ALS; participated on a Data Safety Monitoring Board or Advisory Board for Biogen, uniQure, and Mitsubishi; serves as a secretary for Western ALS study group, unpaid; Chair, Home Health Medical Standard for ALS, unpaid. The other authors report no competing interests.
Ethics Statement
A total of 1442 patients with ALS aged 21 years or older at the time of diagnosis were included in our study (Mayo Clinic Institutional Review Board protocols # 07-005711 and 1246-03). All study participants in #07-005711 gave written informed consent, and an anonymized database was also used for analysis (Institutional Review Board protocol #1246-03).
Acknowledgments
We would like to thank Kevin B. Boylan, MD, Emeritus member, Department of Neurology, Mayo Clinic, Jacksonville, Florida, who started this project. Importantly, we would like to thank all patients and their family members who participated in this amyotrophic lateral sclerosis clinical research study. The Scientific Publications staff at Mayo Clinic provided copyediting, proofreading, administrative, and clerical support. Drs Erica Engelberg-Cook and Jaimin S. Shah contributed equally to this work.
Footnotes
Data Previously Presented: These data were presented at KI-Mayo 2022, The Mayo Clinic and Karolinska Institute Collaboration Annual Scientific Research Meeting, Neurodegeneration Breakout Session, September 26th, 2022.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Oskarsson B., Gendron T.F., Staff N.P. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc. 2018;93(11):1617–1628. doi: 10.1016/j.mayocp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Wobst H.J., Mack K.L., Brown D.G., Brandon N.J., Shorter J. The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med Res Rev. 2020;40(4):1352–1384. doi: 10.1002/med.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., Raymond J., Punjani R., et al. Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(1-2):108–116. doi: 10.1080/21678421.2022.2059380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 US Neurological Disorders Collaborators. Feigin V.L., Vos T., et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165–176. doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbalho I., Valentim R., Júnior M.D., et al. National registry for amyotrophic lateral sclerosis: a systematic review for structuring population registries of motor neuron diseases. BMC Neurol. 2021;21(1):269. doi: 10.1186/s12883-021-02298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcia P., Lunetta C., Vourc’h P., Pradat P.-F., Blasco H. Time for optimism in amyotrophic lateral sclerosis. Eur J Neurol. 2023;30(5):1459–1464. doi: 10.1111/ene.15738. [DOI] [PubMed] [Google Scholar]
- 7.Logroscino G., Piccininni M. Amyotrophic lateral sclerosis descriptive epidemiology: the origin of geographic difference. Neuroepidemiology. 2019;52(1-2):93–103. doi: 10.1159/000493386. [DOI] [PubMed] [Google Scholar]
- 8.Longinetti E., Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol. 2019;32(5):771–776. doi: 10.1097/WCO.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceroni M., Malaspina A., Poloni T.E., et al. Clustering of ALS patients in central Italy due to the occurrence of the L84F SOD1 gene mutation. Neurology. 1999;53(5):1064–1071. doi: 10.1212/wnl.53.5.1064. [DOI] [PubMed] [Google Scholar]
- 10.Chiò A., Borghero G., Pugliatti M., et al. Large proportion of amyotrophic lateral sclerosis cases in Sardinia due to a single founder mutation of the TARDBP gene. Arch Neurol. 2011;68(5):594–598. doi: 10.1001/archneurol.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oskarsson B., Horton D.K., Mitsumoto H. Potential environmental factors in amyotrophic lateral sclerosis. Neurol Clin. 2015;33(4):877–888. doi: 10.1016/j.ncl.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traxinger K., Kelly C., Johnson B.A., Lyles R.H., Glass J.D. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313–320. doi: 10.1212/CPJ.0b013e3182a1b8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamakawa M., Dwyer S., Song X., Statland J. Demographics, clinical characteristics, and prognostic factors of amyotrophic lateral sclerosis in Midwest. Muscle Nerve. 2022;65(2):217–224. doi: 10.1002/mus.27450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cellura E., Spataro R., Taiello A.C., La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2012;114(6):550–554. doi: 10.1016/j.clineuro.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Richards D., Morren J.A., Pioro E.P. Time to diagnosis and factors affecting diagnostic delay in amyotrophic lateral sclerosis. J Neurol Sci. 2020;417 doi: 10.1016/j.jns.2020.117054. [DOI] [PubMed] [Google Scholar]
- 16.Brooks B.R., Miller R.G., Swash M., Munsat T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 17.Labra J., Menon P., Byth K., Morrison S., Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87(6):628–632. doi: 10.1136/jnnp-2015-310998. [DOI] [PubMed] [Google Scholar]
- 18.Kimura F., Fujimura C., Ishida S., et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66(2):265–267. doi: 10.1212/01.wnl.0000194316.91908.8a. [DOI] [PubMed] [Google Scholar]
- 19.Kjældgaard A.-L., Pilely K., Olsen K.S., et al. Prediction of survival in amyotrophic lateral sclerosis: a nationwide, Danish cohort study. BMC Neurol. 2021;21(1):164. doi: 10.1186/s12883-021-02187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiello E.N., Greco L.C., La Tona A., et al. Clinimetrics of the cognitive section of the Italian ALS Cognitive Behavioral Screen (ALS-CBS™) Neurol Sci. 2023;44(4):1243–1249. doi: 10.1007/s10072-022-06569-9. [DOI] [PubMed] [Google Scholar]
- 21.Turon-Sans J., Gascon-Bayarri J., Reñé R., et al. Cognitive impairment in ALS patients and validation of the Spanish version of the ALS-CBS test. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(3-4):221–227. doi: 10.3109/21678421.2015.1125500. [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu-Jones B.K., Lavage D.R., Snyder J.W., Moore J.H., Pendergrass S.A., Bauer C.R. Characterizing and managing missing structured data in electronic health records: data analysis. JMIR Med Inform. 2018;6(1) doi: 10.2196/medinform.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radosavljevíc L.S.M.S., Nichols T.E. A generative model for evaluating missing data methods in large epidemiological cohorts. Preprint. Posted online April 23, 2024 doi: 10.1101/2024.04.23.24306030. MedRxiv 24306030. [DOI] [Google Scholar]
- 24.Tremolizzo L., Pellegrini A., Susani E., et al. Behavioural but not cognitive impairment is a determinant of caregiver burden in amyotrophic lateral sclerosis. Eur Neurol. 2016;75(3-4):191–194. doi: 10.1159/000445110. [DOI] [PubMed] [Google Scholar]
- 25.Gouveia M.H., Bentley A.R., Leal T.P., et al. Unappreciated subcontinental admixture in Europeans and European Americans and implications for genetic epidemiology studies. Nat Commun. 2023;14(1):6802. doi: 10.1038/s41467-023-42491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechtman L., Jordan H., Wagner L., Horton D.K., Kaye W. Racial and ethnic differences among amyotrophic lateral sclerosis cases in the United States. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1-2):65–71. doi: 10.3109/21678421.2014.971813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiò A., Logroscino G., Hardiman O., et al. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009;10(5-6):310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannoccaro M.P., Bartoletti-Stella A., Piras S., et al. Multiple variants in families with amyotrophic lateral sclerosis and frontotemporal dementia related to C9orf72 repeat expansion: further observations on their oligogenic nature. J Neurol. 2017;264(7):1426–1433. doi: 10.1007/s00415-017-8540-x. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki N., Nishiyama A., Warita H., Aoki M. Genetics of amyotrophic lateral sclerosis: seeking therapeutic targets in the era of gene therapy. J Hum Genet. 2023;68(3):131–152. doi: 10.1038/s10038-022-01055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariosa D., Hammar N., Malmström H., et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol. 2017;81(5):718–728. doi: 10.1002/ana.24936. [DOI] [PubMed] [Google Scholar]
- 31.Paganoni S., Deng J., Jaffa M., Cudkowicz M.E., Wills A.-M. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44(1):20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falcão de Campos C., Gromicho M., Uysal H., et al. Trends in the diagnostic delay and pathway for amyotrophic lateral sclerosis patients across different countries. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.1064619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knibb J.A., Keren N., Kulka A., et al. A clinical tool for predicting survival in ALS. J Neurol Neurosurg Psychiatry. 2016;87(12):1361–1367. doi: 10.1136/jnnp-2015-312908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorenson E.J., Mandrekar J., Crum B., Stevens J.C. Effect of referral bias on assessing survival in ALS. Neurology. 2007;68(8):600–602. doi: 10.1212/01.wnl.0000254501.58158.e7. [DOI] [PubMed] [Google Scholar]
- 35.Westeneng H.-J., Debray T.P.A., Visser A.E., et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17(5):423–433. doi: 10.1016/S1474-4422(18)30089-9. [DOI] [PubMed] [Google Scholar]
- 36.Vignaroli F., Mele A., Tondo G., et al. The need for biomarkers in the ALS-FTD spectrum: A clinical point of view on the role of proteomics. Proteomes. 2023;11(1) doi: 10.3390/proteomes11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshima J., O’Connor S.A., Marschall E., et al. Molecular subtypes of ALS are associated with differences in patient prognosis. Nat Commun. 2023;14(1):95. doi: 10.1038/s41467-022-35494-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Daele S.H., Masrori P., Van Damme P., Van Den Bosch L. The sense of antisense therapies in ALS. Trends Mol Med. 2024;30(3):252–262. doi: 10.1016/j.molmed.2023.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Hardiman O., van den Berg L.H., Kiernan M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 40.Shefner J.M., Andrews J.A., Genge A., et al. A Phase 2, double-blind, randomized, dose-ranging trial of Reldesemtiv in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(3-4):287–299. doi: 10.1080/21678421.2020.1822410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witzel S., Maier A., Steinbach R., et al. Safety and effectiveness of long-term intravenous administration of Edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121–130. doi: 10.1001/jamaneurol.2021.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaabi A. Modeling amyotrophic lateral sclerosis progression: logic in the logit. Cureus. 2022;14(5) doi: 10.7759/cureus.24887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su W.-M., Cheng Y.-F., Jiang Z., et al. Predictors of survival in patients with amyotrophic lateral sclerosis: A large meta-analysis. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castrillo-Viguera C., Grasso D.L., Simpson E., Shefner J., Cudkowicz M.E. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178–180. doi: 10.3109/17482960903093710. [DOI] [PubMed] [Google Scholar]
- 45.Proudfoot M., Jones A., Talbot K., Al-Chalabi A., Turner M.R. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(5-6):414–425. doi: 10.3109/21678421.2016.1140786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.