Summary
Background
The worldwide geographical and temporal variation in the prevalence of diabetes represents a challenge, but also an opportunity for gaining etiological insights. Encompassing the bulk of East Asians, a large and distinct proportion of the world population, China can be a source of valuable epidemiological insights for diabetes, especially in early life, when pathophysiology begins. We carried out a nationwide, epidemiological survey of Prevalence and Risk of Obesity and Diabetes in Youth (PRODY) in China, from 2017 to 2019, to estimate the population-based prevalence of diagnosed pediatric diabetes and screen for undiagnosed pediatric type 2 diabetes (T2D).
Methods
PRODY was a nation-wide, school population-based, cross-sectional, multicenter survey by questionnaire, fasting urine glucose test and simple oral glucose tolerance test (s-OGTT), among a total number of 193,801 general-population children and adolescents (covered a pediatric population of more than 96.8 million), aged 3–18, from twelve provinces across China. The prevalence of the self-reported pediatric diabetes, the proportion of subtypes, the crude prevalence of undiagnosed T2D and prediabetes in general juvenile population and the main risk factors of type 1 (T1D) and type 2 (T2D) diabetes had been analyzed in the study.
Findings
The prevalence of all self-reported pediatric diabetes was estimated at 0.62/1000 (95% CI: 0.51–0.74), with T1D at 0.44/1000 (95% CI: 0.35–0.54) and T2D at 0.18/1000 (95% CI: 0.13–0.25). For undiagnosed T2D, the crude prevalence was almost ten-fold higher, at 1.59/1000, with an estimated extra 28.45/1000 of undiagnosed impaired glucose tolerance (IGT) and 53.74/1000 of undiagnosed impaired fasting glucose (IFG) by s-OGTT screening. Maternal diabetes history is the major risk factors for all subtypes of pediatric diabetes in China.
Interpretation
The PRODY study provides the first population-based estimate of the prevalence of pediatric diabetes China and reveals a magnitude of the problem of undiagnosed pediatric T2D. We propose a practical screening strategy by s-OGTT to address this serious gap.
Funding
The National Key Research and Development Programme of China, Key R&D Program of Zhejiang, the National Natural Science Foundation of China and the Zhejiang Provincial Key Disciplines of Medicine, Key R&D Program Projects in Zhejiang Province.
Keywords: Prevalence, Pediatric diabetes, Type 1 diabetes, Type 2 diabetes, Prediabetes
Research in context.
Evidence before this study
We searched PubMed for articles published until March 31st, 2024, with MeSH terms of “Diabetes Mellitus/subheading-epidemiology”, “Child” and “Adolescent” with “China” in extra searching. This search confirmed the lack of studies on pediatric diabetes prevalence or environmental effects on risk in China, a country encompassing one-fifth of the world's population. EURODIAB, DIAMOND and SEARCH were well-known epidemiology studies focusing on pediatric diabetes. EURODIAB and DIAMOND had reported the incidence of type 1 diabetes (T1D) in the pediatric population during the 1990s, and found it the lowest in China and Venezuela. SEARCH has been conducted in the U.S. since 2000. It has been keeping on reporting the increasing prevalence as well as the incidence of pediatric T1D and type 2 diabetes (T2D) in the U.S. among multiple ethnic groups. The prevalence of T1D and T2D was 2.15/1000 and 0.67/1000, respectively, in 2017, while non-Hispanic Whites had the highest T1D prevalence at 2.79/1000 and non-Hispanic Black had the highest T2D prevalence at 1.80/1000. Another study by searching MarketScan Multi-State Medical Database in the U.S. has reported a similar prevalence of T1D at 2.34/1000 but a much higher of T2D at 2.12/1000 in 2016. The SEARCH found the American Indians, who shared closer genetic background with Chinese population, had the lowest prevalence for T1D (0.56/1000) and relatively higher one for T2D (1.63/1000). There were several national epidemiological surveys of diabetes in China before PRODY, but none was population-based except for the one we had conducted based on the medical records during 1995–2010. A few other studies had relatively small samples size in limited regions, which could not reflect the integral influence of environmental factors in China. The prevalence of prediabetes in pediatric population had not been systematically investigated in large population, either. A meta-analysis study reviewed 48 studies and provided a pooled prevalence of 8.84% for prediabetes in childhood. It involved 5 studies in the Chinese population with prevalence ranging from 0.7% to 22% among different regions. However, all of them only covered the impaired fasting glucose for prediabetes. Therefore, an updated survey on diabetes and prediabetes in a large-scale sample size of Chinese pediatric population is important.
Added value of this study
We have conducted a school population-based national epidemiological study on diabetes in Chinese pediatric population by in-site survey, including 193,801 participants that sampled a population of more than 96.8 million. The present study updated the prevalence of the self-reported diabetes by questionnaire survey as well as of the undiagnosed diabetes and prediabetes by screening with s-OGTT, a novel screening instrument that permits detection not only of IFG but also of IGT and allowed us to document an alarming and in contrast to the low prevalence of T1D in China as expected, we found a much higher prevalence of undiagnosed T2D and prediabetes. We had also tested the tools of fasting urine glucose (FUG) and s-OGTT in screening for the undiagnosed T2D and prediabetes, which demonstrated the superiority of s-OGTT in such task.
Implications of all the available evidence
Our results revealed that T2D may have composed of an unexpected large fraction of pediatric diabetes in Chinese population, which could be extended to other East Asian populations. The prediabetes in childhood also requires more awareness in clinical practice. The s-OGTT test following multi-step risk assessment displayed good potential in screening for pediatric T2D and prediabetes. It is necessary to test the sensitivity and specificity of it in the future study.
Introduction
Diabetes is a major global health problem, in which multiple etiologies converge to lead to a widespread burden of cardiometabolic and vascular morbidity. A major challenge in better understanding the disease and developing prevention and therapeutics—the considerable heterogeneity of the disease in both geography and time1—also presents an opportunity for insight generation into etiology. The general temporal trend towards increased incidence and prevalence constantly modifies differences between populations and opens a window to the study of gene–environment interactions. China, comprising one-fifth of world's population and experiencing major social and economic changes over the past decades presents an opportunity for such insights. Diabetes of autoimmune etiology (type 1) is known to be several-fold less frequent in East Asians than in European-ancestry populations,2 an underexploited opportunity to study genetic vs. environmental causes and their interactions. Diabetes of metabolic causes (type 2) on the other hand, has shown considerable increase in the past decade, from an estimated 98.4 million cases to 140.9 million (IDF Diabetes Atlas 2021 10th edition), as a result of multifaceted demographic and socioeconomic changes.3, 4, 5, 6 A problem with applying this epidemiology to the generation of etiological and therapeutic insights is that it consists almost exclusively of studies in adult populations, while it is known that clinically detectable pathogenetic mechanisms are already operational much earlier, in the pediatric age group. A population-based incidence study for T1D in China also included children and teens,7 but similar data for T2D are lacking.
It has been long thought that T1D is the primary type among children diagnosed with diabetes, but a fast-increasing incidence of T2D in children and adolescents has been reported in recent decades.8, 9, 10 The prevalence of pediatric T2D based on population surveys is low but, given the asymptomatic nature of early disease, it may be a serious underestimate. In the USA, American Indian had significantly higher pediatric T2D prevalence than Non-Hispanic Whites.11, 12, 13 Genetically, Chinese are closer to this high-prevalence group. Actually, we did find the prevalence of pediatric T2D increased faster than T1D from 1995 to 2010 in our previous study,10 but the clinical diagnosis of pediatric T2D remains low in China.10,14,15 An active, population-based screening study to generate epidemiological data for pediatric diabetes is required to improve etiological understanding and clinical practice.
In the present study, we conducted a national epidemiological study on pediatric diabetes, namely The Prevalence and Risk of Obesity and Diabetes in Youth (PRODY), to estimate the prevalence of pediatric diabetes and search for practical screening method for pediatric T2D. The survey included online questionnaire, physical examination and laboratory testing. During the survey, we practiced the s-OGTT for screening undiagnosed cases on 6428 participants selected for high risk of T2D.
Methods
Participants and study workflow
We applied a multistage sampling method to select a nationally representative sample of youths aged 3–18 years in the general population from 2017 to 2019. The population samples were collected from five regions (Eastern, Western, Southern, Northern, and Middle China) and twelve locations (including provinces, municipalities and autonomous regions; all referred to as “province” for brevity in this paper) in China (Eastern China: Shanghai and Zhejiang; Western China: Chongqing and Xinjiang; Southern China: Guangdong, Fujian and Guangxi; Northern China: Beijing, Tianjin and Jilin; Middle China: Hubei and Henan). The workflow is summarized in Fig. 1 and more details were provided in the supplementary methods-study workflow.
Fig. 1.
Workflow chart of the PRODY. BMI, body mass index; FUG, fasting urine glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; s-OGTT, simple-oral glucose tolerance test; T1D, type 1 diabetes; T2D, type 2 diabetes.
Briefly, we collected data from 193,801 (101,941 males and 91,860 females) participants with integral data of online questionnaire survey (Table S1) or physical examination and 176,086 among them completed the fasting urine glucose (FUG) test at Stage 1 (Supplementary Methods-Study workflow-Line 39). The study participants represented a pediatric population more than 96.8 million in the 12 provinces (refer to the population aged 0–14 years from the national census in 2019).
At Stage 2, a total of 96,044 participants from Fujian, Guangdong, Henan and Zhejiang were enrolled for T2D screening (Supplementary Methods-Study workflow-Line 55). Of 40,858 participants deemed to be high-risk of T2D with at least one risk factors of four (macrosomia, family history of diabetes, body mass index (BMI) over the 95-percentile (P95) or positive FUG results), 6474 participants, whose guardians (and the school administration) agreed to participate, were selected for a s-OGTT and validated data was collected from 6428.
The study was approved by the Institutional Review Board of The Children's Hospital of Zhejiang University School of Medicine (Approval Number 2016-JRB-018) and the institutional review boards or ethics committees of each participating unit. Written informed consent was obtained from each participant and their guardians before the survey.
Study outcome definitions
The major clinical assessment methods were provided in Supplementary Methods-Survey and data collection.
Each type of glucose tolerance status (normoglycemia, at risk for diabetes/prediabetes and diabetes) was defined according to the criteria of the World Health Organization guidelines. T1D diagnosis required at least one positive pancreatic autoantibody (Table S2).
In the T2D risk preliminary screening, macrosomia was defined as the participant's birth weight over 4 kg; family history of diabetes was defined as any major family members of the participant (parents, siblings or grandparents) had been diagnosed with any type of diabetes; BMI over the 95-percentile (P95) was defined as the participant's BMI was higher than the P95 of the peer population (according to quantization table of the Chinese children and adolescents' growth values aged 0–18 year by the Capital Institute of Pediatrics); positive FUG results was defined as the participants had at least one “+” signal reported by the urine analyzer.
Statistical analysis
Sample size was estimated according to the preliminary investigation on T1D we conducted in Hangzhou city of Zhejiang. The known prevalence of T1D in Hangzhou city of Zhejiang was 0.23/1000. According to the confidence intervals for the expectation of Poisson distribution, the expected number of cases should be 4.16 at a prevalence of 0.228/1000 to ensure that we could find at least one case (i.e., that the lower limit of 95% confidence intervals was at least one); at least (4/0.228) × 1000 = 17,500 participants were needed. Assuming 10% attrition, we recruited 19,300 in each province, or a total of 232,600 in 12 provinces.
The prevalence was calculated as the ratio of the number of cases of a given type of diabetes in a sex/age/regional population to the total population number and expressed as cases per 1000. The 95% confidence intervals (CIs) were calculated by Poisson distribution. The crude prevalence based on the s-OGTT data was estimated by the equation shown in Figure S1. Because the high-risk group was selected non-randomly, it was not feasible to calculate the CIs for the prevalence estimated in this way. It was expressed as crude prevalence in this study and represents a minimum (there would be extra cases in low-risk population). The diagnostic rate was calculated as prevalence of known T2D by questionnaire survey to the total prevalence of T2D, which include the known prevalence and unknown crude prevalence estimated by s-OGTT.
The geographic latitude level of each province was defined as the level of the provincial capital. The GDP per capita was calculated as the GDP divided by the total population at the same time in the same region, according to the national GDP statistics and census data in 2019. The risk factor analysis for latitude and socioeconomic development level was based on the populations with known T1D or T2D by questionnaire survey. The analysis for individual's life history risk factors was based on populations with T1D or T2D by either questionnaire survey or s-OGTT. The risk factors analysis was done by stepwise logistic regression, using the Fisher exact test for the theoretic frequency T <5. The statistical significance threshold of P < 0.05 was corrected for the number of hypotheses tested, at 0.00417 for T1D (12 risk factors) and 0.0033 for T2D (15 risk factors).
Statistical analyses were performed using the Stata/MP 17.0 for windows or R-Project for Statistical Computing (version 4.2.3).
Role of funding sourse
The funders had no role in study design, data collection, data analysis, interpretation or writing the manuscript.
Results
Data
Of the 250,000 children who were invited to participate, 231,574 (92.6%) accepted (Fig. 1). Data from 31,902 of these participants, all from one province (Jiangxi), were not used because of concerns about their reliability, for reasons other than the data themselves. Another 6591 questionnaires were not used because of being incomplete (more than 200 items unanswered for the questionnaire or unrecorded for physical examination results). Of these, 176,086 had usable data on physical examination and FUG. Finally, the 96,044 subjects from the four provinces participating in the T2D screening (Henan, Zhejiang, Fujian and Guangdong) we had usable OGTT data from 6428 of the 6474 participants who met the high-risk criteria. Missing data did not correlate with any of the end-points, which led us to classify them as MCAR (missing completely at random). We did not attempt any imputation of missing data.
Prevalence of pediatric diabetes in China by questionnaire survey
PRODY questionnaires identified 120 cases of self-reported diagnosed pediatric diabetes among 193,081 participants, with 85 cases of T1D and 35 cases of T2D, for a prevalence of diagnosed cases of 0.62/1000 (95% CI: 0.51–0.74), of which T1D accounted for 0.44/1000 (95% CI: 0.35–0.54) and T2D for 0.18/1000 (95% CI: 0.13–0.25). As expected, prevalence for all diabetes, T1D and T2D was higher in the 10–19-year age group than in the 3–9-year age group (P = 0.0083 for T1D, P = 0.0009 for T2D). There is no significant difference between sexes in either age groups (Fig. 2a and b and Table S3). The prevalence proportion was 70.83% for T1D and was 29.17% for T2D (Fig. 2c).
Fig. 2.
Prevalence of pediatric diabetes in China. The self-reported prevalence of pediatric diabetes estimated by questionnaire survey in T1D, T2D (a, b). The proportion of self-reported prevalence of T1D and T2D among all pediatric diabetes by questionnaire survey (c). The proportion of the self-reported prevalence of T1D, T2D by questionnaire survey and of the unknown T2D identified by s-OGTT in high-risk population in the four selected provinces (d). KN, known; T1D, type 1 diabetes; T2D, type 2 diabetes; UKN, unknown.
The regional distribution was uneven. Prevalence for both T1D and T2D was much higher in Xinjiang province than anywhere else (T1D: 2.38/1000 (95% CI: 1.23–4.15) and T2D: 1.19/1000 (95% CI: 0.44–2.59)) (Fig. 3 and Table S4).
Fig. 3.
Regional distribution of self-reported prevalence of pediatric diabetes in China. a and b is the reginal distribution of pediatric prevalence of T1D and T2D, respectively (case per 1000). The blue color scale represented the intensity of the prevalence. The prevalence of T1D or T2D in each province was listed at the end of each indicatrix. The prevalence of both T1D and T2D displayed in this figure was based on the self-reported prevalence by questionnaire survey; T1D, type 1 diabetes; T2D, type 2 diabetes.
We found 106 cases positive for FUG among 176,086 valid samples, of which 90 were not in agreement with diabetes on follow-up diagnosis. Of the remaining 16, 8 cases had T1D, all of which had been known by the questionnaire survey. The other 8 had T2D, of which 3 were known cases and 5 were newly diagnosed cases (Fig. 1).
Prevalence of undiagnosed T2D and prediabetes by s-OGTT screening
We selected participants (N = 96,044) in four provinces (Fujian, Guangdong, Henan and Zhejiang) for the search of unknown T2D and prediabetes study. Of the 40,858 who had at least one high-risk criterion 6474 participants were randomly selected for s-OGTT, resulting in 6428 valid s-OGTT results. Among them, 24 cases were identified with T2D, 812 cases with IFG and 430 cases with IGT, of which 94 cases had both IFG and IGT (Fig. 1 and Table S5).
No sex differences in individual provinces withstood correction for multiple-hypothesis testing (Table S5)
The estimated crude prevalence of unknown T2D by s-OGTT, was 1.59/1000 (calculation refer to Figure S1). The total crude prevalence of T2D was 1.77/1000 (known by survey + unknown T2D by s-OGTT), an order of magnitude higher than the known T2D. The estimated crude prevalence of IGT and IFG were 28.46/1000 and 53.74/1000, respectively (Table S6).
The proportion of all T2D cases at all ages among the total crude prevalence of diabetes in the four selected provinces was estimated at 80.77% (7.21% for known cases + 73.56% for study-discovered cases), indicating that most pediatric-age T2D in China remains undiagnosed, 72.35% in the 3–9-year-olds and 83.02% at 10–18 (Fig. 2d and Table S7). It changed the conventional knowledge of the dominant proportion of T1D in pediatrics as shown in Fig. 2c.
IFG only was about two-fold higher of IGT only and seven-fold higher than both in our results (Table S8). FUG test identified few of these cases, especially of the IGT. We also did analysis for a tentative assessment on the FUG efficacy on T2D screening with sensitivity at 4.12%, specificity at 99.95%, positive predictive value at 15.09% and negative predictive value at 99.79% (Table S9). About 52.4% of the cases with a positive result in FUG test were positive in s-OGTT, too (n/N4 in Table S10).
Risk factor analysis
The risk factors we analyzed in pediatric diabetes include latitude, socioeconomic development level and individual's life history.
Previously, and concordant with findings in Europe, T1D incidence had been found higher at high latitude.7,16 Our data do not indicate this, with the exception that the prevalence of T1D in Xinjiang (the province with the highest latitude in this study) was by far the highest. However, the prevalence in Jilin, Beijing and Tianjin, which have a similar latitude level (Xinjiang: 43.5 vs. Jilin: 43.4, Beijing: 39.9 Tianjin: 39), was much lower (Xinjiang: 2.38/1000 vs. Jilin + Beijing + Tianjin: 0.23/1000, P < 0.0001), indicating that genetic factors rather than climate may be the cause, concordant with the known low East Asian admixture in that province. After excluding Xinjiang, the T1D prevalence showed no difference between provinces at latitudes ≥39 and < 39 (≥39: 0.23/1000, SE = 0.09/1000; <39: 0.42/1000, SE = 0.05/1000; P = 0.1531). The prevalence of T2D was not related to the latitude level, either (Figure S2a.). There was no significant relationship between the socioeconomic development in individual provinces and the prevalence in either T1D nor T2D (Figure S2b).
The maternal diabetes history is a potential risk factor for T1D though the evidence by P value was not strong enough (P = 0.00701). The other family history of diabetes was not associated with the risk.
Obesity and large birth weight were the only risk factors for T1D with strong evidence (reached statistical Bonferroni-corrected significance), while parental education played potential protective role with weaker evidence (Fig. 4).
Fig. 4.
Family history risk factors for pediatric type 1 diabetes. The risk factors for T1D were analyzed by stepwise Logistic regression and displayed in forest plots. Horizontal bars represent 95% CIs. CI, confidence interval; OR, odds ratio. The diabetes history referred to any type of diabetes had reported for the related family members in questionnaire survey.
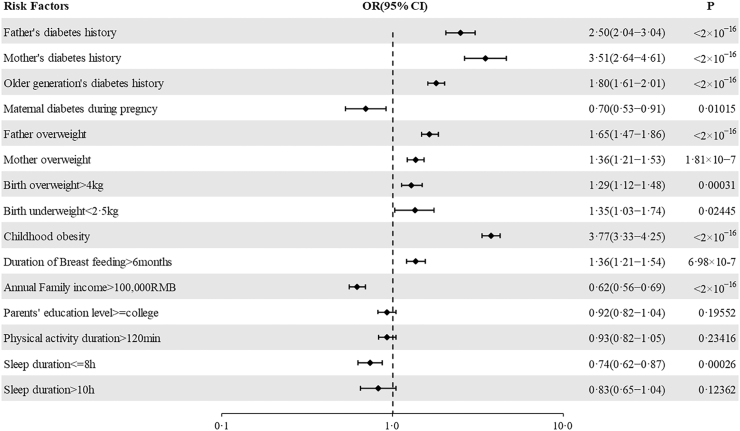
Family history of diabetes or overweight/obesity conferred the highest risk for T2D. Childhood obesity was a risk factor, as was large birth weight, while the evidence for risk from low-birth weight was weak. Exposure to maternal gestational diabetes mellitus (GDM) could not be meaningfully assessed, as in 1020 of 1470 questionnaires that answered YES to maternal diabetes the question of GDM was not answered. Higher family income was significantly protective as was, somewhat surprisingly, shorter duration of sleep (no more than 8 h). Duration of self-reported physical activity had no effect (Fig. 5).
Fig. 5.
Family history risk factors for pediatric type 2 diabetes. The risk factors for T2D were analyzed by stepwise Logistic regression and displayed in forest plots. Horizontal bars represent 95% CIs. CI, confidence interval; OR, odds ratio. The diabetes history referred to any type of diabetes had reported for the related family members in questionnaire survey.
Discussion
Our study is the first population-based systematic evaluation of diabetes prevalence in childhood in China and it adds important information to the existing literature in many respects. Primarily, it indicates an exceptionally high prevalence of undiagnosed T2D and prediabetes. Almost all East Asian epidemiological studies on T2D are based on adult population samples. Our findings reveal an unexpectedly large population of undiagnosed very-early onset T2D, much higher than that found in non-Hispanic whites,11,13,17,18 a goldmine for studies of the metabolic trajectory of T2D in East Asians.
In this study, we have demonstrated the feasibility of the s-OGTT for population screening of pediatric T2D. The numbers, much higher than previously suspected, are alarming and constitute a call to action for development of early intervention strategies. Childhood onset T2D usually has worse prognosis compared with the adult onset one19,20 making timely intervention crucial. Perhaps more strikingly, the estimated prevalence of prediabetes (IFG + IGT) is 82.2/1000, approaching the adult prevalence of T2D. Early intervention in these cases will be a daunting task but may lead to even more effective prevention strategies. The FUG test was not a sensitive screening tool for pediatric T2D and prediabetes. However, it could be a useful preliminary risk-assessing tool as complementary for s-OGTT.
Chen et al. reported the annual prevalence of T1D and T2D among US pediatric population (aged <18 years) in 2016 (chronologically close to our study) as 2.34/1000 and 2.12/1000, respectively.8 The data was from MarketScan Multi-State Medical Database from 2002 to 2016. The prevalence of pediatric T1D in US was 5.3 times of that in China (2.34/1000 vs. 0.44/1000) conforming previous estimation of Caucasian T1D prevalence over 5 times higher than in East Asians.21 The large and somewhat unexpected difference from our findings was in the prevalence of T2D (2.12/1000 vs. 1.77/1000). The increasing prevalence of T2D compared to previous reports in China10 is similar to that reported in the USA during 2009–2014.8 Roony et al. reported the prevalence of IFG in Southeast Asia (SEA) was the highest while that of the IGT was the lowest among all the regions. It was on the opposite in all other regions reported.22 Mohan et al. followed and provided the similar finding of higher prevalence of IFG than IGT in India.23 Herein, we provided evidence representing East Asian population furtherly. These findings implied that Asian population may be more susceptible to IFG than IGT. The present study is the first national population-based survey on pediatric prediabetes, including IFG and IGT, in China. In keeping with the pooled prevalence of prediabetes in childhood at 88.4/1000 estimated in a global meta-analysis,24 our prevalence was about 82.2/1000 (IFG + IGT). The prevalence of childhood prediabetes in China varied from 7/1000 to 220/1000 due to the diverse range of age and regional setting in the previous studies and all of them only covered IFG population.24 The present study provided an insight of the prevalence of IGT and the its distribution among prediabetes.
The risk factors of diabetes associated with individual's life history had been widely studied elsewhere before. Effect of most factors observed in this population-based study conformed with the previous findings, but the importance of maternal diabetes was the major risk factor for T1D at odds with European studies showing less risk from maternal inheritance.25
We found the high birth weight and childhood obesity risk factors for T1D, concordant with several European findings26, 27, 28, 29, 30; but the birth weight at odds with one German study,25 which might be caused by the difference in sample size and study design.
The family history of diabetes was the strongest risk factor for T2D, especially the maternal diabetes with the highest OR of 15.1 (95% CIs: 5.817–33.085), reflecting the known importance of both genetics and shared environment. Longer physical activity duration was a protective factor. Other commonly known risk factors, such as maternal diabetes during pregnancy, lower birth weight, childhood obesity, parental history of overweight or obesity and low sleep duration, have been identified in other studies.20,31,32
Special attention is required for the low sleep duration. We hadn't set lower duration than 8 h in our questionnaire, supposing most of the children and adolescents in China would not have less duration of sleep. However, the results would probably emphasize the shorter duration but lead to neglect of the necessary lower limit of it.
The primary strength of the present study is the national general population-based random sampling for children and adolescents. The in-situ sampling at school guaranteed the quality of the random sampling and data-integrity for such large population. It also ensured the national representativeness of the samples and data.
The large sampling scale also posed several limits. First, we could not practice the s-OGTT screening in all provinces due to the response of schools and guardians to the subsampling with s-OGTT. This left out Xinjiang as an outlier, and a known genetic population isolate. Guardians’ attitude influenced the sample size of 6474 which might introduce sampling bias. The risk factor of BMI over the 95-percentile (P95) was the most frequent one (68.8%) among the 40,858 high-risk group, but shifted to the family history (59.9%) in the 6428 s-OGTT group (Table S10).
Secondly, the questionnaire survey relies on self-report, whose correspondence to factual information is impossible to verify, especially for such factors as physical activity or the consumption of certain foods. This is a common problem in epidemiological studies of this type.
Finally, general population recruitment inevitably limits the sample size compared with studies based on known cases from health-care settings, limiting the statistical power and the precision of the analysis. Further study is required to confirm these results.
T2D accounts for 95% of all diabetes and is usually developed progressively during a long period. Compared with T1D, the onset is relatively later and had good chance to be prevented with timely diagnosis. The present study revealed that the development of T2D might start earlier in pediatric population and be the actual major proportion of all diabetes in children and adolescent in the form of asymptomatic prediabetes in different degrees. It is necessary to do further study to demonstrate if early screening and intervention in pediatric population could reduce the T2D morbidity in elder population. For T1D, our study provides the basis for future etiological studies. T1D genetics explain most of the disease with one of the smallest fractions of “missing heritability”.33,34 However, this is based almost entirely on studies on European ancestry, leaving out the one-third of humanity represented by East and South-East Asians, a crucial omission given their drastically lower incidence of T1D.11,35 Our results will promote planning and interpreting genome studies that are just beginning to emerge,36 to explore genes, environment, and their interactions.
Contributors
Yangxi Li, Jianwei Zhang and Constantin Polychronakos wrote the manuscript. Junfen Fu conceived and designed the study. Wei Wu, Ke Huang, Rui-Min Chen, Mireguli Maimaiti, Jing-Si Luo, Shao-Ke Chen, Di Wu, Min Zhu, Chun-Lin Wang, Zhe Su, Yan Liang, Hui Yao, Hai-Yan Wei, Rong-Xiu Zheng, Hong-Wei Du, Fei-Hong Luo, Pin Li and Ergang Wang participated in the epidemiological survey and collected data. All of the authors reviewed the manuscript.
Data sharing statement
The individual participant data is not available for sharing in principle, since it was part of a national epidemiology survey in pediatrics and will not be open to public online. But please contact the corresponding authors if there was any particular reason to access the data.
Editor note
The Lancet group takes a neutral position with respect to territorial claims in published maps and/or institutional affiliations.
Declaration of interests
The authors have no conflict of interest to disclose.
Acknowledgements
About 4000 clinicians including pediatricians, endocrinologists and school nurses participated in this study with very hard work and many difficulties in the complicated communications with participants, parents, teachers and the school administrators.
The authors appreciated all participants, their guardians.
The authors also appreciated all pediatricians, endocrinologists, technicians, data analysts and schoolteachers participating in this study.
This study is funded by the National Key Research and Development Programme of China (No. 2021YFC2701901, No. 2016YFC1305301), Key R&D Program of Zhejiang (No. 2023C03047), the National Natural Science Foundation of China (No. 81570759, No. 81270938), the Zhejiang Provincial Key Disciplines of Medicine (Innovation Discipline, 11-CX24), Key R&D Program Projects in Zhejiang Province (2024C03154).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101206.
Contributor Information
Constantin Polychronakos, Email: constantin.polychronakos@mcgill.ca.
Jun-Fen Fu, Email: fjf68@zju.edu.cn.
Appendix A. Supplementary data
References
- 1.Danaei G., Finucane M.M., Lu Y., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes epidemiology research international group. Diabetes. 1988;37(8):1113–1119. [PubMed] [Google Scholar]
- 3.Patterson R., McNamara E., Tainio M., et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–829. doi: 10.1007/s10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer A.K., Gustafson B., Kirkland J.L., Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62(10):1835–1841. doi: 10.1007/s00125-019-4934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell K., Westlund H. China's rapid urban ascent: an examination into the components of urban growth. Asian Geogr. 2018;35(1):85–106. [Google Scholar]
- 7.Weng J., Zhou Z., Guo L., et al. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. 2018;360 doi: 10.1136/bmj.j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Wang T., Liu X., Shankar R.R. Prevalence of type 1 and type 2 diabetes among US pediatric population in the MarketScan Multi-State database, 2002 to 2016. Pediatr Diabetes. 2019;20(5):523–529. doi: 10.1111/pedi.12842. [DOI] [PubMed] [Google Scholar]
- 9.Wagenknecht L.E., Lawrence J.M., Isom S., et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for diabetes in youth study. Lancet Diabetes Endocrinol. 2023;11(4):242–250. doi: 10.1016/S2213-8587(23)00025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu J.-F., Liang L., Gong C.-X., et al. Status and trends of diabetes in Chinese children: analysis of data from 14 medical centers. World J Pediatr. 2013;9(2):127–134. doi: 10.1007/s12519-013-0414-4. [DOI] [PubMed] [Google Scholar]
- 11.Liese A.D., D'Agostino R.B., Hamman R.F., et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for diabetes in youth study. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D., Mayer-Davis E.J., Saydah S., et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence J.M., Divers J., Isom S., et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA. 2021;326(8):717–727. doi: 10.1001/jama.2021.11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J., Prasad H.C. Changing epidemiology of metabolic syndrome and type 2 diabetes in Chinese youth. Curr Diab Rep. 2014;14(1):447. doi: 10.1007/s11892-013-0447-z. [DOI] [PubMed] [Google Scholar]
- 15.Wei J.-N., Sung F.-C., Lin C.-C., Lin R.-S., Chiang C.-C., Chuang L.-M. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA. 2003;290(10):1345–1350. doi: 10.1001/jama.290.10.1345. [DOI] [PubMed] [Google Scholar]
- 16.Patterson C.C., Dahlquist G., Soltész G., Green A. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44(Suppl 3):B9–B16. doi: 10.1007/pl00002961. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q., Yin F.-Z., Ma C.-M., et al. Prevalence of impaired fasting glucose and analysis of risk factors in Han adolescents. J Diabetes Complications. 2010;24(5):320–324. doi: 10.1016/j.jdiacomp.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen C.-M., Lou M.-F., Gau B.-S. Prevalence of impaired fasting glucose and analysis of related factors in Taiwanese adolescents. Pediatr Diabetes. 2014;15(3):220–228. doi: 10.1111/pedi.12081. [DOI] [PubMed] [Google Scholar]
- 19.Hannon T.S., Arslanian S.A. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;1353:113–137. doi: 10.1111/nyas.12939. [DOI] [PubMed] [Google Scholar]
- 20.Shah A.S., Zeitler P.S., Wong J., et al. ISPAD clinical practice consensus guidelines 2022: type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23(7):872–902. doi: 10.1111/pedi.13409. [DOI] [PubMed] [Google Scholar]
- 21.Katsarou A., Gudbjörnsdottir S., Rawshani A., et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 22.Rooney M.R., Fang M., Ogurtsova K., et al. Global prevalence of prediabetes. Diabetes Care. 2023;46(7):1388–1394. doi: 10.2337/dc22-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan V., Unnikrishnan R., Anjana R.M. Comment on Rooney et al. global prevalence of prediabetes. Diabetes Care. 2023;46:1388–1394. doi: 10.2337/dc23-1606. [DOI] [PubMed] [Google Scholar]
- 24.Han C., Song Q., Ren Y., Chen X., Jiang X., Hu D. Global prevalence of prediabetes in children and adolescents: a systematic review and meta-analysis. J Diabetes. 2022;14(7):434–441. doi: 10.1111/1753-0407.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonifacio E., Pflüger M., Marienfeld S., Winkler C., Hummel M., Ziegler A.G. Maternal type 1 diabetes reduces the risk of islet autoantibodies: relationships with birthweight and maternal HbA(1c) Diabetologia. 2008;51(7):1245–1252. doi: 10.1007/s00125-008-1022-z. [DOI] [PubMed] [Google Scholar]
- 26.Couper J.J., Beresford S., Hirte C., et al. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care. 2009;32(1):94–99. doi: 10.2337/dc08-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L.L., Lawrence J.M., Davis C., et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for diabetes in youth study. Pediatr Diabetes. 2010;11(1):4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 28.Verbeeten K.C., Elks C.E., Daneman D., Ong K.K. Association between childhood obesity and subsequent type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2011;28(1):10–18. doi: 10.1111/j.1464-5491.2010.03160.x. [DOI] [PubMed] [Google Scholar]
- 29.Cardwell C.R., Stene L.C., Joner G., et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53(4):641–651. doi: 10.1007/s00125-009-1648-5. [DOI] [PubMed] [Google Scholar]
- 30.Harder T., Roepke K., Diller N., Stechling Y., Dudenhausen J.W., Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009;169(12):1428–1436. doi: 10.1093/aje/kwp065. [DOI] [PubMed] [Google Scholar]
- 31.Shan Z., Ma H., Xie M., et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 32.Dabelea D., Mayer-Davis E.J., Lamichhane A.P., et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care. 2008;31(7):1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polychronakos C., Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12(11):781–792. doi: 10.1038/nrg3069. [DOI] [PubMed] [Google Scholar]
- 34.Bradfield J.P., Qu H.-Q., Wang K., et al. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet. 2011;7(9) doi: 10.1371/journal.pgen.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23(8):857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhu M., Xu K., Chen Y., et al. Identification of novel T1D risk loci and their association with age and islet function at diagnosis in autoantibody-positive T1D individuals: based on a two-stage genome-wide association study. Diabetes Care. 2019;42(8):1414–1421. doi: 10.2337/dc18-2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.