Abstract
An infection with the tick-borne Rickettsia helvetica has been associated with a broad spectrum of clinical manifestations in humans, but patients are only seldomly reported. Understanding its disease etiology necessitates well-stablished infection models, improving to recognize and diagnose patients with R. helvetica infection and facilitating the development of effective control strategies. In this study, we used C3H/HeN mice as a model to establish R. helvetica infection, achieving a high infection prevalence (89–100 %). While the liver and the spleen DNA consistently tested positive for infection in all challenged mice, additional infected organs included the kidneys, heart, and the lungs. Notably, a low prevalence of infection was observed in I. ricinus nymphs fed on R. helvetica-challenged mice. In addition, larvae were refractory to infection, suggesting that ticks exhibit low susceptibility to the pathogen. To the best of our knowledge, this is the first study of an animal model for R. helvetica infection. It serves as a valuable tool for advancing research on the interactions among the bacterium and its vertebrate host.
Keywords: Rickettsia helvetica, Pathogen, Virulence, Tick, Animal models of infection
1. Introduction
The genus Rickettsia (order Rickettsiales, family Rickettsiaceae) comprises gram-negative obligate intracellular bacteria that, according to biological and taxonomic characteristics, are classified in the ancestral group (AG), the typhus group (TG), the transitional group (TrG), or the spotted fever group (SFG). It is noteworthy that all groups, except the ancestral group, include species that cause diseases [1]. Rickettsiae within the SFG (SFGR) are transmitted to vertebrate hosts through the bite of infected ticks. Following transmission, SFGR most probably use monocytes as a vehicle for transport through the host body before invasion of the endothelial cells, where the pathogen extensively proliferates [2]. Both invasion and proliferation inside the host cell depend on different components, including virulence factors that are specific to the bacterial strain [e.g., outer membrane protein B (OmpB) structure, secretion of phospholipase D, hemolysin C, and effectors of Type IV secretion system, etc.] [[3], [4], [5]]. SFGR also express RickA [6] and the surface cell antigen 2 (Sca2) [7], which induce the actin polymerization in host cell and formation of filaments that eject the pathogen through the cytoplasm and cell membrane to adjacent cells or extracellular matrix.
One species within the SFG, Rickettsia helvetica, has been associated with development of general and non-specific disease in humans, (i.e., fever, headache, arthralgia, and myalgia) [8] and self-limiting skin lesions [9]. Nevertheless, more severe clinical conditions, such as myocarditis [10], septicemia [11], and meningitis [12] have also been reported, highlighting this bacterium as an emerging human pathogen.
Ixodes ricinus, the most abundant tick in Europe, is commonly found infesting humans, acting as the main vector of tick-borne pathogens in the continent, including R. helvetica [13,14]. Rickettsia helvetica has been also identified in other tick species, such as Ixodes apronophorus, Ixodes trianguliceps [15], and Dermacentor reticulatus [16,17]. However, the role of these ticks in the epidemiology of the disease remains unreported. Rickettsia helvetica has been detected in ticks parasitizing birds in Corsica (France), including migratory species [18]. Moreover, evidence of potential co-feeding transmission of R. helvetica in birds (Parus major) emphasizes the potential dispersion of this pathogen [19]. Furthermore, vertical transmission is believed to be the primary mechanism for maintaining rickettsial endosymbionts within tick populations [13].
Understanding the interactions among pathogens, the tick vector, and the host are essential to better understand the disease and develop effective control strategies. However, these studies require well-stablished infection models. While previous research demonstrated R. helvetica infection in Vero cells (African green monkey kidney cells) [20,21] and L929 cells (murine fibroblasts from connective tissue) [21], attempts to establish R. helvetica infection in animal models were unsuccessful [22].
In this study, we successfully infected C3H/HeN mice with the strain DK2 of R. helvetica, marking the first report on the establishment of an animal model for R. helvetica infection. Of note, I. ricinus was only partially susceptible to infection after an acquisition feeding on infected mice, highlighting the low susceptibility of larvae and nymphs of this tick species to infection through feeding. This model is now available for additional studies on R. helvetica-vertebrate host interactions.
2. Results
2.1. Initial experiment: single mouse challenge
In the first experiment, one C3H/HeN mouse was challenged with the R. helvetica strain DK2, using the same route of infection previously established for R. rickettsii [23,24]. The gDNA extracted from the mouse organs on the fifth DPI served as a template for conventional PCR. The expected 147 bp amplicon was observed in the liver, the spleen, kidneys, and the lungs (Fig. S1).
2.2. Second experiment: confirmation and extension
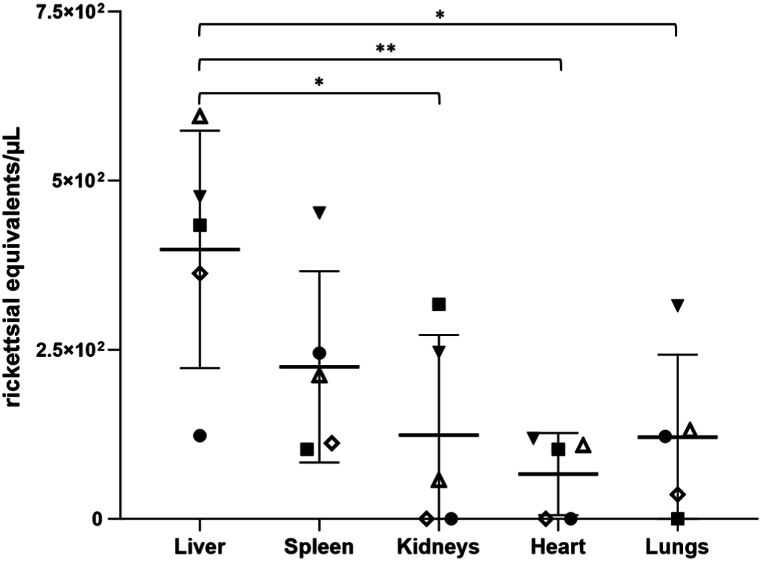
Once the adequacy of the infection route and the inoculum size were confirmed, a second experiment was conducted. Five mice were challenged with R. helvetica, while three mice served as controls. On the fifth DPI, three challenged and one control mice were euthanized for organ collection (Fig. 1). The gDNA extracted from the organs was used for qPCR to detect and quantify R. helvetica (Table 1 and Fig. 2). The liver and spleen of all infected mice were positive, along with the kidneys, the heart and the lungs of two of them (Table 1 and Fig. 2). Subsequently, two challenged and two control mice were infested with I. ricinus nymphs on the first DPI and euthanized for organ collection on the ninth DPI (Fig. 1). The liver, spleen and lungs of both challenged mice were positive for R. helvetica (Table 1). Of note, rickettsiae were not detected in any mice of the control group (Table 1). Despite a 100 % prevalence of infection, classic murine signs of spotted fever were not observed, and no organ alterations were visible during dissection.
Fig. 1.
Schematic representation of mouse groups in experiments. Five naïve mice were challenged with R. helvetica (infected group), while three animals were maintained as a control (noninfected group). On the fifth day post-infection (DPI), one noninfected and two R. helvetica-infected mice were euthanized for organ collection. On the first DPI, two noninfected and two infected mice were infested with I. ricinus nymphs. Fully engorged nymphs were collected daily from the fourth to the eighth DPI. The mice that served as hosts for the ticks were euthanized on the nineth DPI for organ collection. Figure created by BioRender (www.biorender.com).
Table 1.
Molecular detection of R. helvetica in organs of C3H/HeN mice from infected and noninfected groups.
| Organ | Infected Group |
Noninfected group |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5th DPI |
9th DPI |
Total | 5th DPI |
9th DPI |
Total | |||||
| I-1 | I-2 | I-3 | I-4 | I-5 | C-1 | C-2 | C-3 | |||
| Liver | + | + | + | + | + | 100 % (5/5) | ND | ND | ND | 0 % (0/3) |
| Spleen | + | + | + | + | + | 100 % (5/5) | ND | ND | ND | 0 % (0/3) |
| Kidneys | ND | + | + | ND | + | 60 % (3/5) | ND | ND | ND | 0 % (0/3) |
| Heart | ND | + | + | ND | + | 60 % (3/5) | ND | ND | ND | 0 % (0/3) |
| Lungs | + | ND | + | + | + | 80 % (4/5) | ND | ND | ND | 0 % (0/3) |
ND: Not detected.
Fig. 2.
Rickettsia helvetica quantification in mouse organs. The number of genome equivalents of R. helvetica was determined by qPCR using specific primers for the single-copy gene gltA of Rickettsia sp. Solid symbols represent the organs of mice dissected on the fifth DPI and empty symbols those dissected on the nineth DPI. Horizontal lines represent the median of rickettsial equivalents of all animals in a specific organ. ∗p < 0.05; ∗∗p < 0.01 (Mann-Whitney test).
2.3. Third experiment: test of larvae infection
A third experiment was conducted to test the infection of R. helvetica on I. ricinus larvae. Similarly to nymph infection, larvae were fed on mice challenged or not with R. helvetica. The spleen of seven from nine mice inoculated with R. helvetica was positive for infection (Fig. S2). Of note, rickettsiae were not detected in mice of the control group (Fig. S2). Despite an 89 % prevalence of infection, none mouse exhibited the classic murine signs of spotted fever and no organ alterations were visible during dissection, as observed in the previous experiments.
2.4. Molecular confirmation
To further confirm infection, gDNA extracted from the mouse spleen and R. helvetica-infected Vero cells was used for conventional PCR with primers targeting either the gene gltA or rickA of Rickettsia (Table S1). The amplicons for gltA (Fig. S3A) and rickA (Fig. S3B) were observed in all reactions using gDNA from infected mice and Vero cells, but not in those using gDNA from control mice. Sequences obtained in the current study are available in Supplementary File S1.
Phylogenetic analysis of the gltA and rickA sequences obtained in the current study confirmed the species affiliation to R. helvetica (Fig. 3). Derived consensus gltA sequence clustered with others previously reported from European countries including Poland, Romania, Ukraine, Italy, Switzerland and also from Asia, including Iran and Russia (Fig. 3A). The analyzed sequences were characterized by low genetic diversity. As expected, within the study population (R. helvetica infecting Vero cells or mice), only one haplotype was identified within gltA gene (Fig. 3A), confirming the consistency of the R. helvetica strain across the infectious progress. In addition, examined rickA sequence, also confirmed species affiliation to R. helvetica (Fig. 3B).
Fig. 3.
Phylogeny of Spotted Fever Group Rickettsia. The evolutionary history of SFGR was based on gltA (A) and rickA (B) genes and inferred by using the Maximum Likelihood method and the Tamura 3-parameter model (T92) and Kimura 2-paramenter model, respectively. The analysis contains sequences identified in the current study (marked with blue dot and available in Supplementary File S1) and those collected from GenBank database. Accession numbers and country of origin are given. Bootstrap values are represented as percentage of internal branches (1000 replicates), and values lower than 60 are hidden. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Rickettsia bellii sequence AY362703 was used to root the tree for gltA, while R. bellii sequence CP015010 was applied to root the rickA tree. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.5. Tick infection assessment
A total of 17 fully engorged nymphs were obtained from the R. helvetica-infected mice group, and 23 from the control group. Rickettsia helvetica was detected in one tick fed on one mouse of the infected group (Table 2). Additionally, seven engorged nymphs from the control group were randomly selected for qPCR analysis, and all were negative for R. helvetica infection (Table 2). In a third infection experiment, a total of 13 pools of 10 fully engorged larvae each were obtained from the R. helvetica-infected mice and 4 pools from noninfected mice. Rickettsia helvetica was not detected in any of the larvae pools (Table 3).
Table 2.
Number of engorged nymphs collected from infected and noninfected C3H/HeN mice and molecular detection of R. helvetica.
| Infected group |
Noninfected group |
|||
|---|---|---|---|---|
| Dropping day | I-4 | I-5 | C-2 | C-3 |
| 4th DPI | 0 | 2 | 1 | 3 |
| 5th DPI | 4 | 2 | 5 | 5 |
| 6th DPI | 4 | 1 | 5 | 3 |
| 7th DPI | 0 | 3 | 0 | 0 |
| 8th DPI | 1 | 0 | 0 | 1 |
| Prevalence of infected ticks | 0 % (0/9) | 12.5 % (1/8) | 0 % (0/11) | 0 % (0/12) |
Table 3.
Number of pool of engorged larvae collected from infected and noninfected C3H/HeN mice and molecular detection of R. helvetica.
| Infected group |
Noninfected group |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dropping day | I-6 | I-7 | I-8 | I-10 | I-11 | I-13 | I-14 | C-4 | C-5 |
| 4th DPI | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 0 |
| 5th DPI | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Prevalence of infected ticks | 0 % (0/3) | 0 % (0/2) | 0 % (0/1) | 0 % (0/2) | 0 % (0/1) | 0 % (0/1) | 0 % (0/3) | 0 % (0/2) | 0 % (0/2) |
3. Discussion
The C3H/HeN murine model, well-established for studying interactions among vertebrate hosts, ticks, and tick-borne pathogens [[23], [24], [25], [26]], and the most adequate murine model for SFGR, as the endothelial cells are the main infection target [27], was the focus of our investigation into the susceptibility of these mice to R. helvetica. Remarkably, 89 %–100 % prevalence of infection was achieved, contrasting with a previous report indicating Balb/c and C3H/HeN mice as non-susceptible to R. helvetica [22]. In the mentioned study, none Balb/c mouse was positive for infection, and only 20 % (1/5) of C3H/HeN mice became infected after R. helvetica challenge alone or in association with Borrelia afzelii [22]. Discrepancies in outcomes may arise from methodological variations, such as the R. helvetica strain used (isolated from an I. ricinus specimen from Slovakia vs. the strain DK2 from the Netherlands), the number of rickettsiae in the inoculum (8 x 104 vs. 4 x 109 rickettsiae per mouse), the inoculation route [intraperitoneal and subcutaneous vs. intravenous (retro-orbital)], and the time point analyzed after infection (14th DPI vs. 5th and 9th DPI). Of note, a previous attempts of our research group using a smaller number of rickettsiae in the inoculum (i.e., 1 x 107 - 1 x 109 rickettsiae per mouse) and the intraperitoneal infection route resulted in the absence of infection (data not shown). Therefore, introducing a pathogen intravenously seems to be key to bypass dendritic (antigen-presenting) cells in peritoneum and mucosa, making the innate immune response less efficient in the initial moments of infection [28].
In comparison to infection with R. rickettsii, known to induce spotted fever signs (including ruffled fur, shallowed respiration, hunched posture, decreased activity, limited motility or prostration) and mortality in C3H/HeN mice within five days [23,24], mice challenged with R. helvetica, even at a two-orders-of-magnitude higher dose (4.0 x 109 vs 107 in R. rickettsii), did not exhibit disease signs or mortality. In addition, no apparent organ alteration was observed upon gross examination. It was previously shown that the severity of infection in mice [23] and in Guinea pigs (Cavia porcellus) [29] varies according to the strain of R. rickettsii. Therefore, further investigation on the disease evolution in C3H/HeN mice challenged with different R. helvetica strains are warranted. In addition, the murine model established by the current study can also be used for comparative analyses on host responses to infection with R. helvetica and more virulent rickettsial species.
Concerning tick infection, a low number of engorged I. ricinus nymphs was obtained after feeding on C3H/HeN, whether infected with R. helvetica or not. Rickettsia helvetica was detected in only one from 17 nymphs fed on infected mice, yielding 5.9 % infection prevalence. Moreover, none of the pools of larvae fed on infected mice were positive for R. helvetica. This aligns with the prevalence observed in the experimental infection of Amblyomma sculptum ticks with R. rickettsii, which oscillates around 10 % [30,31]. A slightly higher susceptibility is observed when ticks are exposed to autochthonous R. rickettsii [32,33]. The limited susceptibility of A. sculptum to R. rickettsii is attributed to the upregulation of immune factors in the tick midgut upon infection [30]. It was previously shown that the midgut of I. ricinus rapidly clear both gram-positive (Micrococcus luteus) and gram-negative (Pantoea spp.) bacteria ingested by a capillary feeding, possibly due to the presence of antimicrobial factors [34]. Additional studies are warranted to assess the effect of R. helvetica on the expression of immune-related genes of I. ricinus, which may be involved in infection control. The low prevalence of R. helvetica-infected ticks after an experimental infection is also in consonance with the prevalence observed in field-collected ticks [[35], [36], [37], [38], [39], [40], [41], [42]]. For instance, among questing ticks, 1.8 % were positive for R. helvetica in Poland and Bulgaria [35], 4.14 % in Denmark [36], 5.1 % in Siberia [37], and only 0.04 % in Scotland [38], while lightly higher prevalence values were reported in Sweden (22 %) [39] and France (14–27 %) [40]. Among ticks collected from wild and/or domestic animals, 6 % were detected to be infected in Corsica (France) [41] and 11.5–14.4 % in the Netherlands [42]. Previous studies have shown that the presence of R. helvetica in I. ricinus ticks alters tick microbiota, reducing bacterial alpha-diversity [43]. Furthermore, R. helvetica shows a positive correlation with Candidatus Midichloria mitochondrii [43], which influences the feeding fitness of I. ricinus larvae [44]. Therefore, additional studies are warranted to understand the effects of endosymbionts on the acquisition of R. helvetica by ticks.
In conclusion, the murine model of R. helvetica infection established in this study provides a valuable platform for further research into the disease mechanisms and the development of effective control strategies, including vaccines. For example, the vaccination of C3H/HeN mice with rickettsial proteins has previously been used to assess protection against R. rickettsii [[45], [46], [47], [48]]. Given the diverse clinical manifestations of R. helvetica infection in humans, advancing our understanding of its pathogenesis and developing effective control measures are of utmost importance.
4. Material and methods
Ethics approval
Procedures with living animals were performed at the Animal Facility of the Laboratory for Animal Health of the French Agency for Food, Environmental and Occupational Health & Safety (ANSES), Maisons-Alfort, France, according to the French and International Guiding Principles for Biomedical Research Involving Animals (2012). The procedures were reviewed and approved by the Ethics Committee (ComEth, Anses/ENVA/UPEC), with permit number E9404608.
4.1. Rickettsia helvetica cultivation
The R. helvetica strain (DK2) was isolated from a male Ixodes ricinus collected in the costal dunes of the Netherlands (coordinates 52.4347; 4.6258), in 2018. For cultivation, rickettsiae were propagated in Vero cells (ATCCTM CCL-81TM, Manassas, VA, United States). After seven days at 32 °C, infected cells were collected, centrifuged at 175×g for 5 min, and then used to infect additional cells. Subsequent to an additional seven extra days at 32 °C, infected cells were harvested and suspended in Minimal Essential Medium (MEM, ThermoFisher Scientific, USA). The medium was supplemented with 2 mM L-glutamine and 10 % heat-inactivated fetal bovine (FBS) serum, and containing 10 % dimethyl sulfoxide (DMSO). An aliquot of infected Vero cells was used for genomic DNA (gDNA) extraction and quantification of rickettsiae in the inoculum by qPCR, as described below. The R. helvetica inoculum was stored in liquid nitrogen (N2) before use.
4.2. Ticks
The I. ricinus colony (French strain) was maintained at the ANSES animal facility in Maisons-Alfort, France. Larvae, nymphs and adults were fed on 11-week-old New Zealand white rabbits (Oryctolagus cuniculus, Charles River strain code 052), purchased from Charles River Laboratories (Miserey, France). The rabbits were housed in standard cages with unrestricted access to food and water. The room temperature (RT) was maintained at a controlled range of 20–23 °C, and a 12-h light:12-h dark photoperiod was followed. Throughout the entire experimental process, the health and behavior of the animals were carefully monitored twice daily by experienced technicians. During off-host phases, ticks were maintained in an incubator set at 22 °C with a relative humidity (RH) exceeding 97 %, following a 12-h light-dark cycle. Alternatively, larvae of I. ricinus were purchased from IS Insect Services GmbH (Germany).
4.3. Mouse infection and tick infestation
Male C3H/HeN mice (Mus musculus), 7-8-week-old, were purchased from Charles River Laboratories. For inoculum preparation, initially, Vero cells infected with R. helvetica were centrifuged at 3000×g for 5 min. The pellet was resuspended in sterile phosphate buffered saline (PBS) and subjected to three heating cycles at 37 °C, followed by 30 s (s) in liquid N2 to release rickettsiae.
In the first preliminary experiment, one mouse (I-0) was anesthetized with isoflurane (Zoetis, New Jersey, USA) and inoculated with 4.0 x 109 equivalent genomes of R. helvetica (see below) in 50 μL of sterile PBS via the retro-orbital sinus, using an insulin 26-gauge needle and 1 mL syringe (BD Plastipak; Becton, Dickinson and Company, New Jersey, USA). On the fifth day post-infection (DPI), the mouse was euthanized with CO2, and organs (spleen, kidneys, liver, heart, lungs, and skin) were collected and stored at −80 °C.
In a second experiment (see Fig. 1), five mice (I-1, I-2, I-3, I-4, and I-5) were anesthetized and infected as described above. Three naïve mice served as controls (C-1, C-2, and C-3). On the fifth DPI, three challenged mice (I-1, I-2, and I-3) and one control (C-1) mouse were euthanized for organ collection. On the first DPI, two of the five R. helvetica-challenged mice (I-4 and I-5), as well as two control mice (C-2 and C-3), were infested with I. ricinus nymphs (30 nymphs/mouse). Nymphs were placed in EVA-foam (Cosplay Shop, Belgium) capsules glued to the shaved backs of the animals, as previously described [49]. Fully engorged nymphs were collected daily from the fourth to the eighth DPI, washed once in 70 % ethanol and once in sterile PBS, and stored at −80 °C. In a third experiment, nine mice (I-6, I-7, I-8, I-9, I-10, I-11, I-12, I-13, and I-14) were infected with R. helvetica as described above and used as hosts for larvae of I. ricinus. As a control, larvae were fed on two noninfected mice (C-4 and C-5). Fully engorged larvae were collected from three to four DPI, washed and stored as detailed for nymphs. Nine pools of 10 larvae each were obtained from infected mice and four pools from control mice.
The mice hosting the ticks were euthanized on the nineth DPI for organ collection. During the experiments, we monitored disease signs in mice daily, including ruffled fur, shallow breathing, hunched posture, and decreased activity, as outlined by Ref. [23].
4.4. Genomic DNA extraction and absolute quantification of R. helvetica
To extract gDNA from mouse organs (∼20 mg per organ) and fully engorged I. ricinus nymphs and larvae (pools of 10 specimens each), homogenization was performed with sterilized metal beads using a Precellys24 Dual homogenizer (Bertin technologies, France). Three cycles at 5500 rpm for 20 s were applied. gDNA from the homogenized samples or R. helvetica-infected Vero cells was then extracted using the NucleoSpin® Tissue kit (Macherey-Nagel, Germany), according to the manufacturer's instructions.
The resulting gDNA served as a template in quantitative PCR (qPCR) using specific primers for the citrate synthase (gltA) gene and the LightCycler 480 SYBRGreen kit (Roche, Switzerland; for qPCR analyses). The qPCR analyses were carried out in a LightCycler® 480 system II (Roche, Switzerland) with the following thermocycler program: 5 min at 95 °C followed by 45 cycles of 10 s at 95 °C, 15 s at 55 °C, and 15 s at 72 °C. The quantitation cycle (Cq) of each sample was determined by the LightCycler 480 software (Roche, Switzerland) and compared with the Cq of a standard curve constructed with serial dilutions of the gltA amplicon, as detailed by Ref. [50]. The Cq values were subsequently used to calculate the number of genomic equivalents of rickettsiae per μL of gDNA sample. As a control, reactions were carried out in the absence of the gDNA. All samples were analyzed in technical triplicates.
4.5. Nucleotide sequencing
The gDNA extracted from both the spleen of mice and the inoculum of R. helvetica in Vero cells served as the template in conventional PCR, with the specific primers for gltA CS78 and CS283 [51] or rickA [52] (Table S1). The resulting amplicons were separated by electrophoresis on a 2 % agarose gel with ethidium bromide (final concentration of 0.5 μg/mL) and visualized using a transilluminator. Subsequently, selected amplicons were excised from the gel and purified using the NucleoSpin® Gel and PCR Clean-up kit (Macherey Nagel). Nucleotide sequencing was performed by Eurofins (France).
4.6. Phylogenetic analysis
The sequences obtained in the current study were analyzed using the Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on January 23, 2024). In the next step, sequences showing similarity were aligned using the MUSCLE algorithm in MEGA 11. Based on the lowest Bayesian Information Criterion and corrected Akaike Information Criterion, the Tamura 3-parameter model (T92) and Kimura 2-parameter (K2) models were used to construct phylogenetic trees of the Rickettsia gltA and rickA genes, respectively. The sequences representing the rickA gene were obtained by trimming the corresponding nucleotides from the complete genomes of the particular Rickettsia species. The evolutionary history was inferred by using the Maximum Likelihood (ML) method with complete deletion option, bootstrap set at 1000 and analyzed in MEGA 11 [53].
In addition, to determine genetic diversity of sequences clustered together with those obtained in the current study, i.e., R. helvetica gltA were grouped into haplotypes (genotypes) using the DnaSP software (Universitat de Barcelona, Spain, http://www.ub.edu/dnasp, accessed on January 23, 2024).
4.7. Statistical analysis
The statistical analysis of rickettsial loads in mouse organs inoculated with R. helvetica was performed using Mann-Whitney test in GraphPad Prism version 8.0 for Windows (GraphPad Software, USA). Differences between two organs were considered statistically significant if the p-value was <0.05.
Funding
This work was supported by the French Government's Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (Grant ANR–10–LABX–62–IBEID). ACF received a research fellowship from the São Paulo Research Foundation (FAPESP; grant 2022/08257–0) and a research productivity fellowship from the National Council for Scientific and Technological Development (CNPq; grant 309733/2018–9). AM was supported by the ‘Collectivitéde Corse’, grant: ‘Formations superieures’ (SGCE-RAPPORT No. 0300).
Data availability
The nucleotide sequences obtained in this study are available in Supplementary File S1, which can be accessed along with the article.
CRediT authorship contribution statement
Apolline Maitre: Writing – review & editing, Writing – original draft, Visualization, Investigation. Lourdes Mateos-Hernandez: Writing – review & editing, Investigation. Tal Azagi: Writing – review & editing, Methodology, Investigation. Angélique Foucault-Simonin: Writing – review & editing, Investigation. Sabine Rakotobe: Writing – review & editing, Investigation. Zbigniew Zając: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation. Pavle Banović: Writing – review & editing, Writing – original draft. Stefania Porcelli: Writing – review & editing, Investigation. Aurélie Heckmann: Writing – review & editing, Investigation. Clémence Galon: Writing – review & editing, Investigation. Hein Sprong: Writing – review & editing, Supervision, Resources, Investigation, Conceptualization. Sara Moutailler: Writing – review & editing, Supervision, Resources, Conceptualization. Alejandro Cabezas-Cruz: Writing – review & editing, Writing – original draft, Supervision, Resources, Conceptualization. Andrea C. Fogaça: Writing – review & editing, Writing – original draft, Visualization, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37931.
Contributor Information
Alejandro Cabezas-Cruz, Email: alejandro.cabezas@vet-alfort.fr.
Andrea C. Fogaça, Email: deafog@usp.br.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Agarose gel electrophoresis of PCR amplicons from R. helvetica-infected mouse tissues. The gDNA extracted from the liver (Li), spleen (Sp), Kidneys (Kd), heart (H), lungs (Lu), and skin (Sk) of a R. helvetica-infected mouse (I-0) was used as template in conventional PCR using the specific primers for gltA-PCR (Table S1). Amplicons were separated on a 2% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light. DNA marker size (bp) is shown. PC (positive control): fragment of gltA clone in a plasmid; NT: non template
PCR Analysis of R. helvetica infection in mice spleens. The gDNA extracted from the spleen of noninfected (C-4 and C-5) and R. helvetica-infected mice (I-6, I-7, I-8, I-9, I-10, I-11, I-12, and I-14), was used as template in conventional PCR using the specific primers for gltA-PCR (Table S1). Amplicons were separated on a 2% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light. DNA marker size (bp) is shown. RH: inoculum of R. helvetica in Vero cells; NT: non-template control
PCR detection of gltA and rickA genes in R. helvetica-infected mice and Vero cells. The gDNA extracted from the spleen of noninfected (C-1, C-2, and C-3) and R. helvetica-infected mice (I-0, I-1, I-2, I-3, I-4, and I-5), as well as infected Vero cells, was used as template in conventional PCR using the specific primers for either gltA or rickA sequencing (Table S1). Amplicons for gltA (A) or rickA (B) were separated on a 2% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light. DNA marker size (bp) is shown. RH: inoculum of R. helvetica in Vero cells; NT: non-template control
References
- 1.Fang R., Blanton L.S., Walker D.H. Rickettsiae as emerging infectious agents. Clin. Lab. Med. 2017;37:383–400. doi: 10.1016/j.cll.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Pahlson C., Lu X., Ott M., Nilsson K. Characteristics of in vitro infection of human monocytes, by Rickettsia helvetica. Microb. Infect. 2021;23 doi: 10.1016/j.micinf.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Balraj P., Renesto P., Raoult D. Advances in rickettsia pathogenicity. Ann. N. Y. Acad. Sci. 2009;1166:94–105. doi: 10.1111/j.1749-6632.2009.04517.x. [DOI] [PubMed] [Google Scholar]
- 4.Driskell L.O., Yu X.J., Zhang L., Liu Y., Popov V.L., Walker D.H., et al. Directed mutagenesis of the Rickettsia prowazekii pld gene encoding phospholipase D. Infect. Immun. 2009;77:3244–3248. doi: 10.1128/iai.00395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang D., Luo J., OuYang X., Song L. Subversion of host cell signaling: the arsenal of Rickettsial species. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.995933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouin E., Egile C., Dehoux P., Villiers V., Adams J., Gertler F., et al. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature. 2004;427:457–461. doi: 10.1038/nature02318. [DOI] [PubMed] [Google Scholar]
- 7.Haglund C.M., Choe J.E., Skau C.T., Kovar D.R., Welch M.D. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat. Cell Biol. 2010;12:1057–1063. doi: 10.1038/ncb2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier P.E., Allombert C., Supputamongkol Y., Caruso G., Brouqui P., Raoult D. Aneruptive fever associated with antibodies to Rickettsia helvetica in Europe and Thailand. J. Clin. Microbiol. 2004;42:816–818. doi: 10.1128/jcm.42.2.816-818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banovic P., Diaz-Sanchez A.A., Foucault-Simonin A., Mateos-Hernandez L., Wu-Chuang A., Galon C., et al. Emerging tick-borne spotted fever group rickettsioses in the Balkans. Infect. Genet. Evol. 2023;107 doi: 10.1016/j.meegid.2022.105400. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson K., Lindquist O., Pahlson C. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet. 1999;354:1169–1173. doi: 10.1016/s0140-6736(99)04093-3. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson K. Septicaemia with Rickettsia helvetica in a patient with acute febrile illness, rash and myasthenia. J. Infect. 2009;58:79–82. doi: 10.1016/j.jinf.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson K., Wallmenius K., Pahlson C. Coinfection with Rickettsia helvetica and herpes simplex virus 2 in a young woman with meningoencephalitis. Case Rep Infect Dis. 2011 doi: 10.1155/2011/469194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprong H., Wielinga P.R., Fonville M., Reusken C., Brandenburg A.H., Borgsteede F., et al. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasites Vectors. 2009;2(41) doi: 10.1186/1756-3305-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprong H., Azagi T., Hoornstra D., Nijhof A.M., Knorr S., Baarsma M.E., et al. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasites Vectors. 2018;11:145. doi: 10.1186/s13071-018-2744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igolkina Y., Yakimenko V., Tikunov A., Epikhina T., Tancev A., Tikunova N., et al. Novel genetic lineages of Rickettsia helvetica associated with Ixodes apronophorus and Ixodes trianguliceps ticks. Microorganisms. 2023;11 doi: 10.3390/microorganisms11051215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobec M., Golubic D., Punda-Polic V., Kaeppeli F., Sievers M. Rickettsia helvetica in Dermacentor reticulatus ticks. Emerg Infect. 2009;15:98–100. doi: 10.3201/eid1501.080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolf I., Venclikova K., Blazejova H., Betasova L., Mendel J., Hubalek Z., et al. First report of Rickettsia raoultii and Rickettsia helvetica in Dermacentor reticulatus ticks from the Czech Republic. Ticks Tick Borne Dis. 2016;7:1222–1224. doi: 10.1016/j.ttbdis.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Defaye B., Moutailler S., Vollot B., Galon C., Gonzalez G., Moraes R.A., et al. Detection of pathogens and ticks on sedentary and migratory birds in two Corsican wetlands (France, Mediterranean area) Microorganisms. 2023;11 doi: 10.3390/microorganisms11040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heylen D., Fonville M., van Leeuwen A.D., Sprong H. Co-infections and transmission dynamics in a tick-borne bacterium community exposed to songbirds. Env Microbiol. 2015;18:988–996. doi: 10.1111/1462-2920.13164. [DOI] [PubMed] [Google Scholar]
- 20.Elfving K., Lukinius A., Nilsson K. Life cycle, growth characteristics and host cell response of Rickettsia helvetica in a Vero cell line. Exp. Appl. Acarol. 2012;56:179–187. doi: 10.1007/s10493-011-9508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speck S., Kern T., Aistleitner K., Dilcher M., Dobler G., Essbauer S. In vitro studies of Rickettsia-host cell interactions: confocal laser scanning microscopy of Rickettsia helvetica-infected eukaryotic cell lines. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallay B., Vaculova T., Derdakova M., Rusnakova Taragelova V., Spitalska E., et al. Two mice models for transferability of zoonotic bacteria via tick vector. Acta Virol. 2017;61:372–376. doi: 10.4149/av_2017_319. [DOI] [PubMed] [Google Scholar]
- 23.Esteves E., Fongsaran C., Langohr I.M., Riley S.P., Labruna M.B., Daffre S., et al. Comparative analysis of infection by Rickettsia rickettsii sheila smith and taiacu strains in a murine model. Pathogens. 2020;9 doi: 10.3390/pathogens9090744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley S.P., Cardwell M.M., Chan Y.G., Pruneau L., Del Piero F., Martinez J.J. Failure of a heterologous recombinant Sca5/OmpB protein-based vaccine to elicit effective protective immunity against Rickettsia rickettsii infections in C3H/HeN mice. Pathog Dis. 2015;73:ftv101. doi: 10.1093/femspd/ftv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escudero R., Barral M., Perez A., Vitutia M.M., Garcia-Perez A.L., Jimenez S., et al. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 2000;38:4026–4033. doi: 10.1128/jcm.38.11.4026-4033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porcelli S., Heckman A., Lagree A.C., Galon C., Mountailler S., Deshuillers P.L. Exploring the susceptibility of C3H mice to tick-borne encephalitis virus infection: implications for Co-infection models and understanding of the disease. Viruses. 2023;15 doi: 10.3390/v15112270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker D.H., Popov V.L., Wen J., Feng H.M. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab. Invest. 1994;70:358–368. [PubMed] [Google Scholar]
- 28.Kim H.K. Rickettsia-host-tick interactions: knowledge advances and gaps. Infect. Immun. 2022;90 doi: 10.1128/iai.00621-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galletti M., Paddock C.D., Hecht J.A., Biggerstaff B.J., Ritter J.M., Karpathy S.E. Isolate-dependent differences in clinical, pathological, and transcriptional profiles following in vitro and in Vivo infections with Rickettsia rickettsii. Infect. Immun. 2021;89 doi: 10.1128/iai.00626-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins L.A., Galletti M., Ribeiro J.M., Fujita A., Costa F.B., Labruna M.B., et al. The distinct transcriptional response of the midgut of Amblyomma sculptum and Amblyomma aureolatum ticks to Rickettsia rickettsii correlates to their differences in susceptibility to infection. Front. Cell. Infect. Microbiol. 2017;7:129. doi: 10.3389/fcimb.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labruna M.B., Ogrzewalska M., Martins T.F., Pinter A., Horta M.C. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J. Med. Entomol. 2008;45:1156–1159. doi: 10.1603/0022-2585(2008)45[1156:csolso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Gerardi M., Ramirez-Hernandez A., Binder L.C., Krawczak F.S., Gregori F., Labruna M.B. Comparative susceptibility of different populations of Amblyomma sculptum to Rickettsia rickettsii. Front. Physiol. 2019;10:653. doi: 10.3389/fphys.2019.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soares J.F., Soares H.S., Barbieri A.M., Labruna M.B. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Med. Vet. Entomol. 2012;26:139–151. doi: 10.1111/j.1365-2915.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- 34.Guizzo M.G., Dolezelikova K., Neupane S., Frantova H., Hrbatova A., Pafco B., et al. Characterization and manipulation of the bacterial community in the midgut of Ixodes ricinus. Parasites Vectors. 2022;15(1):248. doi: 10.1186/s13071-022-05362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polsomboon Nelson S., Ergunay K., Bourke B.P., Reinbold-Wasson D.D., Caicedo-Quiroga L., Kirkitadze G., et al. Nanopore-based metagenomics reveal a new Rickettsia in Europe. Ticks Tick-borne Dis. 2024;15(2) doi: 10.1016/j.ttbdis.2023.102305. [DOI] [PubMed] [Google Scholar]
- 36.Jensen B.B., Andersen N.S., Wölfel S., Chen M., Paarup H.M., Olesen C.R., et al. Rickettsiosis in Denmark : a nation-wide survey. Ticks Tick-borne Dis. 2023;14(6) doi: 10.1016/j.ttbdis.2023.102236. [DOI] [PubMed] [Google Scholar]
- 37.Rakov A.V., Chekanova T.A., Petremgvdlishvili K., Timonin A.V., Valdokhina A.V., Shirokostup S.V., et al. High prevalence of Rickettsia raoultii found in dermacentor ticks collected in Barnaul, Altai Krai, Western Siberia. Pathogens. 2023;12(7):914. doi: 10.3390/pathogens12070914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsthoorn F., Sprong H., Fonville M., Rocchi M., Medlock J., Gilbert L., et al. Occurrence of tick-borne pathogens in questing Ixodes ricinus ticks from wester ross, Northwest Scotland. Parasites Vectors. 2021;14(1):430. doi: 10.1186/s13071-021-04946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjær L.J., Klitgaard K., Soleng A., Edgar K.S., Lindstedt H.E.H., Paulsen K.M., et al. Spatial patterns of pathogen prevalence in questing Ixodes ricinus nymphs in southern Scandinavia, 2016. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-76334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lejal E., Moutailler S., Šimo L., Vayssier-Taussat M., Pollet T. Tick-borne pathogen detection in midgut and salivary glands of adult Ixodes ricinus. Parasites Vectors. 2019;12(1):152. doi: 10.1186/s13071-019-3418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grech-Angelini S., Stachurski F., Vayssier-Taussat M., Devillers E., Casabianca F., Lancelot R., et al. Tick-borne pathogens in ticks (Acari : Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 2020;67(2):745–757. doi: 10.1111/tbed.13393. [DOI] [PubMed] [Google Scholar]
- 42.Kooyman F.N.J., Zweerus H., Nijsse E.R., Jongejan F., Wagenaar J.A., Broens E.M. Monitoring of ticks and their pathogens from companion animals obtained by the « tekenscanner » application in The Netherlands. Parasitol. Res. 2022;121(7):1887–1893. doi: 10.1007/s00436-022-07518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maitre A., Wu-Chuang A., Mateos-Hernandez L., Foucault-Simonin A., Moutailler S., Paoli J.C., et al. Rickettsia helvetica infection is associated with microbiome modulation in Ixodes ricinus collected from humans in Serbia. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-15681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guizzo M.G., Hatalova T., Frantova H., Zurek L., Kopacek P., Perner J. Ixodes ricinus ticks have a functional association with Midichloria mitochondrii. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong W., Qi Y., Xiong X., Jiao J., Duan C., Wen B. Rickettsia rickettsii outer membrane protein YbgF induces protective immunity in C3H/HeN mice. Hum. Vaccines Immunother. 2015;11(3):642–649. doi: 10.1080/21645515.2015.1011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong W., Wang P., Xiong X., Jiao J., Yang X., Wen B. Enhanced protection against Rickettsia rickettsii infection in C3H/HeN mice by immunization with a combination of a recombinant adhesin rAdr2 and a protein fragment rOmpB-4 derived from outer membrane protein B. Vaccine. 2015;33(8):985–992. doi: 10.1016/j.vaccine.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Gong W., Xiong X., Qi Y., Jiao J., Duan C., Wen B. Surface protein Adr2 of Rickettsia rickettsii induced protective immunity against Rocky Mountain spotted fever in C3H/HeN mice. Vaccine. 2014;32(18):2027–2033. doi: 10.1016/j.vaccine.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 48.Riley S.P., Cardwell M.M., Chan Y.G.Y., Pruneau L., Del Piero F., Martinez J.J. Failure of a heterologous recombinant Sca5/OmpB protein-based vaccine to elicit effective protective immunity against Rickettsia rickettsii infections in C3H/HeN mice. Pathog Dis. 2015;73(9) doi: 10.1093/femspd/ftv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mateos-Hernandez L., Rakotobe S., Defaye B., Cabezas-Cruz A., Simo L. A capsule-based model for immature hard tick stages infestation on laboratory mice. J. Vis. Exp. 2020;161 doi: 10.3791/61430. [DOI] [PubMed] [Google Scholar]
- 50.Galletti M.F., Fujita A., Nishiyama M.Y., Jr Malossi C.D., Pinter A., Soares J.F., et al. Natural blood feeding and temperature shift modulate the global transcriptional profile of Rickettsia rickettsii infecting its tick vector. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labruna M.B., Whitworth T., Horta M.C., Bouyer D.H., McBride J.W., Pinter A., et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004;42:90–98. doi: 10.1128/jcm.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balraj P., ElKarkouri K., Vestris G., Espinosa L., Raoult D., Renesto P. RickA expression is not sufficient to promote actin-based motility of Rickettsia raoultii. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1371/journal.pone.0002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Agarose gel electrophoresis of PCR amplicons from R. helvetica-infected mouse tissues. The gDNA extracted from the liver (Li), spleen (Sp), Kidneys (Kd), heart (H), lungs (Lu), and skin (Sk) of a R. helvetica-infected mouse (I-0) was used as template in conventional PCR using the specific primers for gltA-PCR (Table S1). Amplicons were separated on a 2% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light. DNA marker size (bp) is shown. PC (positive control): fragment of gltA clone in a plasmid; NT: non template
PCR Analysis of R. helvetica infection in mice spleens. The gDNA extracted from the spleen of noninfected (C-4 and C-5) and R. helvetica-infected mice (I-6, I-7, I-8, I-9, I-10, I-11, I-12, and I-14), was used as template in conventional PCR using the specific primers for gltA-PCR (Table S1). Amplicons were separated on a 2% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light. DNA marker size (bp) is shown. RH: inoculum of R. helvetica in Vero cells; NT: non-template control
PCR detection of gltA and rickA genes in R. helvetica-infected mice and Vero cells. The gDNA extracted from the spleen of noninfected (C-1, C-2, and C-3) and R. helvetica-infected mice (I-0, I-1, I-2, I-3, I-4, and I-5), as well as infected Vero cells, was used as template in conventional PCR using the specific primers for either gltA or rickA sequencing (Table S1). Amplicons for gltA (A) or rickA (B) were separated on a 2% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light. DNA marker size (bp) is shown. RH: inoculum of R. helvetica in Vero cells; NT: non-template control
Data Availability Statement
The nucleotide sequences obtained in this study are available in Supplementary File S1, which can be accessed along with the article.