Abstract
Background
Macrophage migration inhibitory factor (MIF) is a highly conserved cytokine with pleiotropic properties, mainly pro-inflammatory. MIF seems to exert its pro-inflammatory features by binding to its transmembrane cellular receptor CD74. MIF also has CXCR4, which acts as a co-receptor in this inflammatory process. Apart from MIF, D-dopachrome tautomerase (DDT) or MIF2, which belongs to the MIF superfamily, also binds to receptor CD74. Therefore, these molecules, MIF, CD74, DDT and CXCR4 are suggested to work together orchestrating an inflammatory process. Diabetes mellitus is characterised by chronic low-grade inflammation. Therefore, the aim of the present study was to evaluate serum and urinary levels of the aforementioned molecules among patients with type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) and among healthy controls.
Methods
We enrolled 13 patients with T1DM, 74 patients with T2DM and 25 healthy individuals as controls. Levels of CD74, CXCR4, DDT, and MIF were measured using ELISA Kits according to the manufacturer's instructions.
Results
We documented increased serum MIF levels together with higher urinary CD74 levels among patients with T1DM, when compared to patients with T2DM and healthy adults. In particular, patients with T1DM showed significantly increased levels of MIF compared to T2DM (p = 0.011) and healthy controls (p = 0.0093). CD74 in urine were significantly higher in patients with T1DM compared to those affected with T2DM (p = 0.0302) and healthy group (p = 0.0099). On the contrary, serum CD74 were similar among the three groups. No statistical differences were identified in CXCR4 levels both in serum and in urine of all groups. Patients with T2DM and overweight/obesity had increased urinary levels of CD74, when compared to lean patients with T2DM.
Conclusion
The increased serum MIF levels and urinary CD74 levels among patients with T1DM may be attributed to the autoimmune milieu, which characterises patients with T1DM, when compared to patients with T2DM. These two findings merit further attention as they could pave the way for further research regarding the potential beneficial effects of inhibitors of MIF among patients with T1DM, especially in the early stages of T1DM. Finally, the role of inhibitors of MIF could be further explored in the context of obesity among patients with T2DM.
Keywords: Autoimmunity, Cytokine, Diabetes mellitus, D-dopachrome tautomerase, Inflammation, Macrophage migration inhibitory factor
1. Introduction
Diabetes mellitus (DM) comprises a chronic metabolic disorder characterised mainly by increased serum glucose levels. It constitutes a major public health issue. According to the International Diabetes Federation (IDF), by 2030 643 million people are expected to have diabetes, while by 2050, 783 million people are projected to live with diabetes [1]. More than 90 % of patients with DM have type 2 DM (T2DM), which has been associated with insulin resistance. Besides, T2DM is related to the obesity pandemic, the sedentary lifestyle, urbanisation, the Western diet and the ageing process [[1], [2], [3]]. On the contrary, patients with type 1 DM (T1DM) constitute less than 10 % of people with DM and they are insulinopenic. T1DM is considered to be autoimmune in its origin and has been related to the presence of various autoantibodies, such as insulin autoantibodies (IAA), glutamic acid decarboxylase antibodies (GADA), tyrosine phosphatase like protein IA2 (IA-2A) and zinc transporter 8 antibody (ZnT8A) [4,5].
Macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine with a molecular weight of 12.5 kDa [6]. MIF is a highly conserved cytokine with pleiotropic functions being also implicated in the pathogenesis of autoimmune disorders by binding to its receptor CD74. Apart from acting as a MHC class II chaperone, CD74 serves as a cell transmembrane receptor with high affinity for MIF and D-dopachrome tautomerase (DDT) or MIF2 [[7], [8], [9], [10]]. In addition, MIF binds to CXCR4, which is a co-receptor for MIF, but does not appear to be specific solely for MIF [[10], [11], [12], [13], [14], [15], [16], [17], [18]].
As both T1DM and T2DM are metabolic disorders characterised by chronic low-grade inflammation, we hypothesised a potential association between MIF, CD74, DDT and CXCR4 in patients with T1DM and T2DM. Thus, the aim of the present study was to measure the serum and urinary levels of MIF, CD74, CXCR4 and DDT among patients with T1DM, T2DM and healthy individuals in order to assess whether there are any differences in their levels between these three distinct groups.
2. Materials and methods
For the purpose of the present study, between April 2019 and May 2022, we enrolled 13 patients with T1DM, 74 patients with T2DM and 25 healthy individuals as controls. Controls were consecutive healthy volunteers that agreed to participate in the study. The retrieved data were confidential, and the study followed the ethical considerations provided by the World Medical Association (52nd WMA General Assembly, Edinburgh, Scotland, October 2000). Moreover, the Institutional review board approved the design, procedures and aims of the study (GA 23/May 14, 2009). All participants were informed about the procedures of the study and agreed to participate providing written informed consent.
For the T2DM patients, the most frequently prescribed medication was Metformin, either alone or in combination with other drugs. Specifically, Metformin was used as monotherapy in 12 patients (20.3 %). The combination of Metformin with Sitagliptin was the most common, appearing in 12 cases (20.3 %). Metformin combined with other oral hypoglycemic agents, such as Vildagliptin, Empagliflozin, Alogliptin, and Dapagliflozin, was used in 13 patients (22 %). Additionally, Metformin was combined with insulin therapies (e.g., Insulin Glargine, Insulin Degludec, Insulin Lispro) in 8 patients (13.5 %). Insulin-based therapies, including various insulin formulations (e.g., Insulin Glargine, Insulin Lispro, Insulin Degludec, Insulin Aspart, Insulin Glulisine), were utilised in 13 patients (22 %), either alone or in combination with oral medications. GLP-1 receptor agonists (e.g., Dulaglutide and Liraglutide) were used in 6 patients (10.2 %), often in combination with Metformin or insulin. SGLT2 inhibitors (such as Empagliflozin) appeared in 3 cases (5 %), either as monotherapy or in combination with Metformin. Notably, 4 patients (6.8 %) were not on any active antidiabetic treatment.
2.1. Laboratory evaluation
Blood collection took place early in the morning. Serum and urine from participants were centrifuged for 20 min at 1000 g. Then, the supernatants were collected and stored in aliquots at −20 °C till the analyses. Protein levels (in pg/mL) of CD74, CXCR4, DDT, and MIF were measured using Human CD74 ELISA Kit (SEB369Hu, Cloud-Clone Corp., Houston, TX, USA), Human CXCR4 ELISA Kit (SEA940Hu, Cloud-Clone Corp., Houston, TX, USA), Human DDT ELISA Kit (SED777Hu, Cloud-Clone Corp., Houston, TX, USA), and Human MIF ELISA Kit (SEA698Hu, Cloud-Clone Corp., Houston, TX, USA) respectively, according to the manufacturer's instructions.
2.2. Transcriptomic analysis
We performed a comprehensive search of the GEO database to identify datasets generated from peripheral blood mononuclear cells (PBMCs) of Type 1 Diabetes and Type 2 Diabetes patients. For T2DM, we identified one microarray dataset, GSE9006, which included samples from 12 T2DM patients and 24 healthy controls. For T1DM, we retrieved three datasets: GSE9006, GSE72377, and GSE55100 [19,20]. The GSE9006 dataset contained data from 81 T1DM patients and 24 healthy controls [19]. The GSE72377 dataset provided mRNA gene expression profiles from unstimulated PBMCs of 15 T1DM patients and 20 healthy controls. The GSE55100 dataset included mRNA gene expression profiles from PBMCs of 12 T1DM patients and 10 healthy controls [20].
To identify differentially expressed genes in the pancreas of T2DM patients, we obtained the datasets GSE20966, GSE25724, and GSE38642, which collectively comprised data from 25 T2DM patients and 71 control islets [[21], [22], [23]]. For T1DM, we analysed the datasets GSE72492 and E-MEXP-1140, which included whole-genome mRNA data from a total of 24 T1DM patients and 13 controls [24,25].
Additionally, we selected the GSE1009 and GSE199838 datasets, which contained transcriptomic data from kidney tissues of patients with diabetic nephropathy and control cases [26,27].
Significant genes were identified using the R package LIMMA (Linear Models for Microarray Data), with a threshold of False Discovery Rate (FDR) < 0.05. For the meta-analysis, we utilised the Network Analyst web utility tool [28,29]. The normalisation of the datasets was performed using the variance stabilising normalisation algorithm, followed by quantile normalisation [30]. To adjust for batch effects in the meta-analysis, the ComBat procedure, embedded in the Network Analyst tool, was applied. The meta-analysis was then conducted using Fisher's P-value combination method.
2.3. Statistical analysis
Data are presented as Mean ± S.D. (Standard Deviation). Kolmogorov–Smirnov test was used to determine the distribution of the data. Based on the results from the normality test, the non-parametric Kruskal-Wallis test followed by Uncorrected Dunn's test was used to compare the differences among the groups. Spearman correlation coefficients (r) were used as measurements of correlation for continuous variables. To evaluate the effects of insulin and metformin on the levels of MIF, DDT, CXCR4, and CD74, we accounted for potential confounding factors, which included all drug treatments other than insulin and metformin. We corrected the ELISA data using the General Linear Model (GLM) to adjust for these confounding drug treatments and derived residuals that reflect the adjusted biomarker levels. Patients were categorised into four groups: those receiving Insulin only, those receiving Metformin only, those receiving Metformin + Insulin, and a fourth group representing all other treatments. The residuals were then compared across these four groups using the Kruskal-Wallis test. Differences were considered statistically significant with a p-value <0.05. Statistical analyses were performed using Graph-Pad Prism 7.0 software (La Jolla, CA, USA).
3. Results
To evaluate whether DM disease is associated with altered levels of pro-inflammatory proteins, we measured the protein levels of CD74, CXCR4, DDT, and MIF in the serum and in the urine of patients with T1DM, T2DM, and healthy subjects (see Table 1). The results of these analyses showed significantly higher MIF levels in serum of patients with DM compared to healthy donors (Fig. 1A). In particular, patients with T1DM showed significantly increased levels of MIF compared to T2DM (p = 0.011) and healthy controls (p = 0.0093) (Table 2). However, in contrast to serum levels, urine MIF levels (Fig. 1B) were found to be significantly lower in T2DM patients compared to the healthy group (p = 0.0233). Furthermore, serum levels of DDT (Fig. 1C) were higher in patients with T1DM compared to healthy donors (p = 0.038). On the other hand, no statistical differences were observed in urine DDT levels among the three examined groups (Fig. 1D).
Table 1.
Depicts the main demographic and clinical characteristics of study participants.
| Patients with T1DM (n = 13) | Patients with T2DM (n = 73) | Controls (n = 25) | |
|---|---|---|---|
| Number of Patients | 13 | 64 | 25 |
| Gender (Male/Female in number) | 9/4 | 44/20 | 8/17 |
| Age (years, mean ± SD) | 43.3 ± 12 | 60.2 ± 11.5 | 38.9 ± 11.4 |
| Duration of DM (months, mean ± SD) | 259.1 ± 183.1 | 109 ± 120.9 | N/A |
| Diabetic Nephropathy (n, %) | 2 (15.4 %) | 15 (20.5 %) | N/A |
| Weight (Kg, mean ± SD) | 79.4 ± 17.4 | 88.5 ± 18.1 | 66.7 ± 7.7 |
| BMI (Kg/m2, mean ± SD) | 26.6 ± 5.2 | 31.2 ± 10.8 | 24.2 ± 5.2 |
Fig. 1.
Analysis of the MIF and DDT levels in the serum or urine from Healthy donors (Control), Type 1 Diabetes Mellitus (T1DM), and Type 2 Diabetes Mellitus (T2DM) patients. Data are presented as mean concentrations ± standard deviation. The statistical significance in the figures has been indicated by asterisks (∗P < 0.05 and ∗∗P < 0.01).
Table 2.
Statistical comparison analyses.
| Uncorrected Dunn's test | ||
|---|---|---|
| Serum MIF | Mean rank diff. | Individual P value |
| Control vs. T1DM | −11.62 | 0.0093 |
| Control vs. T2DM | −1.6 | 0.6055 |
| T1DM vs. T2DM | 10.02 | 0.011 |
| Urine MIF | ||
| Control vs. T1DM | 14.68 | 0.1148 |
| Control vs. T2DM | 15.08 | 0.0233 |
| T1DM vs. T2DM | 0.3944 | 0.9609 |
| Serum DDT | ||
| Control vs. T1DM | −17.74 | 0.038 |
| Control vs. T2DM | −10.35 | 0.081 |
| T1DM vs. T2DM | 7.392 | 0.327 |
| Urine DDT | ||
| Control vs. T1DM | 0 | 1 |
| Control vs. T2DM | 0 | 1 |
| T1DM vs. T2DM | 0 | 1 |
| Serum CD74 | ||
| Control vs. T1DM | 2.146 | 0.5312 |
| Control vs. T2DM | −0.2003 | 0.9328 |
| T1DM vs. T2DM | −2.346 | 0.4377 |
| Urine CD74 | ||
| Control vs. T1DM | −11.46 | 0.0099 |
| Control vs. T2DM | −3.079 | 0.3274 |
| T1DM vs. T2DM | 8.382 | 0.0302 |
| Serum CXCR4 | ||
| Control vs. T1DM | −3.965 | 0.4699 |
| Control vs. T2DM | −1.626 | 0.6691 |
| T1DM vs. T2DM | 2.338 | 0.629 |
| Urine CXCR4 | ||
| Control vs. T1DM | −1.579 | 0.7983 |
| Control vs. T2DM | −1.063 | 0.8116 |
| T1DM vs. T2DM | 0.5336 | 0.9212 |
Levels of CD74 in urine (Fig. 2B) were found to be significantly higher in patients with T1DM compared to those affected with T2DM (p = 0.0302) and healthy group (p = 0.0099). On the contrary, serum CD74 levels were similar in the three groups (Fig. 2A). No statistical differences were identified in CXCR4 levels both in serum and in urine of all selected groups (Fig. 2C–D).
Fig. 2.
Analysis of the CD74 and CXCR4 levels in the serum or urine from healthy donors (Control), Type 1 Diabetes Mellitus (T1DM), and Type 2 Diabetes Mellitus (T2DM) patients. Data are presented as mean concentrations ± standard deviation. The statistical significance in the figures has been indicated by asterisks (∗P < 0.05 and ∗∗P < 0.01).
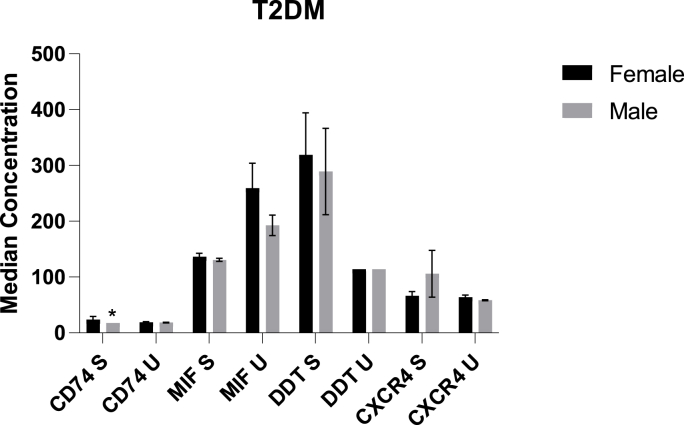
By doing a correlation analysis with BMI, we observed that in patients with T2DM, levels of CD74 in urine correlated positively with BMI (Fig. 3, Table 3). Furthermore, among patients with T2DM, CD74 levels in the blood were higher in females, when compared to males (Fig. 4). In addition, in patients with T2DM, we found an inverse relationship between urinary MIF levels and glycated haemoglobin (HbA1c) as well as erythrocyte sedimentation rate (ESR) (p < 0.05) (Fig. 5).
Fig. 3.
Urinary levels of CD74 varied according to BMI among patients with T2DM. U denotes urinary.
Table 3.
Correlation analysis with BMI among patients with T2DM. U denotes urinary and S denotes serum.
| T2DM | BMI vs. CD74 S | BMI vs. CD74 U | BMI vs. MIF S | BMI vs. MIF U | BMI vs. DDT S | BMI vs. DDT U | BMI vs. CXCR4 S | BMI vs. CXCR4 U |
|---|---|---|---|---|---|---|---|---|
| P (two-tailed) | 0.347 | 0.040 | 0.894 | 0.937 | 0.428 | NA | 0.981 | 0.281 |
Fig. 4.
This histogram depicts sex variations of the various serum and urinary levels of cytokines among patients with T2DM. U denotes urinary and S denotes serum.
Fig. 5.
Correlation analysis between MIF, DDT, CD74 and CXCR4 and blood biochemical-clinical data. A. Heatmap showing the Spearman's correlation analysis for all patients and healthy subjects. The heatmap is colour-coded based on Spearman's r; B. Heatmap showing the Spearman's correlation analysis for T1D patients. The heatmap is colour-coded based on Spearman's r; C. Heatmap showing the Spearman's correlation analysis for T2DM patients. The heatmap is colour-coded based on Spearman's r; D. Correlation between MIF U and ESR in T2D patients. E. Correlation between MIF U and HbA1c in T2DM patients.
Our cohort of T2DM patients showed a predominant use of metformin-based therapies (63.5 %), either as monotherapy or in combination with other agents. There was also a notable use of insulin-based therapies (22 %) and combinations that included newer agents such as DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors. When comparing the four patient groups—Insulin only, Metformin only, Metformin + Insulin, and all other treatments—no significant differences were observed in the levels of MIF, DDT, CD74, and CXCR4. This suggests that none of the treatments had a distinct impact on these biomarker levels.
With regard to the transcriptomic datasets, none of the genes of interest (MIF, DDT, CD74 and CXCR4) were significantly modulated in PBMCs (peripheral blood mononuclear cells) of patients with type 2 (GSE9006) and type 1 diabetes (meta-analysis of the datasets GSE9006, GSE72377, GSE55100). No significant differences were also found in the pancreas (meta-analysis of the datasets GSE20966, GSE25724 and GSE38642 for T2DM; meta-analysis of GSE72492 and E-MEXP-1140 for T1DM). For the kidney, we analysed the GSE199838 and GSE1009 datasets for T2DM, and again the genes of interest were not modulated.
4. Discussion
In this study, we documented a higher level of serum MIF among patients with T1DM, when compared to patients with T2DM. Furthermore, serum concentrations of MIF were increased among patients with T1DM as well as T2DM, when compared to healthy individuals. As already mentioned, MIF is a pleiotropic cytokine considered to be involved in autoimmune and inflammatory diseases, such as rheumatoid arthritis, systemic lupus erythematosus, sepsis and cancer. In particular, there are several lines of evidence suggesting involvement of MIF in the pathogenesis of T1DM [[31], [32], [33], [34], [35], [36], [37]]. Insulitis, which is the cornerstone of T1DM, has been suggested to be mediated-among other factors-via MIF. More specifically, MIF is implicated in autoantigen presentation, in the increased production of other inflammatory cytokines, such as interleukin-1β and Tumor Necrosis Factor (TNF)-a and in beta cell apoptosis in Langerhans islets [[31], [32], [33], [34], [35], [36], [37], [38], [39]]. MIF seems to be involved in the progression of T1DM as well. According to Korf et al. inhibition of MIF results in a delay in the onset of autoimmune diabetes [31]. Therefore, it would be useful to better identify the exact stage in the pathogenesis of T1DM, where inhibition of MIF would be beneficial. In this context, inhibition of MIF could lead to a delay in the onset of T1DM. However, due to the complexity of the pathways and the yet unknown stage that research should focus in order to impede onset of T1DM, results regarding MIF inhibition are yet inconclusive. Nevertheless, Korf et al. have demonstrated that blocking the MIF/CD74 pathway may be an effective way to delay the onset of T1DM, by interfering with macrophage cytokine secretion [31]. Notably, in our study, all patients with T1DM but one, had long lasting T1DM, i.e. of more than 12 months’ duration. Even though the duration of diabetes mellitus among patients with T1DM was long enough, serum levels of MIF were still high in these patients.
In our study, apart from serum MIF levels, urinary levels of CD74 were elevated among patients with T1DM, when compared to patients with T2DM and healthy controls. Urinary levels of CD74 among patients with T1DM were statistically significantly increased, whereas no statistical significant relationship was noted regarding serum levels of CD74 among the three groups examined. This dissociation could be a random effect or could be attributed to a potential increased production of CD74 in the kidney of patients with T1DM. Notably, Valina-Rivas et al. have proposed that TNF-like weak inducer of apoptosis (TWEAK) upregulates CD74 and its ligands MIF and DDT in renal tubular cells [40]. TWEAK belongs to the TNF superfamily and is responsible for the activation of inflammatory cells in conjunction with the downregulation of the anti-inflammatory klotho factor, leading to acute or chronic kidney injury [41]. In our opinion, the aforementioned discrepancy requires further investigation in the near future.
Regarding DDT, we have documented a significant increase of its serum levels in patients with T1DM, when compared to healthy controls. Despite the fact that no statistically significant difference was noted between the three examined groups in terms of its urinary concentrations, we cannot overlook the elevated serum levels of DDT among patients with T1DM. As DDT or MIF2 belongs to the MIF superfamily and shares the same ligand with MIF, i.e. CD74 with substantial affinity, Merk et al. have proposed that MIF2 acts synergistically with MIF [42]. In this context, the elevated serum levels of DDT in patients with T1DM, when compared to healthy controls, could be attributed to the increased inflammatory process that is associated with T1DM.
T2DM and obesity are also characterised by chronic low-grade inflammation [[43], [44], [45], [46], [47], [48], [49], [50], [51], [52]]. However, T2DM lacks the autoimmunity feature, which is indigenous to T1DM. T2DM and its complications are strongly associated with chronic low-grade inflammation, especially due to obesity and the well-known adipogenic-related inflammatory milieu [[53], [54], [55], [56], [57], [58], [59], [60]]. It is noteworthy that MIF has been documented to stimulate the release of inflammatory adipocytokines, such as resistin and interleukin-6 [61,62]. These inflammatory adipocytokines are deeply implicated in the development of insulin resistance, which is a characteristic component of T2DM and related comorbidities including obesity and associated disorders [[63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]]. Nevertheless, T1DM in comparison to T2DM, apart and beyond the inflammatory process, exhibits an autoimmune profile, which accounts for the pathogenesis of T1DM and its complications [5,76,77]. Therefore, the highly elevated levels of serum MIF and urinary CD74, that we found among patients with T1DM, when compared with patients with T2DM, could be due to the inflammation together with the autoimmune milieu in T1DM. It is noteworthy that serum MIF and urinary CD74 levels were significantly decreased in healthy controls, when compared with patients with DM, either T1DM or T2DM. This finding points towards the inflammatory features of MIF and CD74. Indeed, as already mentioned above, MIF is a cytokine with pleiotropic inflammatory properties, which acts via its receptor CD74 on a plethora of cells and not solely on macrophages [[78], [79], [80], [81], [82]]. In addition, in this study we documented a statistically significant association between BMI and urinary levels of CD74 among patients with T2DM. More specifically, increased BMI was correlated to increased urinary levels of CD74 in patients with T2DM. This positive relationship could be attributed to the chronic low-grade inflammation, which is associated with overweight/obesity [[78], [79], [80], [81], [82]]. Only recently, it has been suggested that MIF downregulates the adipose tissue hormone sensitive lipase (HSL), thus contributing to the development of obesity [83]. This observation by Chen et al. may further shed light upon the pathogenetic mechanisms of obesity and could be useful in investigating inhibitors of MIF as potential agents for the prevention and treatment of obesity [83]. Therefore, the MIF/CD74 pathway merits further attention in terms of overweight/obesity, especially in the context of T2DM.
This study has several limitations. First, the number of participants in this study was limited to 102 people, i.e. the sample size was rather small. However, the ratio of patients with T1DM to patients with T2DM was representative of the ratio in the general population. In addition, this study was performed among individuals from Athens in Greece. Therefore, we cannot draw any safe conclusions about people of other origin and not Caucasian.
5. Conclusion
In conclusion, we have documented a statistically significant increase in serum levels of MIF, DDT and urinary concentrations of CD74 among patients with DM, when compared to healthy controls. These differences were even more obvious among patients with T1DM, when compared to patients with T2DM. We have attributed these substantial differences in the autoimmune microenvironment apart from the inflammatory milieu in patients with T1DM, when compared to T2DM. Undoubtedly, MIF, DDT and CD74 are implicated in the pathogenesis of T1DM as well as of T2DM. Future studies could shed light on the potential role of inhibition of MIF in the delay of onset of T1DM. The inhibition of MIF should be further explored as a candidate for managing inflammation, atherosclerosis and overweight/obesity in patients with T2DM.
Declaration of interest statement
There is no conflict of interest regarding this manuscript.
Declaration of funding statement
No funding was received for the preparation of this manuscript.
CRediT authorship contribution statement
Katia Mangano: Formal analysis, Conceptualization. Aristidis Diamantopoulos: Resources, Investigation, Data curation. Natalia G. Vallianou: Writing – original draft, Resources. Theodora Stratigou: Validation, Project administration. Fotis Panagopoulos: Validation, Data curation. Dimitris Kounatidis: Writing – original draft, Visualization. Maria Dalamaga: Writing – review & editing, Formal analysis. Paolo Fagone: Software, Methodology, Investigation. Ferdinando Nicoletti: Writing – review & editing, Supervision, Project administration, Conceptualization.
Contributor Information
Katia Mangano, Email: katia.mangano@unict.it.
Aristidis Diamantopoulos, Email: aris_diamad@yahoo.gr.
Natalia G. Vallianou, Email: natalia.vallianou@hotmail.com.
Theodora Stratigou, Email: theodorastratigou@yahoo.gr.
Fotis Panagopoulos, Email: fotis_1992@hotmail.com.
Dimitris Kounatidis, Email: dimitriskounatidis82@outlook.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
Paolo Fagone, Email: paolofagone@yahoo.it.
Ferdinando Nicoletti, Email: ferdinic@hotmail.com.
References
- 1.Facts and figures. tenth ed. 2021. IDF diabetes atlas. [Google Scholar]
- 2.Taheri S. Type 2 diabetes remission: a new mission in diabetes care. Diabetes Care. 2024;47(1):47–49. doi: 10.2337/dci23-0062. [DOI] [PubMed] [Google Scholar]
- 3.Ruze R., Liu T., Zou X., Song J., Chen Y., Xu R., Yin X., Xu Q. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1161521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasaki E. Anti-Islet autoantibodies in type 1 diabetes. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241210012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallianou N.G., Stratigou T., Geladari E., Tessier C.M., Mantzoros C.S., Dalamaga M. Diabetes type 1: can it be treated as an autoimmune disorder? Rev Endocr Metab Disord. 2021;22(4):859–876. doi: 10.1007/s11154-021-09642-4. [DOI] [PubMed] [Google Scholar]
- 6.Sparkes A., De Baetselier P., Roelants K., Detrez C., Magez S., Van Ginderachter J.A., Raes G., Bucala R., Stijlemans B. The non-mammalian MIF superfamily. Immunobiology. 2017;222(3):473–482. doi: 10.1016/j.imbio.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang I., Bucala R. The immunobiology of MIF: function, genetics and prospects for precision medicine. Nat Rev Rheumatol. 2019;15(7):427–437. doi: 10.1038/s41584-019-0238-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen E., Reiss K., Shah D., Manjula R., Allen B., Murphy E.L., Murphy J.W., Batista V.S., Bhandari V., Lolis E.J., Lisi G.P. A structurally preserved allosteric site in the MIF superfamily affects enzymatic activity and CD74 activation in D-dopachrome tautomerase. J Biol Chem. 2021;297(3) doi: 10.1016/j.jbc.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilstam P.V., Pantouris G., Corman M., Andreoli M., Mahboubi K., Davis G., Du X., Leng L., Lolis E., Bucala R. A selective small-molecule inhibitor of macrophage migration inhibitory factor-2 (MIF-2), a MIF cytokine superfamily member, inhibits MIF-2 biological activity. J Biol Chem. 2019;294(49):18522–18531. doi: 10.1074/jbc.RA119.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caltabiano R., De Pasquale R., Piombino E., Campo G., Nicoletti F., Cavalli E., Mangano K., Fagone P. Macrophage migration inhibitory factor (MIF) and its homologue d-dopachrome tautomerase (DDT) inversely correlate with inflammation in discoid lupus erythematosus. Molecules. 2021;26(1):184. doi: 10.3390/molecules26010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-Zuno G.A., Bucala R., Hernández-Bello J., Román-Fernández I.V., García-Chagollán M., Nicoletti F., Matuz-Flores M.G., García-Arellano S., Esparza-Michel J.A., Cerpa-Cruz S., Pérez-Guerrero E.E., Muñoz-Valle J.F. Canonical (CD74/CD44) and non-canonical (CXCR2, 4 and 7) MIF receptors are differentially expressed in rheumatoid arthritis patients evaluated by DAS28-ESR. J Clin Med. 2021;11(1):120. doi: 10.3390/jcm11010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández D.A.Z., Chagollán M.G., Zuno G.A.S., Arellano S.G., Bello J.H., Palma L.A.H., Cruz S.C., Bonilla G.M., Nicoletti F., Valle J.F.M. Expression of transcriptional factors of T helper differentiation (T-bet, GATA-3, RORγt, and FOXP3), MIF receptors (CD44, CD74, CXCR2, 4, 7), and Th1, Th2, and Th17 cytokines in PBMC from control subjects and rheumatoid arthritis patients. Curr Mol Med. 2023 doi: 10.2174/0115665240260976230925095330. [DOI] [PubMed] [Google Scholar]
- 13.Jankauskas S., Wong D.W.L., Bucala R., Djudjaj S., Boor P. Evolving complexity of MIF signalling. Cell Signal. 2019;57:76–88. doi: 10.1016/j.cellsig.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Ebert S., Zang L., Ismail N., Otabil M., Fröhlich A., Egea V., Ács S., Hoeberg M., Berres M.L., Weber C., Moreira J.M.A., Ries C., Bernhagen J., El Bounkari O. Tissue inhibitor of metalloproteinases-1 interacts with CD74 to promote AKT signalling, monocyte recruitment responses, and vascular smooth muscle cell proliferation. Cells. 2023;12(14):1899. doi: 10.3390/cells12141899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong Y., Yang N., Luo Z., Huang R., Li Q. Key cell types and biomarkers in heart failure identified through analysis of single-cell and bulk RNA sequencing data. Mediat Inflamm. 2023;2023 doi: 10.1155/2023/8384882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djudjaj S., Lue H., Rong S., Papasotiriou M., Klinkhammer B.M., Zok S., Klaener O., Braun G.S., Lindenmeyer M.T., Cohen C.D., Bucala R., Tittel A.P., Kurts C., Moeller M.J., Floege J., Ostendorf T., Bernhagen J., Boor Pl. Macrophage migration inhibitory factor mediates proliferative GN via CD74. J Am Soc Nephrol. 2016;27(6):1650–1664. doi: 10.1681/ASN.2015020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernhagen J., Krohn R., Lue H., Gregory J.L., Zernecke A., Koenen R.R., Dewor M., Georgiev I., Schober A., Leng L., Kooistra T., Fingerle-Rowson G., Ghezzi P., Kleemann R., McColl S.R., Bucala R., Hickey M.J., Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13(5):587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J.Q., Zhu Y., Qi M., Zeng Y., Liu Z.J., Ding C., Zhang T., Li X.L., Han D.D., He Q. Granzyme B+ B cells detected by single-cell sequencing are associated with prognosis in patients with intrahepatic cholangiocarcinoma following liver transplantation. Cancer Immunol Immunother. 2024;73(3):58. doi: 10.1007/s00262-023-03609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaizer E.C., Glaser C.L., Chaussabel D., Banchereau J., Pascual V., White P.C. Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab. 2007;92(9):3705–3711. doi: 10.1210/jc.2007-0979. [DOI] [PubMed] [Google Scholar]
- 20.Yang M., Ye L., Wang B., Gao J., Liu R., Hong J., Wang W., Gu W., Ning G. Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients 1miR-146. J Diabetes. 2015;7(2):158–165. doi: 10.1111/1753-0407.12163. [DOI] [PubMed] [Google Scholar]
- 21.Marselli L., Thorne J., Dahiya S., Sgroi D.C., Sharma A., Bonner-Weir S., Marchetti P., Weir G.C. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez V., Raimondi C., Somanath S., Bugliani M., Loder M.K., Edling C.E., Divecha N., da Silva-Xavier G., Marselli L., Persaud S.J., Turner M.D., Rutter G.A., Marchetti P., Falasca M., Maffucci T. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J Biol Chem. 2011;286(6):4216–4225. doi: 10.1074/jbc.M110.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beveren N.J., Buitendijk G.H., Swagemakers S., Krab L.C., Röder C., de Haan L., van der Spek P., Elgersma Y. Marked reduction of AKT1 expression and deregulation of AKT1-associated pathways in peripheral blood mononuclear cells of schizophrenia patients. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toro-Domínguez D., Martorell-Marugán J., López-Domínguez R., García-Moreno A., González-Rumayor V., Alarcón-Riquelme M.E., Carmona-Sáez P. ImaGEO: integrative gene expression meta-analysis from GEO database. Bioinformatics. 2019;35(5):880–882. doi: 10.1093/bioinformatics/bty721. [DOI] [PubMed] [Google Scholar]
- 25.Rau A., Gallopin M., Celeux G., Jaffrézic F. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics. 2013;29(17):2146–2152. doi: 10.1093/bioinformatics/btt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baelde H.J., Eikmans M., Doran P.P., Lappin D.W., de Heer E., Bruijn J.A. Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis. 2004;43(4):636–650. doi: 10.1053/j.ajkd.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Hu J., Wang Q., Fan X., Zhen J., Wang C., Chen H., Liu Y., Zhou P., Zhang T., Huang T., Wang R., Lv Z. Long noncoding RNA ENST00000436340 promotes podocyte injury in diabetic kidney disease by facilitating the association of PTBP1 with RAB3B. Cell Death Dis. 2023;14(2):130. doi: 10.1038/s41419-023-05658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen K.D., Irizarry R.A., Wu Z. Removing technical variability in RNA-seq data using conditional quantile normalisation. Biostatistics. 2012;13(2):204–216. doi: 10.1093/biostatistics/kxr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7) doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou G., Soufan O., Ewald J., Hancock R.E.W., Basu N., Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47(W1):W234–W241. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korf H., Breser L., Van Hoeck J., Godoy J., Cook D.P., Stijlemans B., De Smidt E., Moyson C., Monteiro Carvalho Mori Cunha J.P., Rivero V., Gysemans C., Mathieu C. MIF inhibition interferes with the inflammatory and T cell-stimulatory capacity of NOD macrophages and delays autoimmune diabetes onset. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187455. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Zamora Y.I., Rodriguez-Sosa M. The role of MIF in type 1 and type 2 diabetes mellitus. J Diabetes Res. 2014;2014 doi: 10.1155/2014/804519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong C., Morrison A., Yan X., Zhao P., Yeung E.D., Wang J., Xie J., Li J. Macrophage migration inhibitory factor deficiency augments cardiac dysfunction in Type 1 diabetic murine cardiomyocytes. J Diabetes. 2010;2(4):267–274. doi: 10.1111/j.1753-0407.2010.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Zamora Y.I., Juarez-Avelar I., Vazquez-Mendoza A., Hiriart M., Rodriguez-Sosa M. Altered macrophage and dendritic cell response in mif-/- mice reveals a role of mif for inflammatory-Th1 response in type 1 diabetes. J Diabetes Res. 2016;2016 doi: 10.1155/2016/7053963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ismail N.A., Abd El Baky A.N., Ragab S., Hamed M., Hashish M.A., Shehata A. Monocyte chemoattractant protein 1 and macrophage migration inhibitory factor in children with type 1 diabetes. J Pediatr Endocrinol Metab. 2016;29(6):641–645. doi: 10.1515/jpem-2015-0340. [DOI] [PubMed] [Google Scholar]
- 36.Prashanth G., Vastrad B., Tengli A., Vastrad C., Kotturshetti I. Identification of hub genes related to the progression of type 1 diabetes by computational analysis. BMC Endocr Disord. 2021;21(1):61. doi: 10.1186/s12902-021-00709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stosic-Grujicic S., Stojanovic I., Maksimovic-Ivanic D., Momcilovic M., Popadic D., Harhaji L., Miljkovic D., Metz C., Mangano K., Papaccio G., Al-Abed Y., Nicoletti F. Macrophage migration inhibitory factor (MIF) is necessary for progression of autoimmune diabetes mellitus. J Cell Physiol. 2008;215(3):665–675. doi: 10.1002/jcp.21346. [DOI] [PubMed] [Google Scholar]
- 38.Stojanovic I., Saksida T., Nikolic I., Nicoletti F., Stosic-Grujicic S. Macrophage migration inhibitory factor deficiency protects pancreatic islets from cytokine-induced apoptosis in vitro. Clin Exp Immunol. 2012;169(2):156–163. doi: 10.1111/j.1365-2249.2012.04607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cvetkovic I., Al-Abed Y., Miljkovic D., Maksimovic-Ivanic D., Roth J., Bacher M., Lan H.Y., Nicoletti F., Stosic-Grujicic S. Critical role of macrophage migration inhibitory factor activity in experimental autoimmune diabetes. Endocrinology. 2005;146(7):2942–2951. doi: 10.1210/en.2004-1393. [DOI] [PubMed] [Google Scholar]
- 40.Valiño-Rivas L., Cuarental L., Grana O., Bucala R., Leng L., Sanz A., Gomez G., Ortiz A., Sanchez-Nino M.D. TWEAK increases CD74 expression and sensitises to DDT proinflammatory actions in tubular cells. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanz A.B., Ruiz-Andres O., Sanchez-Niño M.D., Ruiz-Ortega M., Ramos A.M., Ortiz A. Out of the TWEAKlight: elucidating the role of Fn14 and TWEAK in acute kidney injury. Semin Nephrol. 2016;36 doi: 10.1016/j.semnephrol.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Merk M., Mitchell R.A., Endres S., Bucala R. D-dopachrome tautomerase (D-DT or MIF-2): doubling the MIF cytokine family. Cytokine. 2012;59(1):10–17. doi: 10.1016/j.cyto.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xourafa G., Korbmacher M., Roden M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. 2024;20(1):27–49. doi: 10.1038/s41574-023-00898-1. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Wang J., Tao S.Y., Liang Z., Xie R., Liu N.N., Deng R., Zhang Y., Deng D., Jiang G. Mitochondrial damage-associated molecular patterns: a new insight into metabolic inflammation in type 2 diabetes mellitus. Diabetes Metab Res Rev. 2024;40(2) doi: 10.1002/dmrr.3733. [DOI] [PubMed] [Google Scholar]
- 45.Dumont B.L., Neagoe P.E., Charles E., Villeneuve L., Tardif J.C., Räkel A., White M., Sirois M.G. Low-density neutrophils contribute to subclinical inflammation in patients with type 2 diabetes. Int J Mol Sci. 2024;25(3):1674. doi: 10.3390/ijms25031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domazet S.L., Olesen T.B., Stidsen J.V., Svensson C.K., Nielsen J.S., Thomsen R.W., Jessen N., Vestergaard P., Andersen M.K., Hansen T., Brøns C., Jensen V.H., Vaag A.A., Olsen M.H., Højlund K. Low-grade inflammation in persons with recently diagnosed type 2 diabetes: the role of abdominal adiposity and putative mediators. Diabetes Obes Metabol. 2024 doi: 10.1111/dom.15514. [DOI] [PubMed] [Google Scholar]
- 47.Marouga A., Dalamaga M., Kastania A.N., Kroupis C., Lagiou M., Saounatsou K., Dimas K., Vlahakos D.V. Circulating resistin is a significant predictor of mortality independently from cardiovascular comorbidities in elderly, non-diabetic subjects with chronic kidney disease. Biomarkers. 2016;21(1):73–79. doi: 10.3109/1354750X.2015.1118536. [DOI] [PubMed] [Google Scholar]
- 48.Kazanis K., Dalamaga M., Nounopoulos C., Manolis A.S., Sakellaris N., Jullien G., Dionyssiou-Asteriou A. Ischemia modified albumin, high-sensitivity c-reactive protein and natriuretic peptide in patients with coronary atherosclerosis. Clin Chim Acta. 2009;408(1–2):65–69. doi: 10.1016/j.cca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Hroussalas G., Kassi E., Dalamaga M., Delimaris I., Zachari A., Dionyssiou-Asteriou A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas. 2008;59(4):339–349. doi: 10.1016/j.maturitas.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Dalamaga M., Karmaniolas K., Papadavid E., Pelekanos N., Sotiropoulos G., Lekka A. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause. 2013;20(8):845–851. doi: 10.1097/GME.0b013e31827f06dc. [DOI] [PubMed] [Google Scholar]
- 51.Dalamaga M., Karmaniolas K., Chamberland J., Nikolaidou A., Lekka A., Dionyssiou-Asteriou A., Mantzoros C.S. Higher fetuin-A, lower adiponectin and free leptin levels mediate effects of excess body weight on insulin resistance and risk for myelodysplastic syndrome. Metabolism. 2013;62(12):1830–1839. doi: 10.1016/j.metabol.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Marouga A., Dalamaga M., Kastania A.N., Antonakos G., Thrasyvoulides A., Kontelia G., Dimas C., Vlahakos D.V. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin Lab. 2013;59(9–10):1121–1128. doi: 10.7754/clin.lab.2012.121112. [DOI] [PubMed] [Google Scholar]
- 53.Vilariño-García T., Polonio-González M.L., Pérez-Pérez A., Ribalta J., Arrieta F., Aguilar M., Obaya J.C., Gimeno-Orna J.A., Iglesias P., Navarro J., Durán S., Pedro-Botet J., Sánchez-Margalet V. Role of leptin in obesity, cardiovascular disease, and type 2 diabetes. Int J Mol Sci. 2024;25(4):2338. doi: 10.3390/ijms25042338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan P.C., Wu T.N., Chen Y.C., Lu C.H., Wabitsch M., Tian Y.F., Hsieh P.S. Targeted inhibition of CD74 attenuates adipose COX-2-MIF-mediated M1 macrophage polarisation and retards obesity-related adipose tissue inflammation and insulin resistance. Clin Sci (Lond) 2018;132(14):1581–1596. doi: 10.1042/CS20180041. [DOI] [PubMed] [Google Scholar]
- 55.Dalamaga M., Karmaniolas K., Nikolaidou A., Chamberland J., Hsi A., Dionyssiou-Asteriou A., Mantzoros C.S. Adiponectin and resistin are associated with risk for myelodysplastic syndrome, independently from the insulin-like growth factor-I (IGF-I) system. Eur J Cancer. 2008;44(12):1744–1753. doi: 10.1016/j.ejca.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Dalamaga M., Nikolaidou A., Karmaniolas K., Hsi A., Chamberland J., Dionyssiou-Asteriou A., Mantzoros C.S. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: a case-control study. Oncology. 2007;73(1–2):26–32. doi: 10.1159/000120995. [DOI] [PubMed] [Google Scholar]
- 57.Stratigou T., Dalamaga M., Antonakos G., Marinou I., Vogiatzakis E., Christodoulatos G.S., Karampela I., Papavassiliou A.G. Hyperirisinemia is independently associated with subclinical hypothyroidism: correlations with cardiometabolic biomarkers and risk factors. Endocrine. 2018;61(1):83–93. doi: 10.1007/s12020-018-1550-3. [DOI] [PubMed] [Google Scholar]
- 58.Spyrou N., Vallianou N., Kadillari J., Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune checkpoint inhibitors therapy era. Semin Cancer Biol. 2021;73:356–376. doi: 10.1016/j.semcancer.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Kassi E., Dalamaga M., Hroussalas G., Kazanis K., Merantzi G., Zachari A., Giamarellos-Bourboulis E.J., Dionyssiou-Asteriou A. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas. 2010;67(1):72–77. doi: 10.1016/j.maturitas.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Karamanakos G., Kokkinos A., Dalamaga M., Liatis S. Highlighting the role of obesity and insulin resistance in type 1 diabetes and its associated cardiometabolic complications. Curr Obes Rep. 2022;11(3):180–202. doi: 10.1007/s13679-022-00477-x. [DOI] [PubMed] [Google Scholar]
- 61.Morrison M.C., Kleemann R. Role of macrophage migration inhibitory factor in obesity, insulin resistance, type 2 diabetes, and associated hepatic Co-morbidities: a comprehensive review of human and rodent studies. Front Immunol. 2015;6:308. doi: 10.3389/fimmu.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumaiya K., Langford D., Natarajaseenivasan K., Shanmughapriya S. Macrophage migration inhibitory factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. 2022;233 doi: 10.1016/j.pharmthera.2021.108024. [DOI] [PubMed] [Google Scholar]
- 63.Dalamaga M., Christodoulatos G.S. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm Mol Biol Clin Invest. 2015;23(1):5–20. doi: 10.1515/hmbci-2015-0016. [DOI] [PubMed] [Google Scholar]
- 64.Tsilingiris D., Vallianou N.G., Karampela I., Dalamaga M. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metabol Open. 2021;11 doi: 10.1016/j.metop.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalamaga M., Karmaniolas K., Arsenis G., Pantelaki M., Daskalopoulou K., Papadavid E., Migdalis I. Cedecea lapagei bacteremia following cement-related chemical burn injury. Burns. 2008;34(8):1205–1207. doi: 10.1016/j.burns.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Dalamaga M., Karmaniolas K., Matekovits A., Migdalis I., Papadavid E. Cutaneous manifestations in relation to immunologic parameters in a cohort of primary myelodysplastic syndrome patients. J Eur Acad Dermatol Venereol. 2008;22(5):543–548. doi: 10.1111/j.1468-3083.2007.02520.x. [DOI] [PubMed] [Google Scholar]
- 67.Papadavid E., Vlami K., Dalamaga M., Giatrakou S., Theodoropoulos K., Gyftopoulos S., Stavrianeas N., Papiris S., Rigopoulos D. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venereol. 2013;27(7):820–826. doi: 10.1111/j.1468-3083.2012.04580.x. [DOI] [PubMed] [Google Scholar]
- 68.Pavlidou A., Dalamaga M., Kroupis C., Konstantoudakis G., Belimezi M., Athanasas G., Dimas K. Survivin isoforms and clinicopathological characteristics in colorectal adenocarcinomas using real-time qPCR. World J Gastroenterol. 2011;17(12):1614–1621. doi: 10.3748/wjg.v17.i12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalamaga M., Karmaniolas K., Nikolaidou A., Papadavid E. Hypocalcemia, hypomagnesemia, and hypokalemia following hydrofluoric acid chemical injury. J Burn Care Res. 2008;29(3):541–543. doi: 10.1097/BCR.0b013e3181711152. [DOI] [PubMed] [Google Scholar]
- 70.Papadavid E., Gazi S., Dalamaga M., Stavrianeas N., Ntelis V. Palmoplantar and scalp psoriasis occurring during anti-tumour necrosis factor-alpha therapy: a case series of four patients and guidelines for management. J Eur Acad Dermatol Venereol. 2008;22(3):380–382. doi: 10.1111/j.1468-3083.2007.02335.x. [DOI] [PubMed] [Google Scholar]
- 71.Vallianou N., Kounatidis D., Christodoulatos G.S., Panagopoulos F., Karampela I., Dalamaga M. Mycobiome and cancer: what is the evidence? Cancers. 2021;13(13):3149. doi: 10.3390/cancers13133149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papadavid E., Katsimbri P., Kapniari I., Koumaki D., Karamparpa A., Dalamaga M., Tzannis K., Βoumpas D., Rigopoulos D. Prevalence of psoriatic arthritis and its correlates among patients with psoriasis in Greece: results from a large retrospective study. J Eur Acad Dermatol Venereol. 2016;30(10):1749–1752. doi: 10.1111/jdv.13700. [DOI] [PubMed] [Google Scholar]
- 73.Karampela I., Kandri E., Antonakos G., Vogiatzakis E., Christodoulatos G.S., Nikolaidou A., Dimopoulos G., Armaganidis A., Dalamaga M. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: a prospective study. J Crit Care. 2017;41:78–85. doi: 10.1016/j.jcrc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Dalamaga M., Papadavid E., Basios G., Vaggopoulos V., Rigopoulos D., Kassanos D., Trakakis E. Ovarian SAHA syndrome is associated with a more insulin-resistant profile and represents an independent risk factor for glucose abnormalities in women with polycystic ovary syndrome: a prospective controlled study. J Am Acad Dermatol. 2013;69(6):922–930. doi: 10.1016/j.jaad.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Kassi E., Dalamaga M., Faviou E., Hroussalas G., Kazanis K., Nounopoulos Ch, Dionyssiou-Asteriou A. Circulating oxidised LDL levels, current smoking and obesity in postmenopausal women. Atherosclerosis. 2009;205(1):279–283. doi: 10.1016/j.atherosclerosis.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Vallianou N.G., Geladari E.V., Kounatidis D., Geladari C.V., Stratigou T., Dourakis S.P., Andreadis E.A., Dalamaga M. Diabetes mellitus in the era of climate change. Diabetes Metab. 2021;47(4) doi: 10.1016/j.diabet.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Asare Y., Schmitt M., Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemostasis. 2013;109(3):391–398. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- 78.Vallianou N.G., Tsilingiris D., Kounatidis D., Lempesis I.G., Karampela I., Dalamaga M. Sodium-glucose cotransporter-2 inhibitors in obesity and associated cardiometabolic disorders: where do we stand? Pol Arch Intern Med. 2022;132(10) doi: 10.20452/pamw.16342. [DOI] [PubMed] [Google Scholar]
- 79.Frühbeck G., Gómez-Ambrosi J., Ramírez B., Becerril S., Rodríguez A., Mentxaka A., Valentí V., Moncada R., Reina G., Baixauli J., Casado M., Silva C., Escalada J., Catalán V. Decreased expression of the NLRP6 inflammasome is associated with increased intestinal permeability and inflammation in obesity with type 2 diabetes. Cell Mol Life Sci. 2024;81(1):77. doi: 10.1007/s00018-024-05124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weber C., Habenicht A.J.R., von Hundelshausen P. Novel mechanisms and therapeutic targets in atherosclerosis: inflammation and beyond. Eur Heart J. 2023;44(29):2672–2681. doi: 10.1093/eurheartj/ehad304. [DOI] [PubMed] [Google Scholar]
- 81.Klimontov V.V., Mavlianova K.R., Orlov N.B., Semenova J.F., Korbut A.I. Serum cytokines and growth factors in subjects with type 1 diabetes: associations with time in ranges and glucose variability. Biomedicines. 2023;11(10):2843. doi: 10.3390/biomedicines11102843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lempesis I.G., Tsilingiris D., Liu J., Dalamaga M. Of mice and men: considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol Open. 2022;15 doi: 10.1016/j.metop.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen L., Li L., Cui D., Huang Y., Tong H., Zabihi H., Wang S., Qi Y., Lakowski T., Leng L., Liu S., Wu H., Young L.H., Bucala R., Qi D. Extracellular macrophage migration inhibitory factor (MIF) downregulates adipose hormone-sensitive lipase (HSL) and contributes to obesity. Mol Metabol. 2024;79 doi: 10.1016/j.molmet.2023.101834. [DOI] [PMC free article] [PubMed] [Google Scholar]