Abstract
Background
Saussurea costus (S. costus) is a critically endangered medicinal plant that has been extensively studied for its chemical composition, significance, and therapeutic potential as traditional phytomedicine. This comprehensive review aims to provide a thorough understanding of S. costus, including its biological activities, chemical makeup, and potential therapeutic uses in biotechnology.
Objectives
This study investigated the pharmacological properties of S. costus, including its antimicrobial, antioxidant, and antifungal properties, and its usefulness in treating conditions such as thyroid disorders and liver injury. This study also aimed to assess and improve the techniques used to extract bioactive compounds and to develop effective methods for harvesting these compounds from medicinal plants.
Methods
This review analyzed the available literature on the phytochemical makeup and bioactivity of S. costus extract using techniques such as molecular docking against SARS-CoV-2 protease, green extraction methods, and phytochemical analysis.
Results
This review revealed that S. costus possesses various pharmacological properties, including antimicrobial, antiviral, anti-inflammatory, and anticancer activities. It is effective in combating fungal infections, reducing inflammation, treating cancer, and inhibiting viral replication, and has the potential to control Candida species. Moreover, S. costus has been explored for its capacity to synthesize nanoparticles with antimicrobial properties and for its potential in treating thyroid disorders and liver injury.
Recommendations
Despite promising results, additional research is necessary to fully comprehend the benefits of S. costus and validate its effectiveness in clinical settings. Future research should focus on standardized methodologies and rigorous clinical trials to confirm the safety and effectiveness of S. costus in various medical fields as well as further investigate its biotechnological and pharmaceutical applications.
Keywords: Plant-based treatments, Plant-derived medicines, Modern and traditional medicine, COVID-19
Graphical abstract
Highlights
-
•
S. costus is a critically endangered medicinal plant with significant therapeutic potential.
-
•
It contains compounds with antioxidant, antimicrobial, anti-inflammatory, and anticancer properties.
-
•
S. costus shows antiviral, antifungal, and anti-inflammatory activities.
-
•
It has potential for controlling Candida species and synthesizing antimicrobial nanoparticles.
-
•
S. costus has been studied for treating thyroid disorders and liver injuries.
1. Introduction
Saussurea costus (S. costus) (Falc.) Lipsch, also known as Indian costus, has a long-standing history in traditional Saudi Arabian medicine and is a medicinal plant [1]. It shows diverse biological activities, including anti-inflammatory, anti-urolithiasis, and antimicrobial properties [2]. It has the potential to improve thyroid tissue damage caused by carbazole-induced hypothyroidism [3]. These findings highlight their significance in traditional and modern medicine. Similarly, other medicinal plants, such as saffron [[4], [5], [6]], costus speciosus [7], Solanum torvum [8], Saururus [9], Swertia chirata [10], and Rhus coriari [11] have been extensively studied for their therapeutic properties, further highlighting the potential of natural products in modern medicine. These findings underscore the historical and cultural relevance of S. costus in supporting its traditional use and showcasing its potential in modern medicine.
A plant called S. costus has been widely studied for its ability to treat various health issues and provides multiple therapeutic benefits, making it a promising candidate for medicinal use. Its anti-inflammatory and anti-urolithiatic properties and ability to address thyroid disorders and tissue damage have been studied [3,12,13]. Pathogenic bacteria and fungi can be controlled by plant extracts with antimicrobial activities [2]. It also exhibits hepatoprotective, antitumor, and anti-inflammation impact [14,15]. The possibility of using S. costus, a related species, in drug discovery has also been emphasized [16]. Similarly, the therapeutic potential of S. costus in thyroid treatment has been explored [1]. However, the lack of propagation techniques for many Saussurea species, including S. costus, calls for research on sustainable cultivation methods [17]. Conservation of the genetic pool of this plant species is also crucial [18]. Therefore, the study of S. costus as a versatile and valuable medicinal plant has the potential to contribute to sustainable cultivation methods, conservation efforts, and impact on various health conditions.
S. costus, a critically endangered medicinal plant, has been extensively studied for its chemical composition and traditional significance [19,20]. The therapeutic potential of this plant extract has been demonstrated in traditional medicine, where it has been used to treat a range of conditions [21]. The main components of the plant are volatile constituents such as costunolide, dehydrocostus lactone, and cynaropicrin [20]. In addition to other bioactive components, these compounds possess antioxidant, antimicrobial, anti-inflammatory, antidiabetic, anti-ulcer, anti-cancer, and hepatoprotective properties [7]. Thus, the value of S. costus in traditional medicine and its preservation are crucial for future medical research.
Comprehensive reviews of the phytochemical profiles of plants and their traditional uses have provided a thorough understanding of their potential contributions to medicine and conservation [22]. This dual focus underscores the potential of S. costus to address emerging health challenges, including its inhibitory effect on SARS-CoV-2's main protease, which indicates its potential role in treating COVID-19 [21,23]. Despite extensive research, there are still considerable gaps in our understanding of this subject. Although previous studies have demonstrated antimicrobial properties, a thorough understanding of the specific mechanisms and active components involved remains elusive [2,24,25]. Molecular docking against the SARS-CoV-2 protease highlights its antiviral potential, but further validation and optimization are necessary [21]. Therefore, although S. costus shows promise against COVID-19, further research is required.
This study aimed to gain a comprehensive understanding of S. costus, including its biological activity, chemical composition, and possible therapeutic uses. This study investigated the pharmacological properties of S. costus, including its antimicrobial, antioxidant, and antifungal properties, as well as its usefulness in treating conditions such as thyroid disorders and liver injury. By focusing on S. costus, this study aimed to address emerging health challenges, such as COVID-19, and to uncover bioactive compounds for medicinal and therapeutic purposes. This study suggests that S. costus has significant pharmacological properties, making it a potential therapeutic agent for thyroid disorders, liver injury, and COVID-19.
This study aimed to assess and improve the techniques used to extract bioactive compounds and to develop effective methods for harvesting these compounds from medicinal plants. This review highlights the immense historical, cultural, and medicinal significance of S. costus, and demonstrates its diverse biological activities and potential therapeutic applications. The study's comprehensive approach, focused research question, and hypothesis position S. costus as a promising subject for further exploration and potential contributions to medicine and conservation while acknowledging gaps in knowledge.
2. Ethnobotanical description and distribution
The therapeutic potential of S. costus has been extensively documented in Indian medicinal systems such as Ayurveda, Siddha, and Unani, owing to its diverse medicinal properties. The roots of S. costus are commonly used as antispasmodic agents to treat a variety of conditions, including asthma, persistent cough, cholera, chronic skin disorders, and rheumatism [21]. This medication is often employed to treat a variety of health concerns, including coughs and colds, malaria, leprosy, hiccups, rheumatism, and stomach and toothache [21]. It is crucial for gout, erysipelas, and enhancement of spermatogenesis [21]. The plant has been traditionally employed in diverse indigenous medical practices to treat an array of ailments, such as rheumatism, common cold, cough, headache, stomachache, and throat infections [26]. S. costus is known for its antimicrobial, cytotoxic, and photocatalytic potential [24]. The potential anti-inflammatory properties of this substance could explain its historical use in treating rheumatism [21].
The potential benefits of S. costus include its ability to protect the liver, act as an antioxidant, and prevent kidney stones [12,14,27]. It has anti-hepatotoxic, anti-hypercholesterolemic, and anti-thyroidal effects [3,28,29]. The therapeutic potential of the plant is further demonstrated by its ability to alleviate reproductive, kidney, pulmonary, and splenic tissue damage, and reproductive toxicity [[30], [31], [32]]. S. costus has antifungal properties against Candida species [2,33]. The diverse biological activities of S. costus, including its phytochemical constituents, antimicrobial activity, and antifeedant potential, have been extensively evaluated [34]. S. costus exhibits diverse therapeutic properties, thus validating its use in Ayurveda medicine.
The scientific community has provided substantial evidence to support the use of S. costus in the treatment of many ailments. In line with its traditional use for addressing rheumatism, Idriss et al. S. costus possesses anti-inflammatory properties [21]. The hepatoprotective, antioxidative, and anti-urolithiatic properties of S. costus have been confirmed by Mammate [12] and Marei [29]. S. costus exhibits anti-hepatotoxic, anti-hypercholesterolemic, and anti-thyroidal effects, as reported by Fekry et al. [3] and Elgharabawy et al. [32]. S. costus possesses antifungal activity against Candida species, as exhibited by Soliman et al. [2] and Karim et al. [35]. The diverse biological activities of S. costus, including its phytochemical constituents, antimicrobial activity, and antifeedant potential, have been extensively evaluated [34]. These results emphasize the extensive range of therapeutic qualities associated with S. costus, lending credence to its traditional application in healing practices in India (Table 1). S. costus has been used by individuals of diverse ethnic backgrounds in northern India to address various health issues. Table 1 summarizes the medical applications of S. costus categorized by geographical region and Table 2 describes the various approaches to using this plant.
Table 1.
Region-wise ethnomedical uses of Saussurea costus.
| Part | Region | Biomedical and biopharmaceutical uses | Reference |
|---|---|---|---|
| Root |
|
|
Pachiyappan et al. [63], |
| Decoction of roots |
|
|
Liu et al. [96], |
| Root/root powder |
|
|
Lunz et al. [123], |
| Decoction of root |
|
|
Awoke [211] |
| Root |
|
|
Priyadarshi et al. [212], |
| Root |
|
|
Pachiyappan et al. [63], |
| Root |
|
|
Tousson et al. [58], |
| Root |
|
|
Mujammami [37], |
| Root |
|
|
Pachiyappan et at. [63], |
| Leaves |
|
|
Samant et al. [213], |
Table 2.
Traditional methods of application of Saussurea costus.
| Conditions | Traditional application | Reference |
|---|---|---|
| Stomachache |
|
Pachiyappan et al., [63]. |
| Headache |
|
Fekry et al., [214]. |
| Respiratory disorders |
|
Badeggi et al., [215]. |
| Symptoms associated with coughing and colds |
|
Lade and Patil, [216]. |
| Throat infection |
|
[63]. |
| Symptoms of backache and chest pain |
|
Pachiyappan et al., [63]. |
| ||
| Inflammation of joints and rheumatism |
|
Jimoh and Aina [217]. |
| Minimal urination |
|
Abdel-Hafez [218]. |
| ||
| Insect bites can cause skin rashes |
|
Kolahalam et al., [64]. |
| Fatigue |
|
Mujammami, [37]. |
| Enhancement of hair's luster and growth |
|
Sinclair [219]. |
| Inflammations and pustules |
|
Li and Kasal [220]. |
| ||
| Experiencing fainting spells |
|
Tousson et al., [58]. |
| Weakness and fatigue generally |
|
Abdel-Hafez [218]. |
| Stacks of piles |
|
Pachiyappan et al., [63]. |
| Epilepsy |
|
Qais et al., [221]. |
| Headache |
|
Zamudio et al., [222]. |
| The general weakness of Rasayana |
|
Pachiyappan et al., [63]. |
2.1. Physical characteristics and habitat
S. costus, also known as kuth or costus, is a plant species of the Asteraceae family, with therapeutic properties demonstrated by Mujammami et al. in traditional medicine [36]. The plant, native to the Indian Himalayan region, has been extensively documented by Butola and Samant [17]. Its botanical and medicinal properties are diverse. The assertion that the primary contributions to the Asteraceae family in Indian regions were made by two British botanists is supported by the “Flora of British India," which provides a taxonomic account of approximately 608 species of Asteraceae [37]. Considerable progress has been made in characterizing the genus Saussurea, particularly S. cuspidata, in India. These investigators surveyed various macromorphological traits, including plant habits, size, leaf shape, and the nature of phyllaries. Additionally, they have provided valuable information on the dimensions and configuration of leaves and capitula [21]. Hence, S. costus is crucial in Indian medicine and research on the Asteraceae family.
Many scientific investigations have revealed that the Asteraceae family has emerged as the most species-rich family in the Indian alien flora, as shown in a study by Khuroo et al. [38]. This finding underscores the substantial contribution of non-British botanists in understanding Asteraceae in the Indian region. Debnath et al.'s study reveals that Asteraceae is the most common alien family in Tripura, India, indicating its extensive presence and impact in multiple regions of India beyond the Flora of British India [39]. Zhang et al. [40] also provided insights into the phylogenomics of Saussurea, offering a wider comprehension of the genus beyond the contributions of British botanists. Xu et al. shed light on the phylogeny, origin, and dispersal of Saussurea, expanding the knowledge base beyond the contributions of British botanists [41]. Therefore, while the contributions of British botanists are significant, a wealth of research and contributions from various other sources have expanded our understanding of Asteraceae and the genus Saussurea in Indian regions.
S. costus has been extensively studied for its medicinal applications. Studies have explored its use in thyroid treatment [36], antifungal agents [2], and hypercholesterolemia [29]. In addition, several studies have investigated the antioxidant, antimicrobial, and anti-inflammatory effects of S. costus [13,27,42,43]. Their potential to reduce reproductive toxicity and thyroid tissue damage has also been explored [30,32]. Hence, the findings of these studies support the paragraph's claim regarding the therapeutic benefits of S. costus and its potential application in diverse medical treatments.
Recently, several studies have focused on the hepatoprotective properties of S. costus root extract, making this a topic of significant interest. Rajan et al. investigated the flavonoid content of the ethanolic root extract and its hepatoprotective effects [44]. These results suggest that Saussurea species may have therapeutic value because of their promising hepatoprotective properties. These results are consistent with those found by Yaeesh et al., who showed that S. costus extract has anti-hepatotoxic effects in mice with D-galactosamine and lipopolysaccharide-induced hepatitis. The treatment groups exhibited improved liver architecture and reduced cellular swelling associated with the toxin cluster [28]. Liu et al. provided a comprehensive overview of the active components, pharmacology, conventional uses, and obstacles to the preservation and sustainable utilization of S. costus, highlighting its therapeutic potential [45]. However, it is essential to consider contrasting evidence.
Eldaim et al. highlighted that S. costus root aqueous extract improved some aspects of reproductive toxicity in male rats, significantly increasing sperm abnormalities, testicular tissue and DNA damage, and protein expression associated with testicular damage [30]. Evidence from various sources appears contradictory, implying the need for additional research to explore the negative effects of S. costus root extracts on reproductive well-being. Furthermore, research conducted by Abbasi et al. [46] in the Lesser Himalayas of Pakistan has focused on botanical ethnoveterinary therapies, emphasizing the enduring nature of local knowledge in this area. Although this knowledge may not be related to the hepatoprotective properties of S. costus root extract, it offers valuable insights into the region's broader traditional uses of plant-based remedies.
Another study examined the potential of bioactive compounds derived from S. costus to curb the growth of the microorganisms responsible for food spoilage. These findings indicate that these compounds may function as environmentally friendly alternatives to synthetic preservatives, which may have harmful health impacts [47]. Furthermore, S. costus has been found to have anti-cancer properties, suggesting that it is a natural source. A study of Moroccan medicinal plants for cancer treatment revealed the significant anti-cancer properties of S. costus [48]. Phylogenetic analysis showed that S. costus is more closely related to Frolovia and Dolomiaea than to other Saussurea species. This finding supports the proposal to include S. costus in the expanded Dolomiaea genus based on the molecular phylogenetic context [49]. Table 3 presents the selection of ethnobotanically significant Indian species from the genus Saussurea that are common.
Table 3.
Some common, ethnomedicinally important Indian species of genus Sauss
| Name | Uses | Distribution | References |
|---|---|---|---|
| The species of Saussurea auriculata is found in Europe and North America. |
|
|
Gezahegn et al. [223] |
|
|
Semwal et al., [224] | |
| Saussurea ceratocarpa Decne. |
|
|
Rees [225] |
| The Saussurea costus species (Falc.) Lipsch. |
|
|
Idriss et al. [22], |
| Saussurea gossypiphora D. Don. |
|
|
Aryal et al., [226] |
| Saussurea heteromalla (Don). |
|
|
Rozina et al., [227] |
| Saussurea obvallata (DC.) Edgew. |
|
|
Semwal et al., [224] |
| Simpsoniana (Field. & Gard.) Lipsch. |
|
|
Sharma et al., [228] |
2.2. Global distribution and conservation status of S. costus
The conservation status and global distribution of S. costus are of great interest because of its medicinal properties and threatened existence. S. costus is a plant species of the Asteraceae family that is found in the Himalayan region of India, Pakistan, and other Arab countries [17,50]. The growing need for raw materials in industry has prompted calls for their preservation in natural habitats and controlled environments [21]. This plant has been extensively used in many medical systems worldwide because it has been shown to effectively combat a range of health issues. Its anti-inflammatory, anti-ulcer, anti-cancer, and hepatoprotective properties have been shown [12,32,51]. Superoxide Dismutase (SOD) has been the focus of several investigations, which have highlighted its promising capabilities in the areas of antimicrobial, antifungal, and antiviral functions, as well as its role as a mediator in the synthesis of iron-oxide nanoparticles and the production of compounds rich in antioxidants [3,24,50]. The benefits of this plant in combating heart problems and DNA damage in female mice have been reported, and it may also help improve thyroid tissue under unfavorable conditions [31,52]. Plant roots have pinpointed and evaluated active ingredients as potent antifungal agents against Candida albicans and non-albicans species [24]. S. costus has also been identified as a source of inulin and has been found to contain anti-trypanosomal sesquiterpene lactones [53]. Plant extracts have shown potential for the green synthesis of magnesium oxide (MgO) nanoparticles [3]. Thus, S. costus has therapeutic potential but faces population decline and industrial threats.
However, there are differing opinions regarding this. Bheel et al. stated that C. englerianus bagasse, a related plant, does not acquire significant traction as a partial alternative to cement in concrete production [54]. This suggests a scarcity of interest in the industrial applications of S. costus and its associated species. This paragraph describes S. costus as a vulnerable medicinal plant in the Himalayan region and explores its ethnopharmacology [21]. S. costus has been shown to exhibit therapeutic effects in the treatment of cardiac toxicity and DNA damage caused by Ehrlich solid tumors [32]. These findings contribute to the understanding of the global distribution and conservation of S. costus, emphasizing its presence in the Indian Himalayan region, traditional uses, and potential therapeutic properties [52,55]. These studies highlight the urgent need for conservation efforts to protect S. costus, a medicinal plant that is endangered and found in Himalayan biodiversity hotspots [56]. This aligns with the priority placed on conserving medicinal flora in this region. Research supports that medicinal plants, including S. costus, can be valuable sources for new drug discovery targets [57] and provide a thorough understanding of the conservation status, traditional uses, and therapeutic potential of S. costus, emphasizing the critical importance of conservation efforts to protect this endangered species. Fig. 1 depicts the global distribution and conservation status of S. costus, illustrating its status worldwide.
Fig. 1.
Detailed illustration of Indian Maps, specifically highlighting the regions where the high-altitude plant Saussurea costus can be found.Source:Adapted by the Authors under license(5706810004116).
2.3. Chemical composition and bioactive compounds of S. costus
Commercially important S. costus roots contain essential oils, sesquiterpenoids, and flavonoids, which contribute to their anti-cancer effects [58,59]. S. costus is a medicinally important traditional plant that grows at altitudes between 2500 and 4300 m in the Himalayan region, Cuba, Brazil, and Indonesia [60]. Different bioactivities, such as anti-cancer, antidiabetic, carminative, stimulant, and aromatic properties, have been reported owing to different plant chemical constituents [61]. The different bioactivities of S. costus are due to the presence of different bioactive constituents, such as volatile oils, alkaloids, flavonoids, di-O-β-D-glucosides of oleanolic acid, CO2-soluble extractives, synergizes, and gentiopicrosides [62]. S. costus roots are the most commercially exploited for their different bioactivities, followed by flowers, rhizomes, and whole herbs. Different constituents, such as α-costal, β-costal, nemophila-1 (10)-7(11)-diene, p-cymene, costunolide, α-pinene, α-caryophyllene, and an open-chain sesquiterpene have been reported in S. costus herbs, and their contributions to their bioactivities have been studied [63], the wide range of bioactive compounds in S. costus highlights its potential for medicinal use, warranting further pharmacological research to maximize its therapeutic benefits.
3. Current biological applications
3.1. Medicinal properties and pharmacology
Plant S. costus, a member of the Asteraceae family frequently used in medicinal applications, has garnered substantial attention from researchers because of its possible pharmacological and therapeutic benefits. Studies have shown that this plant displays antimicrobial, anti-inflammatory, anti-cancer, antioxidant, and antiviral properties [42,55]. Bioactive compounds, such as sesquiterpenes, lactones, flavonoids, and alkaloids, have medicinal properties [35,55]. Historically, S. costus has been used for various purposes, including management of thyroid disorders [3,36]. It has shown potential for the management of urolithiasis, diabetes, and candidiasis [12,35]. Plant extracts have also shown protective effects on thyroid tissues and thyroid hormone levels [15]. Briefly, S. costus has a broad spectrum of pharmaceutical properties and can potentially serve as a therapeutic solution for many medical conditions [64]. Thus, S. costus is a potent medicinal plant with diverse therapeutic applications.
The biological activities of S. costus have been extensively studied, revealing its diverse pharmacological potential. Studies have shown anti-inflammatory, hepatoprotective, anti-ulcer, and anti-cancer activities of the plant [42,65]. The potential of Sapium discolor S. costus as a natural bioactive source for combating pathogenic fungi has been demonstrated by its significant antifungal activity against both Candida albicans and non-albicans species [51]. S. costus root extract showed hepatoprotective activity in vivo by regulating various cellular cytokines and miRNAs, highlighting its potential to protect liver function [65]. Plant extracts have been used as mediators in the synthesis of iron nanoparticles, exhibiting their ability to combat microbes [42]. These findings support the traditional claims of S. costus in various medicinal systems and provide a scientific basis for its potential therapeutic applications [42]. Plant extracts have shown efficacy in inhibiting the viability of Echinococcus granulosus protoscoleces, demonstrating their potential for combating parasitic infections [50]. Furthermore, the crude root extract of S. costus exhibits various biological activities, including phytochemical constituents, antimicrobial activity, and antifeedant potential, highlighting its pharmacological significance [34]. Thus, studies have identified promising drug targets and therapeutic applications of S. costus.
The biological impact of S. costus has been recognized for specific compounds found in plants, such as lactonized oil and 12-methoxy dihydrocostunolide, which have shown potent effects [66]. These compounds have been found to exhibit hypotensive action through direct peripheral vasodilation and cardiac depression, indicating their potential role in cardiovascular regulation. Furthermore, the protective effects of different fractions of S. costus against experimentally induced bronchospasm by histamine and acetylcholine aerosols suggest a potential therapeutic effect on asthma, highlighting their significance in respiratory health [67]. Costunolide, derived from the roots of S. costus, inhibits aortic contractions in rabbits, specifically those induced by potassium chloride, suggesting a possible calcium antagonistic action. Similarly, dehydrocostuslactone exhibits inhibitory effects on the aorta, particularly against potassium chloride-induced contractions, suggesting its potential to modulate vascular tone [68]. Therefore, S. costus compounds can potentially be used to treat cardiovascular and respiratory conditions.
Based on the findings of Kim and Choi [69], the current study examined the effect of costunolide, a chemical obtained from the roots of S. costus. The study findings showed that costunolide inhibited the production and expression of proinflammatory mediators, particularly cytokines, which aligns with the observed reduction in interleukin-1 (IL-1) gene expression in LPS-stimulated RAW 264.7 cells. Costunolide's mechanism of action, as detailed by Kang et al. [70]. As shown by Liu et al., the influence of costunolide can be neutralized through pretreatment with sulfhydryl compounds, as per the outcomes presented [71]. According to Kang et al. [70], the function of AP-1 transcription factor and mitogen-activated protein kinase phosphorylation was hindered by costunolide, which aligns with the findings of Bruna et al., who emphasized the interaction of glucocorticoid receptors with AP-1 and the inhibition of the JNK pathway by glucocorticoids [72]. Hence, investigating the anti-inflammatory properties and therapeutic potential of costunolide provides a comprehensive understanding of its potential effects [70]. The results align with the findings of Kang et al., which showed a decrease in both iNOS protein and mRNA levels, consistent with the observed inhibition of inducible nitric oxide synthase (iNOS) by the root extract of S. costus [70]. Thus, S. costus exhibits anti-inflammatory and anti-arthritic properties under inflammatory conditions.
3.1.1. Anti-inflammatory activities of S. costus
Extensive research has highlighted the medicinal potential of S. costus, particularly its anti-inflammatory properties. Various laboratory and animal studies have demonstrated this phenomenon [55,64]. According to Mammate et al. [43], S. costus has various pharmacological properties, including anti-ulcer, anti-cancer, hepatoprotective, and anti-inflammatory effects. These results were consistent with the traditional medicinal uses of these plants. The antimicrobial activity of the plant against pathogens such as Candida, herpes, and SARS-CoV-2 further underscores its therapeutic versatility [55]. S. costus is a versatile medicinal plant with numerous health benefits.
The phytochemical composition of S. costus, particularly its sesquiterpene lactones, is a key contributor to its pharmacological activity [73]. Tag [74] and Idriss et al. [21] provided empirical support for the anti-inflammatory properties of the plant, with further corroborative findings from Yoo et al. [75] and Jia et al. [76], highlighting its anti-angiogenic and anti-nociceptive properties. The potential of S. costus as an antioxidant has been highlighted, particularly its ability to neutralize free radicals [77]. Thus, S. costus phytochemicals are key to their various medicinal properties. Pinedo-Guerrero et al. further advanced the understanding of S. costus 's anti-inflammatory potential through detailed studies on its methanol extract and isolated sesquiterpene lactones [78]. Their research showed significant inhibition of cytokine-induced neutrophil chemotactic factors and suppression of nitric oxide production in LPS-activated cells. Hence, S. costus has pharmacological effects and potential as an NF-kappa B inhibitor.
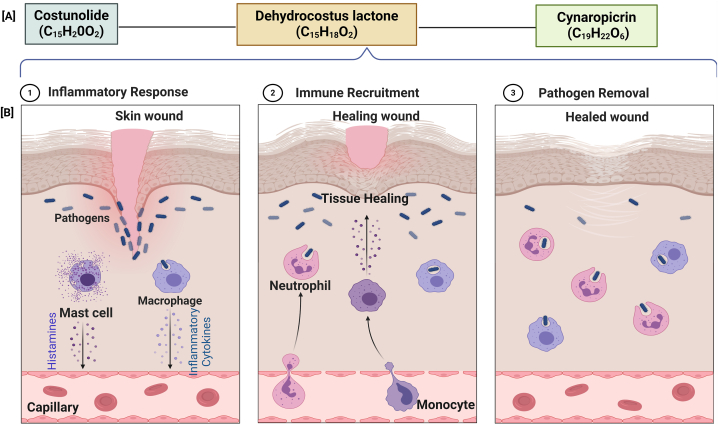
The ethanol extract of S. costus has shown promising results, particularly in inhibiting the production of tumor necrosis factor (TNF)-alpha in LPS-stimulated cells, consistent with its traditional use in Korean prescriptions for treating inflammatory disorders [78]. Kolahalam et al. provided insights into the botanical, chemical, and pharmacological aspects of S. costus, further emphasizing its relevance in inflammatory diseases [68]. Extensive research has been conducted on S. costus, including the studies by Cho et al. [79], Aldieri et al., [80], and Mathema [81], the anti-inflammatory properties and potential therapeutic applications of this substance have been demonstrated, as seen in sesquiterpene lactones such as cynaropicrin, which effectively suppress inflammatory mediators. However, conflicting findings regarding the exact process [64,[82], [83], [84], [85]] supported the importance of conducting additional research to gain a comprehensive understanding of the therapeutic potential of S. costus. The use of this plant in traditional medicine to treat inflammatory conditions has been supported by the anti-inflammatory and anti-arthritic properties demonstrated by Gokhale et al., which serve as a strong basis for future pharmacological research [77]. Fig. 2 illustrates the mechanisms by which natural bioactive compounds exert their anti-inflammatory activities in various scenarios, as previously explained.
Fig. 2.
Illustrates the stages of wound healing facilitated by natural compounds, detailing the inflammatory response, immune recruitment, and pathogen removal, which are mediated by specific chemical compounds: costunolide (C15H20O2), dehydrocostus lactone (C15H18O2), and cynaropicrin (C19H22O6). It shows a cascade from the initial pathogen invasion at the wound site, eliciting an inflammatory response from mast cells and macrophages, through the recruitment of immune cells such as monocytes and neutrophils, which aid in tissue regeneration, to the final healing stage with complete pathogen clearance and tissue restoration. The panels effectively depict the cellular activities and signaling involved, highlighting the dynamic interplay between immune responses and the molecular mechanisms of natural compounds in promoting wound healing.
3.1.2. Antioxidant activities of S. costus
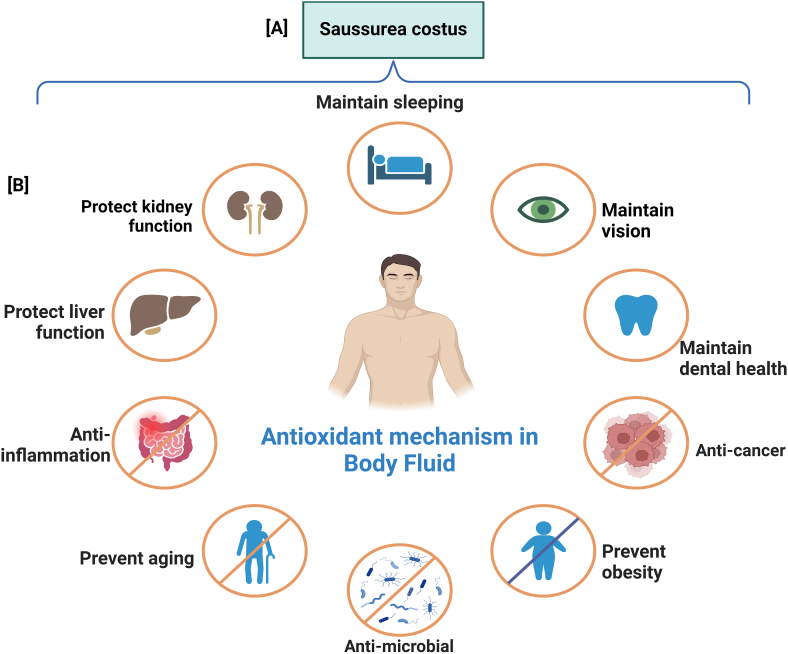
Medicinal applications of S. costus rely heavily on its antioxidant properties. S. costus is a plant rich in flavonoids, phenolic compounds, polyphenols, sesquiterpene lactones, and other active antioxidants that have been shown to have antioxidant properties in several studies [73,[86], [87], [88], [89]]. These antioxidants play a significant role in protecting the cells from oxidative stress, reducing lipid peroxidation, and promoting cellular health [3,90]. In addition to its antioxidant, S. costus exhibits anti-inflammatory, anti-ulcer, anti-cancer, hepatoprotective, and antimicrobial properties [21,60,91,92]. S. costus root extract contains flavonoids, steroids, chlorogenic acid, and other bioactive compounds that neutralize free radicals and reduce oxidative damage in the kidneys, liver, and thyroids [73,87]. Furthermore, S. costus has shown promise in protecting against toxicity caused by various substances such as diazinon, chloropyrofosethyl, and carbimazole, indicating its potential as a hepatoprotective and neuroprotective substance [3,73,88]. S. costus also alleviates blood biochemical derangements, thyroid tissue damage, and toxic-induced cardiac effects, further emphasizing its therapeutic potential [3,15]. Hence, owing to its antioxidant activity, S. costus is a valuable natural resource with diverse medicinal properties. Its biological impact makes it a strong contender for further studies and the development of therapeutic interventions. Fig. 3 illustrates the mechanisms of action by which antioxidants impact body fluids.
Fig. 3.
Shows the effects of antioxidants on body fluids and summarizes the multifaceted health benefits of Saussurea costus which enhances kidney and liver health and provides anti-inflammatory, anti-aging, and antimicrobial activities. Dehydrocostus lactone helps maintain healthy sleep patterns, offers antioxidant protection within body fluids, and exhibits anti-cancer properties. Cynaropicrin supports vision and dental health and contributes to obesity prevention. The therapeutic potential of each compound was visualized around a central human figure, highlighting its significant role in promoting overall human health across various systems and conditions.
3.1.3. Hepatoprotective of S. costus
The hepatoprotective effects of S. costus root extracts have been previously reported. This extract consists of sesquiterpene lactones, which have a range of pharmacological effects including hepatoprotection [73]. Research indicates that S. costus may exhibit powerful hepatoprotective properties by regulating the expression of cellular cytokines and miRNAs [65]. According to various reports, the antioxidant activity of root extracts can contribute to their hepatoprotective effects [12]. The effectiveness of S. costus in preventing liver damage has been examined in various induced liver toxicities, and the outcomes have shown its potential as an anti-hepatotoxic agent has been demonstrated [28]. The protective effect of the S. costus root extract on the liver can be ascribed to its diverse phytochemicals, including sesquiterpene lactones, flavonoids, and terpenoids. These compounds have potent hepatoprotective properties [3]. Bioactive compounds protect the liver from harmful effects and promote general well-being. Furthermore, the traditional use of S. costus in various forms of medicine has been validated in laboratory and animal model studies, confirming its hepatoprotective potential [64].Thus, S. costus root extract protects the liver from phytochemicals that affect cytokines, miRNAs, and oxidative stress, validating its traditional use.
Extensive research has been conducted on S. costus, particularly on its ability to protect the liver. El-Gizawy et al. demonstrated that the root extract of the plant exhibits hepatoprotective effects by regulating the levels of certain cellular cytokines, including miRNA-34a and miRNA-223 [14]. Researchers, including Mammate et al., have highlighted the antioxidant and anti-urolithiatic effects of both aqueous and ethanolic extracts of S. costus, providing additional evidence supporting its hepatoprotective properties [12]. In 2010, scientists examined the hepatoprotective properties of S. costus extract and demonstrated its ability to reduce hepatitis caused by D-galactosamine and lipopolysaccharide in mice [28]. Idriss et al. highlighted the potent hepatoprotective properties of this plant, validating its long-standing use in traditional medicinal practices [64]. Consequently, the sulfated polysaccharide S. costus defends the liver by exhibiting hepatoprotective properties.
The primary reason for the protective effects of S. costus on the liver is its active components, costunolide, and dehydrocostus lactone. These compounds have been shown to inhibit the expression of the hepatitis B surface antigen (HBsAg) in human liver cancer cells (Hep3B) without affecting cell viability [44]. Dehydrocostus lactone has been shown to exhibit anti-angiogenic properties by inhibiting the Akt/GSK-3β/cyclin D1 and mTOR signaling pathways, which are well-known mechanisms for suppressing angiogenesis [93]. Both costunolide and dehydrocostus lactone have been shown to trigger apoptosis and cell cycle arrest in various cancer cells, indicating their potential as anti-cancer agents [94]. The findings of this study align with the previously documented impact of dehydrocostus lactone, particularly its capacity to inhibit cell growth and initiate programmed cell death in laryngeal carcinoma cells [95]. Hence, owing to its potent components, S. costus can safeguard the liver and treat cancer.
Various scientific studies have explored the consequences of costunolide and dehydrocostus lactone use, and these results have been reinforced by a significant amount of research. Costunolide and dehydrocostus lactone possess hepatoprotective properties and prevent the degradation of the extracellular matrix. Together, these properties suggest that these compounds may relieve hepatoprotective reactions [69,93,[96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106]]. Costunolide and dehydrocostus lactone have been shown to possess hepatoprotective activities, which include reducing hepatitis caused by macrophages, suppressing allergic airway inflammation, and limiting the production of pro-inflammatory cytokines [102,103,105]. The inhibition of cell proliferation, tumor invasion, angiogenesis, and the induction of apoptosis in different cancer cells have demonstrated the anti-cancer activity of these compounds [104]. Numerous substances are recognized for their ability to combat microorganisms and protect against the detrimental effects of free radicals owing to their inherent hepatoprotective activities [101]. The broad spectrum of hepatoprotective effects associated with costunolide and dehydrocostus lactone make them promising candidates for further therapeutic exploration [69]. Therefore, costunolide and dehydrocostus lactone possess multiple therapeutic properties.
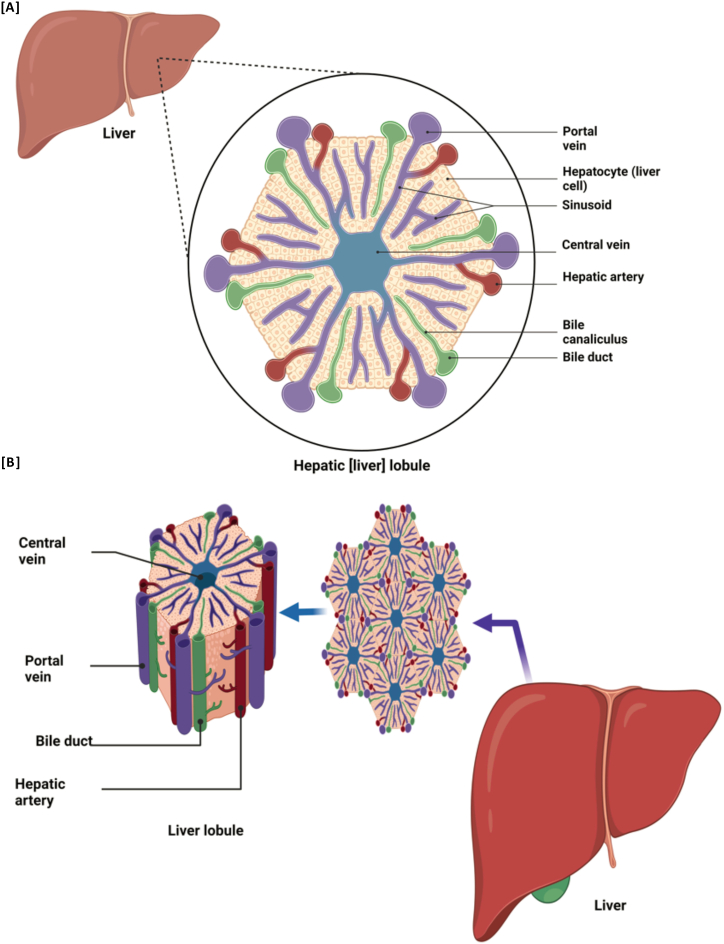
Several studies support the notion that costunolide and dehydrocostus lactone can decrease the levels of hepatitis B surface antigen (HBsAg) at the mRNA level, as reported by Idriss et al. [21]. They confirmed that both costunolide and dehydrocostus lactone significantly reduce HBsAg expression in human hepatoma cells, indicating their potential as anti-HBV therapeutic agents. Peña-Oyarzún et al. [107] reported that these substances hinder the Wnt/β-catenin pathway in colon cancer cells, indicating their potential ability to control cell cycle and programmed cell death (apoptosis). Jeong et al. demonstrated that costunolide can inhibit the VEGFR KDR/Flk-1 signaling pathway, suggesting its potential to modulate cellular signaling pathways [108]. Lee et al. showed that dehydrocostus lactone reduced the production of inducible nitric oxide synthase and TNF-α in macrophages activated by LPS, suggesting its potential anti-inflammatory effects [109]. Research has shown that costunolide and dehydrocostus lactone are potential anti-HBV drugs that can induce apoptosis and regulate cell cycle progression. Fig. 4 provides a detailed illustration of the horizontal and vertical structures of a hepatic liver lobule, delineating its cross-sectional and longitudinal anatomy.
Fig. 4.
Illustrates the hepatic lobule, a key structural and functional unit of the liver, detailing its cross-section and vertical structure. This highlights how the bioactive compounds costunolide, Dehydrocostus lactone, and cynaropicrin affect liver health. Costunolide and Dehydrocostus lactone promote hepatoprotection through anti-inflammatory and antioxidant activities, thereby reducing liver disease progression. Dehydrocostus lactone also has anti-cancer properties, which are critical for targeting liver cancer. Cynaropicrin supports liver health through its immunomodulatory and antimicrobial effects, which are essential for managing autoimmune disorders and preventing infection. Collectively, these compounds enhance the essential functions of the liver in metabolism, detoxification, protein synthesis, and bile production, offering significant therapeutic potential for liver health maintenance and disease intervention.
3.1.4. Anti-ulcer and cholagogic of S. costus
Extracts of S. costus containing costunolide have shown both anti-ulcer and anti-cholagogic effects in mice, validating their traditional anti-ulcer activity [13,29,30,57,110]. Scientists have shown that S. costus exhibits anti-inflammatory, anti-hepatotoxic, and immunomodulatory characteristics, suggesting its potential to combat ulcers [13,26,30]. The anti-hepatotoxic properties of the root extract of S. costus were further demonstrated, providing additional evidence of its potential to protect against liver damage, which is frequently linked to ulcer formation [30,57]. S. costus possesses antioxidant and anti-inflammatory properties relevant to its potential anti-ulcer effects [29,111]. Thus, the anti-ulcer properties of S. costus confirm its potential for ulcer treatment.
S. costus extract and its main component, costunolide, have been shown to stimulate the release of bile and suppress the formation of gastric ulcers in mice, particularly when restrained in water [112]. S. costus decoction has been shown to exhibit potential in the modulation of gastric function, as some can enhance gastric emptying and promote the release of endogenous motilin in patients with severe superficial gastritis [83]. The anti-ulcer properties of UL-409, which is rich in S. costus, have been documented, potentially by enhancing gastric cytoprotection and modulating protective mechanisms [24]. Amino acid-sesquiterpene adducts, particularly those found in the dried roots of Chinese S. costus, have shown anti-ulcer effects in rats with HCl/ethanol-induced lesions. Additionally, Saussurea mine A displays inhibitory activity against stress-induced ulcer formation in mice [113]. Thus, S. costus and its constituents may mitigate gastric ulcer symptoms and regulate gastric functions.
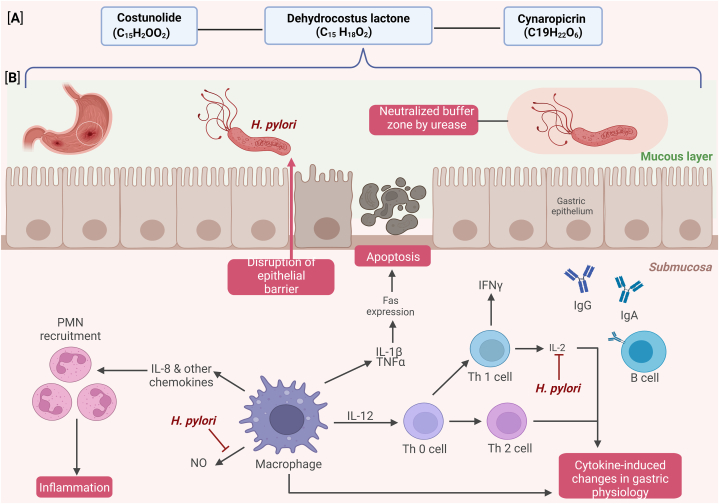
The synthesis of saussure amines and amino acid-sesquiterpene conjugates from the roots of S. costus involves a Michael-type reaction, in which amino acids are added to the α-methylene-γ-lactone portion of sesquiterpene [114]. Experiments with these substances have shown their capacity to prevent gastric mucosal damage caused by acidified ethanol in rats. Additionally, the ethanol extract of S. costus exhibited antibacterial activity against Helicobacter pylori in vitro, suggesting its potential use in the treatment of gastritis-like conditions (Fig. 5). This effect was observed against both standard and clinical strains of H. pylori, with a minimum inhibitory concentration of 40 μg/ml [114]. Thus, S. costus effectively treats gastric and bacterial infections, and provides therapeutic benefits.
Fig. 5.
Shows the interactions within the gastric epithelium during Helicobacter pylori infection and its role in ulcer formation. [A] presents the chemical structures and formulas of the bioactive compounds involved in inflammation: costunolide (C15H20O2), dehydrocostus lactone (C15H18O2), and cynaropicrin (C19H22O6). While [B] illustrates the pathogenesis sequence, H. pylori penetrates the mucous layer, alters the local pH via urease, and triggers an immune response. These include chemokine-mediated Polymorphonuclear neutrophils (PMNs) recruitment and cytokine release (IL-1β, TNFα, IL-12, and IFNγ) by activated macrophages and T-cells. These events lead to disrupted epithelial barriers and apoptosis mediated by Fas expression and cytokine signaling, whereas B cells produce specific antibodies (Immunoglobulin G (IgG) and Immunoglobulin A (IgA)).
The production of sesquiterpene conjugates frequently involves Michael-type addition reactions, which have been extensively documented in literature [115]. The synthesis of natural products such as sesquiterpenes has extensively employed reactions involving the addition of nucleophiles to conjugated systems [116]. Sesquiterpenes are often recognized for their wide range of biological effects, including their ability to inhibit cancer cell growth [117]. Thus, Saussureamines and α-methylene-γ-interlocution exhibited antibacterial and gastroprotective properties. They form sesquiterpene conjugates via Michael-type addition reactions. The process by which Helicobacter pylori contributes to ulcer formation is illustrated in Fig. 5, and the essential stages of this mechanism are highlighted.
3.1.5. Antiparasitic of S. costus
S. costus has shown significant cytotoxicity against the extracellular bloodstream form of Trypanosoma brucei, with a potent effect observed at concentrations between 0.2 and 0.5 mg/mL [118]. Additionally, it has demonstrated anti-trypanosomal effects against Trypanosoma brucei and Trypanosoma equinum [85]. Furthermore, S. costus extract can enhance growth and prevent apoptotic changes in macrophages infected with Trypanosoma cruzi [119]. In vitro, studies have indicated concentration-dependent activity of S. costus root extract against various parasites, including Herpetomonas Samuel Pessoa, Leishmania tarentolae, and Leptomonas Seymour, with minimum inhibitory concentration values of 50, 25, and 12.5 μg/mL respectively [33,120]. Notably, positive results were also observed for both amastigote and promastigote forms of Leishmania. In addition, S. costus has shown significant activity against parasites such as P. falciparum and T. cervi as well as against intestinal tapeworms, hookworms, earthworms, and roundworms [121,122]. S. costus demonstrated ovicidal and larvicidal activities against parasites like Fasciola hepatica, Schistosoma mansoni, Hymenolepis nana, Raillietina spiralis, Ascaris suum, and Setaira digitata [123]. These findings highlight the potential of S. costus as a highly effective and novel antiparasitic agent with proven safety and efficacy [124]. The root extract of S. costus, also known as C. surea, contains various active compounds including sesquiterpene terpenes, anthraquinones, alkaloids, and flavonoids [125]. These compounds possess various pharmacological effects, including antifungal, antidiabetic, anthelminthic, antitumor, anti-ulcer, immunomodulatory, anti-inflammatory, and anti-hepatotoxic properties [126]. Extensive research has supported traditional medicinal claims regarding the antiparasitic efficacy of S. costus, sparking interest in its potential therapeutic applications for combating parasitic infections [122]. Thus, scientific advancements have elucidated the mechanisms by which S. costus root extract acts against parasites, providing new insights into its therapeutic use.
The utilization of S. costus with resistance to microbes has received significant attention from researchers, particularly those that exhibit resistance to bacteria, Candida, and other viruses [85]. Various in vivo and in vitro investigations have been conducted to explore the potential gastroprotective, anthelmintic, parasiticidal, antimicrobial, larvicidal, antioxidative, and antiallergic properties of the fractions extracted from defatted spirals of Saussurea costus [123]. These investigations have revealed the harmful effects of Lactobacillus acidophilus, the causative agent of cryptosporidiosis, as well as moderate inhibition of highly resistant dengue virus and SARS-CoV-2 replication in preliminary studies [127]. With escalating resistance to conventional antiparasitic medications, herbal-based remedies are promising alternatives with fewer side effects [128]. S. costus, an ancient traditional Asian medicine, has been used in Indian, Chinese, and Arabian traditional medicine to treat various ailments [119]. The root of S. costus possesses a potent and long-lasting fragrance, and is the primary source of its distinct essential oil, comprising ketonic sesquiterpenes such as costus root oil, costus acid, costunolide, and dehydrocostus lactone [125,129]. S. costus root exhibits many biological properties, including anti-inflammatory, antimicrobial, antimalarial, antiviral, anti-cancer, antiparasitic, and antihyperglycemic effects, which can be harnessed to manage diverse metabolic disorders [122]. According to accumulated evidence, wisdom, and experiences of AITA, roots play a vital role in the prevention, diagnosis, and treatment of lifestyle-related chronic diseases according to the accumulated evidence, wisdom, and experiences of AITA [21,130]. S. costus is effective against Clonorchis sinensis and nematodes, making it a promising antiparasitic agent (Fig. 6). Based on the information provided, it can be inferred that the phytochemical makeup and diverse biological effects of S. costus offer valuable opportunities for further scientific investigation in this field. Fig. 7 illustrates the dominant bioactive compound in S. costus.
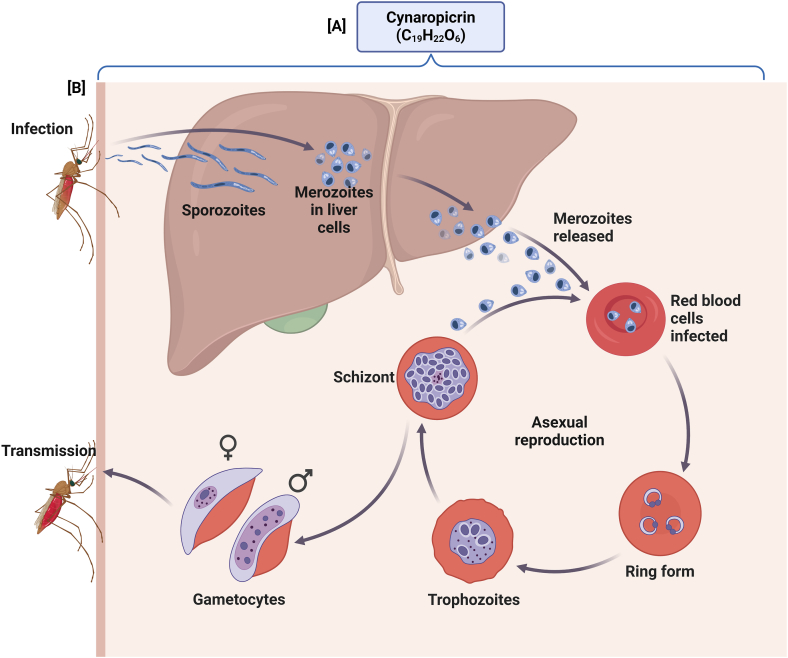
Fig. 6.
Depicts the lifecycle of the malaria parasite Plasmodium within the human host and its interaction with bioactive compounds. [A] shows the chemical structures and formulas of cynaropicrin (C19H22O6), suggesting potential targets for therapeutic interventions. where [B] depicts the sequential stages of Plasmodium infection and propagation: sporozoites transmitted by the mosquito enter the liver cells where they mature into merozoites. These merozoites are then released into the bloodstream, infecting red blood cells and progressing through several stages, including the ring form, trophozoites, and schizonts, before culminating in gametocyte production. These gametocytes are taken up by another mosquito to complete the transmission cycle.
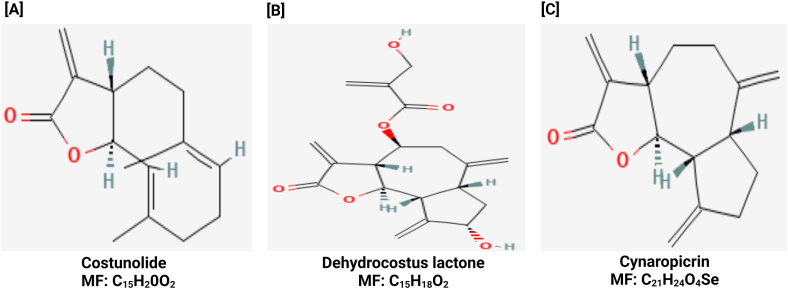
Fig. 7.
Depicts Saussurea costus, a plant species that is rich in bioactive molecules such as [A] costunolide, [B] dehydrocostus lactone, and [C] cynaropicrin.
3.1.6. Antimicrobial activities of S. costus
The antimicrobial properties of S. costus have been extensively investigated in several studies [34,35,51,64,131]. Studies have shown that S. costus prevents the spread and growth of microorganisms such as bacteria, fungi, and viruses. S. costus has also shown several beneficial biological effects, including anti-inflammatory, anti-trypanosomal, anti-cancer, and hepatoprotective impact [13,64]. These results suggest that S. costus may be a viable therapeutic option for treating various illnesses.
The study carried out by Yu et al. [132] explored the suppressive influence of the ethanol extract of S. costus on Streptococcus mutans, revealing substantial impairment of bacterial proliferation, acid secretion, attachment, and water-insoluble glucan synthesis. These results indicated that the ethanol extract of S. costus exerted a considerable inhibitory effect on Streptococcus mutans. The development and secretion of lactic acid by Streptococcus mutans was hindered, and its adherence to surfaces was reduced in a concentration-dependent manner by the extract, which was tested at levels ranging from 0.5 to 4 mg/mL. In particular, the extract displayed a concentration-dependent effect, with diminished growth and acid production at concentrations of 0.25–4 mg/mL and decreased adherence at concentrations of 0.25–4 mg/mL. The results of the water-insoluble glucan synthesis test revealed that when administered at concentrations ranging from 2 to 4 mg/mL, the extract effectively prevented the formation of water-insoluble glucans. These findings suggest that S. costus may be a promising therapy for oral health issues, such as bad breath, tooth decay, and gum disease, as it has been shown to limit the detrimental impact of Streptococcus mutans, which causes cavities [133]. Therefore, the S. costus extract is a promising solution for managing oral health issues.
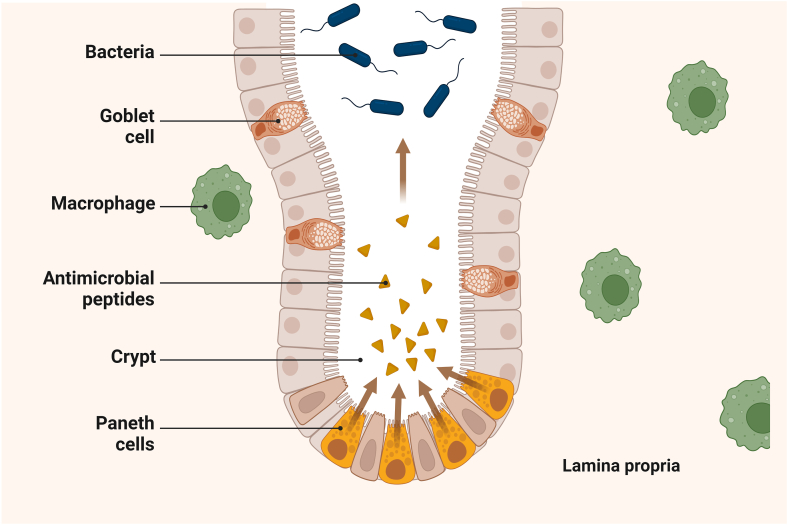
The antimicrobial properties of S. costus are attributed to its phytochemical composition. Various phytochemicals in S. costus, including terpenoids and lactones, have been identified and characterized [64]. They possess antimicrobial properties that contribute to the overall antimicrobial activity of S. costus. In addition, S. costus has been evaluated for antifungal activity against Candida species [51]. S. costus root extracts possess antifungal properties and are, therefore, promising therapeutic agents. Further research is needed to explain the molecular processes underlying the impact of the bioactive compounds found in the solid dispersions of S. costus. This can be achieved by investigating the release of antimicrobial peptides from Paneth cells, as illustrated in Fig. 8.
Fig. 8.
Shows the release of antimicrobial peptides by Paneth cells and the interaction of the bioactive compounds costunolide (C15H20O2), Dehydrocostus lactone (C15H18O2), and cynaropicrin (C19H22O6) with the immune components of the intestinal mucosa. This shows how these compounds influence cellular responses in the gut. Goblet cells produce mucus that captures bacteria, whereas macrophages and Paneth cells actively engage in the destruction of these pathogens. Positioned at the base of the intestinal crypts, Paneth cells release antimicrobial peptides that strengthen the mucosal barrier.
3.1.7. Hypoglycemic properties of S. costus
The efficacy of S. costus as a potent hypoglycemic therapy in obese patients with diabetes was evaluated through a comprehensive survey and clinical examination of plants from diverse regions of India. However, the cited sources do not directly support this assertion. Therefore, Bauza et al. [134], Kolahalam et al. [68] and Bheel et al. [54]did not recommend specific claims based on the efficacy of S. costus density in comprehensive clinical studies of post-tent hypoglycemic plants in India. The potential of S. costus in the treatment of obesity has been explored in several studies. However, these investigations do not explicitly support the assertion that S. costus is the most effective remedy for obesity, based on a comprehensive survey and clinical examination of potent hypoglycemic plants in India [43,135]. According to a study conducted by El-Gizawy et al. [65] the S. costus root extract exhibited in vivo hepatoprotective activity, suggesting its potential in modulating the expression of cellular cytokines and miRNAs, contributing to its hypoglycemic effect. Mujammami et al. [36] highlighted the flavonoid and antioxidant properties of S. costus, indicating their potential role in supporting its medicinal use, including hypoglycemic properties. Fekry et al. investigated the role of S. costus root extract in alleviating carbimazole-induced hypothyroidism, highlighting its potential in managing thyroid-related disorders via its hypoglycemic impact [3]. These studies support the traditional use of S. costus in diabetes management.
3.1.8. Anti-cancer properties of S. costus
S. costus has been extensively studied for its potential therapeutic and anti-cancer properties. Several studies have focused on exploring these effects. For example, Shati et al. [136] highlighted the anti-cancer activity of secondary metabolites from the roots of S. costus and demonstrated its potential as a promising anti-cancer agent. Similarly, Idriss [21] emphasized the strong anti-cancer properties of S. costus, suggesting its potential in cancer treatment. One of the key compounds found in S. costus roots is costunolide, which has been extensively studied for its ability to induce apoptosis in various cancer cell lines. Lee et al. [112] discovered that costunolide induces apoptosis by producing reactive oxygen species (ROS), leading to mitochondrial permeability transition and the release of cytochrome C. Rasul et al. [137] supported these findings by revealing that costunolide triggers apoptosis in bladder cancer cells through ROS production and mitochondrial disruption. Choi and Lee [138] found that costunolide activated c-Jun N-Terminal Kinase, leading to apoptosis in human leukemia cells. Other sesquiterpene lactones found in S. costus, such as dehydrocostus lactone, also exhibit significant anti-cancer properties [[139], [140], [141]]. Hua et al. [142] explained that dehydrocostuslactone causes G1/S phase arrest and initiates mitochondria-mediated apoptotic pathways in human lung squamous carcinoma cells. Hu et al. [143] dehydrocostuslactone inhibits osteoclastogenesis and osteoclast-induced bone loss, suggesting its potential benefits in cancer treatment. Hoesel and Schmid [144] reported that dehydrocostuslactone inhibits cell proliferation and promotes apoptosis in various cancer cells by targeting the PI3K/Akt signaling pathway. Lu et al. [145] demonstrated the ability of the compound to reduce osteoclastogenesis and osteoclast-mediated bone loss by modulating the NF-κB signaling pathway.
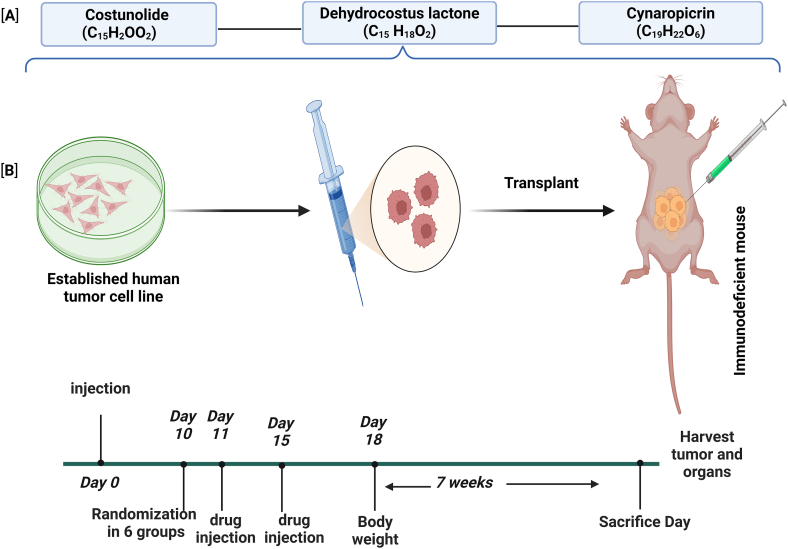
Cynaropicrin, another compound in S. costus, has also shown promising anti-cancer properties. Lau et al. [146]. found that cynaropicrin effectively inhibits the proliferation of leukocyte cancer cells and promotes apoptosis and cell cycle arrest. Zimmermann et al. [147] suggested that cynaropicrin exhibited cytotoxicity against various cell lines, indicating its potential as a cytotoxic compound. Cicco et al. [148]. explored the suppression of human melanoma growth by cynaropicrin, and highlighted its possible cytotoxic effects. Pulito et al. [149] further supported the cytotoxic effects of cynaropicrin by demonstrating its role in promoting apoptosis. Fig. 9 presents a proposed Cell Line-Derived Xenograft Model (CDX) incorporating S. costus-purified bioactive compounds.
Fig. 9.
outlines an experimental protocol used to assess the efficacy of the bioactive compounds costunolide (C15H20O2), dehydrocostus lactone (C15H18O2), and cynaropicrin (C19H22O6) in tumor suppression using an immunodeficient mouse model. [A] shows the chemical structures of these compounds. [B] depicts the experimental flow: established human tumor cell lines were cultured and then injected into immunodeficient mice. The treatment timeline showed drug injections on days 10, 11, and 15, with body weight measurements extending over 7 weeks. The study concluded with the harvesting of tumors and organs on the day of sacrifice.
3.1.9. Antifeedant of S. costus
The rhizomes of S. costus exhibited significant repellent qualities against the red flour beetle Tribolium castaneum (Herbst). They also showed antifeedant effects against the lesser grain borer, Rhyzopertha dominica (F.) [83]. The results of scientific research have shown that the essential oil derived from S. costus can repel adult insects of Tribolium castaneum and suppress larval development [21]. The effectiveness of the essential oil of S. costus and its separate elements as natural larvicides against the larvae of Aedes albopictus has been proven [45]. These findings emphasize the potential of Saussurea species in pest management, particularly for controlling stored-product insects and mosquitoes.
Moreover, monoterpenoid odors repel Tribolium castaneum and Rhyzopertha dominica, highlighting the potential of these natural compounds in pest control [150]. Plant sources have been studied for their insecticidal and repellent activities against the pest Tribolium castaneum, revealing their potential for pest control. Essential oils are among the natural products studied for their insecticidal and repellent activities against pest [[151], [152], [153], [154]]. Thus, monoterpenoid odors and essential oils can combat pests such as Tribolium castaneum and Rhyzopertha dominica.
Numerous studies have investigated the ability of certain substances to inhibit the growth of microorganisms, focusing on limiting the emergence of adults. Among these compounds, S. costus showed impressive repellent properties at 0.1 % and 0.5 % concentrations. After 24 h, it achieved 85.25 % and 85.71 % repellency, respectively, and maintained these levels at 85.22 % and 88.10 % after 48 h [155]. In contrast, adult emergence remained unaffected when the growth-inhibitory effects of S. costus were tested. This study explored the repellent properties of S. costus against the red flour beetle Tribolium castaneum and its capacity to deter feeding by the lesser grain borer Rhyzopertha dominica.
The potential of natural products, particularly S. costus essential oils, for pest control has been extensively studied [156]. The insecticidal and repellent properties of essential oil against Tribolium castaneum demonstrate their potential in pest management. Additionally, monoterpenoid odors from Tribolium castaneum and Rhyzopertha dominica have shown promising results in repelling pests [157]. Essential oils derived from plants have been explored for their ability to repel and kill T. castaneum, demonstrating the versatile applications of natural substances in pest management [21]. S. costus, a plant with therapeutic properties, has been used in traditional medicine to address various health concerns such as asthma and inflammation [158]. Thus, S. costus essential oils and other plant-derived oils have the potential to manage pests, offering a natural alternative to synthetic insecticides.
3.1.10. Imunomodulator of S. costus
Plant S. costus from the Asteraceae family has immunomodulatory impacts recognized in traditional Saudi Arabian medicine [36]. Bioactive compounds found in the roots of S. costus plants, including alkaloids, flavonoids, phenols, and terpenoids, are abundant. The primary components of essential oils derived from these plants include costunolide and dehydrocostus lactone [159]. These compounds have been associated with anti-inflammatory, anti-ulcer, anti-cancer, and hepatoprotective properties [160]. S. costus has shown significant anti-cancer properties in various cell lines, demonstrating its potential as an anti-cancer agent [48]. S. costus has been proposed as a potential therapeutic option for COVID-19 owing to its ability to regulate the immune system [161]. The roots of S. costus have been widely used for healing throughout history, indicating a long-standing recognition of their safety and effectiveness [161]. The standard dose of S. costus has been reported to be 3 g/day, further emphasizing its potential therapeutic use [162]. Immunomodulation has been observed in S. costus owing to its bioactive constituents. For example, vitamin D, which plays a major role in immunomodulation, has been found to have receptors present in various immune-competent cells, suggesting its involvement in modulating immune responses [163]. Thus, S. costus has immunomodulatory effects and potential therapeutic uses in COVID-19 and cancer.
The study conducted by Насухова et al. provides valuable information concerning costunolide and dehydrocostuslactone in laurel leaves, which has garnered attention because of their potential biological activity [164]. The study conducted by Secchi et al. [165] was unsuitable, as it explored the signaling response to redox stress in T-cells and the function of Syk kinase, IL2 receptor, and L-selectin, but did not specifically address the inhibitory effects of costunolide and dehydrocostus lactone on cytotoxic T-lymphocyte-killing activity.
A study conducted by Pachiyappan et al. primarily focused on the synthesis and analysis of MgO nanoparticles while simultaneously exploring their potential use in the photocatalytic degradation of dyes [67]. This research explores the synthesis and characteristics of magnesium oxide nanoparticles without analyzing the structure-activity relationship of Mokko lactone, dehydrocostus lactone, and specific guaianolides. Consequently, the findings of Pachiyappan et al. [67] were irrelevant because their information is not crucial for understanding the relationship between the structure of guaianolides and their ability to inhibit CTL function and induce ICAM-1 expression.
3.1.11. Central nervis system depressant and S. costus
The impact of S. costus as a depressant of the Central Nervous System has been studied. Studies have shown that compounds from S. costus, such as dehydrocostus lactone and costunolide, depress the central nervous system by increasing sleep duration when induced by hexobarbital, thereby reducing nociception and spontaneous locomotion [166]. In particular, costunolide has been highlighted as an active ingredient that contributes to these effects [167]. S. costus also has anti-inflammatory properties owing to its sesquiterpene-lactone fractions, which stabilize lysosomal membranes and inhibit cell proliferation [168]. S. costus also contains complement-inhibitor substances that may be useful for treating diseases related to complement activation, such as respiratory distress [23]. Moreover, the plant possesses antioxidant properties and can prevent and treat many ailments, including cancer, diabetes, and hemorrhoids [60,169]. According to previous studies, S. costus may also have therapeutic potential against conditions such as COVID-19 [60]. Its roots, which contain alkaloids, flavonoids, terpenoids, and sesquiterpenoids, have been studied for their therapeutic and synergistic effects against leukemia [159]. S. costus exhibits antifungal and hepatoprotective properties [169,170]. Therefore, S. costus has many biological applications, including central nervous system depression. This finding supports the claim that costus oil, which is rich in sesquiterpene lactones, handles numerous cases of allergic contact dermatitis.
3.1.12. Bronchitis and S. costus
S. costus, commonly referred to as costus or kuth, has been extensively used in Ayurvedic medicine to treat respiratory issues, particularly bronchitis, for a long time. Some studies have suggested that S. costus possesses biological properties that may be beneficial for the management of bronchitis. For instance, Yu et al. demonstrated the significant impact of biological pesticides on bacterial and fungal activity, indicating the potential biological effects of substances such as S. costus in combating respiratory infections [171]. Middleton et al. emphasized the significance of the One Health approach in managing disease outbreaks, emphasizing the deep interconnectedness between human, animal, and environmental health [172]. This interconnectedness underscores the potential relevance of botanical interventions, such as S. costus, in managing diseases, such as bronchitis, which can have zoonotic implications.
Several studies have explored the biological effects of S. costus on bronchitis and asthma. For instance, Sclare et al. investigated the impact of various S. costus extracts on chronic bronchitis and asthma [173]. The alkaloid fraction exhibited potent spasmolytic and antispasmodic properties in guinea pig smooth and tracheal muscles and perfused lungs in response to histamine stimulation. These findings suggest a possible therapeutic benefit of S. costus for the treatment of bronchitis and asthma. Kim and Choi. discussed the diverse therapeutic potential of costunolide, a bioactive sesquiterpene-lactone found in S. costus [69]. A study by Karim et al. [36] emphasized the anti-inflammatory, antioxidant, and antineoplastic properties of compounds found in S. costus, indicating their potential to impact respiratory conditions such as bronchitis and asthma. Furthermore, we investigated the in vitro anticandidal activity of the secondary metabolites extracted from the roots of S. costus. Therefore, S. costus roots show potential against Candida, with broad-spectrum activity against respiratory infections.
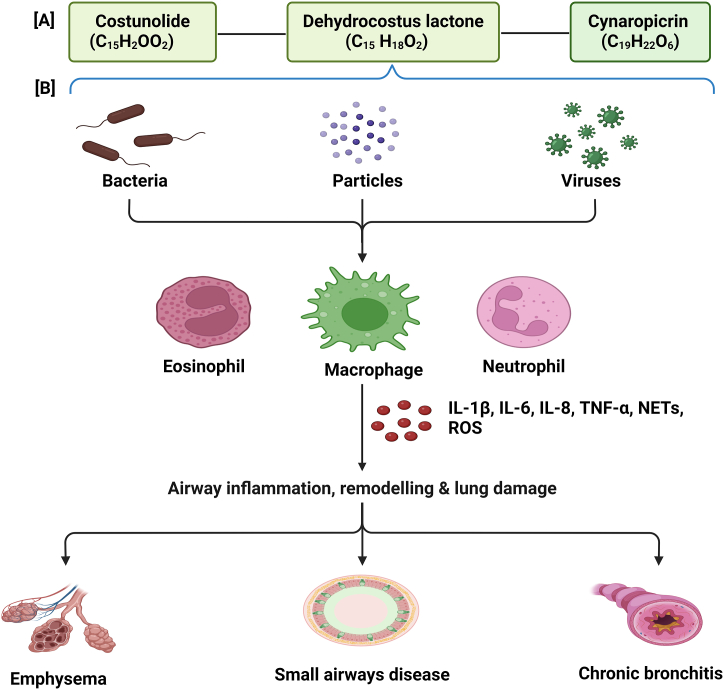
The study performed by Sastry and Dutta [174] used Tincture Saussurea (TS) with petroleum ether extract and TS made from defatted roots, aligned with the findings of Abdallah et al. [34] and Karim et al. (2022b), as demonstrated by the assessment of the biological properties of the crude root extract [34]. The antifungal properties of the secondary metabolites extracted from S. costus roots were examined in a laboratory setting [35]. Research indicates that S. costus extracts can be used to manage chronic bronchitis and asthma, a finding supported by initial trials showing the efficacy of the medication in addressing these conditions. El-Gizawy et al. highlighted the potential therapeutic benefits of S. costus extract, which extend beyond respiratory conditions [65]. Development of chronic obstructive pulmonary disease Fig. 10.
Fig. 10.
depicts the interaction of bioactive compoundsd, such as costunolidee (C15H20O2) dehydrocostuss-lactonee (C15H18O2), and cynaropicrinn (C19H22O6) with immune responses to pathogens in the respiratory system, which contributese to the development of COPD. [A] shows the chemical structures of the compounds. [B] illustrates the initiation of an immune response to different pathogens (bacteria, particles, and viruses) including eosinophils, macrophages, and neutrophils. These cells release inflammatory mediators, such as IL-1β, IL-6, IL-8, TNF-α, neutrophil extracellular traps (NETs), and reactive oxygen species (ROS), leading to airway inflammation, remodeling, and subsequent lung damage, which are critical processes in the pathogenesis of COPD. This figure highlights the potential therapeutic roles of these bioactive compounds in modulating detrimental immune responses.
3.2. Nutritional value and other uses S. costus
S. costus, also known as costus or S. costus, has traditionally been used in various forms of medicine to treat a range of diseases [57,126]. This plant exhibits diverse biological and therapeutic effects. Studies have shown that this plant possesses anti-inflammatory, anti-trypanosomal, anti-cancer, antibacterial, antifungal, and antiviral properties [64]. It also exhibits antioxidant and hepatoprotective impacts [65,175]. Studies have shown that S. costus exhibits anti-ulcer, anti-cancer, and anti-urolithiatic properties [175]. This plant's potential application in treating thyroid disorders has been explored.
S. costus is clinically relevant for the treatment of thyroid conditions and for the maintenance of thyroid hormone levels in tissues [15,36]. The ameliorative effects of S. costus have been demonstrated in thyroid tissues under the negative impact of hypothyroidism caused by carbimazole [3]. The potential of S. costus to exhibit antimicrobial properties was examined, and it was found to possess in vitro anti-cancer activity, particularly against Candida species [35]. Their antimicrobial activity and antifeedant potential were also evaluated [34]. Thus, S. costus has therapeutic potential in thyroid conditions, antimicrobial benefits, and anti-cancer properties.
S. costus, commonly known as Qustal Hindi or S. costus, is a medicinal plant used in traditional medicine to address various health issues [36]. It has numerous pharmaceutical characteristics, including anti-inflammatory, anti-ulcer, hepatoprotective, and antioxidant properties [64,175]. S. costus roots have effectively treated thyroid disorders such as hypothyroidism and have demonstrated clinical importance in thyroid treatment [36]. S. costus has shown a variety of biological and therapeutic effects, including antimicrobial, antifungal, and antiviral effects against Candida spp [35], therapeutic effects by reducing cholesterol levels [29], and protective effects against kidney toxicity [57]. The medicinal properties of plants are attributed to their bioactive compounds, including flavonoids, terpenoids, alkaloids, and lactones [21]. S. costus has been conventionally employed in various medical contexts and has shown promise in managing thyroid ailments [35]. Although notable advancements have been achieved in the medical field of S. costus, additional research is necessary to fully understand its underlying mechanisms and potential applications. S. costus presents a range of therapeutic possibilities and holds promise for further investigation in natural medicine.
4. Chemical constituents of S. Costus
4.1. Leading bioactive compounds
S. costus, also known as costus or S. costus, has been traditionally used in various forms of medicine owing to its medicinal properties [67]. Several studies have investigated bioactive compounds in S. costus and their potential therapeutic applications. Karim et al. found that S. costus contains terpenoid and alkaloid compounds that effectively control Candida species, making it a promising candidate for antifungal treatment [35]. For example, Salooja et al. [176] isolated a major constituent, S. costus Aussurea lactone (C15H2O2), from the roots of S. costus and costunolide from costus root oil of S. costus (Rao and Verma, 1951; Rao et al., 1960). Several compounds have been identified in S. costus [42]. These chemicals included costunolide, dehydrocostuslactone, costic acid, palmitic acid, linoleic acid, β-sitosterol, β-cyclocostunolide, alantolactone, β-cyclocostunolide, isoalantolactone, isodehydrocostus-lactone, iso-zaluzanin C, and guiainolides. These chemicals have been used to produce derivatives such as α-, β-, and γ-cyclocostunolides, with β-cyclocostunolide being the most abundant product resulting from the transformation of costunolide [177]. These compounds are derived from naturally occurring costunolides [178]. Their identification was based on NMR spectra, GC-MS, and comparisons with literature [179]. Furthermore, the biosynthesis of these compounds involves the P450 enzyme CYP71DD6 [180]. Therefore, the diverse pharmacological compounds in S. costus validate its traditional use and underscore its therapeutic potential, warranting further detailed research.
The scientific literature references a variety of compounds, including 12-methoxy dihydro dehydrocostuslactone, 4-methoxydehydrocostus lactone, saussure aldehyde, isodehydrocostus-lactone-15-aldehyde, sesquiterpene lactones, 11,13-epoxydehydrocostus-lactone, 11,13-epoxy-isozazulanin C, 11,13-epoxydehydroiso-zaluzamine, 11,13-epoxy-3-keto dehydro-costus–lactone, cynaropicrin, reynosin, santamarine, 6,10-dimethyl-9-methyleneunused-S-E-en-2-one, (+)-germacrene A, germacra-1(10),4,11(13)-trien-12-al, germacra-1(10), 4,11(13)-trien-12-al, and germacra-1 (10), 4,11(13)-trine-12-oic acid. These compounds are part of the sesquiterpene biosynthetic pathway and exhibit anti-cancer properties. These compounds were identified and produced in previous studies [40,45,[181], [182], [183], [184]]. These compounds are important in a range of biological processes and exhibit promising prospects for medical and biotechnological applications.
The root oil of S. costus pictus is abundant in 22-dihydrostigmasterol and sesquiterpenoids, including saussureal, steroids, pregnenolone, -sitosterol, daucosterol, a phenylpropanoid glycoside, syrine, a lignan glycoside, 1-hydroxypinoresinol-1—d-glucopyranoside, 12-octadecadienoic acid, (Z,Z)-9,12-octadienoic acid-2-hydroxy-1,3-propanedinyl ester, and seven non-cytotoxic compounds, two of which are unique sesquiterpenes: a guaianolide-type compound with a C17 skeleton, a lappalone, and 1,6-dihydroxycostic acid ethyl ester [156]. Thus, the rich and varied composition of S. costus pictus root oil underscores its therapeutic potential, inviting further pharmacological study.
Jung et al. [185] carried out a study investigated the cytotoxic compounds present in the roots of S. costus, known as shikokiols. During their investigation, the researchers isolated five new amino acid-sesquiterpene adducts and identified them as Saussureamines A, B, C, D, and E, as well as a lignan glycoside called (−)-massoniresinol 4∗∗-O—d-glucopyranoside [186]. Matsuda et al. [187] subsequently synthesized these compounds. The production of dihydrocostunolide requires farnesyl pyrophosphate (FPP) as the precursor, which ultimately results in the formation of dihydrocostunolide [183]. Additionally, multifunctional triterpene synthases contribute to the simultaneous presence of ursane- and oleanane-type triterpenes in plants [188]. In addition, dihydrocostunolide occurs naturally [189]. The transformation of costunolide into dihydrocostunolide involves multiple enzymes [183]. These findings provide evidence for the synthesis and natural occurrence of dihydrocostunolide. Two sesquiterpene lactones, santamarine and reynosin, were isolated from the roots of S. costus by HPTLC and HPLC analysis. Their molecular structures and arrangements were determined using spectroscopic methods [190]. According to Singh and Sitaramaiah [191], various techniques can be used to extract oil from S. costus root. Similarly, Bose et al. [192] conducted a chemical analysis of S. costus and found that it contains reducing sugars, tannins, resins, essential oils (1.39 %), and alkaloids (0.05 %). Researchers, including Namboodiripad [193], have successfully isolated two chemical compounds from costus leaves: taraxasterol and taraxasterol acetate. Omer et al. [194] extracted the active compounds costunolide and dehydrocostus lactone from the methanolic portion of crude drug S. costus. On the other hand, Viswanathan and Kulkarni [195]reported that S. costus (Kuth) is a new source of inulin.
The roots of S. costus have been found to contain chlorogenic acid and saussurine, as determined using HPTLC and HPLC, respectively [20]. Two novel compounds, 13-sulfo-dihydrosantamarine and 13-sulfo-dihydroreynosin, were isolated from the roots and their chemical structures and unique arrangements of atoms were identified using advanced analytical techniques [196]. Oil extraction from roots can be achieved using various methods, as reported by Dawood et al. [197]. Bose et al. [192] conducted a chemical analysis of S. costus and discovered reducing sugars, tannins, resins, essential oils (1.39 %), and alkaloids (0.05 %). Researchers, including Namboodiripad, have successfully isolated taraxasterol and taraxasterol acetate from costus leaves [193]. A team, including Chaturvedi, discovered the pharmacologically active compounds costunolide and dehydrocostus lactone in the methanolic extract of the crude drug S. costus [198]. In conclusion, Viswanathan and Kulkarni's [195] research disclosed a new source of inulin, S. costus (Kuth).
Another study demonstrated the anti-inflammatory, anti-cancer, antibacterial, antifungal, and antiviral effects of S. costus, lending credence to its traditional medicinal use [21]. The antioxidant, anti-ulcer, and hepatoprotective effects of S. costus have been well-established [12]. The bioactive ingredients of S. costus include terpenes, alkaloids, flavonoids, and anthraquinones [3]. These compounds contribute to the pharmacological effects of plants and may explain their wide range of therapeutic uses. S. costus has shown potential for the treatment of COVID-19, with some studies suggesting that plant roots can be used for healing purposes and may play a role in the treatment of the disease [161]. The use of bioactive compounds in S. costus has been shown to offer a range of therapeutic benefits, including anti-inflammatory, anti-cancer, and potential treatments for COVID-19.
The in vitro anticandidal activity of S. costus has been demonstrated to be significant against various Candida species, such as Candida albicans and non-albicans species [35,51]. S. costus exhibits antifungal properties against both Candida albicans and non-C. Candida albicans species [51]. S. costus displays antiviral properties against SARS-CoV-2's main protease and other viruses [21]. S. costus also exposes anti-inflammatory influences [51]. The following sentence indicates that the substance in question exhibits potent anti-cancer properties and exerts cytostatic effects by regulating cyclins and pro-apoptotic molecules [125]. Current evidence indicates that S. costus exhibits various pharmacological properties, including antimicrobial, antiviral, anti-inflammatory, and anti-cancer effects. Several studies have shown its potential to combat fungal infections, reduce inflammation, treat cancer, combat bacterial infections, and inhibit viral replication, among other effects, as an antioxidant, anti-ulcer, and hepatoprotective agent [34]. Hence, S. costus's wide-ranging pharmacological effects from antimicrobial to anticancer highlight its therapeutic potential, emphasizing the need for further research.
4.2. Extraction and analysis methods of bioactive compounds in S. costus
S. costus, also known as costus, has been the subject of extensive research because of its numerous biological effects and potential therapeutic benefits. Various studies have investigated the phytochemical makeup and bioactivity of S. costus extract. One such study by Mammate et al. focused on the antioxidant and anti-urolithiatic properties of the aqueous and ethanolic extracts of S. costus [12]. The ethanolic extract displayed the greatest antioxidant capacity, as determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) technique, whereas the aqueous extract showed considerable reducing power, as assessed using the ferric reducing ability of plasma (FRAP) method. In another study, Idriss and colleagues [21]. This analysis aimed to differentiate between S. costus phytochemicals obtained through a water extraction process at room temperature.
The researchers employed a green extraction method to identify various phytochemicals in S. costus extract. Idriss et al. comprehensively reviewed this plant, emphasizing its botanical, chemical, and pharmacological properties [21]. This review identifies several compounds in S. costus, including lactones, sesquiterpenes, and fatty acids. Elshabrawy et al. examined the potential therapeutic and collaborative benefits of S. costus extract in treating leukemia in rats [159]. This study aimed to investigate using S. costus as a natural chemotherapeutic agent. The investigation conducted by Karim et al. assessed the in vitro antifungal activity of secondary compounds from the roots of S. costus [35]. The research showed that the isolated compounds exhibited considerable anti-Candida activities against various Candida species. Albrahim et al. [42] explored the antimicrobial properties of iron-oxide nanoparticles synthesized using an aqueous extract of S. costus.
The potential protective effects of S. costus root extract on the thyroid gland in carbimazole-induced hypothyroidism have been investigated [3]. El-Gizawy et al. [65] analyzed the compounds present in the extract of S. costus roots and evaluated their hepatoprotective effects. Al-obaidy and Esmaeel. explored how S. costus extracts affect the viability of Echinococcus granulosus protoscoleces [50]. Thus, the ethanolic extract of S. costus displays strong efficacy against these parasites.
Farani et al. recently conducted a study that explored the formation of magnesium oxide nanoparticles using S. costus plant roots and evaluated their antimicrobial and antifungal properties [199]. Researchers have also investigated the possibility of using S. costus to create nanoparticles with photocatalytic characteristics. S. costus, also known as costus, is a medicinal plant that has been widely researched for its numerous properties and potential applications [200]. Several studies have explored the antioxidant and anti-urolithiatic activities of [43]. The ethanolic extract of S. costus had the highest IC50 value in the DPPH assay, indicating strong antioxidant activity. The aqueous extract of S. costus exhibited significant reducing power in an FRAP assay [12]. A phytochemical analysis of S. costus extracted with water at room temperature was also performed [21]. This green extraction procedure allows the identification of phytochemicals present in plants. The potential ameliorative role of S. costus in carbimazole-induced hypothyroidism has been previously investigated [3]. This study aimed to determine whether S. costus root extract could reduce the harmful toxic changes in the thyroid gland caused by carbimazole.
Elgharabawy et al. reported that S. costus is a medicinally important plant with a range of therapeutic properties [201]. The roots of this plant have been studied for various potential medicinal uses, and it is one of the few species of Saussurea for which propagation and cultivation techniques have been documented [17]. This critical information is dependent on the conservation and sustainable use of this endangered species. Soliman et al. [51] examined the antifungal activity of S. costus root extracts against Candida albicans and non-albicans species. Therefore, S. costus is a valuable botanical resource with diverse therapeutic properties.
Investigation of antifungal properties of polar and non-polar crude extracts. This plant has previously been studied using botanical, chemical, and pharmacological methods [21]. This review provides a comprehensive overview of plant characteristics, chemical makeup, and therapeutic potential. S. costus is known for its therapeutic properties that are attributed to its flavonoid content. This plant has been studied for its endophytic fungal species and antibacterial activities [202]. Parts of the plants, including their roots, stems, and leaves, were utilized to study endophytic fungal species. Moreover, the traditional use of this plant as a treatment for thyroid-related problems in Saudi Arabia by Mujammami [36] further validated its potential as a therapeutic agent.
In addition, Albrahim et al. demonstrated the potential of S. costus extract as a biomediator for the synthesis of iron-oxide nanoparticles with antimicrobial properties [42]. Using S. costus as a natural antimicrobial source is a viable option. The medicinal plant S. costus has been extensively studied for its antioxidant, anti-urolithiatic, ameliorative, antifungal, antibacterial, and antimicrobial properties. Plant phytochemicals have therapeutic effects and are used to treat thyroid-related conditions in traditional medicine. Additional research is needed to comprehensively understand the uses and operational processes of S. costus extract.
In summary, S. costus possesses antioxidant, anticandidal, antimicrobial, hepatoprotective, and anti-cancer properties. The phytochemical composition of the S. costus extract includes lactones, sesquiterpenes, fatty acids, and other bioactive compounds. These findings support the potential therapeutic applications of S. costus in various fields, including medicine and nanotechnology.
5. Potential future applications
S. costus has gained attention in various studies owing to its potential applications. According to Idriss et al., S. costus has anti-inflammatory, anti-trypanosomal, anti-cancer, antibacterial, antifungal, and antiviral effects, which supports its use in the medical field [21]. Soliman et al. further examined the antifungal properties of S. costus root extracts against Candida albicans and non-albicans species, underscoring their promising medicinal potential [51]. The effectiveness of S. costus in the treatment of specific diseases has been explored in several studies. According to a survey and clinical study conducted by Elgharabawy et al. in India, S. costus has emerged as the most effective plant for obese diabetic patients [201]. Mujammami et al. [36] also highlighted the clinical significance of S. costus in the treatment of thyroid disorders, emphasizing its use as a traditional medicine.
S. costus has been studied not only for its medicinal properties but also for its antimicrobial activity. Ibrahim [43] explored the antimicrobial potential of S. costus extract in synthesizing iron-oxide nanoparticles with promising results. Al-obaidy and Esmaeel conducted a study to evaluate the effectiveness of S. costus extracts in maintaining the vitality of Echinococcus granulosus protoscoleces, demonstrating its potential use as an antimicrobial agent [50]. Previous studies investigated the phytochemical composition of S. costus. Idriss et al. [21] reviewed the botanical, chemical, and pharmacological aspects of S. costus and identified sesquiterpenes and lactones as its significant constituents.
Recent studies of S. costus have shown that this herb has many biological effects and therapeutic potential. Various laboratory and animal model experiments have uncovered its anti-inflammatory, anti-trypanosomal, anti-cancer, antibacterial, antifungal, and antiviral properties [64]. The hepatoprotective properties of S. costus have been observed along with its ability to regulate the expression of cellular cytokines and miRNAs such as miRNA-34a and miRNA-223 [65]. It also exhibits antioxidant, anti-ulcer, and anti-cancer effects [12]. S. costus has traditionally been used in various forms of medicine, as supported by pharmacological experiments [175]. The Asteraceae family includes S. costus, found in India, Pakistan, and certain regions of the Himalayas [42]. The Himalayas are home to this species, which is limited in its geographical range and is unfortunately facing critical endangerment [203]. Thus, S. costus offers diverse therapeutic potentials and requires urgent conservation efforts.
Studies on S. costus have shown that this herb has many biological effects and therapeutic potential. Recent research and preliminary findings have revealed its anti-inflammatory, anti-trypanosomal, anti-cancer, antibacterial, antifungal, and antiviral properties in laboratory and animal model experiments [21]. S. costus exhibits hepatoprotective properties and alters the levels of cellular cytokines and microRNAs, including miRNA-34a and miRNA-223 [65]. The following sentence shows the antioxidant qualities and exhibits anti-ulcer and anti-cancer impacts [12]. Traditional use of S. costus has been demonstrated through pharmacological experiments in various forms of medicine [12]. The plant species S. costus is a member of the Asteraceae family and is native to regions including India, Pakistan, and certain areas of the Himalayas [42]. The species in question is typically found in specific regions of the Himalayas and currently faces a critical threat of extinction [200]. Consequently, owing to its significant endangerment, S. costus has therapeutic value and requires immediate conservation.
The therapeutic potential of S. costus as a medicinal plant has been demonstrated through recent research and initial findings showing its anti-inflammatory, anti-cancer, antifungal, antiviral, and antimicrobial properties. Its effectiveness in treating specific diseases such as obesity and thyroid disorders has also been explored. The phytochemical composition of S. costus, including sesquiterpenes and lactones, has been identified as contributes to its therapeutic effects. Further research is necessary to fully understand the mechanisms of action and potential uses of plants, but this remains an interesting topic for investigation.
5.1. Areas of interest for further exploration
Many studies have shown the promising potential of S. costus for various medical applications, owing to its bioactive components, which possess antimicrobial, antioxidant, and anti-cancer properties [3,13,35,42,51,65,130,[204], [205], [206]]. Research has shown that S. costus possesses therapeutic qualities and has been used to treat conditions such as thyroid disorders, cancer, and renal diseases [21,32,36,57]. This plant has shown potential as a natural source of inhibitors to combat SARS-CoV-2's main protease, highlighting its potential role in managing viral infections [21,130]. The protective effects of S. costus on the liver, heart, and inflammation have been investigated [65]. Therefore, S. costus displays healthcare potential with antimicrobial, antioxidant, and COVID-19 management properties.
Preclinical research has yielded promising results regarding the ability of S. costus to synthesize nanoparticles and regulate oxidative stress and apoptosis [42,207,208]. Further research is necessary to elucidate the specific modes of operation and substantiate their effectiveness in clinical contexts. The diverse results reported in these studies emphasize the necessity of implementing standardized research methodologies and conducting rigorous clinical trials to confirm the safety and effectiveness of S. costus in various medical fields. It is crucial to approach the study of S. costus cautiously and emphasize the need for a thorough investigation to substantiate its potential in clinical settings.
5.2. Potential biotechnological and pharmaceutical uses
The potential of S. costus in biotech and pharma has been extensively studied and documented. It is commonly used to treat various conditions, including chronic gastritis, stomach ulcers, rheumatoid arthritis, asthma, bronchitis, and inflammation-related diseases [36]. Studies in laboratory and animal models have demonstrated anti-inflammatory, anti-cancer, antibacterial, antifungal, and antiviral effects, supporting its traditional use in medicine [64]. S. costus exhibits antioxidant, anti-ulcer, anti-cancer, and hepatoprotective effects [13]. This plant has also been investigated for its potential use in synthesizing nanoparticles with antimicrobial properties [24]. S. costus has shown promise in controlling Candida species, particularly through terpenoids and alkaloids [35]. Thus, our results indicate promising prospects of S. costus for a range of biotechnological and pharmaceutical applications.
6. Challenges and limitations
6.1. Cultivation and sustainability issues
The difficulties and restrictions associated with developing and maintaining Senecio cineraria, a plant belonging to the Asteraceae family that grows annually or biennially, are noteworthy. Despite its widespread use in traditional medicine owing to its therapeutic properties, the cultivation and sustainability of this herb present significant challenges [208]. However, propagation and cultivation techniques for most Saussurea species, including S. costus, are lacking, hindering their sustainable cultivation [17]. The biological effects of the plant, such as its curative and preventive effects against various diseases including cancer, diabetes, and hemorrhoids, are well documented [12]. S. costus has been acknowledged for its capacity to produce cytostatic effects facilitated by the regulation of cyclins and pro-apoptotic molecules. This implies that it may be a promising therapeutic option for cancer treatment [21]. Despite these promising attributes, challenges persist in cultivating and sustaining S. costus because cultivation techniques are not well-established, limiting their availability for potential biological use.
6.2. Side effects and safety concerns of S. costus
S. costus, also known as Mu-Xiang or Guang Xi-Xin in Chinese medicine, is widely used in traditional Tibetan medicine to treat conditions, such as hyperlipidemia and scrofula [119,209]. It belongs to the Compositae family and grows mainly in high-altitude regions of China such as Tibet, Yunnan, and Sichuan [210]. The plant's long, cold winters, and moist conditions allow it to accumulate significant amounts of active substances [211]. Despite itexhibitednal uses, S. costus can cause severe liver injury and can even lead to acute hepatic failure or death [212]. Research has identified 329 compounds in S. costus with various pharmacological activities, including antimicrobial, antitumor, anti-inflammatory, analgesic, immunomodulatory, antidiabetic, antioxidant, and hepatoprotective effects [125]. However, these extracts are toxic to normal liver cells and can have significant side effects during long-term oral use [213]. Clinical reports have documented adverse effects of S. costus for several years. It has been linked to subacute azotemia and liver, kidney, and other organ toxicities as well as multiple organ failure and death [119,214]. S. costus oil has also been used topically to treat various conditions, including hand ulcers, paronychia, abscesses, and burns [215]. S. costus, which has been known for centuries for the treatment of various ailments, can produce essential oils with different medicinal values [125]. However, its main component, Saussurea-lactone, has been associated with significant toxicity [85]. Safety concerns and the potential for severe side effects underscore the need for careful dosage recommendations and further research on the toxic mechanisms of herbs [216]. The primary focus of extensive research on the biological applications of S. costus has been its conventional medical use, including the treatment of chronic gastritis, stomach ulcers, rheumatoid arthritis, asthma, and bronchitis [126]. Studies have shown that S. costus has anti-inflammatory, anti-trypanosomal, potent anti-cancer, antibacterial, antifungal, and antiviral properties, supporting its historical use in therapeutic applications [217]. Incorporating this plant has shown its capacity to exert cytostatic effects, which can be attributed to its ability to regulate cyclins and pro-apoptotic molecules and suppress anti-apoptotic molecules [217]. The benefits of S. costus include antioxidant, anti-ulcer, anti-cancer, and hepatoprotective effects [60]. Nevertheless, it is crucial to consider the careful use of S. costus because of its impact on protoscoleces viability, which depends on the concentration and exposure time [50]. Despite these considerations, S. costus has shown potential therapeutic effects and its use in medicine warrants further investigation.
6.3. Strengths and weaknesses
-
1.
Comprehensive review: This study provides a thorough overview of the biological activities, chemical compositions, and potential therapeutic uses of S. costus, a critically endangered medicinal plant.
-
2.
Diverse applications: This review highlights the wide range of pharmacological properties of S. costus, including its antimicrobial, anti-inflammatory, antioxidant, anti-cancer, antiviral, and hepatoprotective effects, demonstrating its versatility as a potential therapeutic agent.
-
3.
Detailed analysis: This study investigated the specific bioactive compounds present in S. costus, such as costunolide, dehydrocostus lactone, and cynaropicrin, and explored their mechanisms of action and contribution to the plant's diverse biological activities.
-
4.
Conservation importance: This comprehensive review emphasizes the critically endangered status of S. costus and the urgent need for conservation efforts to protect this valuable medicinal species.
-
5.
Interdisciplinary approach: This study integrates information from various fields, including traditional medicine, phytochemistry, pharmacology, and biotechnology, to provide a comprehensive understanding of S. costus.
-
6.
Limited cultivation and propagation techniques: This review identifies the lack of well-established propagation and cultivation techniques for S. costus as a significant challenge that hinders the sustainable utilization and availability of this plant.
-
7.
Gaps in understanding: Despite the extensive research summarized in the review, there are still considerable gaps in the understanding of the specific mechanisms of action and full therapeutic potential of S. costus, particularly in clinical settings.
Overall, our comprehensive study of S. costus presents a robust and informative review, highlighting the significant potential of this medicinal plant and acknowledging the need for further research, standardized methodologies, and conservation efforts to fully realize its therapeutic applications.
7. Conclusion and future directions
Extensive research on S. costus has revealed its significant potential for addressing various health issues and diverse biological applications. The medicinal properties of this plant have been thoroughly investigated, including its antimicrobial, antioxidant, and anti-inflammatory effects, and its potential in treating conditions such as thyroid disorders, liver injury, and COVID-19. The findings highlight the historical and cultural importance of S. costus in supporting traditional use and demonstrate its potential in modern medicine. However, despite these promising results, there are still considerable gaps in our understanding of S. costus. Although this research has provided valuable insights into the biological impacts and therapeutic potential of plants, further validation and optimization are necessary to confirm its effectiveness in clinical contexts. The conflicting evidence obtained from various sources emphasizes the need for additional research to explore the complete range of benefits of S. costus and its potential negative impacts. Future research should focus on validating the potential of S. costus in clinical settings and further investigate its biotechnological and pharmaceutical applications. Standardized research methodologies and rigorous clinical trials are vital for confirming its effectiveness and safety in various medical fields. Moreover, the lack of propagation techniques for several Saussurea species, including S. costus, necessitates research on sustainable cultivation methods and conservation of the genetic pool of this plant species. The diverse outcomes reported in these studies emphasize the importance of implementing standardized research methodologies and conducting rigorous clinical trials to ensure the safety and effectiveness of S. costus in a range of medical fields. It is essential to approach the study of S. costus cautiously and to emphasize the need for extensive investigation to validate its potential in clinical settings. In conclusion, this review of S. costus has highlighted its significant potential in diverse medical treatments and its promising biological uses. However, further research, standardized methodologies, and rigorous clinical trials are necessary to confirm their effectiveness and safety in various medical fields. This study provides a foundation for future research to validate the potential of S. costus in clinical settings and to explore its biotechnological and pharmaceutical applications.
Availability of data and materials
The datasets used in this study are available for future research.
Funding and resources
None.
CRediT authorship contribution statement
Ahmed A.M. Elnour: Writing – original draft, Visualization, Validation, Software, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Nour Hamid Abdurahman: Writing – review & editing, Validation, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Abdurahman Hamid Nour reports financial support was provided by Eastern Unity Technology which funded this research under Grant No. UIC 190806. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors express their gratitude to the Faculty of Chemical and Process Engineering Technology and the Center of Excellence for Advanced Research in Fluid Flow (CARIFF) at Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA) for their indispensable assistance in carrying out this research. We extend our deep appreciation to Dr. Khalid Hamid from Qassim University for his critical contributions in proofreading, critical thinking, and brainstorming, particularly in addressing crucial questions and responding to reviewers' challenges.
Contributor Information
Ahmed A.M. Elnour, Email: adamhassan@umpsa.edu.my, ahmedrashma@gmail.com.
Nour Hamid Abdurahman, Email: abrahman@umpsa.edu.my.
Abbreviations
- CDX
Cell Line-Derived Xenograft
- COPD
Chronic Obstructive Pulmonary Disease
- ROS
Reactive oxygen species
- TS
Tincture Saussurea
- UMPSA
Universiti Malaysia Pahang Al-Sultan Abdullah
- CINC
Cytokine-induced neutrophil chemotactic
- CNS
Central nervous system
- MAPK
Mitogen-activated protein kinase
- MIC
Minimum inhibitory concentration
- MPT
Mitochondrial permeability transition
- ROS
Reactive oxygen species
- S. costus
Saussurea costus
References
- 1.Mujammami M. Clinical significance of Saussurea Costus in thyroid treatment. Saudi Med. J. 2020;41(10):1047–1053. doi: 10.15537/smj.2020.10.25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soliman M.F., Shetaia Y.M., Tayel A.A., Munshi A.M., Alatawi F.A., Alsieni M.A., Al-Saman M.A. Exploring the antifungal activity and action of saussurea costus root extracts against Candida albicans and non-albicans species. Antibiotics (Basel) 2022;11(3) doi: 10.3390/antibiotics11030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fekry E., Awny M., Refaat G., Arafat H. Ameliorative role of saussurea lappa root extract "Costus" on thyroid tissue under the toxic effect of carbimazole induced hypothyroidism. Zagazig Journal of Forensic Medicine. 2023;21(1):172–189. doi: 10.21608/zjfm.2023.188310.1139. [DOI] [Google Scholar]
- 4.Abdullaev F.I., Espinosa-Aguirre J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004;28(6):426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Christodoulou E., Kadoglou N.P., Kostomitsopoulos N., Valsami G. Saffron: a natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015;67(12):1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 6.Samarghandian S., Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014;6(2):99. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behera A., Devi R.S., Pradhan S., Biswal S., Jena P.K., Biswal S.K., Kumar S. Phytochemical analysis and antioxidant potential of Costus speciosus L. Eur. J. Med. Plants. 2020:64–72. [Google Scholar]
- 8.Jaiswal B., Mohan M. Effect of solanum torvum on the contractile response of isolated tissues preparation in fructose fed rat. Int J Pharm Bio Sci. 2012;3(3):161–169. [Google Scholar]
- 9.Zhen X., Choi H.S., Kim J.-H., Kim S.-L., Liu R., Yun B.-S., Lee D.-S. Machilin D, a lignin derived from Saururus chinensis, suppresses breast cancer stem cells and inhibits NF-κB signaling. Biomolecules. 2020;10(2):245. doi: 10.3390/biom10020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha P., Das S. Highlighting the anti-carcinogenic potential of an ayurvedic medicinal plant, Swertia Chirata. Asian Pac. J. Cancer Prev. 2010;11(6):1445–1449. [PubMed] [Google Scholar]
- 11.Abu-Reida I.M., Jamous R.M., Ali-Shtayeh M.S. Phytochemistry , pharmacological properties and industrial applications of Rhus coriaria L. (sumac) Jordan J. Biol. Sci. 2014;7(4):233–244. [Google Scholar]
- 12.Mammate N., El Oumari F.E., Imtara H., Belchkar S., Lahrichi A., Alqahtani A.S., Noman O.M., Tarayrah M., Houssaini T.S. Antioxidant and anti-urolithiatic activity of aqueous and ethanolic extracts from saussurea costus (falc) lispich using scanning electron microscopy. Life. 2022;12(7) doi: 10.3390/life12071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammate N., El Oumari F.E., Imtara H., Belchkar S., Benjelloun Touimi G., Al-Zharani M., Rudayni H.A., Ahmed Qurtam A., Aleissa M.S., Nasr F.A. Anti-struvite, antimicrobial, and anti-inflammatory activities of aqueous and ethanolic extracts of saussurea costus (falc) lipsch Asteraceae. Molecules. 2023;28(2):667. doi: 10.3390/molecules28020667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Gizawy H.A., El-Haddad A.E., Saadeldeen A.M., Boshra S.A. Tentatively identified (UPLC/T-TOF–MS/MS) compounds in the extract of saussurea Costus roots exhibit in vivo hepatoprotection via modulation of HNF-1α, sirtuin-1, C/ebpα, MiRNA-34a and MiRNA-223. Molecules. 2022;27(9):2802. doi: 10.3390/molecules27092802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud M.S. Costus root extract preserves thyroid hormones levels, thyroglobulin expression and thyroid tissues in rats receiving valproate sodium. The Indonesian Biomedical Journal. 2020;12(4):304–312. doi: 10.18585/inabj.v12i4.1145. [DOI] [Google Scholar]
- 16.Amara U., Mashwani Z.u.R., Khan A., Laraib S., Wali R., Sarwar U., Ain Q.T., Shakeel S. Rahimullah, sohail, conservation status and therapeutic potential of & lt;i& gt;Saussurea lappa& lt;/i& gt;: an overview. Am. J. Plant Sci. 2017;8(3):602–614. [Google Scholar]
- 17.Butola J.S., Samant S.S. Saussurea species in Indian himalayan region: diversity, distribution and indigenous uses. Int. J. Plant Biol. 2010;1(1) doi: 10.4081/pb.2010.e9. [DOI] [Google Scholar]
- 18.Pawar V., Pawar P. Costus speciosus: an important medicinal plant. Int. J. Sci. Res. 2014;3(7):28–33. [Google Scholar]
- 19.Gwari G., Bhandari U., Andola H.C., Lohani H., Chauhan N. Volatile constituents of saussurea costus roots cultivated in uttarakhand Himalayas, India. Pharmacogn. Res. 2013;5(3):179–182. doi: 10.4103/0974-8490.112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathore S., Debnath P., Kumar R. Kuth saussurea costus (falc.) lipsch.: a critically endangered medicinal plant from himalaya. Journal of Applied Research on Medicinal and Aromatic Plants. 2021;20 [Google Scholar]
- 21.Idriss H., Siddig B., Maldonado P.G., Elkhair H.M., Alakhras A.I., Abdallah E.M., Torres P.H.S., Elzupir A.O. Phytochemical discrimination, biological activity and molecular docking of water-soluble inhibitors from saussurea costus herb against main protease of SARS-CoV-2. Molecules. 2022;27(15) doi: 10.3390/molecules27154908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bano H., Bhat J.I.A., Siddique M.A.A., Noor F., Ashraf Bhat M., Mir S.A. Seed germination studies of saussurea costus clarke, a step towards conservation of a critically endangered medicinal plant species of north western Himalaya. Plant Arch. 2018;18(1):963–968. [Google Scholar]
- 23.Saif-Al-Islam M. Saussurea Costus may help in the treatment of COVID-19. Sohag Medical Journal. 2020;0(0) doi: 10.21608/smj.2020.31144.1163. 0-0. [DOI] [Google Scholar]
- 24.Amina M., Al Musayeib N.M., Alarfaj N.A., El-Tohamy M.F., Oraby H.F., Al Hamoud G.A., Bukhari S.I., Moubayed N.M.S. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BinShabaib M.S., Ss A.L., Helaby B.S., AlHefdhi M.H., Mohammed A.E., Aabed K. Comparison of the anti-bacterial efficacy of saussurea costus and melaleuca alternifolia against porphyromonas gingivalis, Streptococcus mutans, and Enterococcus faecalis: an in-vitro study. Front Oral Health. 2022;3 doi: 10.3389/froh.2022.950840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tousson E., El-Atrsh A., Mansour M., Abdallah A. Modulatory effects of Saussurea lappa root aqueous extract against ethephon-induced kidney toxicity in male rats. Environ. Toxicol. 2019;34(12):1277–1284. doi: 10.1002/tox.22828. [DOI] [PubMed] [Google Scholar]
- 27.El-Rahman G.I.A., Behairy A., Elseddawy N.M., Batiha G.E., Hozzein W.N., Khodeer D.M., Abd-Elhakim Y.M. Saussurea lappa ethanolic extract attenuates triamcinolone acetonide-induced pulmonary and splenic tissue damage in rats via modulation of oxidative stress, inflammation, and apoptosis. Antioxidants. 2020;9(5) doi: 10.3390/antiox9050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaeesh S., Jamal Q., Shah A.J., Gilani A.H. Antihepatotoxic activity of saussurea lappa extract on D‐galactosamine and lipopolysaccharide‐induced hepatitis in mice. Phytother Res. 2010 doi: 10.1002/ptr.3089. [DOI] [PubMed] [Google Scholar]
- 29.Marei A. The therapeutic effects of Costus (saussurea lappa) against hypercholesterolemia in male albino rats. Egypt. J. Zool. 2022 doi: 10.21608/ejz.2022.121913.1074. [DOI] [Google Scholar]
- 30.Eldaim M.A.A., Tousson E., Sayed I.E.T.E., Awd W.M. Ameliorative effects of saussurea lappa root aqueous extract against ethephon‐induced reproductive toxicity in male rats. Environ. Toxicol. 2018 doi: 10.1002/tox.22669. [DOI] [PubMed] [Google Scholar]
- 31.Habotta O.A., Ateya A., Saleh R.M., El-Ashry E.S. Thiamethoxam-induced oxidative stress, lipid peroxidation, and disturbance of steroidogenic genes in male rats: palliative role of Saussurea lappa and Silybum marianum. Environ. Toxicol. 2021;36(10):2051–2061. doi: 10.1002/tox.23322. [DOI] [PubMed] [Google Scholar]
- 32.Elgharabawy R.M., El Tantawy El Sayed I., Abd-Allah Rezk N., Tousson E. Therapeutic impact of Costus (saussurea lappa) against Ehrlich solid tumor-induced cardiac toxicity and DNA damage in female mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.708785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karim Z.M., Hussein H.J., Al-Rubaye A.F. In vitro anticandidal activity of the secondary metabolites extracted from saussurea Costus (falc.) lipschitz roots. Int. J. Health Sci. 2022:7461–7470. doi: 10.53730/ijhs.v6ns6.12093. [DOI] [Google Scholar]
- 34.Abdallah E.M., Qureshi K.A., Ali A.M.H., Elhassan G.O. Evaluation of some biological properties of Saussurea costus crude root extract. Bioscience Biotechnology Research Communications. 2017;10(4):601–611. doi: 10.21786/bbrc/10.4/2. [DOI] [Google Scholar]
- 35.Karim Z.M., Hussein H.J., Al-Rubaye A.F. In vitro anticandidal activity of the secondary metabolites extracted from saussurea Costus (falc.) lipschitz roots. Int. J. Health Sci. 2022 doi: 10.53730/ijhs.v6ns6.12093. [DOI] [Google Scholar]
- 36.Mujammami M. Clinical significance of saussurea Costus in thyroid treatment. Saudi Med. J. 2020 doi: 10.15537/smj.2020.10.25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silveira F.S., Azzolini M., Divan A.M. Scanning cadmium photosynthetic responses of Elephantopus mollis for potential phytoremediation practices. Water, Air, Soil Pollut. 2015;226:1–12. [Google Scholar]
- 38.Khuroo A.A., Reshi Z.A., Malik A.H., Weber E., Rashid I., Dar G. Alien flora of India: taxonomic composition, invasion status and biogeographic affiliations. Biol. Invasions. 2012;14:99–113. [Google Scholar]
- 39.Debnath A., Paul C., Debnath B. Eight new additions of plant species to the flora of foot Himalayan state Tripura, north east India: distributional range extension, geographic map and their less known ethno medicine. NeBIO. 2017;8(4):246–254. [Google Scholar]
- 40.Zhang X., Deng T., Moore M.J., Ji Y., Lin N., Zhang H., Meng A., Wang H., Sun Y., Sun H. Plastome phylogenomics of saussurea (Asteraceae: cardueae) BMC Plant Biol. 2019;19(1):1–10. doi: 10.1186/s12870-019-1896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L.-S., Herrando-Moraira S., Susanna A., Galbany-Casals M., Chen Y.-S. Phylogeny, origin and dispersal of Saussurea (Asteraceae) based on chloroplast genome data. Mol. Phylogenet. Evol. 2019;141 doi: 10.1016/j.ympev.2019.106613. [DOI] [PubMed] [Google Scholar]
- 42.Al-Brahim J.S. Saussurea costus extract as bio mediator in synthesis iron oxide nanoparticles (IONPs) and their antimicrobial ability. PLoS One. 2023;18(3) doi: 10.1371/journal.pone.0282443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mammate N., oumari F.E.E., Imtara H., Belchkar S., Lahrichi A., Alqahtani A.S., Noman O.M., Tarayrah M., Houssaïni T.S. Antioxidant and anti-urolithiatic activity of aqueous and ethanolic extracts from saussurea Costus (falc) lispich using scanning electron microscopy. Life. 2022 doi: 10.3390/life12071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajan R.G., Pahlavan S., Tejaswi J.K.D. Study of hepatoprotective activity of saussurea lappa root extract. International Journal of Trend in Scientific Research and Development. 2018 doi: 10.31142/ijtsrd18390. [DOI] [Google Scholar]
- 45.Liu Z.L., He Q., Chu S.S., Wang C.F., Du S.S., Deng Z.W. Essential oil composition and larvicidal activity of saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol. Res. 2011 doi: 10.1007/s00436-011-2738-0. [DOI] [PubMed] [Google Scholar]
- 46.Abbasi A.M., Khan S.M., Ahmad M., Khan M.A., Quave C.L., Pieroni A. Botanical ethnoveterinary therapies in three districts of the Lesser Himalayas of Pakistan. J. Ethnobiol. Ethnomed. 2013;9:84. doi: 10.1186/1746-4269-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deabes M.M., Fatah S.I.A.-E., Salem S., Naguib K. Antimicrobial activity of bioactive compounds extract from saussurea Costus against food spoilage microorganisms. Egypt. J. Chem. 2021 doi: 10.21608/ejchem.2021.69572.3528. [DOI] [Google Scholar]
- 48.Benkhaira N., Ferioun M., Kandsi F., Jeddi M., Chebat A., Addi M., Hano C., Fikri-Benbrahim K. Moroccan medicinal plants used to treat cancer: ethnomedicinal study and insights into pharmacological evidence. Evid. base Compl. Alternative Med. 2022 doi: 10.1155/2022/1645265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y.-J., Liu J.-Q., Miehe G. Phylogenetic origins of the himalayan endemic Dolomiaea, diplazoptilon and xanthopappus (Asteraceae: Cardueae) based on three DNA regions. Ann. Bot. 2007;99(2):311–322. doi: 10.1093/aob/mcl259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-obaidy A., Esmaeel F. Effect of Saussurea costus extracts in the viability of Echinococcus granulosus protoscoleces of sheep origin in vitro. Journal of Education and Science. 2021;30(3):73–82. doi: 10.33899/edusj.2021.168656. [DOI] [Google Scholar]
- 51.Soliman M.F., Shetaia Y.M., Tayel A.A., Munshi A.M., Alatawi F.A., Alsieni M., Al-Saman M.A. Exploring the antifungal activity and action of saussurea Costus root extracts against Candida albicans and non-albicans species. Antibiotics. 2022 doi: 10.3390/antibiotics11030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soliman M.F., Shetaia Y.M., Tayel A.A., Munshi A.M., Alatawi F.A., Alsieni M.A., Al-Saman M.A. Exploring the antifungal activity and action of saussurea costus root extracts against Candida albicans and non-albicans species. Antibiotics. 2022;11(3) doi: 10.3390/antibiotics11030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Julianti T., Hata Y., Zimmermann S., Kaiser M., Hamburger M., Adams M. Antitrypanosomal sesquiterpene lactones from saussurea Costus. Fitoterapia. 2011 doi: 10.1016/j.fitote.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Bheel N., Kennedy C., Awoyera P.O., Sohu S., Abbasi S.A. Comparative study on mechanical properties of concrete blended with Costus englerianus bagasse ash and bagasse fibre as partial replacement for lime and cement. Adv. Civ. Eng. 2022 doi: 10.1155/2022/8900167. [DOI] [Google Scholar]
- 55.Idriss H., Siddig B., González-Maldonado P., Elkhair H.M., Alakhras A.I., Abdallah E.M., Elzupir A.O., Sotelo P.H. Inhibitory activity of saussurea Costus extract against bacteria, Candida, herpes, and SARS-CoV-2. Plants. 2023 doi: 10.3390/plants12030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tali B.A., Khuroo A.A., Nawchoo I.A., Ganie A.H. Prioritizing conservation of medicinal flora in the himalayan biodiversity hotspot: an integrated ecological and socioeconomic approach. Environ. Conserv. 2018 doi: 10.1017/s0376892918000425. [DOI] [Google Scholar]
- 57.Tousson E., El-Atrsh A., Mansour M., Assem A. Modulatory effects of saussurea lappa root aqueous extract against ethephon‐induced kidney toxicity in male rats. Environ. Toxicol. 2019 doi: 10.1002/tox.22828. [DOI] [PubMed] [Google Scholar]
- 58.Charkravarty A., Ramasamy G. Profiling of volatiles in tissues of salacia reticulata wight. With anti-diabetic potential using GC-MS and molecular docking. arXiv preprint arXiv:2307.04109. 2023 [Google Scholar]
- 59.Niemann F., Reining C. 2024. Kontextbasierte Aktivit\" atserkennung--Synergie von Mensch und Technik in der Social Networked Industry. arXiv preprint arXiv:2402.05480. [Google Scholar]
- 60.Mammate N., El oumari F.E., Imtara H., Belchkar S., Lahrichi A., Alqahtani A.S., Noman O.M., Tarayrah M., Houssaini T.S. Antioxidant and anti-urolithiatic activity of aqueous and ethanolic extracts from saussurea costus (falc) lispich using scanning electron microscopy. Life. 2022;12(7) doi: 10.3390/life12071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed H.Y., Kareem S.M., Ahmed A., Safwat N.A., Shehata R.M., Yosri M., Youssef M., Baakdah M.M., Sami R., Baty R.S., Alsubhi N.H., Alrefaei G.I., Shati A.A., Elsaid F.G. Optimization of supercritical carbon dioxide extraction of saussurea Costus oil and its antimicrobial, antioxidant, and anticancer activities. Antioxidants. 2022;11(10):1960. doi: 10.3390/antiox11101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alaidarous H.S., Bhassu S., Yusoff I., Jindal H.M., Chandramathi S. Analysis of bioactive elements and antibacterial potential of ethanolic extracts from the COSTUS plant. SAUSSUREA LAPPA, Biochemical & Cellular Archives. 2021;21(1) [Google Scholar]
- 63.Gwari G., Bhandari U., Andola H.C., Lohani H., Chauhan N. Volatile constituents of saussurea costus roots cultivated in uttarakhand Himalayas, India. Pharmacogn. Res. 2013;5(3):179. doi: 10.4103/0974-8490.112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Idriss H., Siddig B., González-Maldonado P., Elkhair H.M., Alakhras A.I., Abdallah E.M., Sotelo P.H., Elzupir A.O. Phytochemical discrimination, biological activity and molecular docking of water-soluble inhibitors from saussurea Costus herb against main protease of SARS-CoV-2. Molecules. 2022 doi: 10.3390/molecules27154908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-gizawy H.A.-E., El-Haddad A.E., Saadeldeen A.M., Boshra S.A. Tentatively identified (UPLC/T-TOF–MS/MS) compounds in the extract of saussurea Costus roots exhibit in vivo hepatoprotection via modulation of HNF-1α, sirtuin-1, C/ebpα, miRNA-34a and miRNA-223. Molecules. 2022 doi: 10.3390/molecules27092802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Razgonova M.P., Zakharenko A.M., Erċışlı S., Grudev V., Golokhvast K.S. Comparative analysis of far east sikhotinsky Rhododendron (Rh. Sichotense) and east siberian Rhododendron (Rh. Adamsii) using supercritical CO2-extraction and HPLC-ESI-MS/MS spectrometry. Molecules. 2020 doi: 10.3390/molecules25173774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pachiyappan J., Gnanasundaram N., Sivamani S., Sankari N.P.B.P., Senthilnathan N., Kerga G.A. Preparation and characterization of magnesium oxide nanoparticles and its application for photocatalytic removal of rhodamine B and methylene blue dyes. J. Nanomater. 2022 doi: 10.1155/2022/6484573. [DOI] [Google Scholar]
- 68.Kolahalam L.A., Prasad K.R.S., Krishna P.M., Supraja N., Shanmugan S. The exploration of bio-inspired copper oxide nanoparticles: synthesis, characterization and in-vitro biological investigations. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e09726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim D.Y., Choi B.Y. Costunolide—a bioactive sesquiterpene lactone with diverse therapeutic potential. Int. J. Mol. Sci. 2019 doi: 10.3390/ijms20122926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang J.S., Yoon Y.D., Lee K.H., Park S.-K., Kim H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochemical and biophysical research communications. 2004;313(1):171–177. doi: 10.1016/j.bbrc.2003.11.109. [DOI] [PubMed] [Google Scholar]
- 71.Liu Q., Majdi M., Cankar K., Goedbloed M., Charnikhova T., Verstappen F.W., De Vos R.C., Beekwilder J., Van der Krol S., Bouwmeester H.J. Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruna A., Nicolàs M., Múñoz A., Kyriakis J., Caelles C. Glucocorticoid receptor-JNK interaction mediates inhibition of the JNK pathway by glucocorticoids. The Embo Journal. 2003 doi: 10.1093/emboj/cdg590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Saggaf M.S., Tayel A.A., Ghobashy M.O.I., Alotaibi M.A., Alghuthaymi M.A., Moussa S.H. Phytosynthesis of selenium nanoparticles using the Costus extract for bactericidal application against foodborne pathogens. Green Process. Synth. 2020;9(1):477–487. doi: 10.1515/gps-2020-0038. [DOI] [Google Scholar]
- 74.Tag H.M., Khaled H.E., Ismail H.A., El-Shenawy N.S. Evaluation of anti-inflammatory potential of the ethanolic extract of the Saussurea lappa root (costus) on adjuvant-induced monoarthritis in rats. J. Basic Clin. Physiol. Pharmacol. 2016;27(1):71–78. doi: 10.1515/jbcpp-2015-0044. [DOI] [PubMed] [Google Scholar]
- 75.Yoo H.-J., Kang H.-J., Jung H.-J., Kim K., Lim C.-J., Park E.-H. Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of Saururus chinensis extract. J. Ethnopharmacol. 2008;120(2):282–286. doi: 10.1016/j.jep.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Jia J.M., Wu C.F., Liu W., Yu H., Hao Y., Zheng J.H., Ji Y.R. Antiinflammatory and analgesic activities of the tissue culture of saussurea involucrata. Biol. Pharm. Bull. 2005;28(9):1612–1614. doi: 10.1248/bpb.28.1612. [DOI] [PubMed] [Google Scholar]
- 77.Gokhale A.B., Damre A.S., Kulkarni K.R., Saraf M.N. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine. 2002;9(5):433–437. doi: 10.1078/09447110260571689. [DOI] [PubMed] [Google Scholar]
- 78.Pinedo-Guerrero Z.H., Hernández-Fuentes A.D., Ortega-Ortíz H., Benavides-Mendoza A., Cadenas‐Pliego G., Juárez-Maldonado A. Cu nanoparticles in hydrogels of chitosan-pva affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules. 2017 doi: 10.3390/molecules22060926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho J.Y., Park J., Yoo E.S., Baik K.U., Jung J.H., Lee J., Park M.H. Inhibitory effect of sesquiterpene lactones from Saussurea lappa on tumor necrosis factor-α production in murine macrophage-like cells. Planta Med. 1998;64(7):594–597. doi: 10.1055/s-2006-957528. [DOI] [PubMed] [Google Scholar]
- 80.Aldieri E., Atragene D., Bergandi L., Riganti C., Costamagna C., Bosia A., Ghigo D. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-kB activation. FEBS Lett. 2003;552(2–3):141–144. doi: 10.1016/s0014-5793(03)00905-0. [DOI] [PubMed] [Google Scholar]
- 81.Mathema V.B., Koh Y.S., Thakuri B.C., Sillanpää M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2011 doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]
- 82.Cho J.Y., Park J., Yoo E.S., Baik K.U., Jung J.H., Lee J.-S., Park M.H. Inhibitory effect of sesquiterpene lactones FromSaussurea lappaon tumor necrosis factor-Α production in murine macrophage-like cells. Planta Med. 1998 doi: 10.1055/s-2006-957528. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y.F., Ni Z.Y., Dong M., Cong B., Shi Q., Gu Y.C., Kiyota H. Secondary metabolites of plants from the genus saussurea: chemistry and biological activity. Chem. Biodivers. 2010 doi: 10.1002/cbdv.200900406. [DOI] [PubMed] [Google Scholar]
- 84.Jin T. Cynaropicrin averts the oxidative stress and neuroinflammation in ischemic/Reperfusion injury through the modulation of NF-kB. Appl. Biochem. Biotechnol. 2022 doi: 10.1007/s12010-022-04060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Idriss H., Siddig B., González-Maldonado P., Elkhair H., Alakhras A.I., Abdallah E.M., Elzupir A.O., Sotelo P.H. Inhibitory activity of Saussurea costus extract against bacteria, candida, herpes, and SARS-CoV-2. Plants. 2023;12(3):460. doi: 10.3390/plants12030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Habotta O.A., Ateya A., Saleh R.M., El-Ashry E. Thiamethoxam‐induced oxidative stress, lipid peroxidation, and disturbance of steroidogenic genes in male rats: palliative role of saussurea lappa and <scp>Silybum marianum</Scp>. Environ. Toxicol. 2021;36(10):2051–2061. doi: 10.1002/tox.23322. [DOI] [PubMed] [Google Scholar]
- 87.Eldaim M.A.A., Tousson E., Sayed I.E.T.E., Awd W.M. Ameliorative effects of saussurea lappa root aqueous extract against ethephon‐induced reproductive toxicity in male rats. Environ. Toxicol. 2018;34(2):150–159. doi: 10.1002/tox.22669. [DOI] [PubMed] [Google Scholar]
- 88.El-Rahman G.I.A., Behairy A., Elseddawy N.M., Batiha G.E.S., Hozzein W.N., Khodeer D.M., Abd‐Elhakim Y.M. Saussurea lappa ethanolic extract attenuates triamcinolone acetonide-induced pulmonary and splenic tissue damage in rats via modulation of oxidative stress, inflammation, and apoptosis. Antioxidants. 2020;9(5):396. doi: 10.3390/antiox9050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marzook E.A., El-Bayoumy A.S., Marzook F.A. Preclinical evaluation of carnosine and Costus as hematological protective agents against gamma radiation. Journal of Radiation Research and Applied Sciences. 2019;12(1):304–310. doi: 10.1080/16878507.2019.1649931. [DOI] [Google Scholar]
- 90.Zaky K., Abdelaal Y., Ashour A., Zeid T., Hussien T.A., Ahmed A.A. Pharmacognostical studies on roots of saussurea Costus (falc.) lipsch. Sphinx Journal of Pharmaceutical and Medical Sciences. 2022;4(1):15–25. doi: 10.21608/sjpms.2022.164141.1015. [DOI] [Google Scholar]
- 91.Tousson E., El-Atrsh A., Mansour M., Assem A. Modulatory effects of saussurea lappa root aqueous extract against ethephon‐induced kidney toxicity in male rats. Environ. Toxicol. 2019;34(12):1277–1284. doi: 10.1002/tox.22828. [DOI] [PubMed] [Google Scholar]
- 92.Elgharabawy R.M., Sayed I.E.T.E., Rezk N.A.-A., Tousson E. Therapeutic impact of Costus (saussurea lappa) against Ehrlich solid tumor-induced cardiac toxicity and DNA damage in female mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.708785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong G.-Z., Shim A.-R., Hyeon J.S., Lee H.J., Ryu J.H. Inhibition of WNT/Β-Catenin pathway by dehydrocostus lactone and costunolide in colon cancer cells. Phytother Res. 2015 doi: 10.1002/ptr.5299. [DOI] [PubMed] [Google Scholar]
- 94.Yuan Y., Hu Q., Liu L., Xie F., Yang L., Li Y., Zhang C., Chen H., Tang J., Shen X. Dehydrocostus lactone suppresses dextran sulfate sodium-induced colitis by targeting the ikkα/β-NF-κb and keap1-Nrf2 signalling pathways. Front. Pharmacol. 2022 doi: 10.3389/fphar.2022.817596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choudhary M., Kumar V., Malhotra H., Singh S. Medicinal plants with potential anti-arthritic activity. Journal of Intercultural Ethnopharmacology. 2015 doi: 10.5455/jice.20150313021918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ahmad F., Dixit D., Sharma V., Kumar A., Joshi S.D., Sarkar C., Sen E. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. 2016;7(5) doi: 10.1038/cddis.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghashghaeinia M., Kořalková P., Giustarini D., Mojzíková R., Fehrenbacher B., Dreischer P., Schaller M., Mrowietz U., Martínez‐Ruiz A., Wieder T., Divoký V., Rossi R., Läng F., Köberle M. The specific PKC-α inhibitor chelerythrine blunts costunolide-induced eryptosis. Apoptosis. 2020 doi: 10.1007/s10495-020-01620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rasul A., Parveen S., Ma T. Costunolide: a novel anti-cancer sesquiterpene lactone. Bangladesh J. Pharmacol. 2012 doi: 10.3329/bjp.v7i1.10066. [DOI] [Google Scholar]
- 99.Duraipandiyan V., Al-Harbi N.A., Ignacimuthu S., Muthukumar C. Antimicrobial activity of sesquiterpene lactones isolated from traditional medicinal plant, Costus speciosus (koen ex.Retz.) Sm. BMC Compl. Alternative Med. 2012 doi: 10.1186/1472-6882-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lohberger B., Rinner B., Stuendl N., Kaltenegger H., Steinecker-Frohnwieser B., Bernhart E., Rad E.B., Weinberg A.M., Leithner A., Bauer R., Kretschmer N. Sesquiterpene lactones downregulate G2/M cell cycle regulator proteins and affect the invasive potential of human soft tissue sarcoma cells. PLoS One. 2013 doi: 10.1371/journal.pone.0066300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi D.L., Kim J., An J., Hong S., Song Y., Kong H. Amelioration of benign prostatic hyperplasia by costunolide and dehydrocostus lactone in wistar rats. The World Journal of Men S Health. 2021 doi: 10.5534/wjmh.190053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pae H.O., Jeong G.S., Kim H.S., Woo W.-H., Rhew H.Y., Kim H.S., Sohn D.H., Kim Y.C., Chung H.T. Costunolide inhibits production of tumor necrosis factor-Α and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflamm. Res. 2007 doi: 10.1007/s00011-007-7015-4. [DOI] [PubMed] [Google Scholar]
- 103.Seo C.S., Lim H.S., Jeong S.J., Shin H.K. Anti-allergic effects of sesquiterpene lactones from the root of aucklandia lappa decne. Mol. Med. Rep. 2015 doi: 10.3892/mmr.2015.4342. [DOI] [PubMed] [Google Scholar]
- 104.Long H., Huang Q., Yu Y., Zhang Z., Yao Z., Chen H., Feng J.-w. Dehydrocostus lactone inhibits in vitro gastrinoma cancer cell growth through apoptosis induction, sub-G1 cell cycle arrest, DNA damage and loss of mitochondrial membrane potential. Arch. Med. Sci. 2019 doi: 10.5114/aoms.2018.73128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nie Y., Wang Z., Chai G., Xiong Y., Li B., Zhang H., Xin R., Qian X., Tang Z., Wu J., Zhao P. Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-κB signaling pathway mediated by P38 MAPK and Akt. Molecules. 2019 doi: 10.3390/molecules24081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu F., Wu X., Zhang X., Yang L., Liu Y., Song Z. <scp>Co‐use</Scp> of organic herbal residue and red mud waste for syngas production by chemical looping gasification. Int. J. Energy Res. 2020 doi: 10.1002/er.5912. [DOI] [Google Scholar]
- 107.Peña-Oyarzún D., Flores T., Torres V.A., Quest A.F., Lobos-González L., Kretschmar C., Contreras P., Maturana-Ramírez A., Criollo A., Reyes M. Inhibition of PORCN blocks Wnt signaling to attenuate progression of oral carcinogenesis. Clin. Cancer Res. 2024;30(1):209–223. doi: 10.1158/1078-0432.CCR-23-0318. [DOI] [PubMed] [Google Scholar]
- 108.Jeong S.J., Itokawa T., Shibuya M., Kuwano M., Ono M., Higuchi R., Miyamoto T. Costunolide, a Sesquiterpene Lactone From Saussurea Lappa, Inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002 doi: 10.1016/s0304-3835(02)00361-0. [DOI] [PubMed] [Google Scholar]
- 109.Lee H.J., Kim N.Y., Jang M.K., Son H.J., Kim K.M., Sohn D.H., Lee S.H., Ryu J.-H. A sesquiterpene, dehydrocostus lactone, Inhibits the expression of inducible nitric oxide synthase and TNF-α in LPS-activated macrophages. Planta Med. 1999;65(2):104–108. doi: 10.1055/s-1999-13968. [DOI] [PubMed] [Google Scholar]
- 110.Wei S., Mao H., Wang C., Yang N., Zhang Z., Han J. Dehydrocostus lactone enhances chemotherapeutic potential of doxorubicin in lung cancer by inducing cell death and limiting metastasis, medical science monitor. 2018. [DOI] [PMC free article] [PubMed]
- 111.Wei M., Li J., Qiu J., Yan Y., Wang H., Wu Z.-B., Liu Y., Shen X., Su C., Guo Q., Pan Y.-R., Zhang P., Zhang J. Costunolide induces apoptosis and inhibits migration and invasion in H1299 lung cancer cells. Oncol. Rep. 2020 doi: 10.3892/or.2020.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee M.G., Lee K.T., Chi S.G., Park J. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol. Pharm. Bull. 2001 doi: 10.1248/bpb.24.303. [DOI] [PubMed] [Google Scholar]
- 113.Min Y., Choi S.U., Choi W.S., Kim S.Y., Lee K.R. Guaiane sesquiterpene lactones and amino acid-sesquiterpene lactone conjugates from the aerial parts of saussurea pulchella. Journal of Natural Products. 2008 doi: 10.1021/np800005r. [DOI] [PubMed] [Google Scholar]
- 114.Torres-Mantilla H.A., Cuesta-Herrera L., Andrades-Grassi J.E., Bianchi G. Simulation of inference test performance for minimum inhibitory concentration censored data. J. Phys. Conf. 2022 doi: 10.1088/1742-6596/2153/1/012013. [DOI] [Google Scholar]
- 115.Gallier F., Martel A., Dujardin G. Enantioselective access to robinson annulation products and Michael adducts as precursors. Angew. Chem. 2017 doi: 10.1002/anie.201701401. [DOI] [PubMed] [Google Scholar]
- 116.Barbero M., Prandi C. Pseudoguaianolides: recent advances in synthesis and applications. Nat. Prod. Commun. 2018;13(3) 1934578X1801300303. [Google Scholar]
- 117.Bosco A., Golsteyn R.M. Emerging anti-mitotic activities and other bioactivities of sesquiterpene compounds upon human cells. Molecules. 2017;22(3):459. doi: 10.3390/molecules22030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elso O.G., Bivona A.E., Sanchez Alberti A., Cerny N., Fabian L., Morales C., Catalán C.A., Malchiodi E.L., Cazorla S.I., Sülsen V.P. Trypanocidal activity of four sesquiterpene lactones isolated from Asteraceae species. Molecules. 2020;25(9):2014. doi: 10.3390/molecules25092014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nadda R.K., Ali A., Goyal R.C., Khosla P.K., Goyal R. Aucklandia costus (syn. Saussurea costus): ethnopharmacology of an endangered medicinal plant of the Himalayan region. J. Ethnopharmacol. 2020;263 doi: 10.1016/j.jep.2020.113199. [DOI] [PubMed] [Google Scholar]
- 120.Soliman M.F., Shetaia Y.M., Tayel A.A., Munshi A.M., Alatawi F.A., Alsieni M., Al-Saman M.A. Exploring the antifungal activity and action of saussurea Costus root extracts against Candida albicans and non-albicans species. Antibiotics. 2022;11(3):327. doi: 10.3390/antibiotics11030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hegazy M.M., Mostafa R.M., El-Sayed Y.A., Baz M.M., Khater H.F., Selim A., El-Shourbagy N.M. 2022. The Efficacy of Saussurea costus Extracts against Hematophagous Arthropods of Camel and Cattle. [Google Scholar]
- 122.Mir F.H., Tanveer S., Bharti P., Para B.A. Anthelmintic activity of saussurea costus (falc.) lipsch. Against ascaridia galli, a pathogenic nematode in poultry: in vitro and in vivo studies. Acta Parasitol. 2024:1–9. doi: 10.1007/s11686-024-00837-8. [DOI] [PubMed] [Google Scholar]
- 123.Hegazy M.M., Mostafa R.M., El-Sayed Y.A., Baz M.M., Khater H.F., Selim A., El-Shourbagy N.M. The efficacy of saussurea costus extracts against hematophagous arthropods of camel and cattle. Pak. Vet. J. 2022;42(4):547–553. doi: 10.29261/pakvetj/2022.064. [DOI] [Google Scholar]
- 124.Maji P., Dhar D.G., Misra P., Dhar P., speciosus Costus, Koen ex Retz Sm.: current status and future industrial prospects. Industrial crops and products. 2020;152 [Google Scholar]
- 125.Kumar J., Pundir M. Phytochemistry and pharmacology of saussurea genus (saussurea lappa, saussurea costus, saussurea obvallata, saussurea involucrata) Mater. Today Proc. 2022;56:1173–1181. doi: 10.1016/j.matpr.2021.11.145. [DOI] [Google Scholar]
- 126.Mujammami M. Clinical significance of Saussurea Costus in thyroid treatment. Saudi Med. J. 2020;41(10):1047. doi: 10.15537/smj.2020.10.25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paul A., Chakraborty N., Sarkar A., Acharya K., Ranjan A., Chauhan A., Srivastava S., Singh A.K., Rai A.K., Mubeen I. Ethnopharmacological potential of phytochemicals and phytogenic products against human RNA viral diseases as preventive therapeutics. BioMed Res. Int. 2023;2023 doi: 10.1155/2023/1977602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Urvashi, R. Kaur, Saussurea Lappa CB Clarke: Kushta/Kut, Immunity Boosting Medicinal Plants of the Western Himalayas, Springer2023, pp. 433-462.
- 129.Dawood H.M., Barghouth N.M., El-Mezayen N.S., Ibrahim R.S., Shawky E. Metabolomic insights into the therapeutic mechanisms of costus (Saussurea costus (Falc.) Lipsch.) root extract in propylthiouracil-induced hypothyroidism rat model. J. Ethnopharmacol. 2024;324 doi: 10.1016/j.jep.2024.117784. [DOI] [PubMed] [Google Scholar]
- 130.Hajji H., Alaqarbeh M., Lakhlifi T., Ajana M.A., Alsakhen N., Bouachrine M. Computational approach investigation bioactive molecules from Saussurea Costus plant as SARS-CoV-2 main protease inhibitors using reverse docking, molecular dynamics simulation, and pharmacokinetic ADMET parameters. Comput. Biol. Med. 2022;150 doi: 10.1016/j.compbiomed.2022.106209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qiu J., Wang J., Luo H., Du X., Li H., Luo M., Dong J., Chen Z., Deng X. The effects of subinhibitory concentrations of costus oil on virulence factor production in Staphylococcus aureus. J. Appl. Microbiol. 2011;110(1):333–340. doi: 10.1111/j.1365-2672.2010.04888.x. [DOI] [PubMed] [Google Scholar]
- 132.Yu H.-H., Lee J.-S., Lee K., Kim K.Y., You Y.O. Saussurea lappa inhibits the growth, acid production, adhesion, and water-insoluble glucan synthesis of Streptococcus mutans. J. Ethnopharmacol. 2007 doi: 10.1016/j.jep.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 133.Livne S., Simantov S., Rahmanov A., Jeffet U., Sterer N. Hydroxyethyl cellulose promotes the mucin retention of herbal extracts active against Streptococcus mutans. Materials. 2022 doi: 10.3390/ma15134652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bauza C., Martin R., Yeatts S.D., Borg K., Magwood G., Selassie A., Ford M.E. Determining the joint effect of obesity and diabetes on all-cause mortality and cardiovascular-related mortality following an ischemic stroke. Stroke Res. Treat. 2018;2018 doi: 10.1155/2018/4812712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan A., Shah A.H., Ali N. In-vitro propagation and phytochemical profiling of a highly medicinal and endemic plant species of the himalayan region (saussurea Costus) Sci. Rep. 2021 doi: 10.1038/s41598-021-03032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shati A.A., Alkahtani M.A., Alfaifi M., Elbehairi S.E.I., Elsaid F.G., Rajagopalan P., Mir M.A. Secondary metabolites of saussurea Costus leaf extract induce apoptosis in breast, liver, and colon cancer cells by caspase-3-dependent intrinsic pathway. BioMed Res. Int. 2020;2020:1–11. doi: 10.1155/2020/1608942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rasul A., Bao R., Malhi M., Zhao B., Tsuji I., Li J., Li Y. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules. 2013 doi: 10.3390/molecules18021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi H.-W. Evaluation of anticancer activity of dehydrocostuslactone in vitro. Mol. Med. Rep. 2009 doi: 10.3892/mmr_00000238. [DOI] [PubMed] [Google Scholar]
- 139.Kumar R., Bhardwaj P., Soni M., Singh R., Choudhary S., Virmani N., Asrani R., Patial V., Sharma D., Gupta V. Modulation of mammary tumour progression using murine model by ethanol root extract of Saussurea costus (falc.) lipsch. J. Ethnopharmacol. 2024;319 doi: 10.1016/j.jep.2023.117302. [DOI] [PubMed] [Google Scholar]
- 140.Kumar R., Bhardwaj P., Soni M., Choudhary S., Singh R., Kumar A., Asrani R.K., Virmani N., Gupta V., Tripathi B. Lipsch Root Extract against DMBA Induced Mammary Tumors in Female Sprague Dawley Rats. 2022. Cytopathological, ultrasonographic and hematological studies on the ameliorative effects of Saussurea costusFalc. [Google Scholar]
- 141.Kumar R., Tripathi B., Rana J., Bhardwaj P. Prevention of DMBA-induced mammary tumors from pulmonary metastases by saussurea costus (falc.) lipsc root ethanolic extract in sprague dawley rats. Indian J. Anim. Res. 2024;1:8. [Google Scholar]
- 142.Hua P., Zhang G., Zhang Y., Sun M., Cui R., Li X., Li B., Zhang X. Costunolide induces G1/S phase arrest and activates mitochondrial-mediated apoptotic pathways in SK-mes 1 human lung squamous carcinoma cells. Oncol. Lett. 2016 doi: 10.3892/ol.2016.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu B., Wu F., Shi Z., He B., Zhao X., Wu H., Yan S. Dehydrocostus lactone attenuates osteoclastogenesis and osteoclast‐induced bone loss by modulating NF‐κB signalling pathway. J. Cell Mol. Med. 2019 doi: 10.1111/jcmm.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013 doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu K., Tang H., Wang Y., Wang L., Zhou M., He G., Lu H., Tang C., Chen W., Ma X., Tang K., Deng Z. Micropattern silk fibroin film facilitates tendon repair in vivo and promotes tenogenic differentiation of tendon Stem/Progenitor cells through the Α2β1/Fak/Pi3k/Akt signaling pathway in vitro. Stem Cell. Int. 2023 doi: 10.1155/2023/2915826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lau A.T.Y., Wang Y., Chiu J.F. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J. Cell. Biochem. 2008 doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 147.Zimmermann S., Kaiser M., Brun R., Hamburger M., Adams M. Cynaropicrin: the First plant natural product WithIn VivoActivity AgainstTrypanosoma brucei. Planta Med. 2012 doi: 10.1055/s-0031-1298241. [DOI] [PubMed] [Google Scholar]
- 148.Cicco P.D., Busà R., Ercolano G., Formisano C., Allegra M., Taglialatela‐Scafati O., Ianaro A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting <scp>MAPK</Scp>, <scp>NF‐κB,</Scp> and Nrf‐2 signaling pathways in vitro. Phytother Res. 2020 doi: 10.1002/ptr.6906. [DOI] [PubMed] [Google Scholar]
- 149.Pulito C., Mori F., Sacconi A., Casadei L., Ferraiuolo M., Valerio M., Santoro R., Goeman F., Maidecchi A., Mattoli L., Manetti C., Agostino S.D., Muti P., Blandino G., Strano S. Cynara Scolymus affects Malignant Pleural Mesothelioma by promoting apoptosis and restraining invasion. Oncotarget. 2015 doi: 10.18632/oncotarget.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ukeh D.A., Umoetok S.B.A. Repellent effects of five monoterpenoid Odours against Tribolium castaneum (Herbst) and Rhyzopertha Dominica (F.) in calabar, Nigeria. Crop Protect. 2011 doi: 10.1016/j.cropro.2011.05.016. [DOI] [Google Scholar]
- 151.Abdelrazik A., Sallam A., Salem H., Hafez E., Abdel Razik E. Potential insecticidal activity of some medicinal plants essential oils against red flour beetle ,Tribolium castaneum (herbst) (Coleoptera:tenebrionidae) Journal of Plant Protection and Pathology. 2018;9(4):253–259. doi: 10.21608/jppp.2018.41398. [DOI] [Google Scholar]
- 152.Islam N., Khan A.R., Saleh D., Kumar Pk A., Abdullah M. vol. 30. University Journal of Zoology, Rajshahi University; 2012. pp. 25–28. (Insecticidal and Repellent Activities of the Chloroform Extracts of Urena Sinuata L. Against Tribolium castaneum (Herbst) Adults). [DOI] [Google Scholar]
- 153.Yang F.-L., Zhu F., Lei C. Garlic essential oil and its major component as fumigants for controlling Tribolium castaneum (herbst) in chambers filled with stored grain. J. Pest. Sci. 2010 doi: 10.1007/s10340-010-0300-y. [DOI] [Google Scholar]
- 154.Chaubey M.K. International Journal of Zoology and Applied Biosciences; 2023. Red Flour Beetle, Tribolium Castaneum (Herbst): Biology and Management. [DOI] [Google Scholar]
- 155.Ngufor C., Agbevo A., Fagbohoun J., Fongnikin A., Rowland M. Efficacy of royal guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci. Rep. 2020 doi: 10.1038/s41598-020-69109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lunz K., Stappen I. Back to the roots—an overview of the chemical composition and bioactivity of selected root-essential oils. Molecules. 2021 doi: 10.3390/molecules26113155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mokhtar M.M., Li J., Du Z., Cheng F. Preliminary phytochemical analysis and biological evaluation of some medicinal Chinese plant extracts against Tribolium castaneum. Sains Malays. 2021 doi: 10.17576/jsm-2021-5008-12. [DOI] [Google Scholar]
- 158.Zhang J.S., Zhao N., Liu Q.Z., Liu Z.L., Du S.S., Zhou L., Deng Z.W. Repellent constituents of essential oil of cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011 doi: 10.1021/jf202266n. [DOI] [PubMed] [Google Scholar]
- 159.Elshabrawy A., Salem M., Ahmed F., Nabeeh A., Abdel-Wahhab K.G. Egyptian Academic Journal of Biological Sciences C Physiology and Molecular Biology. 2023. The therapeutic and synergistic effect of saussurea Costus extract against induced leukemia in adult male rats. [DOI] [Google Scholar]
- 160.Tsenguun T., Altanchime A., Soyolmaa G., Otgonsugar P., Byambajav T., Batkhuu J., Davaapurev B.-O. Extract of scabiosa comosa exhibits an anti-inflammatory effect on carrageenan and lipopolysaccharide-induced acute inflammation in rats. Int. J. Pharmacol. 2023 doi: 10.3923/ijp.2023.157.165. [DOI] [Google Scholar]
- 161.Saif-Al-Islam M. Sohag Medical Journal; 2020. Saussurea Costus May Help in the Treatment of COVID-19. [DOI] [Google Scholar]
- 162.Karbalaiee M., Daneshpajooh A., Khanjani N., Sohbati S., Mehrabani M., Mehrbani M., Mehrabani M. Efficacy of frankincense‐based herbal product in urinary incontinence: a randomized, double‐blind, placebo‐ and active‐controlled clinical trial. Phytother Res. 2022 doi: 10.1002/ptr.7691. [DOI] [PubMed] [Google Scholar]
- 163.Chandrakar A.K., Alexander A., Rajappa M., Rajendiran K.S., Ramasamy K. International Archives of Otorhinolaryngology; 2019. 25-Hydroxyl Vitamin D Deficiency in Nasal Polyposis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Насухова Н.М., Konovalov D.A., Orobinskaya V.N., Galdin E.V. Determination of biologically active compounds (costunolide and dehydrocostuslactone) in the leaves of some forms of laurel noble (sweet bay) IOP Conf. Ser. Earth Environ. Sci. 2021 doi: 10.1088/1755-1315/941/1/012015. [DOI] [Google Scholar]
- 165.Secchi C., Orecchioni M., Carta M., Galimi F., Turrini F.M., Pantaleo A. Signaling response to transient redox stress in human isolated T cells: molecular sensor role of Syk kinase and functional involvement of IL2 receptor and L-selectine. Sensors. 2020 doi: 10.3390/s20020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Okugawa H., Ueda R., Matsumoto K., Kawanishi K., Kato A. Effect of dehydrocostus lactone and costunolide from saussurea root on the central nervous system in mice. Phytomedicine. 1996 doi: 10.1016/s0944-7113(96)80028-6. [DOI] [PubMed] [Google Scholar]
- 167.Salminen A., Lehtonen M., Suuronen T., Kaarniranta K., Huuskonen J. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008;65(19):2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Y., Ni Z., Dong M., Cong B., Shi Q., Gu Y., Kiyota H. Secondary metabolites of plants from the genus saussurea: chemistry and biological activity. Chem. Biodivers. 2010;7(11):2623–2659. doi: 10.1002/cbdv.200900406. [DOI] [PubMed] [Google Scholar]
- 169.Al-Duais M.A.H., Al-Awthan Y.S. Hepatoprotective effect of Costus roots extract against carbon tetrachloride (CCl4)-induced liver injury in Guinea pigs. J. Life Sci. 2017;11(4) doi: 10.17265/1934-7391/2017.04.002. [DOI] [Google Scholar]
- 170.Al-Nafie F.S., Hussein H.J., Al-Rubaye A.F. Antifungal Efficacy of the Crude Alkaloid, Flavonoid, and Terpenoid of Saussurea Costus (Falc.) Lipschitz Roots Against Aspergillus Species Isolated From Rice Seeds. 2024;11(2):392. doi: 10.62940/als.v11i2.2403. [DOI] [Google Scholar]
- 171.Yu R., Liu Y., Qu R., Patel M. Experimental study on spraying performance of biological pesticides in aerial rotary cage nozzle. Int. J. Agric. Biol. Eng. 2020 doi: 10.25165/j.ijabe.20201306.5511. [DOI] [Google Scholar]
- 172.Middleton D., Pallister J., Klein R., Feng Y., Haining J., Arkinstall R., Frazer L., Huang J.C., Edwards N., Wareing M.D., Elhay M.J., Hashmi Z., Bingham J., Yamada M., Johnson D.A., White J.R., Foord A.J., Heine H.G., Marsh G.A., Broder C.C., Wang L.F. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 2014 doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sclare A.B. Chapter 6: stress and asthma. Int. J. Clin. Pract. 1959 doi: 10.1111/j.1742-1241.1959.tb04487.x. [DOI] [PubMed] [Google Scholar]
- 174.Sastry M., Dutta N. A method for preparing tincture saussurea. Indian J. Pharm. 1961;23:247–249. [Google Scholar]
- 175.Mammate N., Imtara H., Belchkar S., Lahrichi A., Aliqahtani S., Noman M., Tarayrah M., Sqalli Houssaini T. 2022. Anti-Urolithiatic Activity of Aqueous and Ethanolic Extracts from Saussurea costus (Falc) Lispich Using Scanning Electron Microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Salooja K., Sharma V., Siddiqui S. Chemical examination of the roots of Saussurea lappa, Clarke-Part I: on the reported isolation of the alkaloid" Saussurine. J Sci Industr Res B. 1950;9:1–4. [Google Scholar]
- 177.Cala A., Zorrilla J.G., Rial C., Molinillo J.M.G., Varela R.M., Macías F.A. Easy access to alkoxy, amino, carbamoyl, hydroxy, and thiol derivatives of sesquiterpene lactones and evaluation of their bioactivity on parasitic weeds. J. Agric. Food Chem. 2019 doi: 10.1021/acs.jafc.9b03098. [DOI] [PubMed] [Google Scholar]
- 178.Baker P.M., Fortes C.C., Fortes E.G., Gazzinelli G., Gilbert B., Lopes J.N., Pellegrino J., Tomassini T.C., Vichnewski W. Chemoprophylactic agents in schistosomiasis: eremanthine, costunolide, -cyclocostunolide and bisabolol. J. Pharm. Pharmacol. 1972;24(11):853–857. doi: 10.1111/j.2042-7158.1972.tb08903.x. [DOI] [PubMed] [Google Scholar]
- 179.Silva L.B., Strapasson R.L.B., Riva D., Salvador M.J., Stefanello M.É.A. Triterpenes from the flowers of gochnatia polymorpha subsp. Floccosa. Revista Brasileira De Farmacognosia. 2011 doi: 10.1590/s0102-695x2011005000031. [DOI] [Google Scholar]
- 180.Frey M., Schmauder K., Pateraki I., Spring O. Biosynthesis of Eupatolide—a metabolic Route for sesquiterpene lactone formation involving the P450 enzyme CYP71DD6. ACS Chem. Biol. 2018 doi: 10.1021/acschembio.8b00126. [DOI] [PubMed] [Google Scholar]
- 181.Szymański P., Ahmed S.A., Radwan M.M., Khalifa S., Fahmy H. Evaluation of anti-melanoma activities of 8,12-epoxy-2,6-Cembradiene-4,11-Diol, epoxy-4,12-epoxy-2,6-Cembradiene and)-Cembra-2 rien-4,13-Diol from the red Sea Soft coral Sarcophyton Glaucum. Nat. Prod. Commun. 2014 doi: 10.1177/1934578x1400900821. [DOI] [PubMed] [Google Scholar]
- 182.Cankar K., Houwelingen A.v., Bosch D., Sonke T., Bouwmeester H.J., Beekwilder J. A Chicory cytochrome P450 Mono-Oxygenase CYP71AV8 for the oxidation of (+)-Valencene. FEBS Lett. 2010 doi: 10.1016/j.febslet.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 183.Kraker J.-W.d., Franssen M.C.R., Joerink M., Groot A.d., Bouwmeester H.J. Biosynthesis of costunolide, dihydrocostunolide, and Leucodin. Demonstration of cytochrome P450-Catalyzed formation of the lactone ring present in sesquiterpene lactones of Chicory. Plant Physiology. 2002 doi: 10.1104/pp.010957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li J., Chen C., Wan X., Ge Y., Bao S., Wang F., Wang K., Song T., Han P., Jiang H. Identification of the sesquiterpene synthase AcTPS1 and high production of (–)-Germacrene D in Metabolically Engineered Saccharomyces Cerevisiae. Microb. Cell Factories. 2022 doi: 10.1186/s12934-022-01814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jung J.H., Kim Y., Lee C.-O., Kang S.S., Park J.-H., Im K.S. Cytotoxic constituents of Saussurea lappa. Arch Pharm. Res. (Seoul) 1998;21:153–156. doi: 10.1007/BF02974020. [DOI] [PubMed] [Google Scholar]
- 186.Yoshikawa M., Hatakeyama S., Inoue Y., Yamahara J., Saussureamines A. B, C, D, and E, new anti-ulcer principles from Chinese Saussureae Radix. Chemical and pharmaceutical Bulletin. 1993;41(1):214–216. doi: 10.1248/cpb.41.214. [DOI] [PubMed] [Google Scholar]
- 187.Matsuda H., Kageura T., Inoue Y., Morikawa T., Yoshikawa M. Absolute stereostructures and syntheses of saussureamines A, B, C, D and E, amino acid–sesquiterpene conjugates with gastroprotective effect, from the roots of Saussurea lappa. Tetrahedron. 2000;56(39):7763–7777. [Google Scholar]
- 188.Ikuta A., Tomiyasu H., Morita Y., Yoshimura K. Ursane-and oleanane-type triterpenes from ternstroemia g ymnanthera callus tissues. Journal of natural products. 2003;66(8):1051–1054. doi: 10.1021/np030069v. [DOI] [PubMed] [Google Scholar]
- 189.Cava M.P., Hwang B.-H., Meter J.P.V. A stable Naphtho derivative of Cyclobutadiene. J. Am. Chem. Soc. 1963 doi: 10.1021/ja00907a029. [DOI] [Google Scholar]
- 190.Zhang J., Chen L., Yin H., Jin S., Liu F., Chen H. Mechanism study of humic acid functional groups for Cr (VI) retention: two-dimensional FTIR and 13C CP/MAS NMR correlation spectroscopic analysis. Environmental Pollution. 2017;225:86–92. doi: 10.1016/j.envpol.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 191.Singh R., Sitaramaiah K. Control of plant parasitic nematodes with organic soil amendments. PANS Pest Artic. News Summ. 1970;16(2):287–297. [Google Scholar]
- 192.Bose B., Saifi A., Vijayvargiya R., Sharma S. Some aspects of phytochemical and pharmacological study of Saussurea lappa clarke. J. Pharmaceut. Sci. 1961;50(8):679–681. [Google Scholar]
- 193.Namboodiripad C., Kulkarni G., Kelkar G. Isolation of taraxasterol and its acetate from the leaves of costus, Saussurea lappa clarke. Curr. Sci. 1968;37(19):550–551. [Google Scholar]
- 194.Omer R.E., Koua F.H.M., Abdelhag I.M., Ismail A.M. Gas chromatography/mass spectrometry profiling of the costus plant Saussurea lappa (Decne.) CB Clarke root extracts and their anti-bacterial activity. J. Appl. Pharmaceut. Sci. 2019;9(5):73–81. [Google Scholar]
- 195.Viswanathan P., Kulkarni P. Properties and application of inulinase obtained by fermentation of costus (Saussurea lappa) root powder with Aspergillus Niger. Food Nahrung. 1995;39(4):288–294. [Google Scholar]
- 196.Semwal R., Joshi K., Pandian A., Badoni P., Semwal D. Biological applications and secondary metabolites of Saussurea costus. Falc.) Lipsch, J. Conv. Knowl. Holist. Health. 2020;4:1–8. [Google Scholar]
- 197.Dawood H.M., Barghouth N.M., El-Mezayen N.S., Ibrahim R.S., Shawky E. Metabolomic insights into the therapeutic mechanisms of costus (Saussurea costus (Falc.) Lipsch.) root extract in propylthiouracil-induced hypothyroidism rat model. J. Ethnopharmacol. 2024 doi: 10.1016/j.jep.2024.117784. [DOI] [PubMed] [Google Scholar]
- 198.Chaturvedi P., Shukla S., Tripathi P., Chaurasia S., Singh S., Tripathi Y. Comparative study of Inula Racemosa and saussurea lappa on the glucose level in Albino rats. Ancient Sci. Life. 1995;15(1):62. [PMC free article] [PubMed] [Google Scholar]
- 199.Ramezani Farani M., Farsadrooh M., Zare I., Gholami A., Akhavan O. Green synthesis of magnesium oxide nanoparticles and Nanocomposites for photocatalytic antimicrobial, Antibiofilm and antifungal applications. Catalysts. 2023;13(4):642. [Google Scholar]
- 200.Khan A., Shah A.H., Ali N. In-vitro propagation and phytochemical profiling of a highly medicinal and endemic plant species of the Himalayan region (Saussurea costus) Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-03032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Elgharabawy R.M., El Tantawy El Sayed I., Abd-Allah Rezk N., Tousson E. Therapeutic impact of Costus (saussurea lappa) against Ehrlich solid tumor-induced cardiac toxicity and DNA damage in female mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.708785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sagar A., Chauhan V., Prakash V. Studies on endophytes and antibacterial activity of Saussurea costus (falc.) J. Drug Deliv. Therapeut. 2017;7(2):5–10. [Google Scholar]
- 203.Khan A., Shah A.H., Ali N. In-vitro propagation and phytochemical profiling of a highly medicinal and endemic plant species of the Himalayan region (Saussurea costus) Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-03032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ahmed H.Y., Kareem S.M., Atef A., Safwat N.A., Shehata R.M., Yosri M., Youssef M., Baakdah M.M., Sami R., Baty R.S., Alsubhi N.H., Alrefaei G.I., Shati A.A., Elsaid F.G. Optimization of Supercritical Carbon Dioxide extraction of saussurea costus oil and its antimicrobial, antioxidant, and anticancer activities. Antioxidants. 2022;11(10) doi: 10.3390/antiox11101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.El-Nashar H.A.S., Eldahshan O.A., Fattah N.F.A., Loutfy S.A., Abdel-Salam I.M. HPLC-ESI/MS-MS characterization of compounds in Dolomiaea costus extract and evaluation of cytotoxic and antiviral properties: molecular mechanisms underlying apoptosis-inducing effect on breast cancer. BMC Complement Med Ther. 2023;23(1):354. doi: 10.1186/s12906-023-04164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Abdul M.R., Rahim S.M., Saleh A.H. Cardioprotective activity of Costus root ethanol extract in experimentally-induced hypothyroidism in female Albino rats. HAYATI Journal of Biosciences. 2023;30(6):1054–1060. [Google Scholar]
- 207.Taha A. Microwave-sample-Preparation-system-assisted Biogenic synthesis of Copper oxide Nanoplates using root aqueous extract and its environmental Catalytic activity. Catalysts. 2022;12(10) ARTN1115 10.3390/catal12101115. [Google Scholar]
- 208.El-Rahman G.I.A., Behairy A., Elseddawy N.M., Batiha G.E.S., Hozzein W.N., Khodeer D.M., Abd‐Elhakim Y.M. Saussurea lappa ethanolic extract Attenuates Triamcinolone Acetonide-induced pulmonary and splenic tissue damage in rats via modulation of oxidative stress, inflammation, and apoptosis. Antioxidants. 2020 doi: 10.3390/antiox9050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kamilijiang M., Zang D., Abudukelimu N., Aidarhan N., Liu G., Aisa H.A. Anti-melanogenesis effect of polysaccharide from Saussurea involucrata on forskolin-induced melanogenesis in B16F10 melanoma cells. Nutrients. 2022;14(23):5044. doi: 10.3390/nu14235044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Herrando‐Moraira S., Group C.R., Calleja J.A., Chen Y.S., Fujikawa K., Galbany‐Casals M., Garcia‐Jacas N., Kim S.C., Liu J.Q., López‐Alvarado J. Generic boundaries in subtribe Saussureinae (Compositae: Cardueae): insights from Hyb‐Seq data. Taxon. 2020;69(4):694–714. [Google Scholar]
- 211.Holfus C.M., Boyd C.S., Rios R.C., Davies K.W., Copeland S.M., Mata-González R. Wyoming Big Sagebrush Transplant Survival and growth affected by Age, Season of planting, and Competition. Rangel. Ecol. Manag. 2024;92:1–11. [Google Scholar]
- 212.Al-Zayadi Z., Shanan H., Akool S. Evaluation of the anticancer effect of Saussurea costus root extract against induced hepatic and renal cancer in white mice: a histopathological study. Adv. Anim. Vet. Sci. 2023;11(7):1065–1076. [Google Scholar]
- 213.Deabes M., Aboulthana W., Marzouk E.E.-D., Mohamed M.I., Ahmed K.A. Evaluation of hepato-and neuroprotective effect of chemical constituents in Saussurea costus extract against the toxicity induced by Chloropyrifos ethyl in rats. Egypt. J. Chem. 2021;64(2):631–647. [Google Scholar]
- 214.Elshaer S.E., Hamad G.M., Hafez E.E., Baghdadi H.H., El-Demerdash F.M., Simal-Gandara J. Root extracts of Saussurea costus as prospective detoxifying food additive against sodium nitrite toxicity in male rats. Food Chem. Toxicol. 2022;166 doi: 10.1016/j.fct.2022.113225. [DOI] [PubMed] [Google Scholar]
- 215.Mohsen E., El-Far A.H., Godugu K., Elsayed F., Mousa S.A., Younis I.Y. SPME and solvent-based GC–MS metabolite profiling of Egyptian marketed Saussurea costus (Falc.) Lipsch. concerning its anticancer activity. Phytomedicine. 2022;2(1) [Google Scholar]
- 216.Bellinato F., Maurelli M., Gisondi P., Girolomoni G. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J. Clin. Med. 2021;10(22):5344. doi: 10.3390/jcm10225344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Idriss H., Siddig B., Maldonado P.G., Elkhair H., Alakhras A., Abdallah E.M., Torres P.H.S., Elzupir A.O. Phytochemical Discrimination, biological activity and molecular docking of water-soluble inhibitors from saussurea costus herb against main protease of SARS-CoV-2. Molecules. 2022;27(15):4908. doi: 10.3390/molecules27154908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are available for future research.