Abstract
While it is well established that cellular activation can increase human immunodeficiency virus (HIV) replication in T lymphocytes, it is also clear that both activated CD8+ and CD4+ T lymphocytes mediate anti-HIV activity. To assess the relative importance of these contrary effects on HIV replication in vivo, we evaluated the consequences of Mycobacterium bovis BCG and staphylococcal enterotoxin B (SEB) inoculation in vivo in rhesus monkeys chronically infected with simian immunodeficiency virus of macaques (SIVmac). BCG inoculation induced as much as a 2.5-log reduction of plasma and intracellular SIV RNA in SIVmac-infected monkeys. This down-regulation of virus replication persisted as long as 4 weeks after BCG inoculation. Similarly, SEB injection resulted in up to a 3-log decrease in plasma and intracellular SIV RNA in SIVmac-infected macaques. Interestingly, the short-term reduction of viremia in these monkeys correlated with the peak in vivo production of SEB- and BCG-induced cytokine responses. However, no long-term clinical benefit was observed in the SIVmac-infected macaques. These studies provide in vivo evidence that potent T-cell stimulation driven by antigens other than the virus itself can, under some circumstances, mediate short-term reduction of viremia in AIDS virus-infected individuals.
It has long been appreciated that the activation of the immune system in the absence of antiretroviral therapy can increase viral replication and accelerate disease progression in an AIDS virus infection (6, 16, 23, 24, 30). In previous studies, it was demonstrated that inoculation of macaques with Mycobacterium bovis BCG or staphylococcal enterotoxin B (SEB) superantigen resulted in the activation and expansion of T-cell subpopulations (10, 30). Moreover, the prolonged stimulation of T cells caused by persistent BCG infection was associated with an increase in simian immunodeficiency virus (SIV) loads and accelerated progression of clinical AIDS in monkeys coinfected with SIV of macaques (SIVmac) and BCG (30).
It is also true that T lymphocytes play an important role in regulating AIDS virus replication in infected individuals. Virus-specific, major histocompatibility complex class I-restricted CD8+ T cells have been shown to suppress human immunodeficiency virus type 1 (HIV-1) or SIV replication in vitro (7, 11). The in vivo expansion of populations of CD8+ cytotoxic T lymphocytes (CTL) correlates temporally with the clearance of viral antigenemia during primary infection of humans with HIV and macaques with SIV or simian-human immunodeficiency virus (1). Moreover, in chronically HIV-1-infected humans, high levels of circulating CD8+ CTL are associated with low viral loads (17). Finally, depletion of CD8+ T cells in monkeys resulted in marked increases in plasma SIV RNA during primary and chronic infection (8, 14, 22).
CD4+ T cells also probably contribute to antiviral activity in AIDS virus-infected individuals (20, 27). Potent proliferative responses of HIV-1-specific CD4+ T cells are associated with the control of viremia in HIV-1-infected humans (20). In a Hu-SCID mouse model, the adoptive transfer of human CD4+ T cells can contribute to the control of HIV-1 infection (26). It has been suggested that intermittent interleukin-2 (IL-2) treatment may be able to enhance the anti-HIV-1 activity of highly active antiretroviral therapy in HIV-1-infected humans (3). While it has been presumed that IL-2 exerts its antiviral effect in this setting by increasing CD4+-T-cell numbers, the mechanism underlying this activity remains poorly understood.
In the present study, we assessed the virologic consequences of T-cell activation in AIDS virus-infected individuals, evaluating the potential conflicting T-cell-mediated increased viral replication and antiviral activity. We examined the magnitude of T-cell activation driven by BCG and SEB, as well as the association of this activation with the changes in viral loads in SIVmac-infected macaques. We found that early T-cell activation driven by BCG or SEB is associated with the short-term reduction of viral replication in SIVmac-infected macaques.
MATERIALS AND METHODS
Animals and virus.
Rhesus monkeys (Macaca mulatta), 3 to 5 years old, were used in these studies. The animals were maintained in accordance with the guidelines of the Committee on Animals for Harvard Medical School and the Guide for the Care and Use of Laboratory Animals. All monkeys were inoculated intravenously with SIVmac strain 251, as described previously (1). Six macaques, designated here as SIVmac-infected monkeys, were infected with SIVmac for 3 months prior to BCG infection or BCG reinfection. All of these monkeys had CD4+ peripheral blood lymphocyte (PBL) counts greater than 900/μl before BCG infection or BCG reinfection. For SEB challenge, four chronically SIVmac-infected macaques were evaluated. The CD4+ PBL counts of these animals were between 500 and 700/μl at the time they received SEB.
M. bovis BCG coinfection.
M. bovis BCG (Pasteur strain) was generously provided by Scott Koenig, MedImmune Inc. BCG was stored in liquid nitrogen and thawed immediately before inoculation. Monkeys 148, 269, 220, and 276 were inoculated sequentially with BCG, then SIVmac, and finally BCG at 2-month intervals. For BCG infection or reinfection (second inoculation), the macaques were inoculated intravenously with 108 CFU of BCG. After BCG inoculation, the monkeys were monitored for signs of clinical illness.
SEB inoculation.
SIVmac-infected monkeys were injected intramuscularly with SEB (Toxin Technology, Sarasota, Fla.) at a dose of 0.3 μg/kg of body weight (10). Following SEB inoculation, collection of blood samples and lymph node biopsies were done as previously described (10).
Isolation and fractionation of lymphocyte populations in blood and lymph nodes.
Peripheral blood mononuclear cells were isolated from EDTA-anticoagulated blood of the monkeys using Ficoll-diatrizoate gradient centrifugation. Peripheral lymph nodes were obtained by standard biopsy procedures before and after BCG inoculation and were carefully teased to generate single-cell suspensions. CD4+ or CD8+ lymphocytes were purified using anti-CD4 or anti-CD8 antibody-conjugated Dynabeads (Dynal, Inc., Great Neck, N.Y.), as described previously (30). Peripheral blood mononuclear cells or lymph node cells were incubated with these immunomagnetic beads for 30 min at room temperature and then selected in two cycles with a magnetic particle concentrator. Monocytes/macrophages in blood or tissues were purified either by anti-CD14 (Immunotech, Westbrook, Maine)-coupled immunomagnetic beads, as described above, or by adherence to culture flasks in a 1-h incubation, as described previously (19). Monocytes/macrophages purified by these methods contained less than 5% CD4+ lymphocytes.
mRNA extraction and cDNA synthesis.
mRNA was extracted from unfractionated or fractionated lymphocytes using guanidinium thiocyanate- and oligo(dT)-spun columns (mRNA extraction kit; Pharmacia, Piscataway, N.J.). The first-strand cDNA was synthesized in a 20-μl volume at 420C for 1 h using approximately 0.2 to 1 μg of mRNA, 1 μg of random hexanucleotides, and 5 U of reverse transcriptase (Promega, Madison, Wis.). The samples were heated for 5 min at 95°C to terminate the reaction.
Proliferation assay.
Conventional proliferation assays were carried out as described previously. Briefly, macaque PBL (105 cells per well) were cultured in triplicate in 96-well plates in the presence of BCG purified protein derivative (PPD) (three different concentrations), concanavalin A, bovine serum albumin, or medium alone. Five days later, cells were pulsed with [3H]thymidine at 1.0 μCi per well, and uptake was measured 8 h later using a 1450 Microbeta scintillation counter (Wallac, Gaithersburg, Md.). The stimulation index was defined as the ratio of the mean counts per minute of PPD-, concanavalin A-, or bovine serum albumin-stimulated wells relative to the mean counts per minute of control wells (medium alone).
ELISA quantitation of plasma cytokines.
IL-2, IL-4, IL-10, and gamma interferon (IFN-γ) were measured in plasma samples using enzyme-linked immunosorbent assay (ELISA) kits that are commercially available from Biosource, International (Camarillo, Calif.). Plasma samples from 10 uninfected macaques were included as controls.
Quantitative measurement of plasma SIV RNA.
Measurement of plasma SIV RNA was done using QC-PCR as described previously (18, 30). Briefly, viral RNA was extracted by following the instructions of the RNA extraction kit from Qiagen (Valencia, Calif.). The extracted RNA was aliquoted into six different tubes, which contained defined numbers of copies of SIVmac gag competitor RNA. The RNA mixtures were reverse transcribed to cDNA and competitively amplified by a 35-cycle PCR using a pair of SIVmac gag-specific primers (30). The amplified PCR products containing the wild type and competitor were separated on 2% agarose gels and measured for their densities in a GS 700 imaging densitometer (Bio-Rad). Quantitation was achieved by data analysis using Molecular Analyst system software (Bio-Rad). The coefficient of variation of intra- and interassays using this protocol was less than 20%. The sensitivity of the QC-PCR was 4 × 102 RNA copies in 1 ml of plasma. As a complementary study, plasma SIV RNA was also quantitated by the branched-DNA (bDNA) assay (Chiron, Emeryville, Calif.). This assay allows detection of a minimum of 104 RNA copies/ml. As controls, two SIVmac-infected monkeys were injected intravenously with tissue culture supernatant from an uninfected CEM T-cell line and assessed for changes in plasma SIV RNA (1).
PCR-based semiquantitation of intracellular SIVmac RNA.
To analyze intracellular SIVmac RNA expression, purified CD4+ lymphocytes and CD14+ cells, as well as adherent monocytes, were subjected to mRNA extraction and cDNA synthesis. The PCR-based semiquantitation was performed as described previously (30). Briefly, the PCR products were separated on a 2% agarose gel, transferred onto a nylon membrane, and then hybridized to a 32P-labeled internal oligonucleotide. Defined numbers of copies of SIV gag cDNA were always included as standards for the semiquantitation. Quantitation was based on the radioactivity measured by an AMBIS 100 (1, 30) or on the density determined using a GS 700 imaging densitometer (Bio-Rad). To normalize SIVmac RNA expression levels, the β-actin housekeeping gene was similarly quantitated using the same amount of cellular cDNA. Semiquantitation was achieved by calculating the number of copies of SIV gag RNA in 1.66 × 10−16 M actin-containing cellular RNA (approximately 106 cells).
Statistical analysis.
The paired Student t test, as described previously (5), was employed to examine whether the BCG- or SEB-associated decrease in plasma SIV RNA was statistically significant. The log scales of plasma SIV RNA and the log reduction after BCG coinfection or SEB injection were calculated and compared as described previously (5).
RESULTS
BCG coinfection induced a transient decrease in plasma SIV RNA in SIVmac-infected macaques.
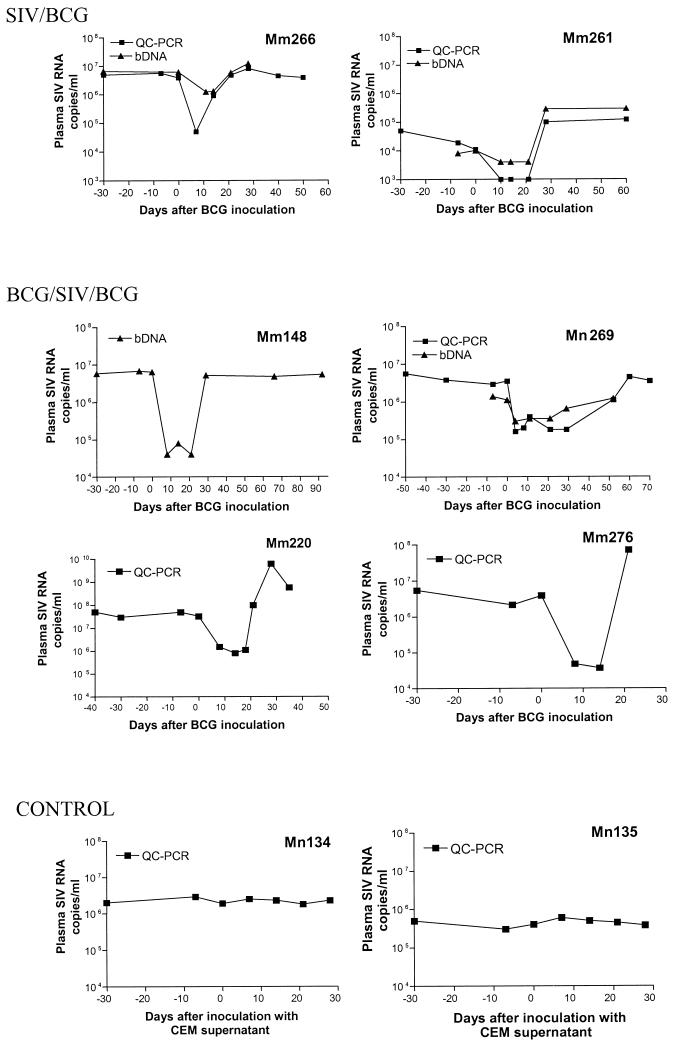
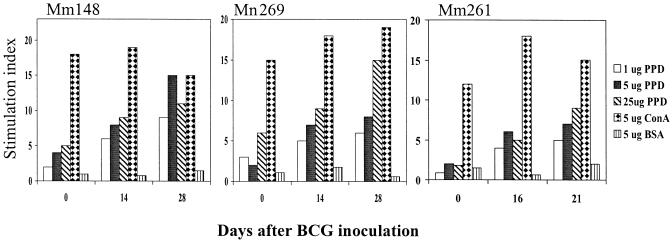
To determine the effect on SIV replication of a BCG-induced stimulation of the immune system, two groups of SIVmac-infected monkeys were inoculated intravenously with BCG and assessed for changes in viral loads. In the first group of SIVmac-infected macaques, animals that were naïve to BCG, the BCG coinfection resulted in a transient decrease in the level of plasma SIV RNA (Fig. 1). Similarly, the BCG coinfection induced a decrease in plasma SIV RNA in the group of macaques that were sequentially infected with BCG, followed by SIVmac, and finally again by BCG. Up to a 2.5-log reduction in plasma SIV RNA was detected in the SIVmac-infected monkeys 4 to 21 days after BCG inoculation, as measured by bDNA assay and QC-PCR (Fig. 1). In contrast, the control SIVmac-infected macaques did not show significant changes in plasma SIV RNA levels after injection with culture supernatant from the CEM cell line (Fig. 1). The BCG-associated decrease in plasma SIV RNA was statistically significant (P < 0.05 by a paired t test). Furthermore, the reduction in plasma SIV RNA coincided with an enhanced proliferative response of BCG PPD-specific T cells after BCG reinfection (Fig. 2). These results, therefore, demonstrate that BCG-induced immune stimulation can result in an early decrease in SIV viremia in SIVmac-infected macaques.
FIG. 1.
BCG coinfection resulted in a transient decrease in virus loads in the macaques primarily infected with SIVmac. Shown in the upper panel (SIV/BCG) is the change in the copy numbers of plasma SIV RNA on different days following the BCG inoculation. The inoculation was done on day 0. Days 0 and −30 after the BCG inoculation were equivalent to the days 86 and 56, respectively, after the SIVmac infection. The middle panel (BCG/SIV/BCG) shows the change in the copy numbers of plasma SIV RNA following BCG reinfection of SIVmac-infected macaques. These macaques were inoculated with BCG before the SIVmac infection. Shown in the lower panel (CONTROL) is the change in the copy numbers of plasma SIV RNA in the control SIVmac-infected monkeys that received CEM supernatant (Reference 1 and Materials and Methods). Plasma SIV RNA was measured by bDNA (triangles) and QC-PCR (squares), as described previously (29).
FIG. 2.
BCG reinfection stimulated proliferative T-cell responses in macaques infected early with SIVmac. Shown are the results of T-cell proliferation in response to BCG PPD antigens following the first (Mm261) and second (Mm148 and Mm269) BCG inoculations. ConA, concanavalin A; BSA, bovine serum albumin.
The BCG-associated decrease in plasma SIV RNA appeared to result from decreased viral replication rather than accelerated decay of plasma virus.
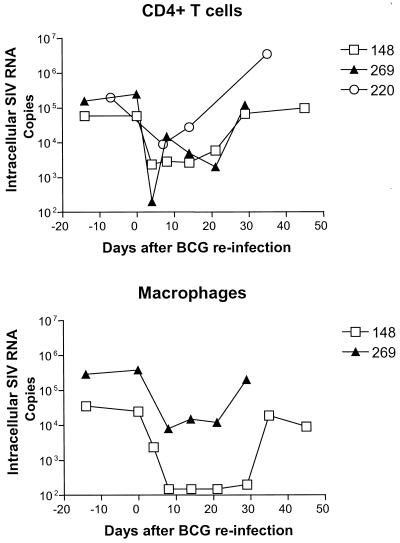
We then sought to determine whether the decrease in plasma SIV RNA reflected an enhanced decay of plasma virions or a decrease in virion production by infected cells. To address this issue, we prospectively measured both the plasma SIV RNA and the intracellular SIV RNA expression in CD4+ T cells and macrophages in lymph nodes, the main major reservoir for the AIDS virus. Coincident with the reduction in plasma SIV RNA in these monkeys during acute BCG reinfection was a marked decrease in intracellular SIV RNA in lymph nodes (Fig. 3). A 2-log decrease in viral RNA was observed in the CD4+ T cells from lymph nodes of these monkeys at the time the plasma SIV RNA was at its nadir. In addition, an associated decrease in intracellular SIV RNA expression was seen in the macrophages derived from the lymph nodes of the SIVmac- and BCG-coinfected macaques (Fig. 3). These results, therefore, suggest that BCG-induced immune stimulation can transiently control SIV replication and reduce viral loads in SIVmac-infected monkeys.
FIG. 3.
Early BCG coinfection induced a consistent decrease in intracellular SIV RNA expression in CD4+ lymphocytes and macrophages purified from the cells in lymph nodes. Intracellular SIV RNA expression was measured by semiquantitative methods as described previously (29). The intracellular SIV RNA was quantitated as copies in 1.66 × 10−16 M actin-containing RNA.
The predominant Th1-like T-cell response driven by BCG coincided with the transient reduction of plasma SIV RNA in SIVmac-infected macaques.
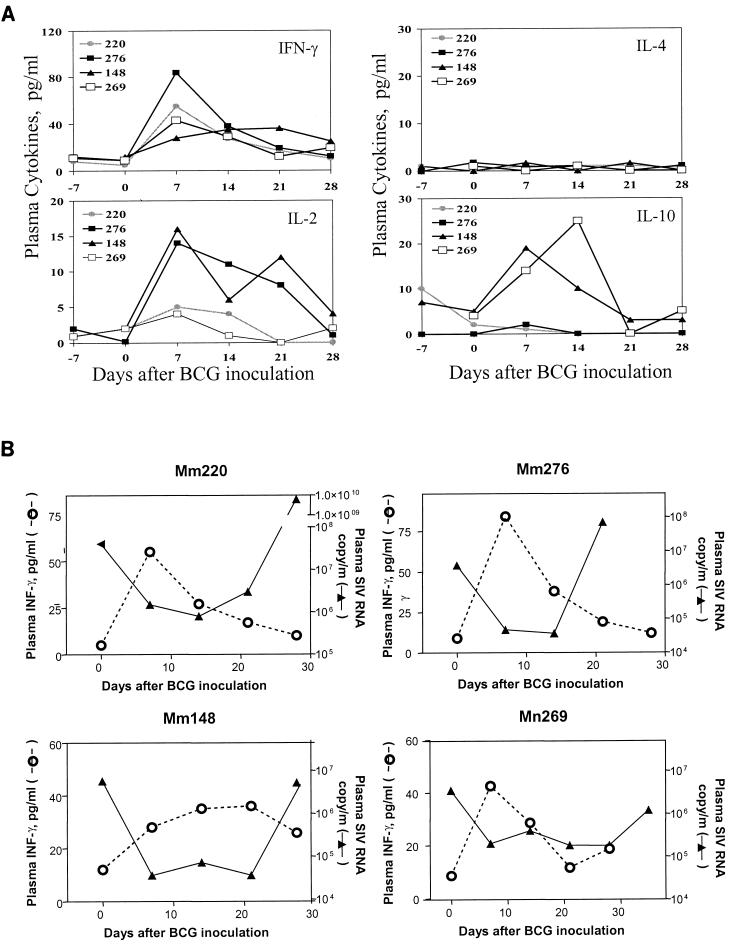
To explore the mechanism responsible for the BCG-induced damping of SIVmac replication, we examined whether the short-term containment of SIVmac replication correlated temporally with the potency of the BCG-driven T-cell activation in the macaques. To address this issue, in vivo cytokine responses were assessed as surrogate markers for the potency of T-cell activation driven by BCG. Following BCG inoculation, an increase in the production of cytokines was observed in the plasma of the SIVmac-infected macaques. An increase in plasma IFN-γ and IL-2 was observed 1 to 2 weeks after BCG inoculation (Fig. 4A), suggesting a BCG-driven T-cell response. No significant change in plasma IL-4 was seen, and an increase in plasma IL-10 was detected in only two monkeys after BCG coinfection (Fig. 4A). Interestingly, the peak of this cytokine response coincided temporally with the early, transient containment of SIVmac replication (Fig. 4B). As the plasma cytokine levels decreased, an increase in plasma SIV RNA was seen in the SIVmac- and BCG-coinfected monkeys (Fig. 4B). These results suggest that an initial potent T-cell response driven by BCG is associated with the transient reduction of viral replication in SIVmac-infected monkeys.
FIG. 4.
Short-term containment of SIVmac replication coincided with the peak phase of the BCG-driven T-cell response. (A) Changes in plasma cytokines IFN-γ, IL-2, IL-4, and IL-10 after BCG inoculation. Plasma cytokines were measured using the ELISA kits for measuring macaque IFN-γ, IL-2, and IL-10, as well as human IL-4. The ELISA kit for measuring human IL-4 can detect macaque IL-4 (data not shown). (B) Correlation between an increase in plasma IFN-γ and a decrease in plasma SIV RNA.
While this transient containment of SIV replication was observed, the long-term clinical consequences of BCG coinfection were clearly detrimental. Viral loads returned to baseline levels or even increased further 3 to 4 weeks after BCG inoculation in the SIVmac-infected monkeys (Fig. 1). A profound decline in CD4+ PBL counts was observed, and fatal BCG dissemination occurred as a result of the chronic coinfection with SIVmac and BCG. All monkeys except for animal 276 died within 4 months of the BCG coinfection (data not shown). While the clinical outcomes in these monkeys confirmed our previous observation that chronic BCG coinfection accelerates the progression of SIV-induced disease, the observations during the period of hyperacute BCG infection indicate that BCG coinfection results in a biphasic change in viral loads in SIVmac-infected monkeys.
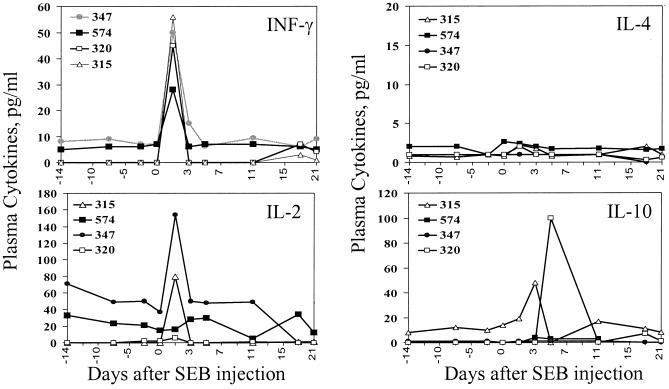
SEB-induced stimulation of IFN-γ-secreting T cells coincided with the short-term control of SIVmac infection.
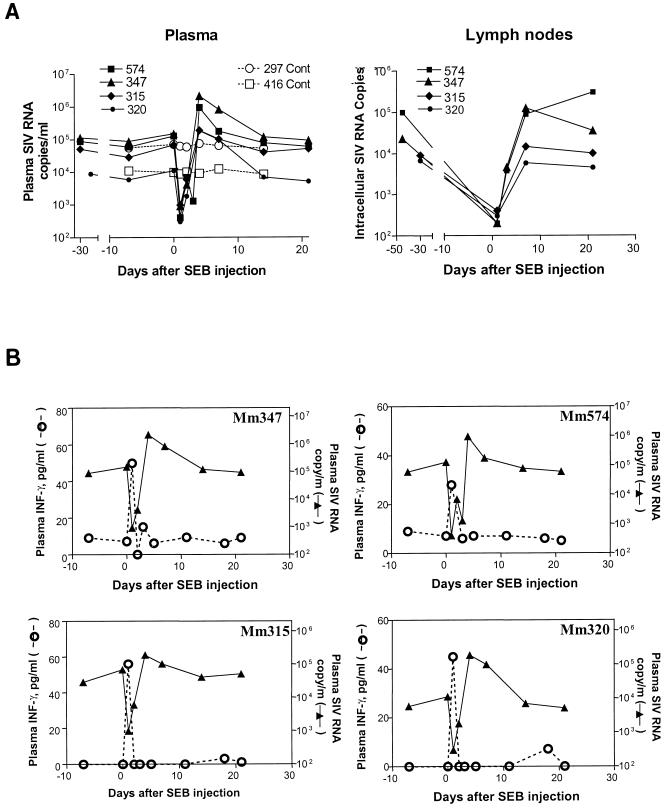
We then sought to confirm that the reduction in SIV replication seen early after BCG coinfection was associated with T-lymphocyte activation. Our previous studies had indicated that the in vivo challenge of macaques with SEB stimulated and expanded approximately 65% the population of their T cells (10). We reasoned that the activation of this diverse population of T cells might down-regulate AIDS virus replication. SIVmac-infected macaques were injected with the T-lymphocyte superantigen SEB to stimulate T cells and were assessed for changes in their viral loads. This exposure induced a T-cell response evidenced by the abrupt increase in their plasma cytokine levels. An increase in production of IFN-γ was seen 1 day after SEB injection of the SIVmac-infected macaques; some of the animals also exhibited an increase in the production of IL-2 and IL-10 (Fig. 5). Consistent with the findings seen in the SIVmac- and BCG-coinfected monkeys, the SEB-induced stimulation of T cells resulted in a striking decrease in SIVmac replication in these monkeys. Up to a 3-log decrease in plasma SIV RNA was seen in the SIVmac-infected macaques 1 to 3 days after SEB injection (Fig. 6A). In addition, consistent decreases in SIV RNA expression were detected in purified CD4+ T cells from lymph nodes of the SEB-injected macaques (Fig. 6A). Importantly, the increase in plasma IFN-γ correlated temporally with a profound decrease in plasma SIV RNA following the SEB injection in the SIVmac-infected macaques (Fig. 6B). As seen in the setting of BCG coinfection, the waning of the IFN-γ cytokine response was associated with a rebound increase in plasma SIV RNA in the SIVmac-infected macaques (Fig. 6B). These results provide additional evidence suggesting that potent T-cell stimulation can result in the short-term reduction of viremia in SIVmac-infected macaques.
FIG. 5.
SEB superantigen induced the apparent cytokine responses in the SIVmac-infected macaques. Shown is an increase in plasma cytokines after the SEB injection of the macaques. The detection of plasma cytokines was the same as that described in the legend to Fig. 4.
FIG. 6.
Initial cytokine response stimulated by SEB correlated with the transient control of viremia. (A) Changes in the copy numbers of plasma SIV RNA and intracellular SIV RNA in lymph node cells. The intracellular SIV RNA was quantitated as copies in 1.66 × 10−16 M actin-containing RNA. (B) Correlation between the abrupt rise of plasma IFN-γ and the sharp decline in plasma SIV RNA in the macaques challenged with SEB.
DISCUSSION
The present studies demonstrate that a potent stimulation of T cells in vivo can be associated with a decrease in viral replication during the hyperacute phase of BCG coinfection or SEB challenge in SIVmac-infected monkeys. Biphasic changes in the control of virus, i.e., an initial decrease followed by an increase in viral replication, correlated temporally with the increase and subsequent decrease in BCG- or SEB-induced T-cell activation in the SIVmac-infected monkeys. The increase in SIV replication seen in the second phase of the response following SEB challenge or BCG coinfection represents the increase in viral replication that has been described following vaccination or opportunistic infections in HIV-1-infected humans (6, 16, 23, 24). The undetectable down-regulation of viral replication following immune activation in those HIV-1-infected humans can perhaps be explained by the limited magnitude of T-cell activation. The T-cell activation that has been studied for HIV-1-infected humans to date is antigen specific and, therefore, restricted to discrete lymphocyte subpopulations. The activation of T cells induced in the present studies in monkeys by inoculation of SEB superantigen and by intravenous injection of a large inoculum of BCG is much greater in magnitude than that occurring in humans who have recently been vaccinated or have a local infection. The early and frequent sampling of blood may help to demonstrate this suppression of AIDS virus replication following immune stimulation in humans. This scenario is supported by a recent study demonstrating that acute scrub typhus coinfection can result in a transient suppression of HIV-1 replication (28).
The difference in SIV replication seen in the hyperacute and chronic phases of BCG coinfection may be explained by the potency of the BCG-driven T-cell activation. The intravenous inoculation of SIVmac-infected monkeys with a large bolus of BCG likely stimulates substantial T-cell activation during the hyperacute phase of BCG coinfection. Our results showing the production of cytokines during BCG coinfection indicate that BCG-mediated T-cell activation is most potent in the first 2 weeks after BCG inoculation, probably during the period of BCG antigenemia. This T-cell activation may reach a threshold that perhaps supersedes T-cell-activation-related enhancement of SIV replication and exerts antiviral activity. In contrast, the magnitude of T-cell activation driven by BCG during the chronic phase of BCG infection may be lower as a result of the control of the mycobacterial load in the setting of an effective anti-BCG immune response. This decreased activation of T cells may result in the waning of T-cell-mediated antiviral activity. As a result, viral replication can rebound to elevated levels in the SIVmac- and BCG-coinfected monkeys. On the other hand, chronic SIVmac and BCG coinfection may lead to the persistence of T-cell activation that is below the antiviral threshold. The low-level and persistent T-cell activation that occurs during chronic SIVmac and BCG coinfection may accelerate both SIV- and BCG-induced diseases (30).
The SEB-induced reduction in SIVmac replication in SIVmac-infected macaques was striking but shorter in duration than that seen in the setting of BCG coinfection. The brief duration of the SEB-induced reduction in viremia may simply reflect the fact that only a single injection of SEB protein was delivered and that protein was rapidly cleared from the body. Nevertheless, the almost 3-log decrease in viral load that persisted for 3 days during the hyperacute phase of SEB stimulation is likely to be biologically significant, since the SIV virions appear to have only a 3.3-min half-life in plasma (29). The finding that the SEB-induced increase in plasma IFN-γ correlated temporally with the duration of the control of viremia provides additional support for the possibility that the initial potent stimulation of T cells contributed to the antiviral activity in these monkeys. The longer duration of immune stimulation driven by BCG antigens during BCG coinfection presumably reflects the length of time BCG replicates in vivo prior to immune control of the mycobacteria (data not shown).
Several factors may contribute to the immune stimulation-associated decreases in viral loads in these monkeys. SEB and BCG both stimulate the activation of CD4+ and CD8+ T-cell subpopulations in macaques (10, 30). These stimulated CD4+ and CD8+ T cells may act in concert to contain viral replication in SIVmac-infected monkeys. The likelihood of cooperation between CD4+ and CD8+ T cells in this regard is supported by the observation that both CD8+ T cells and a vigorous HIV-1-specific CD4+ T-cell response are associated with control of viremia in HIV-1-infected individuals (20). In addition, certain chemokines and cytokines produced during the hyperacute phase of BCG- or SEB-induced T-cell stimulation may contribute to the control of viral infection in SIVmac-infected monkeys. In fact, a cytokine-induced antiviral effect has recently been observed in HIV-1-infected cell lines and CD4+ lymphocytes (9, 12, 21). Furthermore, T-cell activation driven by BCG or SEB may both directly and indirectly augment SIVmac-specific CD8+ T-cell responses in SIVmac-infected monkeys. BCG- or SEB-driven T-cell stimulation may enhance SIV-specific precursor CTL activity as well as their CTL production of chemokines and other antiviral cytokines (3, 15). In studies of SEB-driven T-cell responses in normal macaques, we have observed a marked increase in expression of RANTES and other chemokines after SEB stimulation (data not shown). We certainly cannot exclude the possibility that macrophage-derived cytokines also contribute to the transient down-regulation of SIVmac replication. Further studies are needed to elucidate the precise mechanisms responsible for the antiviral effect exerted by BCG- and SEB-induced immune stimulation.
Therefore, we have demonstrated that the initial potent activation of T cells driven by BCG or SEB coincides temporally with a short-term inhibition of viral replication in SIVmac-infected monkeys. The results of these studies provide in vivo evidence that T-cell activation driven by antigens other than the virus itself can, under some circumstances, contribute to the containment of the viral replication in AIDS virus-infected individuals.
ACKNOWLEDGMENTS
We thank Andrew Lackner, Patricia Fultz, and Andre Namias for critical reviews of the manuscript.
This work was supported by NIH R01 grants RR13601 (to Z.W.C.), HL64560 (to Z.W.C.), and AI20729 (to N.L.L.).
REFERENCES
- 1.Chen Z W, Kou Z, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin N L. T cell receptor Vβ repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric of simian-human immunodeficiency virus. J Exp Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z W, Zhou D, Chalifoux L, Lee-Parritz D, Letvin N L. Desseminated granulomatous disease in a SIVmac- and BCG-infected rhesus monkey. AIDS. 1997;11:266–267. [PubMed] [Google Scholar]
- 3.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Dunn O J, Clark V A. Applied statistics: analysis of variance and regression. 2nd ed. New York, N.Y: Wiley Interscience; 1987. pp. 214–235. [Google Scholar]
- 6.Fultz P N, Gluckman J C, Muchmore E, Girard M. Transient increases in numbers of infectious cells in an HIV-infected chimpanzee following immune stimulation. AIDS Res Hum Retrovir. 1992;8:313–317. doi: 10.1089/aid.1992.8.313. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh F W, Walker C M, Blackbourn D J, Levy J A. Suppression of HIV replication by CD8+ cell clones derived from HIV-infected and uninfected individuals. Cell Immunol. 1994;159:271–279. doi: 10.1006/cimm.1994.1313. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinter A L, Bende S M, Hardy E C, Jackson R, Fauci A S. Interleukin 2 induces CD8+ T cell-mediated suppression of human immunodeficiency virus replication in CD4+ T cells and this effect overrides its ability to stimulate virus expression. Proc Natl Acad Sci USA. 1995;92:10985–10989. doi: 10.1073/pnas.92.24.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kou Z, Halloran M, Lee-Parritz D, Shen L, Simon M, Shen Y, Chen Z W. In vivo effects of a bacterial superantigen on macaque T cell receptor repertoires. J Immunol. 1998;160:5170–5178. [PubMed] [Google Scholar]
- 11.Letvin N L. Animal models for AIDS. Immunol Today. 1990;11:322–326. doi: 10.1016/0167-5699(90)90127-u. [DOI] [PubMed] [Google Scholar]
- 12.Levy J A, Mackewicz C E, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 13.Mackewicz C E, Blackbourn D C, Levy J A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriuchi H, Moriuchi M, Combadiere C, Murphy P M, Fauci A S. CD8+ T-cell-derived soluble factor(s), but not beta-chemokines RANTES, MIP-1 alpha, and MIP-1 beta, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien W A, Grovit-Ferbas K, Namazi A, Ovcak-Derzic S, Wang H J, Park J, Yeramian C, Mao S H, Zack J A. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 17.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 18.Piatak M, Luk K, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–80. [PubMed] [Google Scholar]
- 19.Polyak S, Chen H, Hirsch D, George I, Hershberg R, Sperber K. Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J Immunol. 1997;159:2177–2188. [PubMed] [Google Scholar]
- 20.Rosenberg E S, Billingsley J M, Caliendo M A, Broswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg Z F, Fauci A S. Immunopathogenic mechanisms of HIV infection: cytokine induction of HIV expression. Immunol Today. 1990;11:176. doi: 10.1016/0167-5699(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 23.Stanley S K, Ostrowski M A, Justement J S, Gantt K, Hedayati K S, Mannix M, Roche K, Schwartzentruber D J, Fox C H, Fauci A S. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 24.Staprans S L, Hamilton B L, Follansbee S E, Elbeik T, Barbosa P, Grant R M, Feinberg M B. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsubota H, Lord C I, Watkins D I, Morimoto C, Letvin N L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieillard V, Jouveshomme S, Leflour N, Jean-Pierre E, Debre P, De Maeyer T, Autran B. Transfer of human CD4+ T lymphocytes producing beta interferon in Hu-PBL-SCID mice controls human immunodeficiency virus infection. J Virol. 1999;73:10281–10288. doi: 10.1128/jvi.73.12.10281-10288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasik T J, Wierzbicki A, Whiteman V E, Trinchieri G, Lischner H W, Kozbor D. Association between HIV-specific T helper responses and CTL activities in pediatric AIDS. Eur J Immunol. 2000;30:117–127. doi: 10.1002/1521-4141(200001)30:1<117::AID-IMMU117>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Watt G, Kantipong P, de Souza M, Chanbancherd P, Jongsakul K, Ruangweerayud R, Loomis-Price L D, Polonis V, Myint K S, Birx D L, Brown A E, Krishna S. HIV-1 suppression during acute scrub-typhus infection. Lancet. 2000;356:475–479. doi: 10.1016/S0140-6736(00)02557-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Dailey P J, He T, Gettie A, Bonhoeffer S, Perelson A S, Ho D D. Rapid clearance of simian immunodeficiency virus particles from plasma of rhesus macaques. J Virol. 1999;73:855–860. doi: 10.1128/jvi.73.1.855-860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Shen Y, Chalifoux L, Halloran M, Lee-Parritz D, Simon M, Sehgal P, Chen Z W. Mycobacterium bovis BCG enhances the pathogenicity of simian immunodeficiency virus infection in macaques. J Immunol. 1999;162:2204–2216. [PubMed] [Google Scholar]