Abstract
OBJECTIVES
Extracorporeal circulation induces pronounced effects on haemostasis and rheology. To study these, an ex vivo simulation model is an attractive alternative but often requires large amounts of blood. We sought to create a miniaturized roller pump circuit requiring minimal amounts of blood and to test if the circuit could be used to compare coagulation, platelet function and blood rheology between a dextran-based and a crystalloid-based priming solution.
METHODS
A miniaturized roller pump circuit requiring only 27 ml of blood was created. Blood samples from 8 cardiac surgery patients were mixed with either a dextran-based or a crystalloid-based solution and circulated for 60 min. Coagulation was assessed by rotational thromboelastometry, and platelet function by impedance aggregometry and flow cytometry, before and at 5 and 60 min of circulation.
RESULTS
A time-dependent impairment of coagulation was observed in both groups. Maximum clot firmness was lower with dextran-based than with crystalloid-based priming at 5 min (HEPTEM 37 ± 4 vs 43 ± 4 mm, P < 0.001; EXTEM 37 ± 4 vs 43 ± 4 mm, P < 0.001; FIBTEM 3 ± 2 vs 9 ± 2 mm, P < 0.001) and at 60 min (HEPTEM 29 ± 9 vs 38 ± 5 mm, P < 0.001; EXTEM 30 ± 7 vs 39 ± 5 mm, P < 0.001; FIBTEM 3 ± 2 vs 8 ± 3 mm, P = 0.002). The EXTEM clotting time was longer with dextran-based solution at 5 (109 ± 19 vs 63 ± 7 sec, P < 0.001) and at 60 min (176 ± 72 vs 73 ± 7 sec, P = 0.004).
CONCLUSIONS
The novel miniaturized roller pump circuit can be used to mimic extracorporeal circulation for selected research questions. Dextran-based priming caused a significant impairment in haemostasis compared with a standard crystalloid solution.
Keywords: Cardiopulmonary bypass, Coagulation, Ex vivo simulation, Platelet aggregation, Priming solution Thromboelastometry
Cardiopulmonary bypass (CPB) induces pronounced effects both on haemostasis and on rheology primarily by affecting cellular and soluble blood components [1].
Graphical Abstract
INTRODUCTION
Cardiopulmonary bypass (CPB) induces pronounced effects both on haemostasis and on rheology primarily by affecting cellular and soluble blood components [1]. The mechanisms involved include activation of the coagulation and inflammation cascades, haemodilution, haemolysis and heparinization [1]. To study interactions during CPB, an ex vivo simulation model is an attractive low-cost alternative. Previously, ex vivo simulation has mainly been performed with paediatric oxygenators [2, 3] often requiring more than 500 ml of blood [4, 5]. Furthermore, to mimic the circumstances during CPB, ideally blood from patients with cardiovascular disease would be used, but it may not be feasible or safe to procure the required quantities from these patients. To mimic the environment of blood circulating through an oxygenator while minimizing the need for donor blood, a miniaturized roller pump circuit that can mimic CPB would be desirable.
The optimal priming solution for CPB is debated. The most commonly used prime is a balanced crystalloid solution, such as Ringer’s acetate with additives such as potassium and mannitol [6]. Colloid prime solutions are not as frequently used but they are more effective than crystalloid solutions as intravascular volume expanders. They also are less prone to contribute to interstitial oedema [7]. However, artificial colloids have a negative impact on coagulation. Dextran is considered to have the highest impact on coagulation and, therefore, the clinical use of dextran has declined [8].
The aim of this study was, first, to create a miniaturized roller pump circuit requiring small amounts of patient blood. Second, the novel miniature circuit was then used to compare coagulation, platelet function and blood rheology between 2 different priming solutions.
MATERIALS AND METHODS
Ethical statement
The study was approved by the Regional Research Ethics Committee (registration number 298–11, with amendment T343-17).
The miniaturized roller pump circuit
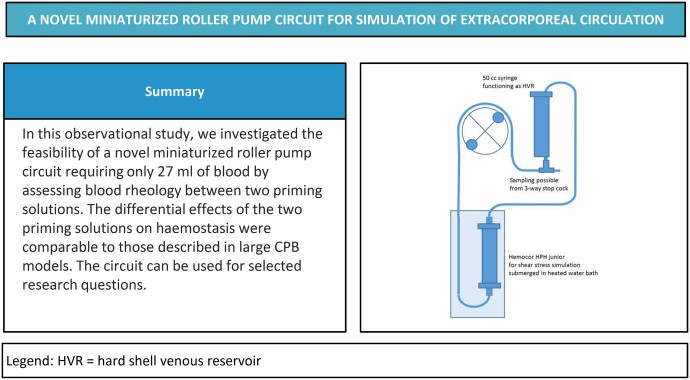
The miniaturized roller pump circuit (Fig. 1) was custom-made (LivaNova, Mirandola, Italy). The circuit consisted of a Hemocor HPH junior (Minntech, Minneapolis, MN, USA) dialysis filter acting as shear stress simulator instead of an oxygenator and a 60 ml syringe with the plunger removed acting as a hard-shell venous reservoir. The tubing consisted of 3/16 × 1/16″ polyvinyl chloride tube and as pump segment a 1/4 × 1/16″ silicon tube. The total prime volume of the circuit was 24 ml.
Figure 1:
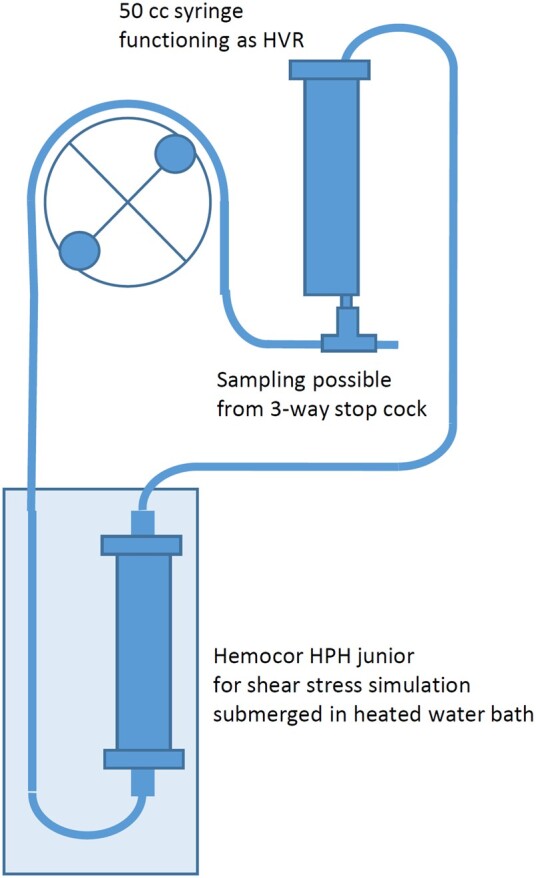
Schematic diagram of the novel miniaturized roller pump circuit. HVR: hard shell venous reservoir.
A Stöckert S5 roller pump 85 (LivaNova, Munich, Germany) was used to drive the circuit. To keep the perfusate at ∼35°C the Hemocor HPH junior was submerged in a water bath heated to 38.5°C using a Thermomix 1419 (Braun Biotech, Melsungen, Germany). To avoid loss of fluid and electrolytes from the circuit, the filtrate compartment of the dialysis filter was filled with Hemosol B0 solution (Gambro Lundia AB, Lund, Sweden).
After priming the circuits, the prime was circulated for 10 min before addition of blood. The S5 roller pump was then stopped and the syringe with priming solution removed. The syringe containing the blood samples was then connected to the circuit. To minimize haemodilution a prime reduction was performed by discarding 10 ml of prime before the return tube was placed in the open syringe. The blood pump was then slowly increased to a flow of 300 ml/min during the first 20 sec. At the start of circuit circulation, 0.5 ml of sodium bicarbonate 50 mg/ml (Fresenius Kabi, Uppsala, Sweden) was added to the circuit to normalize the pH and sodium levels.
Study design
Blood donated from eight patients was used. A total of 60 ml of blood from each patient was collected from a central venous line after the induction of general anaesthesia. For pre-circulation testing, 6 ml was used; the remaining 54 ml was divided into 2 syringes. The 2 syringes were prepared with 0.025 ml of heparin 5000 U/ml (LEO Pharma, Ballerup, Copenhagen, Denmark) per syringe, amounting to ∼5 U/ml blood collected, which is the heparin concentration used during clinical CPB. The 2 syringes were then connected to 2 miniaturized roller pump circuits. One was primed with the crystalloid standard prime used at our institution, and the other with a dextran-based priming solution (PrimEEC; Xvivo AB, Gothenburg, Sweden).
The standard institutional priming solution used for clinical CPB consists of 1000 ml Ringer’s acetate (Baxter, Deerfield, IL, USA), 200 ml mannitol 150 mg/ml (Baxter, Deerfield, IL, USA) and 10 000 U heparin (LEO Pharma, Ballerup, Copenhagen, Denmark). These proportions were kept in the novel miniaturized roller pump circuit. Altogether 50 ml of Ringer’s acetate, 10 ml of mannitol 150 mg/ml and 0.1 ml of heparin 5000 U/ml were mixed. The dextran-based solution is a hyper-oncotic priming solution based on dextran 40. The solution has an electrolyte composition that mimics extracellular fluid to reduce the fluid shift, and is balanced with lactate [9]. An amount of 60 ml of the solution and 0.1 ml of heparin 5000 U/ml was mixed and used to prime the circuit in the dextran-based solution group. Blood samples were collected before circulation and from the two miniaturized circuits at 5 and 60 min of circulation. Whole blood rheology, coagulation, and platelet function were analysed in the blood samples, as described below.
Laboratory analyses
Cell counts and haemolysis
Blood was collected in 4 ml ethylenediaminetetraacetic acid (EDTA) tubes (K2EDTA 18 mg; Becton Dickinson, Franklin Lakes, NJ, USA) for the measurement of haemoglobin (Hb) and haematocrit, and cell counts. The analyses were performed on an automated cell counter (Cell-Dyn Sapphire; Abbott Diagnostics Division, SantaClara, CA, USA). Free Hb was measured with a HemoCue Plasma/low Hb instrument (HemoCue, Ängelholm, Sweden); plasma was obtained by centrifugation and recentrifuged before analysis, according to standard procedure.
Platelet aggregometry
Platelet aggregation was analysed with multiple-electrode aggregometry (Multiplate; Roche Diagnostics, Basel, Switzerland). Technical details and the evaluation of the method have been reported elsewhere [10]. Whole blood samples were collected in hirudin tubes (0.15 mg l−1; Roche Diagnostics GmbH, Mannheim, Germany) and incubated for 30 min before analysis of adenosine diphosphate (ADP) high-sensitive (HS) test (ADP 6.3 µmol/l, prostaglandin E1 9 nmol/l), aspirin-induced platelet inhibition (ASPI) test (arachidonic acid 0.48 mmol/l), and thrombin receptor-activating peptide (TRAP) test (TRAP-6 32 µmol/l). The area under the curve was used as a measure of platelet aggregation and is reported in aggregation units.
Flow cytometry
Platelet activation was evaluated by measuring expression of platelet activation marker P‐selectin (CD62p) with flow cytometry. Blood (100 ul) collected in EDTA tubes at the different time points was fixed in 1% paraformaldehyde for at least 2 h. Samples were washed with phosphate-buffered saline (PBS) (140 mM NaCl and 10 mM Na3PO4, pH 7.4) and then resuspended in PBS containing 2% foetal calf serum. Next, the monoclonal antibodies CD61-FITC (20 ul) (platelet surface marker; GPIIIa) (BD Biosciences, San José, CA, USA) and CD62p-PE (20 ul) (BD Biosciences) were added and incubated for 15 min. The samples were analysed on a flow cytometry system (BD FACS Lyric; BD Biosciences) within 30 min. A total of 10 000 platelet events were acquired. The BD FACSuite was used to analyse the percentage of platelets expressing P-selectin.
Rotational thromboelastometry
Modified rotational thromboelastometry (ROTEM; Pentapharm GmbH, Munich, Germany) was used to analyse whole blood coagulation. Technical details and evaluation of the method have been reported elsewhere [11, 12]. Whole blood samples were collected in citrate tubes (9NC sodium citrate 0.109M; Becton Dickinson, Franklin Lakes, NJ, USA). Samples were analysed at 37°C. The HEPTEM, EXTEM and FIBTEM assays were utilized. For HEPTEM and EXTEM, clotting time (CT) and maximum clot firmness (MCF) are reported; for FIBTEM, only MCF is reported.
Statistical methods
Continuous data are presented as mean and standard deviation. To assess the effect of the crystalloid and dextran-based priming solution between the 3 different time points a 2-factor analysis of variance (ANOVA) with repeated-measures in both factors was performed. The factors priming solution, time and the interaction term priming solution and time was used.
Normal distribution was assessed with Shapiro–Wilk test and visually with a QQ plot. To identify extreme outliers, the box plot method vas used [13]. To assess sphericity Mauchly’s test was used [14]. If significant the Greenhouse-Geisser sphericity correction was applied [15]. If the assumptions of outliers and normal distribution were violated calculations were performed without the patients producing the extreme datapoints. When excluded none of the calculations became non-significant, therefore, they were included in the final model. If the 2-factor ANOVA interaction term was significant, post hoc ANOVA analysis for a simple main effect was performed using a 1-factor ANOVA with repeated measures on the effects of prime at every timepoint and second on the effect of time in every group. If the interaction term was non-statistically significant individual factors was evaluated. If either of the factors time or prime were statistically significant post hoc analysis was performed for a main effect with the statistically significant factor. Finally, a paired t test was performed were there was a significant one-factor ANOVA effect. To correct for multiple testing, the Holm-Bonferroni method was used [16]. For all analyses, a P-value of <0.05 was considered statistically significant. Data were analysed using R version 4.0.2 [17] and RStudio version 1.3.1093 [18].
RESULTS
Patient characteristics
The characteristics of the patients who donated blood are presented in Table 1. Five of the patients underwent coronary artery bypass graft (CABG) surgery and three underwent valve surgery. Five patients were treated with acetylsalicylic acid at the time of surgery. None of the patients were treated with purinergic receptor type Y, subtype 12 (P2Y12), inhibitors, warfarin or direct oral anticoagulants <5 days before surgery.
Table 1:
Preoperative patient characteristics in 8 cardiac surgery patients
| Characteristic | n = 8 |
|---|---|
| Female sex | 0 (0.0%) |
| Age (years) | 70 (9) |
| Body surface area (m2) | 2.10 (0.13) |
| Diabetes | 3 (37.5%) |
| ASA (0–5 days preoperatively) | 5 (62.5%) |
| Smoker | 1 (12.5%) |
| Procedures | |
| CABG surgery | 5 (62.5%) |
| Dual valve surgery | 2 (25.0%) |
| Aortic valve surgery | 1 (12.5%) |
Data are given as number and percentages or mean and standard deviation.
ASA: acetylsalicylic acid; CABG: coronary artery bypass graft.
Thromboelastometry
Clotting time
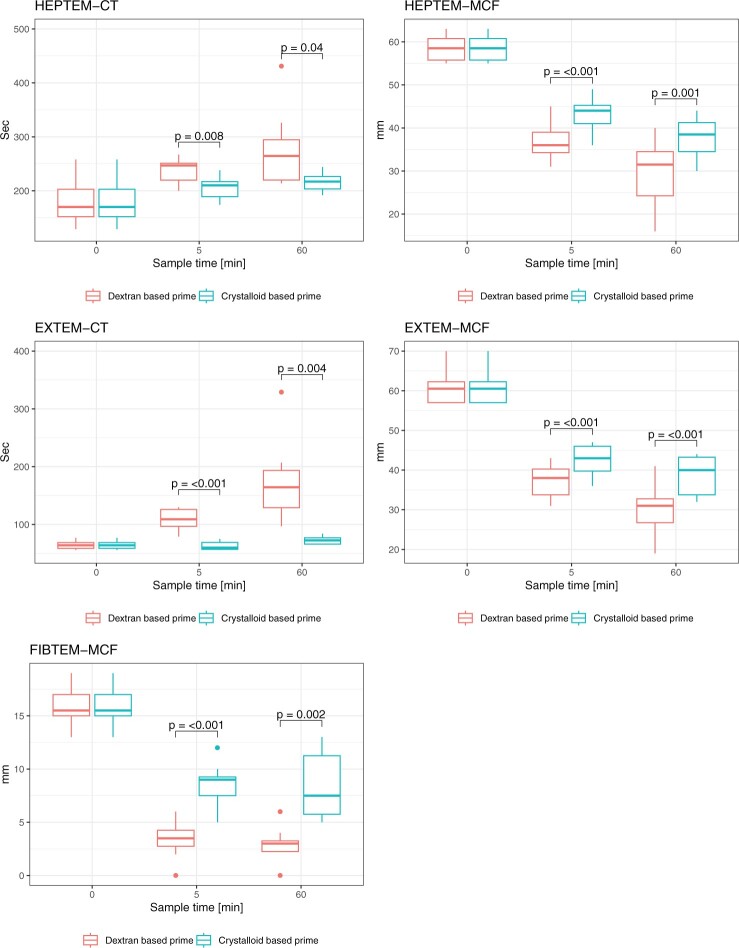
Regarding thromboelastometry (Fig. 2, Supplementary Material, Tables S1–S7), EXTEM-CT increased over time, both with dextran-based prime and with crystalloid-based prime (pre-circulation 65 ± 7 sec). There were significant differences between the dextran-based and the crystalloid-based prime both at 5 min (109 ± 19 vs 63 ± 7 sec, mean difference [MD] 46.4, 95% confidence interval [CI] 31.6–61.1, P < 0.001) and at 60 min (176 ± 72 vs 73 ± 7 sec, MD 102 CI 46.1–159, P = 0.004). Likewise, HEPTEM-CT increased over time with dextran-based prime, but did not change significantly with crystalloid-based prime (Pre-circulation 184 ± 48 sec). There were significant differences between the dextran-based and the crystalloid-based prime both at 5 min (237 ± 23 vs 206 ± 23 sec, MD 31.6 CI 11.2–52.1, P = 0.008) and at 60 min (278 ± 73 vs 217 ± 18 sec, MD 61 CI 3.74–118, P = 0.04).
Figure 2:
Thromboelastometry variables in blood samples from eight cardiac surgery patients during circulation in a miniaturized roller pump circuit primed with either a crystalloid- or a dextran-based priming solution. CT: clotting time; MCF: maximum clot firmness.
Maximum clot firmness
Over time, EXTEM-MCF was reduced, both with the dextran-based prime and with the crystalloid-based prime (Pre-circulation 61 ± 4 mm). There were significant differences between the dextran-based and the crystalloid-based prime, both at 5 min (37 ± 4 vs 43 ± 4 mm, MD -5.25 CI −7.29 to −3.21, P < 0.001) and at 60 min (30 ± 7 vs 39 ± 5 mm, MD −8.75 CI −11.8 to −5.73, P < 0.001). Similarly, HEPTEM-MCF was reduced over time with dextran-based and crystalloid-based prime (pre-circulation 59 ± 3 mm). There were significant differences between the dextran-based and the crystalloid-based prime both at 5 min (37 ± 4 vs 43 ± 4 mm, MD -6.62 CI −9.11 to −4.14, P < 0.001) and at 60 min (29 ± 9 vs 38 ± 5 mm, MD −8.25 CI −12.1 to −4.39, P < 0.001). Likewise, FIBTEM-MCF was reduced over time with both primes (pre-circulation 16 ± 2 mm). There were significant differences between the dextran-based and the crystalloid-based prime, both at 5 (3 ± 2 vs 9 ± 2 mm, MD −5.12 CI −6.99 to −3.26, P < 0.001) and at 60 min (3 ± 2 vs 8 ± 3 mm, MD -5.62 CI −8.41 to −2.84, P = 0.002).
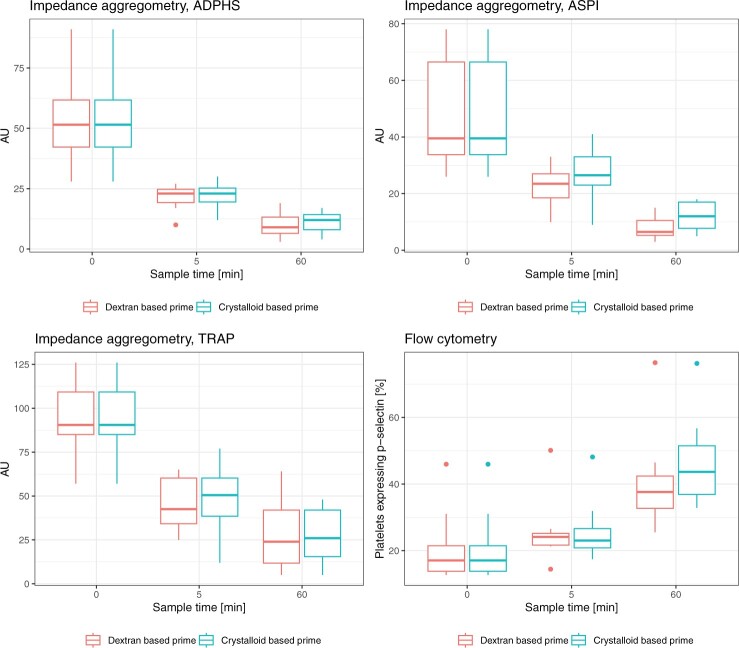
Impedance aggregometry
With regard to impedance aggregometry (Fig. 3, Supplementary Material, Tables S1–S7), ADP-HS, ASPI and TRAP tests were reduced over time with both dextran-based and crystalloid-based prime. There were no significant differences between the primes at 5 or 60 min with ADP-HS and TRAP test. With ASPI test there was a significant difference between the dextran-based and the crystalloid-based prime at 60 min (8 ± 5 vs 12 ± 5 AU, MD −4 CI −7.57 to −0.43, P = 0.033), but not at 5 min.
Figure 3:
Platelet function test in blood samples from eight cardiac surgery patients during circulation in a miniaturized roller pump circuit primed with either a crystalloid- or a dextran-based priming solution. ADP-HS: adenosine diphosphate high-sensitive (test); ASPI: aspirin-induced platelet inhibition; AU: aggregation units; TRAP: thrombin receptor-activating peptide (test).
Flow cytometry
Flow cytometry (Fig. 3, Supplementary Material, Tables S1–S7) showed that platelets expressing P‐selectin increased over time both with dextran-based prime and with crystalloid-based prime. The time by group ANOVA indicated a significant difference (P = 0.024) between the dextran- and crystalloid-based primes. However, direct comparisons at 5 (P = 0.96) and 60 min (P = 0.072) were not statistically significant.
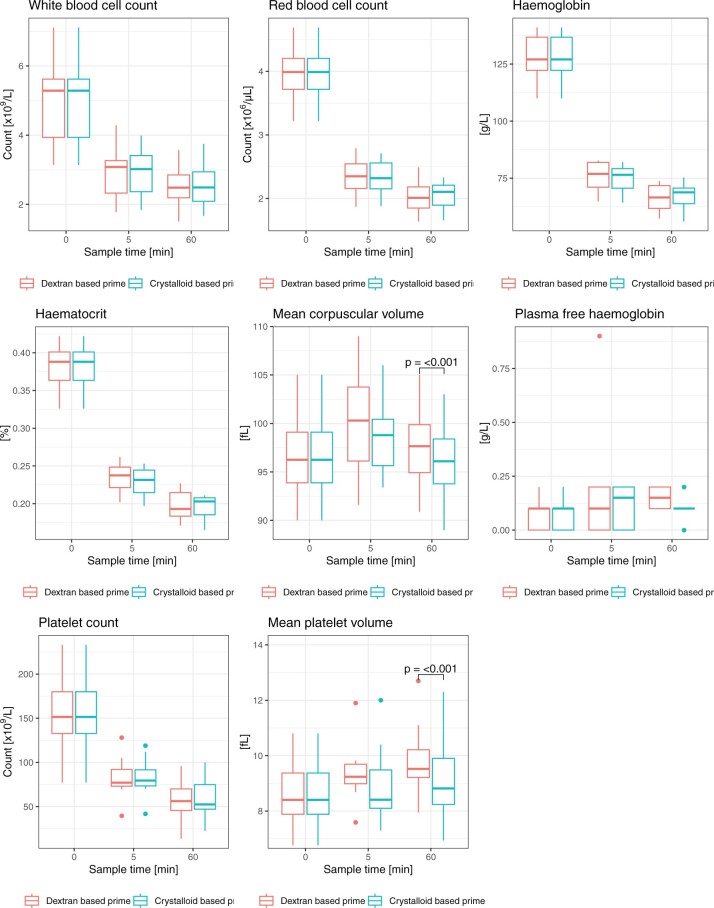
Cell counts and haemolysis
White and red blood cell counts, Hb, haematocrit and platelet counts (Fig. 4, Supplementary Material, Tables S1–S7) were reduced at 5 and 60 min with both the dextran- and the crystalloid-based primes. There were no intergroup differences. Mean corpuscular volume (MCV) increased at 5 min and continued to increase from 5 to 60 min in both groups. At 60 min, MCV was significantly higher with the dextran-based prime (97.6 ± 4.5 vs 96.0 ± 4.4 fl, MD 1.59 CI 1.29–1.89, P < 0.001). Compared with pre-circulation measurements, mean platelet volume was significantly increased at 5 and 60 min with the dextran-based prime. This pattern was not observed with crystalloid prime. At 60 min, we observed a statistically significant difference in mean platelet volume between the dextran-based and crystalloid priming solutions (9.9 ± 1.5 vs 9.1 ± 1.7 fl, MD 0.739 CI 0.442–1.04, P = 0.001). Plasma-free Hb did not change significantly over time with either priming solution and there were no significant intergroup differences.
Figure 4:
Cell counts and haemolysis in blood samples from eight cardiac surgery patients during circulation in a miniaturized roller pump circuit primed with either a crystalloid- or a dextran-based priming solution. fl: femtolitre.
DISCUSSION
In this study of an ex vivo model using a novel miniaturized roller pump circuit, we tested the feasibility of the circuit by comparing two different priming solutions. The dextran-based priming solution caused a significant reduction in clot stability, elongation of CT, and reduced platelet aggregability at 60 min of circulation in a miniaturized roller pump circuit. We also observed time-dependent changes in coagulation, platelet function and cell counts during circulation, independent of priming solution.
In this study, we used a novel miniaturized roller pump circuit that requires only 27 ml of blood, which is markedly lower than most other ex vivo CPB models [3, 4]. The small priming volume reduces the need for donated whole blood facilitating the use of blood from patients with cardiovascular disease. As with all ex vivo models the more standardized setting minimizes interindividual differences and the ethical considerations associated with animal studies are avoided [19]. In addition, the components used in the circuit are in comparison to a paediatric equipment less expensive and, therefore, the costs are lower. The disadvantages of an ex vivo model is that it cannot simulate the cellular and inflammatory response observed during clinical CPB [1, 20, 21] as it lacks the patient’s vascular bed. Also, cardiotomy suction is not present which is a major source of haemolysis [1]. Therefore, the novel miniaturized roller pump circuit model may be useful for studying some CPB objectives, but not all. Further, the circuit does not include an oxygenator, making studies related to oxygenation impossible.
In this study, we chose to compare the effect of 2 priming solutions on coagulation, platelet function and blood rheology. We decided to perform this research first because the influence of CPB on haemostasis is incompletely understood [22]. Second, changes in coagulation and platelet function are an example of interactions that can be assessed in a CPB model without oxygenator. Finally, we have recently performed a randomized clinical study comparing dextran-based and crystalloid priming solutions on coagulation and platelet function [23]. Hence, we have some information about how the priming solutions influence haemostasis during clinical CPB and the results of the present study can be benchmarked against these findings. However, it should be noted that in the clinical study we only assessed coagulation and platelet function before and 2 h after CPB, while in this study we performed measurements before, at 5 min and again at 60 min of circulation. This refines the assessment of the effects of priming solutions and circulation but makes direct comparisons between the studies difficult.
During circulation in the novel miniaturized roller pump circuit we observed a significant reduction in clot stability, as measured by MCF. These results are congruent with our findings in the clinical study and can be explained by dextran-induced inhibition of erythrocyte aggregation [24], and enhanced fibrinolysis by an increase in plasma concentrations of tissue type plasminogen activator and a decrease in concentrations of its inhibitor plasminogen activator inhibitor-1 [25].
We also observed a significant reduction in CT with dextran-based priming solution. In the clinical study there was a trend towards longer CT with dextran-based prime but the difference did not reach statistical significance. The prolonged CT in the present study may have been due to the anticoagulation effect of dextran, caused primarily by a decrease in von Willebrand factor and coagulation factor VIII [26–28]. Von Willebrand factor is the ligand between the platelet surface receptor protein glycoprotein Ib (GPIb) and subendothelial collagen. A reduction in von Willebrand factor, therefore, causes a reduction in platelet adhesion to the vessel wall and, in turn, impaired primary haemostasis [25]. Taken together, the reduction in primary haemostasis, the clot stability and the enhanced fibrinolysis with dextran-based prime are the results we observed in the thromboelastometry tests.
These results are in accordance with the findings of Kam et al. [24] who tested different doses of dextran compared with dilution with saline in an experimental setup. They observed an increased CT and a reduced MCF in the EXTEM test and a reduced MCF in the FIBTEM test. Contrary to our results they did not find any changes in platelet function tests with impedance aggregometry. Sigurjonsson et al. [29] also found that dextran impairs EXTEM clot firmness and that the platelet count decreased postoperatively in a clinical study. However, they observed no differences in impedance aggregometry when comparing dextran with albumin as the primary volume substitution during major gynaecological surgery. In other research, Robless et al. [30] found that dextran inhibited platelet function. Using flow cytometry and platelet aggregometry they showed a reduction in P-selectin and GPIIb–IIIa in line with our findings of a trend towards lower cell surface expression of P-selectin.
The observed time-dependent changes in coagulation, platelet function and cell counts during circulation in the novel miniaturized roller pump circuit are similar to other investigations in larger ex vivo models of CPB. Teligui et al. [3] used heparin-coated paediatric components to create a circuit with a priming volume of ∼150 ml and then added 450 ml of donated human blood. They examined primarily the activation of the complement system but they also performed cell counts. They found similar changes to ours in pre-circulation tests compared with start of circulation, but thereafter they only observed changes in haematocrit. This difference was probably related to the uncoated system used in our study. Surface coatings are known not only to cause a reduction in coagulation and systemic inflammatory processes but also to reduce platelet activation [31]. Baksaas et al. [4] used uncoated adult components to create a circuit primed with 800 ml and then added 500 ml donated human blood. In accordance with our study, they observed a great decline in pre-circulation tests compared with start of circulation; they also observed a reduction over time in Hb, haematocrit, and platelet count, similar to our results. The differences we observed with the novel miniaturized roller pump circuit appear to be in concordance with larger ex vivo models.
However, our findings suggest that the circuit can be used to detect coagulation changes also observed during clinical CPB. When evaluating the performance of the novel miniaturized roller pump circuit we detected changes in coagulation capacity between the 2 different priming solutions similar to those we observed in our clinical study.
Limitations
The limitations of an ex vivo model of CPB is that it cannot simulate the cellular and inflammatory response observed during clinical CPB [1, 20, 21]. Also, cardiotomy suction is not present which is a major source of haemolysis [1] but this is true for the majority of ex vivo models. The statistical limitations of this study are primarily the small sample size.
CONCLUSIONS
In this observational study, we conclude that the novel miniaturized roller pump circuit can be used to compare priming solutions and also to follow coagulation changes during extracorporeal circulation. The dextran-based priming solution caused a significant impairment of coagulation and platelet function compared with a standard crystalloid solution. Further studies are needed to evaluate the miniaturized roller pump circuit and confirm our results.
Supplementary Material
Glossary
ABBREVIATIONS
- ASA
Acetylsalicylic acid
- ADP
Adenosine diphosphate
- AU
Aggregation units
- ANOVA
Analysis of variance
- AUC
Area under the curve
- ASPI
Aspirin-induced platelet inhibition
- CPB
Cardiopulmonary bypass
- CT
Clotting time
- CABG
Coronary artery bypass grafting
- DOACs
Direct oral anticoagulants
- EDTA
Ethylenediaminetetraacetic acid
- fL
Femtolitre
- GP
Glycoprotein
- Hb
Haemoglobin
- HVR
Hard-shell venous reservoir
- HS
High-sensitive
- MCF
Maximum clot firmness
- MCV
Mean corpuscular volume
- PBS
Phosphate-buffered saline
- P2Y12
Purinergic receptor type Y, subtype 12
- ROTEM
Rotational thromboelastometry
- TRAP
Thrombin receptor-activating peptide
Contributor Information
Anders K Hjärpe, Department of Perfusion, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Anaesthesia and Intensive Care, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Anders Jeppsson, Department of Cardiothoracic Surgery, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Lukas Lannemyr, Department of Anaesthesia and Intensive Care, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Cardiothoracic Anaesthesia, Sahlgrenska University Hospital, Gothenburg, Sweden.
Fredrik Pernbro, Department of Anaesthesia and Intensive Care, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Paediatric Anaesthesia and Intensive Care, Queen Silvia Children’s Hospital, Gothenburg, Sweden.
Camilla Hesse, Department of Clinical Immunology and Transfusion Medicine (KITM), Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Laboratory Medicine, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Birgitta Romlin, Department of Anaesthesia and Intensive Care, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Paediatric Anaesthesia and Intensive Care, Queen Silvia Children’s Hospital, Gothenburg, Sweden.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
FUNDING
This work was not supported by any external funding source.
Conflict of interest: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr Anders Jeppsson has received honorarium for consultancy and/or lectures from AstraZeneca, Werfen, Novo Nordisk, Bayer, Boehringer-Ingelheim and LFB Biotechnologies, outside the present work. Dr Lukas Lannemyr has received honorarium for consultancy from Xvivo Perfusion. Otherwise, none of the authors report any conflict of interests.
DATA AVAILABILITY
Data will be available from the corresponding author on reasonable request.
Author contributions
Anders Karl Hjärpe: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Validation; Visualization; Writing—original draft. Anders Jeppsson: Conceptualization; Funding acquisition; Methodology; Project administration; Writing—review & editing. Lukas Lannemyr: Methodology; Validation; Writing—review & editing. Fredrik Pernbro: Validation; Writing—review & editing. Camilla Hesse: Conceptualization; Investigation; Resources; Writing—review & editing. Birgitta Romlin: Methodology; Supervision; Validation; Writing—review & editing.
Reviewer information
Reviewer information Interactive CardioVascular and Thoracic Surgery thanks Anne Beukers and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Pape A, Zacharowski K.. Hematologic effects and coagulopathy. In: Gravlee GP, Davis RF, Hammon JKussman B (eds). Cardiopulmonary Bypass and Mechanical Support: Principles and Practice, 4th edn. Philadelphia: Wolters Kluwer, 2015, 433–47. [Google Scholar]
- 2. Nadtochiy SM, Stefanos T, Angona RE, Lebedko N, Baldzizhar A, Feng C et al Rivaroxaban reduces the dabigatran dose required for anticoagulation during simulated cardiopulmonary bypass. Anesth Analg 2022;135:52–9. [DOI] [PubMed] [Google Scholar]
- 3. Teligui L, Dalmayrac E, Corbeau J-J, Bouquet E, Godon A, Denommé A-S. et al. Ex vivo simulation of cardiopulmonary bypass with human blood for hemocompatibility testing. Perfusion 2016;31:376–83. [DOI] [PubMed] [Google Scholar]
- 4. Baksaas ST, Videm V, Mollnes TE, Pedersen T, Karlsen H, Svennevig JL et al Effects on complement, granulocytes and platelets of a leukocyte-depletion filter during in vitro extracorporeal circulation. Scand Cardiovasc J 1997;31:73–7. [DOI] [PubMed] [Google Scholar]
- 5. Videm V, Fosse E, Mollnes TE, Ellingsen O, Pedersen T, Karlsen H. et al. Different oxygenators for cardiopulmonary bypass lead to varying degrees of human complement activation in vitro. J Thorac Cardiovasc Surg 1989;97:764–70. [PubMed] [Google Scholar]
- 6. Miles LF, Coulson TG, Galhardo C, Falter F.. Pump priming practices and anticoagulation in cardiac surgery: results from the global cardiopulmonary bypass survey. Anesth Analg 2017;125:1871–7. [DOI] [PubMed] [Google Scholar]
- 7. Young R. Perioperative fluid and electrolyte management in cardiac surgery: a review. J Extra Corpor Technol 2012;44:P20–6. [PMC free article] [PubMed] [Google Scholar]
- 8. Farrugia A. Safety of plasma volume expanders. J Clin Pharmacol 2011;51:292–300. [DOI] [PubMed] [Google Scholar]
- 9. Kolsrud O, Barbu M, Dellgren G, Björk K, Corderfeldt A, Thoren A. et al. Dextran‐based priming solution during cardiopulmonary bypass attenuates renal tubular injury—a secondary analysis of randomized controlled trial in adult cardiac surgery patients. Acta Anaesthesiol Scand 2022;66:40–7. [DOI] [PubMed] [Google Scholar]
- 10. Toth O, Calatzis A, Penz S, Losonczy H, Siess W.. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost 2006;96:781–8. [PubMed] [Google Scholar]
- 11. Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol 2005;27:81–90. [DOI] [PubMed] [Google Scholar]
- 12. Lang T, Bauters A, Braun SL, Pötzsch B, von Pape K-W, Kolde H-J. et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis 2005;16:301–10. [DOI] [PubMed] [Google Scholar]
- 13. Tukey JW. Exploratory Data Analysis. London: Pearson, 1977. [Google Scholar]
- 14. Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Ann Math Stat 1940;11:204–9. [Google Scholar]
- 15. Greenhouse SW, Geisser S.. On methods in the analysis of profile data. Psychometrika 1959;24:95–112. [Google Scholar]
- 16. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 17. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2022. [Google Scholar]
- 18. RStudio Team. RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC, 2020. [Google Scholar]
- 19. Kiani AK, Pheby D, Henehan G, Brown R, Sieving P, Sykora P, et al. Ethical considerations regarding animal experimentation. J Prev Med Hyg 2022;63:E255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983;86:845–57. [PubMed] [Google Scholar]
- 21. Day JR, Taylor KM.. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005;3:129–40. [DOI] [PubMed] [Google Scholar]
- 22. Paparella D, Brister SJ, Buchanan MR.. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med 2004;30:1873–81. [DOI] [PubMed] [Google Scholar]
- 23. Barbu M, Kolsrud O, Radulovic V, Dellgren G, Björk K, Thorén A. et al. Hemostatic effects of a dextran-based priming solution for cardiopulmonary bypass: a secondary analysis of a randomized clinical trial. Thromb Res 2023;223:139–45. [DOI] [PubMed] [Google Scholar]
- 24. Kam P, Liou J, Yang K.. In vitro evaluation of the effect of haemodilution with dextran 40 on coagulation profile as measured by thromboelastometry and multiple electrode aggregometry. Anaesth Intensive Care 2017;45:562–8. [DOI] [PubMed] [Google Scholar]
- 25. de Jonge E, Levi M.. Effects of different plasma substitutes on blood coagulation: a comparative review. Crit Care Med 2001;29:1261–7. [DOI] [PubMed] [Google Scholar]
- 26. Ts'ao CH, Krajewski DV.. Effect of dextran on platelet activation by polymerizing fibrin. Am J Pathol 1982;106:1–7. [PMC free article] [PubMed] [Google Scholar]
- 27. Sobel M, Adelman B, Greenfield LJ.. Dextran 40 reduces heparin-mediated platelet aggregation. J Surg Res 1986;40:382–7. [DOI] [PubMed] [Google Scholar]
- 28. Aberg M, Hedner U, Bergentz SE.. Effect of dextran on factor viii (antihemophilic factor) and platelet function. Ann Surg 1979;189:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sigurjonsson J, Hedman D, Bansch P, Schott U.. Comparison of dextran and albumin on blood coagulation in patients undergoing major gynaecological surgery. Perioper Med (Lond) 2018;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robless P, Okonko D, Mikhailidis DP, Stansby G.. Dextran 40 reduces in vitro platelet aggregation in peripheral arterial disease. Platelets 2004;15:215–22. [DOI] [PubMed] [Google Scholar]
- 31. Murphy GS, Hessel EA 2nd, Groom RC.. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009;108:1394–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available from the corresponding author on reasonable request.