Abstract
Luteinizing hormone (LH), a heterodimeric glycoprotein produced by pituitary gonadotrope cells, regulates gonadal function. Hypothalamic gonadotropin-releasing hormone (GnRH) stimulates LH synthesis and secretion. GnRH induces LHβ subunit (Lhb) expression via the transcription factor, early growth response 1 (EGR1), acting on the Lhb promoter. In contrast, overexpression of zinc finger E-box binding homeobox 1 (ZEB1) represses LH production in mice, but the underlying mechanism was not previously elucidated. Here, we observed that ZEB1 inhibited GnRH-stimulated but not basal Lhb mRNA expression in homologous murine LβT2 cells. Moreover, ZEB1 blocked GnRH and/or EGR1 induction of murine Lhb but not human LHB promoter-reporter activity in these cells. Using chimeric reporters, we mapped the species-specific ZEB1 sensitivity to sequence differences, including in Z- and E-boxes, in the proximal Lhb/LHB promoters, immediately upstream of the transcription start sites. ZEB1 bound to the murine Lhb promoter with higher affinity than to the human LHB promoter in this region. To examine ZEB1's physiological role in LH synthesis, we characterized gonadotrope-specific Zeb1 knockout mice. Loss of ZEB1 in gonadotropes did not affect LH production or secretion. Collectively, the data suggest that ZEB1, when overexpressed, can inhibit GnRH/EGR1 induction of murine Lhb transcription but does not play a necessary role in LH synthesis in mice.
Keywords: transcription, pituitary, knockout, gonadotrope, ZEB1
In vertebrates, reproduction is regulated by hormones in the hypothalamic-pituitary-gonadal axis. Luteinizing hormone (LH) is a heterodimeric glycoprotein synthesized and secreted by gonadotrope cells of the anterior pituitary gland that regulates gonadal function in both sexes (1). LH is composed of an α subunit common to pituitary glycoprotein hormones, and a hormone-specific β subunit, LHβ, which confers the ligand's biological specificity. Production of LHβ is rate-limiting in LH synthesis. Lhb transcription is regulated by hypothalamic gonadotropin-releasing hormone (GnRH). GnRH stimulates the expression of the immediate-early gene, early growth response 1 (EGR1) (2, 3). EGR1 acts in concert with 2 constitutively expressed transcription factors, steroidogenic factor 1 (SF-1 or NR5A1) and paired-like homeodomain transcription factor 1 (PITX1), to stimulate Lhb transcription (2, 4-6). All 3 transcription factors bind to cis-elements in the proximal Lhb promoter to mediate their effects. This mechanism appears to be conserved across mammalian species, including humans (2, 3).
Selective LH deficiency, anovulation, and infertility were observed in female mice lacking 2 members of the miR-200 family of micro-RNAs (miRNAs), miR-200b and miR-429 (7). The diminution in LH was attributed to increased protein levels of the transcription factor, zinc finger E-box binding homeobox 1 (ZEB1), a miR-200 family target, in the pituitaries of these mice. Consistent with this idea, overexpression of ZEB1 in gonadotropes of transgenic mice recapitulated the reproductive phenotypes observed in the micro-RNA double knockouts (miR-dKO) (7). Collectively, these data suggested that ZEB1 may repress murine Lhb expression; however, the underlying mechanism was not reported.
ZEB1 is 1 of 5 core epithelial-mesenchymal transition transcription factors, which function as both activators or repressors depending on cellular context (8). ZEB1 is characterized by 2 end-terminal clusters of DNA-binding C2H2 zinc finger domains, a non-DNA-binding central homeodomain, and various cofactor interaction sites, including SMAD-, CtBP-, and p300-P/CAF interaction domains (9-13). The ZEB1 end-terminal zinc finger clusters bind to bipartite E-box (CANNTG) and Z-box (ATACGTGT) cis-elements to repress transcription (10, 14). The murine Lhb promoter contains several E-box-like cis-elements that could bind ZEB1 in gel shift assays (7). It was therefore suggested, but not demonstrated, that ZEB1 represses Lhb transcription by binding to one or more of these sites. Indeed, ZEB1 reportedly repressed rat Lhb promoter-reporter activity by binding to a conserved E-box (15). Nevertheless, the functional relevance, if any, of these E-boxes in ZEB1 repression of murine Lhb transcription was not reported.
Whereas increased ZEB1 protein levels can inhibit LH synthesis in mice in vivo, physiological roles for ZEB1 in gonadotropes have not been established. Lhb mRNA levels were reportedly increased in the murine gonadotrope-like LβT2 cell line following small interfering RNA (siRNA)-mediated knockdown of endogenous ZEB1 (7). Single nucleus multi-omics analyses also revealed high ZEB1 regulon activity in murine gonadotropes (16). Thus, ZEB1 may regulate LH production and other processes in these cells.
Here, we demonstrate that ZEB1 inhibits GnRH and/or EGR1 induction of murine Lhb transcription via species-specific cis-elements located in the proximal Lhb promoter that are distinct from the previously characterized E-boxes. Furthermore, we show that ZEB1 is dispensable for LH synthesis and secretion in vivo.
Methods
DNA Constructs
The FLAG-HA-tagged and untagged ZEB1 expression vectors in pCX were from Dr. Hidetoshi Hasuwa (Keio University School of Medicine, Tokyo, JPN). FLAG-tagged EGR1 expression vector, murine −232/+5 Lhb promoter-luciferase reporter, and human −196/+9 LHB promoter-luciferase reporter were previously described (3, 17, 18). The murine −435/+5, −495/+5, and −968/+5 Lhb promoter-luciferase reporters were produced by PCR amplification of wild-type mixed background (MGI:3028467 and MGI:5651461) mouse genomic DNA and ligated into pA3-luc as previously described (see Table 1 for primers) (18). Mutant promoter-reporter constructs were generated using the QuikChange II site-directed mutagenesis protocol (see Table 1 for primers). Chimeric promoter-reporter constructs were produced by PCR amplification of synthetic gene fragments (Twist Bioscience, South San Francisco, California, USA) using primers provided by the manufacturer and ligated into pA3-luc as previously described (18). All constructs were verified by sequencing (Génome Québec, Montreal, Canada).
Table 1.
Primers
| Genotyping primers | |
| Zeb1 F | CGTGATGGAGCCAGAATCTGACCCC |
| Zeb1 R | GCCCTGTCTTTCTCAGCAGTGTGG |
| Zeb1 R (recombinant) | GCCATCTCACCAGCCCTTACTGTGC |
| GRIC F | GGACATGTTCAGGGATCGCCAGGC |
| GRIC R | GCATAACCAGTGAAACAGCATTGCTG |
| Murine Lhb promoter amplification | |
| +5 promoter amplification rv | CGGAAGCTTTTGATACCTTCCCTACCTTGG |
| −435/+5 promoter amplification fw | CTAGGTACCCTTGAGTCCCCTGGAATCTG |
| −495/+5 promoter amplification fw | CAGGGTACCTCCAGGCGCAATTTACTGAT |
| −968/+5 promoter amplification fw | TTTGGTACCCCAATCCAGGCATCCTGATT |
| Lhb promoter mutagenesis | |
| −183E mLhb mut fw | GCTTCCAATGTCAGCTAAGCCCTGATGCCGAGGCCGAGTGTGAG |
| −183E mLhb mut rv | CTCACACTCGGCCTCGGCATCAGGGCTTAGCTGACATTGGAAGC |
| −30Z mLhb mut fw | ACCCCCACAACCCGTAGGCATAAAGCCAGGTGC |
| −30Z mLhb mut rw | GCACCTGGCTTTATGCCTACGGGTTGTGGGGGT |
| −30E mLhb mut fw | ACAACCCGCAGGTATAAAGCTGGGGACCCAAGGTAGGGAAGGTATC |
| −30E mLhb mut rw | GATACCTTCCCTACCTTGGGTCCCCAGCTTTATACCTGCGGGTTGT |
| qPCR primers | |
| Fshb F | GTGCGGGCTACTGCTACACT |
| Fshb R | CAGGCAATCTTACGGTCTCG |
| Gnrhr F | TTCGCTACCTCCTTTGTCGT |
| Gnrhr R | CACGGGTTTAGGAAAGCAAA |
| Lhb F | ACTGTGCCGGCCTGTCAACG |
| Lhb R | AGCAGCCGGCAGTACTCGGA |
| Rpl19 F | CGGGAATCCAAGAAGATTGA |
| Rpl19 R | TTCAGCTTGTGGATGTGCTC |
| Tgfrb3l F | CCTGACACCAGTGCCTTTGA |
| Tgfbr3l R | CTAGGGGACGGACGAGGTAT |
| Nr5a1 F | AGGAGTTCGTCTGTCTCAAGTTCCT |
| Nr5a1 R | ACAAGGTGTAATCCAACAGGGCAG |
| Zeb1 F | ATTCCCCAAGTGGCATATACA |
| Zeb1 R | GAGCTAGTGTCTTGTCTTTCATCC |
Cell Lines
Immortalized murine gonadotrope-like LβT2 cells (RRID: CVCL_0398; provided by Dr. Pamela Mellon, University of California, San Diego, CA, USA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (319-005-EL, Wisent, St-Bruno, QC, Canada) supplemented with 10% (v/v) fetal bovine serum (FBS) (098150, Wisent) (19). Human embryonic kidney (HEK) 293T cells (RRID: CVCL_0063, provided by Dr. Terence Hébert, McGill University, Montreal, QC, Canada) were cultured in DMEM supplemented with 5% (v/v) FBS. AD-293 cells (RRID: CVCL_9804, Agilent, Santa Clara, CA, USA) were cultured in DMEM supplemented with 10% (v/v) FBS and 1X antibiotic-antimycotic solution (450-115-EL, Wisent). All cells were cultured in a humidified incubator at 37 °C with 5% CO2.
Adenovirus Production and Adenoviral Infection
Recombinant adenoviruses were generated as described in the AdEasy protocol (20). Briefly, to produce adenovirus that co-expressed ZEB1 and green fluorescent protein (GFP), the murine Zeb1 coding sequence was excised from the untagged ZEB1 construct in pCX and ligated into the shuttle vector, pAdTrack-CMV, using KpnI and XhoI (20). The shuttle vector was then linearized with PmeI and transformed into chemically competent AdEasier cells to produce recombinant plasmids (20). AD-293 packaging cells were transfected with the recombinant plasmid to produce recombinant adenovirus, further amplified in AD-293 cells, and titered via quantitative polymerase chain reaction (qPCR). The same protocol was followed to generate the recombinant adenovirus expressing GFP alone.
LβT2 cells were seeded at a density of 150 000 cells/well in 48-well plates. The following day, cells were transduced overnight in 2% (v/v) FBS DMEM containing adenovirus expressing GFP or co-expressing ZEB1 and GFP at a multiplicity of infection (MOI) of 50. Approximately 16 hours post-transduction, medium was replaced with serum-free DMEM, and cells were serum starved for 24 hours. Following starvation, cells were treated with pulses of either vehicle (H2O) or GnRH (10 nM; L7134, Sigma-Aldrich, St. Louis, MO, USA) for the first 5 minutes of every hour for a total of 6 hours. Following the treatment period, total RNA was extracted using TRIzol reagent following the manufacturer's protocol for qPCR analysis (15596026, ThermoFisher).
Antibodies
Antibodies used were: mouse anti-human HA (Sigma-Aldrich Cat# H9658, RRID:AB_260092); mouse IgG (Santa Cruz Biotechnology Cat# sc-2025, RRID:AB_737182); rabbit anti-human ZEB1 (Sigma-Aldrich Cat# HPA027524, RRID:AB_1844977); goat anti-rat LHβ (Santa Cruz Biotechnology Cat# sc-7824, RRID:AB_2249859); donkey anti-goat IgG Alexa Fluor 647 conjugated antibody (Thermo Fisher Scientific Cat# A-21447, RRID: AB_2535864); donkey anti-rabbit IgG Alexa Fluor 405 Plus conjugated antibody (Thermo Fisher Scientific Cat# A48258, RRID: AB_2890547); follicle-stimulating hormone (FSH) enzyme-linked immunosorbent assay (ELISA) capture antibody (A.F. Parlow National Hormone and Peptide Program Cat# AFP1760191, RRID: AB_2665512); FSH ELISA detection antibody (A.F. Parlow National Hormone and Peptide Program Cat# AFP-C0972881; RRID: AB_2687903); horseradish peroxidase–conjugated anti-rabbit antibody (Millipore Cat# AP182P, RRID: AB_92591); LH ELISA capture antibody (Dr. Janet Roser, Department of Animal Science, University of California, Davis Cat# 518B7, RRID:AB_2665514); LH ELISA detection antibody (A.F. Parlow National Hormone and Peptide Program Cat# rLH, RRID:AB_2665533); and ISA horseradish peroxidase–conjugated antibody (Agilent Cat# P0448, RRID:AB_2617138).
Promoter-Reporter Assays
Promoter-reporter assays were performed as described previously (18). Briefly, LβT2 cells were seeded at a density of 150 000 cells/well in 48-well plates. The following day, cells were transfected using Lipofectamine 3000 (L3000015, ThermoFisher Scientific, Burlington, ON, Canada) as indicated in the manufacturer's protocol. Twenty-four hours later, transfection medium was replaced with serum-free DMEM, and cells were serum starved for 24 hours. Following starvation, cells were treated for 6 hours with either vehicle (H2O) or GnRH (100 nM). After treatment, cells were lysed with 50 μL/well of passive lysis buffer (25 mM Tris-phosphate [pH 7.8], 10% [v/v] glycerol, 1% [v/v] Triton X-100, 1 mg/mL bovine serum albumin, 2 mM EDTA) for 15 minutes at room temperature with agitation. Twenty μL of cell lysate were combined with 100 μL of luciferase assay buffer (15 mM potassium phosphate [pH 7.8], 25 mM glycylglycine, 15 mM MgSO4, 4 mM EDTA, 2 mM adenosine triphosphate, 1 mM dithiothreitol, 0.04 mM D-luciferin), and luciferase activity was measured on an Orion II microplate luminometer (Berthold Detection Systems, Oak Ridge, TN, USA). All experiments were performed in technical triplicates. Experiments were repeated as indicated in the figure legends.
Electrophoretic Mobility Shift Assays
HEK 293T cells were seeded at a density of 3 × 106 cells in 10-cm dishes. The following day, cells were transfected with 10 μg of empty vector (pcDNA3.0) or ZEB1-HA-FLAG using polyethylenimine (PEI; PolyScience Inc. cat. #23966) at a ratio of 1:3 (μg DNA to μg PEI) for 2 hours followed by incubation in growth media. Twenty-four hours following transfection, medium was replaced with serum-free DMEM, and cells were serum starved for an additional 24 hours. Following serum starvation, cells were washed twice with phosphate-buffered saline (PBS). Cells were scraped and harvested in 1 mL of PBS and centrifuged at 400g for 5 minutes at 4 °C. The cell pellet was then resuspended in 500 μL of hypotonic lysis buffer (10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid [HEPES] [pH 7.9], 1.5 mM MgCl2, 10 mM KCl) with protease inhibitors (2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL, pepstatin A, 1 mM phenylmethylsulfonyl fluoride [PMSF]) and 1 mM dithiothreitol (DTT) and incubated on ice for 15 minutes. Sixteen μL of 10% (v/v) NP-40 was added to the swollen cells and vortexed for 10 seconds. The lysed cells were then immediately centrifuged at 11 000g for 30 seconds at 4 °C to pellet the nuclei. Supernatant was discarded and the crude nuclei pellet was resuspended in 100 μL of nuclei extraction buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, and 25% [v/v] glycerol) with protease inhibitors (2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstatin A, 1 mM PMSF) and 1 mM DTT. Tubes containing the resuspended nuclei were then mounted to a vortex and lysed via moderate to high agitation for 30 minutes at 4 °C. Lysed nuclei were centrifuged at 21 000g for 5 minutes at 4 °C and the supernatant containing the clarified nuclear extract was stored at −80 °C. Protein concentration of the nuclear extracts was quantified using the Bio-Rad protein assay dye reagent concentrate (5000006, Bio-Rad Laboratories, Hercules, California, USA) as indicated in the manufacturer's protocol.
Nuclear extracts (10 μg) were incubated with 200 fmol biotinylated double-stranded probe corresponding to −38/−15 (see Table 2 for probe sequence) of the murine Lhb promoter in 10 mM Tris (pH 7.5), 50 mM KCl, 2.5% (v/v) glycerol, 1% (v/v) NP-40, 10 μM ZnSO4, 5 mM DTT, and 50 ng/μL sonicated salmon sperm DNA (15632011, Invitrogen) at room temperature for 20 minutes. Where appropriate, unlabeled double-stranded competitor probe (Table 2) or antibodies were added to the binding reactions and incubated at room temperature for 20 minutes prior to the addition of the biotinylated probe.
Table 2.
EMSA probes
| −38/−15 mLhb sense | CCGCAGGTATAAAGCCAGGTGCCC |
| −38/−15 mLhb antisense | GGGCACCTGGCTTTATACCTGCGG |
| −17/+5 mLhb sense | CCCAAGGTAGGGAAGGTATCAA |
| −17/+5 mLhb antisense | TTGATACCTTCCCTACCTTGGG |
| −37/−14 hLHB sense | CCCGAGGTATATAGCCAGATACAC |
| −37/−14 hLHB antisense | GTGTATCTGGCTATATACCTCGGG |
| −16/+9 hLHB sense | CACGAGGCAGGGGATGCACCAAGGA |
| −16/+9 hLHB antisense | TCCTTGGTGCATCCCCTGCCTCGTG |
| −38/−15 mLhb Z-box MUT sense | CCGTAGGCATAAAGCCAGGTGCCC |
| −38/−15 mLhb Z-box MUT antisense | GGGCACCTGGCTTTATGCCTACGG |
| −38/−15 mLhb E-box MUT sense | CCGCAGGTATAAAGCTGGGGACCC |
| −38/−15 mLhb E-box MUT antisense | GGGTCCCCAGCTTTATACCTGCGG |
| −38/−15 mLhb Z-box/E-box MUT sense | CCGTAGGCATAAAGCTGGGGACCC |
| −38/−15 mLhb Z-box/E-box MUT antisense | GGGTCCCCAGCTTTATGCCTACGG |
Binding reactions were resolved on 6% native polyacrylamide gels (37.5:1, acrylamide: bisacrylamide) in 0.5× Tris/Borate/EDTA (TBE), then transferred to Genescreen Plus hybridization transfer membranes (NEF1017001PK, PerkinElmer, Waltham, MA, USA) in 0.5× TBE. Membranes were blocked and detected with the buffers and detection reagents provided in the Pierce Chemiluminescent Nucleic Acid Detection Module (89880, ThermoFisher) according to the manufacturer's protocol. Membranes were imaged on an Amersham Imager 600 (GE Healthcare, Chicago, IL, USA). EMSA band intensities were quantified using the “Gel Analyzer” tool on Fiji (21).
Generation of Zeb1 Conditional Knockout Mice
The Zeb1fl/fl (MGI:5901940) and GnrhrIRES-Cre/IRES-Cre (GRIC) (MGI:3795249) mice were previously described (22, 23). Cre-mediated recombination occurs in the germ line of male GRIC mice. Thus, to prevent global recombination of the floxed allele, the GRIC allele was introduced via the female. Zeb1fl/fl males were crossed with GRIC females to produce Zeb1fl/+; GnrhrGRIC/+ progeny (24). Zeb1fl/fl males were then crossed with Zeb1fl/+; GnrhrGRIC/+ females to produce Zeb1fl/fl; Gnrhr+/+ (control) and Zeb1fl/fl; GnrhrGRIC/+ (conditional knockout; cKO) animals.
Genotyping and assessment of genomic recombination were conducted as previously described (primers listed in Table 1) (25). All animals were housed in a 12 hours light:12 hours dark cycle (lights on at 7:00) and given access to food and water ad libitum. All animal work was conducted in accordance with federal and institutional guidelines and with the approval of the McGill University Facility Animal Care Committee DOW-A (Protocol #5204).
Assessment of Puberty Onset and Female Estrous Cyclicity
Males and females were monitored daily after weaning (postnatal day 21). Puberty onset was determined by the presence of preputial separation in males and vaginal opening in females (26). Starting at 6 weeks of age, vaginal swabs from females were collected daily for 3 weeks to assess estrous cyclicity. Vaginal cells were smeared on glass slides and stained with 0.1% (w/v) methylene blue to determine cycle stage (27). The number of days spent in each stage (proestrus, estrus, or diestrus/metestrus) was counted and divided by the total number of days to determine the relative proportion of time spent in each stage.
Organ Collection and Processing
Testes, seminal vesicles, ovaries, and uteri were dissected from control and cKO males and females at 12-13 weeks of age. Pituitary glands were snap-frozen in liquid nitrogen and stored at −80 °C until RNA extraction, reverse transcription, and quantitative PCR analysis. Females were sampled between 17:00 and 18:00 on diestrus. Reproductive organs were weighed on an analytical balance.
Blood Collection and Hormone Analyses
For terminal sampling, blood was collected by cardiac puncture and then left to coagulate at room temperature for approximately 30 minutes. Blood was then centrifuged at 825g for 10 minutes to obtain serum. Serum was stored at −80 °C until hormone analyses were conducted. To assess LH pulsatility in males, 4 μL of blood were collected from the tail tip every 6 minutes across 3 hours. Prior to blood collection, males were mock-bled every 6 minutes for 1 hour to reduce stress. To assess LH surge amplitude in females, 4 μL of blood were collected from the tail tip over 10 consecutive days at 10:00, 17:00, 18:00, 19:00, and 20:00. For all tail tip blood collections, animals were acclimatized to the procedure by daily massaging for 5 weeks prior to the start of the blood collection. All tail tip blood collections were immediately diluted (1:30) in PBS containing 0.05% (v/v) Tween, gently mixed, and placed on dry ice. Diluted whole blood was stored at −80 °C until hormone analyses were conducted. Serum FSH (FSH ELISA detection range: 0.3215-20 ng/mL; intra-assay coefficient of variation < 10%), serum LH, and whole-blood LH (LH ELISA detection range: 0.117-30 ng/mL; intra-assay coefficient of variation 1%-5%) were assessed by in-house ELISAs (28, 29).
Male whole-blood LH profiles were analyzed using the PULSAR algorithm (30, 31). All PULSAR analyses were conducted with the following parameters; smoothing fraction 0.7, peak split 2.5, level of detection 0.117, amplitude distance 3.0, g(1) 6.2, g(2) 4.9, g(3) 2.5, g(4) 1.5, g(5) 1.2, and assay variability 0, 2.5, and 3.3.
RNA Extraction, Reverse Transcription, and Quantitative PCR
Total RNA was isolated from pituitaries and LβT2 cells with TRIzol reagent following the manufacturer's protocol. RNA concentration was determined using a NanoDrop 2000c spectrophotometer. One-hundred ng of total RNA was reverse-transcribed using MMLV reverse transcriptase (M1701, Promega, Madison, Wisconsin, United States) and random hexamers (C1181, Promega) as previously described (32).
The qPCR analysis was conducted using BlasTaq (G891, Applied Biological Materials Inc., Richmond, BC, Canada) and primers (listed in Table 1) on a Corbett Rotorgene 600 instrument (Corbett Life Science, Sydney, NSW, Australia). mRNA levels were calculated using the 2−ΔΔCT method. Gene expression was normalized to ribosomal protein L19 (Rpl19). All primers were validated for efficiency and specificity.
Immunofluorescence
The 12- to 13-week-old mice were perfused with 4% paraformaldehyde (PFA, P6148, Millipore Sigma) in PBS (pH 7.4) and dissected pituitaries were post-fixed in 4% PFA/PBS (pH 7.4) at 4 °C for 24 hours. Samples were washed once with PBS, then cryoprotected in a sequential sucrose gradient in PBS (10% to 20% to 30%) at 4 °C; samples were incubated in each concentration of sucrose for 15 minutes or until they sank, then incubated in 30% sucrose in PBS overnight at 4 °C. The next day, samples were washed once with Optimal Cutting Temperature (OCT) compound (95057-838, VWR International, Radnor, PA, USA) and then embedded in OCT. Frozen tissue was sectioned at a thickness of 8 μm using a Leica CM3050S cryostat, mounted on Fisherbrand Superfrost Plus slides (22-037-246, ThermoFisher Scientific), and stored at −80 °C until use. Slides were warmed to room temperature for 10 minutes, then washed 3 times for 5 minutes with PBS with 0.1% Tween (PBST) at room temperature. Sections were blocked in blocking buffer (0.15% glycine, 2 mg/mL BSA, 0.1% Triton X in PBS) with 10% donkey serum (035110, Wisent) for 1 hour at room temperature. Sections were then incubated overnight at 4 °C with antibodies against ZEB1 (1:200; 1 μg/mL) and LHβ (1:500; 0.4 μg/mL) (described above) (diluted in blocking buffer with 1% donkey serum). The next day, sections were washed 3 times for 5 minutes with PBST and incubated in the dark at room temperature with Alexa Fluor 405 Plus (1:250; 8 µg/mL) and Alexa Fluor 647 (1:500; 4 µg/mL) (described above) (diluted in blocking buffer with 1% donkey serum) for 1 hour. Sections were then washed 3 times for 5 minutes with PBST, and mounted in Prolong Diamond Antifade Mountant (P36961, Invitrogen). Fluorescent images were captured using a Nikon CSU-X1 spinning disk confocal microscope with a CFI Plan Apochromat Lambda D 20X water objective.
Histochemical Staining
Ovaries from females at 12-13 weeks of age were fixed in 10% neutral buffered formalin (HT501128, Sigma) overnight at room temperature. The following day, samples were transferred to 70% ethanol and stored at 4 °C until processing. Samples were dehydrated in a series of graded ethanol baths (once in 80% for 1 hour, once in 95% for 1 hour, and twice in 100% for 1 hour), cleared twice for 30 minutes with Histoclear (NDIHS-200, Diamed, Mississauga, ON, Canada), and then embedded in paraffin (18-604-991, ThermoFisher Scientific). Sections were cut at a thickness of 5 μm using a Shandon Finesse 325 microtome (ThermoFisher Scientific).
For hematoxylin and eosin (H&E) staining, tissue sections were cleared twice for 5 minutes with Histoclear and rehydrated in a series of graded ethanol baths (100% and 70%, for 5 minutes each). Slides were stained with hematoxylin (Gill No.3, GHS332, Sigma-Aldrich) and eosin (AC611815000, Fisher Scientific), dehydrated in graded ethanol baths (70% and 100%, 5 minutes each), cleared twice for 5 minutes with Histoclear, and mounted with Permount (SP15-100, Fisher Scientific). Sections were imaged using a Zeiss Axio Imager.M2 microscope with a 10×/0.3 EC Plan-Neofluar objective.
Statistical Analyses
Luciferase assays and EMSA quantifications were analyzed by one-way or two-way analysis of variance (ANOVA), followed by post hoc Holm–Sidak multiple comparisons tests. Where applicable, differences between control and cKO animals were assessed by unpaired t tests with Welch's correction. All statistical analyses were conducted using GraphPad Prism (Version 10). The α level was set at P < .05.
Results
ZEB1 Blocks GnRH-Stimulated Murine Lhb Expression
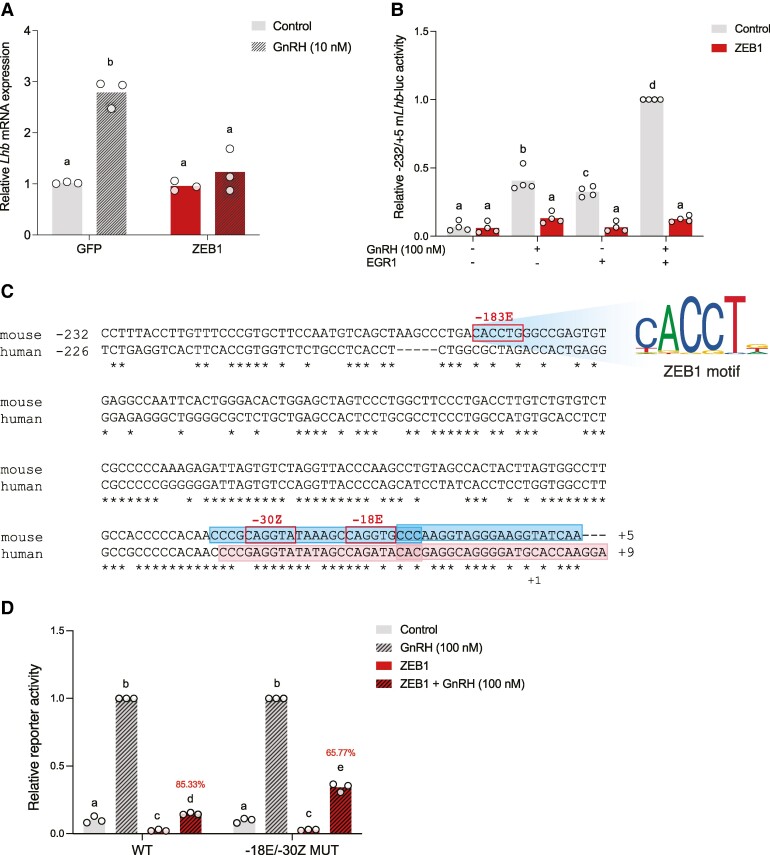
Zeb1 knockdown with siRNAs reportedly increased basal Lhb mRNA levels in LβT2 cells (7). Here, we overexpressed ZEB1 in LβT2 cells using a recombinant adenovirus and then treated with hourly 5-minute GnRH pulses for 6 hours. GnRH stimulated endogenous Lhb expression, which was blocked by ZEB1 overexpression (Fig. 1A). In contrast, ZEB1 did not affect basal Lhb levels (Fig. 1A).
Figure 1.
ZEB1 represses GnRH- and/or EGR1-stimulated murine Lhb expression/transcription. (A) LβT2 cells were transduced with GFP or ZEB1 expressing adenovirus. Cells were treated with pulsatile GnRH. Lhb mRNA was measured by qPCR. N = 3 independent experiments. (B) LβT2 cells were transfected with 225 ng/well −232/+5 mLhb-luc reporter, and 25 ng/well ZEB1 and/or 50 ng/well EGR1 expression vector. Cells were treated with 100 nM GnRH for 6 hours. N = 4 independent experiments. (C) Alignment of the murine Lhb and human LHB promoters. In both cases, +1 refers to the transcription start site (TSS). Consensus ZEB1 cis-elements (E- and Z-box motifs shown) are outlined in red boxes. Positions of murine Lhb probes and human LHB probes used in electrophoretic mobility shift assays (EMSAs) are outlined in filled blue boxes and pink boxes, respectively. (D) LβT2 cells were transfected with 225 ng/well of the indicated −232/+5 mLhb-luc reporter and 25 ng/well ZEB1 expression vector.
Abbreviations: WT, wild-type; −30Z/-18E MUT, mutated site −30Z, and −18E. Cells were treated with GnRH as in panel B. N = 3 independent experiments. In B and D, protein lysates were collected and reporter activity measured by luciferase assays. White circles represent experimental replicates. Percent repression of GnRH-induced promoter-reporter activity by ZEB1 is shown in red. Data were analyzed by two-way ANOVA followed by Holm–Sidak multiple comparisons test. Bars with different letters differ significantly.
ZEB1 Attenuates GnRH- and EGR1-Stimulated Murine Lhb Promoter-Reporter Activity
To examine whether and how ZEB1 regulates Lhb transcription, we generated luciferase reporter constructs containing varying lengths of the murine Lhb promoter (−232/+5, −435/+5, −495/+5, and −968/+5). GnRH induction of murine Lhb transcription is EGR1-dependent (2, 3, 17). Consistent with previous observations, GnRH and/or EGR1 overexpression (17) induced Lhb promoter-reporters of all lengths in LβT2 cells (Fig. 1B, Supplementary Fig. S1A-S1C) (33). These effects were inhibited by overexpressed ZEB1, which did not impact basal Lhb promoter-reporter activity (Fig. 1B, Supplementary Fig. S1A-S1C) (33). As all reporters responded similarly, we used the minimal Lhb promoter (−232/+5) in subsequent analyses. Similarly, as ZEB1 repressed the stimulatory effects of both GnRH and its downstream effector, EGR1, we used GnRH to induce Lhb promoter-reporter activity in most of the subsequent analyses.
ZEB1 Actions Are Independent of Previously Characterized E-boxes in the Murine Lhb Promoter
Prior gel shift analyses indicated that ZEB1 could bind to at least 3 E-box-like cis-elements in the Lhb promoter (7). The most proximal of these sites is a canonical E-box (CANNTG) located in the minimal promoter-reporter at −188 to −183 (CACCTG, hereafter −183E) (Fig. 1C). Mutation of −183E did not affect ZEB1 inhibition of GnRH-stimulated Lhb promoter-receptor activity (Supplementary Fig. S2A) (33). We therefore analyzed the proximal promoter and identified 2 additional ZEB1 cis-elements: a Z-box located at −35 to −30 (CAGGTA, −30Z) and an E-box located at −23 to −18 (CAGGTG, −18E) (Fig. 1C). Mutations of these sites alone did not significantly affect ZEB1 inhibition of GnRH-stimulated Lhb promoter-reporter activity (Supplementary Fig. S2B-S2C) (33). However, when −30Z and −18E were mutated in combination, ZEB1 repression of GnRH-stimulated Lhb transcription was significantly attenuated (Fig. 1D).
Regulatory Sequence in the Proximal Murine Lhb Promoter Mediates ZEB1's Repressive Activity
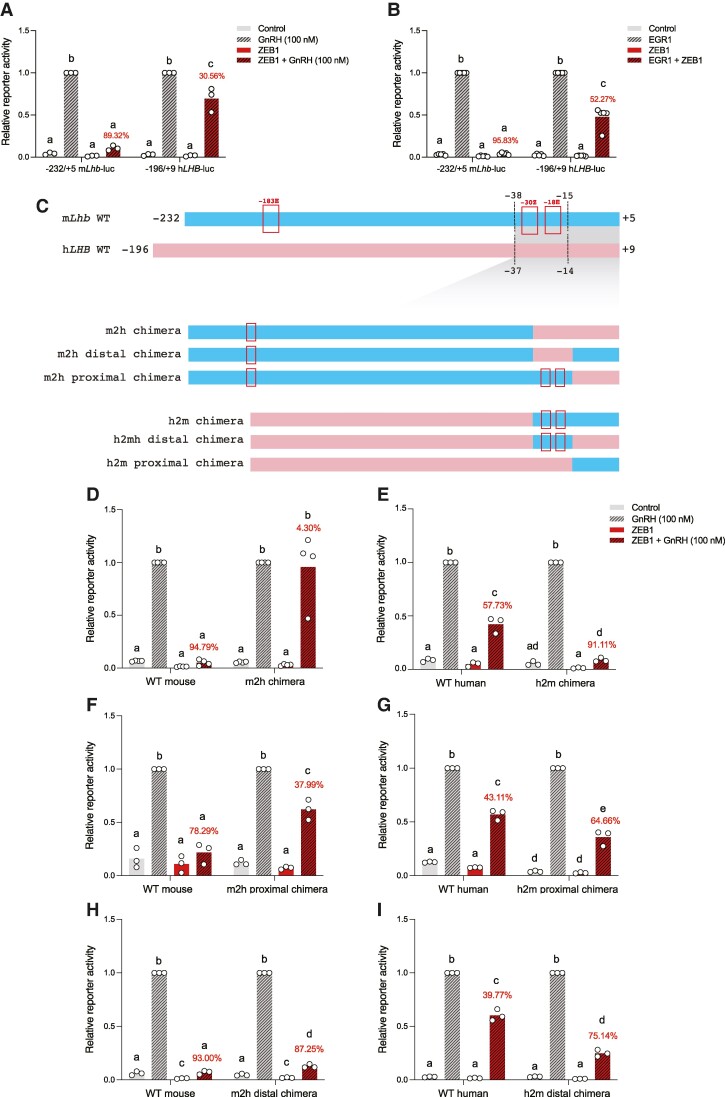
The murine Lhb and human LHB promoters are highly conserved (Fig. 1C). Therefore, we next examined ZEB1 regulation of human LHB promoter-reporter activity in LβT2 cells. As previously reported (2, 3), GnRH and/or EGR1 overexpression induced the human LHB promoter-reporter (Fig. 2A and 2B). Unexpectedly, ZEB1 repression of human LHB was relatively modest (∼31%-52%) compared to its effects on the murine Lhb promoter (∼89%-96%; Fig. 2A and 2B).
Figure 2.
ZEB1 modestly represses human LHB transcription. (A) LβT2 cells were transfected with 225 ng/well of WT −232/+5 murine Lhb-luc or WT −196/+9 human LHB-luc reporter, as well as 25 ng/well ZEB1 expression vector. Cells were treated with GnRH as in Fig. 1. (B) LβT2 cells were transfected with 225 ng/well WT −232/+5 mLhb-luc or WT −196/+9 hLHB-luc reporter, and 25 ng/well ZEB1 and/or 50 ng/well EGR1 expression vector. (C) Schematic of the WT murine Lhb (blue) and WT human LHB (pink) promoters. Putative ZEB1 cis-elements are boxed. Chimeric murine Lhb and human LHB promoters are depicted. Dashed lines at the top indicate the sequence comprising swapped regions in the chimeric reporters. (D-I) LβT2 cells were transfected with 225 ng/well of the indicated reporters, 25 ng/well ZEB1 expression vector, and treated with GnRH. In A-B and D-I, luciferase assays were performed as in Fig. 1. Data represent the mean of 3 or more independent experiments performed in triplicate. Percent repression of GnRH-induced promoter-reporter activity by ZEB1 is shown in red. White circles represent experimental replicates. Data were analyzed by two-way ANOVA followed by Holm–Sidak multiple comparisons test. Bars with different letters differ significantly.
Abbreviations: m2h, mouse-to-human; h2m, human-to-mouse.
To gain greater insight into the differential effects of ZEB1 on murine Lhb and human LHB promoter activities, we performed reporter assays in LβT2 cells using mouse-to-human (m2h) and human-to-mouse (h2m) promoter chimeras (schematized in Fig. 2C). Swapping the murine and human promoter sequences close to the transcription start sites (TSS) inverted ZEB1's inhibition of GnRH-induced murine Lhb (Fig. 2D) and human LHB (Fig. 2E) promoter-reporter activities. That is, ZEB1 inhibition of GnRH-induced murine Lhb promoter-reporter activity was effectively lost in the mouse-to-human (m2h) chimera (∼95% to ∼4% inhibition, Fig. 2D). In contrast, ZEB1 repression of GnRH-induced human LHB promoter-reporter activity was increased in the human-to-mouse (h2m) chimera (∼58% to ∼91%, Fig. 2E).
To further refine the relevant DNA sequences, we divided the murine Lhb and human LHB promoters near the TSS into proximal (−15/+5 mouse; −14/+9 human) and distal (−38/−15 mouse; −37/−14 human) halves (schematized in Fig. 2C). The distal half contains the newly identified and functionally relevant Z- and E-boxes in the murine promoter (Fig. 1D). Notably, these cis-elements are not perfectly conserved in the 2 species (Fig. 1C). ZEB1 inhibition of GnRH-induced reporter activity was attenuated in the murine Lhb promoter m2h proximal chimera (∼78% to ∼38%, Fig. 2F). In contrast, there was a moderate increase in ZEB1 inhibition of GnRH-stimulated human LHB promoter-reporter activity in the h2m proximal chimera (∼43% to ∼65%, Fig. 2G). Despite differences in the Z- and E-boxes contained within, we observed only a subtle decrease in ZEB1 inhibition of GnRH stimulation of the murine m2h distal chimera (∼93% to ∼87%, Fig. 2H). In contrast, ZEB1 repression of GnRH-induced human LHB promoter-reporter activity was enhanced in the h2m distal chimera (∼40% to ∼75%, Fig. 2I).
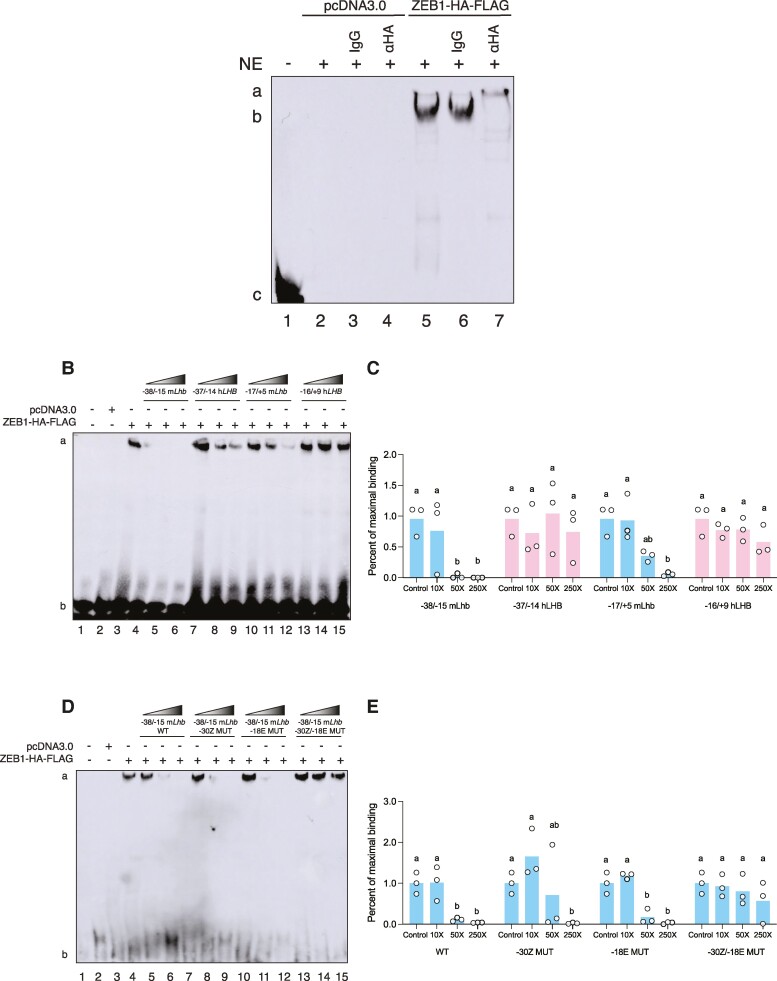
ZEB1 Binds With Higher Affinity to the Murine Lhb Than the Human LHB Promoter
We next examined ZEB1 binding to the proximal promoters in the 2 species. We performed electrophoretic mobility shift assays (EMSAs) using a biotinylated probe corresponding to −38 to −15 of the murine Lhb promoter (the “distal” region containing the Z- and E-boxes; see Table 2 for probe sequences). ZEB1-HA-FLAG expressed in heterologous HEK293T cells bound to the probe (Fig. 3A, lane 5, labeled “b”). The complex was super-shifted by an HA antibody (Fig. 3A, lane 7, labeled “a”), but not by control IgG (Fig. 3A, lane 6). Binding was competed completely by 50× and 250× molar excess of unlabeled homologous −38/−15 murine Lhb probe (Fig. 3B, lanes 4-6; quantified in Fig. 3C). Binding was also competed by unlabeled −15/+5 murine Lhb (proximal) probe, but completely only at 250× (Fig. 3B, lanes 10-12, and Fig. 3C). In contrast, ZEB1 binding to the labeled −38/−15 murine Lhb probe was not significantly displaced by unlabeled human −37/−14 (distal; Fig. 3B, lanes 7-9, and Fig. 3C) or −14/+9 (proximal; Fig. 3B, lanes 13-15, and Fig. 3C) LHB probes.
Figure 3.
ZEB1 binds with higher affinity to the murine Lhb than human LHB promoter. (A) Nuclear extract (NE) from HEK293T cells transfected with pcDNA3.0 or ZEB1-HA-FLAG was incubated with a biotinylated probe corresponding to −38/−15 of the murine Lhb promoter. Where indicated, NE was pre-incubated with control (IgG) or α-HA antibody. (B) NE was pre-incubated with unlabeled competitor probes corresponding to the indicated regions of the murine Lhb or human LHB promoter. (C) Quantification of EMSA assays in B. (D) NE was pre-incubated with unlabeled competitor probes corresponding to the indicated wild-type (WT) or Z-box or E-Box mutant (MUT) murine Lhb probes. (E) Quantification of EMSA assays in D. For B and D, increasing concentrations of unlabeled competitor probes are denoted by the gradient-filled slopes; 10X, 50X, and 250X molar excess. One representative experiment of 3 is shown. Data are the mean of 3 independent experiments. White circles represent experimental replicates. Data were analyzed by two-way ANOVA followed by Holm–Sidak multiple comparisons test. Bars with different letters differ significantly.
Mutations to the E-box at −23 to −18 (−18E) and Z-box located at −35 to −30 (−30Z) attenuated ZEB1 activity in murine Lhb reporter assays (Fig. 1D). We therefore examined the effects of these mutations on ZEB1 binding to the labeled murine −38/−15 promoter probe. Binding was competed moderately at 50× and completely by 250× molar excess of unlabeled −38/−15 murine Lhb probe containing a mutation in site −30Z (Fig. 3D, lanes 7-9; quantified in Fig. 3E). Similarly, binding was competed by 50× and 250× molar excess, but not 10×, of unlabeled −38/−15 murine Lhb probe containing a mutation in site −18E (Fig. 3B, lanes 10-12; quantified in Fig. 3E). In contrast, the unlabeled −38/−15 murine Lhb probe containing mutations in both site −30Z and −18E failed to compete for binding at all concentrations (Fig. 3B, lanes 13-15, quantified in Fig. 3E).
Generation of Zeb1 Conditional Knockout Mice
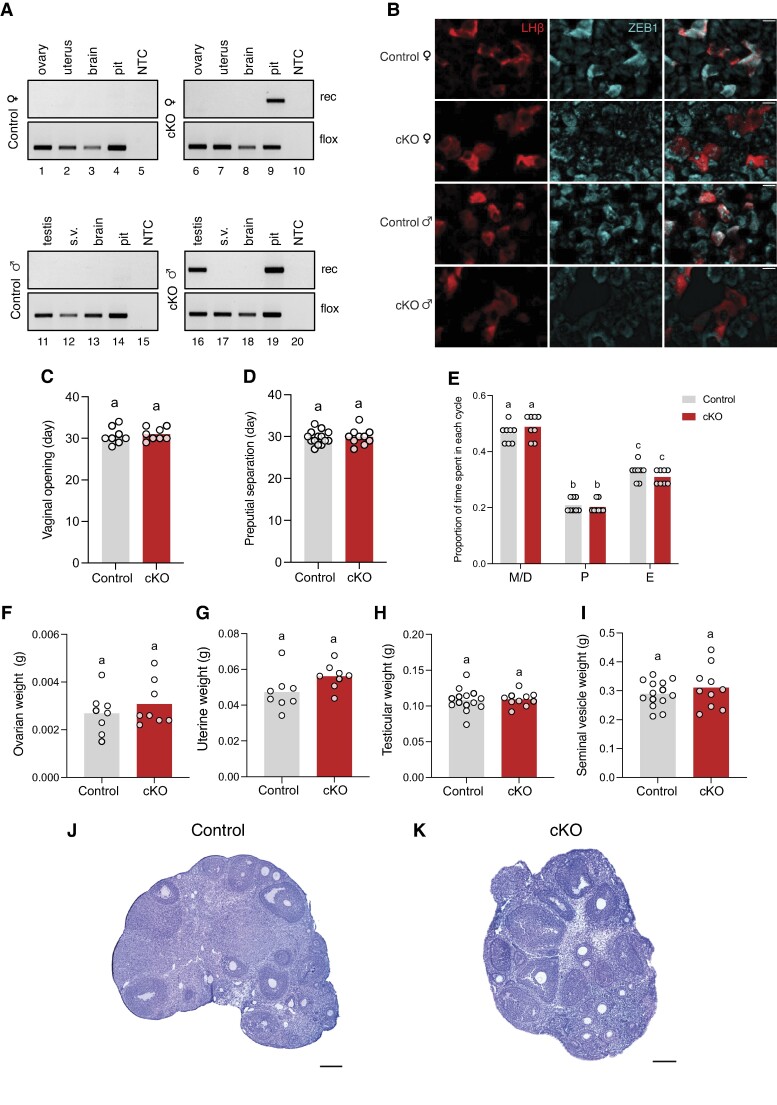
The above in vitro analyses indicated that ZEB1 binds to the proximal murine Lhb promoter to inhibit GnRH-dependent transcription, which could explain LH deficiency in mice with ZEB1 overexpression (gain of function) in gonadotropes (7). To investigate whether ZEB1 plays a physiological role in gonadotropes, we generated Zeb1 conditional knockout (cKO) mice (loss of function) by crossing floxed Zeb1 (Zeb1fl/fl) and GnRH receptor-Cre (GnrhrGRIC) animals. We observed recombination of the floxed Zeb1 allele in the pituitaries of cKO animals of both sexes (Fig. 4A, lanes 9 and 19) and in the testes of cKO males (Fig. 4A, lane 16). The GRIC Cre-driver is active in pituitary gonadotropes and in male germ cells (22, 34-37). There was no recombination in the absence of the GRIC allele in control animals of either sex (Fig. 4A, lanes 1-4 and 11-14). Ablation of ZEB1 protein in gonadotropes was demonstrated by immunofluorescence on pituitaries of cKOs compared with controls (Fig. 4B).
Figure 4.
Normal puberty onset and gonad weights in gonadotrope-specific Zeb1 knockout mice. (A) Genomic DNA was extracted from the indicated tissues of Zeb1fl/fl (control; gray) and Zeb1fl/fl; GnrhrGRIC/+ (cKO; red) mice and analyzed by PCR for the presence of the floxed or recombined (rec) Zeb1 alleles. (B) Pituitary sections from control and cKO mice were analyzed for ZEB1 by immunofluorescence (blue); LHβ (red) was used to label gonadotropes. Scale bars: 20 μm. (C) Age of vaginal opening (days) in female control and cKO mice. (D) Age of preputial separation (days) in male control and cKO mice. (E) Proportion of time spent in each stage of the estrous cycle (over 21 days) in female control and cKO females; metestrus (M)/diestrus (D), proestrus (P), estrus (E). (F) Ovarian weights, (G) uterine weights, (H) testicular weights, and (I) seminal vesicle weights. Bar heights are group means. Each white circle represents an individual mouse. Data were analyzed by two-tailed unpaired t tests with Welch's correlation. Bars with different letters differ significantly. Ovarian sections stained with hematoxylin and eosin (H&E) from (J) control and (K) cKO females. Scale bars: 200 μm.
Normal Puberty Onset and Reproductive Organ Weights in Zeb1 cKO Mice
Puberty onset, marked by vaginal opening or preputial separation in females and males, respectively, occurred at similar ages in control and cKO animals (Fig. 4C and 4D). Estrous cyclicity, as determined by vaginal cytology, was normal in adult Zeb1 cKO females (Fig. 4E). In females sampled at diestrus, ovarian (Fig. 4F) and uterine (Fig. 4G) weights were comparable between genotypes. Testicular (Fig. 4H) and seminal vesicle (Fig. 4I) weights did not differ between adult control and cKO males. Control and cKO ovaries appeared morphologically normal, with follicles at all stages, including corpora lutea (Fig. 4J and 4K). Although we did not conduct fertility trials, cKO females were used to maintain the colony and routinely gave birth to 6-8 pups/litter.
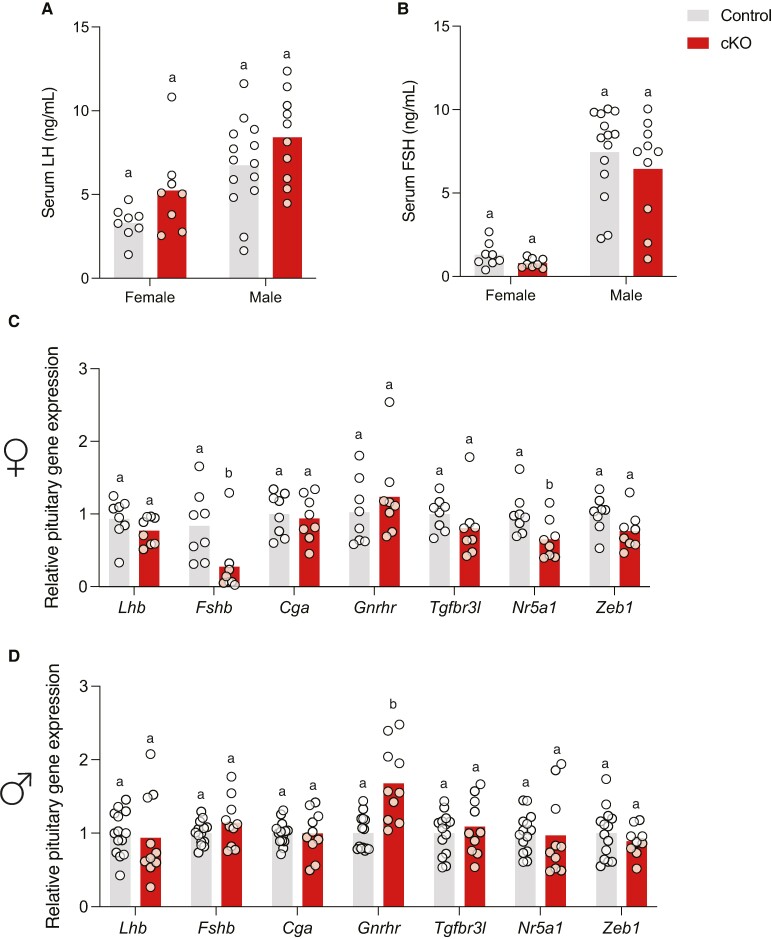
LH and FSH Production Are Normal in Zeb1 cKO Mice
Blood samples were collected from adult (diestrus) females and males in the evening (17:00-18:00). Serum LH levels (Fig. 5A) and FSH levels (Fig. 5B) did not differ between genotypes of either sex. Pituitary Lhb, Fshb, Cga, Tgfbr3l, and Zeb1 mRNA levels were equivalent between genotypes in males, while pituitary Fshb and Nr5a1 mRNA levels were significantly decreased in cKO females (Fig. 5C and 5D). In contrast, pituitary Gnrhr mRNA levels were equivalent between genotypes in females but significantly elevated in male Zeb1 cKO animals compared to littermate controls (Fig. 5C and 5D).
Figure 5.
Normal gonadotropin production in Zeb1 conditional knockout mice. Serum (A) LH and (B) FSH levels in female and male control and cKO mice. Pituitary gene expression in (C) female and (D) male control and cKO mice. Bar heights are group means. Each white circle represents an individual mouse. Data were analyzed by two-tailed unpaired t tests with Welch's correlation. Bars with different letters differ significantly.
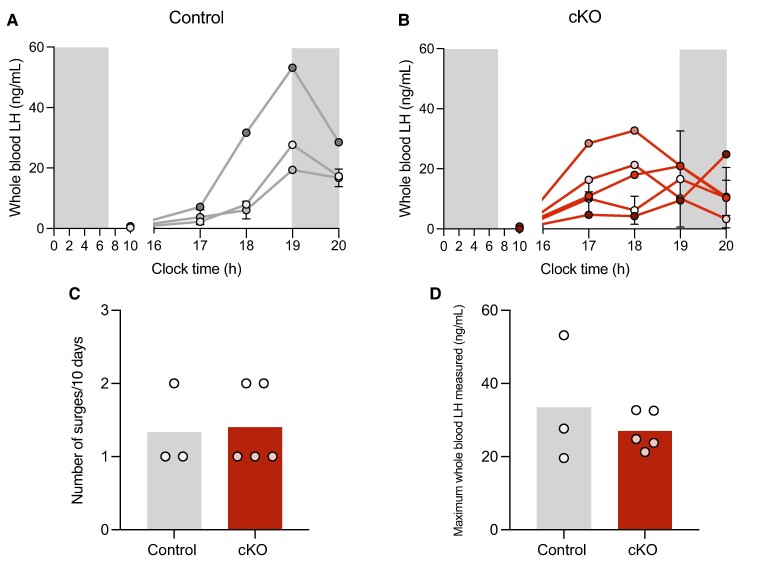
Preovulatory LH Surges Are Normal in Zeb1 cKO Females
ZEB1 overexpression in gonadotropes led to a block in ovulation (7). Therefore, we next examined the effect of ZEB1 ablation in gonadotropes on the LH surge. Profiles of LH surges in control and cKO females are shown in Fig. 6A and 6B, respectively. Three control females (out of 9 sampled) and 5 cKO females (out of 8 sampled) surged at least once during the 10-day sampling period (see Methods). One control and 2 cKOs surged twice (Fig. 6C). The number of surges in a 10-day period was comparable between genotypes (Fig. 6C). Likewise, the maximum LH levels measured appeared to be equivalent between cKO and control females (Fig. 6D).
Figure 6.
Normal preovulatory LH surges in cKO females. Profiles of the LH secretion obtained on proestrus from 3 control (A) and 5 cKO (B) females that exhibited LH surges. Different-colored circles and lines indicate different mice. Gray areas represent the dark phase of the light/dark cycle. For mice that surged more than once in the sampling period, their whole-blood LH levels are represented as the mean ± standard error of the mean [SEM]. (C) Number of surges observed in each mouse during the sampling period. (D) Maximal whole-blood LH levels (ng/mL) measured in each mouse. White circles in (C) and (D) represent individual mice. Data represent the subset of females that had 1 ≥ LH surge in the sampling period.
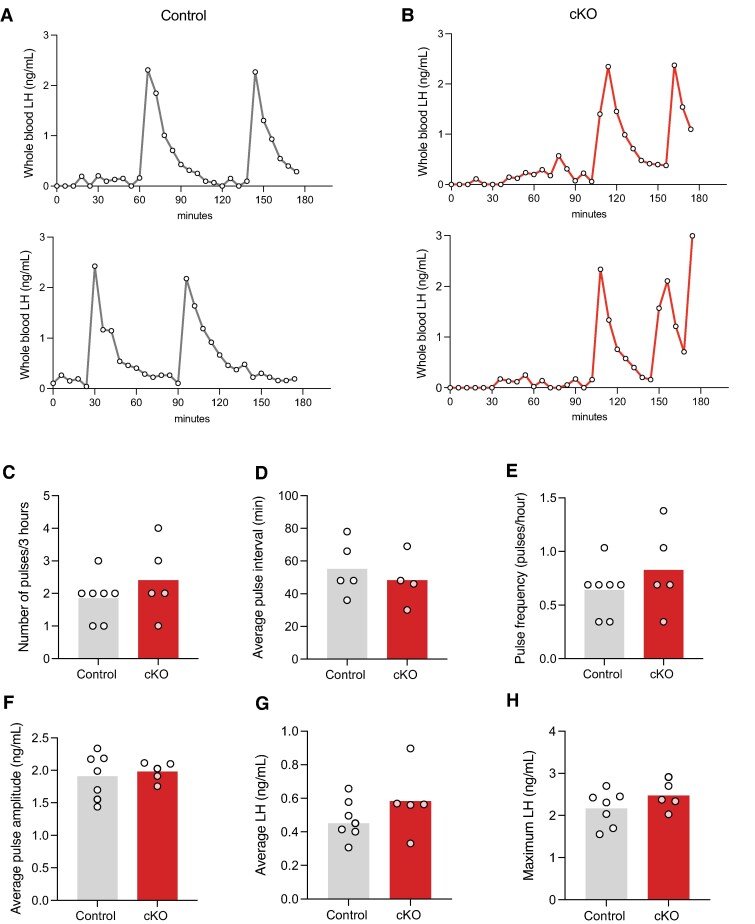
Pulsatile LH Is Normal in Zeb1 cKO Males
Finally, we examined LH pulsatility in control and cKO mice. As LH pulses vary in females depending on estrous cycle stage, we limited our analysis to males (38, 39). Representative profiles of control and cKO males that pulsed regularly are shown in Fig. 7A and 7B, respectively. We observed no genotype-dependent differences in LH pulse number, pulse interval, pulse frequency, pulse amplitude, average LH level, or maximal LH level (Fig. 7C-7H).
Figure 7.
Normal LH pulses in cKO males. Profiles of LH secretion from (A) 2 control (gray) and (B) 2 cKO (red) males. Quantification of the (C) number of pulses, (D) pulse interval, (E) pulse frequency, (F) average pulse amplitude (ng/mL), (G) average LH (ng/mL), and (H) maximum LH (ng/mL) in the sampling period. Bar heights are group means. Each white circle represents an individual mouse. Data represent the subset of males that exhibited 1≥ LH pulse (as determined by PULSAR analysis) in the sampling period.
Discussion
It was previously hypothesized that overexpressed ZEB1 inhibits LH synthesis in miR-200b and miR-429 knockout mice by repressing transcription of the LHβ-subunit gene (Lhb) (7). The data presented here both support this idea and provide mechanistic insight into ZEB1's actions. We observed that ZEB1 inhibits GnRH-stimulated Lhb transcription in homologous murine gonadotrope-like LβT2 cells via species-specific cis-element(s) in the proximal promoter. The data also show that, while its overexpression may inhibit LH synthesis in vivo, ZEB1 does not play an essential role in the physiological regulation of LH (or FSH) in mice.
Canonically, ZEB1 acts via E-box [CANNTG] and Z-box [A(C/T)A(C/G)GT(A/G)T] cis-elements in target gene promoters to repress transcription (10, 14). We discovered closely spaced Z- and E-box elements in the proximal murine Lhb promoter that mediated, at least in part, ZEB1 suppression of GnRH-stimulated transcription. The E-box in mice (CAGGTG) differs by 2 base pairs in the human LHB promoter (CAGATA). The Z-boxes also differ between species (CAGGTA in mouse vs GAGGTA in human). A human LHB chimeric reporter containing the murine E- and Z-boxes had increased ZEB1 sensitivity (Fig. 2I). Thus, species differences in these 2 elements contribute to ZEB1's greater repression of murine Lhb than human LHB transcription. Nevertheless, ZEB1 activity also depends on additional sequence differences in the most proximal promoter, as revealed in reporter assays using chimeric murine/human reporters (Fig. 2F and 2G). However, we were not able to identify the relevant cis-regulatory sequences given the relatively weak binding of ZEB1 to this region, at least under the EMSA conditions used here (Fig. 3B and 3C).
ZEB1 overexpression inhibits LH, but not FSH production in vivo (7). Based on our results, we hypothesize that this is explained by ZEB1 antagonism of EGR1 action. Not only did overexpressed ZEB1 attenuate EGR1-induced murine Lhb promoter-reporter activity, but FSH production is EGR1-independent (40). We employed chromatin immunoprecipitation-PCR along with other protein-DNA interaction assays to examine whether ZEB1 interferes with EGR1 binding to the Lhb promoter; however, the results of these experiments were inconclusive. As a result, the precise mechanism underlying ZEB1 inhibition of EGR1 action on the murine Lhb promoter is unresolved. It is unlikely that ZEB1 disrupts EGR1 interactions with SF1 or PITX1 given the limited effects of ZEB1 on human LHB transcription, which also depends on the combined actions of these transcription factors.
Although the results of our in vitro analyses provide greater insight into how ZEB1 overexpression led to LH deficiency in miR-200b and -429 knockout mice (7), our in vivo observations indicate that ZEB1 is not an essential physiological regulator of LH. The lack of notable reproductive phenotypes in gonadotrope-specific Zeb1 knockout mice could, in principle, result from compensation by other transcriptional repressors, including ZEB2, SNAIL, SLUG, and TWIST, or by incomplete Cre-mediated recombination. We consider these possibilities unlikely, however. First, recent snRNA-seq analyses reveal that Zeb2, Snai1, Snai2, Twist1, and Twist2 are expressed at low to undetectable levels across murine pituitary cell types, including gonadotropes, in both sexes (16). In addition, these proteins do not typically functionally overlap (8, 41-43). Second, the GRIC Cre-driver mouse line is specific and efficient in recombining floxed alleles in gonadotropes (34, 35, 37), and we confirmed loss of the ZEB1 protein in gonadotropes in the cKO mice.
In summary, overexpressed ZEB1 inhibits LH production and secretion in mice by attenuating GnRH/EGR1-stimulated Lhb transcription. ZEB1 actions depend upon species-specific regulatory sequences, including E- and Z-boxes, near the transcription start site in the murine Lhb promoter. Conversely, at physiological levels, ZEB1 is unlikely to be a necessary regulator of LH in mice or humans.
Acknowledgments
Reagents were provided by Drs. Terry Hébert (HEK293T cells; McGill University), Pamela Mellon (LβT2 cells; UCSD), and Hidetoshi Hasuwa (ZEB1 expression vectors; Keio University School of Medicine).
Abbreviations
- cKO
conditional knockout
- DMEM
Dulbecco’s Modified Eagle Medium
- DTT
dithiothreitol
- EGR1
early growth response 1
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine serum
- FSH
follicle-stimulating hormone
- GFP
green fluorescent protein
- GnRH
gonadotropin-releasing hormone
- GRIC
gonadotropin-releasing hormone receptor-internal ribosome entry site-Cre
- h2m
human-to-mouse
- HEK
human embryonic kidney
- LH
luteinizing hormone
- m2h
mouse-to-human
- miRNA
micro-RNA
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PITX1
paired-like homeodomain transcription factor 1
- qPCR
quantitative polymerase chain reaction
- SF-1
steroidogenic factor 1
- siRNA
small interfering RNA
- TSS
transcription start site
- ZEB1
zinc finger E-box binding homeobox 1
Contributor Information
Hailey Schultz, Department of Anatomy and Cell Biology, McGill University, Montréal, Québec H3G 1Y6, Canada.
Xiang Zhou, Department of Pharmacology and Therapeutics, McGill University, Montréal, Québec H3G 1Y6, Canada.
Carlos Agustín Isidro Alonso, Department of Pharmacology and Therapeutics, McGill University, Montréal, Québec H3G 1Y6, Canada.
Luisina Ongaro, Department of Pharmacology and Therapeutics, McGill University, Montréal, Québec H3G 1Y6, Canada.
Yeu-Farn Lin, Department of Pharmacology and Therapeutics, McGill University, Montréal, Québec H3G 1Y6, Canada.
Mary Loka, Integrated Program in Neuroscience, McGill University, Montréal, Québec H3G 1Y6, Canada.
Thomas Brabletz, Department of Experimental Medicine 1, Nikolaus-Fiebiger Center for Molecular Medicine, FAU University Erlangen-Nürnberg, Erlangen 91054, Germany.
Simone Brabletz, Department of Experimental Medicine 1, Nikolaus-Fiebiger Center for Molecular Medicine, FAU University Erlangen-Nürnberg, Erlangen 91054, Germany.
Marc P Stemmler, Department of Experimental Medicine 1, Nikolaus-Fiebiger Center for Molecular Medicine, FAU University Erlangen-Nürnberg, Erlangen 91054, Germany.
Ulrich Boehm, Department of Experimental Pharmacology, Center for Molecular Signaling, Saarland University School of Medicine, Homburg 66421, Germany.
Daniel J Bernard, Department of Anatomy and Cell Biology, McGill University, Montréal, Québec H3G 1Y6, Canada; Department of Pharmacology and Therapeutics, McGill University, Montréal, Québec H3G 1Y6, Canada; Integrated Program in Neuroscience, McGill University, Montréal, Québec H3G 1Y6, Canada.
Funding
Canadian Institutes of Health Research project grant PJT-169184 (D.J.B.). Natural Sciences and Engineering Research Council of Canada Master’s and Doctoral Graduate Scholarships (H.S. and Y.F.L.). Fonds de Recherche du Québec Nature et technologies Doctoral Graduate Scholarship (H.S. and Y.F.L.).
Author Contributions
H.S. and D.J.B. were responsible for experimental design, data analyses, and manuscript preparation. H.S. conducted the in vitro experiments. H.S. and C.A.I.A. designed the murine Lhb promoter-reporter constructs. T.B., S.B., and M.P.S. provided the Zeb1 floxed mice. U.B. provided the GRIC mice. H.S. and X.Z. performed tissue collection, mouse colony management, and analyses of the ZEB1 mice. L.O. performed the FSH ELISAs. Y.F.L. and M.L. embedded and sectioned ovaries.
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the References.
References
- 1. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004;101(49):17294‐17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol. 1999;19(4):2567‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone β subunit transcription. Mol Hum Reprod. 2009;15(2):77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-β promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol. 1999;13(5):752‐763. [DOI] [PubMed] [Google Scholar]
- 5. Halvorson LM, Ito M, Jameson JL, Chin WW. Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone β-subunit gene expression*. J Biol Chem. 1998;273(24):14712‐14720. [DOI] [PubMed] [Google Scholar]
- 6. Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone β gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1 (∗). J Biol Chem. 1996;271(12):6645‐6650. [DOI] [PubMed] [Google Scholar]
- 7. Hasuwa H, Ueda J, Ikawa M, Okabe M. MiR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science. 2013;341(6141):71‐73. [DOI] [PubMed] [Google Scholar]
- 8. Stemmler MP, Eccles RL, Brabletz S, Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21(1):102‐112. [DOI] [PubMed] [Google Scholar]
- 9. Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66(5):773‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Xu L, Li A, Han X. The roles of ZEB1 in tumorigenic progression and epigenetic modifications. Biomed Pharmacother. 2019;110:400‐408. [DOI] [PubMed] [Google Scholar]
- 11. Postigo AA, Dean DC. ZEB represses transcription through interaction with the corepressor CtBP. Proc Natl Acad Sci U S A. 1999;96(12):6683‐6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Postigo AA, Dean DC. Differential expression and function of members of the zfh-1 family of zinc finger/homeodomain repressors. Proc Natl Acad Sci U S A. 2000;97(12):6391‐6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22(10):2453‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drápela S, Bouchal J, Jolly MK, Culig Z, Souček K. ZEB1: a critical regulator of cell plasticity, DNA damage response, and therapy resistance. Front Mol Biosci. 2020;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowase T, Walsh HE, Darling DS, Shupnik MA. Estrogen enhances gonadotropin-releasing hormone-stimulated transcription of the luteinizing hormone subunit promoters via altered expression of stimulatory and suppressive transcription factors. Endocrinology. 2007;148(12):6083‐6091. [DOI] [PubMed] [Google Scholar]
- 16. Ruf-Zamojski F, Zhang Z, Zamojski M, et al. Single nucleus multi-omics regulatory landscape of the murine pituitary. Nat Commun. 2021;12(1):2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fortin J, Bernard DJ. SMAD3 and EGR1 physically and functionally interact in promoter-specific fashion. Cell Signal. 2010;22(6):936‐943. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Fortin J, Lamba P, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149(11):5577‐5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122(10):3319‐3329. [DOI] [PubMed] [Google Scholar]
- 20. Luo J, Deng Z-L, Luo X, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236‐1247. [DOI] [PubMed] [Google Scholar]
- 21. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen S, Schwarz JR, Niculescu D, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701‐2711. [DOI] [PubMed] [Google Scholar]
- 23. Brabletz S, Losada ML, Schmalhofer O, et al. Generation and characterization of mice for conditional inactivation of Zeb1. Genesis. 2017;55(4):e23024. [DOI] [PubMed] [Google Scholar]
- 24. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107(37):16372‐16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou X, Wang Y, Ongaro L, et al. Normal gonadotropin production and fertility in gonadotrope-specific Bmpr1a knockout mice. J Endocrinol. 2016;229(3):331‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann HM. Determination of reproductive competence by confirming pubertal onset and performing a fertility assay in mice and rats. J Vis Exp. 2018;(140):58352. Doi: 10.3791/58352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix-4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ongaro L, Alonso CAI, Zhou X, et al. Development of a highly sensitive ELISA for measurement of FSH in serum, plasma, and whole blood in mice. Endocrinology. 2021;162(4):bqab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939‐4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243(4):E310‐E318. [DOI] [PubMed] [Google Scholar]
- 31. Porteous R, Haden P, Hackwell ECR, et al. Reformulation of PULSAR for analysis of pulsatile LH secretion and a revised model of estrogen-negative feedback in mice. Endocrinology. 2021;162(11):bqab165. [DOI] [PubMed] [Google Scholar]
- 32. Turgeon M-O, Silander TL, Doycheva D, et al. TRH action is impaired in pituitaries of male IGSF1-deficient mice. Endocrinology. 2017;158(4):815‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schultz H, Zhou X, Alonso CA, et al. Supplementary material for “ZEB1 Inhibits LHβ Subunit Transcription When Overexpressed, But Is Dispensable for LH Synthesis in Mice”. Figshare Digital Repository. 2024. 10.6084/m9.figshare.25833862.v1 [DOI]
- 34. Lin Y-F, Schang G, Buddle ERS, et al. Steroidogenic factor 1 regulates transcription of the inhibin B coreceptor in pituitary gonadotrope cells. Endocrinology. 2022;163(11):bqac131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toufaily C, Schang G, Zhou X, et al. Impaired LH surge amplitude in gonadotrope-specific progesterone receptor knockout mice. J Endocrinol. 2020;244(1):111‐122. [DOI] [PubMed] [Google Scholar]
- 36. Ongaro L, Zhou X, Cui Y, Boehm U, Bernard DJ. Gonadotrope-specific deletion of the BMP type 2 receptor does not affect reproductive physiology in mice†‡. Biol Reprod. 2020;102(3):639‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schang G, Ongaro L, Schultz H, et al. Murine FSH production depends on the activin type II receptors ACVR2A and ACVR2B. Endocrinology. 2020;161(7):bqaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology. 2019;160(6):1480‐1491. [DOI] [PubMed] [Google Scholar]
- 39. Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794‐4802. [DOI] [PubMed] [Google Scholar]
- 40. Lee SL, Sadovsky Y, Swirnoff AH, et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science. 1996;273(5279):1219‐1221. [DOI] [PubMed] [Google Scholar]
- 41. Sánchez-Tilló E, Siles L, de Barrios O, et al. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am J Cancer Res. 2011;1(7):897‐912. [PMC free article] [PubMed] [Google Scholar]
- 42. Almotiri A, Alzahrani H, Menendez-Gonzalez JB, et al. Zeb1 modulates hematopoietic stem cell fates required for suppressing acute myeloid leukemia. J Clin Invest. 2021;131(1):e129115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bruneel K, Verstappe J, Vandamme N, Berx G. Intrinsic balance between ZEB family members is important for melanocyte homeostasis and melanoma progression. Cancers (Basel). 2020;12(8):2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Schultz H, Zhou X, Alonso CA, et al. Supplementary material for “ZEB1 Inhibits LHβ Subunit Transcription When Overexpressed, But Is Dispensable for LH Synthesis in Mice”. Figshare Digital Repository. 2024. 10.6084/m9.figshare.25833862.v1 [DOI]
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the References.