Abstract
Introduction:
The monarchE trial demonstrated improved outcomes with the use of adjuvant abemaciclib in patients with high-risk HR+/HER2− breast cancer defined as those with ≥4 positive lymph nodes (+LNs) or 1–3 +LNs with one or more additional high-risk features. We sought to investigate the proportion of patients with 1–2 positive sentinel lymph nodes (+SLNs) without HRF who had ≥4 +LNs at the time of axillary lymph node dissection (cALND), and therefore qualify for receipt of abemaciclib.
Methods:
From the National Cancer Database (2018–19), we identified females with pN+ non-metastatic HR+ HER2− breast cancer and stratified by number of +SLNs, +LNs and the presence of one or more HRF. We assessed the proportion of patients meeting criteria for abemaciclib both before and after ALND.
Results:
Of the 22,048 patients identified, 1,578 patients underwent upfront surgery, had 1–2 +SLNs without HRF, and went on to cALND. Only 213 (13%) of these patients had ≥4 +LNs: thus cALND performed solely to determine abemaciclib candidacy would have constituted surgical overtreatment in 1,365 (87%) patients. When stratified by number of +SLNs, only 10% of those with 1 +SLN and 24% of those with 2 +SLN had ≥4 +LNs after cALND, meeting criteria for abemaciclib.
Conclusion:
Patients with 1 +SLN without HRF are unlikely to have ≥4 +LNs and should not be subjected to the morbidity of ALND in order to inform candidacy for abemaciclib. Individualized multidisciplinary discussion should be undertaken about the risk:benefit of ALND and abemaciclib for those with 2 +SLN.
Keywords: breast cancer, sentinel lymph nodes, axillary lymph node dissection, abemaciclib
Précis:
Among patients with HR+/HER2− breast cancer with no other high-risk features, a limited sentinel node disease burden is unlikely to predict a large enough overall nodal burden to qualify for adjuvant abemaciclib. A multi-disciplinary discussion weighing the morbidity of axillary dissection and the potential benefit of abemaciclib is critical.
Introduction
Despite many advances in the adjuvant treatment of hormone receptor-positive (HR+)/ human epidermal growth factor receptor 2-negative (HER2−) breast cancer, the risk of distant recurrence remains substantial. A 2017 meta-analysis of 88 studies found that 20-year rates of distant disease after 5 years of adjuvant endocrine therapy ranged from 13% in those with T1N0 cancers to 41% in those with T2 cancer and 4–9 positive lymph nodes (+ LNs).1 Many of these patients recur in the first few years. Therefore, additional strategies are needed to identify and treat those at high risk for relapse.
The monarchE trial was a randomized, phase 3 trial in which patients with high risk HR+/HER2− early breast cancer were randomized to standard adjuvant endocrine therapy (ET) with or without 2 years of abemaciclib, an oral cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitor.2 The mechanism of action of CDK 4/6 inhibitors relies upon the suppression of phosphorylation of the retinoblastoma tumor suppressor protein (a pathway enriched in luminal A cancers), preventing progression from G1 to S phase in the cell cycle, and thus inhibiting cell proliferation.3 The monarchE trial defined high risk patients as those having ≥4 +LNs or those having 1–3 +LNs and at least one of the following high-risk features (HRFs): a tumor that was ≥5 cm, grade 3 or had a Ki-67 ≥20%. The trial accrued 5,637 patients, and at a median follow up of 42 months, the addition of adjuvant abemaciclib was associated with improved invasive disease-free survival (IDFS; 85.8% vs. 79.4%; hazard ratio [HR] 0.664, 95% confidence interval [CI] 0.578–0.762, p<0.001).4 Overall survival (OS) are still immature, but there was no apparent difference at the most recent analysis with 94.4% of those treated with abemaciclib + ET still alive compared with 93.9% of those treated with ET alone (HR 0.929, 95% CI 0.748–01.153, p=0.50). The monarchE trial led to Food and Drug Administration (FDA) approval for adjuvant abemaciclib in 2021 for patients with high-risk disease.
In clinical practice, the study criteria leave a gray zone; specifically, patients with 1–3 positive sentinel nodes (+SLNs) without HRFs in whom candidacy for abemaciclib relies upon meeting the threshold of 4 +LNs.5 When considering the guidelines for the surgical management of the axilla, patients with 1–2 +SLNs pose an even larger dilemma. In clinically node-negative patients undergoing upfront surgery, axillary staging is performed with sentinel lymphadenectomy (SLNB), and a completion axillary lymph node dissection (cALND) is only indicated in those with ≥3 +SLNs regardless of surgical approach, or any number of +LNs among patients undergoing mastectomy and do not receive adjuvant nodal radiotherapy (RNI).6–8 Thus, patients with 3 +SLNs are recommended to undergo cALND regardless of whether this information is needed to inform adjuvant treatment decisions. However, in patients with 1–2 +SLNs, in whom cALND is not required, the proportion of patients who have no HRFs and have additional axillary disease such that they would meet criteria for adjuvant abemaciclib is unknown. Additionally, reports of the monarchE data do not indicate what axillary surgical approach was used in the patients enrolled in the trial, leaving surgeons and medical oncologists without clear guidance about how to approach patients who may meet criteria for abemaciclib. This study has consequently been designed to determine the proportion of patients with 1–2 +SLNs, no HRFs and ≥4 +LNs at the time of cALND, meeting criteria for adjuvant abemaciclib, and, conversely, the extent to which this constitutes surgical overtreatment.
Methods
After Institutional Review Board (IRB) approval, we performed a retrospective analysis of the National Cancer Database (NCDB). The NCDB is a joint collaboration between the American College of Surgeons and the American Cancer Society in which patient-level data are collected from all cancer patients seen at Commission on Cancer accredited programs,9 representing approximately 70% of United States cancer cases.10
From the NCDB breast cancer participant user file (PUF), we identified female patients with non-metastatic, HR+/HER2− breast cancer that was pathologically node positive (pN+). We defined HR+ as positive for the estrogen receptor (ER), progesterone receptor (PR) or both, with positivity as defined by the College of American Pathologists in the year of diagnosis (e.g. currently ≥1%). Complete exclusion criteria are outlined in Figure 1. We included patients diagnosed in 2018–2019, as the Ki-67 variable used to identify high risk patients was added to the database in 2018. Pertinent to determining candidacy for abemaciclib, we excluded patients with an unknown number of positive SLNs or total lymph nodes, and among those with 1–3 +LNs, any patients with unknown values for all three HRFs (tumor size, grade and Ki-67).
FIGURE 1.
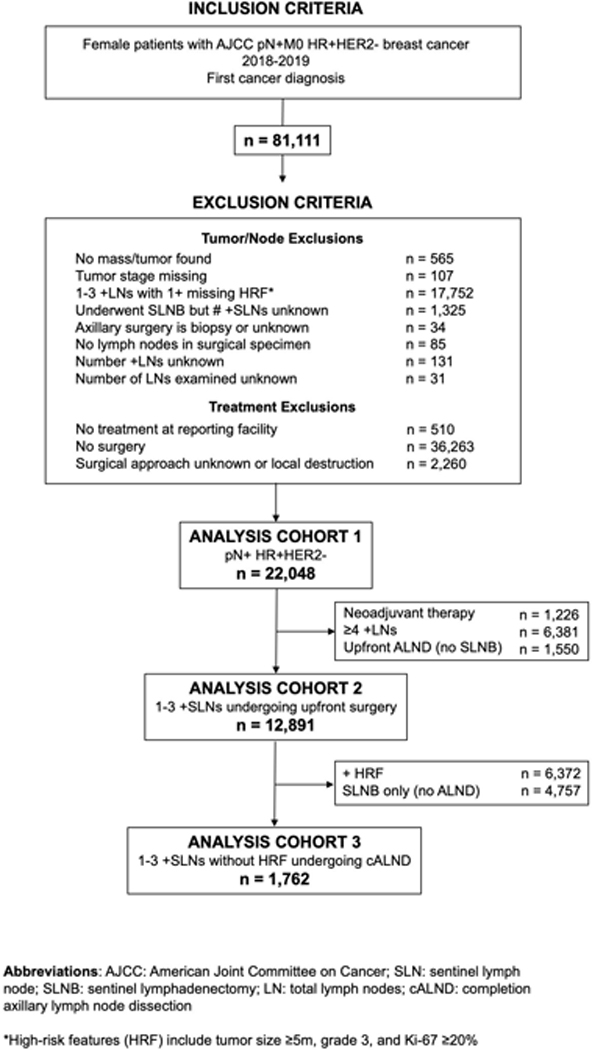
Analysis cohort inclusion and exclusion criteria. AJCC indicates American Joint Committee on Cancer; cALND, completion axillary lymph node dissection; HR, hormone receptor; HRF, high-risk feature; LN, lymph node; pN+, pathologically node-positive; SLN, sentinel lymph node; SLNB, sentinel lymphadenectomy. *HRFs include tumor size ≥5 cm, grade 3, and Ki-67 ≥20%.
In order to assess the proportion of patients who meet criteria for abemaciclib based on current surgical practice, the overall cohort was stratified into three groups: 1) ≥4 +LNs, 2) 1–3 +LNs with at least one high risk feature, and 3) 1–3 +LNs and no high-risk features. These groups were analyzed regardless of the type of axillary surgery that was performed. For this first analysis we did not exclude patients who had received neoadjuvant therapy as this was not an exclusion criterion in the monarchE trial, and these patients were found to derive a similar benefit to patients treated with adjuvant chemotherapy.11
We then constructed two analysis cohorts for our primary objective. First, we excluded patients who underwent neoadjuvant therapy (because cALND is standard for any patients with residual nodal disease), those with ≥4 +LNs and those who underwent upfront ALND without SLNB, which yielded a cohort of patients with 1–3 +SLNs undergoing upfront surgery. We stratified this cohort by presence of HRFs to determine what proportion of these patients would meet criteria for abemaciclib. Finally, after excluindg patient with HRF and those who had SLNB alone, yielding a cohort of patients with 1–3 +SLNs without HRF who underwent SLNB and cALND to assess the number of +LNs, and ultimate abemaciclib candidacy. Since cALND is also standard among patients with 3 +SLNs, we performed analyses on the overall group (1–3 +SLNs) and then subset analyses focused on only patients with 1–2 +SLNs. After compared demographic and clinicopathologic features by number of positive SLNs, we calculated the number needed to treat (NNT) for each SLN group which we defined as the number of patients who require cALND to identify one patient who meets criteria for abemaciclib.
Statistical analyses
We used descriptive statistics (frequencies, percents, means, standard deviations) to summarize demographic, tumor and treatment characteristics, and compared categorical variables across groups using Chi-square tests or Fisher’s exact tests, and continuous variables using Wilcoxon rank sum tests (2 groups) or Kruskal-Wallis tests (>2 groups). Our comparison groups were eligibility categories for abemaciclib, based on the number of positive LNs and number of HRFs. Similarly, within the primary analytic cohort, we compared characteristics by number of positive sentinel lymph nodes (1,2 or 3). Within each SLN strata, NNT was calculated as the reciprocal of the proportion of patients who met the criterion of having ≥4 +LNs after cALND. Confidence intervals for NNT were calculated using the Wilson score method.12 In supplementary analyses, we compared characteristics by HRF status (any vs none) in those with 1–3 + SLNs. All p-values reported were from two sided tests. Analyses were performed using SAS/STAT software, version 9.4 (Cary, NC).
Results
In total, 22,048 female patients with non-metastatic pN+ HR+/HER2− breast cancer were identified from the NCDB (Supplementary Table 1). Within this cohort, 6% of patients had undergone neoadjuvant therapy, 54% underwent mastectomy and 45% had SLNB alone and a mean of 9.4 total LNs were removed. We assessed the proportion of patients that met criteria for abemaciclib based on complete pathologic data (including ALND if one was performed). Of these patients, 6,948 (32%) had ≥4 +LNs and 7,678 (35%) had 1–3 +LNs with at least one high risk feature, and thus qualified for abemaciclib. The remaining 7,422 (34%) had 1–3 +LNs without any HRFs and did not qualify for abemaciclib.
We then focused on the 12,891 patients with 1–3 +SLNs undergoing upfront surgery, stratified by whether HRFs were present (Table 1). Patients with ≥1 HRF constituted 49% of this group with 3,934 (31)% having only 1 HRF [581 (5%) had a tumor >5cm, 529 (4%) had a grade 3 tumor, and 2,824 (22%) had a Ki-67 ≥20%]. When compared to those without any HRFs, patients with ≥1 HRF were younger, more likely to be non-White, and to have private insurance (all p<0.001). Aside from the pre-defined HRFs, other tumor characteristics also differed between the groups: having ≥1 high risk feature was associated with the presence of lymphovascular invasion, ER or PR negative subtype, and a higher number of lymph nodes removed and lymph nodes positive (all p<0.001). Patients with HRFs were also more likely to have undergone mastectomy (50% vs. 38%, p<0.001) and cALND (34% vs. 27%, p<0.001).
Table 1.
Clinicopathologic features of females with HR+HER2- non-metastatic invasive breast cancer and 1–3 positive sentinel lymph nodes undergoing upfront surgery.
| Overall | No high-risk features | ≥1 high-risk features | p | ||
|---|---|---|---|---|---|
| n | 12,891 | 6,519 | 6,372 | ||
| Age (years) | 59.3 ± 12.6 | 60.3 ± 11.8 | 58.4 ± 13.2 | <0.001 | |
| Race/Ethnicity | |||||
| White | 10709 (83.1) | 5543 (85.0) | 5166 (81.1) | ||
| Black | 1266 (9.8) | 524 (8.0) | 742 (11.6) | <0.001 | |
| Asian | 545 (4.2) | 257 (3.9) | 288 (4.5) | ||
| Other/Unknown | 371 (2.9) | 195 (3.0) | 176 (2.8) | ||
| Charlson/Deyo Score | |||||
| 0 | 10699 (83.0) | 5414 (83.0) | 5285 (82.9) | ||
| 1 | 1557 (12.1) | 782 (12.0) | 775 (12.2) | 0.98 | |
| 2 | 377 (2.9) | 190 (2.9) | 187 (2.9) | ||
| ≥3 | 258 (2.0) | 133 (2.0) | 125 (2.0) | ||
| Insurance Status | |||||
| Not Insured | 225 (1.7) | 126 (1.9) | 99 (1.6) | ||
| Private Insurance | 6931 (53.8) | 3399 (52.1) | 3532 (55.4) | ||
| Medicaid | 915 (7.1) | 441 (6.8) | 474 (7.4) | <0.001 | |
| Medicare | 4549 (35.3) | 2424 (37.2) | 2125 (33.3) | ||
| Other Government | 176 (1.4) | 79 (1.2) | 97 (1.5) | ||
| Unknown | 95 (0.7) | 50 (0.8) | 45 (0.7) | ||
| Institution Type | |||||
| Community Cancer Center | 951 (7.4) | 512 (7.9) | 439 (6.9) | ||
| Comprehensive Community Cancer Program | 5340 (41.4) | 2711 (41.6) | 2629 (41.3) | ||
| Academic/Research Program | 3288 (25.5) | 1716 (26.3) | 1572 (24.7) | <0.001 | |
| Integrated Network Cancer Program | 2594 (20.1) | 1342 (20.6) | 1252 (19.6) | ||
| Unknown | 718 | 238 | 480 | ||
| Pathologic Tumor Size (mm) | 25.6 ± 25.3 | 19.8 ± 9.5 | 31.5 ± 33.7 | <0.001 | |
| Pathologic Tumor Stage | |||||
| T1 | 6274 (48.6) | 3887 (59.6) | 2387 (37.4) | <0.001 | |
| T2 | 5577 (43.3) | 2589 (39.7) | 2988 (46.9) | ||
| T3 | 954 (7.4) | 16 (0.2) | 938 (14.7) | ||
| T4 | 86 (0.7) | 27 (0.4) | 59 (0.9) | ||
| Tumor Grade | |||||
| 1 | 2414 (18.7) | 1945 (29.8) | 469 (7.4) | <0.001 | |
| 2 | 7748 (60.1) | 4574 (70.2) | 3174 (49.8) | ||
| 3 | 2729 (21.2) | 0 (0.0) | 2729 (42.8) | ||
| Ki-67 | |||||
| <20% | 7685 (59.6) | 6519 (100.0) | 1166 (18.3) | <0.001 | |
| ≥20% | 5206 (40.4) | 0 (0.0) | 5206 (81.7) | ||
| Lymphovascular Invasion | |||||
| Absent | 6908 (53.6) | 3988 (61.2) | 2920 (45.8) | <0.001 | |
| Present | 4676 (36.3) | 1843 (28.3) | 2833 (44.5) | ||
| Unknown | 1307 (10.1) | 688 (10.6) | 619 (9.7) | ||
| Estrogen Receptor | |||||
| Negative | * | * | * | <0.001 | |
| Positive | 12834 (99.6) | 6514 (99.9) | 6320 (99.2) | ||
| Unknown | * | * | * | ||
| Progesterone Receptor | |||||
| Positive | 11816 (91.7) | 6121 (93.9) | 5695 (89.4) | <0.001 | |
| Negative/Unknown | 1075 (8.3) | 398 (6.1) | 677 (10.6) | ||
| Breast Surgery | |||||
| Partial Mastectomy | 7256 (56.3) | 4046 (62.1) | 3210 (50.4) | <0.001 | |
| Mastectomy | 5635 (43.7) | 2473 (37.9) | 3162 (49.6) | ||
| Axillary surgery | |||||
| SLNB alone | 8963 (69.5) | 4757 (73.0) | 4206 (66.0) | <0.001 | |
| SNLB then ALND | 3928 (30.5) | 1762 (27.0) | 2166 (34.0) | ||
| Number of SLN removed | 3.2 ± 2.7 | 3.0 ± 2.6 | 3.3 ± 2.8 | <0.001 | |
| Number of positive SLN | 1.3 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.6 | <0.001 | |
| Number of total LN removed | 6.1 ± 6.4 | 5.6 ± 6.1 | 6.6 ± 6.7 | <0.001 | |
| Number of total positive LN | 1.8 ± 2.2 | 1.6 ± 1.7 | 2.0 ± 2.5 | <0.001 | |
Abbreviations: SLNB: sentinel lymphadenectomy; ALND: axillary lymph node dissection; SLN: sentinel lymph nodes; LN: total lymph nodes
Results suppressed: NCDB does not permit aggregate results for cell sizes < 10
Note: Table cells show frequency (column percent) or mean ± standard deviation.
Next, we created an analysis cohort of 1,762 patients undergoing upfront surgery who had 1–3 +SLNs, underwent cALND and had no HRF identified on final pathology (Table 2). The majority of patients (66%) had only 1 +SLN. When comparing the clinicopathologic features of the groups after stratifying by number of +SLNs, the groups were very similar. Most patients in this cohort (65%) had undergone mastectomy. As expected, larger tumor size, higher tumor stage, the presence of lymphovascular invasion, a higher number of SLNs removed, total LNs removed and +LNs were all associated with more positive SLNs, (all p<0.05). We then categorized patients by whether they had ≥4 +LNs after ALND and found that 1,456 (83%) had <4 +LNs and 306 (17%) had ≥4 +LNs and met criteria for abemaciclib (Table 3).
Table 2.
Clinicopathologic features of females with HR+HER2- non-metastatic invasive breast cancer and 1–3 positive sentinel lymph nodes without high-risk features who underwent upfront surgery and completion axillary lymph node dissection.
| Overall | Number of positive sentinel lymph nodes | p | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| n | 1,762 | 1,159 | 419 | 184 | ||
| Pathologic Tumor Size (mm) | 22.0 ± 10.2 | 21.2 ± 10.0 | 23.2 ± 10.2 | 24.3 ± 11.0 | <0.001 | |
| Pathologic Tumor Stage | ||||||
| T1 | 857 (48.6) | 603 (52.0) | 180 (43.0) | 74 (40.2) | 0.001 | |
| T2 | 883 (50.1) | 544 (46.9) | 235 (56.1) | 104 (56.5) | ||
| T3/4 | 22 (1.2) | * | * | * | ||
| Tumor Grade | ||||||
| 1 | 430 (24.4) | 293 (25.3) | 94 (22.4) | 43 (23.4) | 0.48 | |
| 2 | 1332 (75.6) | 866 (74.7) | 325 (77.6) | 141 (76.6) | ||
| Lymphovascular Invasion | ||||||
| Absent | 983 (55.8) | 680 (58.7) | 218 (52.0) | 85 (46.2) | <0.001 | |
| Present | 591 (33.5) | 354 (30.5) | 153 (36.5) | 84 (45.7) | ||
| Unknown | 188 (10.7) | 125 (10.8) | 48 (11.5) | 15 (8.2) | ||
| Estrogen Receptor | ||||||
| Positive | 1,760 (99.9) | * | * | * | 0.06 | |
| Negative/Unknown | 1 (0.1) | * | * | * | ||
| Progesterone Receptor | ||||||
| Positive | 1,647 (93.5) | 1075 (92.8) | 399 (95.2) | 173 (94.0) | 0.20 | |
| Negative/Unknown | 115 (6.5) | 84 (7.2) | 20 (4.8) | 11 (6.0) | ||
| Breast Surgery | ||||||
| Partial Mastectomy | 623 (35.4) | 407 (35.1) | 146 (34.8) | 70 (38.0) | 0.72 | |
| Mastectomy | 1,139 (64.6) | 752 (64.9) | 273 (65.2) | 114 (62.0) | ||
| Number of SLN removed | 3.3 ± 3.4 | 2.8 ± 3.0 | 4.0 ± 3.9 | 4.9 ± 3.2 | <0.001 | |
| Number of total LN removed | 12.3 ± 7.6 | 11.3 ± 7.3 | 13.6 ± 7.8 | 15.8 ± 10.0 | <0.001 | |
| Number of total positive LN | 2.6 ± 3.0 | 1.9 ± 2.4 | 3.4 ± 3.1 | 5.3 ± 3.8 | <0.001 | |
Abbreviations: SLN: sentinel lymph nodes; LN: total lymph nodes
Results suppressed: NCDB does not permit aggregate results for cell sizes < 10
Note: Table cells show frequency (column percent) or mean ± standard deviation. No differences were seen when comparing demographic information between the cohorts, and are therefore not shown.
Table 3.
Number needed to treat with cALND to determine abemaciclib candidacy among females with HR+HER2- non-metastatic invasive breast cancer and 1–3 positive sentinel lymph nodes without high-risk features who underwent upfront surgery and completion axillary lymph node dissection.
| Total number of positive LNs after cALND [n (row %)] | Number Needed to Treat [n (95% CI)] | ||||
|---|---|---|---|---|---|
| Overall | 1–3 | ≥4 | |||
| Number of positive SLNs | Overall | 1,762 | 1,456 (82.6) | 306 (17.4) | |
| 1 | 1,159 | 1,048 (90.4) | 111 (9.6) | 11 (9, 13) | |
| 2 | 419 | 317 (75.7) | 102 (24.3) | 5 (4, 5) | |
| 3 | 184 | 91 (49.5) | 93 (50.5) | 2 (2, 3) | |
Abbreviations: cALND: completion axillary lymph node dissection; CI: confidence interval; SLN: sentinel lymph node; LN: total lymph nodes
Since current guidelines recommend that patients with 3 +SLNs undergo cALND, we then focused on the group of 1,578 patients having 1–2 +SLNs for whom cALND is not typically required. Within this group, only 213 (13%) had ≥4 +LNs after cALND and met criteria for abemaciclib while the remaining 1,365 patients (87%) had only1–3 +LNs. When stratified by number of +SLNs, only 10% of those with 1 +SLN had ≥4 +LNs at the time of cALND compared with 24% of those with 2 +SLNs (Table 3).
Finally, we calculated the NNT for each SLN group (Table 3). For patients with 1 +SLN, the NNT was 11 (95% CI 9 – 13), 5 (95% CI 4 – 5) for patients with 2 +SLN, and 2 (95% CI 2 – 3) for those with 3 +SLNs.
Discussion
In this national population-based sample, we found that for patients having 1–2 +SLNs without HRFs, the likelihood of finding ≥4+LNs at the time of cALND such that they would meet criteria for adjuvant abemaciclib is only 13%. For the remaining 87% of patients, cALND to determine candidacy for abemaciclib results in surgical overtreatment, needlessly exposing these patients to the morbidities conferred by ALND for no benefit.
The major risk of ALND is the development of lymphedema. Rates of lymphedema following axillary surgery vary from 4–8% when SLNB is performed alone (and ~10% if RNI is performed) to 20–25% for ALND and ~30% for ALND +RNI. 13–15 Additional morbidity, such as pain, infection, numbness and weakness are also experienced by up to two-third of patients undergoing ALND, further exacerbating the risk of surgery.13 In our study, we found that for patients having 1+ SLN, 11 patients would require cALND to determine abemaciclib candidacy for one patient. Similarly, for patients having 2+ SLN, 5 patients would require cALND to determine candidacy for one patient. Using these data, and assuming a rate of lymphedema around 20% after cALND, 2 patients (≈20% of 11) would develop lymphedema for every patient meeting criteria for adjuvant abemaciclib in those with 1 +SLN, demonstrating that the surgical risk may outweigh the medical benefit in this group. For those with 2 +SLN, 1 patient (20% of 5) would develop lymphedema for every patient meeting criteria for adjuvant abemaciclib, equalizing the perceived risk:benefit ratio for this group.
Simultaneously, the advantages of adjuvant abemaciclib must also be included in the risk:benefit analysis for these patients. The monarchE trial demonstrated a 6.4% increase in IDFS at 42 months when abemaciclib is added to standard adjuvant ET.4 We found in our analysis that patients with 1 +SLN have only a 10% likelihood of having ≥4 +LNs at the time of cALND, and the expected IDFS gain is therefore only 0.6% for these patients. Similarly, since patients with 2 +SLNs have only a 24% likelihood of having ≥4 +LNs at the time of cALND, the expected IDFS gain becomes only 1.5%. It is worth noting that there was no difference in OS between the study arms, although OS data for the trial are still immature. Taken together, patients with 1–2 +SLNs have very little expected benefit when contemplating cALND to inform candidacy for adjuvant abemaciclib and should be appropriately counseled prior to taking on its morbidity.
The 2022 update of the monarchE trial demonstrated that Ki-67 was not a predictive biomarker for abemaciclib as both high (≥20%) and low (20%) Ki-67 groups derived similar benefit from the addition of abemaciclib, and overall patients with high Ki-67 expression had worse prognosis.4 The initial approval of abemaciclib required a high Ki-67 for eligibility, but as a result of these updated data, the FDA issued an expanded indication for abemaciclib by removing the Ki-67 requirement.14 We modeled our analysis on the original inclusion and exclusion criteria of the monarchE trial and included Ki-67 ≥20% as a HRF. In practice, the expansion of criteria to include patients who have low Ki-67 will only serve to increase the number of patients for whom discussion about risk:benefit of cALND and adjuvant abemaciclib becomes relevant. Additionally, abemaciclib is currently the only FDA approved adjuvant CDK4/6 inhibitor, however, there is encouraging early data from the NATALEE study, which is evaluating adjuvant ribociclib in addition to ET in a broader population which even includes select patients with node negative disease.15 Thus far, only 20% of the study population have completed the planned 3-year treatment course with ribociclib. If we continue to see a benefit over time, there may be even less benefit to performing cALND as patients may at minimum have access to an alternate CDK4/6 inhibitor in addition to ET.
Our study has several strengths and limitations. The primary strength is our use of the NCDB which is a large, prospectively-collected population sample that is diverse and representative of current surgical practice at Commission on Cancer-accredited facilities. This dataset includes granular details including tumor characteristics and the numbers of lymph nodes (sentinel and total) removed and positive, permitting this analysis. Another is that our study period was prior to the 2021 approval of adjuvant abemaciclib. This means that the surgical decision-making represented by the dataset was performed without without bias related to the findings and practice implications of the trial. One limitation is that there may be some unquantifiable selection bias related to the patients with 1–2 +SLNs who underwent completion ALND, since ALND is not standard practice in patients with limited nodal disease after the publication of ACOSOG Z0011 and AMAROS. This can be appreciated when noting that an outsized proportion of the primary analysis cohort underwent mastectomy (65%) likely since some of these patients may have been treated with cALND rather than RNI. If clinicians were recommending cALND for patients with more unfavorable tumor characteristics and who were therefore more likely to have additional +LNs, our results may actually overestimate the utility of cALND in finding patients who meet criteria for abemaciclib.
Conclusions
Patients with 1 +SLN without HRFs are unlikely to have ≥4 +LNs and should not be subjected to the morbidity of ALND in order to inform candidacy for abemaciclib, due to its limited benefit. Individualized multidisciplinary discussion should be undertaken about the risk:benefit of ALND and abemaciclib for those with 2 +SLN.
Supplementary Material
Acknowledgements
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identifed NCDB file. The American College of Surgeons and the Commission on Cancer have not verifed and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigators.
This work was supported by United States Public Health Services grant P30CA006927 for analysis of the data via support of our biostatistics facility and by generous private donor support from the Marlyn Fein Chapter of the Fox Chase Cancer Center Board of Associates for analysis and interpretation of the data.
This work was presented as a Parallel Session oral presentation at the 2023 Society of Surgical Oncology International Conference on Surgical Cancer Care.
Footnotes
Conflict of Interest:
The authors report no relevant disclosures.
References
- 1.Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. New England Journal of Medicine. 2017;377(19):1836–1846. doi: 10.1056/NEJMOA1701830/SUPPL_FILE/NEJMOA1701830_DISCLOSURES.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). Journal of Clinical Oncology. 2020;38(34):3987. doi: 10.1200/JCO.20.02514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Research 2022 24:1. 2022;24(1):1–11. doi: 10.1186/S13058-022-01510-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SRD, Toi M, O’Shaughnessy J, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24(1):77–90. doi: 10.1016/S1470-2045(22)00694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittendorf EA, King TA, Tolaney SM. Impact of RxPONDER and monarchE on the Surgical Management of the Axilla in Patients with Breast Cancer. Journal of Clinical Oncology. 2022;40(29):3361–3364. doi: 10.1200/JCO.22.00173 [DOI] [PubMed] [Google Scholar]
- 6.NCCN Guidelines Version 4.2023 Breast Cancer. Published April 2023. Accessed June 4, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 7.Giuliano AE, Ballman KV., McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918–926. doi: 10.1001/JAMA.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981–22023 AMAROS Trial. Journal of Clinical Oncology. Published online April 20, 2022. doi: 10.1200/jco.22.01565 [DOI] [PubMed] [Google Scholar]
- 9.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3(12):1722–1728. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 10.Mallin K, Browner A, Palis B, et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol. 2019;26(6):1604–1612. doi: 10.1245/s10434-019-07213-1 [DOI] [PubMed] [Google Scholar]
- 11.Martin M, Hegg R, Kim SB, et al. Treatment With Adjuvant Abemaciclib Plus Endocrine Therapy in Patients With High-risk Early Breast Cancer Who Received Neoadjuvant Chemotherapy: A Prespecified Analysis of the monarchE Randomized Clinical Trial. JAMA Oncol. 2022;8(8):1190–1194. doi: 10.1001/JAMAONCOL.2022.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender R Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22(2):102–110. doi: 10.1016/S0197-2456(00)00134-3 [DOI] [PubMed] [Google Scholar]
- 13.Peintinger F, Reitsamer R, Stranzl H, Ralph G. Comparison of quality of life and arm complaints after axillary lymph node dissection vs sentinel lymph node biopsy in breast cancer patients. Br J Cancer. 2003;89(4):648. doi: 10.1038/SJ.BJC.6601150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA expands early breast cancer indication for abemaciclib with endocrine therapy | FDA. Accessed June 4, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-early-breast-cancer-indication-abemaciclib-endocrine-therapy
- 15.Slamon DJ, Stroyakovskiy D, Yardley DA, et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: Primary results from the phase III NATALEE trial. Accessed June 5, 2023. https://meetings.asco.org/abstracts-presentations/218407
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


