Abstract
We recently found a novel cell-cell adhesion system at cadherin-based adherens junctions (AJs), consisting at least of nectin, a Ca2+-independent homophilic immunoglobulin-like adhesion molecule, and afadin, an actin filament-binding protein that connects nectin to the actin cytoskeleton. Nectin is associated with cadherin through afadin and α-catenin. The cadherin-catenin system increases the concentration of nectin at AJs in an afadin-dependent manner. Nectin constitutes a family consisting of three members: nectin-1, -2, and -3. Nectin-1 serves as an entry and cell-cell spread mediator of herpes simplex virus type 1 (HSV-1). We studied here a role of the interaction of nectin-1α with afadin in entry and/or cell-cell spread of HSV-1. By the use of cadherin-deficient L cells overexpressing the full length of nectin-1α capable of interacting with afadin and L cells overexpressing a truncated form of nectin-1α incapable of interacting with afadin, we found that the interaction of nectin-1α with afadin increased the efficiency of cell-cell spread, but not entry, of HSV-1. This interaction did not affect the binding to nectin-1α of glycoprotein D, a viral component mediating entry of HSV-1 into host cells. Furthermore, the cadherin-catenin system increased the efficiency of cell-cell spread of HSV-1, although it also increased the efficiency of entry of HSV-1. It is likely that efficient cell-cell spread of HSV-1 is caused by afadin-dependent concentrated localization of nectin-1α at cadherin-based AJs.
Herpes simplex viruses (HSVs) are members of the neurotropic subfamily (alphaherpesviruses) of the herpesvirus family. Infection with HSV type 1 (HSV-1) is prevalent. HSV-1 infects cells through initial attachment to the plasma membrane and subsequent fusion of the viral envelope with the plasma membrane or through contiguous cell-cell spread. The entry pathway of HSV-1 is divided into three major processes: binding, fusion, and capsid penetration. These processes require several viral envelope glycoproteins (49–51). The initial attachment is mediated through glycoprotein C (gC) and/or gB to cell surface heparan sulfate proteoglycans (17, 18, 45), but this attachment is not sufficient for virus penetration (3, 11, 22, 27). The fusion of the viral envelope with the plasma membrane requires gD, gB, gH, and gL (49–51). These four viral glycoproteins also participate in cell-cell spread (3, 11, 20, 28, 36, 40). Cell-cell spread furthermore requires gE and gI (1, 8–10), but these glycoproteins are not required for entry (8). Thus, entry and cell-cell spread of HSV-1 share similar processes, but these pathways also differ in some significant aspects.
Recently, expression cloning has led to the identification and isolation of HSV entry mediators (5, 12, 33, 46, 51). The human receptors identified include a lymphotoxin receptor (31), designated HVEM (33, 65) or HSV entry mediator A (HveA), which belongs to the tumor necrosis factor receptor family; two members of the immunoglobulin (Ig) superfamily (5, 12, 48, 62); and 3-O-sulfated heparan sulfate (46). The two members of the Ig superfamily are closely related to the poliovirus receptor and named poliovirus receptor-related protein 1 (PRR1) and PRR2 (12, 62). Based on their ability to promote entry into cells, PRR1 and PRR2 are also called HveC and HveB, respectively (12, 62). PRR1/HveC is active as an entry mediator for all alphaherpesviruses tested so far (HSV-1, HSV-2, pseudorabies virus, and bovine herpesvirus 1) (12). PRR2/HveB enhances entry of a restricted number of mutant strains of HSV-1 (those carrying mutations in gD, such as rid1, rid2, and ANG), some HSV-2 strains, and pseudorabies virus (62).
We recently found that PRR1/HveC and PRR2/HveB are components of a novel cell-cell adhesion system at cadherin-based adherens junctions (AJs) (53). We therefore renamed PRR1/HveC and PRR2/HveB nectin-1 and -2, respectively. Nectin-1 consists of two splicing variants, named nectin-1α and -1β/HIgR (5, 53), and nectin-2 also consists of two splicing variants, named nectin-2α and -2δ (53). Thus, nectin constitutes a family. We furthermore isolated a third member, named nectin-3, consisting of three splicing variants, nectin-3α, -3β, and -3γ (44). Nectin-3 may also serve as a receptor for some virus(es), but it has not been identified. Each member of the nectin family consists of three extracellular Ig-like domains, one transmembrane segment, and one cytoplasmic region. Nectin-1α, -2α, -2δ, and -3α have been shown to be Ca2+-independent homophilic cell-cell adhesion molecules at AJs that are directly associated with afadin, an actin filament (F-actin)-binding protein that connects nectins to the actin cytoskeleton (29, 30, 32, 44, 53). These members form cis homodimers where the monomers are aligned in a parallel orientation. They show cell-cell adhesion activities through trans homointeractions where the monomers interact with each other from opposing cell surfaces in an antiparallel orientation. Nectin-3α furthermore shows trans heterointeraction with nectin-1α or -2α, whereas nectin-1α does not show trans heterointeraction with nectin-2α (44).
AJs constitute a junctional complex with tight junctions and desmosomes in polarized epithelial cells. Cadherin is a key cell-cell adhesion molecule at cell-cell AJs (14, 55, 56). The cytoplasmic region of cadherin is associated with the actin cytoskeleton through three F-actin-binding proteins: α-catenin, α-actinin, and vinculin (23, 39, 64). α-Catenin indirectly interacts with cadherin through β-catenin (35, 38), whereas α-actinin and vinculin indirectly interact with cadherin through α-catenin (23, 63, 64). The cadherin-catenin system plays essential roles in the formation and maintenance of cell-cell AJs (14, 55, 56) and is also required for the organization of tight junctions (15, 16, 63). Our recent studies of the role of afadin using afadin−/− mice and embryoid bodies have shown that afadin plays a key role in the proper organization of AJs and tight junctions (21). We have furthermore shown that nectin and cadherin interact through afadin and α-catenin and that the nectin-afadin and cadherin-catenin systems cooperatively organize AJs (52). The interaction of nectin with afadin is necessary for their clustering at cell-cell contact sites (32). Thus, evidence is accumulating that the nectin-afadin and cadherin-catenin systems interact and cooperatively play key roles at cell-cell AJs.
Evidence is accumulating that nectin-1 mediates entry of HSV-1 by interaction with gD (4, 24, 25, 41). gD contains a binding domain specific for the first Ig-like domain, called the V domain, of nectin-1 (4, 25). The interaction of nectin-1 with gD has been proposed to activate the membrane-fusing activity of gB or gH-gL that, in addition to gD, are known to be required for this fusion (51). However, the V domain is sufficient to mediate entry of HSV-1, and the second and third Ig-like domains, called the C2 domains, increase the efficiency of HSV-1 entry (4). The cytoplasmic region of nectin-1 is not required for entry of HSV-1 (4, 5), suggesting that the interaction of nectin-1α with afadin does not affect entry. Recently, nectin-1 and -2 have been shown to mediate cell-cell spread of HSV-1 (6). However, it remains to be determined whether the interaction of nectin-1α with afadin is involved in cell-cell spread of HSV-1. Little is known, either, about the role of the cadherin-catenin system in entry and cell-cell spread of HSV-1.
In this paper, we examined the role of afadin in entry and/or cell-cell spread of HSV-1 and found that the interaction of nectin-1α with afadin is required for efficient cell-cell spread, but not entry, of this virus. We have also found that the cadherin-catenin system is involved in both entry and cell-cell spread of HSV-1.
MATERIALS AND METHODS
Viruses and cells.
A strain of HSV-1, HSV-1(KOS)tk12 (also designated KOS) (62), which expresses β-galactosidase from a lacZ cassette in its genome, was kindly supplied by P. G. Spear (Northwestern University, Chicago, Ill.), and the titer was determined with Vero cells. Where indicated, another strain of HSV-1, Seibert (37), was used. Various L-cell lines were cloned by introduction of the following cDNAs: EL cells, the full length of E-cadherin (34); nectin-1α-L cells, the full length of human nectin-1α (amino acids [aa] 1 to 518); and nectin-1α-ΔC-L cells, the COOH-terminal 4-aa-deleted form of human nectin-1α (aa 1 to 514). L and EL cells were kindly supplied by S. Tsukita and A. Nagafuchi (Kyoto University, Kyoto, Japan). Nectin-1α-L and nectin-1α-ΔC-L cells were prepared as previously described (32, 52, 53). Briefly, L cells were transfected with the pPGKIH construct described below with Lipofectamine reagent (GIBCO BRL). The cells were then cultured for 1 day, replated, and selected by culturing in the presence of 500 μg of hygromycin (GIBCO BRL)/ml. These cells were cultured in Dulbecco's modified Eagle's medium (DME) supplemented with 10% fetal calf serum (FCS). L cells were similarly transfected with the empty pPGKIH vector and used as a control.
Constructs, expression, and purification.
The cDNAs of human nectin-1α, human IgG Fc, and HSV-1 gD were obtained by PCR using a human brain cDNA, a human spleen cDNA, and the HSV-1 genome as templates, respectively. The sequence of gD from the HSV-1 Seibert strain was identical to that from the HSV-1 KOS strain. The sequences of gE and gI from the Seibert strain were not determined, but the sequences in the Seibert and KOS strains were assumed to have no important differences. Expression vectors for human nectin-1α were constructed with pPGKIH (32) and pGEX-KG (13) by standard molecular biology methods (43). Constructs of human nectin-1α contained the following amino acids: pPGKIH-nectin-1α, aa 1 to 518 (full length); pPGKIH-nectin-1α-ΔC, aa 1 to 514 (deletion of the COOH-terminal 4 aa residues); and GST-nectin-1α-CPN, aa 379 to 438. The glutathione S-transferase (GST) fusion protein was purified by use of glutathione-Sepharose beads (Amersham-Pharmacia Biotech, Ltd.). A baculovirus transfer vector for the chimeric protein of gD(306t) (1 to 306 aa) or gD(285t) (1 to 285 aa) (41) fused with human IgG Fc was constructed as follows: pFastBac1-Msp-Fc was first constructed by subcloning the inserts encoding the honeybee melittin signal peptide (ATGAAATTCT TAGTCAACGTTGCCCTTGTTTTTATGGTCGTGTACATTTCTTACATC TATGCG) (59) and human IgG Fc into pFastBac1 (GIBCO BRL). A fragment of gD(306t) or gD(285t) was then inserted into pFastBac1-Msp-Fc to express the chimeric protein fused with the NH2-terminal signal peptide and the COOH-terminal IgG Fc [gD(306t)-Fc or gD(285t)-Fc]. A baculovirus bearing the cDNA of gD(306t)-Fc or gD(285t)-Fc was prepared according to the manufacturer's protocol. High Five insect cells (Invitrogen) were grown in serum-free EX-CELL 400 medium (JRH Biosciences), infected with the baculovirus, and cultured at 26°C for 72 h. Culture supernatants were collected, subjected to a protein A-Sepharose column, and then eluted with 20 mM glycine-HCl at pH 2.5. The eluted proteins were immediately neutralized with 1 M Tris and dialyzed against phosphate-buffered saline (PBS). gD(306t)-Fc and gD(285t)-Fc were used as gDl and gDs, respectively.
Assays for entry and cell-cell spread of HSV-1.
To assay entry of HSV-1, confluent cells grown on 35-mm dishes were incubated at 4°C for 2 h with an indicated concentration of HSV-1(KOS)tk12 (62). The cells were then incubated at 37°C for various periods of time (0, 10, 20, 30, 40, 50, or 60 min). They were washed with PBS, treated for 1 min with buffer A (100 mM citrate at pH 3.0, 10 mM KCl, and 135 mM NaCl) for inactivation of extracellular viruses (19), and then washed three times with PBS. The cells were further cultured in DME supplemented with 5% FCS. At 6 h after the inoculation, the cells were fixed with 0.2% glutaraldehyde in PBS and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (33). The number of blue cells was determined by microscopy.
Cell-cell spread of HSV-1 was assayed with plaque formation. Confluent cells on 35-mm dishes were incubated at 37°C for 30 min with the same virus and washed with PBS and buffer A. The cells were then overlaid with DME supplemented with 5% FCS containing 0.5% agarose. At 2 days after inoculation, the cells were fixed and stained with X-Gal, and the plaque number and size were determined by microscopy.
Antibodies.
A rabbit antiserum against human nectin-1α was raised against GST–nectin-1α–CPN (53). This serum was affinity purified by use of the GST fusion protein covalently coupled to NHS-activated Sepharose beads (Amersham-Pharmacia Biotech, Ltd.) and used as anti-nectin-1α antibody 1 (Ab1). Another rabbit antiserum was raised against a 19-mer synthetic peptide (corresponding to aa 450 to 468; ERKVGGPHPKYDEDAKRPY, a conserved sequence of nectin-1α between human and mouse) coupled via cysteine at the NH2-terminal residue to keyhole limpet hemocyanin. This serum was used as the anti-nectin-1α Ab2. A monoclonal mouse anti-afadin Ab was prepared as described previously (42).
gD-binding assay.
A gD-binding assay was performed as previously described (57). Briefly, confluent cells on 96-well plates were fixed with 3.7% formaldehyde for 15 min. After the cells were extensively washed with PBS, they were blocked with a blocking buffer (Block Ace; Dainihon Pharmaceuticals). The cells were incubated with various concentrations of gDl at room temperature for 1 h, washed three times with a washing buffer (0.1% Tween 20 in PBS), and then incubated with horseradish peroxidase-conjugated anti-human IgG Fc Ab (American Qualex) at room temperature for 30 min. The samples were washed three times with the washing buffer and once with 50 mM citrate at pH 4.5. After the addition of o-phenylenediamine (Wako Pure Chemicals, Osaka, Japan) as the substrate for horseradish peroxidase, the binding of gDl to cells was determined by enzyme-linked immunosorbent assay (ELISA) of absorbance at 490 nm.
gD affinity chromatography.
gDs was immobilized on protein A-Sepharose beads. Cells were sonicated in buffer B (20 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride, and 1 μg of pepstatin A/ml), followed by ultracentrifugation. The supernatant was incubated for 60 min with the gDs affinity beads equilibrated with buffer B, followed by centrifugation. After the beads were collected and extensively washed with buffer B, the bound proteins were eluted with 20 mM glycine-HCl at pH 2.5. The eluate was immediately neutralized with 1 M Tris and boiled in an sodium dodecyl sulfate (SDS) sample buffer (60 mM Tris-HCl at pH 6.7, 3% SDS, 2% [vol/vol] 2-mercaptoethanol, and 5% glycerol). The sample was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (8% polyacrylamide gel), followed by Western blotting with the monoclonal anti-afadin Ab and the polyclonal anti-nectin-1α Ab2.
Cell aggregation assay.
A cell aggregation assay was done as previously described (54). Briefly, to obtain a single-cell suspension, cells were washed with PBS, incubated with 0.2% trypsin and 1 mM EDTA at 37°C for 5 min, and dispersed by gentle pipetting. Nectin-1α was resistant to trypsin, and this treatment with trypsin did not induce proteolysis. The cells were then suspended in Hanks' balanced salt solution (106 cells/ml) in the presence or absence of gDl or HSV-1 (Seibert), placed in 12-well plates precoated with bovine serum albumin, and rotated on a gyratory shaker at 37°C for indicated periods of time. Aggregation was stopped with the addition of 2% glutaraldehyde. It has been shown that the addition of glutaraldehyde does not cause any artificial aggregation or dissociation of aggregates (61). The extent of aggregation of cells was represented by the ratio of the total particle number N at time t of incubation (Nt) to the initial particle number (No).
Chemical cross-linking.
Chemical cross-linking was done as described previously (29, 32, 44). Briefly, a single-cell suspension (106 cells/ml) obtained as described above was incubated in PBS with 1 mM bis-(sulfosuccinimidyl)-suberate cross-linker (Pierce) in the presence or absence of 2 μM gDl. After incubation at 14°C for 15 min, the reaction was stopped with the addition of 10 mM Tris-HCl at pH 7.5. The cells were washed with PBS and counted to confirm that there was no aggregation in the cell suspension.
Other procedures.
Immunofluorescence microscopy of cultured cells was done as described previously (30, 53). Protein concentrations were determined with bovine serum albumin as a reference protein (2). SDS-PAGE was done as described previously (26).
RESULTS
Interaction of nectin-1α with afadin.
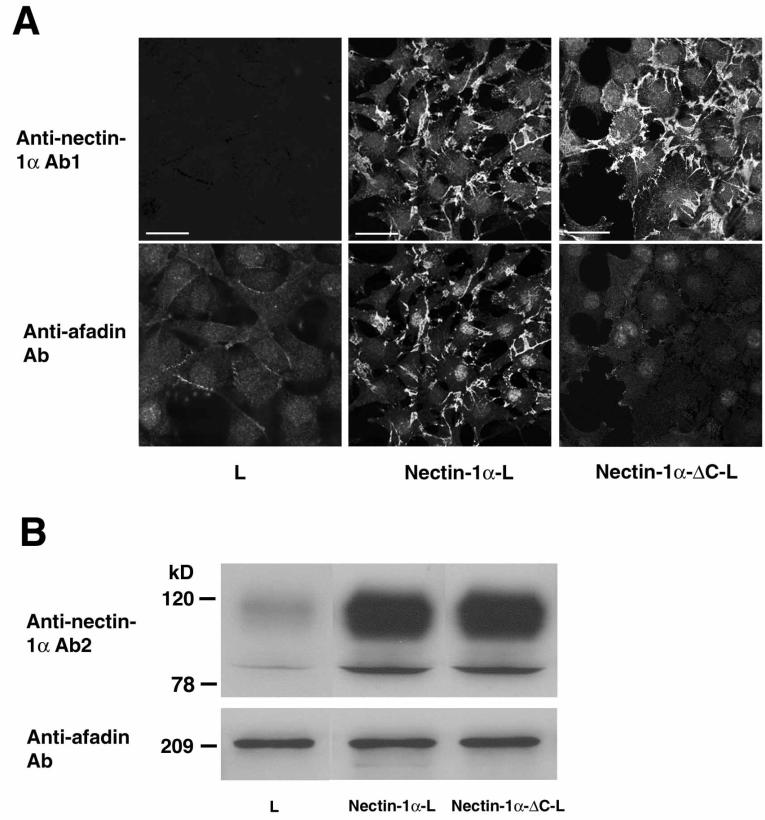
To examine whether the interaction of nectin-1α with afadin is involved in entry and/or cell-cell spread of HSV-1, we used L cells stably expressing the full length of human nectin-1α (nectin-1α-L cells) or the COOH-terminal 4-aa-deleted mutant (nectin-1α-ΔC-L cells). L cells lack the cadherin that is required to assemble cell-cell AJs (34) where nectin-2 and afadin are localized (30, 53). We first confirmed whether nectin-1α, but not nectin-1α-ΔC, interacts with afadin. When the cell lysates of nectin-1α-L and -1α-ΔC-L cells were subjected to gD affinity chromatography, the bound amounts of nectin-1α and -1α-ΔC were similar, but afadin was coeluted with nectin-1α, not with nectin-1α-ΔC (data not shown). We then compared the localization of afadin in nectin-1α-L and -1α-ΔC-L cells. In nectin-1α-L cells, afadin was concentrated at nectin-1α-based cell-cell contact sites, whereas in nectin-1α-ΔC-L cells, afadin was diffusely distributed and not concentrated at nectin-1α-ΔC-based cell-cell contact sites (Fig. 1A). This diffuse distribution of afadin results in weak staining intensity. These results are consistent with our previous reports that nectin-2α interacts with afadin through the COOH-terminal 4 aa (32, 52, 53). Nectin-1α-L cells sometimes adhered to each other through long and thin cellular processes (data not shown). These processes were observed by electron microscopy but were hardly visible by light microscopy. Therefore, even if the cell membranes where the nectin-afadin system is localized appear not to contact adjacent cells, these membranes are cell-cell contact sites.
FIG. 1.
Interaction of afadin with nectin-1α, but not with nectin-1α-ΔC. (A) Immunofluorescence microscopy. L, nectin-1α-L, and nectin-1α-ΔC-L cells were doubly stained with the polyclonal anti-nectin-1α Ab1 and the monoclonal anti-afadin Ab. There was nuclear staining with this monoclonal anti-afadin Ab, but its significance is not clear. Bars, 10 μm. (B) Western blotting. Each cell lysate of L, nectin-1α-L, and nectin-1α-ΔC-L cells (20 μg of protein each) was subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting with the polyclonal anti-nectin-1α Ab2 and the monoclonal anti-afadin Ab. These results are representatives of three independent experiments.
The expression levels of the afadin protein in the two cell lines were similar, and the expression level of the nectin-1α or -1α-ΔC protein in these cell lines was about 10-fold higher than that of the endogenous mouse nectin-1α protein in L cells, as estimated by Western blotting (Fig. 1B). Both nectin-1α and nectin-1α-ΔC showed two bands. The upper band was more broadly observed, and the ratio of the upper band to the lower band was about 10:1. Their relationship is not clear, but this may be due to different levels of posttranslational modifications such as glycosylation.
No requirement of the interaction of nectin-1α with afadin for its gD-binding activity.
To examine whether the interaction of nectin-1α with afadin affects the gD-binding activity of nectin-1α, we compared the activities among nectin-1α-L, nectin-1α-ΔC-L, and L cells. The gD-binding activities of nectin-1α-L cells and nectin-1α-ΔC-L cells were similar but remarkably higher than that of L cells (Fig. 2). It has recently been shown that mouse nectin-1α has gD-binding activity for entry of HSV-1 (48). Consistently, mouse nectin-1α bound to the gD affinity beads when the cell lysate of L cells was subjected to affinity chromatography (data not shown). It is likely that the gD-binding activity of L cells is derived from endogenous mouse nectin-1α. The difference in the gD-binding activities between L and nectin-1α-L or -1α-ΔC-L cells is undoubtedly due to exogenously expressed human nectin-1α or -1α-ΔC, respectively. These results indicate that the interaction of nectin-1α with afadin is not required for gD binding to host cells.
FIG. 2.
gD-binding activities of L-cell lines. Confluent cells on 96-well plates were subjected to a gD-binding assay. The binding was determined by ELISA. ●, L cells; ▪, nectin-1α-L cells; ▴, nectin-1α-ΔC-L cells. These results are representative of three independent experiments.
Requirement of the interaction of nectin-1α with afadin for efficient cell-cell spread, but not entry, of HSV-1.
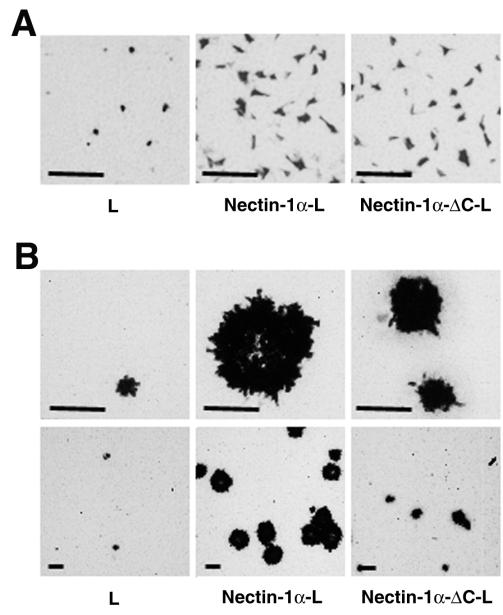
We next examined whether the interaction of nectin-1α with afadin affects entry and/or cell-cell spread of HSV-1. L, nectin-1α-L, and nectin-1α-ΔC-L cells were incubated for various periods of time (0 to 60 min) with a strain of HSV-1, [HSV-1(KOS)tk12] (62), which expresses β-galactosidase from a lacZ cassette in its genome. At 6 h after inoculation, the infected cells were detected by staining with X-Gal. The number of infected cells of each L-cell line reached a plateau for the first 30-min inoculation (data not shown). At 30 min, the numbers of infected cells of nectin-1α-L and -1α-ΔC-L cells were similar (Fig. 3A and Table 1). However, the numbers of infected cells of nectin-1α-L or -1α-ΔC-L cells were about fourfold higher than that of L cells. Essentially the same results were obtained with two other pairs of nectin-1α-L and -1α-ΔC-L cell clones (data not shown). These results indicate that entry of HSV-1 is dependent on the expression level of the nectin-1α or -1α-ΔC protein and that the interaction of nectin-1α with afadin is not required for entry of HSV-1.
FIG. 3.
Requirement of the interaction of nectin-1α with afadin for efficient cell-cell spread, but not entry, of HSV-1. L, nectin-1α-L, and nectin-1α-ΔC-L cells were incubated at 37°C for 30 min with 105 PFU of HSV-1(KOS)tk/well. At 6 h (A) or 2 days (B) after inoculation, the cells were fixed and stained with X-Gal. Upper panel, high magnification; lower panel, low magnification; bars, 200 μm. These results are representative of three independent experiments.
TABLE 1.
Quantitative analysis of entry and cell-cell spread of HSV-1 in various L-cell linesa
| Cell line | Time after inoculation
|
||
|---|---|---|---|
| 6 h
|
2 days
|
||
| No. of infected cells (per 35-mm dish) | No. of plaques (per 35-mm dish) | Plaque diameter (μm) | |
| L | 30 ± 10 | <10 | 100 ± 50 |
| Nectin-1α-L | 130 ± 30 | 130 ± 40 | 320 ± 70 |
| Nectin-1α-ΔC-L | 120 ± 30 | 60 ± 20 | 160 ± 60 |
| EL | 90 ± 20 | 80 ± 20 | 230 ± 70 |
Confluent cells on 35-mm dishes were incubated at 37°C for 30 min with 105 PFU of HSV-1(KOS)tk12 per well. At 6 h or 2 days after inoculation, the cells were fixed and stained with X-Gal. The number of infected cells or the plaque number and size were determined by microscopy. The values are averages ± standard error of three independent experiments.
When plaque formation in these L-cell lines was assayed at 2 days after inoculation for 30 min with HSV-1(KOS)tk12, the plaque size of nectin-1α-L cells was about twice as large as that of nectin-1α-ΔC-L cells (Fig. 3B and Table 1). The number of plaques in nectin-1α-L cells was also higher than that in nectin-1α-ΔC-L cells. The number of plaques in nectin-1α-ΔC-L cells was higher than that in L cells. Essentially the same results were obtained with two other pairs of nectin-1α-L and -1α-ΔC-L cell clones (data not shown). These results indicate that cell-cell spread of HSV-1 is dependent on the expression level of the nectin-1α or -1α-ΔC protein and that the interaction of nectin-1α with afadin is required for efficient cell-cell spread of HSV-1.
Requirement of the cadherin-catenin system for efficient entry and cell-cell spread of HSV-1.
To examine the effect of the cadherin-catenin system on entry and cell-cell spread of HSV-1, we used EL cells, which are L cells stably expressing E-cadherin (34). The gD-binding activities of L and EL cells were similar (Fig. 4A). The expression levels of the afadin protein in L and EL cells were similar and those of the nectin-1α protein were also similar as estimated by Western blotting (Fig. 4B). When the cell lysate of EL cells was subjected to gD affinity chromatography, mouse nectin-1α bound to the affinity beads (data not shown). The localization of endogenous mouse nectin-1α in EL cells remains to be clarified because no Ab capable of detecting the localization is available at present. However, we have previously shown that endogenous mouse nectin-2 is colocalized with E-cadherin at cell-cell AJs in EL cells and that when exogenous human nectin-1α is expressed in EL cells, it is also localized there (53). It is most likely that endogenous nectin-1α is also localized with E-cadherin at cell-cell AJs.
FIG. 4.
gD-binding activities of L and EL cells. (A) gD-binding activities. Confluent cells were subjected to a gD-binding assay. The binding was determined by ELISA. ○, L cells; ●, EL cells. (B) Western blotting. Each cell lysate of L and EL cells (20 μg of protein each) was subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting with the polyclonal anti-nectin-1α Ab2 and the monoclonal anti-afadin Ab. These results are representative of three independent experiments.
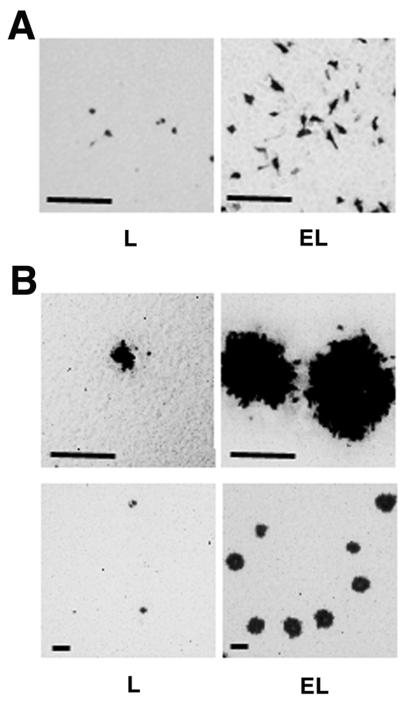
L and EL cells were incubated for various periods of time (0 to 60 min) with HSV-1(KOS)tk12. At 6 h after the inoculation, the infected cells were detected by staining with X-Gal. The number of infected cells in each cell line reached a plateau for the first 30-min inoculation (data not shown). At 30 min, the infected cell number of EL cells was about threefold higher than that of L cells (Fig. 5A and Table 1). At 2 days after the inoculation for 30 min with HSV-1(KOS)tk12, the plaque size of EL cells was larger than that of L cells (Fig. 5B and Table 1). The number of plaques in EL cells was much higher than in L cells. These results indicate that the cadherin-catenin system is involved in both entry and cell-cell spread of HSV-1.
FIG. 5.
Requirement of the cadherin-catenin system for efficient entry and cell-cell spread of HSV-1. L and EL cells were incubated at 37°C for 30 min with 105 PFU of HSV-1(KOS)tk/well. At 6 h (A) or 2 days (B) after inoculation, the cells were fixed and stained with X-Gal. Upper panel, high magnification; lower panel, low magnification; bars, 200 μm. These results are representative of three independent experiments.
Inhibition of cell-cell adhesion activity of nectin-1α by HSV-1 and gD.
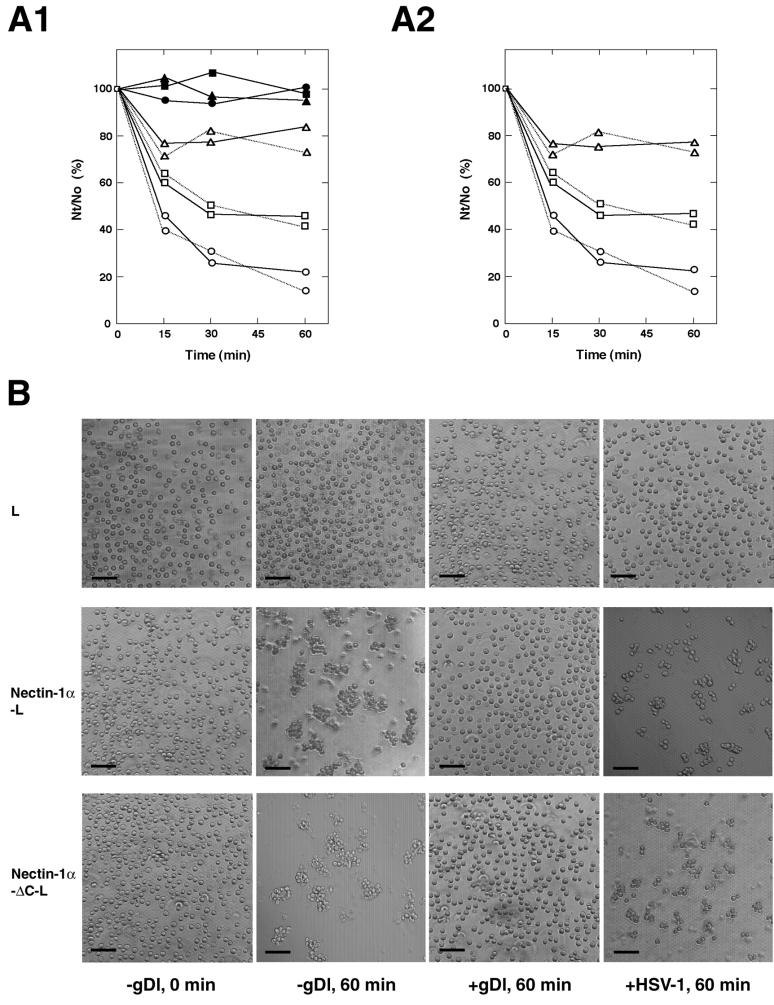
In the last set of experiments, we examined the effects of HSV-1 (Seibert) and the recombinant gD, gDl, on cell-cell adhesion activity of nectin-1α. Nectin-1α and -1α-ΔC showed similar cell aggregation activities in a time-dependent manner (Fig. 6, A1 and B). HSV-1 itself and gDl inhibited this aggregation activity. gDl inhibited this activity in a dose-dependent manner (Fig. 6A2). It may be noted that there is a difference in the inhibition of cell aggregation activity between HSV-1 and gDl. Small cell aggregates were still observed with HSV-1, whereas no aggregate was observed with gDl. The exact reason for this difference is not known, but the small aggregates may be induced by the multivalent binding activity of the viral particles.
FIG. 6.
Inhibition of cell-cell adhesion activity of nectin-1α by HSV-1 and gD. (A) Cell aggregation activities of nectin-1α and -1α-ΔC. A single-cell suspension of each L-cell line was rotated for indicated periods of time in the presence or absence of 1 μM gDl, 2 μM gDl, or 107 PFU of HSV-1 (Seibert)/well) . The extent of aggregation of cells was represented by the ratio of the total particle number at time t of incubation (Nt) to the initial particle number (No). (A1) In the presence of gDl or HSV-1. ●—●, L cells alone; ▴—▴, L cells in the presence of 2 μM gDl; ▪—▪, L cells in the presence of HSV-1; ○—○, nectin-1α-L cells alone; ▵—▵, nectin-1α-L cells in the presence of 2 μM gDl; □—□, nectin-1α-L cells in the presence of HSV-1; ○. . .○, nectin-1α-ΔC-L cells alone; ▵. . .▵, nectin-1α-ΔC-L cells in the presence of 2 μM gDl; □. . .□, nectin-1α-ΔC-L cells in the presence of HSV-1. These results are representative of three independent experiments. (A2) In the presence of various concentrations of gDl. ○—○, nectin-1α-L cells alone; ▵—▵, nectin 1-α-L cells in the presence of 2 μM gDl; □—□, nectin-1α-L cells in the presence of 1 μM gDl; ○. . .○, nectin-1α-ΔC-L cells alone; ▵. . .▵, nectin-1α-ΔC-L cells in the presence of 2 μM gDl; and □. . .□, nectin-1α-ΔC-L cells in the presence of 1 μM gDl. These results are representative of three independent experiments. (B) Cell aggregation of L, nectin-1α-L, and nectin-1α-ΔC-L cells. A single-cell suspension of each L-cell line was rotated for the indicated periods of time in the presence or absence of 2 μM gDl or 107 PFU of HSV-1 (Seibert)/well. Bars, 100 μm. These results are representative of three independent experiments.
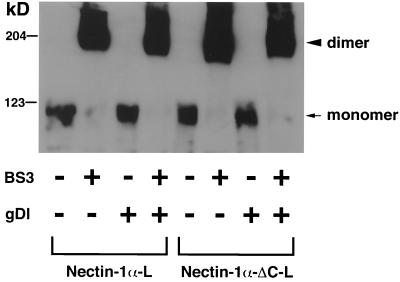
It has previously been shown that nectin-2α forms a cis dimer (29, 32). We recently found that this cis dimerization of nectin-2α is independent of the interaction with afadin and suggested that the trans interaction (cell-cell adhesion) of nectin-2α follows the cis dimerization (32). gDl did not inhibit this cis dimerization of nectin-1α (Fig. 7). These results indicate that gD inhibits the trans interaction of nectin-1α.
FIG. 7.
Inability of gD to inhibit cis dimerization of nectin-1α and -1α-ΔC. A single-cell suspension of nectin-1α-L or -1α-ΔC-L cells was incubated with various combinations of 2 μM gDl and 1 mM cross-linker bis-(sulfosuccinimidyl)-suberate. Each cell lysate was subjected to SDS-PAGE (8% polyacrylamide gel), followed by Western blotting using the polyclonal anti-nectin-1α Ab2. Arrowhead, dimer; arrow, monomer. These results are representative of three independent experiments.
DISCUSSION
We have shown here that the interaction of nectin-1α with afadin does not affect the gD-binding activity of nectin-1α or entry of HSV-1 but increases the efficiency of cell-cell spread of this virus. These results suggest that the fusion process by which HSV-1 spreads from cell to cell is mechanistically different from that by which the virus enters into cells. The mechanism by which interaction of nectin-1α with afadin increases the efficiency of cell-cell spread of HSV-1 remains to be elucidated, but one possible explanation is that the afadin-dependent association of nectin-1α with the actin cytoskeleton is involved. Another possibility is that denser and more-focused localization of nectin-1α and afadin at cell-cell contact sites, caused by their interaction, increases the efficiency of cell-cell spread. During cell-cell spread, attachment of HSV-1 to concentrated nectin-1α may trigger membrane fusion that utilizes the actin cytoskeleton activity mediated by afadin to bring the lipid domains of the viral envelope and the plasma membrane into apposition. Further studies are necessary for our understanding of the role of afadin in the mechanism of cell-cell spread of HSV-1.
We have next shown here that the cadherin-catenin system increases efficiency of entry and cell-cell spread of HSV-1. The mechanism of the stimulatory effect of the cadherin-catenin system remains to be clarified. As to the stimulatory effect on entry, however, one possible explanation is that the cadherin-catenin system affects viral components other than gD and/or their cellular receptors to increase efficiency of entry. As to the stimulatory effect on cell-cell spread, one possibility is that the higher concentration of nectin-1α and afadin at cell-cell AJs, caused by their interaction with the cadherin-catenin system, increases efficiency of cell-cell spread. The nectin-afadin system is colocalized with the cadherin-catenin system at cell-cell AJs in various epithelial and nonepithelial cells (30, 53). Nectin and cadherin interact through their cytoplasmic domain-associating proteins, afadin and α-catenin (52, 53). The concentration of nectin-1α is higher when it is colocalized with the cadherin-catenin system through afadin than when the truncated form of nectin-1α incapable of interacting with afadin is localized independent of the cadherin-catenin system (52). Thus, the cadherin-catenin system-dependent higher concentrations of nectin-1α and afadin may be related to higher efficiency of cell-cell spread of HSV-1. This interpretation is consistent with the result that the efficiency of cell-cell spread of HSV-1 in L cells overexpressing the full-length nectin-1α is higher than that in L cells overexpressing truncated nectin-1α. However, we cannot exclude another possibility, that other viral components, such as gE and gI, bind to cadherin and thereby increase efficiency. This possibility is consistent with a previous report that gE and gI are colocalized with β-catenin (10). Further studies are necessary to elucidate the mechanism of the stimulatory effect of the cadherin-catenin system on entry and cell-cell spread of HSV-1.
L and EL cells endogenously express at least nectin-1α and -2α (32, 52, 53). There is a possibility that HVEM/HveA is also involved in entry and/or cell-cell spread of HSV-1 in these cell lines, although we have not determined whether the L-cell lines express HVEM/HveA. This possibility is unlikely, however, because nectin-1α-mediated entry and cell-cell spread are enhanced by the cadherin-catenin system, but HVEM/HveA-mediated entry or cell-cell spread is not expected to be affected by the cadherin-catenin system. It has recently been shown that human nectin-2 mediates cell-cell spread of HSV-1 (6), although human or mouse nectin-2 has been shown not to mediate entry of HSV-1 (47, 62). However, it is unlikely that endogenous mouse nectin-2α is involved in entry or cell-cell spread of HSV-1 in the L-cell lines, because entry and cell-cell spread in L cells stably overexpressing mouse nectin-2α are similar to those in L cells (data not shown). Thus, nectin-1α is the most relevant entry and cell-cell spread mediator in the L-cell lines used here.
Finally, we have shown here that gD inhibits cell-cell adhesion activity (trans interaction) but not cis dimerization of nectin-1α. We have previously found that the cis dimerization of nectin-2α may be prerequisite for its trans interaction (32), as described for cadherin (58, 60), and that the V domain is likely to be responsible for the trans interaction (32). Taken together with previous reports that gD contains a binding domain specific for the V domain of nectin-1 (4, 25), it is likely that gD interacts with this domain and inhibits the trans interaction of nectin-1α.
HSV replicates in tissues of epithelial origin, such as the oral and genital mucosa and corneal epithelium, and spreads efficiently through these tissues, gaining access to sensory neurons, which eventually become the site of latency (7). In epithelial tissues, cell-cell adhesion is mediated by a junctional complex comprised of tight junctions, cell-cell AJs, and desmosomes, and HSV spreads across these cell-cell junctions. By spreading rapidly from cell to cell through a space that is isolated by tight junctions, HSV must spread rapidly to avoid neutralization by anti-HSV Abs made in host animals. HSV can cause secondary lesions in the mucosa of individuals who produce high titers of anti-HSV Abs, and the severity of disease does not correlate with Ab titers (7). Thus, Abs cannot stop cell-cell spread in epithelium. These observations indicate that direct cell-cell spread is a primary mode of HSV transmission. Our present results, that the interaction of nectin-1α with afadin and the cadherin-catenin system increase efficiency of cell-cell spread of HSV-1, are consistent with these earlier observations. Further studies of the nectin-afadin and cadherin-catenin systems will lead us to a better understanding of rapid cell-cell spread of HSV-1.
ACKNOWLEDGMENTS
We thank P. G. Spear (Northwestern University, Chicago, Ill.) for helpful discussions and for providing HSV-1(KOS)tk12. We are also grateful to S. Tsukita and A. Nagafuchi (Kyoto University, Kyoto, Japan) for L and EL cells and to J. Koga (JCR Pharmaceuticals Co., Ltd., Kobe, Japan) for the HSV-1 genome.
The work performed at the Department of Molecular Biology and Biochemistry was supported by grants-in-aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, Sports, and Culture, Japan (1999 and 2000). The work performed at the Department of Microbiology was supported by grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan (1999 and 2000).
REFERENCES
- 1.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI, or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Gu B, Person S. Role of glycoprotein B of herpes simplex type 1 in viral entry and cell fusion. J Virol. 1988;62:2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpes virus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attribute of a bona fide receptor for herpes simplex virus type 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocchi F, Menotti L, Dubreuil P, Lopez M, Campadelli-Fiume G. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR/HveC/HIgR) and nectin2 (PRR/HveB) J Virol. 2000;74:3909–3917. doi: 10.1128/jvi.74.8.3909-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corey L, Spear P G. Infection with herpes simplex viruses (1) N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 8.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwell K S, Johnson D C. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester A, Farrel H, Wilkinson G, Kayne J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequence detected. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geraphty R J, Krummenacher C, Eisenberg R J, Cohen G H, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor related protein 1 and polio virus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 13.Guan K L, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 15.Gumbiner B M, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumbiner B M, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the absorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold B C, Visalli R J, Sumarski N, Brandt C, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulfate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 19.Highlander S L, Sutherland S L, Gage P J, Johnson D C, Levine M, Glorioso J C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987;61:3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T, Campadelli-Fiume G. Anti-idiotypic antibodies mimicking glycoprotein D of herpes simplex virus identify a cellular protein required for virus spread from cell to cell and virus-induced polykaryocytosis. Proc Natl Acad Sci USA. 1996;93:1836–1840. doi: 10.1073/pnas.93.5.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y. Afadin: a key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen K A, Soler A P, Johnson K R, Wheelock M J. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummenacher C, Rux A H, Whitbeck J C, Ponce-de-Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty R J, Spear P G, Eisenberg R J, Cohen G H. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol. 1999;73:8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee W C, Fuller A O. Herpes simplex type 1 and pseudorabies virus bind to a common saturable receptor on Vero cells that is not heparan sulfate. J Virol. 1993;67:5088–5097. doi: 10.1128/jvi.67.9.5088-5097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;70:8402–8410. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- 30.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauri D N, Ebner R, Kochel K D, Montgomery R I, Cheung T C, Yu G-L, Murphy M, Eisenberg R J, Cohen G H, Spear P G, Ware C F. LIGHT, a new member of the TNF superfamily, and lymphotoxin (LT) α are ligands for herpesvirus entry mediator (HVEM) Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 32.Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 34.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- 35.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- 36.Navarro D, Paz P, Pereira L. Domains of herpes simplex virus glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992;186:99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- 37.Ogino T, Rapp F. Differences in thermal stability of deoxythymidine kinase activity in extracts from cells infected with herpes simplex virus type 1 or type 2. Virology. 1971;46:953–955. doi: 10.1016/0042-6822(71)90094-8. [DOI] [PubMed] [Google Scholar]
- 38.Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rimm D L, Koslov E R, Kebriaei P, Cianci C D, Morrow J S. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rux A H, Willis S H, Nicola A V, Hou W, Peng C, Lou H, Cohen G H, Eisenberg R J. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to herpes virus entry mediator. J Virol. 1998;72:7091–7098. doi: 10.1128/jvi.72.9.7091-7098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakisaka T, Nakanishi H, Takahashi K, Mandai K, Miyahara M, Satoh A, Takaishi K, Takai Y. Different behavior of l-afadin and neurabin-II during the formation and destruction of cell-cell adherens junctions. Oncogene. 1999;18:1609–1617. doi: 10.1038/sj.onc.1202451. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3: a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- 45.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans J. Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla D, Liu J, Blaiklock P, Schworak N W, Bai X, Esko J D, Cohen G H, Eisenberg R J, Rosenberg R D, Spear P G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 47.Shukla D, Rowe C L, Dong Y, Racaniello V R, Spear P G. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol. 1999;73:4493–4497. doi: 10.1128/jvi.73.5.4493-4497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla D, Dal Canto M C, Rowe C L, Spear P G. Striking similarity of murine nectin-1α to human nectin-1α (HveC) in sequence and activity as a gD receptor for alphaherpesvirus entry. J Virol. 2000;74:11773–11781. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 50.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- 51.Spear P G, Eisenberg R J, Cohen G H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 52.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1176. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junction through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 56.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 57.Tal-Singer R, Peng C, Ponce de Leon M, Abrams W R, Banfield B W, Tufaro F, Cohen G H, Eisenberg R J. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura K, Shan W-S, Hendrickson W A, Coleman D R, Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 59.Tessier D C, Thomas D Y, Khouri H E, Laliberte F, Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98:177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- 60.Tomoschy A, Fauser C, Landwher R, Engel J. Homophilic adhesion of E-cadherin occurs by a cooperative two-step interaction of N-terminal domains. EMBO J. 1996;15:3507–3514. [PMC free article] [PubMed] [Google Scholar]
- 61.Ueda M J, Takeichi M. Two mechanisms in cell adhesion revealed by effects of divalent cations. Cell Struct Funct. 1976;1:377–388. [Google Scholar]
- 62.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 63.Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson E D, Takeichi M. α-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss E E, Kroemker M, Rüdiger A-H, Jockusch B M, Rüdiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between α-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]