Abstract
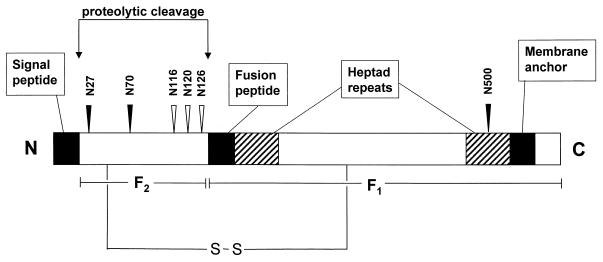
The human respiratory syncytial virus (Long strain) fusion protein contains six potential N-glycosylation sites: N27, N70, N116, N120, N126, and N500. Site-directed mutagenesis of these positions revealed that the mature fusion protein contains three N-linked oligosaccharides, attached to N27, N70, and N500. By introducing these mutations into the F gene in different combinations, four more mutants were generated. All mutants, including a triple mutant devoid of any N-linked oligosaccharide, were efficiently transported to the plasma membrane, as determined by flow cytometry and cell surface biotinylation. None of the glycosylation mutations interfered with proteolytic activation of the fusion protein. Despite similar levels of cell surface expression, the glycosylation mutants affected fusion activity in different ways. While the N27Q mutation did not have an effect on syncytium formation, loss of the N70-glycan caused a fusion activity increase of 40%. Elimination of both N-glycans (N27/70Q mutant) reduced the fusion activity by about 50%. A more pronounced reduction of the fusion activity of about 90% was observed with the mutants N500Q, N27/500Q, and N70/500Q. Almost no fusion activity was detected with the triple mutant N27/70/500Q. These data indicate that N-glycosylation of the F2 subunit at N27 and N70 is of minor importance for the fusion activity of the F protein. The single N-glycan of the F1 subunit attached to N500, however, is required for efficient syncytium formation.
Human respiratory syncytial virus (HRSV), a member of the genus Pneumovirus within the family Paramyxoviridae, contains three envelope glycoproteins, designated F, G, and SH. Among these glycoproteins, the F protein is the most conserved molecule, demonstrating a homology of 80% or more to F proteins of different HRSV and bovine respiratory syncytial virus (BRSV) serotypes. The F protein contains important T-cell epitopes and is the major virus antigen inducing neutralizing antibodies (53, 57, 69). The F protein plays a central role in virus entry. It mediates the fusion of the virus and the cellular membrane, thereby allowing the nucleocapsid to enter the cytoplasm of the host cell. The fusion process is independent of pH. Therefore, virus bound to the cell surface can directly fuse with the plasma membrane and does not require prior uptake by endocytic vesicles. In addition, cells infected with HRSV can fuse with adjacent cells, resulting in giant, multinucleated syncytia. Syncytium formation can also be observed with cells transfected with the gene encoding F protein. Coexpression of F protein together with G and/or SH protein greatly enhances fusion activity (27, 55). However, the presence of both the G and the SH proteins appears to be nonessential for virus replication in cell culture (5, 30, 70). Recent studies suggest that certain glycosaminoglycans on the cell surface are required for HRSV infection (24, 25, 34, 44). The G protein, as well as the fusion protein, has been demonstrated to bind to these carbohydrate structures (14, 15, 34).
The F protein is a type I integral membrane protein that is synthesized as a precursor, F0, of 69 kDa, which is postranslationally cleaved by a cellular protease at a multibasic sequence into two disulfide-linked subunits, F2 (19 kDa) and F1 (50 kDa) (23). A stretch of hydrophobic amino acids at the N terminus of the F1 subunit is supposed to form a fusion peptide that interacts with the host membrane, thereby initiating the fusion process. As with other paramyxoviruses, there are two heptad repeats in the F1 subunit; heptad A is adjacent to the fusion peptide, and heptad B is adjacent to the transmembrane region. Other posttranslational modifications of the F protein include acylation of C550 (1), oligomerization (8), and N-glycosylation (6, 22). The F protein of HRSV strain Long contains six potential N-glycosylation sites: N27, N70, N116, N120, N126, and N500 (Fig. 1). The latter site is part of heptad repeat B, adjacent to the membrane anchor; the other five are located on the F2 subunit. Except for N120, all potential N-glycosylation sites are conserved among different HRSV strains, suggesting that N-glycosylation at these sites might be important for the structural and functional integrity of the fusion protein. For many other viral glycoproteins, it has been shown that N-glycans are important structural components that affect the folding and transport (12) as well as the activity (39, 49, 51, 52, 60, 68), stability (54), and immunological properties (10, 19, 20, 48) of these molecules. In contrast, there is only little information available about the importance of N-linked oligosaccharides for the function and activity of the HRSV F glycoprotein. Total inhibition of N-glycosylation by the drug tunicamycin indicated that cell surface transport of the F protein does not depend on the presence of carbohydrates (8). Treatment of purified virions with a glycosidase that cleaves off N-linked oligosaccharides resulted in a significant reduction of virus infectivity (37). Since all three envelope glycoproteins, G, F, and SH, contain N-linked oligosaccharides, it is not understood whether this effect is due to the deglycosylation of a certain glycoprotein or a combination of two or all three proteins. The role of the four potential N-glycosylation sites in the cell surface transport of the related BRSV fusion protein has been previously investigated by site-directed mutagenesis (56). However, it is still unknown how the N-glycans affect the fusion activity of the protein. In this study, we determined the number and location of N-linked oligosaccharides in the fusion protein of HRSV (strain Long) and analyzed their role in cell surface transport, proteolytic activation, and fusion activity.
FIG. 1.
Schematic diagram of the HRSV (strain Long) fusion protein. The two disulfide-linked F protein subunits, F1 and F2, are indicated; the arrows point to proteolytic cleavage sites. The black boxes represent the signal peptide, the fusion peptide, and the membrane anchor. Heptad repeats A and B are shown as hatched boxes. The locations of the six potential N-glycosylation sites are indicated by arrowheads. Closed arrowheads point to sites that in this study were shown to contain oligosaccharides.
MATERIALS AND METHODS
Cells and virus.
BSR-T7/5 cells were a generous gift of K.-K. Conzelmann (Max-von-Pettenhofer-Institut, Munich, Germany). The cells were grown in Eagle's minimal essential medium with Earle's salts supplemented with nonessential amino acids and 10% fetal calf serum. Vero cells and primary chicken fibroblasts were maintained in Dulbecco's modified Eagle medium with 5 and 10% fetal calf serum, respectively. HRSV strain Long, a generous gift of H.-J. Streckert (Ruhr-Universität, Bochum, Germany), was propagated in Vero cells. Recombinant vaccinia virus MVA-T7 was provided by G. Sutter (Technische Universität, Munich, Germany) and was propagated in primary chicken fibroblasts.
Cloning and mutagenesis of the F gene.
Cloning of the F gene started with the reverse transcription of total RNA that was prepared from Vero cells infected with HSRV (strain Long). The open reading frame of the F protein was amplified from the cDNA by PCR and was cloned into the pTM1 vector downstream of the T7 promoter (47), creating plasmid pTM1-F. The entire open reading frame of F was sequenced, and the sequence obtained was compared with the published sequence (40). The following differences were found: V76E, P101S, T152I, S211N, and A442V. The glycosylation mutants were generated by replacing the asparagine residue at position N27, N70, N116, N120, N126, or N500 with glutamine. A QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, Calif.) was used to substitute the triplet CAG or CAA (both of which encode glutamine) for the triplet AAC or AAT (both of which encode asparagine) by PCR. The nucleotide exchanges were confirmed by DNA sequencing.
Cell surface biotinylation and immunoprecipitation.
Transient expression of the F protein was performed in BSR-T7/5 cells, a subline of BHK-21 cells stably expressing T7 RNA polymerase under the control of the cytomegalovirus promoter (3). The cells were grown in 35-mm-diameter dishes to 90% confluence and were infected with 5 focus-forming units of MVA-T7, a recombinant vaccinia virus (modified vaccinia virus Ankara [MVA]) encoding T7 RNA polymerase (66), per cell. Infection with the recombinant vaccinia virus resulted in a higher expression level than that of uninfected BSR-T7/5 cells. One hour postinfection, the cells were washed twice with phosphate-buffered saline (PBS) and transfected with 5 μg of plasmid DNA in 10 μl of Lipofectamine 2000 transfection reagent (Life Technologies, Karlsruhe, Germany). Twenty hours posttransfection, the cell surface proteins of the transfectants were labeled with N-hydroxysuccinimide ester of biotin (sulfo-NHS-biotin; Pierce, Rockford, Ill.) as described previously (71). The cells were lysed in 1 ml of NP-40 lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5% sodium deoxycholate; 1% Nonidet P-40; protease inhibitor cocktail), and insoluble material was removed by centrifugation (16,000 × g for 30 min at 4°C). To 500 μl of each supernatant were added 50 μl of a 50% slurry of protein A-Sepharose (Sigma, Deisenhofen, Germany) and 2.5 μl of the RSV3216 monoclonal antibody, which is directed against the HRSV F protein (Serotec, Oxford, United Kingdom). After agitation for 90 min at 4°C, the immunoprecipitates were collected by centrifugation (16,000 × g for 3 min), washed three times with NP-40 lysis buffer, and eluted by boiling the beads in twofold-concentrated sodium dodecyl sulfate (SDS) sample buffer. The immunoprecipitates were electrophoresed on an SDS–10% polyacrylamide gel under reducing and nonreducing conditions and transferred to nitrocellulose by the semidry blotting technique (35). The membrane was incubated with blocking reagent (Roche Diagnostics, Mannheim, Germany) overnight at 4°C, washed three times with PBS containing 0.1% Tween 20, and incubated with streptavidin-peroxidase (1:1,000; Amersham, Braunschweig, Germany) for 1 h at room temperature. The nitrocellulose was washed as described above and incubated for 1 min with a chemiluminescent peroxidase substrate (BM; Roche Diagnostics, Mannheim, Germany). The resulting light emission was visualized by short-term exposure of the membrane to Biomax autoradiography film (Kodak, Rochester, N.Y.).
Radioimmunoprecipitation.
BSR-T7/5 cells grown in 35-mm-diameter dishes to 90% confluence were infected with recombinant vaccinia virus MVA-T7 and transfected with plasmid DNA as described above. At 20 h posttransfection, the cells were starved for 1 h in methionine- and cysteine-deficient minimal essential medium and were then cultured for 3 h in 1 ml of the same medium supplemented with 100 μCi of [35S]methionine-[35S]cysteine (Tran35S-Label; ICN, Eschwege, Germany). Immunoprecipitation of F protein was performed as described in the previous section. The immunoprecipitates were analyzed under reducing conditions by Tricine-SDS-polyacrylamide gel electrophoresis (61).
Detection of carbohydrates.
For detection of total carbohydrates by periodate oxidation, F protein was immunoprecipitated as described above except that the transfected cells were not labeled with biotin. The immunoprecipitates were separated by SDS–10% polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, Mass.). The membrane was incubated in PBS for 10 min and then in 100 mM acetate buffer (pH 5.5) containing 10 mM sodium metaperiodate for 20 min (at room temperature in the dark). The membrane was washed thoroughly in PBS and then incubated in 100 mM acetate buffer (pH 5.5) containing 0.025 μM biotin hydrazide (Amersham Pharmacia Biotech, Freiburg, Germany) for 60 min at room temperature. The membrane was washed and incubated in blocking reagent overnight at 4°C. Biotin groups were detected with streptavidin-peroxidase as described for the cell surface biotinylation procedure (see above).
Flow cytometry.
BSR-T7/5 cells grown in 35-mm-diameter dishes to 90% confluence were infected with recombinant vaccinia virus MVA-T7 and transfected with 5 μg of plasmid DNA as described above. At 20 h after transfection, the cells were washed twice with PBS containing 1 mM EDTA and incubated in the same buffer for 15 min at 37°C. The cells were suspended, pelleted by centrifugation, and resuspended in PBS containing 0.5% bovine serum albumin. Each sample was incubated with a fluorescein isothiocyanate (FITC)-conjugated bovine anti-BRSV serum at a 1:100 dilution for 30 min on ice. This serum was tested for its reactivity with the HRSV fusion protein. Subsequently, the cells were washed twice with PBS and immediately analyzed with a flow cytofluorometer (FACScan; Becton Dickinson, Heidelberg, Germany), using 20,000 cells per analysis. The Cell Quest (Becton Dickinson) and WinMDI (Salk Institute, San Diego, Calif.) software packages were used for calculations. Three independent transfection procedures were analyzed in this way.
Immunofluorescence analysis and syncytium formation.
BSR-T7/5 cells grown on 12-mm-diameter coverslips to 80 to 90% confluence were infected with the recombinant vaccinia virus (MVA-T7) and transfected with 1.5 μg of plasmid DNA with 3 μl of Superfect transfection reagent (Qiagen, Hilden, Germany). Twenty hours after transfection, the cells were fixed with 3% paraformaldehyde for 20 min at room temperature. For detection of intracellular antigen in a parallel sample, the fixed cells were permeabilized with 0.2% Triton X-100. The cells were stained by incubation with a monoclonal antibody directed against the HRSV F protein followed by incubation with an FITC-conjugated antibody directed against mouse immunoglobulin (Amersham, Braunschweig, Germany). Both antibodies were used at a dilution of 1:200. Conventional epifluorescence microscopy was performed with a Zeiss Axioplan 2 microscope. Digital photographs were taken with a digital video camera (Focus Imager; INTAS, Göttingen, Germany). For quantitative analysis of syncytium formation, we determined the average syncytium size by counting the nuclei in at least 20 fluorescent polykaryons (i.e., cells containing more than two nuclei). The frequency of syncytium formation was determined by counting the number of polykaryons in 100 fluorescent cells. The product of syncytium frequency and syncytium size was calculated (fusion index). The percentage value (relative fusion index) was determined by setting the value of parental F at 100%. Mean values were determined for data from three independently performed transfection experiments.
RESULTS
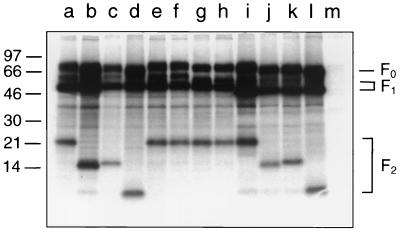
The fusion protein of the HRSV Long strain contains six potential N-glycosylation sites (-N-X-S/T-) (Fig. 1). To elucidate which of them are utilized for attachment of N-glycans, we constructed six F protein mutants in which the asparagine of each glycosylation motif was replaced by a glutamine. Glutamine was chosen because of its similarity to asparagine. The parental and mutated F protein genes were cloned into the pTM1 plasmid, a vector designed for gene expression under the control of the T7 promoter. This plasmid also contains an internal ribosomal entry site from encephalomyocarditis virus to allow cap-independent translation of the transcripts (47). Transient expression of the F protein was achieved by transfection of BSR-T7/5 cells, a cell line that stably expresses the T7 RNA polymerase (3). In addition to undergoing transfection, the cells were infected with a recombinant vaccinia virus containing the T7 RNA polymerase gene because this procedure significantly enhanced the level of F protein expression. For analysis of the recombinant F protein, the transfected cells were metabolically labeled with [35S]methionine-[35S]cysteine and the F protein was immunoprecipitated from the cell lysates with a monoclonal antibody. Figure 2 shows an autoradiograph of the immunoprecipitates separated by Tricine-SDS-polyacrylamide gel electrophoresis under reducing conditions. The parental F protein (lane a) appeared as three dominant bands: an uncleaved precursor, F0, of about 72 kDa; a large subunit, F1, of 50 kDa; and a small subunit, F2, of 22 kDa. Both mutation N27Q (lane b) and mutation N70Q (lane c) resulted in a significant reduction of the apparent molecular mass of the F2 subunit, which appeared as a band of about 15 or 16 kDa, respectively. No differences between the electrophoretic mobility of the parental protein and those of mutants N116Q (lane e), N120Q (lane f), and N126Q (lane h) were observed. The mutation N500Q caused a shift of the F1 subunit from 50 kDa to about 46 kDa (lane i). This difference in the apparent molecular mass of the F1 subunit was more obvious when a lower polyacrylamide concentration was chosen (cf. Fig. 4). We then generated four double-site mutants and one triple-site mutant by introducing into the F gene the mutations N27Q, N70Q, and N500Q in different combinations. When the mutations N27Q and N70Q were combined, the F2 subunit of the resulting N27/70Q mutant migrated much faster in the gel than did the F2 of either of the single-site mutants (lane d). Its apparent molecular mass was estimated to be about 10 kDa. A similar additive effect was observed with the mutants N27/500Q (lane j), N70/500Q (lane k), and N27/70/500Q (lane l). The changes in the electrophoretic mobility of the F subunit are explained by the loss of one and more N-linked oligosaccharides, respectively. Our results therefore indicate that the mature HRSV F protein contains three N-linked oligosaccharides that are attached to N27 and N70 of the F2 subunit and to N500 of the F1 subunit. In addition, the elimination of any single N-linked oligosaccharide did not appear to affect proteolytic activation of the F protein. Even in the absence of all three N-glycans, cleavage of the F0 precursor into the F1 and F2 subunits was as efficient as in the parental protein.
FIG. 2.
Electrophoretic mobilities of the F protein mutants. MVA-T7-infected BSR-T7/5 cells were transfected with recombinant pTM1 plasmids (lane a, parental F; lane b, N27Q; lane c, N70Q; lane d, N27/70Q; lane e, N116Q; lane f, N120Q; lane g, N116/120Q; lane h, N126Q; lane i, N500Q; lane j, N27/500Q; lane k, N70/500Q; lane l, N27/70/500Q; and lane m, pTM1). The cells were metabolically labeled with [35S]methionine-[35S]cysteine, F protein was immunoprecipitated from the cell lysates, and the immunoprecipitates were separated by Tricine–SDS–10% polyacrylamide gel electrophoresis under reducing conditions. The relative positions of standard proteins of the indicated molecular masses (in kilodaltons) are shown on the left.
FIG. 4.
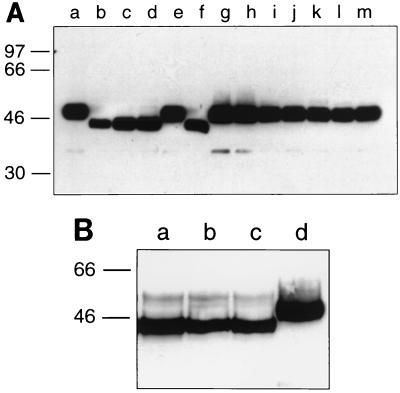
Detection of cell surface-biotinylated F protein. Transfected BSR-T7/5 cells were labeled with sulfo-NHS-biotin at 4°C, and F protein was immunoprecipitated from the cell lysates by using a monoclonal antibody. The immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis under reducing conditions, transferred to nitrocellulose membranes, and probed with streptavidin-peroxidase. (A) Lanes a and m, parental F; lane b, N27/70/500Q; lane c, N27/500Q; lane d, N70/500Q; lane e, 27/70Q; lane f, N500Q; lane g, N27Q; lane h, N70Q; lane i, N116Q; lane j, N120Q; lane k, N116/120Q; lane l, N126Q). (B) Lane a, N500A; lane b, S502A; lane c, N500Q; lane d, parental F. The relative positions of standard proteins of the indicated molecular masses (in kilodaltons) are shown on the left.
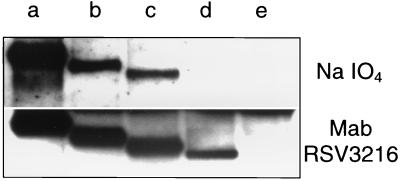
To confirm the absence of N-linked oligosaccharides in the F protein mutants, we applied an approach that involves the biotinylation of the oligosaccharides. The parental F protein and the mutants N500Q, N27/500Q, and N27/70/500Q were immunoprecipitated from transfected, unlabeled cells and treated with sodium metaperiodate. In this way, the carbohydrate portions of the proteins were oxidized to aldehyde groups that were conjugated with biotin hydrazide (11). The biotinylated carbohydrates were then detected with streptavidin-peroxidase (Fig. 3, upper panel). The carbohydrate content of the mutants was progressively reduced in the order parental F protein (lane a) > N500Q (lane b) > N27/500Q (lane c) > N27/70/500Q (lane d), with no carbohydrate being detectable on the triple mutant. As a control, an aliquot of each sample was immunostained with a monoclonal antibody directed against the F protein (lower panel). The stepwise reduction of the apparent molecular masses of the mutant proteins reflects the loss of one, two, or three N-linked oligosaccharides. The signals of the mutant F protein bands showed a somewhat lower intensity than the signal obtained from the parental protein. However, in contrast to the carbohydrate labeling approach, a protein band was detected with the triple mutant. These results indicate that the triple mutant N27/70/500Q is devoid of any N-linked carbohydrates. The presence of O-linked oligosaccharides in the F protein can also be ruled out, because the periodate oxidation approach does not discriminate between N- and O-linked carbohydrates. Thus, the results obtained by the periodate approach confirm the observation that the mature F protein contains three oligosaccharides, attached to N27, N70, and N500.
FIG. 3.
Detection of carbohydrates by periodate oxidation. Parental F protein (lane a) and mutants N500Q (lane b), N27/500Q (lane c), and N27/70/500Q (lane d) were expressed in BSR-T7/5 cells, immunoprecipitated with a monoclonal antibody, and separated by SDS-polyacrylamide gel electrophoresis. Cells transfected with a nonrecombinant vector plasmid (lane e) were used as a control. (Upper panel) The F proteins were transferred to a polyvinylidene difluoride membrane and treated with sodium metaperiodate. The oxidized carbohydrates were conjugated with biotin-hydrazide and detected with streptavidin-peroxidase. (Lower panel) The F proteins were transferred to a nitrocellulose membrane and detected by sequential incubation with a monoclonal antibody (Mab) directed against the HRSV F protein, a biotinylated anti-mouse immunoglobulin serum, and streptavidin-peroxidase.
Cell surface expression of the glycosylation mutants was analyzed using a biotinylation approach. The transfected cells were labeled at 4°C with a water-soluble biotinylation reagent prior to lysis and immunoprecipitation. This reagent does not penetrate the plasma membrane and therefore reacts only with proteins at the cell surface (38). The immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis under reducing conditions and transferred to nitrocellulose, and biotinylated F protein was stained with a streptavidin-peroxidase complex (Fig. 4A). Using this approach, the F1 subunits of the parental protein and almost all the glycosylation mutants were detected with signals of comparable intensity. The band representing the triple mutant N27/70/500 (lane b), however, appeared to be weaker than those of the other mutants (lanes c to l) and the parental protein (lanes a and m). A prolonged incubation of the cell lysates with the antibody during immunoprecipitation was found to further reduce the signal (data not shown). Probably, the elimination of all three N-glycans resulted in an increased sensitivity of the mutant to protease attack. The electrophoretic mobility of the F1 subunit was changed in the case of those mutants in which had occurred an amino acid exchange at N500, i.e., N27/70/500Q (lane b), N27/500Q (lane c), N70/500Q (lane d), and N500Q (lane f). The reduced apparent molecular mass is consistent with the absence of the N500-glycan in the respective mutants and has also been observed in radioimmunoprecipitation (cf. Fig. 2). In contrast to F1, we were not able to detect a band of biotinylated F2. When the polyacrylamide gel was run under nonreducing conditions, the biotinylated F protein migrated as a 72-kDa band (data not shown), indicating that the F1-linked F2 subunit was transported to the cell surface but was not labeled with biotin. The uncleaved precursor protein F0 that we observed in the radioimmunoprecipitation assay (cf. Fig. 2) was not detectable by the biotinylation approach. It appears that only the proteolytically cleaved fusion protein is transported to the plasma membrane.
Cell surface expression of the glycosylation mutants was also quantitatively determined by flow cytometry. Transfected cells were detached without trypsin treatment and were stained with an FITC-conjugated bovine anti-BRSV serum that recognizes HRSV F protein as well. As a negative control, cells were transfected with pTM1 plasmid and stained in the same way. Table 1 summarizes the results of the flow-cytometric analysis for three independent transfections. It shows that all of the glycosylation mutants analyzed in this experiment exhibited similar mean fluorescence intensities. The percentage of cells expressing F protein on their surfaces differed to some extent (left column). While the values for mutants N500Q, N27/500Q, and N27/70/500Q were similar to that of the parental F protein, more fluorescent cells were found in the case of mutants N27Q and N70Q, suggesting that these mutations facilitated cell surface transport. In contrast, the proportions of cells expressing the mutants N27/70Q and N70/500Q were found to be reduced to 80 and 70% of the parental F values, respectively. Taken together, cell surface biotinylation and flow cytometry provide evidence that all glycosylation mutants of the HRSV F protein are efficiently transported to the cell surface, with only slight differences in the rate of transport.
TABLE 1.
Flow-cytometric analysis of BSR-T7/5 cells expressing HRSV F protein glycosylation mutantsa
| HRSV strain | % Fluorescent cellsb | Mean fluorescence intensity (% of parental F) |
|---|---|---|
| HRSV F (parental) | 35 | 100 |
| N27Q | 49 | 113 |
| N70Q | 44 | 99 |
| N500Q | 38 | 100 |
| N27/70Q | 28 | 96 |
| N27/500Q | 36 | 108 |
| N70/500Q | 25 | 93 |
| N27/70/500Q | 32 | 96 |
BSR-T7/5 cells transfected with the pTM1 plasmid encoding either the parental HRSV F protein or one of the indicated F mutants were suspended 20 h after transfection with an FITC-conjugated polyclonal serum directed against BRSV and incubated for 30 min at 4°C. The cells (2 × 104 per analysis) were washed and analyzed by flow cytometry.
Cells with a fluorescence intensity between 1 × 101 and 2 × 103 arbitrary units were gated and used for the calculation.
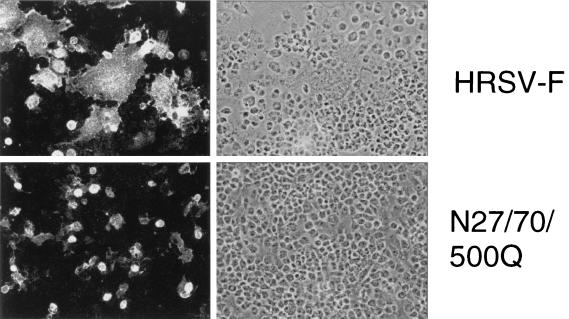
The ability of the glycosylation mutants to induce formation of syncytia in transfected cells was studied by indirect immunofluorescence analysis of nonpermeabilized cells (Fig. 5). The parental F protein induced the formation of large syncytia, showing that the recombinant protein has maintained its fusion activity. Most of the mutants formed syncytia, although clear differences were observed with regard to syncytium size and frequency (Table 2). For example, about 40% of the cells expressing the parental F protein induced formation of syncytia, with an average size of about nine nuclei per syncytium. The mutant N27Q showed the same fusion characteristics as the parental F protein, whereas the mutant N70Q had somewhat larger syncytia which occurred more frequently. About 50% of the cells expressing this mutant induced syncytium formation. When both N-glycans of the F2 subunit were removed (N27/70Q), fusion activity was impaired; the syncytium size was reduced to six nuclei, and their frequency was reduced to about 30% of the parental value. The mutant N500Q, which lacks the single N-linked oligosaccharide in the F1 subunit, was characterized by the formation of syncytia that were half the size of those induced by the parental protein. The frequency of syncytium formation was reduced even more; only 10% of the cells expressing this mutant exhibited any syncytia. The double mutant N70/500Q had fusion characteristics similar to those of N500Q, whereas a lower fusion activity was found in the case of N27/500Q. Hardly any syncytia were observed with the triple mutant N27/70/500Q. Although the N116Q, N120Q, and N126Q mutations were not found to be associated with a change in the N-glycosylation status of the F protein, only the N116Q and N120Q mutants exhibited fusion characteristics similar to those of the parental protein. The mutant N126Q, on the other hand, showed an enhanced fusion activity, with larger and more frequent syncytia. It appears that glutamine is a more favorable amino acid in this position than asparagine in terms of promoting fusion activity. To take into account both syncytium size and frequency, the product of these values was calculated and analyzed in relation to the parental fusion activity. The data demonstrate that among the three glycosylation sites that are used for attachment of N-glycans, N500 is the most important for the fusion activity of the HRSV F protein. To confirm that the phenotype associated with the N500Q mutation is due to the elimination of the N-linked oligosaccharide rather than to the amino acid exchange, we constructed and analyzed two more mutants. In the first mutant, N500 was replaced by an alanine rather than a glutamine. In the second case, a different amino acid position, S502, was mutated to an alanine. Since serine 502 is essential for N-glycosylation, this mutation should also lead to elimination of the N500-glycan. The mutants were analyzed by immunoprecipitation after lysis of transfected, cell surface-biotinylated BSR-T7/5 cells (Fig. 4B). Mutants N500A (lane a) and S502A (lane b) showed the same mobility shift as the N500Q mutant (lane c) in comparison to the parental F1 subunit, which migrated as a 50-kDa band in the reducing SDS-polyacrylamide gel (lane d), indicating that all three mutations resulted in the elimination of the N500-glycan. In addition, all mutants were efficiently transported to the cell surface, as indicated by their identical levels of biotinylation. Cell surface immunofluorescence studies revealed that both N500A and S502A mutants have a phenotype like the N500Q mutant, i.e., smaller and less frequent syncytia than the parental F protein (Table 2).
FIG. 5.
Indirect surface immunofluorescence analysis of HRSV F protein mutants. Recombinant vaccinia virus MVA-T7-infected BSR-T7/5 cells were transfected with pTM1 plasmid encoding the parental or mutant HRSV F protein as indicated. Twenty hours after transfection, the cells were fixed with 3% paraformaldehyde. F protein was visualized using a monoclonal antibody directed against the HRSV F protein and an FITC-conjugated anti-mouse immunoglobulin serum. The cells were examined at 200× magnification with a Zeiss Axioplan 2 microscope equipped for epifluorescence (left panels) and phase-contrast (right panels) studies.
TABLE 2.
Size and frequency of syncytia induced by HRSV F protein mutantsa
| HRSV strain | Sizeb | Frequencyc | Fusion indexd | Relative fusion index (%)e |
|---|---|---|---|---|
| Parental F | 8.7 | 0.39 | 3.4 | 100 |
| N27Q | 8.8 | 0.37 | 3.3 | 97 |
| N70Q | 9.4 | 0.51 | 4.8 | 141 |
| N116Q | 9.1 | 0.41 | 3.7 | 109 |
| N120Q | 8.0 | 0.33 | 2.6 | 76 |
| N126Q | 10.3 | 0.54 | 5.6 | 165 |
| N500Q | 4.3 | 0.10 | 0.4 | 12 |
| N500A | 4.6 | 0.11 | 0.5 | 15 |
| S502A | 4.6 | 0.12 | 0.6 | 17 |
| N27/70Q | 6.0 | 0.31 | 1.9 | 56 |
| N116/120Q | 8.9 | 0.44 | 3.9 | 115 |
| N27/500Q | 3.6 | 0.07 | 0.3 | 9 |
| N70/500Q | 4.1 | 0.09 | 0.4 | 12 |
| N27/70/500Q | 3.4 | 0.03 | 0.1 | 3 |
BSR-T7/5 cells transiently expressing parental or mutated HRSV F protein were analyzed by immunofluorescence (see Fig. 5). Results are mean values of data from three independently performed transfections.
The size of syncytia was determined by counting their nuclei.
The frequency of syncytium formation is the relation between the number of syncytia and the number of fluorescent cells.
The fusion index represents the product of syncytium size and syncitium frequency.
The relative fusion index was estimated by setting the fusion index of the parental F protein to 100%.
DISCUSSION
N-glycosylation and protein folding are closely interconnected processes that take place in the endoplasmic reticulum (ER). The addition of N-linked oligosaccharides occurs cotranslationally before or while the nascent polypeptide chain folds into its proper three-dimensional conformation. The folding process is assisted by a number of chaperones. Two of them, calnexin and calreticulin, are lectins that bind to almost all glycoproteins synthesized in the ER by recognizing monoglycosylated trimming intermediates (26, 67). Chaperones not only catalyze folding and assembly of polypeptides but also prevent the transport of misfolded proteins out of the ER. Thus, the transport-competent form of a protein usually corresponds to the correctly folded and processed native conformation (13). Inhibition of N-glycosylation by using the drug tunicamycin or by mutagenesis of the consensus sequence N-X-S/T has been shown to cause misfolding and aggregation of several viral glycoproteins which do not exit the ER in this form (12). In some cases, elimination of individual N-glycosylation sites leads to the generation of a temperature-sensitive phenotype: at nonpermissive temperatures, the underglycosylated glycoproteins are misfolded and retained in the ER, whereas at permissive temperatures, folding and transport are normal (16, 17, 21, 33, 43, 50). Individual N-linked oligosaccharides may differ in terms of their importance for folding of the glycoprotein in the ER, because some oligosaccharides can be eliminated with little or no consequence while others appear to be essential for folding (2, 28, 46, 50, 58, 59, 62, 64, 65). Sometimes the removal of any single N-glycan in a viral glycoprotein is well tolerated, while the elimination of more N-linked oligosaccharides often impairs or even blocks correct folding (18, 42, 43). Thus, there are many examples of viral glycoproteins that depend on N-glycosylation for proper folding in the ER. The fusion protein of HRSV (strain Long) appears to be a notable exception on this list. We have shown that the mature F protein contains three N-linked oligosaccharides and that elimination of all three does not impair transport of the protein to the cell surface. In accordance with these data, total deglycosylation of the HRSV F protein by use of the drug tunicamycin was reported to have no effect on its cell surface expression (8). The related BRSV fusion protein (from strain A51908) contains four potential N-glycosylation sites: N27, N70, N120, and N500. Replacement of the asparagine residues at N27, N70, and N120 by the amino acid alanine did not affect the transport of the BRSV F protein to the cell surface, whereas the mutant protein N500A was not detected at the cell surface (56). This indicates that the BRSV and HRSV fusion proteins, though highly homologous, have different requirements with respect to N-glycosylation.
Proteolytic activation of the F protein takes place at a stretch of basic amino acids that serve as a cleavage site for furin-like endoproteases (32). In the case of other viral glycoproteins, it has been demonstrated that N-linked oligosaccharides can affect the efficiency of this process. For example, a carbohydrate side chain near the cleavage site of the H5-subtype influenza virus hemagglutinin (HA) interfered with protease accessibility (31). The loss of this oligosaccharide resulted in enhancement of both HA cleavability and pathogenicity of the influenza virus. Interestingly, the HRSV F protein contains three potential N-glycosylation sites—N116, N120, and N126—in close proximity to the proteolytic cleavage site. However, in the mature F protein, N-glycans attached to these sites were not detected. In accordance with this observation, N120 is not conserved among different HRSV isolates and N116 and N126 are not found in the otherwise highly homologous BRSV F protein. Nevertheless, the last two potential N-glycosylation sites have been found in all HRSV F proteins sequenced so far, indicating that they may be of some importance. Our finding that N126Q is more fusogenic than the parental F protein suggests that the asparagine in this position negatively controls fusion activity.
Our results indicate that among the three N-glycans attached to the mature F protein, the N500-glycan is the most important with respect to fusion activity. All mutants in which the N500Q exchange took place showed a drastic reduction of the ability to form syncytia. The same phenotype was observed with the mutants N500A and S502A. Thus, it is more likely that the elimination of the N-glycan, rather than the particular amino acid exchange, is responsible for this effect. N500 is located within heptad repeat region B. Heptad repeats form triple-stranded coiled coils consisting of three α-helices and are common structures of the paramyxovirus, orthomyxovirus, and retrovirus fusion proteins (29, 36, 45). Heptad repeats not only are involved in the formation of the oligomers but are also of major importance for the fusion activity of these viruses (4, 29, 63). Some paramyxoviruses, such as simian virus 5, Newcastle disease virus, and mumps virus, also contain a single potential N-glycosylation site within the heptad repeat B region, whereas other members of the paramyxoviruses, such as measles virus, do not contain any N-glycosylation sites in the F1 subunit. How N-linked oligosaccharides influence the conformation and function of triple-stranded coiled coils has not been studied to date. Mutagenesis of the potential N-glycosylation sites of the simian virus 5 and BRSV F1 subunits had deleterious effects on cell surface transport, making it impossible to analyze the fusion activity of these mutants (2, 56). In contrast, the HRSV N500Q mutant was transported to the cell surface as efficiently as the parental protein. However, syncytium formation by this mutant was drastically reduced, although not totally abolished. Thus, it is possible that the N500-glycan in the stem region of the HRSV F protein is directly involved in the fusion process. Alternatively, the N-glycan may be needed to maintain a fusion-competent conformation or to allow a conformational change—for example, after binding of the F protein to the target membrane. A similar function has been proposed for the three conserved oligosaccharides located in the stem region of influenza virus HA (52). Interestingly, the fusion protein of a more distantly related member of the genus Pneumovirus, pneumonia virus of mice, contains no potential N-glycosylation sites in its F2 subunit but has two glycosylation motifs in the F1 subunit that, like N500, are located proximal to the membrane anchor (7).
In contrast to the N500-glycan, the two N-glycans located in the HRSV F2 subunit, N27 and N70, could be eliminated without reducing syncytium formation. Although the fusion activity of the mutant lacking both of these oligosaccharides (N27/70Q) was impaired, this mutant was much more fusogenic than the single-site mutant N500Q. This indicates that N-glycosylation of the F2 subunit is of minor importance for fusion activity. Nevertheless, N27 and N70 are highly conserved. N27 is found in all known HRSV and BRSV strains, and N70 exists in all HRSV strains and in most BRSV strains. This suggests that both oligosaccharides are of some functional importance. We observed that the triple mutant and, to a lesser extent, the double mutants were more sensitive to proteolytic attack than the parental protein or the single-site mutants. For that reason, flow-cytometric analysis could not be performed with cells that were suspended in a solution containing trypsin. Thus, protection from proteolytic degradation may be one function of the N-linked oligosaccharides. Another important role of the N27- and N70-glycans may be protection of the F protein from antibody recognition. Interestingly, all known antigenic epitopes on the HRSV F protein have been mapped to the F1 subunit and none has been mapped to the F2 subunit (41). It is possible that the antigenic epitopes on the F2 subunit are masked by the two oligosaccharide chains. With the availability of reverse-genetics procedures for respiratory syncytial viruses (3, 9), it should now be possible to generate HRSV mutants with F proteins devoid of individual N-glycans. It will be interesting to see whether the antigenicity of the resulting HRSV mutants is changed. In addition, recombinant viruses with F protein mutants that have reduced fusion activity are expected to be attenuated. Thus, glycosylation mutants of the RSV F protein may be an interesting new approach for the generation of live, attenuated vaccine candidates.
ACKNOWLEDGMENTS
We thank Karl-Klaus Conzelmann for providing the BSR-T7/5 cells. We also acknowledge the help of H.-Jürgen Streckert and Gerd Sutter, who provided HRSV and MVA-T7, respectively. We are grateful to Marion Heuer and Thomas Tschernig for their assistance in flow-cytometric analysis.
This work was supported by a grant from Deutsche Forschungsgemeinschaft (HE 1168/11-1/2) to G.H.
REFERENCES
- 1.Arumugham R G, Hildreth S W, Paradiso P R. Fatty acid acylation of the fusion protein of respiratory syncytial virus. J Biol Chem. 1989;264:10339–10342. [PubMed] [Google Scholar]
- 2.Bagai S, Lamb R A. Individual roles of N-linked oligosaccharide chains in intracellular transport of the paramyxovirus SV5 fusion protein. Virology. 1995;209:250–256. doi: 10.1006/viro.1995.1251. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz U J, Finke S, Conzelmann K-K. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckland R, Malvoisin E, Beauverger P, Wild F. A leucine zipper structure present in the measles virus fusion protein is not required for its tetramerization but is essential for fusion. J Gen Virol. 1992;73:1703–1707. doi: 10.1099/0022-1317-73-7-1703. [DOI] [PubMed] [Google Scholar]
- 5.Bukreyev A, Whitehead S S, Murphy B R, Collins P L. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cash P, Pringle C R, Preston C M. The polypeptides of human respiratory syncytial virus: products of cell-free protein synthesis and post-translational modifications. Virology. 1979;92:375–384. doi: 10.1016/0042-6822(79)90142-9. [DOI] [PubMed] [Google Scholar]
- 7.Chambers P, Pringle C R, Easton A J. Sequence analysis of the gene encoding the fusion glycoprotein of pneumonia virus of mice suggests possible conserved secondary structure elements in paramyxovirus fusion glycoproteins. J Gen Virol. 1992;73:1717–1724. doi: 10.1099/0022-1317-73-7-1717. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, Mottet G. Post-translational processing and oligomerisation of the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1991;72:3095–3101. doi: 10.1099/0022-1317-72-12-3095. [DOI] [PubMed] [Google Scholar]
- 9.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng R, Wang Z, Glickman R L, Iorio R M. Glycosylation within an antigenic site on the HN glycoprotein of Newcastle disease virus interferes with its role in the promotion of membrane fusion. Virology. 1994;204:17–26. doi: 10.1006/viro.1994.1506. [DOI] [PubMed] [Google Scholar]
- 11.Dennis J W, Laferte S, Waghorne C, Breitman M L, Kerbel R S. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 12.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 13.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 14.Feldman S A, Hendry R M, Beeler J A. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol. 1999;73:6610–6617. doi: 10.1128/jvi.73.8.6610-6617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman S A, Audet S, Beeler J A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol. 2000;74:6442–6447. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felkner R H, Roth M J. Mutational analysis of the N-linked glycosylation sites of the SU envelope protein of Moloney murine leukemia virus. J Virol. 1992;66:4258–4264. doi: 10.1128/jvi.66.7.4258-4264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher P, Henneberry J, Wilson I, Sambrook J, Gething M-J. Addition of carbohydrate side chains at novel sites on influenza hemagglutinin can modulate the folding, transport, and activity of the molecule. J Cell Biol. 1988;107:2059–2073. doi: 10.1083/jcb.107.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher P J, Henneberry J M, Sambrook J F, Gething M-J H. Glycosylation requirements for intracellular transport and function of the hemagglutinin of influenza virus. J Virol. 1992;66:7136–7145. doi: 10.1128/jvi.66.12.7136-7145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Beato R, Franci C, Real F X, Garcia-Barreno B, Melero J A. Host cell effect upon glycosylation and antigenicity of human respiratory syncytial virus G glycoprotein. Virology. 1996;221:301–309. doi: 10.1006/viro.1996.0379. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Beato R, Melero J A. The C-terminal third of human respiratory syncytial virus attachment (G) protein is partially resistant to protease digestion and is glycosylated in a cell-type-specific manner. J Gen Virol. 2000;81:919–927. doi: 10.1099/0022-1317-81-4-919. [DOI] [PubMed] [Google Scholar]
- 21.Gibson R, Schlesinger S, Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979;254:3600–3607. [PubMed] [Google Scholar]
- 22.Gruber C, Levine S. Respiratory syncytial virus polypeptides. IV. The oligosaccharides of the glycoproteins. J Gen Virol. 1985;66:417–432. doi: 10.1099/0022-1317-66-3-417. [DOI] [PubMed] [Google Scholar]
- 23.Gruber C, Levine S. Respiratory syncytial virus polypeptides. V. The kinetics of glycoprotein synthesis. J Gen Virol. 1985;66:1241–1247. doi: 10.1099/0022-1317-66-6-1241. [DOI] [PubMed] [Google Scholar]
- 24.Hallak L K, Collins P L, Knudson W, Peeples M E. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology. 2000;271:264–275. doi: 10.1006/viro.2000.0293. [DOI] [PubMed] [Google Scholar]
- 25.Hallak L K, Spillmann D, Collins P L, Peeples M E. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74:10508–10513. doi: 10.1128/jvi.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heminway B R, Yu Y, Tanaka Y, Perrine K G, Gustafson E, Bernstein M, Galinski M S. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology. 1994;200:801–805. doi: 10.1006/viro.1994.1245. [DOI] [PubMed] [Google Scholar]
- 28.Hu A, Cathomen T, Cattaneo R, Norrby E. Influence of N-linked oligosaccharide chains on the processing, cell surface expression and function of the measles virus fusion protein. J Gen Virol. 1995;76:705–710. doi: 10.1099/0022-1317-76-3-705. [DOI] [PubMed] [Google Scholar]
- 29.Joshi S B, Dutch R E, Lamb R A. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology. 1998;248:20–34. doi: 10.1006/viro.1998.9242. [DOI] [PubMed] [Google Scholar]
- 30.Karron R A, Buonagurio D A, Georgiu A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for virus replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaoka Y, Naeve C W, Webster R G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984;139:303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- 32.Klenk H-D, Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 33.Kotwal G J, Buller R M L, Wunner W W, Pringle C R, Gosh H P. Role of glycosylation in transport of vesicular stomatitis virus envelope glycoprotein. A new class of mutant defective in glycosylation and transport of G protein. J Biol Chem. 1986;261:8936–8943. [PubMed] [Google Scholar]
- 34.Krusat T, Streckert H-J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 35.Kyhse-Anderson J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 36.Lamb R A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 37.Lambert D M. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology. 1988;164:458–466. doi: 10.1016/0042-6822(88)90560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bivic A, Real F X, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Schulman J, Itamura S, Palese P. Glycosylation of neuraminidase determines the neurovirulence of influenza A/WSN/33 virus. J Virol. 1993;67:6667–6673. doi: 10.1128/jvi.67.11.6667-6673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez J A, Villanueva N, Melero J A, Portela A. Nucleotide sequence of the fusion and phosphoprotein genes of human respiratory syncytial (RS) virus Long strain: evidence of subtype genetic heterogeneity. Virus Res. 1988;10:249–262. doi: 10.1016/0168-1702(88)90020-2. [DOI] [PubMed] [Google Scholar]
- 41.López J A, Bustos R, Örvell C, Berois M, Arbiza J, García-Barreno B, Melero J A. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J Virol. 1998;72:6922–6928. doi: 10.1128/jvi.72.8.6922-6928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machamer C E, Florkiewicz R Z, Rose J K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985;5:3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machamer C E, Rose J K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonds. J Biol Chem. 1988;263:5955–5960. [PubMed] [Google Scholar]
- 44.Martinez I, Melero J A. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J Gen Virol. 2000;81:2715–2722. doi: 10.1099/0022-1317-81-11-2715. [DOI] [PubMed] [Google Scholar]
- 45.Matthews J M, Young T F, Tucker S P, Mackay J P. The core of the respiratory syncytial virus fusion protein is a trimeric coiled coil. J Virol. 2000;74:5911–5920. doi: 10.1128/jvi.74.13.5911-5920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGinnes L W, Morrison T G. The role of individual oligosaccharide chains in the activities of the HN glycoprotein of Newcastle disease virus. Virology. 1995;212:398–410. doi: 10.1006/viro.1995.1497. [DOI] [PubMed] [Google Scholar]
- 47.Moss B, Elroy-Stein O, Mizukami T, Alexander W A, Fuerst T R. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 48.Munk K, Pritzer E, Kretzschmar E, Gutte B, Garten W, Klenk H-D. Carbohydrate masking of an antigenic epitope of influenza virus haemagglutinin independent of oligosaccharide size. Glycobiology. 1992;2:233–240. doi: 10.1093/glycob/2.3.233. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama E E, Shioda T, Tatsumi M, Xin X, Yu D, Ohgimoto S, Kato A, Sakai Y, Ohnishi Y, Nagai Y. Importance of the N-glycan in the V3 loop of HIV-1 envelope protein for CXCR-4- but not CCR-5-dependent fusion. FEBS Lett. 1998;426:367–372. doi: 10.1016/s0014-5793(98)00375-5. [DOI] [PubMed] [Google Scholar]
- 50.Ng D T W, Hiebert S W, Lamb R A. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol Cell Biol. 1990;10:1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohuchi M, Ohuchi R, Feldmann A, Klenk H-D. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J Virol. 1997;71:8377–8384. doi: 10.1128/jvi.71.11.8377-8384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohuchi R, Ohuchi M, Garten W, Klenk H-D. Oligosaccharides in the stem region maintain the influenza virus hemagglutinin in the metastable form required for fusion activity. J Virol. 1997;71:3719–3725. doi: 10.1128/jvi.71.5.3719-3725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olmsted R A, Elango N, Prince G, Murphy B R, Johnson P R, Moss B, Chanock R M, Collins P L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contribution of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papandreou J M, Fenouillet E. Effect of various glycosidase treatments on the resistance of the HIV-1 envelope to degradation. FEBS Lett. 1997;406:191–195. doi: 10.1016/s0014-5793(97)00273-1. [DOI] [PubMed] [Google Scholar]
- 55.Pastey K M, Samal S K. Analysis of bovine respiratory syncytial virus envelope glycoproteins in cell fusion. J Gen Virol. 1997;78:1885–1889. doi: 10.1099/0022-1317-78-8-1885. [DOI] [PubMed] [Google Scholar]
- 56.Pastey K M, Samal S K. Role of individual N-linked oligosaccharide chains and different regions of bovine respiratory syncytial virus fusion protein in cell surface transport. Arch Virol. 1997;142:2309–2320. doi: 10.1007/s007050050245. [DOI] [PubMed] [Google Scholar]
- 57.Pemberton R M, Cannon M J, Openshaw P J, Ball L A, Wertz G W, Askonas B A. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987;68:2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- 58.Pique C, Pham D, Tursz T, Dokhélar M-C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992;66:906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts S R, DeLeon M P, Cohen G H, Eisenberg R J. Analysis of the intracellular maturation of the herpes simplex virus type 1 glycoprotein gH in infected and transfected cells. Virology. 1991;184:609–624. doi: 10.1016/0042-6822(91)90431-a. [DOI] [PubMed] [Google Scholar]
- 60.Saito T, Kawano K. Loss of glycosylation at Asn144 alters the substrate preference of the N8 influenza A virus neuraminidase. J Vet Med Sci. 1997;59:923–926. doi: 10.1292/jvms.59.923. [DOI] [PubMed] [Google Scholar]
- 61.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 62.Segawa H, Yamashita T, Kawakita M, Taira H. Functional analysis of the individual oligosaccharide chains of Sendai virus fusion protein. J Biochem (Tokyo) 2000;128:65–72. doi: 10.1093/oxfordjournals.jbchem.a022731. [DOI] [PubMed] [Google Scholar]
- 63.Sergel-Germano T, McQuain C, Morrison T. Mutations in the fusion peptide and heptad repeat regions of the Newcastle disease virus fusion protein block fusion. J Virol. 1994;68:7654–7658. doi: 10.1128/jvi.68.11.7654-7658.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shakin-Eshleman S H, Remaley A T, Eshleman J R, Wunner W H, Spitalnik S L. N-linked glycosylation of rabies virus glycoprotein: individual sequons differ in their glycosylation efficiencies and influence on cell-surface expression. J Biol Chem. 1992;267:10690–10698. [PubMed] [Google Scholar]
- 65.Sodora D L, Cohen G H, Muggeridge M I, Eisenberg R J. Absence of asparagine-linked oligosaccharides from glycoprotein D of herpes simplex virus type 1 results in a structurally altered but biologically active protein. J Virol. 1991;65:4424–4431. doi: 10.1128/jvi.65.8.4424-4431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sutter G, Ohlmann M, Erfle V. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 1995;371:9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]
- 67.Trombetta E S, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 68.Wagner R, Wolff T, Herwig A, Pleschka S, Klenk H-D. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J Virol. 2000;74:6316–6323. doi: 10.1128/jvi.74.14.6316-6323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wertz G W, Stott E J, Young K K Y, Anderson K, Ball L A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987;61:293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitehead S S, Bukreyev A, Teng M N, Firestone C-Y, St. Claire M, Elkins W R, Collins P L, Murphy B R. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73:3438–3442. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmer G, Klenk H-D, Herrler G. Identification of a 40-kDa cell surface sialoglycoprotein with the characteristics of a major influenza C virus receptor in a Madin-Darby canine kidney cell line. J Biol Chem. 1995;270:17815–17822. doi: 10.1074/jbc.270.30.17815. [DOI] [PubMed] [Google Scholar]