Abstract
Certain human papillomaviruses (HPVs) cause most cervical cancer, which remains a significant source of morbidity and mortality among women worldwide. HPV recombinant virus-like particles (VLPs) are promising vaccine candidates for controlling anogenital HPV disease and are now being evaluated as a parenteral vaccine modality in human subjects. Vaccines formulated for injection generally are more costly, more difficult to administer, and less acceptable to recipients than are mucosally administered vaccines. Since oral delivery represents an attractive alternative to parenteral injection for large-scale human vaccination, the oral immunogenicity of HPV type 11 (HPV-11) VLPs in mice was previously investigated; it was found that a modest systemic neutralizing antibody response was induced (R. C. Rose, C. Lane, S. Wilson, J. A. Suzich, E. Rybicki, and A. L. Williamson, Vaccine 17:2129–2135, 1999). Here we examine whether VLPs of other genotypes may also be immunogenic when administered orally and whether mucosal adjuvants can be used to enhance VLP oral immunogenicity. We show that HPV-16 and HPV-18 VLPs are immunogenic when administered orally and that oral coadministration of these antigens with Escherichia coli heat-labile enterotoxin (LT) mutant R192G (LT R192G) or CpG DNA can significantly improve anti-VLP humoral responses in peripheral blood and in genital mucosal secretions. Our results also suggest that LT R192G may be superior to CpG DNA in this ability. These findings support the concept of oral immunization against anogenital HPV disease and suggest that clinical studies involving this approach may be warranted.
Papillomaviruses are small DNA viruses that infect vertebrate hosts, including humans, and cause the formation of hyperproliferative epithelial lesions (41). More than 80 human papillomaviruses (HPVs) have been identified and classified on the basis of genetic sequence differences (12). Approximately half of HPVs tend to infect cutaneous skin and usually cause only benign disease (e.g., plantar or common warts), while others more often infect oral or anogenital mucosal epithelium (3). Certain mucosal HPVs, including type 16 (HPV-16), HPV-18, HPV-31, HPV-45, and a few others, are known to cause most cervical cancers (48). Worldwide, cancer of the cervix is the second leading cause of cancer death in women (behind breast cancer) and is the most common form of cancer among women in developing countries (4), with an estimated 500,000 cases diagnosed each year, resulting in over 200,000 deaths annually (49). Other consequences of mucosal HPV infection include condyloma acuminatum (i.e., benign anogenital warts) and recurrent respiratory papillomatosis, which are caused primarily by HPV-6 and HPV-11 (3). These and other clinical associations have generated great interest in the development of vaccines capable of preventing HPV infection.
HPV is difficult to study because the virus cannot be grown efficiently in cell culture. The virion consists of a circular double-stranded DNA genome of about 8,000 bp contained within a nonenveloped capsid consisting of major (L1) and minor (L2) structural proteins. When expressed in a recombinant system, L1 self-assembles in the absence of L2 into noninfectious virus-like particles (VLPs), which replicate virion morphology and antigenicity (18, 21, 36). Several groups of investigators have contributed to the development of a rationale for testing VLPs in human volunteers for immunoprophylactic efficacy against anogenital HPV disease. It has been shown, for example, that VLPs of genital HPVs induce antibodies that efficiently neutralize infectious genital HPV virions (10, 38, 47) and that genotype-dependent L1 amino acid sequence variation determines serotype specificity (15, 33–35, 47). Importantly, immunization with VLPs of animal papillomaviruses has been shown to confer protection from experimental challenge in relevant animal models (5, 22, 42). Protection against challenge has also been achieved by passive transfer of VLP postimmune serum to naive animals (42), suggesting that immunity from infection may be antibody mediated.
Most cases of oncogenic HPV infection are sexually transmitted; therefore, protection from infection may depend to some extent on immunity acting at genital mucosal surfaces (28). Mucosal routes of immunization generally are superior to parenteral routes for the induction of mucosal responses (26). Adjuvants are usually required, however, to boost mucosal responses and to prevent the induction of tolerance (26). Cholera toxin (CT), Escherichia coli heat-labile enterotoxin (LT), and their genetically detoxified derivatives are promising mucosal adjuvants for coadministered protein antigens (8). Mutants of LT have been developed in an effort to dissociate adjuvanticity from toxicity. One of these, designated LT R192G, was constructed by site-directed mutagenesis to introduce a single amino acid substitution into the active subunit (13). This mutation rendered the toxin insensitive to trypsin activation and thus greatly diminished toxicity without altering the adjuvanticity of the native molecule. Several recent studies have evaluated LT R192G and found it to be an effective mucosal adjuvant (7, 9, 17, 31). Synthetic oligodeoxynucleotides containing unmethylated CpG dinucleotide motifs (CpG DNA) provide another promising mucosal adjuvant (23, 27). Accumulating evidence has indicated that CpG DNA has potent immunostimulatory properties (23, 25, 45). While the mechanism of action is unclear at present, recent evidence has suggested that CpG DNA acts through a mammalian Toll-like receptor that may have evolved for the purpose of distinguishing between bacterial and mammalian DNA (19).
The induction of a modest systemic neutralizing response in mice immunized orally with HPV-11 VLPs without adjuvant was previously reported (37). Here we investigate whether coadministration of VLPs with the mucosal adjuvant LT R192G or CpG DNA can improve VLP oral immunogenicity.
MATERIALS AND METHODS
Animals.
Female Swiss-Webster mice were obtained from Taconic Laboratories (Germantown, N.Y.). Female BALB/c mice were obtained from Harlan, Inc. (Indianapolis, Ind.). Mice were used at ages ranging from 8 to 12 weeks. All animals were housed and used in accordance with institutional guidelines.
Antigens.
Purified baculovirus-expressed HPV-16 and HPV-18 L1 VLPs were provided by MedImmune, Inc., Gaithersburg, Md., and were produced essentially as described previously (35, 36).
Adjuvants.
E. coli LT R192G was produced as previously described (13) and reconstituted in sterile phosphate-buffered saline (PBS) (1 mg/ml) prior to use. CpG DNA (CpG ODN 1826; 5′-TCCATGACGTTCCTGACGTT-3′) (46) was synthesized with a nuclease-resistant phosphorothioate backbone (Synthetic Genetics, San Diego, Calif.) and resuspended in sterile PBS (1 mg/ml) prior to use.
Immunizations.
To compare the relative immunogenicities of VLPs administered parenterally versus orally, mice were immunized with 0.3 μg of HPV-16 or HPV-18 VLPs by intramuscular injection or with 1, 3, or 9 μg of VLPs by oral gavage as previously described (37). In other experiments, animals were immunized orally with VLP antigens at a single dose level (10 μg), with or without LT R192G (10 μg) or CpG DNA (10 μg).
ELISA.
Pre- and postimmune sera were obtained by retrobulbar collection; vaginal washes were collected with a pipette (0.1 ml of sterile PBS). VLP antibody levels were measured with an enzyme-linked immunosorbent assay (ELISA) as previously described (37). Briefly, Nunc MaxiSorp (Nalgene, Roskilde, Denmark) 96-well microtiter plates were coated with 0.1 μg of antigen in PBS per well, incubated at 4°C overnight, blocked with 2% bovine serum albumin (BSA) (diluent-blocking solution; Kirkegaard & Perry [K&P] Laboratories, Gaithersburg, Md.), and washed four times with 0.05% Tween 20 in PBS. Test sera were diluted 1:50 in BSA diluent-blocking solution, serially diluted twofold down the microtiter plate, and incubated for 90 min at room temperature to permit antibody binding. Control mouse sera with known HPV-16 or HPV-18 VLP antibody titers were added to each plate. Plates were washed as before and reacted for 90 min at room temperature with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) (Southern Biotechnology Associates, Inc., Birmingham, Ala.) diluted 1:5,000 in BSA diluent-blocking solution. IgA antibody levels were determined with goat anti-mouse IgA (Southern Biotechnology Associates, Inc.) as the secondary antibody as previously described (16, 37). The reaction was developed with 100 μl of substrate (p-nitrophenyl phosphate; Sigma Chemical Co., Inc., St. Louis, Mo.) per well for 1 h. Absorbance measurements were obtained at 405 nm using an automated plate reader. End-point titers were calculated as the reciprocal of the highest serum dilution with an absorbance value greater than twice the background absorbance value of each serum sample tested. Nonresponder serum samples were assigned a value of one-half the lowest serum dilution tested.
Serum IgG subclass ELISA.
Mouse IgG subclass standards and corresponding alkaline phosphatase-conjugated antibodies were obtained from Southern Biotechnology Associates. The specificity, sensitivity, and optimal dilution of each reagent were confirmed prior to use. Serial twofold dilutions of test sera were prepared with diluent-blocking solution, added to VLP-coated wells, and incubated for 90 min at room temperature. After four washes, IgG subclass-specific conjugated antibodies were added to plates and incubated for 90 min at room temperature. End-point titers were determined as described above.
Vaginal wash ELISA.
Pre- and postimmune vaginal wash specimens were diluted 1:16 in PBS, added to VLP-coated wells, serially diluted down the microtiter plate, and reacted for 90 min at room temperature. After four washes, 100 μl of alkaline phosphatase-conjugated goat anti-mouse IgG or alkaline phosphatase-conjugated goat anti-mouse IgA (Southern Biotechnology Associates) diluted 1:5,000 in diluent-blocking solution was added to each well in alternate rows of the plate. Conjugated antibodies were incubated for 90 min at room temperature, and levels of VLP-specific vaginal IgG and IgA antibodies were determined by measuring the absorbance at 405 nm. Additional evaluations of the same specimens indicated that concentrations of total IgA were essentially equivalent among groups, with only minor variations (P = 0.80) (data not shown).
Evaluation of VLP polyclonal antibody specificities.
VLP postimmune sera were tested with an ELISA against either native or denatured VLPs of either HPV-16 or HPV-18 as previously described (37). Postimmune sera were also tested with a VLP binding inhibition assay against previously characterized virus-neutralizing polyclonal antibodies as previously described (15).
Preparation of lymphoid cell suspensions.
Single-cell suspensions were obtained from the spleen by gently pressing the organ between two sterile frosted end slides; dissociated cells were then washed into a 60-mm plate containing 5% fetal bovine serum-buffered salt solution (Sigma). Mesenteric and inguinal lymph nodes and Peyer's patches were isolated by careful excision and processed as described above. Cell viability was determined by trypan blue exclusion, and cells were diluted to a density of 2 × 106/ml in complete medium.
Lymphoproliferation assay.
Single-cell suspensions were prepared as described above and plated (2 × 105 cells/well) in a 96-well flat-bottom plate (Costar, Corning, N.Y.) with or without antigen. Test (i.e., stimulated) wells contained the same antigen as that used for immunization at one of three dose levels (i.e., 0.03, 0.3, or 3.0 μg per well); control (unstimulated) wells did not contain antigen. Cultures were maintained in a final volume of 200 μl at 37°C in 5% CO2 for 96 h. The plate was pulsed with l μCi of 3H-thymidine (Amersham Pharmacia Biotech, Piscataway, N.J.) for the final 20 h of incubation. Cultures were harvested using a Packard (Meriden, Conn.) harvester, and incorporated radioactivity was quantitated with a Packard Matrix 96 direct beta counter. Results from triplicate wells were averaged and used to calculate a stimulation index: mean counts per minute of stimulated cells divided by mean counts per minute of unstimulated cells.
Statistical analysis.
A nonparametric (Kruskal-Wallis) rank sum test was used for one-way analysis of variance, followed by a multiple-comparison procedure (Dunn's test) to compare antibody titers between groups. Nonresponders were included in all calculations. Statistical significance was assessed at a P value of <0.05 for all comparisons.
RESULTS
VLP systemic antibody responses.
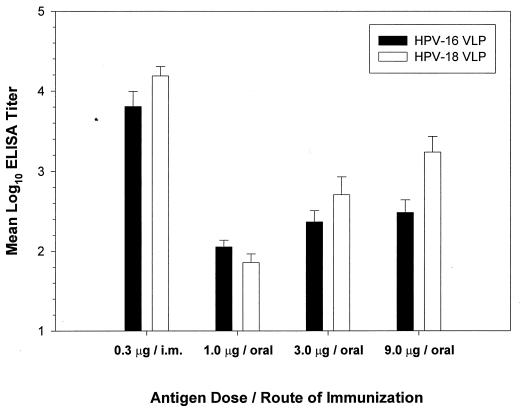
It was previously found that a 100-μg oral dose of HPV-11 VLPs was adequate to induce a serum neutralizing response (37). To characterize further the oral dose response to VLPs, purified VLPs (1, 3, or 9 μg of VLP antigen) of HPV-16 or HPV-18 in 0.1 ml of PBS were administered orally to groups of female Swiss-Webster mice (nine per group). For comparison, other mice received 0.3 μg of the same immunogens by intramuscular injection (nine per group). Boosting was done 2 and 6 weeks after primary immunizations. Consistent with previous results (37), HPV-16 and HPV-18 VLPs were immunogenic when administered orally without adjuvant and induced dose-dependent antibody responses, with ELISA end-point titers of 10−2 to 10−3 (Fig. 1). However, parenteral injection of a smaller amount (i.e., 0.3 μg) of the same immunogens elicited titers (>104) relatively higher than those induced by oral immunization. Consistent with other reported results (24), in this study parenteral administration of VLPs without adjuvant was much (>100-fold) more efficient than oral immunization for the induction of anti-VLP humoral responses.
FIG. 1.
Dose-dependent antibody responses after VLP oral immunization. VLPs of HPV-16 or HPV-18 were administered to nine female Swiss-Webster mice per group. Sera were collected 12 weeks after primary immunization and evaluated in a VLP ELISA against the same antigens used for immunization. Data are reported as mean log10 ELISA titers plus standard errors of the means. i.m., intramuscular.
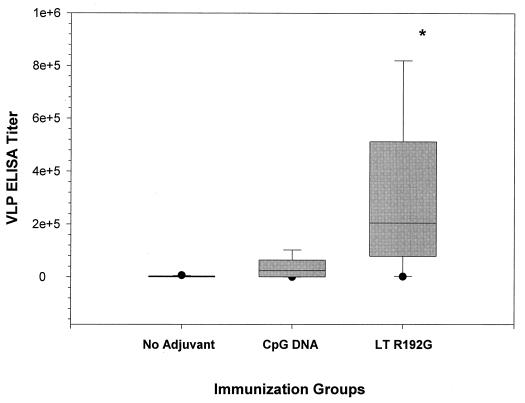
To evaluate whether adjuvant use could enhance VLP oral immunogenicity, we next immunized mice (female Swiss-Webster; nine per group) orally with HPV-16 VLPs at a single dose (10 μg), alone or in combination with LT R192G (10 μg) or CpG DNA (10 μg). Boosting was done at 2 and 6 weeks after primary immunizations. Sera were collected 2 weeks after the second boost and evaluated with an ELISA for anti-VLP antibodies. Mice immunized orally with VLPs in combination with LT R192G had serum IgG titers that were significantly higher (median, 204,800; interquartile range, 78,400 to 512,000) than the titers induced by VLPs alone (median, 1,600; interquartile range, 88 to 3,200) (P < 0.05). Titers obtained in mice immunized with VLPs in combination with CpG DNA (median, 25,600; interquartile range, 400 to 64,000) were not significantly different from those of either the control or the LT R192G adjuvant group (Fig. 2).
FIG. 2.
Serum IgG antibody responses in outbred mice. Nine female Swiss-Webster mice per group were immunized orally as described in Materials and Methods. Sera were collected 2 weeks after the second booster immunization, and ELISA end-point titers were determined. In the box plot analysis shown, each box includes the middle 50% of values, and the horizontal bar within represents the median end-point titer. The short horizontal lines at the ends of the vertical lines extending above and below the boxes are the inner fences (43). The asterisk indicates a P value of <0.05.
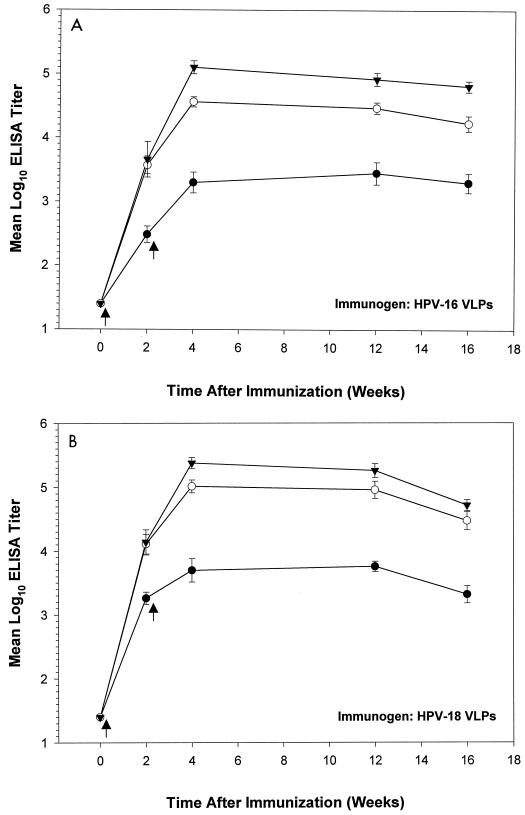
Next, BALB/c mice were used to evaluate the kinetics of anti-VLP serum antibody responses after oral immunization. In this experiment, animals received a single booster inoculation 2 weeks after primary immunizations. The results (Fig. 3) indicated that oral administration of VLPs in combination with either adjuvant significantly increased serum IgG titers over those obtained with VLPs alone (the P value was <0.05 for each group receiving antigen plus adjuvant in comparison with the control [no adjuvant] group at each time point after primary immunizations). Four months after primary immunizations, VLP serum IgG and IgA ELISA titers in all antigen-adjuvant groups were significantly higher than titers in corresponding control groups (Table 1).
FIG. 3.
Kinetics of serum IgG responses after oral administration of VLPs with and without adjuvants. Ten female BALB/c mice per group were immunized orally with HPV-16 (A) or HPV-18 (B) VLPs either alone (filled circles) or with CpG DNA (open circles) or LT R192G (filled triangles). Pre- and postimmune sera were collected at the indicated times and evaluated in an ELISA. Arrows denote immunization times. Group mean end-point titers were calculated from log10-transformed values. Bars indicate standard errors of the means.
TABLE 1.
Serum antibody titers in BALB/c mice 16 weeks postimmunizationa
| Antibody | Immunogen | Adjuvant | Titer
|
|
|---|---|---|---|---|
| Median | Interquartile range | |||
| IgG | HPV-16 VLPs | None | 1,200 | 800–6,400 |
| CpG DNA | 12,800 | 6,400–51,200 | ||
| LT R192G | 102,400 | 44,800–102,400 | ||
| HPV-18 VLPs | None | 1,600 | 800–5,600 | |
| CpG DNA | 38,400 | 12,800–51,200 | ||
| LT R192G | 51,200 | 25,600–102,400 | ||
| IgA | HPV-16 VLPs | None | 100 | 100–200 |
| CpG DNA | 1,200 | 800–3,200 | ||
| LT R192G | 3,200 | 3,200–3,200 | ||
| HPV-18 VLPs | None | 400 | 400–700 | |
| CpG DNA | 3,200 | 3,200–3,200 | ||
| LT R192G | 3,200 | 3,200–3,200 | ||
The P value was <0.05 for all comparisons with control groups.
Serum IgG subclass analysis.
Sera from mice that were positive for VLP-specific IgG were evaluated for the presence of anti-VLP IgG1 and IgG2a antibodies (Table 2). The use of LT R192G was associated with the induction of IgG1 and IgG2a, whereas in animals immunized with CpG DNA, a more Th1-like (IgG2a) pattern emerged (Table 2).
TABLE 2.
Serum antibody isotype analysis
| Immunogen (ELISA antigen) | Adjuvant | Geometric mean titera:
|
|
|---|---|---|---|
| IgG1 | IgG2a | ||
| HPV-16 VLPs | None | 459 | 746 |
| CpG DNA | 7,352 | 19,401 | |
| LT R192G | 51,200 | 47,771 | |
| HPV-18 VLPs | None | 429 | 1,493 |
| CpG DNA | 7,352 | 47,771 | |
| LT R192G | 29,407 | 95,543 | |
Ten mice per group.
VLP mucosal antibody responses.
To determine whether oral immunization could induce anti-VLP humoral responses in genital mucosal secretions, vaginal wash specimens collected from the same mice were tested with an ELISA (Table 3). BALB/c mice immunized orally with HPV-16 VLPs in combination with LT R192G demonstrated significantly higher titers of anti-VLP IgG and IgA antibodies than were obtained with VLPs administered either alone or in combination with CpG DNA (Table 3). Similar results were obtained with HPV-18 VLPs; however, in this instance, the use of either adjuvant significantly enhanced IgA responses over responses in control animals (Table 3). Differences between groups that received VLPs in combination with LT R192G versus CpG DNA were not significant. A similar evaluation of specimens collected from parenterally immunized animals (from the experiment depicted in Fig. 1) revealed the presence of anti-VLP IgG but not IgA antibodies in genital mucosal secretions (data not shown).
TABLE 3.
Mucosal antibody analysis
| Immunogen (ELISA antigen) | Antibody | Adjuvant | Titer
|
P < 0.05a | |
|---|---|---|---|---|---|
| Median | Interquartile range | ||||
| HPV-16 VLPs | IgA | None | 12 | 12–12 | |
| CpG DNA | 36 | 12–192 | No | ||
| LT R192G | 288 | 48–384 | Yes | ||
| IgG | None | 12 | 12–12 | ||
| CpG DNA | 18 | 12–48 | No | ||
| LT R192G | 24 | 12–48 | Yes | ||
| HPV-18 VLPs | IgA | None | 12 | 12–21 | |
| CpG DNA | 192 | 48–192 | Yes | ||
| LT R192G | 576 | 384–768 | Yes | ||
| IgG | None | 12 | 12–12 | ||
| CpG DNA | 24 | 24–48 | No | ||
| LT R192G | 48 | 24–48 | Yes | ||
For comparisons with control groups.
Evaluation of VLP polyclonal antibody specificities.
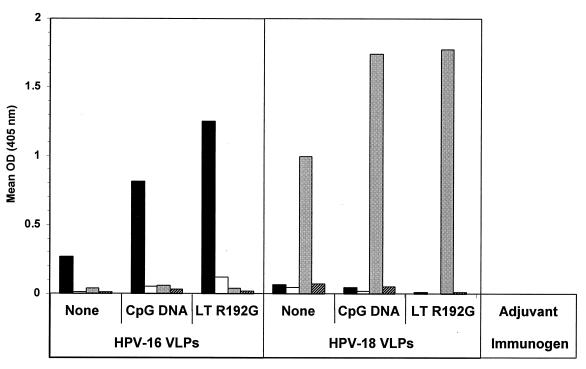
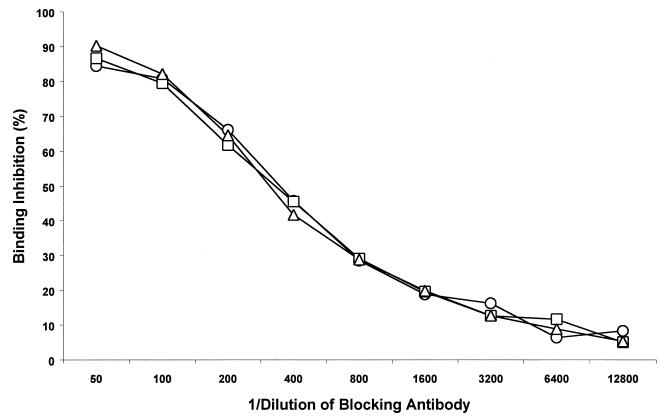
VLP-induced virus-neutralizing polyclonal antibody specificities characteristically exhibit the properties of conformational dependence and virus genotype specificity (40). To examine whether coadministered adjuvants may alter VLP polyclonal antibody specificities, we evaluated VLP postimmune polyclonal sera for conformational dependence, HPV genotype specificity, and susceptibility to VLP binding inhibition by previously characterized neutralizing polyclonal antibodies essentially as previously described (15). A subset of the sera tested above (Fig. 3) was further examined with an ELISA against native and denatured VLPs of HPV-16 or HPV-18. The results indicated that the coadministration of VLPs with either CpG DNA or LT R192G had little effect on the conformational dependence or genotype specificity of the responses (Fig. 4). Moreover, in a VLP binding inhibition assay (15), there were no discernible differences in the ability of a previously characterized HPV-16 virion-neutralizing polyclonal antiserum (47) to inhibit HPV-16 VLP binding by postimmune sera from animals immunized with VLPs alone or in combination with CpG DNA or LT R192G (Fig. 5). Thus, properties characteristically associated with virus-neutralizing antibody specificities appeared to be unaltered by these adjuvants.
FIG. 4.
Conformational dependence and type specificity of orally induced VLP serum IgG. BALB/c mice were immunized as described in Materials and Methods, and a subset of three mice from each group was tested in a VLP ELISA against native HPV-16 VLPs (black bars), denatured HPV-16 VLPs (white bars), native HPV-18 VLPs (gray bars), or denatured HPV-18 VLPs (hatched bars). OD, optical density.
FIG. 5.
VLP binding inhibition assay. BALB/c mice were immunized as described in Materials and Methods. Two serum samples from each immunization group were tested in a VLP binding inhibition ELISA (see Materials and Methods for details) using as a blocking antibody a previously described rabbit polyclonal antiserum (R-079) that efficiently neutralizes HPV-16 virions (47). Mice were immunized orally with either HPV-16 VLPs alone (circles), HPV-16 VLPs in combination with CpG DNA (squares), or HPV-16 VLPs in combination with LT R192G (triangles).
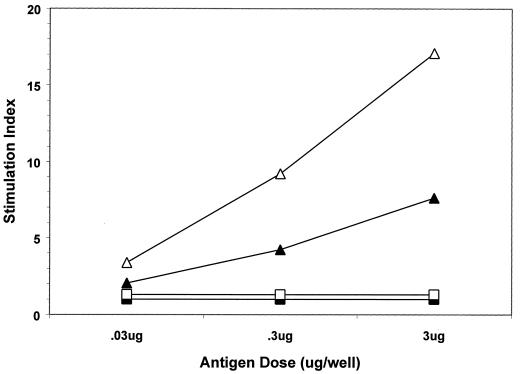
Lymphoproliferative responses.
Peyer's patches, spleens, and inguinal lymph nodes were recovered from mice at 1 and 10 weeks after boosting with VLPs with or without adjuvants. Immune cells were dissociated, restimulated in vitro with increasing amounts of the same antigen as that used for immunization, and then evaluated for proliferation by 3H-thymidine incorporation. Mice that received VLPs in combination with LT R192G demonstrated strong proliferative responses in gut-associated lymphoid tissues (GALT) (i.e., mesenteric lymph nodes, Peyer's patches, and spleens) (Fig. 6 and data not shown). Lymphocytes recovered from non-GALT organs (i.e., inguinal lymph nodes) did not respond to antigen restimulation (data not shown). Only minimal proliferative responses were detected in lymphocytes recovered from animals immunized with VLPs alone (data not shown) or in combination with CpG DNA (Fig. 6). These results confirmed VLP oral delivery to GALT and provided additional evidence that, in these experiments, the adjuvant effect of LT R192G was relatively greater than that of CpG DNA.
FIG. 6.
Mesenteric lymphoproliferative responses after oral administration of VLPs with adjuvant. Mice were immunized and boosted with HPV-16 VLPs in combination with CpG DNA (squares) or LT (R192G) (triangles) as described in Materials and Methods. At 1 week (open symbols) or 10 weeks (filled symbols) after boosting, three mice from each group were sacrificed; gut mesenteric lymphocytes were isolated in vitro and then restimulated with the same antigens as those used for immunization in the amounts indicated. Mesenteric lymphocytes recovered from mice immunized with VLPs alone were not responsive to restimulation (data not shown).
DISCUSSION
HPV VLP oral immunogenicity in mice was assessed with and without mucosal adjuvants. Consistent with previous results (37), VLPs of oncogenic anogenital HPV-16 and HPV-18 were immunogenic when administered orally and induced type-specific IgG and IgA antibody responses in serum and in genital mucosal secretions. Adjuvant use significantly enhanced these responses, and the overall effect of LT R192G on humoral and cellular responses was found to be greater than that of CpG DNA. VLP-adjuvant administration elicited high (104 to 105) and durable (>4 months) humoral responses that were 2 to 3 orders of magnitude higher than responses generated without adjuvant. Strong HPV-16 and HPV-18 anti-VLP responses in BALB/c mice were achieved after two immunizations, whereas a third immunization was required to generate comparable responses in outbred (i.e., Swiss-Webster) mice. LT R192G significantly enhanced anti-VLP IgA responses in mucosal secretions and, to a lesser extent, CpG DNA also enhanced these responses. Antibody isotype analysis revealed that LT R192G was able to enhance the production of both IgG1 and IgG2a antibodies in serum, whereas CpG DNA primarily elicited a more Th1-like (IgG2a) response. Consistent with a somewhat greater ability to enhance humoral responses, the use of the LT R192G mutant was also associated with antigen dose-dependent proliferative responses in mesenteric lymphoid tissues. This result also served to confirm that orally administered VLPs were being delivered to intestinal mucosal immune inductive sites.
Titers elicited by VLPs in combination with either adjuvant were found to exceed titers induced by parenteral vaccination with the same immunogens. The parenterally induced titers depicted in Fig. 1 were elicited with a relatively smaller amount of antigen (0.3 μg); nevertheless, these observations support the potential feasibility of oral immunization against anogenital HPV disease. Although virion neutralization assays were not performed in the present study, the work of several groups has consistently indicated a correlation between VLP ELISA titers and virion-pseudovirion neutralization titers in vitro (2, 33, 37, 39, 44, 47) and in vivo (6, 11, 38). Consequently, the VLP ELISA is now regarded as a good surrogate assay for the detection of virus-neutralizing activity (40). The overall results indicate that VLPs are efficient oral immunogens when coadministered with a potent mucosal adjuvant and suggest furthermore that VLP oral immunization can induce potentially protective immune responses at a level that may prove to be efficacious for controlling anogenital HPV disease.
We previously (37) reported the initial induction of a Th1-like (IgG2a) response in BALB/c mice after oral immunization with HPV-11 VLPs without adjuvant but found that IgG1 antibody titers soon became comparable in magnitude (i.e., within 8 weeks following primary immunizations). Here we obtained a similar antibody profile following coadministration of VLPs with LT R192G. In contrast, and consistent with results reported elsewhere (25), coadministration of VLPs with CpG DNA induced a more Th1-like (IgG2a) response. In the context of anogenital HPV disease, potential advantages or disadvantages of such adjuvant properties are not currently known.
Preclinical studies of VLPs using alternate immunization routes have been limited (2, 14, 24, 29, 30, 37), and an optimal method for mucosal administration has not yet been defined. Other groups investigating alternate immunization strategies have reported results similar to the present results. Induction of anti-VLP serum IgG and vaginal IgA antibody responses has been demonstrated, for example, following intranasal immunization of mice with HPV-16 VLPs coformulated with CT (2, 14). Balmelli et al. (2) found that VLPs were immunogenic when administered intranasally but were poorly immunogenic when administered orally with or without CT (2). Similarly, Dupuy et al. (14) reported anti-HPV-16 VLP serum IgG titers of greater than 104 and vaginal IgA titers of greater than 102 after intranasal administration of VLPs with CT, whereas VLPs administered intranasally without CT were only poorly immunogenic (14). Taken together, the present and previous results (37) indicate that VLPs are good oral immunogens when coadministered with the adjuvants used in the present study (i.e., LT R192G or CpG DNA). Differences in adjuvants used, immunization methods, dosage levels, or sources of antigens or adjuvants may account for the observed discrepancies.
Observed differences in the magnitudes of the immune responses elicited after immunizations with HPV-16 versus HPV-18 capsids suggest the possibility of inherent immunogenic differences between these genotypes (e.g., compare Fig. 3A and B). While it may be interesting to consider how such differences might affect the relative prevalence of one genotype versus another, similar evaluations of alternate lots of immunogens will be required to rule out the possibility that the observed differences instead merely reflect slight variations in preparative methods.
Vaccines represent the most efficient and cost-effective means of preventing disease; however, the full potential of vaccination to improve public health is not yet realized (20). Oral vaccines offer practical and financial advantages over parenterally administered vaccines. From a practical standpoint, mucosal vaccines are easier to administer and less invasive than parenteral vaccines and thus are more likely to facilitate mass vaccination programs in underdeveloped regions. The development of needle-free vaccines has a high priority, in part due to the recognition that blood-borne diseases are often transmitted through the reuse of needles (1, 32). The relative simplicity of oral immunization could very well facilitate vaccine distribution in developing regions, which bear the brunt of genital HPV disease (4).
ACKNOWLEDGMENTS
We thank J. Suzich (MedImmune, Inc., Gaithersburg, Md.) for kindly providing VLPs for these studies. We also thank J. Frelinger (University of Rochester) for helpful comments concerning the manuscript.
This work was supported by grants (to R.C.R.) from the American Cancer Society (RPG-99-265-01-MBC) and the NIH (CA 84105-01).
REFERENCES
- 1.Aylward B, Lloyd J, Zaffran M, McNair-Scott R, Evans P. Reducing the risk of unsafe injections in immunization programmes: financial and operational implications of various injection technologies. Bull W H O. 1995;73:531–540. [PMC free article] [PubMed] [Google Scholar]
- 2.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnez W. Papillomavirus. In: Richman D D, Whitley R J, Hayden F G, editors. Clinical virology. New York, N.Y: Churchill Livingstone; 1997. pp. 569–611. [Google Scholar]
- 4.Bosch F X, Manos M M, Munoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 5.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan J T, Jansen K U, Lowe R S, Fife K H, McClowry T, Glass D, Brown D R. Human papillomavirus type 11 neutralization in the athymic mouse xenograft system: correlation with virus-like particle IgG concentration. J Med Virol. 1997;53:185–188. doi: 10.1002/(sici)1096-9071(199711)53:3<185::aid-jmv1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas-Freytag L, Cheng E, Mayeux P, Domer J E, Clements J D. Effectiveness of a vaccine composed of heat-killed Candida albicans and a novel mucosal adjuvant, LT(R192G), against systemic candidiasis. Infect Immun. 1999;67:826–833. doi: 10.1128/iai.67.2.826-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardenas-Freytag L, Cheng E, Mirza A. New approaches to mucosal immunization. Adv Exp Med Biol. 1999;473:319–337. doi: 10.1007/978-1-4615-4143-1_34. [DOI] [PubMed] [Google Scholar]
- 9.Choi A H, Basu M, McNeal M M, Flint J, VanCott J L, Clements J D, Ward R L. Functional mapping of protective domains and epitopes in the rotavirus VP6 protein. J Virol. 2000;74:11574–11580. doi: 10.1128/jvi.74.24.11574-11580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen N D, Hopfl R, DiAngelo S L, Cladel N M, Patrick S D, Welsh P A, Budgeon L R, Reed C A, Kreider J W. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994;75:2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- 11.Christensen N D, Kirnbauer R, Schiller J T, Ghim S J, Schlegel R, Jenson A B, Kreider J W. Human papillomavirus types 6 and 11 have antigenically distinct strongly immunogenic conformationally dependent neutralizing epitopes. Virology. 1994;205:329–335. doi: 10.1006/viro.1994.1649. [DOI] [PubMed] [Google Scholar]
- 12.de Villiers E-M. Human pathogenic papillomavirus types: an update.Curr. Top Microbiol Immunol. 1994;186:1–12. doi: 10.1007/978-3-642-78487-3_1. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuy C, Buzoni-Gatel D, Touze A, Bout D, Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J Virol. 1999;73:9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giroglou T, Sapp M, Lane C, Fligge C, Christensen N D, Streeck R E, Rose R C. Immunological analyses of human papillomavirus capsids. Vaccine. 2001;19:1783–1793. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- 16.Gray J J, Cunliffe C, Ball J, Graham D Y, Desselberger U, Estes M K. Detection of immunoglobulin M (IgM), IgA, and IgG Norwalk virus-specific antibodies by indirect enzyme-linked immunosorbent assay with baculovirus-expressed Norwalk virus capsid antigen in adult volunteers challenged with Norwalk virus. J Clin Microbiol. 1994;32:3059–3063. doi: 10.1128/jcm.32.12.3059-3063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillobel H C, Carinhanha J I, Cardenas L, Clements J D, de Almeida D F, Ferreira L C. Adjuvant activity of a nontoxic mutant of Escherichia coli heat-labile enterotoxin on systemic and mucosal immune responses elicited against a heterologous antigen carried by a live Salmonella enterica serovar Typhimurium vaccine strain. Infect Immun. 2000;68:4349–4353. doi: 10.1128/iai.68.7.4349-4353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagensee E M, Yaegashi N, Galloway D A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 20.Katz S L. Future vaccines and a global perspective. Lancet. 1997;350:1767–1770. doi: 10.1016/S0140-6736(97)05358-0. [DOI] [PubMed] [Google Scholar]
- 21.Kirnbauer R, Booy F, Cheng N, Lowy D R, Schiller J T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirnbauer R, Chandrachud L M, O'Neil B W, Wagner E R, Grindlay G J, Armstrong A, McGarvie G M, Schiller J T, Lowy D R, Campo M S. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 23.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 24.Liu X S, Abdul-Jabbar I, Qi Y M, Frazer I H, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 25.McCluskie M J, Davis H L. CpG DNA as mucosal adjuvant. Vaccine. 1999;18:231–237. doi: 10.1016/s0264-410x(99)00194-2. [DOI] [PubMed] [Google Scholar]
- 26.McGhee J R, Czerkinsky C, Mestecky J. Mucosal vaccines: an overview. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. London, England: Academic Press Ltd.; 1999. pp. 741–757. [Google Scholar]
- 27.Moldoveanu Z, Love-Homan L, Huang W Q, Krieg A M. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16:1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 28.Murphy B R. Mucosal immunity to viruses. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. London, England: Academic Press Ltd.; 1999. pp. 695–707. [Google Scholar]
- 29.Nardelli-Haefliger D, Roden R, Balmelli C, Potts A, Schiller J, De Grandi P. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J Virol. 1999;73:9609–9613. doi: 10.1128/jvi.73.11.9609-9613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nardelli-Haefliger D, Roden R B, Benyacoub J, Sahli R, Kraehenbuhl J P, Schiller J T, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65:3328–3336. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neal C M, Clements J D, Estes M K, Conner M E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeler A V. Anthropological perspectives on injections: a review. Bull W H O. 2000;78:135–143. [PMC free article] [PubMed] [Google Scholar]
- 33.Roden R B, Greenstone H L, Kirnbauer R, Booy F P, Jessie J, Lowy D R, Schiller J T. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roden R B, Hubbert N L, Kirnbauer R, Christensen N D, Lowy D R, Schiller J T. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J Virol. 1996;70:3298–3301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose R C, Bonnez W, Da Rin C, McCance D J, Reichman R C. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J Gen Virol. 1994;75:2445–2449. doi: 10.1099/0022-1317-75-9-2445. [DOI] [PubMed] [Google Scholar]
- 36.Rose R C, Bonnez W, Reichman R C, Garcea R L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose R C, Lane C, Wilson S, Suzich J A, Rybicki E, Williamson A L. Oral vaccination of mice with human papillomavirus virus-like particles induces systemic virus-neutralizing antibodies. Vaccine. 1999;17:2129–2135. doi: 10.1016/s0264-410x(98)00484-8. [DOI] [PubMed] [Google Scholar]
- 38.Rose R C, Reichman R C, Bonnez W. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J Gen Virol. 1994;75:2075–2079. doi: 10.1099/0022-1317-75-8-2075. [DOI] [PubMed] [Google Scholar]
- 39.Rose R C, White W I, Li M, Suzich J A, Lane C, Garcea R L. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiller J T. Papillomavirus-like particle vaccines for cervical cancer. Mol Med Today. 1999;5:209–215. doi: 10.1016/S1357-4310(99)01463-X. [DOI] [PubMed] [Google Scholar]
- 41.Shah K V, Howley P M. Papillomaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2077–2110. [Google Scholar]
- 42.Suzich J A, Ghim S J, Palmer-Hill F J, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tukey J W. Exploratory data analysis. Reading, Mass: Addison-Wesley Publishing Co.; 1977. [Google Scholar]
- 44.Unckell F, Streeck R E, Sapp M. Generation and neutralization of pseudovirions of human papillomavirus type 33. J Virol. 1997;71:2934–2939. doi: 10.1128/jvi.71.4.2934-2939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–368. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 46.Walker P S, Scharton-Kersten T, Krieg A M, Love-Homan L, Rowton E D, Udey M C, Vogel J C. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White W I, Wilson S D, Bonnez W, Rose R C, Koenig S, Suzich J A. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J Virol. 1998;72:959–964. doi: 10.1128/jvi.72.2.959-964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]
- 49.zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111:581–587. doi: 10.1046/j.1525-1381.1999.99723.x. [DOI] [PubMed] [Google Scholar]