Abstract
Cystic fibrosis (CF) is a genetic disease caused by variants in the gene encoding for the CF transmembrane conductance regulator (CFTR) protein, a chloride and bicarbonate channel. CFTR dysfunction results in a multiorgan disease with the main clinical features being exocrine pancreatic insufficiency and diffuse bronchiectasis with chronic airway infection leading to respiratory failure and premature death. Over the past decades, major progress has been made by implementing multidisciplinary care, including nutritional support, airway clearance techniques and antibiotics in specialised CF centres. The past decade has further seen the progressive development of oral medications, called CFTR modulators, for which around 80% of people with CF are genetically eligible in Europe. CFTR modulators partially restore ion transport and lead to a rapid and major improvement in clinical manifestations and lung function, presumably resulting in longer survival. CFTR modulators have been game-changing in the care of people with CF. However, many questions remain unanswered, such as the long-term effects of CFTR modulators, especially when treatment is started very early in life, or the new CF-related disease emerging due to CFTR modulators. Moreover, severe complications of CF, such as diabetes or cirrhosis, are not reversed on CFTR modulators and around 20% of people with CF bear CFTR variants leading to a CFTR protein that is unresponsive to CFTR modulators. Challenges also arise in adapting CF care to a changing disease. In this review article, we highlight the new questions and challenges emerging from this revolution in CF care.
Shareable abstract
Cystic fibrosis, a very severe genetic disease, has changed dramatically with CFTR modulator therapies. Long-term effects and adaptation of models of care are some of the new questions and challenges arising from this revolution in cystic fibrosis care. https://bit.ly/3V0zxFo
Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CF transmembrane conductance regulator (CFTR) gene [1]. It is the most common life-shortening genetic disease in the Caucasian population, affecting at least 100 000 individuals worldwide [2]. The CFTR gene encodes the CFTR protein, which is a chloride and bicarbonate channel expressed at the cell membrane of many epithelial cells and other cell types, including inflammatory cells [3, 4]. CF is a multisystem disease affecting organs and tissues where CFTR is expressed. The main clinical features are exocrine pancreatic insufficiency and diffuse bronchiectasis with chronic airway infection leading to respiratory failure and premature death [5]. The principles of CF care were established as early as the 1960s and have steadily evolved with a better understanding of the disease and the availability of new drugs. They are based on a holistic approach to care and intensive symptomatic treatment. Specialised CF centres formed by multidisciplinary teams experienced in CF are the established model of care for people with CF (pwCF) [6]. The principles of symptomatic treatment are maintenance of good nutrition, compensation of pancreatic insufficiency with pancreatic enzymes, enhancement of mucociliary clearance with physiotherapy and mucolytic agents, prevention and aggressive treatment of pulmonary infection, and early identification and treatment of complications [6, 7]. As a result of this structured care in dedicated centres, the life expectancy for pwCF has increased from a matter of a few years to around 50 years [8]. Similarly, in several countries, the number of adults with CF is currently larger than the number of children with CF [9].
The CFTR gene was cloned in 1989, around 2100 variants were identified and the various resulting CFTR protein abnormalities were studied. This led to very active research on new treatments termed CFTR modulators, which aim to correct the defective CFTR protein [10]. The first CFTR modulator was approved in 2012 and there are, to date, four approved CFTR modulators with more than 80% of pwCF in Europe genetically eligible for at least one of them. CFTR modulators treat the root cause of the disease and they have been game-changing in the care of pwCF. The goals of this review article are to provide an overview of the new questions and challenges emerging from this revolution in CF care.
Search strategy
We searched PubMed for research related to CF and CFTR modulators to identify relevant articles. We mainly selected recent publications (from the past 5 years) describing randomised-controlled trials or large real-world studies. We also included highly regarded older publications and review articles to provide readers with more details. The reference lists of included studies and relevant reviews were screened for relevant papers and these were added for assessment at the full-text stage.
New drugs for CF
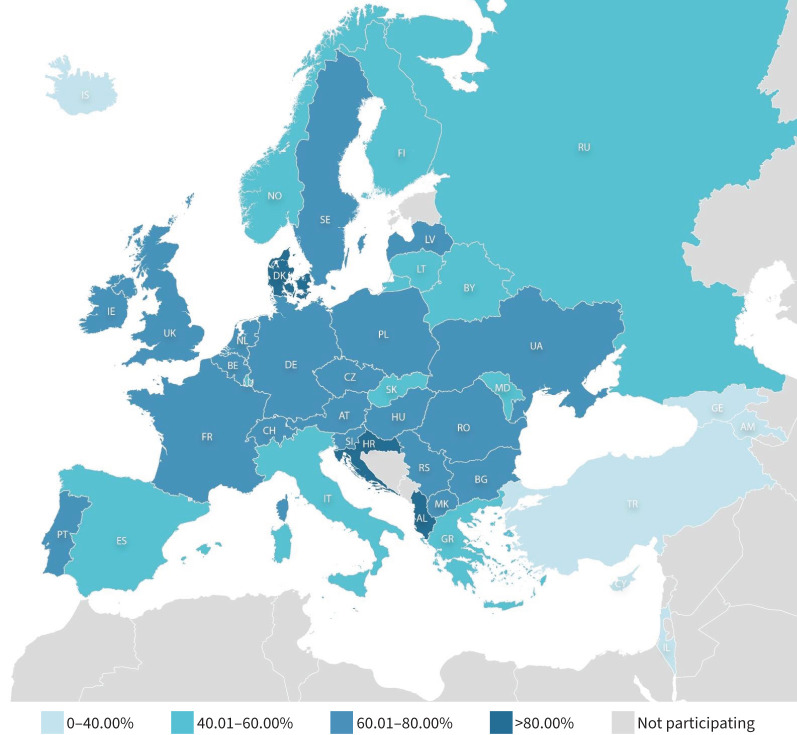
CFTR modulators are small oral drugs that bind to the CFTR protein and improve its function. There are two classes of CFTR modulators, namely potentiators that increase the open probability of the CFTR protein expressed at the cell membrane and correctors that improve the intracellular processing of the CFTR protein. Since 2012, four CFTR modulators have been marketed, ivacaftor, which is a potentiator marketed for specific CFTR variants carried by around 3% of pwCF, and a combination of correctors and ivacaftor: lumacaftor and ivacaftor, tezacaftor and ivacaftor, and elexacaftor, tezacaftor and ivacaftor (ETI). Lumacaftor/ivacaftor and tezacaftor/ivacaftor are mainly marketed for pwCF homozygous for F508del, the most frequent CFTR variant. They are now supplanted by the more effective triple combination, ETI, which is marketed in Europe for pwCF bearing at least one F508del variant. Around 80% of pwCF in Europe bear at least one F508del variant, although there are large disparities between countries due to genetic heterogeneity (figure 1) [11].
FIGURE 1.
Geographical distribution of the F508del variant in countries participating in the European Registry which gathers data for more than 54 000 people with cystic fibrosis. Reproduced from [11], with permission. Names of countries are abbreviated according to the International Organization for Standardization.
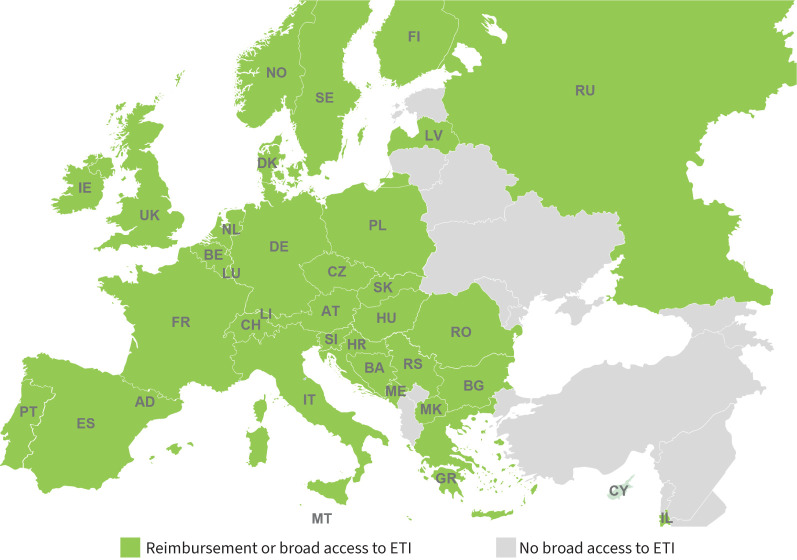
Ivacaftor and ETI were shown to be well tolerated and to have similar responses in pivotal phase 3 placebo-controlled trials in eligible children (≥6 years old) and adults with a sustained and robust improvement in respiratory function (mean increase of 10% or more in predicted forced expiratory volume in 1 s (FEV1)), a gain in weight and a decrease in the rate of pulmonary exacerbations [12–15]. Ivacaftor and ETI are sometimes called “highly effective modulator therapy” to differentiate them from lumacaftor/ivacaftor and tezacaftor/ivacaftor, which are much less effective [16]. In Europe, ivacaftor is currently approved for pwCF aged 4 months and above bearing at least one variant, called a gating variant, and ETI is approved for pwCF aged 2 years and above bearing at least one F508del variant. Most European countries have access to ETI through licensing and reimbursement or through varied special access programmes (figure 2).
FIGURE 2.
Access to elexacaftor/tezacaftor/ivacaftor (ETI) in Europe through licensing and reimbursement or through special access programmes. Names of countries are abbreviated according to the International Organization for Standardization.
Results from real-world studies have confirmed data from clinical trials and showed that even in adults with severe lung disease who were not included in clinical trials, restoring CFTR function with ivacaftor or ETI significantly improved lung function and slowed disease progression [17, 18]. Registry studies of large patient cohorts over a follow-up of 5 years showed sustained favourable effects of ivacaftor therapy on disease progression with better preserved lung function, improved nutritional status and decreased risk of pulmonary exacerbations than in an untreated comparator population [19]. Similarly, analysis of data from clinical trials and registries showed that the clinical benefits of ETI were durable and on average there was no loss of pulmonary function over a 3-year period [20, 21]. Moreover, a major decrease in lung transplantation for end-stage pwCF has been observed after ETI availability [22, 23]. It will take years before the gain in survival of pwCF under long-term treatment with CFTR modulators can be truly observed. Estimates have been generated using different models. An analysis using a person-level microsimulation model predicted that treating pwCF homozygous for the F508del variant with ETI would result in a substantial increase in survival to around 70 years [24]. Conclusions of this model need to be verified by future data. Nevertheless, this impressive increased survival under ETI did not reach the life expectancy of the general reference population, which was more than 80 years.
PwCF on ivacaftor or ETI have seen their daily life and future perspectives transformed. They often no longer cough and/or expectorate, feel physically stronger, and have fewer and less severe exacerbations. These improvements lead to an improved quality of life and new life goals [25]. However, many questions on the long-term use of CFTR modulators remain, especially when treatment is started very early in life and before the occurrence of irreversible lung structural disease. Studies are ongoing in an attempt to answer them. A new CF disease has emerged when on CFTR modulators and CF care has already evolved to monitor, treat and adapt to a large diversity of CF disease severity and to an ageing population. Thus, groundbreaking CFTR modulator therapy has transformed CF disease and CF care, leading to new questions and challenges that we highlight in this review.
How to improve the prescription of CFTR modulators?
Acquire knowledge on long-term safety
CFTR modulators are not a curative treatment. To be effective, they need to be taken daily and their effects disappear rapidly when the treatment is interrupted. Therefore, CFTR modulators are a lifelong treatment and knowledge about their long-term safety is critical. Clinical trials and real-world studies show that they are usually well tolerated. In phase 3 studies on ETI, adverse events were usually mild or moderate, leading to only 1% of drug discontinuations [14, 26]. They mainly consisted of rash, headache, abdominal pain, abnormal liver function tests and elevated creatine kinase level. This good safety profile was confirmed in real-world studies on pwCF treated with ETI. However, the association between ETI and drug-induced liver injury was confirmed in an analysis using the Food and Drug Administration adverse event reporting system [27], confirming the need to periodic liver monitoring as recommended. Possible mitigation strategies, such as dose reduction, need to be studied further. Moreover, neuropsychiatric adverse events such as anxiety, low mood, insomnia or “brain fog” were reported in a minority of pwCF on ETI. Depression, including suicidal ideation and suicide attempts, was also reported, usually occurring within 3 months of treatment initiation and in patients with a history of psychiatric disorders. In some cases, symptoms improved after dose reduction or treatment discontinuation. Although rates of depression-related adverse events on ETI could be consistent with background epidemiology of depression in the CF population, monitoring of depressed mood, suicidal thoughts or changes in behaviour is recommended in pwCF on ETI [28, 29].
The timing of CFTR modulator initiation in pwCF with minimal or no detectable lung disease is still an open question, although the possibility of preserving lung function, or even pancreatic function in infants, with the early use of CFTR modulators is very attractive [30, 31]. Observational studies in the paediatric population are underway, such as the BEGIN study (NCT04509050) in infants and young children and the PROMISE paediatric study (NCT04613128). They are critical to acquire knowledge on biological and clinical effects, including effects on growth and development, of CFTR modulators in the paediatric population. Long-term safety data on the use of CFTR modulators in infants and children will enable risk–benefit analyses to inform decisions on initiating therapy in this population with limited disease.
Assess restoration of CFTR function
Measurement of sweat chloride concentrations is a well-known, easy and standardised method to assess CFTR function in vivo. Data from clinical trials on the different CFTR modulators are in favour of a relationship between the degree of CFTR function improvement, as shown by sweat chloride concentrations, and important clinical outcomes such as gain in respiratory function [32]. This might not be as straightforward in individuals, but many CF centres use sweat chloride concentrations as a tool to monitor the degree of CFTR restoration. However, the degree of sweat chloride improvement to expect varies with the genotype and the CFTR modulator studied, and this needs to be further characterised. Moreover, we do not yet know if the magnitude of improvement in sweat chloride concentrations to normal values (<30 mmol·L−1) or intermediate values (between 30 and 59 mmol·L−1) on CFTR modulators can be predictive of the long time course of CF disease. Similarly, how to interpret and deal with a poor correlation between sweat chloride improvement and clinical disease needs to be further investigated.
Develop tools for therapeutic drug monitoring
After dosing of an oral drug, plasma concentrations are influenced by many factors, such as individual clinical characteristics like malabsorption, renal or liver disease, obesity, gender, or pregnancy, as well as diet, pharmacogenetic variants or concurrent medication use. Altered absorption, specific metabolism, distribution and clearance of drugs are well recognised in pwCF and known to have pronounced impact on drug efficacy. Moreover, diet and concurrent medication are known to influence absorption and metabolism of CFTR modulators. Clinical response depends in part on plasma concentration, which is one variable that can be followed through therapeutic drug monitoring, allowing personalised dose adjustment until optimised outcomes are reached. Although titration of CFTR modulators would be a useful tool to optimise drug response, it is not currently used due to a lack of detailed pharmacokinetic data, assays for monitoring and data on associations between blood concentrations and clinical response and adverse events [33]. Improved access to serum drug-level monitoring of CFTR modulators and their metabolites may help determine whether differences in drug metabolism can account for the occurrence of adverse reactions or low responsiveness to modulator therapy and facilitate dose adjustment in patients with adverse reactions or a poor response [34].
What is the new CF disease on CFTR modulators?
The phenotypic features of CF disease on CFTR modulators and knowledge gaps are summarised in table 1.
TABLE 1.
Phenotypic features of cystic fibrosis (CF) disease on cystic fibrosis transmembrane conductance regulator (CFTR) modulators and knowledge gaps
| CF disease on CFTR modulators | Knowledge gaps on CFTR modulators effects | |
|---|---|---|
| Respiratory disease | Marked reduction of respiratory symptoms Rapid improvement in respiratory function |
Long-term progression of lung function Best monitoring methods and frequency |
| Microbiology and pulmonary exacerbations | Lower frequency of pulmonary exacerbations Reduction but persistence of airway inflammation |
Long-term clearance of airway pathogens Long-term effect on airway inflammation Prevention or delay in airway colonisation Best sampling methods Relevance of current guidelines |
| Exocrine pancreatic insufficiency | Possible reversal of pancreatic insufficiency in young children | Long-term effect on exocrine pancreatic function |
| Gastrointestinal disease and nutrition | No meaningful change in abdominal symptoms Increased number of overweight pwCF No effect on established biliary complications |
Optimal diet and nutrition outcomes Prevention or delay in biliary complications |
| CF-related diabetes | Possible reduction of insulin requirements Improved diabetes control |
Robust longitudinal data to assess improvements Prevention or delay in CF-related diabetes |
| Fertility | No recovery of fertility in males Improved fertility in females No abnormalities in fetal development to date |
Effects on male fertility with very early CFTR modulator treatment Robust longitudinal data to assess mothers and infants when CFTR modulators are used during pregnancy and lactation |
| Chronic rhinosinusitis | Improvement in symptoms and CT scan findings | Effects of early treatment on nasal polyposis development |
| Bone disease | Possible improvement in bone mineral density | Robust longitudinal data to assess improvement |
CT: computed tomography; pwCF: people with CF.
Respiratory disease
When ivacaftor or ETI are started in pwCF with existing respiratory disease, a rapid and marked reduction in cough and expectoration usually occurs, together with an improvement in their respiratory function shown by an increase in FEV1 and a decreased number of exacerbations. Monitoring of this less severe respiratory disease is still possible with the usual outcome measures, such as spirometry and lung imaging [35]. The effects of CFTR modulators on lung function are ascribed to their effects on mucociliary clearance [36]. CFTR modulators are usually thought not to be able to reverse structural lesions such as bronchiectasis [37], although limited reversal has been described in specific cases [38].
When CFTR modulators are started in pwCF with minimal or no respiratory disease, monitoring clinical progression over time can be difficult as more sensitive measures than FEV1 are needed. At present, lung clearance index (LCI), chest computed tomography (CT) and chest magnetic resonance imaging (MRI) are the main methods for detecting and monitoring early lung disease in CF. The pros and cons of these biomarkers for reliably detecting early lung damage have been discussed elsewhere [39–41]. To summarise, LCI is sensitive to early disease and is feasible in the very young. However, it is not an easy technique and requires specialised equipment and trained personnel. CT is the gold standard for imaging pulmonary structures and it is sensitive to early disease and disease progression. However, it is qualitative and scoring is not easy and not yet automatic. Moreover, radiation exposure remains an important consideration, especially in children. MRI, possibly with the addition of inhaled gases, is emerging as an attractive alternative to CT imaging as it is radiation-free. However, standardisation across centres is difficult and it needs further investigation before it can be implemented in routine use [35]. Over the last few years, CF-related patient-reported outcomes captured by many questionnaires and tools were developed. They focus primarily on symptoms rather than objective data. They might enhance our ability to monitor lung disease, but their use in clinical practice is not yet clear.
Microbiology and pulmonary exacerbations
Clinical trials and real-world studies all showed lower frequencies of pulmonary exacerbations on CFTR modulators. A large analysis of US and UK patient registries showed that this drop in pulmonary exacerbation frequency was sustained over 5 years in ivacaftor-treated patients [19]. This lower frequency of pulmonary exacerbations is not clearly linked with clearance of airway pathogens. PwCF with intermittent Pseudomonas aeruginosa-positive sputum cultures tend to stop testing positive for the infection when on ivacaftor. However, for pwCF and chronic P. aeruginosa infection, studies in ivacaftor- or ETI-treated patients showed that after a first drop in sputum bacterial burden, P. aeruginosa abundance tended to return to baseline levels after a few years despite improved FEV1 levels and reduced pulmonary symptoms [42, 43]. In pwCF without airway colonisation with CF-traditional pathogens when starting CFTR modulators, it is not known whether CFTR modulators may delay or prevent airway colonisation and longitudinal studies will answer this question. Neutrophilic inflammation is a key driver of structural lung damage progression in CF. After 1 year on ETI, a reduction in airway inflammation was reported, but residual protease burden was still observed [44]. Long-term data are required to determine the evolution of this residual inflammation and its role on progression of structural lung damage. Monitoring of airway infection is made more complex by the absence of spontaneous sputum in many patients on ETI. Other sampling methods are available and well known to paediatricians who have dealt with nonexpectorating children for many years. Induced sputum, throat swabs and upper airway samples are less invasive than bronchoscopy, but have lower sensitivity to detect pulmonary microbes, with induced sputum having the best concordance with bronchoscopy [45–47]. The use of exhaled breath analyses or serology to identify pathogens of interest are promising methods that require further study [48]. As a new respiratory disease emerges on CFTR modulators with fewer symptoms, little or no spontaneous sputum and fewer exacerbations with possibly different airway pathogens, a whole area of research is opening up to establish the relevance of previous definitions and guidelines for pulmonary exacerbations, bacterial monitoring and treatment of exacerbations.
Exocrine pancreatic insufficiency
Some clinical trials or small paediatric case series suggested that ivacaftor could preserve or improve exocrine pancreatic function in infants and young children [30, 49, 50]. This was not observed in older children and adults. In a large observational US study in pwCF aged 12 years and above, there was no change in pancreatic insufficiency 6 months after ETI treatment [51]. In analyses of the US and UK registries, there was a decline in the use of pancreatic enzyme replacement therapy after ivacaftor licensing in the US CF population, but these results were not replicated in the UK CF population [52]. Longitudinal and large studies in pwCF on ETI are needed to evaluate the possible effects of CFTR modulators on exocrine pancreatic function and requirements of pancreatic enzyme replacement therapy.
Gastrointestinal disease and nutrition
Gastrointestinal symptoms are a regular complaint of pwCF and they impact their quality of life. CFTR modulators have been reported to reduce intestinal inflammation, change proximal intestinal pH and positively impact the gastrointestinal microbiome, thus contributing to improved nutrient absorption and improved intestinal transit [53, 54]. Despite these effects, changes in gastrointestinal symptoms were not clinically meaningful in pwCF after 6 months on ETI [51]. CFTR modulators may contribute to the increased number of pwCF who are overweight or obese. Increase in body mass index was a regular feature in clinical trials on CFTR modulators [12–15] and in real-world studies [55, 56]. To prevent obesity-associated comorbidities, changes in the CF diet and lifestyle are recommended [57]. New studies are needed to define optimal nutrition for pwCF on CFTR modulators. At present, no effect of CFTR modulators was reported on established biliary complications such as cholelithiasis, hepatic steatosis and end-stage liver disease (cirrhosis and portal hypertension). Future studies will tell if CFTR modulators can prevent these complications.
CF-related diabetes (CFRD)
Analyses of the US and the UK CF registries showed a lower prevalence of CFRD in pwCF on ivacaftor versus comparators [19]. Several small observational studies suggest that CFTR modulators reduce insulin requirements and improve diabetes control, possibly through improvement in insulin sensitivity [58, 59]. However, more robust data are needed. Moreover, longitudinal data will determine if CFTR modulators can prevent or reverse CFRD.
Fertility
There has been no report of men on CFTR modulators recovering fertility. Obstruction in the genital tract happens during fetal development and reversal of bilateral absence of vas deferens is unlikely to happen, even if CFTR modulators are prescribed very early in life. However, this needs to be investigated.
In women, there has been a notable rise in the number of pregnancies since the introduction of CFTR modulators [60, 61]. This is thought to be related to improved viscoelastic properties of cervical secretions, favourable uterine pH and a change in nutritional status. CFTR modulators cross the placenta, but to date there has been no evidence that CFTR modulators may cause abnormalities in fetal development. In contrast, some women who stopped CFTR modulators during pregnancy have experienced a decline in lung function. These data come from small observational series or case reports and there is a need for a better overview of the outcomes in mothers and infants when mothers use CFTR modulators during pregnancy and lactation. The prospective MAYFLOWERS (NCT04828382) study will provide some important data on this topic [62].
Chronic rhinosinusitis
Improvement of chronic rhinosinusitis symptoms and/or CT scan findings with ivacaftor and ETI has been reported [63–65]. However, symptom scores remain high in many patients [64]. It is not known if early treatment may prevent nasal polyposis development.
Bone disease
An improvement of bone mineral density upon ivacaftor or ETI were reported in very small case series [66, 67]. CFTR modulators may improve CF bone disease either by a direct effect on CFTR expressed in osteoblasts and osteoclasts, or by improving clinical factors affecting bone health, such as nutritional status and physical activity levels [68]. Larger and longer studies will elucidate the effects of CFTR modulators on bone in pwCF and on their mechanisms of action.
How do we address the burden of treatment in patients receiving CFTR modulators?
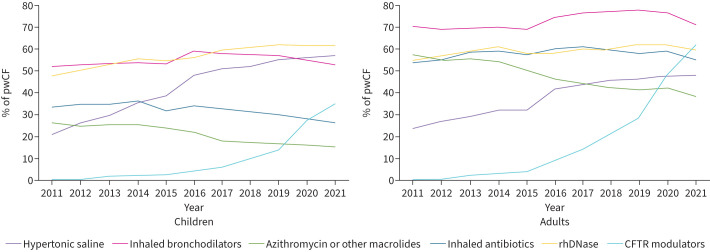
CFTR modulators are usually prescribed on top of all other medications and patients with CF have a high burden of treatment with a median of seven medications per day [69]. As CF disease is less severe on CFTR modulators, it is tempting to withdraw some of the symptomatic medications and some withdrawal studies have begun. In adolescents and adults with CF on ETI with well-preserved lung function (mean baseline FEV1 of 97%), discontinuing mucociliary agents such as hypertonic saline or dornase alfa for 6 weeks did not result in a meaningful difference in lung function when compared with continuing treatment [70]. However, this was a short study in patients with minimal disease. Another ongoing study is investigating the withdrawal of mucoactive drugs. It is a 52-week study in adolescents and adults and enrols patients with an FEV1 as low as 40%. These studies are very important but difficult to run. Data from the European Registry already suggest that the prescription of several symptomatic treatments in children and adults decreases while CFTR modulators prescription increases (figure 3) [11]. However, it is still very important to establish whether therapies designed for CF airway disease before the use of modulators are optimal, effective or necessary in the era of modulator therapy.
FIGURE 3.
Use of therapies among children and adults from 2011 to 2021. Reproduced from [11], with permission. CFTR: cystic fibrosis transmembrane conductance regulator; rhDNase: recombinant human deoxyribonuclease.
What are the effects of CFTR modulators in an ageing population?
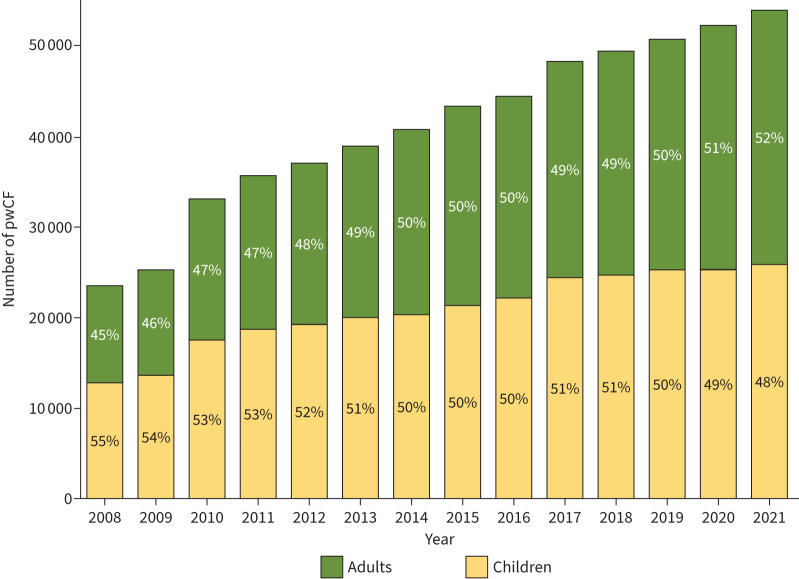
With structured care in dedicated centres and more effective symptomatic therapies, the life expectancy of pwCF has increased over recent last decades and adults have outnumbered children in the European Registry since 2019 (figure 4) [11]. A further increase in survival is expected with the broad use of CFTR modulators [24]. This means that comorbidities usually linked with ageing, such as cardiovascular disease or cancer, may be seen more frequently and the possible effects of CFTR modulators on these comorbidities are still unclear.
FIGURE 4.
Number of people with cystic fibrosis (pwCF) and percentage of adults and children from 2008 to 2021. Reproduced from [11], with permission.
Traditional cardiac risk factors, such as high body mass index, smoking, lipid metabolism, hypertension and ageing, were not usually a concern in CF. However, with increased longevity, CFRD, traditional high-salt high-fat high-carbohydrate diets, relative inactivity, as well as chronic inflammation, pwCF are now recognised as having an increased risk of cardiac disease [71]. This was recently shown in a multinational retrospective cohort study [72]. It is still impossible to predict how CFTR modulators may affect cardiovascular disease. They may increase cardiovascular risk by their contribution in increasing longevity, body mass index, body fat content, cholesterol levels and blood pressure. However, they may also have protective effects though decreases in oxidative stress and systemic inflammation, as well as better glucose control [73].
Several cohort or registry studies have shown an increased risk of cancer, mainly bowel cancer, in nontransplanted pwCF as compared with the general population, and this increases with age [74–76]. The pathogenesis of digestive cancer in CF remains unclear, but inflammation and the role of CFTR have been discussed. With pwCF living longer, this risk of digestive cancer or other cancers may increase further. The relationship between long-term CFTR modulator treatment and cancer risk will be important to evaluate.
How to adapt models of care for a heterogeneous CF population?
There has always been a large diversity in CF disease severity, depending mainly on genetics and the extent of CFTR dysfunction caused by CFTR variants. However, home environment, socioeconomic status, access to healthcare and medication, as well as adherence to treatment are all known to play a role in CF disease severity [77]. When the only therapeutic option was symptomatic therapies and the only course for the disease was aggravation, the goal for the mutidisciplinary team was to slow disease progression. With the advent of CFTR modulators, a larger diversity in CF disease is expected, depending not only on eligibility for these new treatments, but also on starting age and on the severity of the disease at starting age. Moreover, the goal is not only to slow disease progression, but also to possibly prevent the disease from occurring, even though the new CF disease on CFTR modulators is not yet well known. PwCF on CFTR modulators have a less severe disease and feel better, leading to new horizons opening up regarding education, work, family and long-term plans. Models of care need to adapt to satisfy the growing needs of pwCF while also being careful to capture and address events known to trigger disease progression. Some key principles for the care of pwCF are still valid: centre-based care with a multidisciplinary team with expertise for all stages of CF, a close integration of associated specialties and regular visits and assessments based on international and national guidelines. However, new avenues need to be considered and have already been put in place in many centres, including virtual consultations, care closer to home with fewer hospital visits, stronger links with specialties such as obstetrics and with primary care, home monitoring with use of connected devices, and screening for new comorbidities such as cancers and cardiovascular disease [6]. As the field moves on, CF teams face challenges such as the need to maintain severe CF disease expertise, even though severe disease is becoming rarer. It is also critical to continue working closely with patients to identify changing clinical patterns and more subtle presentations, and to stress how adherence to CFTR modulators is paramount.
How to increase eligibility and access to CFTR modulators?
The European Medicines Agency (EMA) has approved ETI for pwCF bearing at least one F508del variant based on pivotal phase 3 studies [14, 15]. However, more pwCF could benefit from the treatment as additional CFTR variants lead to a CFTR protein responsive to ETI. This was shown in in vitro data generated in nonhuman cell lines and led the Food and Drug Administration (FDA) in the US to also approve ETI for pwCF bearing at least one among 177 rare variants. A clinical trial in pwCF bearing some rare non-F508del variants recently showed a statistically significant improvement in respiratory function on ETI compared to placebo [78]. These data have been used to support an application to extend the approval of ETI currently being examined by the EMA. Several real-world reports of a few patients or of small cohorts also supported a clinical benefit of ETI in pwCF bearing some non-F508del variants [79–81]. The French health authorities adopted a more extensive and pragmatic approach with a compassionate programme that was first aimed at pwCF bearing no F508del variant and with severe disease [82]. It was then extended to all pwCF bearing no F508del variant regardless of the severity of their disease. With this programme, pwCF are granted 4–6 weeks of ETI and effectiveness is evaluated by a centralised adjudication committee in terms of clinical manifestations, sweat chloride concentration and respiratory function. Among the first 84 pwCF included in the programme, 45 pwCF (54%) were responders and continued ETI. Of interest, 22 pwCF (49%) bore a rare CFTR variant that was not included on the FDA list of 177 rare variants [82]. Due the scarcity of pwCF bearing rare CFTR variants, it is impossible to conduct clinical trials fulfilling all the requirements of clinical research for each rare variant. The pragmatic and rational French approach is possible because there are strong clinical biomarkers of ETI effectiveness and there are minimal safety concerns associated with ETI. This approach should be advocated as it grants a fair opportunity for all patients to test a truly transformative therapy, addresses an unmet medical need and promotes equity of care.
Need to continue research into curative and symptomatic therapies
Around 10% of CFTR variants result in the absence of CFTR protein and CFTR modulators cannot be effective as they have no target to act upon. For pwCF bearing these variants, other strategies are initiated, such as read-through agents for nonsense variants or nucleic acid-based therapies that benefit all patients. For nucleic acid-based therapies, several approaches have been developed based on DNA or RNA transfer with viral or nonviral vectors. Some of these approaches are currently undergoing early clinical trials [83]. Even with CFTR modulators, CF is not cured and there is still a need to continue developing better symptomatic treatments to improve mucociliary clearance with inhibitors of the epithelial sodium channel, agonists of alternative chloride channels or mucoytics; to decrease airway inflammation with neutrophil elastase inhibitors or other new anti-inflammatories; and to improve anti-infective agents with new antibiotics or novel anti-infective approaches [83].
Conclusion
CFTR modulators that treat the root cause of the disease are now available for more than 80% of pwCF and they represent a paradigm shift for pwCF, who see a rapid and dramatic improvement in their respiratory disease and the alleviation of some extrapulmonary symptoms. The long-term effects of CFTR modulators on both the respiratory system and other affected organs need to be thoroughly evaluated, as well as the possible prevention of the disease with early prescription. Tools should be developed for therapeutic drug monitoring and new methods should be assessed to monitor the new CF disease emerging on CFTR modulators. Models of care need to be rethought in order to maintain the expertise gained in all stages of CF built over decades and to adapt to the new needs of pwCF. All pwCF who could benefit from these revolutionary drugs should have access to them and research should continue so that all pwCF have access to a curative treatment.
Points for clinical practice
CFTR modulators partially restore ion transport and lead to a rapid and major improvement in respiratory symptoms and lung function.
CFTR modulators may also improve pancreatic insufficiency in young children.
CFTR modulators may improve diabetes control.
CFTR modulators improve fertility in females.
CFTR modulators improve chronic rhinosinusitis.
Questions for future research
What will be the extent of improved survival on CFTR modulators?
What will be the long-term progression of lung function on CFTR modulators?
What will be the long-term effect of CFTR modulators on airway pathogens and inflammation?
What will be the CF disease of pwCF when CFTR modulators are started in infancy or early childhood?
How will the usual complications of CF evolve on CFTR modulators?
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: I. Fajac reports grants from AbbVie, Bayer, Boehringer Ingelheim, Insmed, GSK, Vertex Pharmaceuticals and Zambon; consulting fees from AbbVie, Boehringer Ingelheim, Genvade, Kither Biotech and Vertex Pharmaceuticals; lecture honoraria from Vertex Pharmaceuticals; and a leadership role as President of the European Cystic Fibrosis Society, outside the submitted work. P-R. Burgel reports grants from Vertex Pharmaceuticals and GSK; consulting fees from AstraZeneca, Chiesi, GSK, Insmed, Vertex, Viatris and Zambon; and travel support from AstraZeneca and Chiesi, outside the submitted work. C. Martin reports lecture honoraria from AstraZeneca, Chiesi and Zambon; travel support from Chiesi, Sanofi and Zambon; and advisory board participation with Vertex, Zambon and GSK, outside the submitted work.
References
- 1.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–1073. doi: 10.1126/science.2475911 [DOI] [PubMed] [Google Scholar]
- 2.Guo J, Garratt A, Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros 2022; 21: 456–462. doi: 10.1016/j.jcf.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 3.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 1999; 79: S23–S45. doi: 10.1152/physrev.1999.79.1.S23 [DOI] [PubMed] [Google Scholar]
- 4.Mall MA, Criner GJ, Miravitlles M, et al. Cystic fibrosis transmembrane conductance regulator in COPD: a role in respiratory epithelium and beyond. Eur Respir J 2023; 61: 2201307. doi: 10.1183/13993003.01307-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasemann H, Ratjen F. Cystic fibrosis. N Engl J Med 2023; 389: 1693–1707. doi: 10.1056/NEJMra2216474 [DOI] [PubMed] [Google Scholar]
- 6.Southern KW, Castellani C, Lammertyn E, et al. Standards of care for CFTR variant-specific therapy (including modulators) for people with cystic fibrosis. J Cyst Fibros 2023; 22: 17–30. doi: 10.1016/j.jcf.2022.10.002 [DOI] [PubMed] [Google Scholar]
- 7.Burgel P-R, Southern KW, Addy C, et al. Standards for the care of people with cystic fibrosis (CF); recognising and addressing CF health issues. J Cyst Fibros 2024; 23: 187–202. doi: 10.1016/j.jcf.2024.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Coriati A, Ma X, Sykes J, et al. Beyond borders: cystic fibrosis survival between Australia, Canada, France and New Zealand. Thorax 2023; 78: 242–248. doi: 10.1136/thorax-2022-219086 [DOI] [PubMed] [Google Scholar]
- 9.Burgel P-R, Burnet E, Regard L, et al. The changing epidemiology of cystic fibrosis: the implications for adult care. Chest 2023; 163: 89–99. doi: 10.1016/j.chest.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Farinha CM, Callebaut I. Molecular mechanisms of cystic fibrosis – how mutations lead to misfunction and guide therapy. Biosci Rep 2022; 42: BSR20212006. doi: 10.1042/BSR20212006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zolin A, Orenti A, Jung A, et al. ECFSPR Annual Report 2021. Karup, European Cystic Fibrosis Society, 2023. www.ecfs.eu/sites/default/files/Annual%20Report_2021_09Jun2023_ECFSPR_final.pdf [Google Scholar]
- 12.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. doi: 10.1056/NEJMoa1105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013; 187: 1219–1225. doi: 10.1164/rccm.201301-0153OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mall MA, Brugha R, Gartner S, et al. Efficacy and safety of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis heterozygous for F508del and a minimal function mutation: a phase 3b, randomized, placebo-controlled study. Am J Respir Crit Care Med 2022; 206: 1361–1369. doi: 10.1164/rccm.202202-0392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373: 220–231. doi: 10.1056/NEJMoa1409547 [DOI] [PubMed] [Google Scholar]
- 17.Barry PJ, Plant BJ, Nair A, et al. Effects of ivacaftor in patients with cystic fibrosis who carry the G551D mutation and have severe lung disease. Chest 2014; 146: 152–158. doi: 10.1378/chest.13-2397 [DOI] [PubMed] [Google Scholar]
- 18.Burgel P-R, Durieu I, Chiron R, et al. Rapid improvement after starting elexacaftor–tezacaftor–ivacaftor in patients with cystic fibrosis and advanced pulmonary disease. Am J Respir Crit Care Med 2021; 204: 64–73. doi: 10.1164/rccm.202011-4153OC [DOI] [PubMed] [Google Scholar]
- 19.Volkova N, Moy K, Evans J, et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: data from national US and UK registries. J Cyst Fibros 2020; 19: 68–79. doi: 10.1016/j.jcf.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 20.Lee T, Sawicki GS, Altenburg J, et al. Effect of elexacaftor/tezacaftor/ivacaftor on annual rate of lung function decline in people with cystic fibrosis. J Cyst Fibros 2023; 22: 402–406. doi: 10.1016/j.jcf.2022.12.009 [DOI] [PubMed] [Google Scholar]
- 21.Daines CL, Tullis E, Costa S, et al. Long-term safety and efficacy of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis and at least one F508del allele: 144-week interim results from a 192-week open-label extension study. Eur Respir J 2023; 62: 2202029. doi: 10.1183/13993003.02029-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin C, Legeai C, Regard L, et al. Major decrease in lung transplantation for patients with cystic fibrosis in France. Am J Respir Crit Care Med 2022; 205: 584–586. doi: 10.1164/rccm.202109-2121LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringshausen FC, Sauer-Heilborn A, Büttner T, et al. Lung transplantation for end-stage cystic fibrosis before and after the availability of elexacaftor–tezacaftor–ivacaftor, Germany, 2012–2021. Eur Respir J 2023; 61: 2201402. doi: 10.1183/13993003.01402-2022 [DOI] [PubMed] [Google Scholar]
- 24.Lopez A, Daly C, Vega-Hernandez G, et al. Elexacaftor/tezacaftor/ivacaftor projected survival and long-term health outcomes in people with cystic fibrosis homozygous for F508del. J Cyst Fibros 2023; 22: 607–614. doi: 10.1016/j.jcf.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 25.Martin C, Burnet E, Ronayette-Preira A, et al. Patient perspectives following initiation of elexacaftor–tezacaftor–ivacaftor in people with cystic fibrosis and advanced lung disease. Respir Med Res 2021; 80: 100829. doi: 10.1016/j.resmer.2021.100829 [DOI] [PubMed] [Google Scholar]
- 26.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi A, Nguyen H, Kuo CB, et al. Drug-induced liver injury associated with elexacaftor/tezacaftor/ivacaftor: a pharmacovigilance analysis of the FDA adverse event reporting system (FAERS). J Cyst Fibros 2024; 23: 566–572. doi: 10.1016/j.jcf.2024.01.001 [DOI] [PubMed] [Google Scholar]
- 28.VanElzakker MB, Tillman EM, Yonker LM, et al. Neuropsychiatric adverse effects from CFTR modulators deserve a serious research effort. Curr Opin Pulm Med 2023; 29: 603–609. doi: 10.1097/MCP.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey B, Correll CU, DeMaso DR, et al. Elexacaftor/tezacaftor/ivacaftor treatment and depression-related events. Am J Respir Crit Care Med 2024; 209: 299–306. doi: 10.1164/rccm.202308-1525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenfeld M, Wainwright CE, Higgins M, et al. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. Lancet Respir Med 2018; 6: 545–553. doi: 10.1016/S2213-2600(18)30202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szentpetery S, Foil K, Hendrix S, et al. A case report of CFTR modulator administration via carrier mother to treat meconium ileus in a F508del homozygous fetus. J Cyst Fibros 2022; 21: 721–724. doi: 10.1016/j.jcf.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 32.Ramos KJ, Pilewski JM, Taylor-Cousar JL. Challenges in the use of highly effective modulator treatment for cystic fibrosis. J Cyst Fibros 2021; 20: 381–387. doi: 10.1016/j.jcf.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guimbellot JS, Nichols DP, Brewington JJ. Novel applications of biomarkers and personalized medicine in cystic fibrosis. Clin Chest Med 2022; 43: 617–630. doi: 10.1016/j.ccm.2022.06.005 [DOI] [PubMed] [Google Scholar]
- 34.Hisert KB, Birket SE, Clancy JP, et al. Understanding and addressing the needs of people with cystic fibrosis in the era of CFTR modulator therapy. Lancet Respir Med 2023; 11: 916–931. doi: 10.1016/S2213-2600(23)00324-7 [DOI] [PubMed] [Google Scholar]
- 35.Britto CJ, Ratjen F, Clancy JP. Emerging approaches to monitor and modify care in the era of cystic fibrosis transmembrane conductance regulators. Clin Chest Med 2022; 43: 631–646. doi: 10.1016/j.ccm.2022.06.006 [DOI] [PubMed] [Google Scholar]
- 36.Donaldson SH, Laube BL, Mogayzel P, et al. Effect of lumacaftor–ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis: results from the PROSPECT MCC sub-study. J Cyst Fibros 2022; 21: 143–145. doi: 10.1016/j.jcf.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin C, Regard L, Chassagnon G, et al. Change in lung function after initiation of elexacaftor–tezacaftor–ivacaftor: do not forget anatomy! Am J Respir Crit Care Med 2022; 205: 1365–1366. doi: 10.1164/rccm.202112-2852LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cazier P, Chassagnon G, Dhote T, et al. Reversal of cylindrical bronchial dilatations in a subset of adults with cystic fibrosis treated with elexacaftor–tezacaftor–ivacaftor. Eur Respir J 2024; 63: 2301794. doi: 10.1183/13993003.01794-2023. [DOI] [PubMed] [Google Scholar]
- 39.Nissenbaum C, Davies G, Horsley A, et al. Monitoring early stage lung disease in cystic fibrosis. Curr Opin Pulm Med 2020; 26: 671–678. doi: 10.1097/MCP.0000000000000732 [DOI] [PubMed] [Google Scholar]
- 40.Mondéjar-López P, Horsley A, Ratjen F, et al. A multimodal approach to detect and monitor early lung disease in cystic fibrosis. Expert Rev Respir Med 2021; 15: 761–772. doi: 10.1080/17476348.2021.1908131 [DOI] [PubMed] [Google Scholar]
- 41.Ciet P, Booij R, Dijkshoorn M, et al. Chest radiography and computed tomography imaging in cystic fibrosis: current challenges and new perspectives. Pediatr Radiol 2023; 53: 649–659. doi: 10.1007/s00247-022-05522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hisert KB, Heltshe SL, Pope C, et al. restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 2017; 195: 1617–1628. doi: 10.1164/rccm.201609-1954OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols DP, Morgan SJ, Skalland M, et al. Pharmacologic improvement of CFTR function rapidly decreases sputum pathogen density, but lung infections generally persist. J Clin Invest 2023; 133: e167957. doi: 10.1172/JCI167957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaupp L, Addante A, Völler M, et al. Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on sputum viscoelastic properties, airway infection and inflammation in patients with cystic fibrosis. Eur Respir J 2023; 62: 2202153. doi: 10.1183/13993003.02153-2022 [DOI] [PubMed] [Google Scholar]
- 45.Zemanick ET, Wagner BD, Robertson CE, et al. Assessment of airway microbiota and inflammation in cystic fibrosis using multiple sampling methods. Ann Am Thorac Soc 2015; 12: 221–229. doi: 10.1513/AnnalsATS.201407-310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seidler D, Griffin M, Nymon A, et al. Throat swabs and sputum culture as predictors of P. aeruginosa or S. aureus lung colonization in adult cystic fibrosis patients. PLoS One 2016; 11: e0164232. doi: 10.1371/journal.pone.0164232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenn D, Abdel-Aziz MI, Brinkman P, et al. Comparison of microbial composition of cough swabs and sputum for pathogen detection in patients with cystic fibrosis. J Cyst Fibros 2022; 21: 52–60. doi: 10.1016/j.jcf.2021.08.031 [DOI] [PubMed] [Google Scholar]
- 48.Licht J-C, Seidl E, Slingers G, et al. Exhaled breath profiles to detect lung infection with Staphylococcus aureus in children with cystic fibrosis. J Cyst Fibros 2023; 22: 888–893. doi: 10.1016/j.jcf.2023.02.010 [DOI] [PubMed] [Google Scholar]
- 49.Davies JC, Cunningham S, Harris WT, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med 2016; 4: 107–115. doi: 10.1016/S2213-2600(15)00545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols AL, Davies JC, Jones D, et al. Restoration of exocrine pancreatic function in older children with cystic fibrosis on ivacaftor. Paediatr Respir Rev 2020; 35: 99–102. doi: 10.1016/j.prrv.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 51.Schwarzenberg SJ, Vu PT, Skalland M, et al. Elexacaftor/tezacaftor/ivacaftor and gastrointestinal outcomes in cystic fibrosis: report of promise-GI. J Cyst Fibros 2023; 22: 282–289. doi: 10.1016/j.jcf.2022.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calthorpe R, Rosenfeld M, Goss CH, et al. Pancreatic enzyme prescription following ivacaftor licensing: a retrospective analysis of the US and UK cystic fibrosis registries J Cyst Fibros 2024; 23: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ooi CY, Syed SA, Rossi L, et al. Impact of CFTR modulation with ivacaftor on gut microbiota and intestinal inflammation. Sci Rep 2018; 8: 17834. doi: 10.1038/s41598-018-36364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gelfond D, Heltshe S, Ma C, et al. Impact of CFTR modulation on intestinal pH, motility, and clinical outcomes in patients with cystic fibrosis and the G551D mutation. Clin Transl Gastroenterol 2017; 8: e81. doi: 10.1038/ctg.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen MC, Begnel L, Wallendorf M, et al. Effect of elexacaftor–tezacaftor–ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J Cyst Fibros 2022; 21: 265–271. doi: 10.1016/j.jcf.2021.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Proud D, Duckers J. Weight a minute: exploring the effect on weight and body composition after the initiation of elexacaftor/tezacaftor/ivacaftor in adults with CF. J Cyst Fibros 2023; 22: 847–850. doi: 10.1016/j.jcf.2023.06.002 [DOI] [PubMed] [Google Scholar]
- 57.Leonard A, Bailey J, Bruce A, et al. Nutritional considerations for a new era: a CF foundation position paper. J Cyst Fibros 2023; 22: 788–795. doi: 10.1016/j.jcf.2023.05.010 [DOI] [PubMed] [Google Scholar]
- 58.Lurquin F, Buysschaert M, Preumont V. Advances in cystic fibrosis-related diabetes: current status and future directions. Diabetes Metab Syndr 2023; 17: 102899. doi: 10.1016/j.dsx.2023.102899 [DOI] [PubMed] [Google Scholar]
- 59.Salazar-Barragan M, Taub DR. The effects of elexacaftor, tezacaftor, and ivacaftor (ETI) on blood glucose in patients with cystic fibrosis: a systematic review. Cureus 2023; 15: e41697. doi: 10.7759/cureus.41697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain R, Kazmerski TM, Zuckerwise LC, et al. Pregnancy in cystic fibrosis: review of the literature and expert recommendations. J Cyst Fibros 2022; 21: 387–395. doi: 10.1016/j.jcf.2021.07.019 [DOI] [PubMed] [Google Scholar]
- 61.Meiss LN, Jain R, Kazmerski TM. Family planning and reproductive health in cystic fibrosis. Clin Chest Med 2022; 43: 811–820. doi: 10.1016/j.ccm.2022.06.015 [DOI] [PubMed] [Google Scholar]
- 62.Jain R, Magaret A, Vu PT, et al. Prospectively evaluating maternal and fetal outcomes in the era of CFTR modulators: the MAYFLOWERS observational clinical trial study design. BMJ Open Respir Res 2022; 9: e001289. doi: 10.1136/bmjresp-2022-001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheikh SI, Long FR, McCoy KS, et al. Ivacaftor improves appearance of sinus disease on computerised tomography in cystic fibrosis patients with G551D mutation. Clin Otolaryngol 2015; 40: 16–21. doi: 10.1111/coa.12310 [DOI] [PubMed] [Google Scholar]
- 64.Sheikh S, Ho M-L, Eisner M, et al. Elexacaftor–tezacaftor–ivacaftor therapy for chronic sinus disease in cystic fibrosis. JAMA Otolaryngol Head Neck Surg 2023; 149: 1075–1082. doi: 10.1001/jamaoto.2023.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Gioia S, Lucca F, Venditto L, et al. Efficacy of elexacaftor–tezacaftor–ivacaftor on chronic rhinosinusitis in cystic fibrosis. Am J Otolaryngol 2024; 45: 104236. doi: 10.1016/j.amjoto.2024.104236 [DOI] [PubMed] [Google Scholar]
- 66.Sermet-Gaudelus I, Delion M, Durieu I, et al. Bone demineralization is improved by ivacaftor in patients with cystic fibrosis carrying the p.Gly551Asp mutation. J Cyst Fibros 2016; 15: e67–e69. doi: 10.1016/j.jcf.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Gur M, Bar-Yoseph R, Hanna M, et al. Effect of Trikafta on bone density, body composition and exercise capacity in CF: a pilot study. Pediatr Pulmonol 2023; 58: 577–584. doi: 10.1002/ppul.26243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Putman MS, Anabtawi A, Le T, et al. Cystic fibrosis bone disease treatment: current knowledge and future directions. J Cyst Fibros 2019; 18: Suppl. 2, S56–S65. doi: 10.1016/j.jcf.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 69.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009; 8: 91–96. doi: 10.1016/j.jcf.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayer-Hamblett N, Ratjen F, Russell R, et al. Discontinuation versus continuation of hypertonic saline or dornase alfa in modulator treated people with cystic fibrosis (SIMPLIFY): results from two parallel, multicentre, open-label, randomised, controlled, non-inferiority trials. Lancet Respir Med 2023; 11: 329–340. doi: 10.1016/S2213-2600(22)00434-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saunders T, Burgner D, Ranganathan S. Identifying and preventing cardiovascular disease in patients with cystic fibrosis. Nat Cardiovasc Res 2022; 1: 187–188. doi: 10.1038/s44161-022-00030-y [DOI] [PubMed] [Google Scholar]
- 72.Frost F, Nazareth D, Fauchier L, et al. Prevalence, risk factors and outcomes of cardiac disease in cystic fibrosis: a multinational retrospective cohort study. Eur Respir J 2023; 62: 2300174. doi: 10.1183/13993003.00174-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hebestreit H, Thornton CS. Cystic fibrosis and the cardiovascular system: the unexpected heartache. Eur Respir J 2023; 62: 2301253. doi: 10.1183/13993003.01253-2023 [DOI] [PubMed] [Google Scholar]
- 74.Maisonneuve P, Marshall BC, Knapp EA, et al. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst 2013; 105: 122–129. doi: 10.1093/jnci/djs481 [DOI] [PubMed] [Google Scholar]
- 75.Archangelidi O, Cullinan P, Simmonds NJ, et al. Incidence and risk factors of cancer in individuals with cystic fibrosis in the UK; a case–control study. J Cyst Fibros 2022; 21: 302–308. doi: 10.1016/j.jcf.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 76.Rousset-Jablonski C, Dalon F, Reynaud Q, et al. Cancer incidence and prevalence in cystic fibrosis patients with and without a lung transplant in France. Front Public Health 2022; 10: 1043691. doi: 10.3389/fpubh.2022.1043691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8: 65–124. doi: 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vertex . Vertex announces European Medicines Agency validation for marketing authorization application extension for KAFTRIO® in combination with ivacaftor to include people with cystic fibrosis and responsive rare mutations. Date last updated: 24 November 2023. https://news.vrtx.com/news-releases/news-release-details/vertex-announces-european-medicines-agency-validation-marketing
- 79.Huang Y, Paul G, Lee J, et al. Elexacaftor/tezacaftor/ivacaftor improved clinical outcomes in a patient with N1303K-CFTR based on in vitro experimental evidence. Am J Respir Crit Care Med 2021; 204: 1231–1235. doi: 10.1164/rccm.202101-0090LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Livnat G, Dagan A, Heching M, et al. Treatment effects of elexacaftor/tezacaftor/ivacaftor in people with CF carrying non-F508del mutations. J Cyst Fibros 2023; 22: 450–455. doi: 10.1016/j.jcf.2022.10.011 [DOI] [PubMed] [Google Scholar]
- 81.Burgel P-R, Sermet-Gaudelus I, Girodon E, et al. Gathering real-world compassionate data to expand eligibility for elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis with N1303 K or other rare CFTR variants: a viewpoint. Eur Respir J 2024; 63: 2301959. doi: 10.1183/13993003.01959-2023 [DOI] [PubMed] [Google Scholar]
- 82.Burgel P-R, Sermet-Gaudelus I, Durieu I, et al. The French compassionate programme of elexacaftor–tezacaftor–ivacaftor in people with cystic fibrosis with advanced lung disease and no F508del CFTR variant. Eur Respir J 2023; 61: 2202437. doi: 10.1183/13993003.02437-2022 [DOI] [PubMed] [Google Scholar]
- 83.Graeber SY, Mall MA. The future of cystic fibrosis treatment: from disease mechanisms to novel therapeutic approaches. Lancet 2023; 402: 1185–1198. doi: 10.1016/S0140-6736(23)01608-2 [DOI] [PubMed] [Google Scholar]