Abstract

The electrochemical CO2 reduction (eCO2R) in membrane electrode assemblies (MEAs) has brought e-chemical production one step closer to commercialization because of its advantages of minimized ohmic resistance and stackability. However, the current performance of reported eCO2R in MEAs is still far below the threshold for economic feasibility where low overall cell voltage (<2 V) and extensive stability (>5 years) are required. Furthermore, while the production cost of e-chemicals heavily relies on the carbon capture and product separation processes, these areas have received much less attention compared to CO2 electrolysis, itself. In this perspective, we examine the current status of eCO2R technologies from both academic and industrial points of view. We highlight the gap between current capabilities and commercialization standards and offer future research directions for eCO2R technologies with the hope of achieving industrially viable e-chemical production.
Keywords: CO2 electrolysis, Membrane electrode assembly, Carbon capture, Product separation, Commercialization
1. Introduction
The escalating global population and growing energy demand are seriously contributing to climate change with increased carbon dioxide (CO2) emissions. Faced with the impending depletion of fossil fuels, the carbon capture and utilization (CCU) connected to renewable energy is receiving huge attention as an efficient method to mitigate this climate crisis where atmospheric CO2 is directly captured and converted into value-added commodities, such as carbon monoxide, formate, methane, ethylene, and ethanol.1−12 The key technology enabling this field is electrochemical CO2 reduction (eCO2R), which has a history of decades of development. The recent application of the membrane electrode assembly (MEA)-type electrolyzer, which allows for a stack system with significantly reduced ohmic loss, has demonstrated the commercial potential of CO2 electrolysis technology, thereby heightening interest in this field.13,14
Nevertheless, there is a significant gap between the current targets in lab-scale CO2 electrolysis and the commercialization standards. For example, given that eCO2R can produce more than a dozen different products, most current reports prioritize the high selectivity of their target products to evaluate CO2 electrolysis performance. However, the economic viability of CO2 electrolysis is determined not only by product selectivity but also by applied cell voltage and stability of the overall system. Techno-economic analysis indicates that profitable CO2 electrolysis is achievable with a full-cell voltage below 2.5 V and a system stability of at least 5 years.15 Besides, while most lab-scale CO2 electrolysis studies are conducted with relatively small areas (<10 cm2), scaling up CO2 electrolysis in MEA systems must be validated through pilot-scale operations as this can lead to critical changes, such as mass transport or internal temperature.16
Furthermore, the commercialization of eCO2R requires advancements not only in CO2 electrolysis but also in the development and integration of all stages from carbon capturing, which captures CO2 from the emission sources or atmosphere, to purification processes converting resulting e-chemicals from CO2 electrolysis into final products.17,18 However, the absence of upstream and downstream processes in lab-scale CO2 electrolysis studies often leads to idealized assumptions about these excluded parts. For example, in terms of reactant supply, lab-scale CO2 electrolysis typically uses gaseous CO2 with ultrahigh purity, while the industrial carbon capturing processes immobilize atmospheric CO2 in liquid solutions using capture agents, resulting in additional costs for reactant purification unless the CO2-containing solution is directly utilized.19,20 Moreover, although unreacted CO2 is not significantly considered in determining CO2 electrolysis performance in most lab-scale studies, this represents a substantial loss from the perspective of the CO2 capturing process. In addition, despite the necessity of separating and concentrating the mixture of various eCO2R products for productization, the samples obtained in lab-scale experiments are only used to evaluate the selectivity of eCO2R system through gas and liquid chromatography.
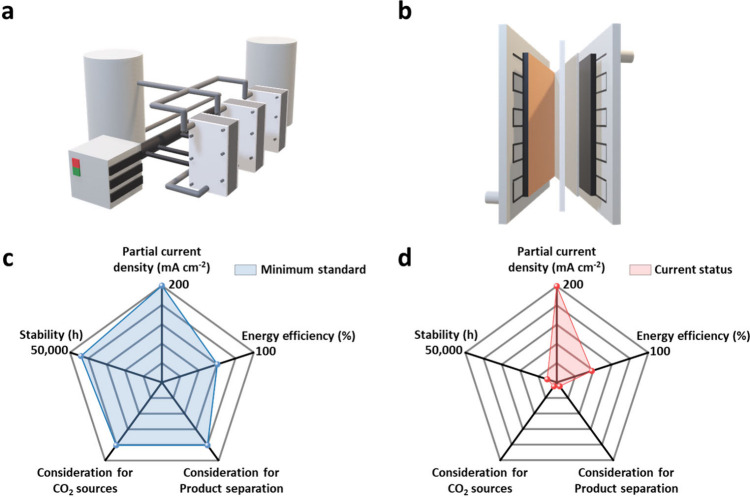
In this perspective, we aim to present research directions for the overall eCO2R field to bridge the gap between the current status and commercialization standards (Figure 1). As a first step, we explore the state-of-the-art progress in eCO2R technology within MEA systems that are closest to commercialization, meeting at least one of the following criteria: a minimum partial current density of 1 A cm–2, stability of more than 1,000 h, or pilot-scale operation. Then, we propose advancements for each component of the MEA system to achieve the commercialization target of high energy efficiency (>60%) and stability (>5 years). Finally, we address the previously overlooked upstream carbon capture and downstream product separation processes, suggesting necessary developments to ensure seamless integration with CO2 electrolysis and the economic viability of the CCU field.
Figure 1.
Illustrations of (a) an industrial-relevant eCO2R system and (b) the current MEA system. (c) Minimum standard for commercialization and (d) current status of eCO2R technology.
2. Current Status of Low-Temperature CO2 Electrolysis
Over the past decades, there has been significant advancement in eCO2R technologies, including numerous studies reporting that the partial current density of their target product surpasses the commercialization benchmark (>200 mA cm–2) and even a few attempts to operate eCO2R at a pilot scale. In this section, we provide a summary of the current state-of-the-art progress in eCO2R within MEA systems, focusing intensively on the production of carbon monoxide (CO) and formic acid (HCOOH). Since our focus in this perspective is commercialization, the investigations presented here are restricted to those that satisfy at least one of the following criteria: a minimum partial current density of 1 A cm–2, stability of more than 1,000 h, or pilot-scale operation.
2.1. Massive and Stable E-Chemical Production
2.1.1. Carbon Monoxide (CO)
Carbon monoxide (CO) is one of the simplest eCO2R products that can be produced through a two-electron transfer (eq 1), and many eCO2R studies targeting CO production have reported that their partial current densities (jCO) already exceed commercialization standards.21 CO can serve as both a feedstock for various chemical processes and a component of syngas which allows for the production of long-chain carbon products by combining it with a well-established Fischer–Tropsch process.22
| 1 |
In eCO2R, gold (Au) and silver (Ag) are well-known catalyst materials for CO production, with Ag-based electrocatalysts being more extensively utilized in state-of-the-art studies due to their cost-effectiveness, and superior stability compared to nonprecious metal catalysts.21,23−26 Edwards et al. achieved a jCO over 1.0 A cm–2 with 73% of FECO (@2.75 V), employing a strong alkaline electrolyte (5 M KOH) and high pressure of 50 bar.27 However, while strong alkaline electrolytes can improve energy efficiency by reducing overall cell voltage, their corrosiveness may negatively affect the long-term stability of the catalyst and cell components. Under dilute alkaline conditions (0.1 M CsOH), Endrődi et al. achieved a jCO of 1.0 A cm–2 with a high FECO of 90% (@3.0 V) at ambient pressure (1 bar), and mildly elevated temperature (60 °C).28 One distinctive feature of this MEA system is the utilization of the PiperION membrane, known for its high carbonate ion conductivity.28 Additionally, the primary crossover species, carbonate, neutralizes the electrolyte irrespective of its initial pH. making a carbonate-based electrolyte more appropriate than an alkaline electrolyte. Wen et al., by employing a neutral electrolyte (0.5 M KHCO3), achieved a remarkable jCO of 1.78 A cm–2 with 92% FECO (@3.5 V), at ambient temperature and pressure (1 bar, 25 °C). Notably, an in situ CO2(g)-liquid-catalyst interface was established by forced convection toward the porous electrode from an aqueous CO2-saturated electrolyte, enhancing the transportation of CO2, electron, proton, and product in this advanced MEA system.29
In terms of stability, the best CO2-to-CO conversions were achieved by companies. Dioxide Materials (USA) showcased their anion exchange membrane (Sustanion), performing stable electrolysis for 6 months (4,380 h) at a current density of 100 mA cm–2, with a 98% FECO (@3.0 V).30 Siemens (Germany) used a zirconium-oxide-based diaphragm membrane for consistent CO2 electrolysis at a commercially relevant current density of 300 mA cm–2 (@7.0–7.5 V) for 1,200 h, and demonstrated the production of butanol and hexanol with high carbon selectivity by connecting the obtained syngas from CO2 electrolysis to a fermentation module.31
2.1.2. Formic Acid (HCOOH)
With the growing interest in the hydrogen economy, there is an expectation of an expansion in the market size of formic acid as it can be utilized as a hydrogen carrier in addition to its conventional applications, such as a precursor to high-energy-density liquids (e.g., methanol) or fuel for power generation.32−35 Similar to CO, HCOOH is an eCO2R product involving a two-electron transfer (eq 2), where Bi, Sn, In, and Pb are well-known catalyst materials for its selective production. One advantage of CO2 to HCOOH conversion is that HCOOH is produced through a mechanistically distinct pathway from other eCO2R products that inevitably involve CO intermediates. This enables the inhibition of CO and beyond-CO products, simplifying subsequent separation processes since formic acid exists as the sole product in the liquid electrolyte.36
| 2 |
However, the presence of formic acid in its conjugate base form, HCOO–, in the alkaline or neutral environment (pKa of HCOOH = 3.745) in which most CO2 electrolyzers operate, necessitates the separation or acidification of the product-containing electrolyte.37−39 Moreover, since the crossover of anionic HCOO– to the anode and its reoxidization to CO2 in anion exchange membrane based MEA systems results in a substantial decrease in CO2 utilization efficiency, strategies to prevent such product loss are also required.
In this regard, an acidic eCO2R system can be an effective alternative. Fang et al. proposed acidic eCO2R for HCOOH production, utilizing a cation exchange membrane (CEM) system with a catalyst derived from waste lead-acid batteries (cathode recycled Pb, r-Pb).40 Given that the r-Pb catalyst, composed of lead and lead sulfate, can be readily prepared in large quantities (from kilograms to tons) from waste batteries, this system could significantly reduce the production cost of the catalyst, bringing the commercialization of e-HCOOH production closer. Furthermore, by employing a hydrogen oxidation reaction (HOR) as an alternative to oxygen evolution reaction (OER) for the anodic reaction, they were able to reduce the overall cell voltage, achieving a current density of 600 mA cm–2 (@2.2 V) for an impressive duration of 5,200 h while maintaining FEHCOOH over 90%.
2.1.3. C2+ Products
eCO2R products with two or more carbons (C2+ products, e.g., C2H4, C2H5OH, CH3COOH, C3H7OH, etc.) have high energy density, making them suitable for storing intermittent renewable electricity. In addition, the significant market size of C2+ products, such as C2H4 (120 MtC/year),41 offers further economic advantages. Nevertheless, producing C2+ products via eCO2R is challenging because their mechanistic pathway involves a range of intermediates and products, making it difficult to achieve high selectivity for a specific product. This complexity necessitates multiple steps of proton-coupled electron transfer, which inevitably leads to high cathodic overpotential. Therefore, to the best of our knowledge, there have been no studies that produce C2+ products within MEA systems that satisfy the three criteria we have previously outlined.
To overcome such limitations, a promising strategy could be first to produce high-purity CO via eCO2R and then produce C2+ products through subsequent electrochemical CO reduction (eCOR) in an eCO2R-eCOR tandem approach. Jiao et al. successfully established an eCO2R-eCOR tandem system consisting of a 500 cm2 CO2 electrolyzer and a 1000 cm2 CO electrolyzer.42 In the CO2 electrolyzer, they employed a carbon black modified Ag catalyst, achieving a jCO of 320 mA cm–2 with an 80% FECO at 3.0 V. This system was able to reduce the remaining unreacted CO2 in the gas-phase product mixture below 10 vol %, with an additional NaOH trap used to prevent CO2 from entering back to the CO electrolyzer. They then introduced fluorinated ethylene propylene (FEP) and carbon black reinforcement layer to the gas diffusion layer (GDL) of the CO electrolyzer and used a Cu catalyst mixed with carbon black and Nafion, achieving approximately 20% FEC2H4 and 50% FECH3COOH at 2.3 V. Through the operation of the stack CO electrolyzer at a total current of 300 A for 125 h, they produced 98 L of acetate solution (1.2 M) with an impressive 98% purity through continuous anolyte recirculation.
2.2. Pilot-Scale MEA Electrolyzers
Despite the significant achievements in lab-scale eCO2R studies, which commonly employ electrodes smaller than 10 cm2, scaling up the dimensions of the electrode and constructing a stack system is much more complex. For example, the uniform supply of CO2 or electrolyte to the electrode surface is not easily achieved in MEA systems with large electrode areas, emphasizing the importance of proper fluid flow channel design to alleviate mass transport issues.43,44 Since the CO2 flow rate can impact the mass transfer, current density, and single-pass CO2 conversion across the entire MEA system,45 and considering that the performance of eCO2R in a stack system is susceptible to gas flow or voltage distribution,46 it is essential to optimize the flow rate and stack design according to the target products and the eCO2R catalyst used. In addition, the pressure difference between the inlet and outlet should be precisely regulated in the stack system, as such differences may induce product or intermediate crossover.47,48 Besides, the internal temperature of the electrolyzer with large-area electrodes can rapidly increase at industrially relevant current densities (∼200 mA cm2) due to the Joule heating effect.16 Since the elevated temperature not only alters product selectivity but also accelerates the degradation of cell components, this necessitates the development of robust catalysts, ionomers, membranes, and other cell components that can withstand increased temperatures, as well as in situ analysis models or management techniques for continuous in-cell monitoring, which can replace post-mortem analysis.
From this point of view, pilot-scale operations with large electrode area electrolyzers and stack systems can offer valuable insights as an intermediate platform to bridge the gap between lab-scale eCO2R studies and their industrial implementation. In a pressurized MEA system, Han et al. demonstrated consistent CO production with 93% FECO at 100 mA cm–2 under 3 barg, regardless of electrode areas from 5 to 250 cm2. Interestingly, their system employed a bipolar membrane (BPM), effectively mitigating the issue of anodic CO2 crossover as an anionic carbonate in CO2 electrolyzers with anion exchange membranes (AEMs), and was able to significantly improve the CO2 single pass conversion up to 70%. Furthermore, Oh et al. successfully demonstrated a stack system that effectively connects three single MEA cells, achieving 50% FEC2H4 at 200 mA cm–2 using a Cu-KOH catalyst, with nearly identical eCO2R performance in terms of FE and unit cell voltage in both single cells and stack system.46
When the pilot-scale operation collaborates with model systems, their synergistic effect can accelerate the scale-up of eCO2R technology. Sinton et al. constructed a semiempirical model of an MEA electrolyzer, inputting operating parameters obtained from a lab-scale electrolyzer with an active area of 5 cm2 to output mass and energy balances for a larger electrolyzer, then validated the model’s predictions by comparing them to results obtained from a pilot-scale single MEA (800 cm2) and stack (10 × 800 cm2) system.49 Surprisingly, their model system was able to accurately predict the outcomes in the pilot-scale operations, with a low absolute error (<16%) for most variables. This included the finding that the carbon loss through anodic CO2 crossover, as well as the Nernstian and ohmic voltage losses in eCO2R system, is directly related with the type of main charge carrier species (hydroxide or (bi)carbonate ion) that changes with current density. Despite the limitation of the range of current density utilized in their predictive model, where higher current density might result in unexpected issues such as cathode flooding or membrane dehydration,50,51 the utilization and advancement of such model systems could prove highly beneficial in scaling eCO2R systems from lab-scale to pilot-scale and potentially to industrial implementation.
3. Scalable MEA-Type CO2 Electrolyzer
To date, most eCO2R studies have primarily focused on achieving high FE and partial current density for the target product. However, many technoeconomic analyses point out that for eCO2R to be economically viable, a minimum energy efficiency (EE) of 60% is needed (with renewable electricity price <0.04 $/kWh), while providing steady electrolysis for an extended duration surpassing 5 years.15,52,53 Unfortunately, as shown in Figure 2, there has been no study that has met these standards, as achieving over 60% EE is challenging in typical MEA systems using OER as the anode reaction and the state-of-the-art stability (5,200 h) remains far below the 5-year target. We summarized the current cutting-edge achievements of eCO2R in the MEA system for CO,27−31,54−64 HCOOH,2,40,59,65−70 and C2H412,71−74 in Table 1. In this section, we briefly review the factors that can hinder EE and stability in each component of the MEA and propose future research directions for the commercialization of eCO2R.
Figure 2.
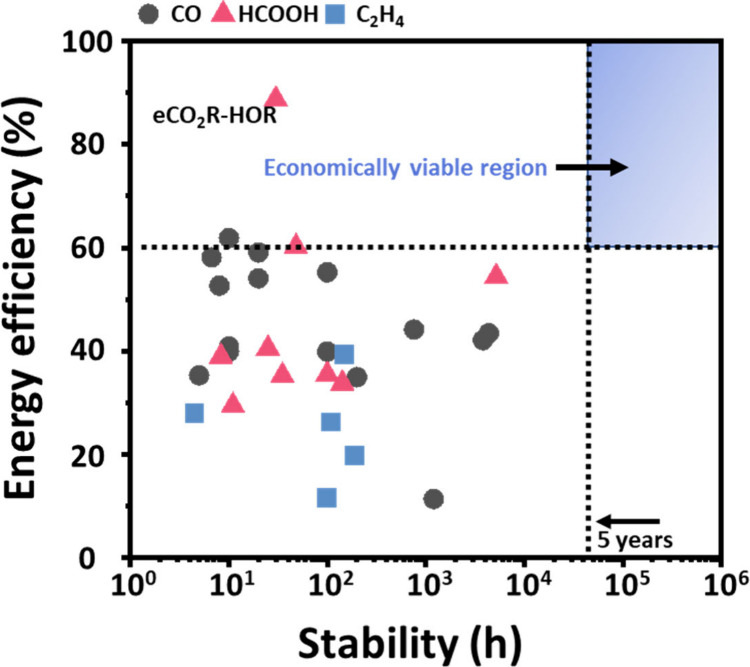
Current cutting-edge achievements of eCO2R in the MEA system regarding stability and energy efficiency.
Table 1. Current Cutting-Edge Achievements of eCO2R in the MEA System for CO, HCOOH, and C2H4.
| product | catalyst | electrolyte | FE (%) | Ecell (V) | EE (%) | stability (h) | reference |
|---|---|---|---|---|---|---|---|
| CO | Ag | 1 M KOH | 98 | 3 | 43.45 | 4,380 | (30) |
| CO | Ag | 0.01 M KHCO3 | 95 | 3 | 42.12 | 3,800 | (54) |
| CO | Ag | 0.1 M K2SO4/1.5 M KHCO3 | 60 | 7 | 11.4 | 1,200 | (31) |
| CO | Ag | 0.01 M KHCO3 | 98 | 2.95 | 44.18 | 760 | (55) |
| CO | Ag | 0.5 M KHCO3 | 92 | 3.5 | 34.96 | 200 | (29) |
| CO | Ag | 0.1 M CsOH | 90 | 3 | 39.9 | 100 | (28) |
| CO | Au | DI | 93 | 2.24 | 55.22 | 100 | (64) |
| CO | Ni–N–C | 0.1 M KHCO3 | 100 | 2.46 | 54.07 | 20 | (57) |
| CO | Ag | 1 M KOH | 99 | 2.23 | 59.04 | 20 | (58) |
| CO | CoPc-CN/CNT | 1 M KOH | 93 | 2 | 61.85 | 10 | (59) |
| CO | Ag | 7 M KOH | 86 | 2.8 | 40.85 | 10 | (60) |
| CO | Ag | 0.01 M KHCO3 | 90 | 3 | 39.9 | 10 | (61) |
| CO | CoPC | 1 M KOH | 95 | 2.4 | 52.65 | 8 | (62) |
| CO | Zn2P2O7 | 1 M KOH | 93.9 | 2.15 | 58.09 | 6.7 | (63) |
| CO | Ag | 5 M KOH | 73 | 2.75 | 35.31 | 5 | (27) |
| HCOOH | r-Pb | 0.5 M K2SO4 | 90 | 2.2 | 54.41 | 5,200 | (40) |
| HCOOH | Sn | DI | 94 | 3.7 | 33.79 | 142 | (65) |
| HCOOH | Bi | 0.1 M KOH | 80 | 3 | 35.47 | 100 | (66) |
| HCOOH | Sn | 1 M KOH | 95 | 2.1 | 60.17 | 48 | (70) |
| HCOOH | SnO2 | 1 M KOH | 61 | 2.3 | 35.27 | 35 | (59) |
| HCOOH | nBuLi-Bi | PSE | 90 | 1.35 | 88.67 | 30 | (2) |
| HCOOH | Sn | 2 M KOH | 81 | 2.7 | 40.5 | 25 | (67) |
| HCOOH | SnO2 | 0.4 M K2SO4 | 80 | 3.61 | 29.47 | 11 | (68) |
| HCOOH | Pb | 0.5 M K2SO4, H2SO4 | 82 | 2.8 | 38.95 | 8.3 | (69) |
| C2H4 | Cu | 0.1 M KHCO3 | 62 | 3.6 | 19.81 | 190 | (71) |
| C2H4 | Cu | 7 M KOH | 70 | 2.4 | 39.38 | 150 | (72) |
| C2H4 | Sputtering Cu | 1 M KOH | 65 | 2.85 | 26.23 | 110 | (73) |
| C2H4 | Cu | 0.1 M KHCO3 | 38 | 3.75 | 11.65 | 100 | (74) |
| C2H4 | Cu(100)-rich film | 0.1 M KHCO3 | 55.8 | 2.3 | 27.9 | 4.5 | (12) |
3.1. Energy Efficiency
The energy efficiency refers to the energy used to produce a specific product over the total energy consumed by the electrolysis system. As shown in eq 3, the higher EE can be achieved with enhanced selectivity for desired products at low cell voltage (Ecell), where EEi and FEi denote energy efficiency and Faradaic efficiency of the specific product, respectively.
| 3 |
In eCO2R, high selectivity has always been prioritized among various performance metrics due to the diversity of products. In particular, when the target eCO2R products are CO and HCOOH, which follow relatively simple two-electron transfer pathways, high Faradaic efficiencies (>90%) are often reported.29,30,57,62,66,75,76 This is observed not only in lab-scale experiments but also in large-scale electrodes and pilot-scale operations, indicating significant potential for commercializing these products. Meanwhile, achieving selective production of C2+ products remains challenging, with the highest reported FE of C2+ products (e.g., ethylene) in MEA systems still around 65%.73 Therefore, to accelerate the commercialization of C2+ products, high selectivity under industrially relevant conditions should be achieved by the collaborative efforts of fundamental research, and demonstrations on the MEA system.77−88
However, Ecell has received much less attention compared to FE when evaluating eCO2R performance, despite being equally crucial in determining EE. Technoeconomic analysis suggests that the production cost of e-chemicals is directly proportional to the cell voltage, where Ecell must not surpass 2.5 V and ideally below 2 V for economical e-chemical production.52,53 However, to the best of our knowledge, there have been no cases that have satisfied this standard in MEA systems using OER as the anode reaction, particularly when the partial current density for the target product exceeds the commercially viable threshold of 200 mA cm–2. This implies that achieving over 60% EE in commercializing CO2 electrolysis requires significant efforts to lower the overall cell voltage, in addition to focusing on improving selectivity. Since the Ecell of the MEA system is influenced by its all components, we will discuss current issues and potential solutions for each cell component to reduce the overall Ecell.
In an MEA system, the cathode typically comprises a catalyst layer, which includes a catalyst and ionomer, coated on a GDL.89 Therefore, Ecell may increase due to ohmic losses arising from the resistance of overall cathodic components, in addition to the overpotential of eCO2R (ηeCO2R). When highly conductive GDLs made of metals or carbon (>105 S m–1) are used, the resistance from the GDL does not significantly affect the overall MEA resistance.90 However, if hydrophobic materials like polytetrafluoroethylene (PTFE) are employed for stability enhancement, additional conductive materials (e.g., graphite layer) are necessary to reduce Ecell.72 In addition, within the catalyst layer, an excessively high or low ratio of catalyst to ionomer, which results in poor ion conductivity or poor electron transfer, respectively,91 or the use of inappropriate solvents leading to agglomeration of catalyst-ionomer composites, can cause additional ohmic losses and increase Ecell. Therefore, when determining the type of GDL or the composition of the catalyst ink, it is essential to consider not only the FE of eCO2R but also its impact on Ecell.
Moreover, in typical CO2 electrolysis coupled with OER as the anodic reaction, the thermodynamic potential required by both sides of the reactions ranges from 1.02 to 1.35 V depending on the target product,41 indicating that the overpotentials of eCO2R (ηeCO2R) and OER (ηOER) must be minimized to achieve a desirable Ecell of below 2.5 V. In this context, developing eCO2R catalysts that can reduce ηeCO2R while maintaining high selectivity is essential. Promising strategies for designing highly active catalysts include single-atom catalysts to enhance the initial proton-coupled electron transfer,56,92 dopant introduction,6,93 oxide-derived Cu-based catalysts to facilitate C–C coupling (particularly for C2+ products).4,94
However, despite the extensive research on OER, there have been only a limited number of studies focused on the development of OER electrocatalysts in eCO2R-relevant environments, especially in MEA systems.95−101 In particular, given that a near pH-neutral local anodic environment is created due to the crossover of a high flux carbonate, the development of highly active OER catalysts in such neutral environments is necessary to reduce ηOER.102 Meanwhile, as an alternative to addressing the challenge of OER in a neutral environment, one approach to achieve a lower Ecell is to utilize alternative organic compound oxidation reactions (e.g., glycerol oxidation) with lower thermodynamic potentials compared to OER.103 Besides, when utilizing alternative oxidation at the anode to coproduce value-added chemicals, targeted by cathodic eCO2R at the same time, it is possible to attain a maximum FE of up to 200%.104 Nevertheless, organic compound oxidation in anode with multiple products inevitably requires product separation process, which can be more severe when the crossover of eCO2R products occurred through the ion-exchange membrane. Moreover, the gradual decrease in the concentration of organic compounds during electrolysis may lead to reduced activity of the anodic reaction, although this can be mitigated by periodic reactant supplementation. Therefore, to effectively contribute to reducing Ecell, operational conditions that carefully balance the trade-off between the activity and reactant conversion are required when considering alternative anodic reactions to OER.
Furthermore, the low conductivity of the membrane used in CO2 electrolysis (4.5–10 S m–1) contributes a significant portion to the overall cell’s ohmic loss.90 Therefore, to improve the low conductivity of the membrane, it is necessary to optimize its chemical composition to increase ion exchange capacity and enhance its water uptake properties to facilitate ion transfer.105−110 Additionally, the inevitable interfacial losses between the cathode, membrane, and anode in MEAs produced via manual pressing contribute significantly to the overall Ecell, in addition to ohmic losses, where strategies from analogous water electrolysis, such as hot pressing, catalyst-coated membranes, and direct membrane deposition, could be adopted to minimize these interfacial losses.
3.2. Stability
Since each component of the CO2 electrolysis system is intimately interconnected, damage to a single component can lead to the failure of the entire system. Therefore, to achieve our target stability of over 5 years, balanced development of all MEA components is required. Generally, the stability of an MEA system encompasses both the mechanical and chemical stability of the components, as well as stable electrolysis performance. Thus, we have considered both types of stability.
To efficiently produce the desired target product, eCO2R catalysts are designed with appropriate surface engineering, which induces structural characteristics such as defects,111 grain boundaries,112 facets,12,113 and confinement,1,114 as well as chemical properties like oxidation states.4,6 However, structures with high catalytic activity generally have high surface energy, resulting in lower thermodynamic stability compared to bulk or flat surfaces.115 Consequently, during the eCO2R, active sites can dissolve and then agglomerate, forming an inactive catalyst surface with low electrochemical active surface area,116 which further decreases the electrolysis performance.117 Additionally, cathodic potential applied to the eCO2R catalyst or residual O2 in the inlet can change the chemical properties of catalyst during the reaction.118−121 Therefore, developing eCO2R catalysts that can maintain their initial characteristics is necessary. Promising strategies include forming continuous interconnected networks based on metal nanoparticles,122,123 using surface capping ligands124,125 to prevent the loss of metal active sites and structural changes, or introducing modifier elements with significantly different electronegativity or orbital occupancy into the elements constituting the active site through alloying4 or doping6 to maintain chemical properties during the reaction, as this method allows electrons to migrate in a single direction continuously.
The GDL serves as the conduit for the reactant (CO2) and gaseous products. Therefore, the failure of the GDL can lead to the mass transfer issue to the catalyst layer. Flooding is the most common phenomenon induced by physical or chemical failure of GDL, where the electrolyte infiltrates the GDL, rendering it nonfunctional as a conduit. Although using PTFE with high mechanical and chemical stability as a GDL can effectively prevent flooding, the trade-off between this improved stability and the low conductivity that may result in energy efficiency losses needs careful consideration. However, permanent damage to typical carbon-based GDLs can result from physical cracks or the formation of salts due to carbonation between CO2 and OH– in the electrolyte,48 while the electrowetting effect from cathodic voltage can weaken the hydrophobicity of the GDL.126 To prevent physical cracks to the GDL, the pressure difference between the inlet and outlet of gas-phase channel in the MEA system should be minimized,47,48 as well as the pressure balance on both sides of the GDL by adjusting the flow rates of the anolyte and CO2.127 Besides, strategies such as applying alternating current voltage during the reaction or flushing DI water through the catalyst layer to remove carbonate salts before they cause permanent damage to the GDL128 can be utilized to mitigate damage caused by carbonation.
Meanwhile, there have been only a few anode catalysts developed specifically for CO2 electrolysis, as it has been assumed that long-developed alkaline water oxidation catalysts could be directly applied for the anodic reaction. However, due to the high flux of carbonate crossover along with the continuous proton release from OER, a stable anodic catalyst that can survive in the near pH-neutral local anodic environment of an MEA system needs to be developed. Besides, given that the most common nonprecious metal anodic catalysts, such as Ni-based catalysts, can easily dissolve in neutral electrolytes,129 it is important to enhance their stability in commercially relevant CO2 electrolysis environments with strategies such as alloying or developing transition metal chalcogenides and phosphides.130−132 Moreover, if organic compound oxidation is employed as the anodic reaction to lower the Ecell, this will leads to more severe damage on anode catalyst as the anodic environment becomes more complex. In this context, incorporating a reference electrode into the existing MEA system could serve as an in situ diagnostic tool for deconvolution of the electrochemical performance of anode catalysts in the commercially viable device. While a significant disparity exists between the typical aqueous three-electrode setup and the local anodic environment in commercial CO2 electrolysis systems, this reference electrode-integrated MEA system is expected to bridge this gap, facilitating the rapid and accurate development of stable anodic catalysts suitable for CO2 electrolysis under industrially relevant environment.102,133,134
The ion-exchange membrane in an MEA system can be damaged by pressure, joule heating, and chemical corrosion.135,136 Specifically, the low mechanical and chemical stability of AEMs poses a significant challenge in the eCO2R operations within the MEA system. Currently available commercial AEMs were originally developed for electrodialysis and generally exhibit chemical stability up to pH 10.137 However, even when using a neutral anolyte, the local pH at the cathode-AEM interface can exceed pH 11 due to the generation of OH– at the cathode during eCO2R, leading to damage of the AEM.138 Furthermore, AEMs designed for electrodialysis are vulnerable to alcohols such as methanol, ethanol, and propanol,137 exacerbating stability issues when targeting alcohol production. However, although CEMs can provide improved mechanical and chemical stability compared to AEMs, solutions must be developed to address the acidic cathode environment due to the H+ transfer from the anode. Therefore, there is an urgent need to develop membranes specifically tailored for eCO2R applications. In this regard, leveraging accelerated stress testing (AST) protocols, which have been used in membrane development for fuel cells,139,140 is a promising approach for eCO2R as well. By subjecting eCO2R membranes to independent, short-term, high-intensity exposures of chemical (ion and product/intermediate concentration), thermal (temperature), and mechanical (stress) factors that could potentially degrade the membrane, it is possible to identify parameters that need reinforcement for stability enhancement, and suggest the future research direction.
4. CO2 Sources
Over the past few decades, most research on eCO2R has used refined gaseous CO2 as a reactant. Although this conventional scenario of separating the carbon capturing process from CO2 electrolysis offers flexibility by allowing the captured CO2 to be used in other fields besides CO2 electrolysis, this approach inevitably requires energy-intensive capturing agent regeneration and CO2 purification processes, which greatly reduce the economic viability of CCU. Hence, there is a growing need for a new scenario where CO2 immobilized in the capture agent is directly electroreduced instead of being purified. Although several pioneering eCO2R studies have been conducted in this regard, there still exists a discrepancy between the industrial carbon capturing process and the current status. In this section, we discuss the three possible sources of CO2, including direct air capture (DAC), amine-based chemical capture, and flue gas conversion, along with the factors that need to be considered when integrating them with eCO2R.
4.1. Direct Air Capture (DAC)
DAC is a method capable of directly capturing CO2 from the atmosphere, regardless of a specific emission source. In contrast to other carbon capture methods that can only be applied in locations with high CO2 concentrations (e.g., factory smokestacks) to achieve “carbon neutrality,″ DAC can be implemented anywhere to capture CO2 that has already been emitted into the atmosphere, thus moving toward “carbon negativity”. DAC is a long-developed technology and has a higher technology readiness level (TRL) compared to CO2 electrolysis. More than 20 companies, including AirCapture (USA), Climeworks (Switzerland), Carbon Engineering (Canada), and Soletair Power (Finland), have successfully completed large-scale pilot operations and are collectively capturing a total of 11,000 tons of CO2 annually. Although specific conditions differ by company, a typical DAC process generally consists of two major loops. In the first loop (loop 1), CO2 captured through an air container is fixed in an aqueous alkaline solution (e.g., KOH), where it exists in the form of carbonate (CO32–). For example, in the case of Carbon Engineering, a 2 M KOH initial aqueous alkaline solution is employed until the OH– concentration drops to 0.68 M, resulting in a solution containing 2.00 M K+, 0.68 M OH–, and 0.66 M CO32– after the first loop.20 Although this solution still retains some capacity to capture additional CO2, the lowered capturing efficiency due to the chemical equilibrium makes it applicable only for high CO2 emission sources, such as flue gas.141 Therefore, to fully utilize the advantage of DAC being location-independent, it is preferable to directly use this solution as a reactant in the eCO2R system, instead of further CO2 capturing. Moreover, while a typical second loop is used to concentrate the obtained solution into high-purity CO2 through multiple chemical steps requiring high temperatures and pressures,20 direct utilization of immobilized CO2 in eCO2R allows the replacement of loop 2, where the substitution of high-temperature, high-pressure processes with ambient conditions makes this integration more promising.18
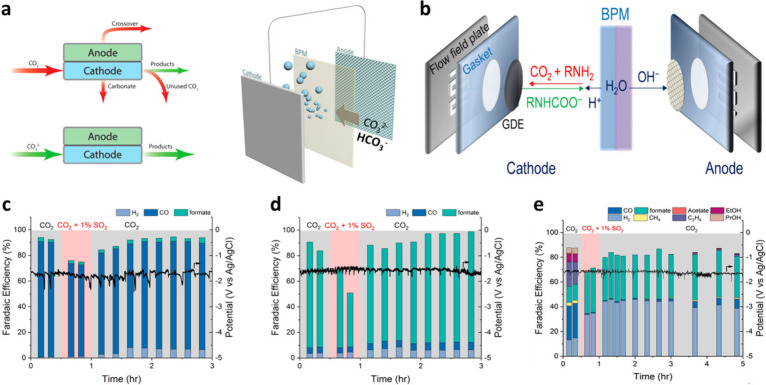
Nevertheless, to directly integrate DAC with eCO2R, several additional considerations need to be addressed. Since direct electroreduction of CO32– is not feasible, in situ generation of CO2 by reacting CO32– with H+ is necessary. Typically, a bipolar membrane (BPM) is employed for this purpose, where reverse bias is applied to induce water dissociation (WD) reactions to produce H+ and OH– ions (Figure 3a). H+ ions are then transported toward the cathode to facilitate local acidification of CO32– to convert it into gaseous CO2, which then can be used as the reactant.142 However, BPM, which consists of a CEM and an AEM with a bipolar junction between them, inevitably leads to higher ohmic and interfacial losses compared to monopolar membranes.143,144 Moreover, since two H+ ions are required to convert CO32– into CO2 via local acidification, the in situ CO2 generation step becomes rate-limiting for the entire system.145 Therefore, to increase the overall reaction rate, it is crucial to enhance the production rate of H+ ions through the development of BPMs with superior WD capabilities. As an alternative to BPM, employing CEM and HOR could potentially facilitate local acidification.146 However, it is important to consider the changes in fluid dynamics at the anode when the reactant transitions to the gaseous phase. Furthermore, the additional process for producing H2 as a reactant not only incurs additional costs for the entire process but also results in significant CO2 emissions, which could cancel out the effort toward achieving carbon negativity.
Figure 3.
Considerations for scenarios that directly connect eCO2R with the carbon capture process. (a) Carbon loss mechanisms in a CO2 electrolysis cell with gas-fed CO2 (left) and illustration of the BPM for the in situ generation of CO2 through the local acidification of (bi)carbonate (right). Reproduced from ref (145). Copyright 2019 American Chemical Society. (b) Schematic illustration of the in situ CO2 generation using BPM from the carbamate ion. Reproduced with permission from ref (152). Copyright 2022 Royal Society of Chemistry. (c) Impact of SO2 in the flue gas on the electrolysis using the (c) Ag, (d) Sn, and (e) Cu catalysts. Reproduced from ref (162). Copyright 2019 American Chemical Society.
Generally, many studies assume that the remaining catholyte after DAC-coupled eCO2R is reused in the capture process, thereby completing the overall capture-and-conversion loop. However, the catholyte, which gradually becomes more acidic due to the local acidification process, has a low CO2 capture efficiency and its direct utilization in the capture process may reduce the CO2 capture fraction to below 1%.147 The introduction of an additional electrodialysis unit can raise the pH of the catholyte to complete the loop, but this can increase both capital and operational costs.148 Therefore, when establishing an environmentally and economically viable DAC coupled eCO2R process, careful consideration, including a life cycle assessment of the entire process, will be essential.
4.2. Amine-Based Chemical Capture
Amine-based chemical capture is one of the wet absorption methods used in postcombustion CO2 capture within various carbon capture and storage (CCS) technologies.149 Unlike the dry absorption method, which stores CO2 in solid form, wet absorption is more suitable for integration with eCO2R electrolyzers as it stores CO2 in liquid form. Amines can be categorized into primary, secondary, and tertiary amines. Primary and secondary amines directly react with CO2 to store it as carbamate ions (RMHCOO–), whereas tertiary amines react with water to form OH–, thereby indirectly store CO2 as bicarbonate through carbonation.150 While the ability of primary and secondary amines to form carbamate ions provides relatively faster kinetics for CO2 capture reactions and allows operation under lower CO2 partial pressures, storing CO2 as carbamate ions involves strong C–N bonds, which require high overpotentials for C–N cleavage when integrated with CO2 electrolysis. Conversely, tertiary amines, which indirectly capture CO2, are rarely used in CCS processes due to their low capture efficiency but offer the advantage of requiring less electrical energy in subsequent CO2 electrolysis.150
When utilizing reactants in the form of carbamate fixed by primary or secondary amines in CO2 electrolysis, there are two main approaches. The first approach involves adding additives, such as alkali cations, to the CO2-captured carbamate solution. These additives can destabilize the strong C–N bond in carbamates, allowing carbamate to directly participate in eCO2R as a reactant, rather than merely serving as an additional anion to enhance the conductivity of the electrolyte.17,151 However, implementing a local acidification using a BPM, similar to the DAC-integrated eCO2R system, could be employed as an alternative approach.152 In this case, the H+ generated from the BPM can react with the carbamate ion (RMHCOO–) to produce CO2 and RNH2, allowing the cathode catalyst to utilize the in situ generated CO2 as a reactant (Figure 3b). Since the overall process will be heavily dependent on the C–N bond cleavage step, efficient local acidification through the development of BPMs will be essential, as discussed in Section 4.1.
When utilizing tertiary amines, CO2 is captured in the solution in the form of bicarbonate, also allowing for the in situ CO2 generation strategy by using BPMs (Figure 3a).153 Since bicarbonate does not have the strong C–N bond as carbamate, the energy required for in situ CO2 generation is much lower compared to when utilizing primary or secondary amines. However, the slow CO2 capture kinetics of tertiary amines result in a longer time needed to fix sufficient amounts of CO2, making these capturing agents suitable only for sources with relatively high CO2 concentrations,150 such as flue gas, and necessitating further research to enhance their CO2 capture efficiency.
Furthermore, it is worth noting the high affinity of amines for metals, which can lead to the formation of amine-metal complexes when amine-containing solution contacts with cathode catalysts.154,155 Since high concentrations of amines are used to maximize carbon capturing efficiency during the carbon capture process,156 it is crucial to develop eCO2R catalysts that can mitigate the binding between amines and metals when directly employing amine-containing solution.
4.3. Flue Gas
Directly utilizing flue gas as a CO2 source presents a promising strategy to bypass the energy-intensive CO2 purification process where industries such as coal-fired power plants, iron and steel manufacturing, and cement production, which emit substantial amounts of CO or CO2, can serve as potential emission sources for this approach. Compared to DAC and amine-based chemical capture, which utilize liquid-captured carbon sources, the direct conversion of flue gas can use gaseous reactants, allowing the use of typical eCO2R MEA electrolyzers, although the numerous impurities contained in the flue gas, aside from the required reactants (CO2/CO), must be pretreated.
Among the impurities present in flue gas, the most electrochemically active species are SO2 and NOx (NO, N2O, etc.). Since their thermodynamic reduction potentials are more positive than those of eCO2R, a high concentration of these impurities will divert the input power to side product formation rather than eCO2R, significantly reducing the current efficiency for e-chemical production. Therefore, desulfurization and denitrification processes must be performed prior to flue gas conversion. With the current technology, desulfurization and denitrification processes can generally remove about 90% of SO2 and 80% of NOx, leaving approximately 100 ppm of residual SO2 and NOx in the outlet.157,158 Fortunately, the reduced NOx concentration achieved through denitrification does not significantly affect the eCO2R reaction.159,160 However, due to the high affinity of sulfur for metals, even trace amounts of SO2 can easily poison the catalyst active sites, reducing the overall activity, selectivity, and stability of the reaction (Figure 3c–e).161 In particular, SO2 poisoning may cause permanent damage to Cu-based catalysts, which are considered the only element capable of producing C2+ products (Figure 3e).162 Therefore, when directly converting flue gas to C2+ products, strategies are necessary for Cu-based catalysts to survive in sulfur environments, such as introducing a passivation layer to block SO2 access to the catalyst.
In addition to SO2 and NOx, O2 can occupy as much as 14% (e.g., cement production industry) of the flue gas.163,164 Although O2 does not directly cause catalyst degradation like SO2, its positive reduction potential (oxygen reduction reaction, E° = 1.23 VRHE) compared to the eCO2R can consume most of the electricity input in the electrolyzer, even in trace amounts.165 Given that the solubility of O2 in water (1.22 mM) is much lower than that of CO2 (33.4 mM), increasing the hydrophilicity near the catalyst to prevent ORR could be considered.166 However, this approach might lead to GDL flooding during long-term operation. Therefore, despite the additional refining costs, a practical approach could involve implementing a deoxygenation process before introducing flue gas into the electrolyzer. Alternatively, restricting the application to industries like iron and steel, where flue gas contains minimal O2 (<1%), could also be viable.
Since flue gas features low reactant concentrations, developing eCO2R electrocatalysts with high activity and selectivity under low CO2 partial pressures is crucial for direct conversion of flue gas. For instance, inert nitrogen (N2), which is predominant in the composition of flue gas, ranges from about 5% to 47% in iron and steel industries,163 and even higher in other emission sources up to 80%.167,168 In addition, the high concentration of CO in flue gas could be beneficial as it can contribute to the reaction as a key intermediate when targeting C2+ products; however, when targeting CO or formate, it serves only as a diluting factor for the reactant CO2, similar to N2. Recognizing this challenge, there have been a few studies focused on addressing eCO2R under low CO2 partial pressure conditions.169−171 However, these studies have primarily focused on producing C1 products, and there have been no successful cases yet of producing C2+ products from CO2 concentrations diluted to less than 10%.
Consequently, we propose three criteria to consider when directly adopting flue gas as a reactant. First, prioritize emission sources with low O2 concentrations to minimize power loss. Second, target C1 products for emission sources with high N2 concentrations. Third, in the case of flue gas with a high concentration of CO, such as blast furnace gas or basic oxygen furnace gas in the iron and steel industry,163,172 aim to produce C2+ products using Cu-based catalysts while also considering the additional impact of SO2 in this scenario.
5. Product Separation Process
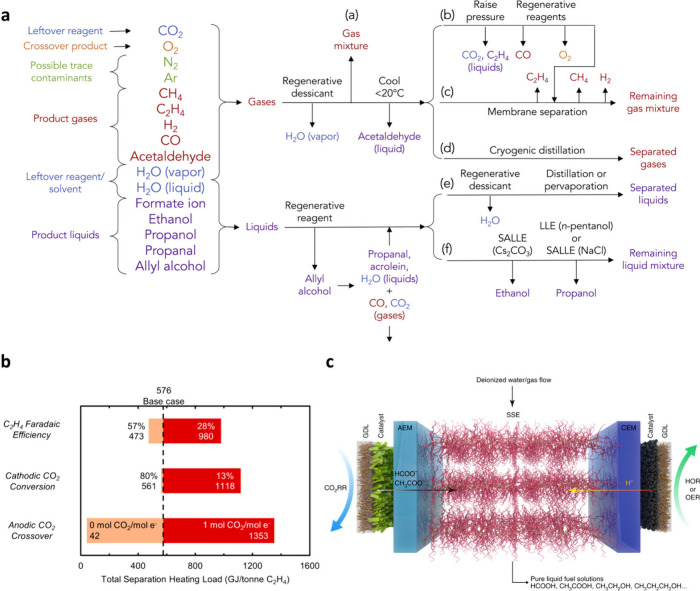
The ultimate goal of CO2 electrolysis is to economically produce high-purity e-chemicals, making the separation and concentration processes of the eCO2R product crucial, although these have been overlooked in most eCO2R studies. Since over a dozen products can be produced through eCO2R,41 separating the target product is not straightforward, and numerous energy analyses predict that the cost of separation and purification may exceed the cost of electrolysis if the concentration of products in the outlet is low.52,173 In this regard, the technology to concentrate individual products from the outlet is necessary and detailed strategies will vary depending on the characteristics of each product (Figure 4a). In this section, we aim to discuss the separation strategies of e-chemicals categorized into gas and liquid phases, and provide our viewpoint on considerations for future research.
Figure 4.
Considerations for the product separation processes. (a) Possible separation approach for a mixture of eCO2R products, reagents, and contaminants. Reproduced with permission from ref (174). Copyright 2018 Elsevier. (b) The impact of unreacted CO2 on the product separation cost. Reproduced from ref (173). Copyright 2021 American Chemical Society. (c) Illustration of porous solid electrolyte (PSE) used to concentrate liquid products. Reproduced with permission from ref (66). Copyright 2019 Springer Nature.
5.1. Separation of Gas Product
The mixture of gas-phase products commonly contains unreacted CO2 and water vapor. Therefore, the mixture gas from the outlet should first pass through CO2 adsorbents to recover unreacted CO2 and desiccants to remove water vapor.173,174 However, since the regeneration process of CO2 constitutes a significant portion of the entire separation process, it is necessary to develop strategies to maximize single-pass CO2 conversion (Figure 4b).173,175 To increase single-pass CO2 conversion, it is necessary to minimize both the unreacted CO2 that forms a mixture with gas-phase products and the loss of CO2 through carbonation. In typical MEA systems that utilize pure CO2 as the reactant, reducing the CO2 flow rate is a common strategy to decrease unreacted CO2.74,176,177 However, excessively low CO2 flow rates may promote competing reactions such as HER, necessitating careful optimization.74 Furthermore, the loss of CO2 through carbonation can be mitigated by conducting electrolysis in an acidic environment. In an ideal scenario where H3O+ is used as the proton source, carbonate formation is inhibited, and the (bi)carbonate formed at high current densities is regenerated into CO2 in the bulk electrolyte before passing through the membrane. Nonetheless, the lower FE and higher Ecell compared to neutral or alkaline eCO2R require improvement. Promising strategies include developing catalysts with enhanced CO2 adsorption178,179 or utilization of neutral buffer layers to mitigate acidic conditions near the catalyst surface.180,181 However, when directly converting immobilized CO2 in solution through DAC and amine-based chemical capture, unreacted CO2 remains in the captured solution,153 which is advantageous for gas-phase product separation. Conversely, in the direct conversion of flue gas containing high levels of impurities, additional process units might be required.
After removing unreacted CO2 and water vapor, the remaining gaseous eCO2R products, such as CO, CH4, and C2H4, along with H2 produced by HER, must be separated and concentrated, considering their specific properties. Furthermore, the gas-phase product mixture originating from the anode side contains not only high concentrations of O2 but also CO2 that has been reoxidized after crossover through the membrane. Additionally, gas-phase product crossover may occur due to leakage within the MEA system.174 Therefore, when designing the separation process, it is crucial to adequately consider not only eCO2R but also the products generated from the anode reaction. Typical gas-phase separation steps include cryogenic distillation, pressure/temperature-swing adsorption (PSA), and membrane-based separation, where the appropriate method depends on the target product to be separated.174 For example, cryogenic distillation could be promising for separating C2H4, as C2H4 (169 K) has a higher boiling point compared to other gas-phase products such as CO (81.5 K) and H2 (20.4 K) at 1.013 bar.173
5.2. Separation of Liquid Product
The typical MEA system for CO2 electrolysis operates with the circulation of liquid electrolytes (anolytes), and the liquid-phase products generated by eCO2R are present within the electrolyte in a diluted state. Regardless of the type of membrane used in an MEA system, all liquid-phase products can crossover through electro-osmotic drag or diffusion.182,183 This crossover is particularly notable when using an AEM under neutral and alkaline conditions, as products existing in anionic forms such as formate and acetate can migrate more extensively due to the electric field effect.184 For example, in an MEA system using an AEM, approximately 75% of the produced ethanol has crossover to the anolyte when electrolysis is conducted at a current density of 100 mA cm–2, resulting in an anolyte ethanol concentration of roughly 0.05 wt %, where the liquid-phase products in the anolyte can undergo reoxidation back to CO2, potentially causing further losses of products.182
Furthermore, although the adjustment of the volume of the electrolyte might increase the liquid product concentration to some extent, there remains a significant disparity between the concentrations achieved at the lab scale and those required to meet industrial purity standards. For example, in commercialized bioethanol production, ethanol with a concentration of 10 wt % is typically found at the outlet,185 suggesting that a minimum target concentration of about 10 wt % ethanol should also be set for e-ethanol production. Moreover, the cost of concentrating dilute products in the electrolyte through subsequent separation processes far surpasses the price of e-chemicals themselves, making this scenario economically challenging.174,186 Therefore, a strategy is needed to sufficiently concentrate the liquid products from eCO2R to achieve high purity before entering the separation process.
In order to concentrate the liquid product, utilizing a porous solid electrolyte (PSE) proposed by Wang et al. offers a promising strategy (Figure 4c).2,66 In this novel MEA system, the PSE is located between the AEM on the cathode side and the CEM on the anode side, capturing the liquid-phase products that pass through the AEM. Specifically, anionic products such as formate and acetate can combine with H+ ions generated from the anode to reform their original molecular forms, such as formic acid and acetic acid, within the PSE layer. These products are then concentrated by the continuous flow of inert gas (N2) passing through it. Using this novel system, state-of-the-art eCO2R liquid products with remarkably high concentrations have been achieved, with 49 wt % for formic acid and 13 wt % for alcohols, demonstrating the effectiveness of this MEA design.2,66,65,182 However, despite the promise of PSE for liquid product separation, the MEA system consisting of AEM/PSE/CEM inevitably suffers from high interfacial losses, resulting in relatively low energy efficiency compared to typical MEA systems. Therefore, further investigation is needed to develop strategies to minimize the Ecell of this novel MEA system.
Meanwhile, integrating the eCO2R system with the carbon capture process requires several considerations depending on the capture method. For example, when directly converting CO2 immobilized in solutions via DAC or amine-based chemicals, it is important to separate unreacted CO2, which exists in the form of carbamate or (bi)carbonate in the liquid electrolyte, as well as the high concentration of remaining capturing agents such as amines. Furthermore, if the eCO2R system directly utilizes flue gas with high impurities, effective methods may be required to remove highly water-soluble impurities such as SO2.
The concentrated liquid product exiting the outlet of the eCO2R system can be further refined for productization through additional separation processes including distillation and pervaporation.174 As with gas phase products, a rational design of the separation process is essential to reduce the overall process cost, while also considering the materials used in the process unit, especially when dealing with corrosive eCO2R products such as formic acid. For instance, distillation columns used for separating liquid products typically operate at high temperatures, where formic acid is known to be corrosive to stainless steel in particular.187 This corrosion of process units not only disrupts the stable operation of the entire process but also release metal cations to induce the decomposition of formic acid, decreasing the overall yield of the process.188 Hence, careful consideration of the characteristics of the possible products at the outlet is crucial when selecting materials for separation units. Furthermore, if the process is designed to simultaneously separate the gas phase and liquid phase outlet for minimal separation steps, the high partial pressure of the gas phase products can cause vacuum destruction of the distillation column, leading to corrosion of the process unit by the corrosive liquid phase products. Therefore, when designing a separation process, all possible failure factors should be considered, while pilot-scale operations can provide valuable information for identifying any overlooked issues during the process design.
6. Summary
In this perspective, we discussed future research directions aimed at bridging the gap between the standards necessary for the commercialization of eCO2R technology and its current status from three perspectives: CO2 electrolysis, CO2 sources, and product separation (Figure 5). As a first step, we explored the most advanced state-of-the-art progress in eCO2R technology within the MEA system reported to date. This exploration highlights the exciting progress made in leveraging successes achieved in lab-scale operations toward recent pilot-scale operations, aiming to pave the way for commercial-scale applications. At the same time, it reveals that even the state-of-the-art lab-scale achievements significantly diverges from the standards required for commercialization. Awareness of this gap emphasizes the need for a balanced development in eCO2R, which has focused heavily on achieving high selectivity and production rates. To bridge this gap, we have evaluated the fundamental hurdles in achieving high energy efficiency and stability in CO2 electrolysis. Faced with these issues, we have identified potential impediments in each component of the MEA system and proposed strategies to overcome them. Then, to enhance overall economic viability, we reviewed scenarios for directly utilizing CO2 immobilized in capture agents without purification or converting CO2 from emission sources, with a special emphasis on examining the distinct properties of captured CO2 via DAC and amine-based chemical capture and the potential threats posed by impurities in flue gas. Finally, the critical role of product separation processes in achieving the ultimate goal of productizing high-purity e-chemicals from eCO2R products is emphasized. We concluded that concentration and separation methods must be tailored to the specific characteristics of each eCO2R product, and potential equipment damage should be thoroughly considered during the process design. In addition, technoeconomic analysis has played a crucial role in assessing the current status of the technology and suggesting development directions from a commercial standpoint in all parts of eCO2R technology covered in this perspective. However, many existing technoeconomic analyses focus only on specific parts of eCO2R technology, assuming no economic losses in other areas. We believe that a comprehensive technoeconomic analysis considering all parts of eCO2R technology is necessary to serve as an accurate evaluation metric for achieving commercialization. We hope that this perspective will stimulate future research efforts addressing the previously overlooked aspects of eCO2R technology and believe that combining these efforts with the extensive knowledge accumulated over the past decades will accelerate the commercialization of eCO2R.
Figure 5.
Schematic illustration for the overall process of the eCO2R technology, including CO2 source (left), CO2 electrolysis (middle), and product separation (right).
Acknowledgments
This work was supported by the National Research Foundation of Korea (RS-2024-00343794 and RS-2024-00431568) funded by the Ministry of Science and ICT.
Author Contributions
§ H.L. and S.K. contributed equally to this work. Y.K. supervised the project. CRediT: Hojeong Lee conceptualization, investigation, methodology, validation, visualization, writing - original draft; Seontaek Kwon conceptualization, methodology, visualization, writing - original draft; Namgyoo Park writing - review & editing; Sun Gwan Cha writing - review & editing; Eunyoung Lee writing - review & editing; Tae-Hoon Kong writing - review & editing; Jihoo Cha writing - review & editing; Youngkook Kwon conceptualization, funding acquisition, project administration, supervision, writing - review & editing.
The authors declare no competing financial interest.
References
- Kim M. K.; Lee H.; Won J. H.; Sim W.; Kang S. J.; Choi H.; Sharma M.; Oh H.-S.; Ringe S.; Kwon Y.; Jeong H. M. Design of less than 1 nm Scale Spaces on SnO2 Nanoparticles for High-Performance. Adv. Funct. Mater. 2022, 32 (8), 2107349. 10.1002/adfm.202107349. [DOI] [Google Scholar]
- Fan L.; Xia C.; Zhu P.; Lu Y.; Wang H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 2020, 11 (1), 3633. 10.1038/s41467-020-17403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra K. K.; Liu Z.; Lee H.; Hong S.; Song H.; Abbas H. G.; Kwon Y.; Ringe S.; Oh J. Boosting Electrochemical CO2 Reduction to Methane via Tuning Oxygen Vacancy Concentration and Surface Termination on a Copper/Ceria Catalyst. ACS Catal. 2022, 12 (17), 10973–10983. 10.1021/acscatal.2c02669. [DOI] [Google Scholar]
- Sultan S.; Lee H.; Park S.; Kim M. M.; Yoon A.; Choi H.; Kong T.-H.; Koe Y.-J.; Oh H.-S.; Lee Z.; et al. Interface rich CuO/Al2CuO4 surface for selective ethylene production from electrochemical CO2 conversion. Energy Environ. Sci. 2022, 15 (6), 2397–2409. 10.1039/D1EE03861C. [DOI] [Google Scholar]
- De Luna P.; Hahn C.; Higgins D.; Jaffer S. A.; Jaramillo T. F.; Sargent E. H. What would it take for renewably powered electrosynthesis to displace petrochemical processes?. Science 2019, 364 (6438), eaav3506 10.1126/science.aav3506. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Che F.; Liu M.; Zou C.; Liang Z.; De Luna P.; Yuan H.; Li J.; Wang Z.; Xie H.; et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 2018, 10 (9), 974–980. 10.1038/s41557-018-0092-x. [DOI] [PubMed] [Google Scholar]
- Li F.; Li Y. C.; Wang Z.; Li J.; Nam D.-H.; Lum Y.; Luo M.; Wang X.; Ozden A.; Hung S.-F.; et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 2020, 3 (1), 75–82. 10.1038/s41929-019-0383-7. [DOI] [Google Scholar]
- Lee J. H.; Jang W.; Lee H.; Oh D.; Noh W. Y.; Kim K. Y.; Kim J.; Kim H.; An K.; Kim M. G.; et al. Tuning CuMgAl-Layered Double Hydroxide Nanostructures to Achieve CH4 and C2+ Product Selectivity in CO2 Electroreduction. Nano Lett. 2024, 24 (30), 9322–9330. 10.1021/acs.nanolett.4c02233. [DOI] [PubMed] [Google Scholar]
- Sang Z.; Tong Y.; Hou F.; Liang J. Recent Progress of Conductive Metal–Organic Frameworks for Electrochemical Energy Storage. Trans. Tianjin Univ. 2023, 29 (2), 136–150. 10.1007/s12209-022-00352-9. [DOI] [Google Scholar]
- Du X.; Zhang P.; Zhang G.; Gao H.; Zhang L.; Zhang M.; Wang T.; Gong J. Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways. Natl. Sci. Rev. 2024, 11 (2), nwad149. 10.1093/nsr/nwad149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D.; Li W.; Wang H.; Wang G.; Cai R. Heterogeneous Catalysis for CO2 Conversion into Chemicals and Fuels. Trans. Tianjin Univ. 2022, 28 (4), 245–264. 10.1007/s12209-022-00326-x. [DOI] [Google Scholar]
- Zhang G.; Zhao Z.-J.; Cheng D.; Li H.; Yu J.; Wang Q.; Gao H.; Guo J.; Wang H.; Ozin G. A.; et al. Efficient CO2 electroreduction on facet-selective copper films with high conversion rate. Nat. Commun. 2021, 12 (1), 5745. 10.1038/s41467-021-26053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L.; Rabiee H.; Li M.; Subramanian S.; Zheng Y.; Lee J. H.; Burdyny T.; Wang H. Electrochemical CO2 reduction in membrane-electrode assemblies. Chem. 2022, 8 (3), 663–692. 10.1016/j.chempr.2021.12.002. [DOI] [Google Scholar]
- She X.; Zhai L.; Wang Y.; Xiong P.; Li M. M.-J.; Wu T.-S.; Wong M. C.; Guo X.; Xu Z.; Li H.; et al. Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A. Nat. Energy 2024, 9 (1), 81–91. 10.1038/s41560-023-01415-4. [DOI] [Google Scholar]
- Shin H.; Hansen K. U.; Jiao F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 2021, 4 (10), 911–919. 10.1038/s41893-021-00739-x. [DOI] [Google Scholar]
- Iglesias van Montfort H.-P.; Burdyny T. Mapping Spatial and Temporal Electrochemical Activity of Water and CO2 Electrolysis on Gas-Diffusion Electrodes Using Infrared Thermography. ACS Energy Lett. 2022, 7 (8), 2410–2419. 10.1021/acsenergylett.2c00984. [DOI] [Google Scholar]
- Lee G.; Li Y. C.; Kim J.-Y.; Peng T.; Nam D.-H.; Sedighian Rasouli A.; Li F.; Luo M.; Ip A. H.; Joo Y.-C.; Sargent E. H. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 2021, 6 (1), 46–53. 10.1038/s41560-020-00735-z. [DOI] [Google Scholar]
- Sullivan I.; Goryachev A.; Digdaya I. A.; Li X.; Atwater H. A.; Vermaas D. A.; Xiang C. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 2021, 4 (11), 952–958. 10.1038/s41929-021-00699-7. [DOI] [Google Scholar]
- Dutcher B.; Fan M.; Russell A. G. Amine-based CO2 capture technology development from the beginning of 2013-A Review. ACS Appl. Mater. Interfaces. 2015, 7 (4), 2137–2148. 10.1021/am507465f. [DOI] [PubMed] [Google Scholar]
- Keith D. W.; Holmes G.; Angelo D. S.; Heidel K. A process for capturing CO2 from the atmosphere. Joule 2018, 2 (8), 1573–1594. 10.1016/j.joule.2018.05.006. [DOI] [Google Scholar]
- Dufek E. J.; Lister T. E.; Stone S. G.; McIlwain M. E. Operation of a pressurized system for continuous reduction of CO2. J. Electrochem. Soc. 2012, 159 (9), F514. 10.1149/2.011209jes. [DOI] [Google Scholar]
- Lee M.-Y.; Park K. T.; Lee W.; Lim H.; Kwon Y.; Kang S. Current achievements and the future direction of electrochemical CO2 reduction: A short review. Crit. Rev. Environ. Sci. Technol. 2020, 50 (8), 769–815. 10.1080/10643389.2019.1631991. [DOI] [Google Scholar]
- Ma M.; Trześniewski B. J.; Xie J.; Smith W. A. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts. Angew. Chem., Int. Ed. 2016, 128 (33), 9900–9904. 10.1002/ange.201604654. [DOI] [PubMed] [Google Scholar]
- Mistry H.; Choi Y. W.; Bagger A.; Scholten F.; Bonifacio C. S.; Sinev I.; Divins N. J.; Zegkinoglou I.; Jeon H. S.; Kisslinger K.; et al. Enhanced carbon dioxide electroreduction to carbon monoxide over defect-rich plasma-activated silver catalysts. Angew. Chem., Int. Ed. 2017, 129 (38), 11552–11556. 10.1002/ange.201704613. [DOI] [PubMed] [Google Scholar]
- Dufek E. J.; Lister T. E.; McIlwain M. E. Bench-scale electrochemical system for generation of CO and syn-gas. J. Appl. Electrochem. 2011, 41, 623–631. 10.1007/s10800-011-0271-6. [DOI] [Google Scholar]
- Verma S.; Lu X.; Ma S.; Masel R. I.; Kenis P. J. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016, 18 (10), 7075–7084. 10.1039/C5CP05665A. [DOI] [PubMed] [Google Scholar]
- Edwards J. P.; Xu Y.; Gabardo C. M.; Dinh C.-T.; Li J.; Qi Z.; Ozden A.; Sargent E. H.; Sinton D. Efficient electrocatalytic conversion of carbon dioxide in a low-resistance pressurized alkaline electrolyzer. Appl. Energy 2020, 261, 114305 10.1016/j.apenergy.2019.114305. [DOI] [Google Scholar]
- Endrődi B.; Kecsenovity E.; Samu A.; Halmágyi T.; Rojas-Carbonell S.; Wang L.; Yan Y.; Janáky C. High carbonate ion conductance of a robust PiperION membrane allows industrial current density and conversion in a zero-gap carbon dioxide electrolyzer cell. Energy Environ. Sci. 2020, 13 (11), 4098–4105. 10.1039/D0EE02589E. [DOI] [Google Scholar]
- Wen G.; Ren B.; Wang X.; Luo D.; Dou H.; Zheng Y.; Gao R.; Gostick J.; Yu A.; Chen Z. Continuous CO2 electrolysis using a CO2 exsolution-induced flow cell. Nat. Energy 2022, 7 (10), 978–988. 10.1038/s41560-022-01130-6. [DOI] [Google Scholar]
- Kutz R. B.; Chen Q.; Yang H.; Sajjad S. D.; Liu Z.; Masel I. R. Sustainion Imidazolium-Functionalized Polymers for Carbon Dioxide Electrolysis. Energy Technol. 2017, 5 (6), 929–936. 10.1002/ente.201600636. [DOI] [Google Scholar]
- Haas T.; Krause R.; Weber R.; Demler M.; Schmid G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 2018, 1 (1), 32–39. 10.1038/s41929-017-0005-1. [DOI] [Google Scholar]
- Tsurusaki A.; Murata K.; Onishi N.; Sordakis K.; Laurenczy G.; Himeda Y. Investigation of Hydrogenation of Formic Acid to Methanol using H2 or Formic Acid as a Hydrogen Source. ACS Catal. 2017, 7 (2), 1123–1131. 10.1021/acscatal.6b03194. [DOI] [Google Scholar]
- Dutta I.; Chatterjee S.; Cheng H.; Parsapur R. K.; Liu Z.; Li Z.; Ye E.; Kawanami H.; Low J. S. C.; Lai Z.; et al. Formic Acid to Power towards Low-Carbon Economy. Adv. Energy Mater. 2022, 12 (15), 2103799 10.1002/aenm.202103799. [DOI] [Google Scholar]
- Papadias D. D.; Peng J.-K.; Ahluwalia R. K. Hydrogen carriers: Production, transmission, decomposition, and storage. Int. J. Hydrogen Energy 2021, 46 (47), 24169–24189. 10.1016/j.ijhydene.2021.05.002. [DOI] [Google Scholar]
- Eppinger J.; Huang K.-W. Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2017, 2 (1), 188–195. 10.1021/acsenergylett.6b00574. [DOI] [Google Scholar]
- Bagger A.; Ju W.; Varela A. S.; Strasser P.; Rossmeisl J. Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem 2017, 18 (22), 3266–3273. 10.1002/cphc.201700736. [DOI] [PubMed] [Google Scholar]
- Zhu P.; Wang H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 2021, 4 (11), 943–951. 10.1038/s41929-021-00694-y. [DOI] [Google Scholar]
- Trivedi G.; Shah B.; Adhikary S.; Indusekhar V.; Rangarajan R. Studies on bipolar membranes. Part II—Conversion of sodium acetate to acetic acid and sodium hydroxide. React. Funct. Polym. 1997, 32 (2), 209–215. 10.1016/S1381-5148(96)00088-0. [DOI] [Google Scholar]
- Huang C.; Xu T.; Zhang Y.; Xue Y.; Chen G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288 (1–2), 1–12. 10.1016/j.memsci.2006.11.026. [DOI] [Google Scholar]
- Fang W.; Guo W.; Lu R.; Yan Y.; Liu X.; Wu D.; Li F. M.; Zhou Y.; He C.; Xia C.; et al. Durable CO2 conversion in the proton-exchange membrane system. Nature 2024, 626 (7997), 86–91. 10.1038/s41586-023-06917-5. [DOI] [PubMed] [Google Scholar]
- Nitopi S.; Bertheussen E.; Scott S. B.; Liu X.; Engstfeld A. K.; Horch S.; Seger B.; Stephens I. E.; Chan K.; Hahn C.; et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119 (12), 7610–7672. 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- Crandall B. S.; Ko B. H.; Overa S.; Cherniack L.; Lee A.; Minnie I.; Jiao F. Kilowatt-scale tandem CO2 electrolysis for enhanced acetate and ethylene production. Nat. Chem. Eng. 2024, 1, 421–429. 10.1038/s44286-024-00076-8. [DOI] [Google Scholar]
- Jung B.; Park S.; Lim C.; Lee W. H.; Lim Y.; Na J.; Lee C.-J.; Oh H.-S.; Lee U. Design methodology for mass transfer-enhanced large-scale electrochemical reactor for CO2 reduction. Chem. Eng. J. 2021, 424, 130265 10.1016/j.cej.2021.130265. [DOI] [Google Scholar]
- Jeanty P.; Scherer C.; Magori E.; Wiesner-Fleischer K.; Hinrichsen O.; Fleischer M. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO2 Util. 2018, 24, 454–462. 10.1016/j.jcou.2018.01.011. [DOI] [Google Scholar]
- Hawks S. A.; Ehlinger V. M.; Moore T.; Duoss E. B.; Beck V. A.; Weber A. Z.; Baker S. E. Analyzing Production Rate and Carbon Utilization Trade-offs in CO2RR Electrolyzers. ACS Energy Lett. 2022, 7 (8), 2685–2693. 10.1021/acsenergylett.2c01106. [DOI] [Google Scholar]
- Lee W. H.; Lim C.; Lee S. Y.; Chae K. H.; Choi C. H.; Lee U.; Min B. K.; Hwang Y. J.; Oh H.-S. Highly selective and stackable electrode design for gaseous CO2 electroreduction to ethylene in a zero-gap configuration. Nano Energy 2021, 84, 105859 10.1016/j.nanoen.2021.105859. [DOI] [Google Scholar]
- Legrand U.; Lee J. K.; Bazylak A.; Tavares J. R. Product Crossflow through a Porous Gas Diffusion Layer in a CO2 Electrochemical Cell with Pressure Drop Calculations. Ind. Eng. Chem. Res. 2021, 60 (19), 7187–7196. 10.1021/acs.iecr.1c01316. [DOI] [Google Scholar]
- Baumgartner L. M.; Koopman C. I.; Forner-Cuenca A.; Vermaas D. A. Narrow Pressure Stability Window of Gas Diffusion Electrodes Limits the Scale-Up of CO2 Electrolyzers. ACS Sustain. Chem. Eng. 2022, 10 (14), 4683–4693. 10.1021/acssuschemeng.2c00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. P.; Alerte T.; O’Brien C. P.; Gabardo C. M.; Liu S.; Wicks J.; Gaona A.; Abed J.; Xiao Y. C.; Young D.; et al. Pilot-Scale CO2 Electrolysis Enables a Semi-empirical Electrolyzer Model. ACS Energy Lett. 2023, 8 (6), 2576–2584. 10.1021/acsenergylett.3c00620. [DOI] [Google Scholar]
- Wheeler D. G.; Mowbray B. A. W.; Reyes A.; Habibzadeh F.; He J.; Berlinguette C. P. Quantification of water transport in a CO2 electrolyzer. Energy Environ. Sci. 2020, 13 (12), 5126–5134. 10.1039/D0EE02219E. [DOI] [Google Scholar]
- Weng L.-C.; Bell A. T.; Weber A. Z. A systematic analysis of Cu-based membrane-electrode assemblies for CO2 reduction through multiphysics simulation. Energy Environ. Sci. 2020, 13 (10), 3592–3606. 10.1039/D0EE01604G. [DOI] [Google Scholar]
- Jouny M.; Luc W.; Jiao F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57 (6), 2165–2177. 10.1021/acs.iecr.7b03514. [DOI] [Google Scholar]
- Masel R. I.; Liu Z.; Yang H.; Kaczur J. J.; Carrillo D.; Ren S.; Salvatore D.; Berlinguette C. P. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 2021, 16 (2), 118–128. 10.1038/s41565-020-00823-x. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Yang H.; Kutz R.; Masel R. I. CO2 electrolysis to CO and O2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 2018, 165 (15), J3371. 10.1149/2.0501815jes. [DOI] [Google Scholar]
- Kaczur J. J.; Yang H.; Liu Z.; Sajjad S. D.; Masel R. I. Carbon dioxide and water electrolysis using new alkaline stable anion membranes. Front. Chem. 2018, 6, 263. 10.3389/fchem.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller T.; Ju W.; Bagger A.; Wang X.; Luo F.; Ngo Thanh T.; Varela A. S.; Rossmeisl J.; Strasser P. Efficient CO2 to CO electrolysis on solid Ni–N–C catalysts at industrial current densities. Energy Environ. Sci. 2019, 12 (2), 640–647. 10.1039/C8EE02662A. [DOI] [Google Scholar]
- Zheng T.; Jiang K.; Ta N.; Hu Y.; Zeng J.; Liu J.; Wang H. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 2019, 3 (1), 265–278. 10.1016/j.joule.2018.10.015. [DOI] [Google Scholar]
- Cheng W.-H.; Richter M. H.; Sullivan I.; Larson D. M.; Xiang C.; Brunschwig B. S.; Atwater H. A. CO2 Reduction to CO with 19% Efficiency in a Solar-Driven Gas Diffusion Electrode Flow Cell under Outdoor Solar Illumination. ACS Energy Lett. 2020, 5 (2), 470–476. 10.1021/acsenergylett.9b02576. [DOI] [Google Scholar]
- Lu X.; Wu Y.; Yuan X.; Huang L.; Wu Z.; Xuan J.; Wang Y.; Wang H. High-Performance Electrochemical CO2 Reduction Cells Based on Non-noble Metal Catalysts. ACS Energy Lett. 2018, 3 (10), 2527–2532. 10.1021/acsenergylett.8b01681. [DOI] [Google Scholar]
- Gabardo C. M.; Seifitokaldani A.; Edwards J. P.; Dinh C.-T.; Burdyny T.; Kibria M. G.; O’Brien C. P.; Sargent E. H.; Sinton D. Combined high alkalinity and pressurization enable efficient CO2 electroreduction to CO. Energy Environ. Sci. 2018, 11 (9), 2531–2539. 10.1039/C8EE01684D. [DOI] [Google Scholar]
- Lee J.; Lim J.; Roh C.-W.; Whang H. S.; Lee H. Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes. J. CO2 Util. 2019, 31, 244–250. 10.1016/j.jcou.2019.03.022. [DOI] [Google Scholar]
- Ren S.; Joulié D.; Salvatore D.; Torbensen K.; Wang M.; Robert M.; Berlinguette C. P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365 (6451), 367–369. 10.1126/science.aax4608. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y.; Li W. J.; Chen J.; Wu X. F.; Liu Y. W.; Mao F.; Yuan H. Y.; Zhu M.; Dai S.; Wang H. F.; et al. In Operando Identification of In Situ Formed Metalloid Zincδ+ Active Sites for Highly Efficient Electrocatalyzed Carbon Dioxide Reduction. Angew. Chem., Int. Ed. 2022, 61 (28), e202202298 10.1002/anie.202202298. [DOI] [PubMed] [Google Scholar]
- Yin Z.; Peng H.; Wei X.; Zhou H.; Gong J.; Huai M.; Xiao L.; Wang G.; Lu J.; Zhuang L. An alkaline polymer electrolyte CO2 electrolyzer operated with pure water. Energy Environ. Sci. 2019, 12 (8), 2455–2462. 10.1039/C9EE01204D. [DOI] [Google Scholar]
- Yang H.; Kaczur J. J.; Sajjad S. D.; Masel R. I. Electrochemical conversion of CO2 to formic acid utilizing Sustainion membranes. J. CO2 Util. 2017, 20, 208–217. 10.1016/j.jcou.2017.04.011. [DOI] [Google Scholar]
- Xia C.; Zhu P.; Jiang Q.; Pan Y.; Liang W.; Stavitski E.; Alshareef H. N.; Wang H. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 2019, 4 (9), 776–785. 10.1038/s41560-019-0451-x. [DOI] [Google Scholar]
- De Mot B.; Ramdin M.; Hereijgers J.; Vlugt T. J. H.; Breugelmans T. Direct Water Injection in Catholyte-Free Zero-Gap Carbon Dioxide Electrolyzers. ChemElectroChem. 2020, 7 (18), 3839–3843. 10.1002/celc.202000961. [DOI] [Google Scholar]
- Chen Y.; Vise A.; Klein W. E.; Cetinbas F. C.; Myers D. J.; Smith W. A.; Deutsch T. G.; Neyerlin K. C. A Robust, Scalable Platform for the Electrochemical Conversion of CO2 to Formate: Identifying Pathways to Higher Energy Efficiencies. ACS Energy Lett. 2020, 5 (6), 1825–1833. 10.1021/acsenergylett.0c00860. [DOI] [Google Scholar]
- Lu X.; Leung D. Y. C.; Wang H.; Xuan J. A high performance dual electrolyte microfluidic reactor for the utilization of CO2. Appl. Energy 2017, 194, 549–559. 10.1016/j.apenergy.2016.05.091. [DOI] [Google Scholar]
- Lee W.; Kim Y. E.; Youn M. H.; Jeong S. K.; Park K. T. Catholyte-Free Electrocatalytic CO2 Reduction to Formate. Angew. Chem., Int. Ed. 2018, 57 (23), 6883–6887. 10.1002/anie.201803501. [DOI] [PubMed] [Google Scholar]
- Li F.; Thevenon A.; Rosas-Hernández A.; Wang Z.; Li Y.; Gabardo C. M.; Ozden A.; Dinh C. T.; Li J.; Wang Y.; et al. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577 (7791), 509–513. 10.1038/s41586-019-1782-2. [DOI] [PubMed] [Google Scholar]
- Dinh C.-T.; Burdyny T.; Kibria M. G.; Seifitokaldani A.; Gabardo C. M.; García de Arquer F. P.; Kiani A.; Edwards J. P.; De Luna P.; Bushuyev O. S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360 (6390), 783–787. 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- Adnan M. A.; Shayesteh Zeraati A.; Nabil S. K.; Al-Attas T. A.; Kannimuthu K.; Dinh C.-T.; Gates I. D.; Kibria M. G. Directly-Deposited Ultrathin Solid Polymer Electrolyte for Enhanced CO2 Electrolysis. Adv. Energy Mater. 2023, 13 (12), 2203158 10.1002/aenm.202203158. [DOI] [Google Scholar]
- Gabardo C. M.; O’Brien C. P.; Edwards J. P.; McCallum C.; Xu Y.; Dinh C.-T.; Li J.; Sargent E. H.; Sinton D. Continuous Carbon Dioxide Electroreduction to Concentrated Multi-carbon Products Using a Membrane Electrode Assembly. Joule 2019, 3 (11), 2777–2791. 10.1016/j.joule.2019.07.021. [DOI] [Google Scholar]
- Wu Z.-Y.; Zhu P.; Cullen D. A.; Hu Y.; Yan Q.-Q.; Shen S.-C.; Chen F.-Y.; Yu H.; Shakouri M.; Arregui-Mena J. D.; et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nat. Synth. 2022, 1 (8), 658–667. 10.1038/s44160-022-00129-x. [DOI] [Google Scholar]
- Zheng T.; Liu C.; Guo C.; Zhang M.; Li X.; Jiang Q.; Xue W.; Li H.; Li A.; Pao C.-W.; et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 2021, 16 (12), 1386–1393. 10.1038/s41565-021-00974-5. [DOI] [PubMed] [Google Scholar]
- Fan L.; Xia C.; Yang F.; Wang J.; Wang H.; Lu Y. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6 (8), eaay3111 10.1126/sciadv.aay3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun M.; Kundu J.; Kim D. H.; Kim M.; Kim D.; Lee K.; Choi S.-I. Strategies to Modulate the Copper Oxidation State Toward Selective C2+ Production in the Electrochemical CO2 Reduction Reaction. Adv. Mater. 2024, 36 (21), 2313028 10.1002/adma.202313028. [DOI] [PubMed] [Google Scholar]
- Yan T.; Chen X.; Kumari L.; Lin J.; Li M.; Fan Q.; Chi H.; Meyer T. J.; Zhang S.; Ma X. Multiscale CO2 Electrocatalysis to C2+ Products: Reaction Mechanisms, Catalyst Design, and Device Fabrication. Chem. Rev. 2023, 123 (17), 10530–10583. 10.1021/acs.chemrev.2c00514. [DOI] [PubMed] [Google Scholar]
- Chang B.; Pang H.; Raziq F.; Wang S.; Huang K.-W.; Ye J.; Zhang H. Electrochemical reduction of carbon dioxide to multicarbon (C2+) products: challenges and perspectives. Energy Environ. Sci. 2023, 16 (11), 4714–4758. 10.1039/D3EE00964E. [DOI] [Google Scholar]
- Xue Y.; Guo Y.; Cui H.; Zhou Z. Catalyst Design for Electrochemical Reduction of CO2 to Multicarbon Products. Small Methods 2021, 5 (10), 2100736 10.1002/smtd.202100736. [DOI] [PubMed] [Google Scholar]
- Ma M.; Seger B. Rational Design of Local Reaction Environment for Electrocatalytic Conversion of CO2 into Multicarbon Products. Angew. Chem., Int. Ed. 2024, 63 (23), e202401185 10.1002/anie.202401185. [DOI] [PubMed] [Google Scholar]
- Xiao C.; Zhang J. Architectural Design for Enhanced C2 Product Selectivity in Electrochemical CO2 Reduction Using Cu-Based Catalysts: A Review. ACS Nano 2021, 15 (5), 7975–8000. 10.1021/acsnano.0c10697. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Yang X.; Li Z.; Yang B.; Zhang Q.; Lei L.; Hou Y. Designs of Tandem Catalysts and Cascade Catalytic Systems for CO2 Upgrading. Angew. Chem., Int. Ed. 2023, 62 (43), e202307283 10.1002/anie.202307283. [DOI] [PubMed] [Google Scholar]
- Xiang K.; Shen F.; Fu Y.; Wu L.; Wang Z.; Yi H.; Liu X.; Wang P.; Liu M.; Lin Z.; Liu H. Boosting CO2 electroreduction towards C2+ products via CO* intermediate manipulation on copper-based catalysts. Environ. Sci. Nano 2022, 9 (3), 911–953. 10.1039/D1EN00977J. [DOI] [Google Scholar]
- Qin Q.; Suo H.; Chen L.; Wang Y.-X.; Wang J.-Z.; Liu H.-K.; Dou S.-X.; Lao M.; Lai W.-H. Emerging Cu-Based Tandem Catalytic Systems for CO2 Electroreduction to Multi-Carbon Products. Adv. Mater. Interfaces 2024, 11 (13), 2301049 10.1002/admi.202301049. [DOI] [Google Scholar]
- Bagchi D.; Roy S.; Sarma S. C.; Peter S. C. Toward Unifying the Mechanistic Concepts in Electrochemical CO2 Reduction from an Integrated Material Design and Catalytic Perspective. Adv. Funct. Mater. 2022, 32 (51), 2209023 10.1002/adfm.202209023. [DOI] [Google Scholar]
- Ma W.; He X.; Wang W.; Xie S.; Zhang Q.; Wang Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50 (23), 12897–12914. 10.1039/D1CS00535A. [DOI] [PubMed] [Google Scholar]
- Rabiee H.; Ge L.; Zhang X.; Hu S.; Li M.; Yuan Z. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review. Energy Environ. Sci. 2021, 14 (4), 1959–2008. 10.1039/D0EE03756G. [DOI] [Google Scholar]
- Lai W.; Qiao Y.; Zhang J.; Lin Z.; Huang H. Design strategies for markedly enhancing energy efficiency in the electrocatalytic CO2 reduction reaction. Energy Environ. Sci. 2022, 15 (9), 3603–3629. 10.1039/D2EE00472K. [DOI] [Google Scholar]
- Romiluyi O.; Danilovic N.; Bell A. T.; Weber A. Z. Membrane-electrode assembly design parameters for optimal CO2 reduction. Electrochem. Sci. Adv. 2023, 3 (1), e2100186 10.1002/elsa.202100186. [DOI] [Google Scholar]
- Zhou Y.; Zhou Q.; Liu H.; Xu W.; Wang Z.; Qiao S.; Ding H.; Chen D.; Zhu J.; Qi Z.; et al. Asymmetric dinitrogen-coordinated nickel single-atomic sites for efficient CO2 electroreduction. Nat. Commun. 2023, 14 (1), 3776. 10.1038/s41467-023-39505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-Y.; Hong D.; Lee J.-C.; Kim H. G.; Lee S.; Shin S.; Kim B.; Lee H.; Kim M.; Oh J.; et al. Quasi-graphitic carbon shell-induced Cu confinement promotes electrocatalytic CO2 reduction toward C2+ products. Nat. Commun. 2021, 12 (1), 3765. 10.1038/s41467-021-24105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoko A. D.; Chan K. W.; Yeo B. S. –CH3 Mediated Pathway for the Electroreduction of CO2 to Ethane and Ethanol on Thick Oxide-Derived Copper Catalysts at Low Overpotentials. ACS Energy Lett. 2017, 2 (9), 2103–2109. 10.1021/acsenergylett.7b00514. [DOI] [Google Scholar]
- Du J.; Chen Z.; Ye S.; Wiley B. J.; Meyer T. J. Copper as a Robust and Transparent Electrocatalyst for Water Oxidation. Angew. Chem., Int. Ed. 2015, 54 (7), 2073–2078. 10.1002/anie.201408854. [DOI] [PubMed] [Google Scholar]
- Meng Y.; Zhang X.; Hung W.-H.; He J.; Tsai Y.-S.; Kuang Y.; Kenney M. J.; Shyue J.-J.; Liu Y.; Stone K. H.; et al. Highly active oxygen evolution integrated with efficient CO2 to CO electroreduction. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (48), 23915–23922. 10.1073/pnas.1915319116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya K. S.; de Groot H. J. M. Controlled Surface-Assembly of Nanoscale Leaf-Type Cu-Oxide Electrocatalyst for High Activity Water Oxidation. ACS Catal. 2016, 6 (3), 1768–1771. 10.1021/acscatal.5b02950. [DOI] [Google Scholar]
- Li F.; Bai L.; Li H.; Wang Y.; Yu F.; Sun L. An iron-based thin film as a highly efficient catalyst for electrochemical water oxidation in a carbonate electrolyte. Chem. Commun. 2016, 52 (33), 5753–5756. 10.1039/C6CC00766J. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Zhang B.; De Luna P.; Liang Y.; Comin R.; Voznyy O.; Han L.; García de Arquer F. P.; Liu M.; Dinh C. T.; et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 2018, 10 (2), 149–154. 10.1038/nchem.2886. [DOI] [PubMed] [Google Scholar]
- Gurudayal; Bullock J.; Srankó D. F.; Towle C. M.; Lum Y.; Hettick M.; Scott M. C.; Javey A.; Ager J. Efficient solar-driven electrochemical CO2 reduction to hydrocarbons and oxygenates. Energy Environ. Sci. 2017, 10 (10), 2222–2230. 10.1039/C7EE01764B. [DOI] [Google Scholar]
- Zhang L.; Wang L.; Wen Y.; Ni F.; Zhang B.; Peng H. Boosting Neutral Water Oxidation through Surface Oxygen Modulation. Adv. Mater. 2020, 32 (31), 2002297 10.1002/adma.202002297. [DOI] [PubMed] [Google Scholar]
- Kwon S.; Kong T.-H.; Park N.; Thangavel P.; Lee H.; Shin S.; Cha J.; Kwon Y. Direction of oxygen evolution reaction electrocatalyst evaluation for an anion exchange membrane CO2 electrolyzer. EES catal. 2024, 2, 911–922. 10.1039/D3EY00314K. [DOI] [Google Scholar]
- Na J.; Seo B.; Kim J.; Lee C. W.; Lee H.; Hwang Y. J.; Min B. K.; Lee D. K.; Oh H.-S.; Lee U. General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation. Nat. Commun. 2019, 10 (1), 5193. 10.1038/s41467-019-12744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Tian B.; Xu X.; Wang X.; Ran P.; Sun Y.; Wu J.; Qiu A.; Wang F.; Tang L.; et al. Ampere-Level Electrolytic Coproduction of Formate with Coupled Carbon Dioxide Reduction and Selective Methanol Oxidation. ACS Catal. 2024, 14, 9476–9486. 10.1021/acscatal.4c01275. [DOI] [Google Scholar]
- Varcoe J. R.; Atanassov P.; Dekel D. R.; Herring A. M.; Hickner M. A.; Kohl P. A.; Kucernak A. R.; Mustain W. E.; Nijmeijer K.; Scott K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7 (10), 3135–3191. 10.1039/C4EE01303D. [DOI] [Google Scholar]
- Pan J.; Lu S.; Li Y.; Huang A.; Zhuang L.; Lu J. High-Performance alkaline polymer electrolyte for fuel cell applications. Adv. Funct. Mater. 2010, 20 (2), 312–319. 10.1002/adfm.200901314. [DOI] [Google Scholar]
- Disabb-Miller M. L.; Johnson Z. D.; Hickner M. A. Ion motion in anion and proton-conducting triblock copolymers. Macromolecules 2013, 46 (3), 949–956. 10.1021/ma301947t. [DOI] [Google Scholar]
- Kim Y. S.; Pivovar B. S. Moving beyond mass-based parameters for conductivity analysis of sulfonated polymers. Annu. Rev. Chem. Biomol. Eng. 2010, 1 (1), 123–148. 10.1146/annurev-chembioeng-073009-101309. [DOI] [PubMed] [Google Scholar]
- Dischinger S. M.; Gupta S.; Carter B. M.; Miller D. J. Transport of neutral and charged solutes in imidazolium-functionalized poly (phenylene oxide) membranes for artificial photosynthesis. Ind. Eng. Chem. Res. 2020, 59 (12), 5257–5266. 10.1021/acs.iecr.9b05628. [DOI] [Google Scholar]
- Krödel M.; Carter B. M.; Rall D.; Lohaus J.; Wessling M.; Miller D. J. Rational design of ion exchange membrane material properties limits the crossover of CO2 reduction products in artificial photosynthesis devices. ACS Appl. Mater. Interfaces. 2020, 12 (10), 12030–12042. 10.1021/acsami.9b21415. [DOI] [PubMed] [Google Scholar]
- Gong Q.; Ding P.; Xu M.; Zhu X.; Wang M.; Deng J.; Ma Q.; Han N.; Zhu Y.; Lu J.; et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 2019, 10 (1), 2807. 10.1038/s41467-019-10819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W.; Chen Z.; Guo W.; Mao W.; Liu Y.; Guo Y.; Chen J.; Huang R.; Kang L.; Ma Y.; et al. Pb-rich Cu grain boundary sites for selective CO-to-n-propanol electroconversion. Nat. Commun. 2023, 14 (1), 4882. 10.1038/s41467-023-40689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C.; Kwon S.; Cheng T.; Xu M.; Tieu P.; Lee C.; Cai J.; Lee H. M.; Pan X.; Duan X.; et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 2020, 3 (10), 804–812. 10.1038/s41929-020-00504-x. [DOI] [Google Scholar]
- Jeong H. M.; Kwon Y.; Won J. H.; Lum Y.; Cheng M.-J.; Kim K. H.; Head-Gordon M.; Kang J. K. Atomic-Scale Spacing between Copper Facets for the Electrochemical Reduction of Carbon Dioxide. Adv. Energy Mater. 2020, 10 (10), 1903423 10.1002/aenm.201903423. [DOI] [Google Scholar]
- Hori Y.; Wakebe H.; Tsukamoto T.; Koga O. Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons. Surf. Sci. 1995, 335, 258–263. 10.1016/0039-6028(95)00441-6. [DOI] [Google Scholar]
- Choi W.; Won D. H.; Hwang Y. J. Catalyst design strategies for stable electrochemical CO2 reduction reaction. J. Mater. Chem. A 2020, 8 (31), 15341–15357. 10.1039/D0TA02633F. [DOI] [Google Scholar]
- Lum Y.; Yue B.; Lobaccaro P.; Bell A. T.; Ager J. W. Optimizing C–C Coupling on Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121 (26), 14191–14203. 10.1021/acs.jpcc.7b03673. [DOI] [Google Scholar]
- Li X.; Wang S.; Li L.; Sun Y.; Xie Y. Progress and perspective for in situ studies of CO2 reduction. J. Am. Chem. Soc. 2020, 142 (21), 9567–9581. 10.1021/jacs.0c02973. [DOI] [PubMed] [Google Scholar]
- Wang J.; Tan H. Y.; Zhu Y.; Chu H.; Chen H. M. Linking the dynamic chemical state of catalysts with the product profile of electrocatalytic CO2 reduction. Angew. Chem., Int. Ed. 2021, 133 (32), 17394–17407. 10.1002/ange.202017181. [DOI] [PubMed] [Google Scholar]
- Mu S.; Lu H.; Wu Q.; Li L.; Zhao R.; Long C.; Cui C. Hydroxyl radicals dominate reoxidation of oxide-derived Cu in electrochemical CO2 reduction. Nat. Commun. 2022, 13 (1), 3694. 10.1038/s41467-022-31498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Yang Y.; Tang Y.; Gao Q. In-situ reconstruction of catalysts in cathodic electrocatalysis: New insights into active-site structures and working mechanisms. J. Energy Chem. 2022, 70, 414–436. 10.1016/j.jechem.2022.02.036. [DOI] [Google Scholar]
- Welch A. J.; DuChene J. S.; Tagliabue G.; Davoyan A.; Cheng W.-H.; Atwater H. A. Nanoporous Gold as a Highly Selective and Active Carbon Dioxide Reduction Catalyst. ACS Appl. Energy Mater. 2019, 2 (1), 164–170. 10.1021/acsaem.8b01570. [DOI] [Google Scholar]
- Kim D.; Kley C. S.; Li Y.; Yang P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2-C3 products. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (40), 10560–10565. 10.1073/pnas.1711493114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.; Hu W.; Pan Q.; Zou L.; Zou Z.; Wen K.; Yang H. Polyvinyl alcohol-modified gold nanoparticles with record-high activity for electrochemical reduction of CO2 to CO. J. CO2 Util. 2019, 34, 108–114. 10.1016/j.jcou.2019.06.002. [DOI] [Google Scholar]
- Cao Z.; Kim D.; Hong D.; Yu Y.; Xu J.; Lin S.; Wen X.; Nichols E. M.; Jeong K.; Reimer J. A.; et al. A Molecular Surface Functionalization Approach to Tuning Nanoparticle Electrocatalysts for Carbon Dioxide Reduction. J. Am. Chem. Soc. 2016, 138 (26), 8120–8125. 10.1021/jacs.6b02878. [DOI] [PubMed] [Google Scholar]
- Li M.; Idros M. N.; Wu Y.; Burdyny T.; Garg S.; Zhao X. S.; Wang G.; Rufford T. E. The role of electrode wettability in electrochemical reduction of carbon dioxide. J. Mater. Chem. A 2021, 9 (35), 19369–19409. 10.1039/D1TA03636J. [DOI] [Google Scholar]
- De Mot B.; Hereijgers J.; Duarte M.; Breugelmans T. Influence of flow and pressure distribution inside a gas diffusion electrode on the performance of a flow-by CO2 electrolyzer. Chem. Eng. J. 2019, 378, 122224 10.1016/j.cej.2019.122224. [DOI] [Google Scholar]
- Xu Y.; Edwards J. P.; Liu S.; Miao R. K.; Huang J. E.; Gabardo C. M.; O’Brien C. P.; Li J.; Sargent E. H.; Sinton D. Self-Cleaning CO2 Reduction Systems: Unsteady Electrochemical Forcing Enables Stability. ACS Energy Lett. 2021, 6 (2), 809–815. 10.1021/acsenergylett.0c02401. [DOI] [Google Scholar]
- Vass A. d.; Endrődi B.; Samu G. F.; Balog A. d.; Kormányos A.; Cherevko S.; Janáky C. Local chemical environment governs anode processes in CO2 electrolyzers. ACS Energy Lett. 2021, 6 (11), 3801–3808. 10.1021/acsenergylett.1c01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Zhao K.; Yu Z.; Sun C.; Wang Z.; Feng N.; Mai L.; Wang Y.; Xia Y. In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction. Energy Environ. Sci. 2020, 13 (7), 2200–2208. 10.1039/D0EE00755B. [DOI] [Google Scholar]
- Liang H.; Gandi A. N.; Xia C.; Hedhili M. N.; Anjum D. H.; Schwingenschlögl U.; Alshareef H. N. Amorphous NiFe-OH/NiFeP Electrocatalyst Fabricated at Low Temperature for Water Oxidation Applications. ACS Energy Lett. 2017, 2 (5), 1035–1042. 10.1021/acsenergylett.7b00206. [DOI] [Google Scholar]
- Chen W.; Wang H.; Li Y.; Liu Y.; Sun J.; Lee S.; Lee J.-S.; Cui Y. In Situ Electrochemical Oxidation Tuning of Transition Metal Disulfides to Oxides for Enhanced Water Oxidation. ACS Cent. Sci. 2015, 1 (5), 244–251. 10.1021/acscentsci.5b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D.; Berlinguette C. P. Voltage Matters When Reducing CO2 in an Electrochemical Flow Cell. ACS Energy Lett. 2020, 5 (1), 215–220. 10.1021/acsenergylett.9b02356. [DOI] [Google Scholar]
- Hansen K. U.; Cherniack L. H.; Jiao F. Voltage Loss Diagnosis in CO2 Electrolyzers Using Five-Electrode Technique. ACS Energy Lett. 2022, 7 (12), 4504–4511. 10.1021/acsenergylett.2c02096. [DOI] [Google Scholar]
- Wu J.; Yuan X. Z.; Martin J. J.; Wang H.; Zhang J.; Shen J.; Wu S.; Merida W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184 (1), 104–119. 10.1016/j.jpowsour.2008.06.006. [DOI] [Google Scholar]
- Ran J.; Wu L.; He Y.; Yang Z.; Wang Y.; Jiang C.; Ge L.; Bakangura E.; Xu T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. 10.1016/j.memsci.2016.09.033. [DOI] [Google Scholar]
- Salvatore D. A.; Gabardo C. M.; Reyes A.; O’Brien C. P.; Holdcroft S.; Pintauro P.; Bahar B.; Hickner M.; Bae C.; Sinton D.; et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 2021, 6 (4), 339–348. 10.1038/s41560-020-00761-x. [DOI] [Google Scholar]
- Larrazábal G. O.; Strøm-Hansen P.; Heli J. P.; Zeiter K.; Therkildsen K. T.; Chorkendorff I.; Seger B. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces. 2019, 11 (44), 41281–41288. 10.1021/acsami.9b13081. [DOI] [PubMed] [Google Scholar]
- Huang B. T.; Chatillon Y.; Bonnet C.; Lapicque F.; Leclerc S.; Hinaje M.; Raël S. Experimental Investigation of Air Relative Humidity (RH) Cycling Tests on MEA/Cell Aging in PEMFC Part I: Study of High RH Cycling Test With air RH at 62%/100%. Fuel Cells 2012, 12 (3), 335–346. 10.1002/fuce.201100060. [DOI] [Google Scholar]
- Rodgers M. P.; Bonville L. J.; Kunz H. R.; Slattery D. K.; Fenton J. M. Fuel Cell Perfluorinated Sulfonic Acid Membrane Degradation Correlating Accelerated Stress Testing and Lifetime. Chem. Rev. 2012, 112 (11), 6075–6103. 10.1021/cr200424d. [DOI] [PubMed] [Google Scholar]
- Borhani T. N. G.; Azarpour A.; Akbari V.; Wan Alwi S. R.; Manan Z. A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. Int. J. Greenhouse Gas Control 2015, 41, 142–162. 10.1016/j.ijggc.2015.06.026. [DOI] [Google Scholar]
- Watkins J. D.; Siefert N. S.; Zhou X.; Myers C. R.; Kitchin J. R.; Hopkinson D. P.; Nulwala H. B. Redox-mediated separation of carbon dioxide from flue gas. Energy Fuels 2015, 29 (11), 7508–7515. 10.1021/acs.energyfuels.5b01807. [DOI] [Google Scholar]
- Mayerhöfer B.; McLaughlin D.; Böhm T.; Hegelheimer M.; Seeberger D.; Thiele S. Bipolar Membrane Electrode Assemblies for Water Electrolysis. ACS Appl. Energy Mater. 2020, 3 (10), 9635–9644. 10.1021/acsaem.0c01127. [DOI] [Google Scholar]
- Giesbrecht P. K.; Freund M. S. Recent Advances in Bipolar Membrane Design and Applications. Chem. Mater. 2020, 32 (19), 8060–8090. 10.1021/acs.chemmater.0c02829. [DOI] [Google Scholar]
- Li Y. C.; Lee G.; Yuan T.; Wang Y.; Nam D.-H.; Wang Z.; García de Arquer F. P.; Lum Y.; Dinh C.-T.; Voznyy O.; Sargent E. H. CO2 Electroreduction from Carbonate Electrolyte. ACS Energy Lett. 2019, 4 (6), 1427–1431. 10.1021/acsenergylett.9b00975. [DOI] [Google Scholar]
- Zhang Z.; Lees E. W.; Ren S.; Mowbray B. A. W.; Huang A.; Berlinguette C. P. Conversion of Reactive Carbon Solutions into CO at Low Voltage and High Carbon Efficiency. ACS Cent. Sci. 2022, 8 (6), 749–755. 10.1021/acscentsci.2c00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almajed H. M.; Kas R.; Brimley P.; Crow A. M.; Somoza-Tornos A.; Hodge B.-M.; Burdyny T. E.; Smith W. A. Closing the Loop: Unexamined Performance Trade-Offs of Integrating Direct Air Capture with (Bi)carbonate Electrolysis. ACS Energy Lett. 2024, 9 (5), 2472–2483. 10.1021/acsenergylett.4c00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino F.; Mehta M.; Grimm A.; Gazzani M.; Gallucci F.; Kramer G. J.; van Sint Annaland M. Evaluation of a Direct Air Capture Process Combining Wet Scrubbing and Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2020, 59 (15), 7007–7020. 10.1021/acs.iecr.9b05641. [DOI] [Google Scholar]
- Siegelman R. L.; Kim E. J.; Long J. R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20 (8), 1060–1072. 10.1038/s41563-021-01054-8. [DOI] [PubMed] [Google Scholar]
- Filburn T.; Helble J. J.; Weiss R. A. Development of Supported Ethanolamines and Modified Ethanolamines for CO2 Capture. Ind. Eng. Chem. Res. 2005, 44 (5), 1542–1546. 10.1021/ie0495527. [DOI] [Google Scholar]
- Chen L.; Li F.; Zhang Y.; Bentley C. L.; Horne M.; Bond A. M.; Zhang J. Electrochemical Reduction of Carbon Dioxide in a Monoethanolamine Capture Medium. ChemSusChem 2017, 10 (20), 4109–4118. 10.1002/cssc.201701075. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Jang H.; Bak G.; Choi W.; Yun H.; Lee E.; Kim D.; Kim J.; Lee S. Y.; Hwang Y. J. The insensitive cation effect on a single atom Ni catalyst allows selective electrochemical conversion of captured CO2 in universal media. Energy Environ. Sci. 2022, 15 (10), 4301–4312. 10.1039/D2EE01825J. [DOI] [Google Scholar]
- Langie K. M. G.; Tak K.; Kim C.; Lee H. W.; Park K.; Kim D.; Jung W.; Lee C. W.; Oh H.-S.; Lee D. K.; et al. Toward economical application of carbon capture and utilization technology with near-zero carbon emission. Nat. Commun. 2022, 13 (1), 7482. 10.1038/s41467-022-35239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflaum R. T.; Brandt W. W. Metal-Amine Coördination Compounds. I. Copper(II) Complexes. J. Am. Chem. Soc. 1954, 76 (24), 6215–6219. 10.1021/ja01653a001. [DOI] [Google Scholar]
- Li K.; van der Poel P.; Conway W.; Jiang K.; Puxty G.; Yu H.; Feron P. Mechanism Investigation of Advanced Metal–Ion–Mediated Amine Regeneration: A Novel Pathway to Reducing CO2 Reaction Enthalpy in Amine-Based CO2 Capture. Environ. Sci. Technol. 2018, 52 (24), 14538–14546. 10.1021/acs.est.8b05179. [DOI] [PubMed] [Google Scholar]
- Heldebrant D. J.; Koech P. K.; Glezakou V.-A.; Rousseau R.; Malhotra D.; Cantu D. C. Water-Lean Solvents for Post-Combustion CO2 Capture: Fundamentals, Uncertainties, Opportunities, and Outlook. Chem. Rev. 2017, 117 (14), 9594–9624. 10.1021/acs.chemrev.6b00768. [DOI] [PubMed] [Google Scholar]
- Lee J.-Y.; Keener T. C.; Yang Y. J. Potential flue gas impurities in carbon dioxide streams separated from coal-fired power plants. JA&WMA 2009, 59 (6), 725–732. 10.3155/1047-3289.59.6.725. [DOI] [PubMed] [Google Scholar]
- Gholami F.; Tomas M.; Gholami Z.; Vakili M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712 10.1016/j.scitotenv.2020.136712. [DOI] [PubMed] [Google Scholar]
- Komatsu S.; Tanaka M.; Okumura A.; Kungi A. Preparation of cu-solid polymer electrolyte composite electrodes and application to gas-phase electrochemical reduction of CO2. Electrochim. Acta 1995, 40 (6), 745–753. 10.1016/0013-4686(94)00325-U. [DOI] [Google Scholar]
- Ko B. H.; Hasa B.; Shin H.; Jeng E.; Overa S.; Chen W.; Jiao F. The impact of nitrogen oxides on electrochemical carbon dioxide reduction. Nat. Commun. 2020, 11 (1), 5856. 10.1038/s41467-020-19731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahner J.; Wassmuth H. W. Molecular and dissociative chemisorption and condensation of SO2 on Cu(111). Surf. Sci. 1993, 287–288, 125–129. 10.1016/0039-6028(93)90755-9. [DOI] [Google Scholar]
- Luc W.; Ko B. H.; Kattel S.; Li S.; Su D.; Chen J. G.; Jiao F. SO2-Induced Selectivity Change in CO2 Electroreduction. J. Am. Chem. Soc. 2019, 141 (25), 9902–9909. 10.1021/jacs.9b03215. [DOI] [PubMed] [Google Scholar]
- Uribe-Soto W.; Portha J.-F.; Commenge J.-M.; Falk L. A review of thermochemical processes and technologies to use steelworks off-gases. Renew. Sustain. Energy Rev. 2017, 74, 809–823. 10.1016/j.rser.2017.03.008. [DOI] [Google Scholar]
- Dai Z.; Fabio S.; Marino N. G.; Riccardo C.; Deng L. Field test of a pre-pilot scale hollow fiber facilitated transport membrane for CO2 capture. Int. J. Greenhouse Gas Control 2019, 86, 191–200. 10.1016/j.ijggc.2019.04.027. [DOI] [Google Scholar]
- Xu Y.; Edwards J. P.; Zhong J.; O’Brien C. P.; Gabardo C. M.; McCallum C.; Li J.; Dinh C.-T.; Sargent E. H.; Sinton D. Oxygen-tolerant electroproduction of C2 products from simulated flue gas. Energy Environ. Sci. 2020, 13 (2), 554–561. 10.1039/C9EE03077H. [DOI] [Google Scholar]
- Lu X.; Jiang Z.; Yuan X.; Wu Y.; Malpass-Evans R.; Zhong Y.; Liang Y.; McKeown N. B.; Wang H. A bio-inspired O2-tolerant catalytic CO2 reduction electrode. Sci. Bull. 2019, 64 (24), 1890–1895. 10.1016/j.scib.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Dai Z.; Fabio S.; Giuseppe Marino N.; Riccardo C.; Deng L. Field test of a pre-pilot scale hollow fiber facilitated transport membrane for CO2 capture. Int. J. Greenhouse Gas Control 2019, 86, 191–200. 10.1016/j.ijggc.2019.04.027. [DOI] [Google Scholar]
- Feraud A.; Marocco L.; Howard T.. CASTOR study on technological requirements for flue gas clean-up prior to CO2-capture; GHGT-8 Conference, Trondheim, Norway, 19–22 June, 2006.
- Jiao L.; Yang W.; Wan G.; Zhang R.; Zheng X.; Zhou H.; Yu S. H.; Jiang H. L. Single-atom electrocatalysts from multivariate metal–organic frameworks for highly selective reduction of CO2 at low pressures. Angew. Chem., Int. Ed. 2020, 59 (46), 20589–20595. 10.1002/anie.202008787. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Hou J.; Kang P. Integrated Capture and Electroreduction of Flue Gas CO2 to Formate Using Amine Functionalized SnOx Nanoparticles. ACS Energy Lett. 2021, 6 (9), 3352–3358. 10.1021/acsenergylett.1c01553. [DOI] [Google Scholar]
- Kim D.; Choi W.; Lee H. W.; Lee S. Y.; Choi Y.; Lee D. K.; Kim W.; Na J.; Lee U.; Hwang Y. J.; Won D. H. Electrocatalytic Reduction of Low Concentrations of CO2 Gas in a Membrane Electrode Assembly Electrolyzer. ACS Energy Lett. 2021, 6 (10), 3488–3495. 10.1021/acsenergylett.1c01797. [DOI] [Google Scholar]
- Hos T.; Herskowitz M. Utilization of CO-rich waste gases from the steel industry for production of renewable liquid fuels. Energy Convers. Manage. 2021, 240, 114233 10.1016/j.enconman.2021.114233. [DOI] [Google Scholar]
- Alerte T.; Edwards J. P.; Gabardo C. M.; O’Brien C. P.; Gaona A.; Wicks J.; Obradović A.; Sarkar A.; Jaffer S. A.; MacLean H. L.; et al. Downstream of the CO2 Electrolyzer: Assessing the Energy Intensity of Product Separation. ACS Energy Lett. 2021, 6 (12), 4405–4412. 10.1021/acsenergylett.1c02263. [DOI] [Google Scholar]
- Greenblatt J. B.; Miller D. J.; Ager J. W.; Houle F. A.; Sharp I. D. The Technical and Energetic Challenges of Separating (Photo)Electrochemical Carbon Dioxide Reduction Products. Joule 2018, 2 (3), 381–420. 10.1016/j.joule.2018.01.014. [DOI] [Google Scholar]
- Ozden A.; García de Arquer F. P.; Huang J. E.; Wicks J.; Sisler J.; Miao R. K.; O’Brien C. P.; Lee G.; Wang X.; Ip A. H.; et al. Carbon-efficient carbon dioxide electrolysers. Nat. Sustain. 2022, 5 (7), 563–573. 10.1038/s41893-022-00879-8. [DOI] [Google Scholar]
- Xu Y.; Miao R. K.; Edwards J. P.; Liu S.; O’Brien C. P.; Gabardo C. M.; Fan M.; Huang J. E.; Robb A.; Sargent E. H.; Sinton D. A microchanneled solid electrolyte for carbon-efficient CO2 electrolysis. Joule 2022, 6 (6), 1333–1343. 10.1016/j.joule.2022.04.023. [DOI] [Google Scholar]
- Huang J. E.; Li F.; Ozden A.; Sedighian Rasouli A.; García de Arquer F. P.; Liu S.; Zhang S.; Luo M.; Wang X.; Lum Y.; et al. CO2 electrolysis to multicarbon products in strong acid. Science 2021, 372 (6546), 1074–1078. 10.1126/science.abg6582. [DOI] [PubMed] [Google Scholar]
- Ringe S.; Clark E. L.; Resasco J.; Walton A.; Seger B.; Bell A. T.; Chan K. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 2019, 12 (10), 3001–3014. 10.1039/C9EE01341E. [DOI] [Google Scholar]
- Resasco J.; Chen L. D.; Clark E.; Tsai C.; Hahn C.; Jaramillo T. F.; Chan K.; Bell A. T. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 2017, 139 (32), 11277–11287. 10.1021/jacs.7b06765. [DOI] [PubMed] [Google Scholar]
- Delacourt C.; Ridgway P. L.; Kerr J. B.; Newman J. Design of an electrochemical cell making syngas (CO+ H2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 2008, 155 (1), B42. 10.1149/1.2801871. [DOI] [Google Scholar]
- Salvatore D. A.; Weekes D. M.; He J.; Dettelbach K. E.; Li Y. C.; Mallouk T. E.; Berlinguette C. P. Electrolysis of Gaseous CO2 to CO in a Flow Cell with a Bipolar Membrane. ACS Energy Lett. 2018, 3 (1), 149–154. 10.1021/acsenergylett.7b01017. [DOI] [Google Scholar]
- Miao R. K.; Xu Y.; Ozden A.; Robb A.; O’Brien C. P.; Gabardo C. M.; Lee G.; Edwards J. P.; Huang J. E.; Fan M.; et al. Electroosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2. Joule 2021, 5 (10), 2742–2753. 10.1016/j.joule.2021.08.013. [DOI] [Google Scholar]
- Zhang J.; Luo W.; Züttel A. Crossover of liquid products from electrochemical CO2 reduction through gas diffusion electrode and anion exchange membrane. J. Catal. 2020, 385, 140–145. 10.1016/j.jcat.2020.03.013. [DOI] [Google Scholar]
- Li Y. C.; Yan Z.; Hitt J.; Wycisk R.; Pintauro P. N.; Mallouk T. E. Bipolar membranes inhibit product crossover in CO2 electrolysis cells. Adv. Sustain. Syst. 2018, 2 (4), 1700187 10.1002/adsu.201700187. [DOI] [Google Scholar]
- Baeyens J.; Kang Q.; Appels L.; Dewil R.; Lv Y.; Tan T. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. 10.1016/j.pecs.2014.10.003. [DOI] [Google Scholar]
- Nabil S. K.; McCoy S.; Kibria M. G. Comparative life cycle assessment of electrochemical upgrading of CO2 to fuels and feedstocks. Green Chem. 2021, 23 (2), 867–880. 10.1039/D0GC02831B. [DOI] [Google Scholar]
- Forest I.-S.The corrosion resistance of nickel-containing alloys in sulfuric acid and related compounds; INCO The International Nickel Company, 1983; pp 51–82.
- Luo Q.; Wang T.; Beller M.; Jiao H. Hydrogen generation from formic acid decomposition on Ni (2 1 1), Pd (2 1 1) and Pt (2 1 1). J. Mol. Catal. A: Chem. 2013, 379, 169–177. 10.1016/j.molcata.2013.08.015. [DOI] [Google Scholar]