Abstract
Most of the early gene therapy trials for cystic fibrosis have been with adenovirus vectors. First-generation viruses with E1a and E1b deleted are limited by transient expression of the transgene and substantial inflammatory responses. Gene transfer is also significantly curtailed following a second dose of virus. In an effort to reduce adenovirus-associated inflammation, capsids of first-generation vectors were modified with various activated monomethoxypolyethylene glycols. Cytotoxic T-lymphocyte production was significantly reduced in C57BL/6 mice after a single intratracheal administration of modified vectors, and length of gene expression was extended from 4 to 42 days. T-cell subsets from mice exposed to the conjugated vectors demonstrated a marked decrease in Th1 responses and slight enhancement of Th2 responses compared to animals dosed with native virus. Neutralizing antibodies (NAB) against adenovirus capsid proteins were reduced in serum and bronchoalveolar lavage fluid of animals after a single dose of modified virus, allowing significant levels of gene expression upon rechallenge with native adenovirus. Modification with polyethylene glycol (PEG) also allowed substantial gene expression from the new vectors in animals previously immunized with unmodified virus. However, gene expression was significantly reduced after two doses of the same PEG-conjugated vector. Alternating the activation group of PEG between doses did produce significant gene expression upon readministration. This technology in combination with second-generation or helper-dependent adenovirus could produce dosing strategies which promote successful readministration of vector in clinical trials and marked expression in patients with significant anti-adenovirus NAB levels and reduce the possibility of immune reactions against viral vectors for gene therapy.
First-generation recombinant adenovirus vectors rendered defective by deletion of the immediate-early genes E1a and E1b have shown great promise as vehicles for somatic gene therapies (3, 47). The natural tropism of the virus is the human airway, which makes it an attractive candidate for gene therapies for lung diseases such as cystic fibrosis and malignant pleural mesothelioma (36, 46). Adenovirus has been shown to be moderately effective for gene transfer to the lung in mice, cotton rats, nonhuman primates, and humans (12, 27, 56, 61). In each model, direct instillation of adenovirus into the airway led to efficient gene transfer into surface airway epithelial cells. Enthusiasm for extensive use of these vectors, however, has diminished because of limited stability of transgene expression due to cellular immune responses generated against cells expressing viral and transgene products (21, 54, 55, 59). Furthermore, transduction efficiency in the lung (2, 37) is severely hampered upon readministration of recombinant adenovirus due to neutralization of viral particles by antibodies generated against the viral proteins (24, 31, 55, 60).
Various strategies have been developed in an effort to circumvent both cellular and humoral immune responses generated against adenovirus vectors. A diverse range of pharmacological agents, such as cyclophosphamide (23), dexamethasone (33), dichloromethylene diphosphonate (clodronate) (45), and recombinant interleukin-12 (IL-12) (60), when administered in combination with adenovirus have been successful in blunting the cellular immune response against both the virus and transgene product, resulting in prolonged gene expression. These regimens significantly reduced overall inflammatory responses but did not inhibit the formation of neutralizing antibodies (NAB), suggesting that vector readministration, though not evaluated, would not have been successful. In addition to their limited efficacy and toxicity, these regimens will impair existing immunity. Administration of monoclonal antibodies which inhibit costimulatory interactions between B cells and T cells, such as anti-CD40 ligand antibody (39, 51, 58) and CTLA4Ig (22), extended the duration of gene expression but did not ablate the formation of cellular and humoral immune responses to the vector, and readministration was unsuccessful. Only when the two inhibitors were administered in concert with the first and second dose of virus were significant levels of gene expression detected (25).
Other attempts to achieve successful readministration involve systematic elimination of adenovirus protein coding sequences responsible for precipitating the immune response. Suppression of the E2a region of the viral genome has significantly reduced inflammation associated with the viral vector but has only modestly extended the length of gene expression beyond that of first-generation vectors (12, 56). Reintroduction of the E3 region, which encodes functions involved in virus escape from the host immune response, can prolong transgene expression in some animal models (18). Deletion of E4 regions of the viral genome has also offered some improvement in the stability of gene expression with a reduction in inflammatory response generated against the vector (1, 6). However, antibodies were still generated against these second-generation viruses, compromising readministration of the vector. Helper-dependent viruses deleted of all adenovirus protein coding sequences have demonstrated long-term, high-level gene expression (29, 34, 40). However, production of amounts of vector necessary for study in vivo is hampered by high levels of contaminating helper virus, low recovery, and poor stability of vector during propagation (13, 16, 28). In addition, these vectors still cannot completely overcome the humoral immune response and ablate production of NAB due to the presence of viral capsid proteins and contaminating helper virus. Significant levels of gene expression upon readministration have only been achieved by alternating the serotype of these “gutted” viruses (35).
Recently, a method for the conjugation of functionalized polyethylene glycol (PEG) to free lysine groups on the adenovirus capsid has been established (8, 32). This technique, termed PEGylation, has been employed since the 1970s in the pharmaceutical industry to protect therapeutic proteins and enzymes from metabolic degradation and shield them from both humoral and cellular immune responses (11, 14, 41). In this report, the immunology of PEGylated adenovirus vectors administered to the lung of immunocompetent animals is characterized. This simple modification of the vector reduces both the humoral and cellular immune response against viral proteins, extends the duration of gene expression, and allows significant levels of gene expression upon readministration without compromising the immune system of the recipient.
MATERIALS AND METHODS
Production of conjugated adenovirus vectors.
First-generation adenovirus type 5 expressing either green fluorescent protein or β-galactosidase under the control of a cytomegalovirus promoter were amplified in 293 cells using a modification of established methods (15) and purified from cell lysates by banding twice on CsCl gradients. Aliquots of virus were desalted on Econo-Pac 10DG disposable chromatography columns (Bio-Rad, Hercules, Calif.) and equilibrated with the respective buffer for optimal conjugation (see below). Viral concentrations were determined by UV spectrophotometric analysis at 260 nm. Protein content of adenovirus preparations was determined by a micoplate assay with Bio-Rad DC protein assay reagents and bovine serum albumin (BSA) as a standard.
Three types of activated monomethoxypolyethylene glycol (MPEG) were used in this study, tresyl-MPEG (TMPEG), succinimidyl succinate MPEG (SSPEG), and cyanuric chloride MPEG (CCPEG), and were obtained from Sigma Chemical Co. (St. Louis, Mo.). Conjugation reactions were performed using a modification of established methods (10, 20, 26). For TMPEG, adenovirus bands were desalted into 10 mM potassium phosphate buffer (pH 7.4). Virus was desalted into 0.2 M sodium phosphate (pH 7.2) and 0.1 M sodium tetraborate (pH 9.2) buffers for conjugation with SSPEG and CCPEG, respectively. A 10:1 PEG-virus ratio (amount of PEG to amount of adenovirus protein) provided the most efficient reaction times and produced minimal loss of infectivity of the virus. All conjugation reactions were performed at 25°C with gentle stirring. Reactions were stopped by addition of 10× l-lysine. Unreacted PEG, excess lysine, and reaction byproducts were eliminated by buffer exchange over a Sephadex G-50 column equilibrated with 10 mM potassium-buffered saline (pH 7.4). Fractions containing virus were identified by UV spectrophotometric analysis at 260 nm and pooled for further study. Virus concentrations were determined from the formula: particles/ml = (optical density at 260 nm) × (dilution factor) × (1012).
Administration of PEGylated vectors to immunocompetent animals.
C57BL/6 (H-2b) mice (6 to 8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, Maine). Preparations were administered intratracheally at a dose of 5 × 1010 particles in 50 μl of phosphate-buffered saline (PBS). Study designs and dosing schedules are detailed in Fig. 1.
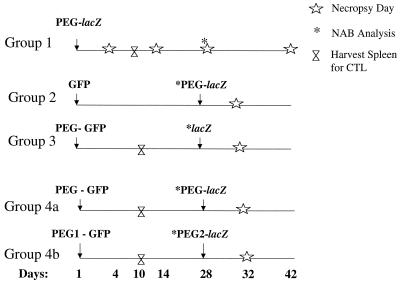
FIG. 1.
Dosing strategies for PEGylated adenovirus vectors. A total of five groups were studied. Animals were immunized by intratracheal injection with vectors expressing green fluorescent protein and rechallenged with vector expressing E. coli β-galactosidase (lacZ). Arrows indicate time of injection, and open stars indicate days of necropsy. Double open triangles indicate when animals were necropsied and splenocytes harvested for assessment of adenovirus-specific CTL. Asterisks indicate time when NAB against adenovirus capsid proteins was assessed. In order to determine if conjugation of PEG to adenovirus capsids compromised transduction efficiency, naive animals received a single dose of PEG-adenovirus, and gene expression was assessed over time (group 1). Group 2 represents animals that were immunized with native virus and rechallenged 28 days later with the conjugated vector. Group 3 represents animals that were immunized with conjugated virions and rechallenged with native vector 28 days later. Group 4a represents animals that received two doses of the same conjugated vector. In group 4b, animals were immunized with adenovirus conjugated by one method and rechallenged with vector conjugated by a different method.
CTL assay.
Cytotoxic T-lymphocyte (CTL) assays were performed as described previously (6, 21). In short, lymphocytes harvested from spleens were cultured for 5 days at 6 × 106 cells/well in RPMI 1640 medium (Gibco-BRL, Grand Island, N.Y.) with 10% fetal bovine serum (FBS) and 50 μM 2-mercaptoethanol in the presence of adenovirus expressing the lacZ gene at a multiplicity of infection (MOI) of 1 PFU/cell in 24-well culture plates. A standard 6-h 51Cr release assay was performed using different ratios of effector to target cells (C57SV, H-2b) in 200 μl of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS in V-bottomed 96-well plates. Prior to mixing with the effector cells, target cells (106) were labeled with 100 μCi of 51Cr after a 24-h infection with adenovirus expressing the lacZ gene at an MOI of 50 PFU/cell and seeded at 5 × 103 cells/well. Six hours after incubation, 100-μl aliquots of supernatant were removed for counting in a gamma counter. Percent specific 51Cr release was calculated as [(cpm of sample − cpm of spontaneous release)/(cpm of maximal release − cpm of spontaneous release)] × 100. Spontaneous release was determined by assaying target cells without the addition of effector cells. Maximum release was determined by the addition of 5% sodium dodecyl sulfate to the target cells during the 6-h incubation time. All sample values represent the averages of 4 to 8 wells.
Cytokine release assays.
Lymphocytes were cultured with or without inactivated adenovirus lacZ at an MOI of 10 PFU/cell for 48 h in 24-well plates. Cell-free supernatants were collected and analyzed for the presence of IL-2, IL-4, gamma interferon (IFN-γ) and IL-10 by enzyme-linked immunosorbent assay (ELISA) as described previously (6).
Neutralization assays.
Mouse serum was incubated at 56°C for 30 min to inactivate complement and diluted in DMEM in twofold increments starting from a 1:20 dilution. Each dilution (100 μl) was mixed with adenovirus expressing GFP (106 PFU), incubated for 1 h at 37°C, and applied to HeLa cells in 96-well plates (2 × 104 cells/well). After 1 h at 37°C, 100 μl of DMEM supplemented with 20% FBS was added to each well. Cells were infected for 24 h. Green fluorescent protein expression was assessed by FluoroImaging (Molecular Dynamics). NAB titers were calculated as the dilution at which fluorescence intensity was reduced by 50%.
Analysis of adenovirus-specific immunoglobulins.
Serum samples from mice were assessed for adenovirus-specific, isotype-specific immunoglobulins (IgG1, IgG2a, IgG2b, IgG3, and IgM) by ELISA as described previously (7). Microtiter plates (MaxiSorp; Nunc) were coated with 100 μl of adenovirus antigen (5 × 1010 particles/ml) in 0.1 M bicarbonate buffer (pH 9.6) overnight at 4°C, washed four times with PBS containing 0.05% Tween 20, and blocked in PBS containing 3% BSA for 3 h at room temperature. Serum samples at a 1:100 dilution were added to the antigen-coated plates and incubated overnight at 4°C. Plates were washed four times with PBS containing 0.05% Tween 20 and incubated with biotin-conjugated rat anti-mouse IgG1, IgG2a, IgG2b, IgG3, and IgM (PharMingen, San Diego, Calif.) at a 1:1,000 dilution for 3 h at room temperature. Plates were washed as above, and a 1:10,000 dilution of alkaline phosphatase-conjugated avidin (Sigma Chemical Company) was added for 2 h at room temperature. After four washes, p-nitophenyl phosphate in diethanolamine buffer was added (Sigma Chemical Company), and optical densities were read at 405 nm on a microplate reader (Dynatech Laboratories, Chantilly, Va.).
BAL.
Mice were sacrificed, and tracheas were exposed. A plastic disposable angiocatheter with a 3-ml syringe attached was inserted through an incision immediately posterior to the larynx. The respiratory tract was irrigated with two separate aliquots of PBS, each of which was infused and withdrawn three times. The resulting bronchoalveolar lavage (BAL) fluid solution was processed and assessed for presence of NAB.
X-Gal histochemistry.
Frozen sections (6 μm) were fixed in 0.5% glutaraldehyde and stained for β-galactosidase activity as described (57). Sections were counterstained with hematoxylin.
β-galactosidase assays.
Treated tissues were excised from freshly euthanized animals and washed twice in cold PBS. When numerous samples were processed, excised tissues were stored for up to 2 h in cold DMEM. Tissues were rinsed in lysis buffer (provided with the β-galactosidase ELISA kit; Boehringer-Mannheim) containing 4 mM Pefablock (Boehringer-Mannheim), 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 0.5 mM EDTA, 0.5 mM dithiothreitol, plus 1 μg of pepstatin, 5 μg of aprotinin (Sigma), and 1 μg of leupeptin per ml. Tissues were homogenized in 1 ml of lysis buffer using a Brinkman Polytron. Following homogenization, extracts were centrifuged at 14,000 rpm for 10 min. The protein concentration of the cleared supernatants was determined by a microplate assay with Bio-Rad DC protein assay reagents and BSA as a standard. Extracts were quick-frozen in a dry ice-ethanol bath and stored at −80°C until assayed. β-galactosidase concentrations were determined by ELISA (Boehringer-Mannheim) according to the manufacturer's instructions.
RESULTS
Administration of PEGylated adenovirus to the lung of immunocompetent animals.
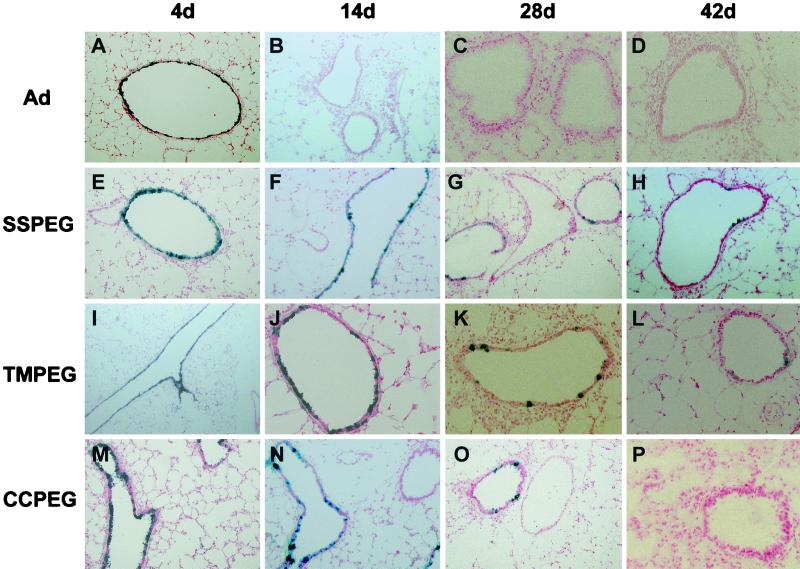
Intratracheal instillation of a first-generation recombinant adenovirus containing the Escherichia coli beta-galactosidase gene resulted in high levels of transgene expression in epithelial cells of the conducting airway 4 days after administration (Fig. 2A) and declined to undetectable levels by day 14 (Fig. 2B and Table 1). PEGylated adenovirus also produced significant levels of gene expression 4 days after instillation into the trachea of C57BL/6 mice (Fig. 2E, 2I, and 2M). Significant levels of gene expression continued for up to 28 days after administration in these animals (Fig. 2G, 2K, and 2O and Table 1). Mice that received SSPEG and TMPEG preparations continued to express the transgene in the small airways 42 days after administration (Fig. 2H, and 2L and Table 1). Gene expression was undetectable at this time point in all animals receiving the CCPEG preparation (Fig. 2P).
FIG. 2.
Gene expression of PEGylated adenovirus in the lung. First-generation adenovirus vectors were conjugated to various forms of MPEG and administered intratracheally (5 × 1010 particles/ml) to C57BL/6 mice. Animals were sacrificed, and lung tissues were evaluated for lacZ expression by X-Gal histochemistry at day 4 (4d, first column), day 14 (second column), day 28 (third column), and day 42 (fourth column). All preparations produced high levels of gene expression 4 days after administration, with many large and small airways staining positive for lacZ expression (panels A, E, I, and M). Fourteen days after administration, gene expression was absent in animals receiving the native virus (panel B) but remained stable in animals treated with the PEGylated preparations (panels F, J, and N). Gene expression in these animals diminished 28 days after administration (panels G, K, and O). Low levels of gene expression could be found in animals treated with the SSPEG and TMPEG preparations 42 days after administration.
TABLE 1.
Quantitative analysis of mouse lung for transduction efficiency of PEGylated adenovirus vectorsa
| Treatment | Avg β-galactosidase (pg/mg of protein × 105) ± SD
|
|||
|---|---|---|---|---|
| 4 days | 14 days | 28 days | 42 days | |
| Native virus | 19.2 ± 0.9 | 0.21 ± 0.06 | ND | ND |
| CCPEG | 22 ± 0.5 | 0.29 ± 0.07 | 0.10 ± 0.05 | 0.002 ± 0.0005 |
| SSPEG | 22 ± 0.5 | 0.25 ± 0.09 | 0.11 ± 0.06 | ND |
| TMPEG | 24 ± 0.9 | 2.01 ± 0.8 | 0.26 ± 0.10 | 0.009 ± 0.0006 |
Data were quantified from a β-galactosidase ELISA of lung homogenates and reflects average values ± standard deviations for six animals. ND transgene expression not detectable.
Characterization of the cellular and humoral response after a single dose of PEGylated adenovirus to the lung.
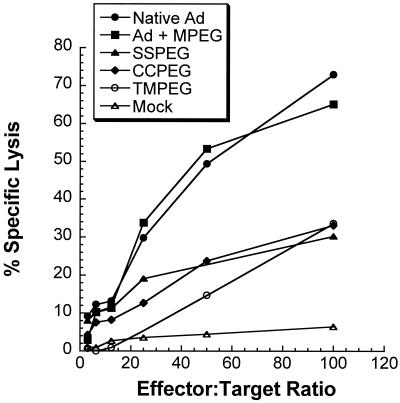
It is known that instillation of first-generation adenovirus into mouse lung elicits significant major histocompatibility complex (MHC) class I CTL responses, leading to loss of transgene expression by mechanisms that destroy virus-infected cells (55, 59). PEGylation of adenovirus extended gene expression beyond that seen with the native virus in immunocompetent animals. The effect of PEGylation on the T-cell response was evaluated by 51Cr release assays using splenocytes of C57BL/6 mice dosed with either the native or modified vector expressing lacZ as the effector. After incubation with H-2b target cells infected with native lacZ virus, substantial cytolysis was detected in samples from animals receiving the unmodified virus (Native Ad, Fig. 3). PEG alone does not blunt the CTL response, as significant cytolysis was also detected in samples from animals receiving the native virus in PBS and 1% unactivated MPEG, a polymer that cannot covalently attach to the virus capsid. Cytolysis was significantly reduced in samples from animals dosed with the PEGylated preparations. No cytolysis was observed with naive restimulated splenocytes tested on adenovirus-infected targets (Mock).
FIG. 3.
CTL responses to PEGylated adenovirus. Splenocytes harvested 10 days after injection of native or PEGylated viruses from C57BL/6 mice were restimulated in vitro for 5 days and tested for specific lysis on C57SV target cells infected with native first-generation adenovirus expressing the lacZ gene in a 6-h 51Cr release assay. Percent specific lysis is expressed as a function of different effector-to-target cell ratios (6:1, 12.5:1, 25:1, 50:1, and 100:1). Adenovirus + MPEG, animals received native virus in 1% unactivated MPEG which was not attached to the virus capsid. Mock, naive, restimulated splenocytes tested on adenovirus-infected targets.
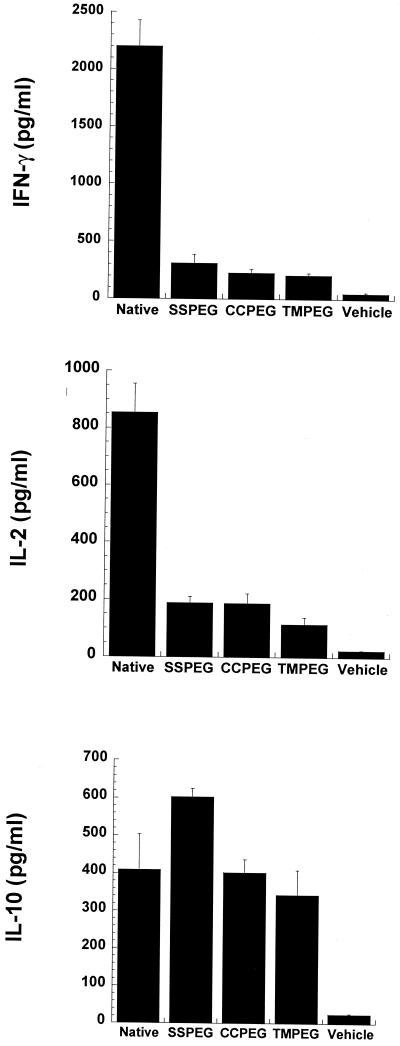
Full Th1-type (IFN-γ and IL-2) and Th2-type (IL-10) responses were observed in samples from mice that received the native adenovirus (Fig. 4). Th1-type responses were significantly reduced in samples from animals given the PEGylated vectors. However, there was no significant difference in IL-10 levels from animals receiving either the native, CCPEG, or TMPEG preparation (Student's t test, P ≤ 0.05). The SSPEG preparation demonstrated a slight increase in IL-10 secretion. Another Th2-specific cytokine, IL-4, could not be detected in the samples.
FIG. 4.
Cytokine secretion profiles. C57BL/6 mice were given either vehicle (PBS) or native or PEGylated viruses at a dose of 5 × 1010 particles/ml intratracheally. Splenocytes harvested 10 days after administration were cultured in the presence or absence of inactivated, unmodified adenovirus for 48 h. Culture supernatants were analyzed for IL-2, IFN-γ, IL-4, and IL-10 by ELISA. Values are averages ± standard deviations for duplicate culture supernatants from spleens of six animals treated in two separate experiments.
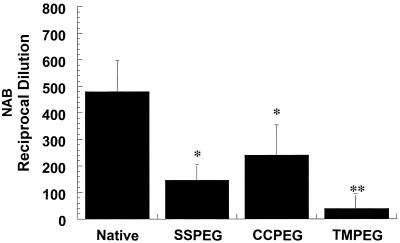
The humoral immune response following intratracheal administration of unmodified and PEGylated preparations was analyzed in serum and BAL fluid 30 days after a single dose of virus. High levels of NAB against adenovirus were detected in the serum of animals receiving unmodified virus (Fig. 5). Titers were significantly lower in animals receiving equivalent doses of the SSPEG and CCPEG preparations (P ≤ 0.05, Student's t test). The TMPEG preparation produced an NAB level that was slightly higher than that in animals receiving a bolus of saline (data not shown).
FIG. 5.
NAB profile of C57BL/6 mice after a single dose of PEGylated adenovirus. Twenty-eight days after injection of native or PEGylated adenovirus, serum from C57BL/6 mice was analyzed for the presence of NAB by its ability to block adenovirus infection of HeLa cells. The reciprocal dilution is plotted according to vector administered. These results represent the means and standard deviations of six animals per group from three separate experiments. ∗, P < 0.05; ∗∗, P < 0.01 (Student's t test).
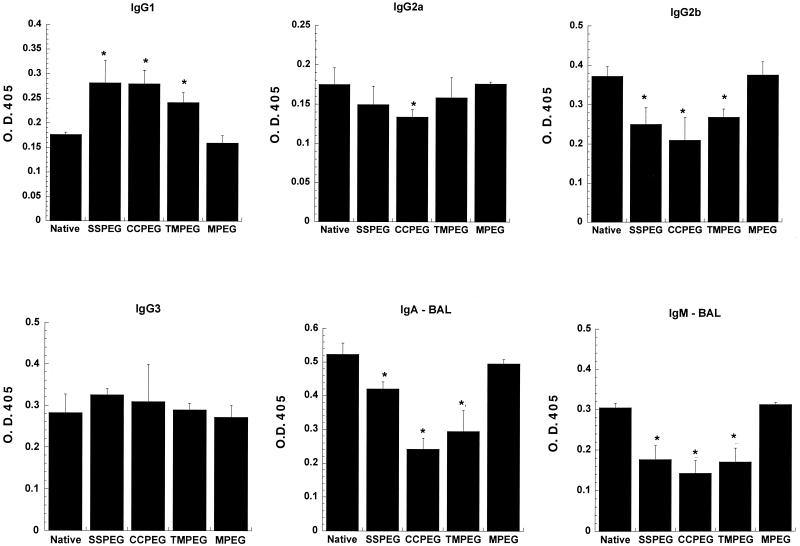
Further characterization of adenovirus-specific Ig isotypes confirmed that Th2-type responses were somewhat enhanced in animals dosed with the PEGylated preparations (Fig. 6), as IgG1 levels were significantly higher that that seen in animals given the native virus (P ≤ 0.05, Student's t test). The Th1- dependent isotype Ig2b was significantly reduced in animals receiving the PEGylated preparations, but Ig2a levels remained normal, as did those for IgG3. PEGylated preparations significantly reduced IgA and IgM levels in BAL fluid compared to those with the native virus. In each analysis, samples were included from animals that received a dose of the native virus in a formulation of PBS and 1% unactivated MPEG. These samples generated responses similar to that seen with the native virus, indicating that PEG alone does not influence the humoral immune response generated against adenovirus vectors in the lung.
FIG. 6.
Anti-adenovirus vector-specific Ig isotypes after a single dose of native and PEGylated adenovirus. Thirty days after administration of 5 × 1010 particles of native adenovirus, PEGylated adenovirus, or native adenovirus in the presence of unactivated PEG (MPEG), serum and BAL fluid from C57BL/6 mice were analyzed for the presence of adenovirus-specific IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA antibodies by ELISA. The optical densities (O. D.) obtained from each sample as a measure of relative concentration are presented. Data are the means and standard deviations for six animals from three separate experiments. ∗, P < 0.05, Student's t test.
PEGylation of adenovirus allows significant levels of gene expression upon readministration to animals exposed to native virus.
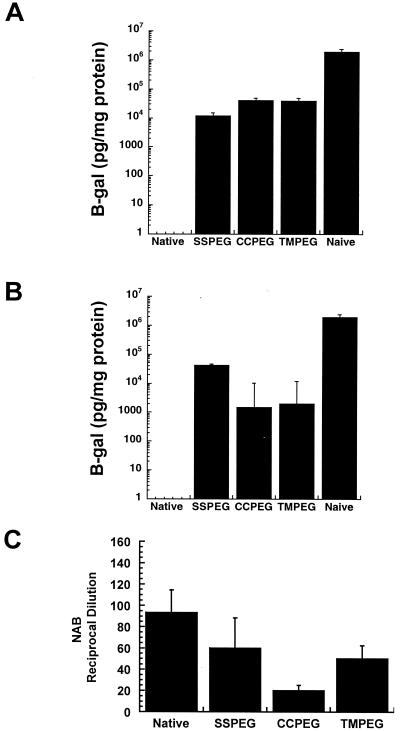
Therapeutic proteins and enzymes have been conjugated with PEGs to protect them from neutralization in patients who must receive repeated doses of protein (17, 19, 48). This application was the driving force for the development of PEGylated adenovirus vectors. After determining that the PEGylation process could effectively protect the virus from neutralization by immune sera in vitro (8), these vectors were tested for their ability to avoid neutralization in vivo by assessing transgene expression in animals immunized with 5 × 1010 particles of unmodified virus. Two doses of the native virus failed to produce significant levels of gene expression (Fig. 7A). Animals that received the SSPEG preparation had β-galactosidase levels equal to 1.2 × 104 pg/mg protein 4 days after the second dose of virus, a level of gene expression 100-fold lower than that in naive animals after a single dose of virus (19.2 × 105 pg β-galactosidase/mg protein, Table 1). The CCPEG and TMPEG preparations produced levels of 4.0 × 104 and 3.82 × 104 pg β-galactosidase/mg protein, respectively.
FIG. 7.
Readministration profiles of PEGylated adenovirus vectors upon delivery to the lung of immunocompetent mice. (A) Levels of β-galactosidase expression obtained with PEGylated preparations after immunization with native adenovirus expressing green fluorescent protein. Prior to readministration, animals had an average titer of 360 (reciprocal dilution), a level sufficient to completely block gene expression after a second dose of native virus. (B) Levels of β-galactosidase expression from native adenovirus after immunization with PEGylated preparations. In panels A and B, the native group represents animals that received two consecutive doses of unconjugated adenovirus. The naive group represents animals that received a single dose of unmodified virus. (C) Anti-adenovirus NAB profile of animals in panel B prior to administration of with the native virus. In each panel, data are averages derived from six animals in two separate experiments.
PEGylation allows significant gene expression by unconjugated adenovirus in immunocompetent animals after exposure to PEGylated virus.
Initial studies revealed that PEGylation reduced production of anti-adenovirus NAB. In order to assess what this meant with respect to dosing strategies for readministration to the lung, animals were given 5 × 1010 particles of each PEGylated preparation and rechallenged 30 days later with an equivalent dose of the native virus. While two doses of the native virus failed to produce detectable levels of β-galactosidase, it did produce significant levels of gene expression in animals immunized with the PEGylated vectors. Native adenovirus produced 4.2 × 104 pg β-galactosidase/mg protein in animals that were immunized with the SSPEG preparation (Fig. 7B). The CCPEG and TMPEG animals had β-galactosidase levels of 1.5 × 103 and 1.8 × 103 pg/mg protein, respectively; approximately 100-fold lower than that seen in naive animals. While each treatment group had different levels of anti-adenovirus NAB present prior to readministration (Fig. 7C), all levels were sufficient to effectively neutralize adenovirus and prevent transduction in vitro.
Administration of two consecutive doses of PEGylated adenovirus to immunocompetent animals fails to produce significant levels of gene expression upon readministration.
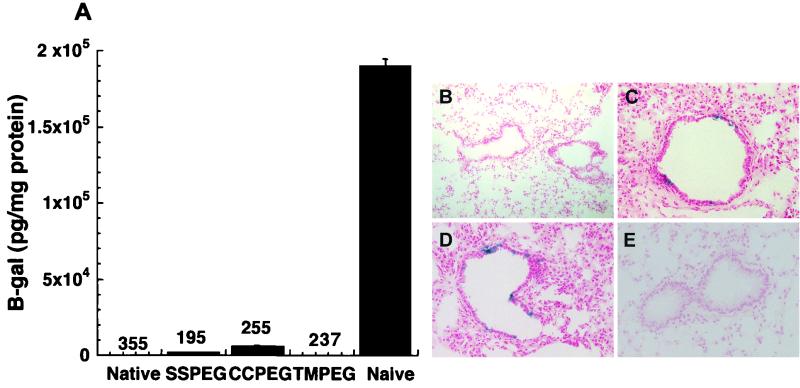
Because previous readministration studies with native and PEGylated adenovirus combinations were favorable, the possibility of successful administration of two doses of PEGylated adenovirus was evaluated. Two doses of either the TMPEG or the native virus failed to produce detectable levels of gene expression (Fig. 8A, B, and E). Low levels of gene expression were detected in sections from six different animals that received two consecutive doses of the SSPEG and CCPEG preparations (Fig. 8C and 8D). Quantification of transgene expression in lung tissue revealed that these preparations did contain significant levels of β-galactosidase but at a level 2 log lower than in naive animals (Fig. 8A).
FIG. 8.
Readministration profiles of C57BL/6 mice after two consecutive doses of the same PEGylated preparation. (A) Levels of β-galactosidase expression from animals administered PEGylated preparations after immunization with adenovirus PEGylated in the same manner but expressing green fluorescent protein. Numbers above each data set represent the average reciprocal dilution of anti-adenovirus NAB present in serum prior to administration of the second dose of vector. The naive group represents animals that received a single dose of unmodified virus. Data are average values for 10 animals from two separate experiments. (B) Cryosection of mouse lung after receiving two doses of native adenovirus. (C) Cryosection of mouse lung after two doses of the SSPEG preparation. (D) Representative airway of an animal that received two doses of adenovirus conjugated to CCPEG. (E) Section of lung from an animal that received two doses of the TMPEG preparation. Magnification, ×140.
Changing activation group allows significant gene expression in animals immunized with PEGylated adenovirus vectors.
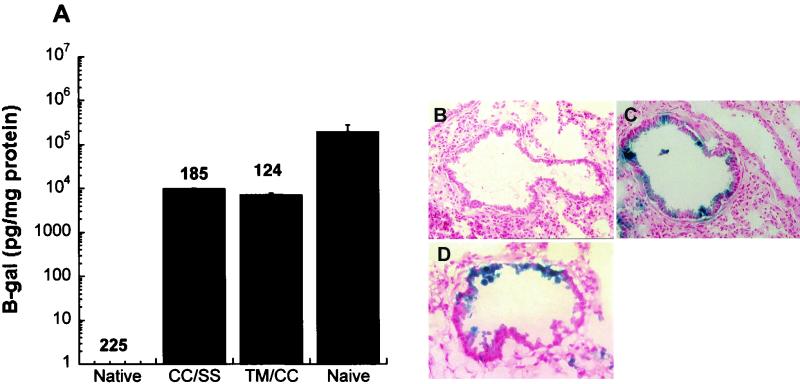
In a final effort to develop dosing strategies with PEGylated adenovirus, animals were immunized with 5 × 1010 particles of adenovirus PEGylated by one method and rechallenged with a similar dose of adenovirus PEGylated by a different method. Rechallenge with SSPEG-conjugated viruses in animals immunized with a CCPEG preparation produced a level of gene expression 2 log lower than in naive animals (Fig. 9A). However, sections from these animals showed concentrated levels of gene expression in the small airways (Fig. 9C). Rechallenge with CCPEG-conjugated virions in animals immunized with a TMPEG preparation produced 7,344 pg β-galactosidase/mg protein. Many tissue sections from these animals also revealed concentrated levels of gene expression in the small airways (Fig. 9D).
FIG. 9.
Readministration profiles of immunocompetent mice after two doses of recombinant adenovirus PEGylated by different methods. (A) β-galactosidase expression from animals after administration of PEGylated adenovirus after immunization with adenovirus PEGylated by a different method. Numbers above data columns are the average reciprocal dilution of anti-adenovirus NAB present in serum prior to dosing with the second vector. The naive group represents animals that received a single dose of unmodified virus. Data (β-galactosidase and NAB) are average values for 10 animals from two separate experiments. Abbreviations: CC, CCPEG; SS, SSPEG; TM, TMPEG. (B) Cryosection of lung from a mouse that received two doses of native adenovirus. (C) Section of lung from an animal that was immunized with a CCPEG preparation and rechallenged with an SSPEG preparation (CC/SS). (D) Representative airway of an animal that was dosed with a TMPEG preparation and rechallenged with the CCPEG preparation (TM/CC). Magnification, ×140
DISCUSSION
In the studies described here, we found that PEGylation of adenovirus vectors slightly enhanced transduction efficiency when administered intratracheally. This effect was somewhat unexpected, as the majority of lysine residues that are present on the viral capsid are concentrated on the fiber and penton proteins, which are necessary for virus binding and entry into target cells (50). However, the new physical characteristics of the PEGylated viruses may contribute to the observed increase in viral transduction (8). Zeta potential measurements have shown that PEGylation effectively masks the groups responsible for the negative surface charge on the viral capsid, producing an environment that would favor nonspecific interaction of the virus with the cell membrane. Particle size measurements of the final PEGylated preparations also revealed that each method produced a suspension of single viral particles which enhance the number of virions that come in contact with cell monolayers and, as a result, can increase transduction efficiency. Partition coefficients for the PEGylated virions indicate that the modified vectors have an increased affinity for hydrophobic environments that would allow them to indiscreetly partition through cell membranes. Initial studies to assess the mechanism by which the transduction efficiency of PEGylated adenoviruses is enhanced support this theory, as the permeability of the PEGylated vectors across differentiated monolayers is significantly enhanced (data not shown).
Immunologic responses to recombinant adenovirus have emerged as a significant issue in the success of in vivo gene therapy (52, 53). Initial prototypes suggest that activation of CTL in response to proteins derived from both the viral genome and the trangene and the formation of NAB to the viral capsid were the basis for transient transgene expression and failure to produce significant levels of gene expression upon readministration.
The role of CTL in vector performance and transgene persistence has been confirmed in multiple studies. Our findings demonstrate that covalent attachment of MPEG to adenovirus capsid proteins is sufficient to reduce CTL responses generated against cells infected with the native vector and, as a result, can extend the length of gene expression beyond that of unmodified virus in immunocompetent animals. While it has been shown that some PEGylated biomolecules fail to elicit significant T-cell responses against the native compound when administered to immunocompetent animals (30, 43, 44), there is very little data to illustrate the basis of this phenomenon. One can envision models in which the polymer inhibits or disrupts proper processing of viral peptides in the endosome of the infected cell. Peptides that are displayed on the cell surface by MHC class I molecules are then left to be unrecognized by circulating CD8+ T cells, allowing significant levels of gene expression upon readministration of an unmodified virus.
It is also important to note that animals that received a single dose of the modified vector produced significant levels of the transgene product approximately 30 days longer than animals given an equivalent dose of the unmodified virus. While PEGylation certainly reduced the CTL response against viral proteins, modification of the viral capsid cannot directly account for modification of the immune response against foreign transgene products such as green fluorescent protein and E. coli β-galactosidase used in these experiments. This modification also cannot prevent the immune response against newly synthesized viral proteins. We believe that CTL directed against both the transgene and newly synthesized viral proteins are responsible for the eventual clearance of cells transduced by the modified vectors. It is also possible that PEG modification redirected the vector away from antigen-presenting cells. In these studies, we chose a first-generation adenovirus as our model vector. Additional studies with PEGylated second-generation and helper-dependent adenovirus expressing a mouse transgene (i.e., mouse erythropoietin) may provide the ultimate solution to achieving stable gene expression with this class of viral vectors.
We also found that T-cell subsets from mice exposed to the conjugated vectors demonstrated a marked decrease in Th1 responses, with slight enhancement of Th2 responses compared to animals dosed with native virus. While these results are consistent with decreased CTL, it suggests that there may be a switch in the qualitative nature of the B-cell response from Th1 to Th2 dependent. There have been documented toxicities associated with PEGs activated by cyanuric chloride in which the triazine rings of the activating group stimulate Th2 responses (9, 49). This type of response could account for the failure of the PEGylated viruses to produce significant levels of gene expression in animals immunized with the same modified virus (Fig. 8). In the experiments described here, only CTL against the unmodified virus were assessed. Further characterization of the nature of the T-cell response against the modified viruses is under way in our laboratories.
The formation of NAB to viral capsid proteins is also a universal finding in most gene therapy experiments involving viral vectors. We and others have shown that coating the virus capsid with activated PEG molecules affords sufficient protection against NAB and allows the modified vector to produce significant levels of gene expression in animals with high levels of anti-adenovirus antibodies (8, 32). Our studies also imply that B-cell-mediated responses (and resultant antibody production) directed against unmodified viral capsid proteins can be somewhat diminished by conjugation of PEG molecules to adenovirus capsids. This finding is consistent with that for PEGylated biomolecules (4, 5, 19, 38, 42) and provides a rationale for the significant level of gene expression attained with the native virus in animals previously exposed to the modified vectors. In this case, attachment of the polymer to viral capsids alters processing of the viral capsid proteins and perhaps provides new antigenic epitopes at the site of polymer attachment. Antibodies are then generated against peptide sequences that were originally viewed as nonimmunogenic. Further assessment of the nature of the epitopes against which these antibodies are directed are under way in our laboratories.
This “antigen switching” pattern is further supported by our results with animals that received two consecutive doses of vector conjugated by the same activated polymer. In this case, readministration of the same vector produced limited levels of gene expression. Similar findings were reported from various studies involving PEGylation of superoxide dismutase and uricase, in which the appearance of a “new” antigenicity and immunogenicity was described (48). After exposure to PEGylated uricase, animals could be subjected to the native enzyme without any immunological consequences but did show cross-reactivity when exposed to superoxide dismutase conjugated with the same activated form of PEG. We have found, however, that this effect can be circumvented by administration of vector modified by one form of PEG followed by an equivalent dose of the same vector modified with another form of the polymer.
In summary, we have shown that modification of viral capsids with various activated MPEGs can reduce the immune response generated against viral proteins. The covalent attachment of polymer to first-generation adenovirus vectors diminishes CTL responses generated against native viral proteins and protects the new vector from neutralization in the presence of anti-adenovirus antibodies. As a result, significant levels of gene expression can be achieved when these viruses are administered to animals previously immunized against the native virus. While this effect can play a significant role in the design of viral vectors for use in the clinic, these modified viruses still failed to produce significant levels of gene expression upon readministration of viruses subjected to the same modification. Thus, the exact nature of the immune response generated from these modified vectors must be extensively characterized. In light of the results reported here, PEGylation of second-generation and helper-dependent adenovirus vectors could be highly effective in enhancing transgene stability and in resolving the problem of readministration of viral vectors without compromising the innate immune system. Studies with modified vectors of this type are under way in our laboratories.
ACKNOWLEDGMENTS
We thank Animal Models Group (Marcia Houston-Leslie, Rosalind Barr, Jeanna Stabinski, Holly Clouse) of the Institute for Human Gene Therapy for expert technical assistance with vector administration. Support from the Immunology Core (George Qian and Ruth Qian) of the Institute for Human Gene Therapy is greatly appreciated. We also thank Chris Munery of the Cell Morphology Core for assistance with tissue processing.
This work was funded by grants from the NIH (P30 DK47757-05), NIH/NIAMS (P01 AR/NS43648-04), the Cystic Fibrosis Foundation, and Genovo, Inc., a biotechnology company that J. M. Wilson founded and has equity in. M.A.C. is a recipient of a National Research Service Award.
REFERENCES
- 1.Armentano D, Zabner J, Sacks C, Sookdeo C, Smith M, St. George J, Wadsworth S, Smith A, Gregory R. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellon G, Michel-Calemard L, Thouvenot D, Jagneaux V, Poitevin F, Malcus C, Accart N, Layani M, Aymard M, Bernon H, Bienvenu J, Courtney M, Doring G, Gilly B, Gilly R, Lamy D, Levrey H, Morel Y, Paulin C, Perraud F, Rodillon L, Sene C, So S, Touraine-Moulin F, Pavirani A, et al. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: a phase I clinical trial. Hum Gene Ther. 1997;8:15–25. doi: 10.1089/hum.1997.8.1-15. [DOI] [PubMed] [Google Scholar]
- 3.Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol. 1999;10:440–447. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 4.Chaffee S, Mary A, Stiehm E, Girault D, Fischer A, Hershfield M. IgG antibody response to polyethylene glycol-modified adenosine deaminase in patients with adenosine deaminase deficiency. J Clin Investig. 1992;89:1643–1651. doi: 10.1172/JCI115761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinol M, Casalini P, Maggiolo M, Canevari S, Omodeo E, Caliceti P, Veronese F, Cremonesi M, Chiolerio F, Nardone E, Siccardi A, Paganelli G. Biochemical modifications of avidin improve pharmacokinetics and biodistribution, and reduce immunogenicity. Br J Cancer. 1998;78:189–197. doi: 10.1038/bjc.1998.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirmule N, Hughes J V, Gao G-P, Raper S E, Wilson J M. Role of E4 in eliciting CD4 T-cell and B-cell responses to adenovirus vectors delivered to murine and nonhuman primate lungs. J Virol. 1998;72:6138–6145. doi: 10.1128/jvi.72.7.6138-6145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirmule N, Truneh A, Haecker S A, Tazelaar J T, Gao G-p, Raper S E, Hughes J V, Wilson J M. Repeated administration of adenoviral vectors in lungs of human CD4 transgenic mice treated with a non-depleting CD4 antibody. J Immunol. 1999;163:448–455. [PubMed] [Google Scholar]
- 8.Croyle M, Yu Q, Wilson J. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther. 2000;11:1721–1730. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- 9.Dearman R, Smith S, Basketter, D. D, Kimber I. Classification of chemical allergens according to cytokine secretion profiles of murine lymph node cells. J Appl Toxicol. 1997;17:53–62. doi: 10.1002/(sici)1099-1263(199701)17:1<53::aid-jat393>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Delgado C, Patel J, Francis G, Fisher D. Coupling of poly(ethylene glycol) to albumin under very mild conditions by activation with tresyl chloride: characterization of the conjugate by partitioning in aqueous two-phase systems. Biotechnol Appl Biochem. 1990;12:119–128. [PubMed] [Google Scholar]
- 11.DeSantis G, Jones J. Chemical modification of enzymes for enhanced functionality. Curr Opin Biotechnol. 1999;10:324–330. doi: 10.1016/S0958-1669(99)80059-7. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt J F, Litzky L, Wilson J M. Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum Gene Ther. 1994;5:1217–1229. doi: 10.1089/hum.1994.5.10-1217. [DOI] [PubMed] [Google Scholar]
- 13.Fisher K J, Choi H, Burda J, Chen S J, Wilson J M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 14.Francis G, Fisher D, Delgado C, Malik F, Gardiner A, Neale D. PEGylation of cytokines and other therapeutic proteins and peptides: the importance of biological optimisation of coupling techniques. Int J Hematol. 1998;68:1–18. doi: 10.1016/s0925-5710(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 15.Graham F, Van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 16.Haecker S E, Stedman H H, Balice-Gordon R J, Smith D B, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. In vivo expression of full-length human dystrophin from adenoviral vectors deleted of all viral genes. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 17.Hershfield M. Enzyme replacement therapy of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase (PEG-ADA) Immunodeficiency. 1993;4:93–97. [PubMed] [Google Scholar]
- 18.Ilan Y, Droguett G, Chowdhury N R, Li Y, Sengupta K, Thummala N R, Davidson A, Chowdhury J R, Horwitz M S. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inada Y, Furukawa M, Sasaki H, Kodera Y, Hiroto M, Nishimura H, Matsushima A. Biomedical and biotechnological applications of PEG- and PM-modified proteins. Trends Biotechnol. 1995;13:86–91. doi: 10.1016/S0167-7799(00)88912-X. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, Charlton C J, Kuzminski K, Lang G, Sehon A. Synthesis, isolation, and characterization of conjugates of ovalbumin with monomethoxypolyethylene glycol using cyanuric chloride as the coupling agent. Anal Biochem. 1987;165:114–127. doi: 10.1016/0003-2697(87)90208-9. [DOI] [PubMed] [Google Scholar]
- 21.Jooss K, Ertl H C, Wilson J M. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jooss K, Turka L A, Wilson J M. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 23.Jooss K, Yang Y, Wilson J M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 24.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 25.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligans antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kita Y, Rohde M, Arakawa T, Fagin K, Fish E, Banerjee K. Characterization of a polyethylene glycol conjugate of recombinant human interferon-gamma. Drug Des Deliv. 1990;6:157–167. [PubMed] [Google Scholar]
- 27.McDonald R, Lukason M, Raabe O, Canfield D, Burr E, Kaplan J, Wadsworth S, St. George J. Safety of airway gene transfer with Ad2/CFTR2: aerosol administration in the nonhuman primate. Hum Gene Ther. 1997;8:411–422. doi: 10.1089/hum.1997.8.4-411. [DOI] [PubMed] [Google Scholar]
- 28.Mitani K, Graham F L, Caskey C T, Kochanek S. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc Natl Acad Sci USA. 1995;92:3854–3858. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morral N, Parks R, Zhou H, Langston C, Schiedner G, Quinones J, Graham F, Kochanek S, Beaudet A. High doses of a helper-dependent adenoviral vector yield supra-physiological levels of alphal-antitrypsin with negligible toxicity. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 30.Murad K, Gosselin E, Eaton J, Scott M. Stealth cells: prevention of major histocompatibility complex class II-mediated T-cell activation by cell surface modification. Blood. 1999;94:2135–2141. [PubMed] [Google Scholar]
- 31.Nunes F, Furth E, Wilson J, Raper S. Gene transfer into the liver of non-human primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- 32.O'Riordan C, Lachapelle A, Delgado C, Parkes V, Wadsworth S, Smith A, Francis G. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 33.Otake K, Ennist D, Harrod K, Trapnell B. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum Gene Ther. 1998;9:2207–2222. doi: 10.1089/hum.1998.9.15-2207. [DOI] [PubMed] [Google Scholar]
- 34.Parks R, Bramson J, Wan Y, Addison C, Graham F. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J Virol. 1999;73:8027–8034. doi: 10.1128/jvi.73.10.8027-8034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 36.Robinson C. Is DNA destiny? A cure for cystic fibrosis. Clin Chest Med. 1998;19:527–534. doi: 10.1016/s0272-5231(05)70098-8. [DOI] [PubMed] [Google Scholar]
- 37.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, Perricaudet M, Guggino W B, Pavirani A, Lecocq J P, Crystal R G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 38.Saito T, Nishimura H, Sekine T, Urushibara T, Kodera Y, Hiroto M, Matsushima A, Inada Y. Tolerogenic capacity of poly(ethylene glycol) (PEG)-modified ovalbumins in relation to their immunoreactivity towards anti-ovalbumin antibody. J Biomater Sci Polym Ed. 1996;8:311–321. doi: 10.1163/156856296x00327. [DOI] [PubMed] [Google Scholar]
- 39.Scaria A, St. George J, Gregory R, Noelle R, Wadsworth S, Smith A, Kaplan J. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997;4:611–617. doi: 10.1038/sj.gt.3300431. [DOI] [PubMed] [Google Scholar]
- 40.Schiedner G, Morral N, Parks R, Wu Y, Koopmans S, Langston C, Graham F, Beaudet A, Kochanek S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 41.Scott M, Murad K. Cellular camouflage: fooling the immune system with polymers. Curr Pharm Des. 1998;4:423–438. [PubMed] [Google Scholar]
- 42.Sehon A. Carl Prausnitz Memorial Lecture: suppression of antibody responses by chemically modified antigens. Int Arch Allergy Appl Immunol. 1991;94:11–20. [PubMed] [Google Scholar]
- 43.So T, Ito H, Koga T, Ueda T, Imoto T. Reduced immunogenicity of monomethoxypolyethylene glycol-modified lysozyme for activation of T cells. Immunol Lett. 1996;49:91–97. doi: 10.1016/0165-2478(95)02488-3. [DOI] [PubMed] [Google Scholar]
- 44.So T, Ito H, Tsujihata Y, Hirata M, Ueda T, Imoto T. The molecular weight ratio of monomethoxypolyethylene glycol (mPEG) to protein determines the immunotolerogenicity of mPEG proteins. Protein Eng. 1999;12:701–705. doi: 10.1093/protein/12.8.701. [DOI] [PubMed] [Google Scholar]
- 45.Stein C, Pemberton J, van Rooijen N, Davidson B. Effects of macrophage depletion and anti-CD40 ligand on transgene expression and redosing with recombinant adenovirus. Gene Ther. 1998;5:431–439. doi: 10.1038/sj.gt.3300616. [DOI] [PubMed] [Google Scholar]
- 46.Sterman D, Kaiser L, Albelda S. Gene therapy for malignant pleural mesothelioma. Hematol Oncol Clin N Am. 1998;12:553–568. doi: 10.1016/s0889-8588(05)70008-3. [DOI] [PubMed] [Google Scholar]
- 47.Stone D, David A, Bolognani F, Lowenstein P, Castro M. Viral vectors for gene delivery and gene therapy within the endocrine system. J Endocrinol. 2000;164:103–118. doi: 10.1677/joe.0.1640103. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji J, Hirose K, Kasahara E, Naitoh M, Yamamoto I. Studies on antigenicity of the polyethylene glycol (PEG)-modified uricase. Int J Immunopharmacol. 1985;7:725–730. doi: 10.1016/0192-0561(85)90158-4. [DOI] [PubMed] [Google Scholar]
- 49.Veronese F, Largajolli R, Boccu E, Benassi C, Schiavon O. Surface modification of proteins: activation of monomethoxy-polyethylene glycols by phenylchloroformates and modification of ribonuclease and superoxide dismutase. Appl Biochem Biotechnol. 1985;11:141–152. doi: 10.1007/BF02798546. [DOI] [PubMed] [Google Scholar]
- 50.Wickham T, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 51.Wilson C, Embree L, Schowalter D, Albert R, Aruffo A, Hollenbaugh D, Linsley P, Kay M. Transient inhibition of CD28 and CD40 ligand interactions prolongs adenovirus-mediated transgene expression in the lung and facilitates expression after secondary vector administration. J Virol. 1998;72:7542–7550. doi: 10.1128/jvi.72.9.7542-7550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson J M. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 53.Wold W, Doronin K, Toth K, Kuppuswamy M, Lichtenstein D, Tollefson A. Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr Opin Immunol. 1999;11:380–386. doi: 10.1016/S0952-7915(99)80064-8. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Jooss K U, Su Q, Ertl H C, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 55.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Raper S E, Cohn J A, Engelhardt J F, Wilson J M. An approach for treating the hepatobiliary disease of cystic fibrosis by somatic gene transfer. Proc Natl Acad Sci USA. 1993;90:4601–4605. doi: 10.1073/pnas.90.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Su Q, Grewal I, Schilz R, Flavell R, Wilson J. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Su Q, Wilson J. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Trinchieri G, Wilson J. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 61.Zuckerman J, Robinson C, McCoy K, Shell R, Sferra T, Chirmule N, Magosin S, Propert K, Brown-Parr E, Hughes J, Tazelaar J, Baker C, Goldman M, Wilson J. A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther. 1999;10:2973–2985. doi: 10.1089/10430349950016384. [DOI] [PubMed] [Google Scholar]