Abstract
A 14‐year‐old spayed female Miniature Pinscher presented with tongue curling, dysphagia, hypersalivation, and sublingual gland swelling. Comprehensive evaluation, including neurologic and musculoskeletal examinations, blood work, and urinalysis, revealed no abnormalities other than tongue‐related signs. Magnetic resonance imaging (MRI) revealed a multilobed cystic structure in the occipito‐atlanto‐axial joint, compressing the right hypoglossal canal. The lesion appeared cerebrospinal fluid (CSF)‐like on T1‐weighted and T2‐weighted images, and hyperintense compared with CSF on fluid‐attenuated inversion recovery T2‐weighted images. The scans suggested mucinous content with enhanced peripheral areas on contrast‐enhanced images. Surgical removal and drainage of this cyst were performed, and clinical signs improved markedly. The dorsal cyst was tentatively diagnosed as a ganglion cyst based on histopathologic and imaging findings. Ganglion cysts should be considered in the differential diagnosis for dogs with similar MRI findings and neurologic signs.
Keywords: canine, dysphagia, hypersalivation, hypoglossal nerve, juxta‐articular cyst, tongue curling
Abbreviations
- CSF
cerebrospinal fluid
- FLAIR
fluid‐attenuated inversion recovery
- MRI
magnetic resonance imaging
- T1W
T1‐weighted
- T2W
T2‐weighted
- TE
echo time
- TR
repetition time
1. INTRODUCTION
Ganglion cysts are benign cysts that arise from the periarticular space owing to fluid leakage from a nearby joint. 1 They are caused by mucinous degeneration of periarticular connective tissues and contain myxoid material with fibrous walls. 2 They usually occur in the lumbar spinal area or extremities and are relatively uncommon in the atlanto‐axial joint. 3 , 4 The clinical signs of ganglion cysts result from compression of the nerve root or spinal cord, leading to signs such as hyperesthesia, proprioceptive ataxia, and tetraparesis. 3 , 4 , 5 , 6
In humans, there have been few reports of ganglion cysts occurring in the atlanto‐axial joint. 7 , 8 , 9 The patients in these reports exhibited clinical signs of hypoglossal nerve dysfunction, such as tongue dysfunction and difficulty swallowing. 7 , 8 , 9 Magnetic resonance imaging (MRI) is an effective method for making a provisional diagnosis by identifying cysts with clear boundaries that appear hyperintense on T2‐weighted (T2W) images and hypo‐ or isointense on T1‐weighted (T1W) images. 10 , 11 Surgery could be considered as a treatment option for ganglion cysts, with the procedure being similar to that for other spinal lesions. 7 , 8 , 9
This case report describes the diagnostic MRI features, surgical treatment, and follow‐up of a multiloculated extraneural ganglion cyst associated with hypoglossal nerve paralysis in a dog.
2. CASE PRESENTATION
A 14‐year‐old, spayed female, Miniature Pinscher, weighing 3.7 kg, presented with a curled tongue, dysphagia, hypersalivation, and swelling of the sublingual gland that began a month earlier. Initially suspected of having sublinguitis at a local hospital, the dog was prescribed antibiotics (metronidazole 10 mg/kg PO q12h), but as the clinical signs did not improve, she was then prescribed prednisolone (0.3 mg/kg PO q12h), which led to temporary improvement in sublingual swelling, but the dysphagia worsened.
During physical examination, the dog showed no pain in the cervical region. Although neurological and musculoskeletal examinations revealed no specific findings, there was dysphagia and hypersalivation because of tongue curling. Other blood tests and urinalysis results were within normal ranges. Thoracic and abdominal radiography, abdominal ultrasonography, and echocardiography revealed no specific findings.
We performed 1.5 Tesla MRI scans (Genesis Signa 1.5 T; GE Healthcare, Chicago, IL) on the brain, cervical, and thoracic spinal regions, with a magnetic field strength of 1.5 T. The dog was anesthetized and positioned in the dorsal recumbent position. Images were acquired using sagittal T2W and T1W sequences with slice thicknesses of 2.5 mm; T2W images were acquired using a fast spin‐echo sequence with a repetition time (TR) of 2416 ms and echo time (TE) of 118 ms, while T1W images were obtained with a TR of 650 ms and TE of 9 ms. Images in the transverse plane included T2W, fluid‐attenuated inversion recovery (FLAIR), T1W, and T1W contrast enhancement images, with a slice thickness of 3.5 mm. We acquired T2W images in this plane using the fast spin echo sequence with a TR of 3600 ms and TE of 118 ms; FLAIR images were acquired with a TR of 7677 ms and TE of 150 ms; T1W images with a TR of 700 ms and a TE of 10 ms; and T1W contrast enhancement images with the same TR and TE of 10, as 0.2 mL/kg of gadolinium was administered before imaging.
The cystic lesion extended from the level of the atlanto‐occipital joint to the level of the atlanto‐axial joint. At the atlanto‐occipital joint level, the lesion was located in the ventral region of the vertebra and was observed to be bilateral, situated around the atlas. The lesion mildly compressed the right hypoglossal canal (Figure 1). Additionally, multiloculated cystic structures were found in the dorsal region of the vertebrae at the level of the atlanto‐axial joint, with the right side of the lesion observed to be larger. Deviation of the muscles around the cysts and compression of the muscles because of the cyst were observed (Figure 2).
FIGURE 1.
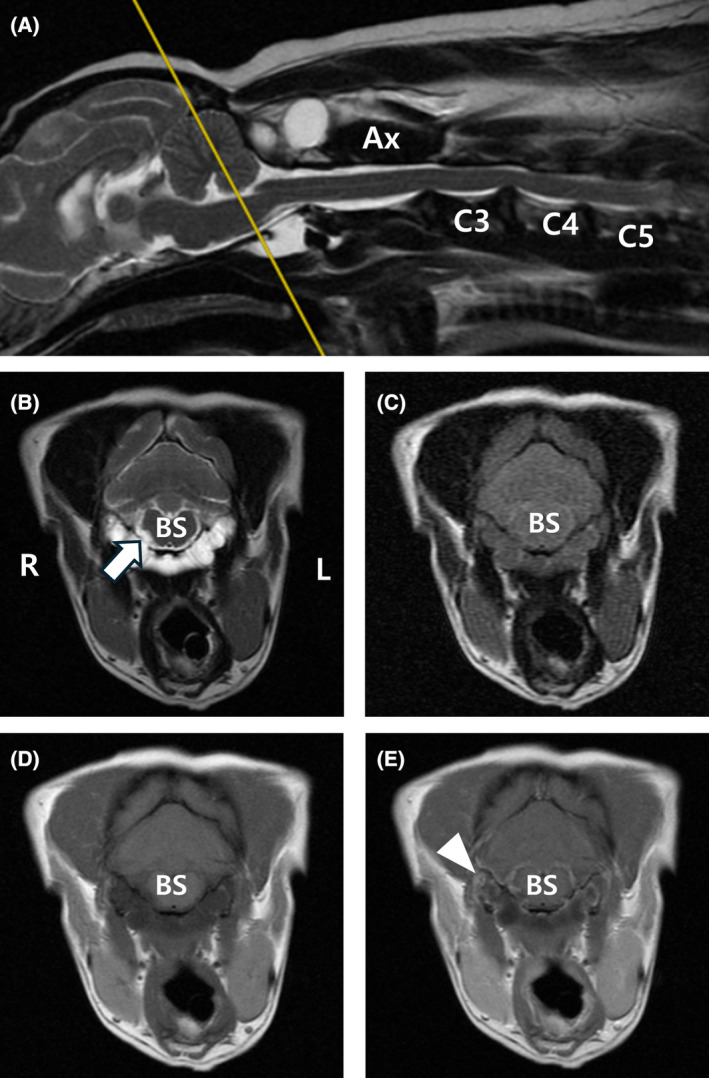
Magnetic resonance images of cystic lesions at the level of the brainstem. Sagittal (A) and transverse (B) T2‐weighted, transverse FLAIR (C), T1‐weighted (D), and T1‐weighted post‐contrast (E) magnetic resonance images. The lesion is observed hyperintense in T2‐weighted images (A, B). In the T2‐weighted FLAIR image, hyperintensity is observed compared with CSF (C), and there is mild peripheral enhancement (white arrowhead) in T1‐weighted post‐contrast images (E). At the brainstem level, the cystic lesion was located in the ventral region of the vertebra and was observed to be bilateral, encircling the atlas and resulting in slight compression of the right‐sided hypoglossal nerve (white arrow). Ax, axis; BS, brainstem; FLAIR, fluid‐attenuated inversion recovery; L, left; R, right.
FIGURE 2.
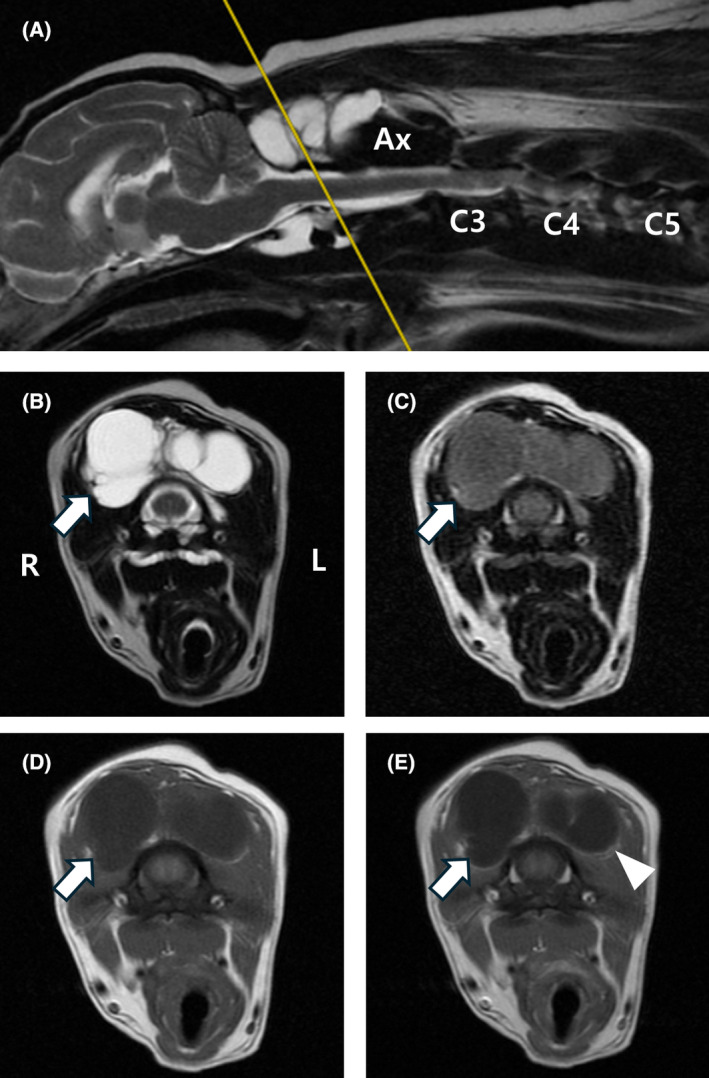
Magnetic resonance images of cystic lesions at the level of the atlanto‐axial joint. Sagittal (A) and transverse (B) T2‐weighted, transverse FLAIR (C), T1‐weighted (D), and T1‐weighted post‐contrast (E) magnetic resonance images. The lesion appears hyperintense in T2‐weighted images (A, B). In the T2‐weighted FLAIR image, hyperintensity is observed compared with CSF (C), and there is mild peripheral enhancement (white arrowhead) in T1‐weighted post‐contrast images (E). At the atlanto‐axial joint level, the lesion in the dorsal region of the vertebra is observed as multiloculated cystic structures, with the right side of the lesion observed to be larger (white arrow). Deviation of the muscles around the cysts and compression of the muscles due to the cysts were observed. Ax, axis; FLAIR, fluid‐attenuated inversion recovery; L, left; R, right.
These lesions appeared hypointense on the T1W images, hyperintense on the T2W images, and hyperintense on FLAIR images compared with cerebrospinal fluid (CSF), with peripheral enhancement observed on contrast‐enhanced images (Figures 1 and 2). These were considered to be fluid‐filled cysts extending to the ventral and dorsal regions of the occipito‐atlanto‐axial joint. This area contains the hypoglossal, vagus, glossopharyngeal, vestibulocochlear, facial, and cranial accessory nerves. The dog's clinical signs could have been associated with the hypoglossal nerves. Additional abnormal findings included disc protrusion, extrusion, and compressed nerve roots affecting the spinal cord at the C2‐C5 level.
Surgery was performed ~1 month after the initial recognition of signs. It was conducted to collect samples for histopathological examination and to decompress the spinal cord to alleviate clinical signs. Before anesthesia induction, the dog was pre‐administered glycopyrrolate (0.01 mg/kg IV) and was pre‐oxygenated for 10 minutes. Anesthesia was induced using midazolam (0.3 mg/kg IV) followed by propofol (2 mg/kg IV). Throughout the surgery, anesthesia was maintained with isoflurane (1.5%‐2.0%) delivered via a ventilator. The perioperative antibiotic cefazolin sodium (22 mg/kg IV) was administered 10 minutes before surgery.
The dog was initially placed in the dorsal recumbent position to access a cyst in the ventral region. The ventral surgical approach to the atlanto‐occipital joint was performed as previously described. 5 A fluid‐filled transparent cystic membrane was observed on the ventral surface of the atlanto‐occipital joint. This thin cystic membrane was attached to the joint capsule of the atlanto‐occipital joint complex. Approximately 1 mL of bloody mucous fluid was aspirated from the cyst, and the remaining fluid within the cyst was aspirated and surgically removed. After suturing the incision in the ventral region, the dog was positioned in sternal recumbency to access the cyst in the dorsal region, and laminectomy was performed at the C1‐C2 level. Beneath the muscle, colorless fluid‐filled sacs were observed, and most of the cysts in the dorsal region were removed The surgical wound was closed using subcutaneous metric 2 polydioxanone sutures for the deeper layers and metric 2 nylon sutures for the skin. The dog recovered without complications, and both aerobic and anaerobic cultures were negative.
Microscopic examination revealed a large cystic structure surrounded by an edematous fibrous connective tissue wall lined with flattened, well‐differentiated cells, and the lumen was empty (Figure 3). Given the absence of visible synovium lining, a ganglion cyst became the more probable diagnosis. Consequently, based on histopathologic and imaging findings, a provisional diagnosis of a ganglion cyst was formulated.
FIGURE 3.
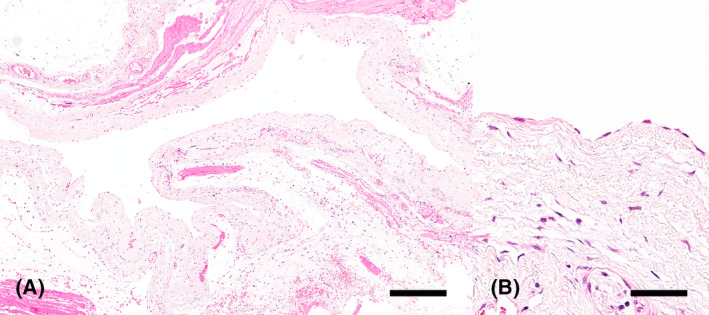
Ganglion cyst, hematoxylin and eosin. Large cystic structure with an empty lumen surrounded by an edematous fibrous connective tissue wall lined by flattened, well‐differentiated cells. Bar = 800 μm (A). Higher magnification of the flattened cells lining the cystic structure. Bar = 100 μm (B).
At postoperative follow‐up, the dog showed improved clinical signs and was able to eat without spilling. At 6‐month follow‐up, there was mild drooling but significant improvement in dysphagia. However, 8 months after surgery, the dog returned to the hospital with signs similar to those previously reported; MRI confirmed the regrowth of the cyst in an area that did not allow for its complete removal. Owing to severe respiratory distress caused by the primary signs, the dog was euthanized at the owner's request.
3. DISCUSSION
Extraspinal cysts are non‐meningeal, extradural fluid‐filled structures that occur in the periarticular joint tissue and are categorized into 2 types. These types do not present notable clinical differences and are differentiated by histopathological examination. 11 A synovial cyst is a protrusion of the synovial membrane through the weakened capsular tissue with an epithelial‐like synovium. A ganglion cyst is caused by the mucous degeneration of the connective tissue around the joint and contains mucinous material with a fibrous wall. The etiology of these cystic lesions is not well understood. 3 , 12 , 13 However, some reports suggest an association between increased intra‐articular pressure because of degenerative joint disease and capsule formation resulting from increased joint motion or trauma. 6 , 14 The suspected mechanisms include extrusion or herniation of synovial joint fluid, myxoid degeneration of collagen tissue, increased secretion of fibroblasts, and nonspecific proliferation of mesenchymal cells. 3 , 13
Cystic lesions around the spinal cord occur in humans. 1 , 7 , 8 , 9 In particular, several cases of cystic lesions with unilateral paralysis or atrophy of the tongue, dysarthria, and difficulty swallowing have been reported. 1 , 7 , 8 , 9 These lesions are located in the atlanto‐axial joint region where mild or severe compression of the hypoglossal nerve has been noted. 1 , 7 , 8 , 9 Most patients show improvement in clinical signs after surgery. 1 , 7 , 8 , 9 Although cystic lesions around the spinal cord are rare in cats, ganglion cysts and cystic lesions in the atlanto‐axial joint region occur. 2 , 15 , 16 Specifically, cats with ganglion cysts in this region exhibit ambulatory signs, showing signs of tetraparesis because of moderate spinal cord compression. 15 , 16
Cystic lesions in the paraspinal region are rare in dogs. Extradural synovial and ganglion cysts occur around the connective tissue of the cervical, thoracolumbar, and lumbar spine, as well as at the atlanto‐axial joint. 3 , 4 , 5 , 10 , 12 , 13 These cysts can cause focal compression of the spinal cord and nerve roots, leading to abnormal neurological signs. 3 , 4 , 5 , 10 , 12 , 13 The clinical signs of cystic lesions vary depending on the location and degree of compression. 3 , 4 , 5 , 10 , 12 , 13 Most dogs present with spinal pain as their chief complaint. 3 , 4 , 5 , 10 , 12 , 13
Notably, MRI examination shows hypointensity on T1W images and hyperintensity on T2W images, as well as hyperintensity relative to CSF on FLAIR images. If the cyst is filled with synovial fluid, its appearance is similar to that of CSF on both T1W and T2W images. 10 , 11 However, if the fluid within the cyst is gelatinous or mucinous, it might appear hyperintense on both T1W and T2W images. 10 , 11 Signal intensity might vary if the cyst is associated with hemorrhage. 10 , 11 The cyst contents were not completely removed in the T2‐FLAIR images, suggesting a protein enrichment consistent with previous reports indicating a gelatinous or mucinous nature. The opacity of the fluid suggested it was either viscous or protein‐rich, indicative of a ganglion cyst.
Compared with previous studies on cystic lesions in the atlanto‐occipital joint region in humans, the anatomical location, clinical signs, macroscopic findings, and MRI findings of the cyst, in this case, were similar, including improvements in clinical signs after surgical removal. 1 , 7 , 9 In a previous study involving cats, the anatomical location of the cyst was similar to that of the atlanto‐occipital joint region. However, unlike the ataxia seen in that cat, the signs in this case were tongue curling, dysphagia, and hypersalivation. 2 , 15 The macroscopic and MRI findings were similar, and clinical signs improved with corticosteroid treatment or surgical removal. 15 , 16 In particular, the cat that underwent surgical removal showed an improvement in clinical signs over 64 months. 15 In most cases, the cysts were surgically removed; however, in some cases that occurred in the lumbar spine, the clinical signs improved over time after drug treatment for multiple cysts. 2 , 3 , 4 , 5 , 10 , 12 , 13 , 14 , 16
The hypoglossal nerve, also known as the twelfth cranial nerve, provides motor innervation to the geniohyoideus muscle and the intrinsic and extrinsic muscles of the tongue, including the styloglossus, hyoglossus, and genioglossus. Neuronal cell bodies are located within the motor nucleus of the medulla oblongata of the caudal brainstem, with axons exiting the caudal aspect of the skull through the hypoglossal canal. Bilateral lesions of this nerve result in an inability to retract the tongue from the mouth, leading to difficulties in grasping food and water. An important differential diagnosis for bilateral hypoglossal nerve dysfunction is tongue paresis/atrophy secondary to a primary myopathy affecting the tongue. 17
In the present case, the dog exhibited clinical signs of hypersalivation and dysphagia, unlike previously reported cases of ganglion cysts in the atlanto‐axial joint in cats. 15 Furthermore, there was a partial improvement in clinical signs after surgery. Considering the location of the cyst and its association with the signs, we considered that cyst compression might have affected the cranial nerves.
Fluid property analysis could have contributed to a more accurate diagnosis. In this case, aspiration of the fluid from the cyst was performed; however, no analysis was performed. Complete removal of the cyst in the ventral region was difficult because of its connection to the surrounding structures, and this later led to the recurrence of the lesion, that became the direct cause of the dog's death.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
No funding was received for this study.
Ji M, Kiupel M, Park H, Lee K, Yoon H. Magnetic resonance imaging features of bilateral multiloculated extraneural ganglion cysts of the occipito‐atlanto‐axial joint causing hypoglossal nerve paralysis in a dog. J Vet Intern Med. 2024;38(5):2675‐2680. doi: 10.1111/jvim.17192
REFERENCES
- 1. Giordano M, Gerganov VM, Samii A, Samii M. Intradural extraneural bilobate ganglion cyst of the atlanto‐occipital joint compressing the hypoglossal nerve. J Clin Neurosci. 2012;19:472‐473. doi: 10.1016/j.jocn.2011.04.035 [DOI] [PubMed] [Google Scholar]
- 2. Strobel F, Taeymans O, Rosati M, et al. Lumbosacral intraspinal extradural ganglion cyst in a cat. JFMS Open Rep. 2015;1:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez B, Rollan E, Ramiro F, et al. Intraspinal synovial cyst in a dog. J Am Anim Hosp Assoc. 2000;36:235‐238. doi: 10.5326/15473317-36-3-235 [DOI] [PubMed] [Google Scholar]
- 4. Ferrarin DA, Polidoro Neto DNP, Schwab ML, et al. Extradural synovial cyst of the cervical spine in a Saint Bernard. Acta Sci Vet. 2021;49:614. doi: 10.22456/1679-9216.101479 [DOI] [Google Scholar]
- 5. Forterre F, Vizcaino Reves NV, Stahl C, Rupp S, Gendron K. Atlantoaxial synovial cyst associated with instability in a Chihuahua. Case Rep Vet Med. 2012;2012:1‐4. doi: 10.1155/2012/898241 [DOI] [Google Scholar]
- 6. Hsu KY, Zucherman JF, Shea WJ, Jeffrey RA. Lumbar intraspinal synovial and ganglion cysts (facet cysts). Ten‐year experience in evaluation and treatment. Spine. 1995;20:80‐89. doi: 10.1097/00007632-199501000-00015 [DOI] [PubMed] [Google Scholar]
- 7. Gambhir S, Mujic A, Hunn A. An intraneural ganglion cyst causing unilateral hypoglossal nerve palsy. J Clin Neurosci. 2011;18:1114‐1115. doi: 10.1016/j.jocn.2010.12.030 [DOI] [PubMed] [Google Scholar]
- 8. Nonaka Y, Grossi PM, Filomena CA, Friedman AH, Fukushima T. Unilateral hypoglossal nerve palsy caused by an intraneural ganglion cyst. J Neurosurg. 2010;113:380‐383. doi: 10.3171/2010.1.JNS091526 [DOI] [PubMed] [Google Scholar]
- 9. Mujic A, Hunn A, Liddell J, Taylor B, Havlat M, Beasley T. Isolated unilateral hypoglossal nerve paralysis caused by an atlanto‐occipital joint synovial cyst. J Clin Neurosci. 2003;10:492‐495. doi: 10.1016/s0967-5868(03)00083-3 [DOI] [PubMed] [Google Scholar]
- 10. Webb AA, Pharr JW, Lew LJ, Tryon KA. MR imaging findings in a dog with lumbar ganglion cysts. Vet Radiol Ultrasound. 2001;42:9‐13. doi: 10.1111/j.1740-8261.2001.tb00897.x [DOI] [PubMed] [Google Scholar]
- 11. Bonelli MA, da Costa RC. Spontaneous regression of extradural intraspinal cysts in a dog: a case report. BMC Vet Res. 2019;15:396. doi: 10.1186/s12917-019-2152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickinson PJ, Sturges BK, Berry WL, Vernau KM, Koblik PD, LeCouteur RA. Extradural spinal synovial cysts in nine dogs. J Small Anim Pract. 2001;42:502‐509. doi: 10.1111/j.1748-5827.2001.tb02458.x [DOI] [PubMed] [Google Scholar]
- 13. Forterre F, Kaiser S, Garner M, et al. Synovial cysts associated with cauda equina syndrome in two dogs. Vet Surg. 2006;35:30‐33. doi: 10.1111/j.1532-950X.2005.00108.x [DOI] [PubMed] [Google Scholar]
- 14. Sale CSH, Smith KC. Extradural spinal juxtafacet (synovial) cysts in three dogs. J Small Anim Pract. 2007;48:116‐119. doi: 10.1111/j.1748-5827.2006.00205.x [DOI] [PubMed] [Google Scholar]
- 15. Aikawa T, Sadahiro S, Nishimura M, Miyazaki Y, Shibata M. Ganglion cyst arising from the composite occipito‐atalanto‐axial joint cavity in a cat. Vet Comp Orthop Traumatol. 2014;27:319‐323. doi: 10.3415/VCOT-13-10-0119 [DOI] [PubMed] [Google Scholar]
- 16. Gutierrez‐Quintana R, Hammond G, Wessmann A. Ventral occipito‐atlanto‐axial fluid‐filled lesion causing dynamic spinal cord compression in a cat. J Feline Med Surg. 2014;16:532‐535. doi: 10.1177/1098612X13507073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freeman PM, Ives E. A Practical Approach to Neurology for the Small Animal Practitioner. 1st ed. Hoboken, NJ: John Wiley & Sons; 2020. [Google Scholar]


