Abstract
Background
Reports of leptospirosis in horses are limited.
Objectives
To describe the clinical and diagnostic findings of acute systemic leptospirosis in horses.
Animals
Eleven client‐owned horses presented to an equine hospital because of acute onset of disease between 2015 and 2023.
Methods
Retrospective case series. Horses diagnosed with leptospirosis by 1 or more of urine PCR, serologic microscopic agglutination test (MAT), and histopathology.
Results
Common clinical signs included lethargy (10), anorexia (10), fever (9), tachypnea (9), abnormal lung sounds (9), and epistaxis (6). Acute kidney injury was present in all cases. Evidence of pulmonary hemorrhage and liver disease was found in 8 (73%) and 6 (55%) horses, respectively. In 6 (55%) horses, kidneys, lungs, and liver were affected. Urine quantitative polymerase chain reaction for detection of pathogenic Leptospira spp. was positive in 6 (55%) cases. On serology Leptospira interrogans serovar Australis, Autumnalis, and Bratislava accounted for 86% of all titers ≥1 : 800. Overall case fatality rate was 4/11 (36%). Main findings on necropsy were tubular necrosis, interstitial nephritis, hemorrhage in the alveoli, pulmonary edema, periportal hepatitis and necrosis, cholestasis, and cholangitis.
Conclusions and Clinical Importance
Leptospirosis should be considered as a differential diagnosis in horses with evidence of acute systemic inflammation and acute renal injury, epistaxis, or hepatic disease. For increased likelihood of identifying positive cases, both MAT serology and urine PCR should be performed.
Keywords: hepatic involvement, Leptospira, lung bleeding, systemic disease
Abbreviations
- AKI
acute kidney injury
- AST
aspartate aminotransferase
- BALF
bronchoalveolar lavage fluid
- BUN
blood urea nitrogen
- ERU
equine recurrent uveitis
- GDH
glutamate dehydrogenase
- GGT
gamma glutamyltransferase
- LDH
lactate dehydrogenase
- LPHS
leptospiral pulmonary hemorrhage syndrome
- MAT
microscopic agglutination test
- NSAID
nonsteroidal anti‐inflammatory drug
- POC
point of care
- qPCR
quantitative polymerase chain reaction
- rDVM
referring doctor of veterinary medicine
- RRT
renal replacement therapy
- SDH
sorbitol dehydrogenase
- TBA
tracheobronchial aspirate
1. INTRODUCTION
Bacteria of the genus Leptospira have a global distribution and can infect and cause disease in most mammalian species. Leptospirosis is a leading zoonosis worldwide in terms of morbidity and mortality with an estimated 1 million human clinical cases and 60 000 deaths yearly. 1 Concerns about increasing incidences in humans and dogs and underestimation of the true case numbers have been raised in recent years with the need for a One Health approach suggested by some. 1 , 2 , 3 , 4 , 5
Exposure to Leptospira spp. is reportedly common in horses in Europe and North America although systemic disease seems to be rare in comparison. Seroprevalences of leptospiral infection in horses varies between countries with 77% and 82% in 2 North American studies, 16.6% in Sweden, 33.2% in Poland, and 58.5% in Switzerland. 6 , 7 , 8 , 9 , 10 Rodents, a variety of wildlife and domestic animals serve as maintenance hosts and harbor leptospires in the renal tubules, from which the bacteria can be shed into the urine intermittently. Horses might come into contact with leptospires through feed or water contaminated with infectious urine, reproductive fluids or contaminated environmental reservoirs. 11 After entry into the body via mucus membranes or breaks in the skin, leptospiremia causes vasculitis and facilitates infection of multiple organs including the kidneys, liver, spleen, central nervous system, and genital tract. 11 , 12 Placentitis, abortions, stillbirths, birth of weak foals, uveitis, and renal disease are clinical syndromes associated with equine leptospirosis. The most common disease being equine recurrent uveitis (ERU), which might develop weeks to months after infection with pathogenic Leptospira. 11 , 13 , 14 Other clinical manifestations of equine leptospirosis such as kidney disease and respiratory failure are sporadic. 15 , 16 , 17 , 18 , 19 One reason for the disparity between the relatively high seroprevalences in the equine population and the infrequent diagnosis of leptospirosis is likely a high percentage of subclinical infections. 11 , 13 Diagnostic challenges could be another explanation for the sparse number of clinical reports and it is possible that the disease is underdiagnosed.
In this retrospective case series, we report on 11 cases of acute leptospirosis where the main clinical manifestations were renal, pulmonary, and hepatic disease. The aims of this study were (a) to expand the limited knowledge on systemic equine leptospirosis, (b) highlight the diagnostic challenges associated with leptospirosis in horses, and (c) increase awareness of the occurrence of this important zoonosis in horses.
2. MATERIALS AND METHODS
Hospital records from horses presented to the Clinic for Equine Internal Medicine, University of Zurich from 2015 to 2023 were retrospectively reviewed. Hospital records were searched by key words leptospirosis, leptospira, and leptospir*. Cases were eligible for inclusion in this study if a diagnosis of leptospirosis was confirmed by 1 or more of: (1) urine PCR positive for pathogenic Leptospira serovars, (2) clinical signs and laboratory findings of fever, acute kidney injury (AKI), renal azotemia, hematuria, icterus, anemia and a positive antibody titer >1 : 100 for 1 or more pathogenic Leptospira serovars, and (3) postmortem histopathological findings consistent with leptospirosis including identification of spirochetes in the renal proximal tubules by silver staining. Cases with only ERU and reproductive tract disease were not included in the study.
Medical records were screened and date of hospital presentation, age, breed, sex, clinical examination findings, diagnostic imaging findings, and further diagnostics that is, tracheal aspirate, bronchoalveolar lavage (BAL), rectal exam, abdominal ultrasonography that is, fast localized abdominal sonography in horses as previously described, 20 abdominocentesis, blood pressure, ECG, results of hematology, serum biochemistry, urinalysis, urine leptospira PCR, and leptospira serology were recorded. In nonsurvivors, postmortem findings were recorded. Medications administered before presentation to the hospital were also recorded.
Acute kidney injury was defined as a 50% or higher increases in serum creatinine concentration above the reference range with exclusion of postrenal azotemia or purely prerenal azotemia. In addition, a 30% or higher increases in blood urea nitrogen (BUN) concentration above the reference range was seen as further evidence of AKI. Post renal azotemia was excluded based on a lack of typical clinical signs. Azotemia of purely prerenal origin was excluded based on lack of rapid improvement of azotemia in response to fluid therapy. The presence of chronic renal failure was excluded based on lack of typical clinical signs such as weight loss, polyuria, polydipsia, ventral edema, and dental tartar. If present in addition to azotemia, isosthenuria, hematuria, hyponatremia, hypochloremia, and hyperkalemia were accounted as further evidence of AKI. Pulmonary hemorrhage was defined as bleeding from the lower airways visualized on endoscopy or intra‐alveolar hemorrhage confirmed during necropsy. Liver disease was defined as a 50% or higher increase in activity of 1 or more liver enzymes above the reference range or histopathologic evidence of hepatitis, cholangitis, cholestasis, necrosis, and/or vasculitis of portal veins. A 50% or higher increase in bilirubin concentration above the reference range, in addition to increased activities of liver enzymes, was considered supportive of liver disease. In cases of repeated measurements of liver enzymes, peak values were reported. Electrolytes were reported from the 1st available measurements after hospital admission, either from venous blood gas analysis or from serum biochemistry.
Survival was defined as discharge from the hospital. Follow‐up information was recorded if available in the medical records.
3. RESULTS
3.1. Animals
Eleven cases met the inclusion criteria. There were 3 foals (ages 3, 6, and 10 weeks) and 8 adult horses (age 6‐23 years). Signalment and seasonal distribution is presented in Table 1 and Figure 1. Nine of 11 horses had received treatment by the referring veterinarian (rDVM) before referral. Treatments consisted of a variety of antimicrobials and nonsteroidal anti‐inflammatory drugs (NSAIDs). Details can be found in Supplementary Information S1. One mare had aborted a 90‐day old fetus 16 days before presentation and developed mild metritis following the abortion which was resolved by the time of presentation. A search for the cause for the abortion was not pursued further by the rDVM.
TABLE 1.
Characteristics and organ affection of 11 horses with systemic leptospirosis.
| Survivors (N = 7) | Nonsurvivors (N = 4) | All horses (N = 11) | |
|---|---|---|---|
| Age in years (median[range]) | 10 (10 wk to 23 y) | 3.6 (3 wk to 13 y) | 7 (3 wk to 23 y) |
| Sex (n [%]) | |||
| Male | 2 (29%) | 3 (75%) | 5 (45%) |
| Female | 5 (71%) | 1 (25%) | 6 (55%) |
| Gelding | 1 (14%) | 1 (25%) | 2 (18%) |
| Mare | 4 (57%) | 1 (25%) | 5 (46%) |
| Stallion | 1 (14%) | 0 | 1 (9%) |
| Colt | 0 | 2 (50%) | 2 (18%) |
| Filly | 1 (14%) | 0 | 1 (9%) |
| Breed | |||
| Warmblood | 6 (86%) | 1 (25%) | 7 (64%) |
| Icelandic Horse | 1 (14%) | 1 (25%) | 2 (18%) |
| Black Forest Horse | 0 | 1 (25%) | 1 (9%) |
| Shetland Pony | 0 | 1 (25%) | 1 (9%) |
| Organ affected | |||
| Kidneys | 7 | 4 | 11 (100%) |
| Lungs | 5 | 3 | 8 (73%) |
| Liver | 3 | 3 | 6 (55%) |
Abbreviation: n, number of samples.
FIGURE 1.
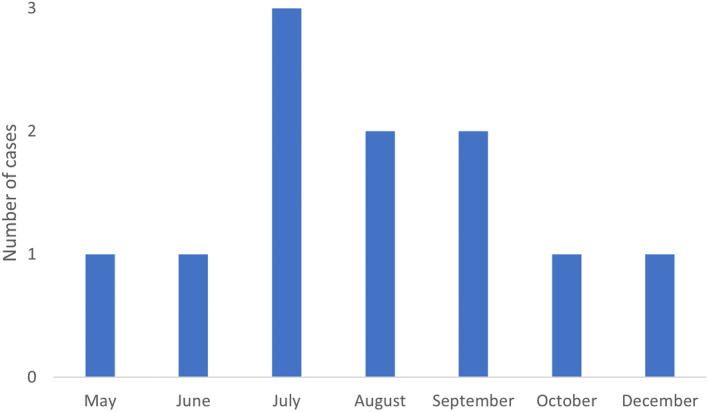
Seasonal distribution of 11 cases of systemic leptospirosis.
3.2. Clinical findings
Lethargy and reduced appetite/anorexia were presenting clinical signs in 10/11 horses. Fever (median, 38.6°C; range, 37.6‐40.6°C) was present in 9/11 horses and epistaxis in 6/11 (55%) horses. In further 2 horses without epistaxis, pulmonary hemorrhage was confirmed on necropsy. In total, pulmonary hemorrhage was identified in 8/11 (73%) cases. Abnormal lung sounds (9/11, 82%, increased vesicular sounds, crackles, wheezes, or a combination of these abnormal lung sounds on both sides of the thorax), tachypnea (9/11; median respiratory rate, 24 breaths/min; range, 20‐44 breaths/min), tachycardia (5/11; median heart rate, 60 beats/min; range, 36‐100 beats/min), coughing (4/11), diarrhea (3/11), icterus (2/11), uveitis (1/11), mild colic signs (1/11), and ataxia (1/11) were recorded. Oliguria which progressed to anuria was present in 3 cases, 2 of which were foals younger than 2 months. All cases with anuria were nonsurvivors. The stallion developed paraphimosis and urinary incontinence during hospitalization. No α‐adrenergic agonists or phenothiazine tranquilizers, known to have the potential to cause penile prolapse, had been administered and there was no history of trauma. Rectal palpation was performed in 4 horses. An unspecific displacement of the ascending colon (n = 1), enlargement of the left kidney (1), distension of the urinary bladder (1), and no abnormal findings (1) were recorded.
3.2.1. Diagnostic imaging findings
Airway endoscopy was performed in 8/11 cases, bleeding from the lungs was confirmed in 6 horses. In 1 case, petechiae were noted during endoscopy of the upper airways. Ultrasonography of the thorax was performed in 7/11 cases. Main findings were irregularities of the pleura (B lines) and in 2 cases mild pleural effusion. Radiography of the thorax in 2 horses with epistaxis and tachypnea showed a generalized mixed lung pattern with increased lung opacity mainly caudodorsally. Representative imaging findings from 2 horses are shown in Figure 2.
FIGURE 2.
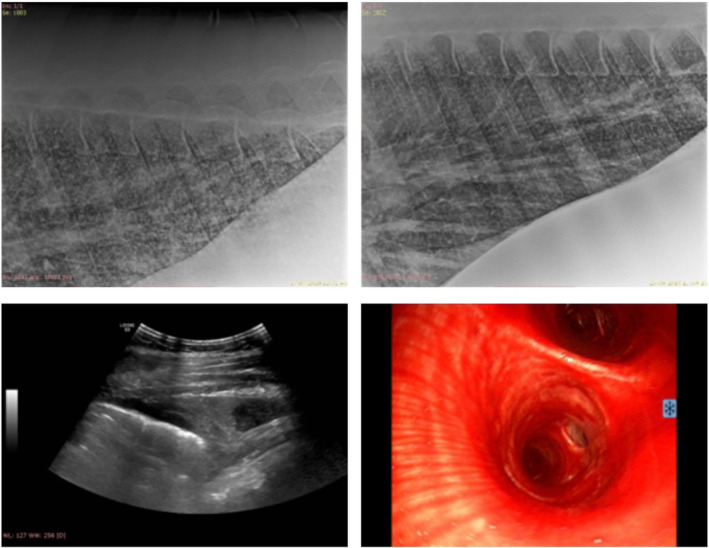
Diagnostic imaging findings from 2 horses with systemic leptospirosis. Top images: thoracic radiographs of a horse with epistaxis and pulmonary hemorrhage. There is a generalized mixed lung pattern caudodorsally with some improvement from Day 2 (left) to Day 10 (right). Bottom left: thoracic ultrasonography of the same horse as above. There is mild irregularity of the pleura and pleural effusion of mixed echogenicity. Bottom right: tracheobronchoscopy of another horse with epistaxis and pulmonary hemorrhage. There is severe hemorrhage in the trachea and main bronchi.
Transabdominal ultrasonography of the kidneys was performed in 6/11 cases. Findings were consistent with AKI and consisted of perirenal edema (4), renomegaly (2), and enlargement of the renal pelvis (2). Fast localized abdominal sonography in horses was performed in 7/11 horses. Abnormal findings consisted of gastric distension in 1 horse and mildly thickened colon walls in 1 foal with diarrhea.
3.3. Laboratory findings
Blood work consisting of complete blood count (10/11) and serum biochemistry panels (9/11) was performed upon admission (Table 2). In 1 horse, a limited serum biochemistry panel was performed and in 1 other horse, blood work had been performed by the rDVM the day before referral. Variable degrees of anemia were seen in 5/11 (45%; median PCV, 23.6; range, 19.6‐27.5) cases with relevant progression observed in 1 case (PCV 16). Marked anemia developed in 1 additional horse within 24 h of admission (PCV 20). Of the 6/11 (55%) cases with thrombocytopenia (median PLT count, 56 × 103/μL; range, 9‐91 × 103/μL), 4 were nonsurvivors including 2 foals who also had microcytic anemia. Mild to severe azotemia and serum amyloid A concentration above the reference range was present in all cases. Serum creatinine concentration was 50% to 624% above the reference range in 10/11 horses. In 1 horse with mild azotemia, serum creatinine concentration and BUN was 16% and 39% above the reference range, respectively, with severe hyponatremia, hypochloremia, and hematuria further testifying to AKI. Fibrinogen was above the reference range in 8/11 (73%) cases, normal in 1 case and not available in 2 others. Venous blood gas analysis was performed in 9/11 (82%) cases on the day of admission. Electrolyte abnormalities were a common finding with hypochloremia, hyponatremia, and hyperkalemia present in 82% (9/11), 73% (8/11), and 55% (6/11) of cases, respectively.
TABLE 2.
Results of hematology, biochemistry, and urinalysis in 11 horses with systemic leptospirosis.
| Variable | No. of horses with data | Reference range | Survivors median (range), N = 7 | Nonsurvivors median (range), N = 4 | All horses median (range), N = 11 |
|---|---|---|---|---|---|
| Hematology | |||||
| Hematocrit (%) | 11 | 30‐47 | 28.6 (23.6‐38) | 26 (19.6‐35) | 29 (19.6‐38) |
| Hemoglobin (g/dL) | 11 | 10.7‐16.5 | 11.1 (9.9‐14.3) | 8.9 (8.7‐13) | 10.9 (8.7‐14.3) |
| RBC (106/μL) | 10 | 6.4‐10.4 | 6.8 (5.87‐9.06) | 7.1 (6.91‐7.59) | 7.0 (5.87‐9.06) |
| WBC (103/μL) | 11 | 4.9‐11.1 | 7.3 (6.4‐10.06) | 11.2 (5.85‐26.9) | 7.3 (5.85‐26.9) |
| Neu (103/μL) | 10 | 2.5‐6.9 | 5.7 (3.2‐8.03) | 7.7 (5.36‐20.58) | 5.8 (3.2‐20.58) |
| PLT (103/μL) | 10 | 100‐250 | 122.5 (58‐174) | 46.5 (9‐83) | 90.5 (9‐174) |
| Biochemistry | |||||
| Creatinine (μmol/L) | 11 | 82‐147 | 580 (291‐1065) | 611 (171‐990) | 580 (171‐1065) |
| Urea (mmol/L) | 11 | 3.5‐7 | 18 (5.2‐34.1) | 16.4 (9.7‐36.5) | 18 (5.2‐36.5) |
| Bilirubin (μmol/L) | 10 | 9‐39 | 48 (30‐124.9) | 122.2 (45‐268) | 48 (30‐268) |
| GGT (IU/L) | 10 | 6‐31 | 15 (9.9‐23) | 19 (18‐20) | 16.5 (9.9‐23) |
| GDH (IU/L) | 10 | 0.5‐2.2 | 18.6 (6.5‐32.7) | 3 (1.2‐14.2) | 14.2 (1.2‐32.7) |
| SDH (IU/L) | 10 | 0.1‐7.6 | 11.4 (4.3‐18.7) | 6.2 (1‐11.5) | 8.8 (1‐18.7) |
| Albumin (g/L) | 10 | 22‐37 | 30 (26‐33) | 22 (21‐25) | 27 (21‐33) |
| Sodium (mmol/L) | 11 | 132‐146 | 129 (125.4‐137.9) | 116.7 (111.4‐120) | 129 (111.4‐137.9) |
| Potassium (mmol/L) | 11 | 2.4‐4.7 | 4.4 (3.3‐5.23) | 5.37 (3.1‐6.57) | 4.9 (3.1‐6.57) |
| Chloride (mmol/L) | 11 | 99‐109 | 94 (86‐111) | 82 (80‐92) | 92 (80‐104) |
| iCalcium (mmol/L) | 9 | 1.50‐1.75 | 1.59 (1.55‐1.98) | 1.29 (1.13‐1.68) | 1.58 (1.13‐1.98) |
| SAA (mg/L) | 11 | 0.5‐1.2 | 1893 (405‐4447) | 3795 (2223‐7865) | 3153 (405‐7865) |
| Fibrinogen (g/L) | 9 | 1.25‐2.85 | 3.6 (2.8‐5.3) | 3; 7.3 a | 3.6 (2.8‐7.3) |
| Urinalysis | |||||
| Specific gravity (g/L) | 10 | 1020‐1045 | 1009 (1005‐1018) | 1021 (1019‐1021) | 1005‐1021 |
| Protein, dipstick | 10 | <2+ | 1+ (0 to 3+) | 3+ (1+ to 3+) | 0 to 3+ |
| Blood/Hgb, dipstick | 10 | Negative | 4+ (1+ to 5+) | 4+ (4+ to 5+) | 1+ to 5+ |
| RBC/HPF | 8 | <5 | 0‐4 to >250/HPF | 0‐4 to >250/HPF | 0‐4 to >250/HPF |
| WBC/HPF | 8 | <5 | 0‐4 to 4‐8 | 0‐4 to 4‐8 | 0‐4 to 4‐8 |
Abbreviations: GGT, γ‐glutamyltransferase; GDH, glutamate dehydrogenase; HPF, high power field; iCalcium, ionized calcium; Neu, neutrophils; PLT, platelets; RBC, red blood cells; SAA, serum amyloid A; SDH, sorbitol dehydrogenase; WBC, white blood cells.
Fibrinogen only available in 2 nonsurvivors.
Urinalysis was available in 10/11 cases. First voided urine was collected in 5 cases before and in 4 cases after IV administration of fluids. In 1 anuric case a small sample was collected via urinary catheter after IV administration of fluids and furosemide administration, in another anuric case no urine could be sampled antemortem. Point of care (POC, urine reagent strip and specific gravity) and sediment analysis was performed in 8/11 cases, whereas only POC results were available in the remaining 2 cases. Pigmenturia on reagent strip analysis was a consistent finding in all cases, ranging from mild (1+) in 2 cases to severe (5+) in 4 cases. In 2 cases with a 5+ blood/hemoglobin on the reagent strip analysis, the red blood cell count per high power field was normal. Combined, these results indicate the presence of hemoglobin in those samples. Mild to moderate proteinuria was present in 5/11 cases.
Liver disease was identified in 6/11 (55%) cases. Findings consisted of above the reference range activities in serum of specific liver‐derived enzymes (sorbitol dehydrogenase [SDH], glutamate dehydrogenase [GDH]) and values of total bilirubin above the reference range (Table 2). Values above the reference range of bile acids and nonspecific liver‐derived enzymes (aspartate aminotransferase [AST], lactate dehydrogenase [LDH]) were seen in some cases. Conjugated bilirubin was measured and found markedly elevated in 2 horses (18.8 and 11.1 μmol/L; reference range, <5 μmol/L). In 1 horse, liver disease was found only on necropsy and histopathology as liver enzymes were not measured antemortem. Gamma glutamyltransferase (GGT) was available in 10/11 cases and was within reference range in all.
3.3.1. Other diagnostic testing
Abdominocentesis was only performed in the horse with the nonspecific displacement of the large colon, an increased lactate concentration (5.1 mmol/L; reference range, <2 mmol/L) and mildly increased total protein (2.2 g/dL; reference range, <2.0 g/dL) was identified.
Noninvasive blood pressure was measured in 3/11 horses. Systolic, diastolic, and mean arterial pressure as well as pulse pressure was normal in 2 horses. Mild hypotension was present in 1 horse (134/104/86 mm Hg, corrected values). Electrocardiograms in 1 foal with severe hyperkalemia showed an irregularly irregular rhythm, broadening, and flattening of the P wave and tall, tented T wave. Tracheobronchial aspirate (TBA) and bronchoalveolar lavage fluid (BALF) were sampled for cytological evaluation in 5/8 and 2/8 cases, respectively. The cytological evaluation showed some to numerous erythrocytes in all cases and macrophages with phagocytized intracytoplasmic erythrocytes in 1 TBA sample and 1 BALF sample. Results were consistent with acute bleeding into the airways. In 1 case, follow‐up BALF cytology was performed approximately 3, 7, 10, 17, and 26 months after initial presentation to the hospital. Few erythrocytes were found in 2 samples whereas all samples contained varying degrees of hemosiderophages. Results were consistent with acute to chronic hemorrhage.
In the horse that presented with ataxia, cerebrospinal fluid was collected from the lumbosacral space for cytologic evaluation. The sample had an increased protein concentration (1.97 g/L) and mild lymphocytic pleocytosis (WBC 11.7/μL, RBC 19/μL, 84% lymphocytes mainly small and mature, 14% monocytes, 2% neutrophils). This horse was a nonsurvivor, systemic leptospirosis with associated sepsis was diagnosed based on postmortem examination and histopathology. Histopathology of the central nervous system showed diffuse, acute hemorrhage mainly into the arachnoid space of the cervical and lumbar spinal cord. These findings were considered most likely traumatic in origin.
Polymerase chain reaction for detection of equine herpesvirus type 1 and type 4 was performed on blood samples and nasal swabs in 7/11 cases. All results were negative. In 3 cases the following diagnostic tests were performed on fecal samples: ELISA for detection of Rotavirus (1/3), bacterial culture for detection of Salmonella, Clostridium difficile, Clostridium perfringens and ELISA for detection of C. difficile toxins A and B (3/3). All tests were negative except for moderate growth of C. perfringens in 1 sample.
3.4. Leptospira testing
Real‐time quantitative polymerase chain reaction (qPCR) 21 for detection of pathogenic Leptospira was performed on urine samples in 10/11 (91%) cases. The test was positive in 3/7 survivors and in all nonsurvivors tested (3/4). Repeat sampling on different days was performed in 2 cases and furosemide bolus was given before sampling in 1 case. Serovars detected by this PCR assay are shown in Table S2.
Serologic results of the microscopic agglutination test (MAT) were available for 6/11 cases (55%), 5 survivors and 1 nonsurvivor. In all but 1 horse, serology was not performed if urine PCR was positive. The majority (12/14, 86%) of all titers ≥1 : 800 was observed for serovars Australis, Autumnalis, and Bratislava (Figure 3). No horse had a positive MAT titer for serovars Canicola and Tarassovi. In 1 horse, follow‐up MAT serology was performed at 2, 3 and 7 months after the 1st sample and showed a steady decline in MAT titers. At the last follow‐up sample, titers had decreased from 1 : 3200 to 1 : 400 for serovar Australis, 1 : 3200 to 1 : 200 for Bratislava and 1 : 800 to 1 : 100 for Autumnalis. In 2 other horses, paired serology samples were taken 3 days and approximately 2 weeks apart, respectively, and showed marked differences in MAT titers between the 1st and 2nd sample (Table S3).
FIGURE 3.
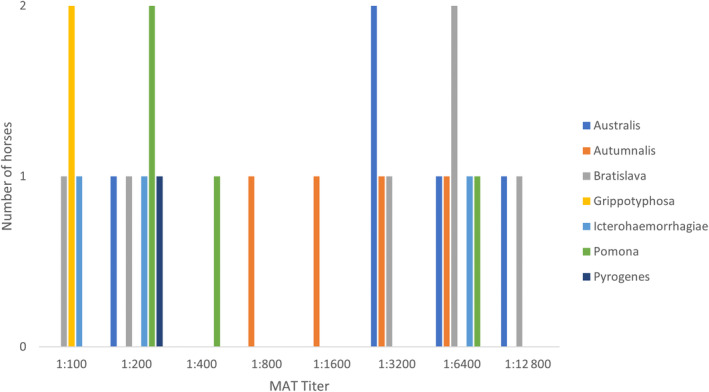
Results of serologic testing, microscopic agglutination test (MAT), for antibody against a panel of pathogenic Leptospira species. A total of 11 samples are depicted. Three horses were tested once, 2 horses were tested twice 3 days and approximately 2 weeks apart. One horse was tested once in the acute stage and on 3 occasions at approximately 2, 3, and 7 months after the 1st sample.
3.5. Treatment
Treatment varied markedly among horses. Details of treatments are given in Supporting Information S1. Isolation measures were upheld until resolution of clinical signs, urine PCR returned negative for pathogenic Leptospira, or a combination of both.
3.6. Outcome
Overall case fatality was 4/11 cases (36%) including 1 instance of sudden death and 3 horses euthanized in hospital. One foal was euthanized because of acute, severe clinical deterioration with tachypnea, dyspnea, tachycardia, and collapse. One other foal became recumbent, tachypneic, tachycardic and was unresponsive to treatment and was euthanized. One horse was euthanized because of formation and progression of ventral edema, ongoing epistaxis, dyspnea, tachycardia. All 3 cases that were euthanized displayed nonresponsive anuria. The ataxic horse became recumbent with seizure activity nonresponsive to anticonvulsant treatment, after which breathing stopped and death was confirmed on further examination. Duration of hospitalization for horses that survived to discharge was 7 to 14 days. One horse that survived to discharge was euthanized 15 months later because of clinical deterioration and a steep increase in serum creatinine concentration compared to the value at the time of discharge from the hospital. The long‐term outcome of the other surviving horses was unknown.
3.7. Postmortem findings
Necropsy results were available in 4 horses. Histopathology of affected organs was performed in 3 cases. Icterus was evident throughout the body in 3 cases. Various degrees of multifocal petechiae, hemorrhages on mucous membranes and serous membranes of lungs, intestine, urinary bladder, or a combination of these findings were present in all cases. Additional petechiae and hemorrhage were present in the epi‐ and myocardium along with pericardial effusion in 1 horse. Mild pleural and peritoneal effusion was found in 3 horses. Findings in the kidneys consisted of swelling with interstitial edema and nephritis (4), tubular necrosis (3), purulent glomerulonephritis (1), purulent nephritis (1), tubular cellular casts (1). In 2 cases, Warthin‐Starry silver staining identified contorted bacteria with Leptospira morphology in the cytoplasm of proximal tubular epithelial cells. Histopathology of the lungs showed severe hemorrhage in the alveoli (3), generalized congestion (3), pulmonary edema (2), accumulation of neutrophils in the alveoli and capillaries (2), acute interstitial pneumonia (1), and hemorrhage in the pulmonary interstitium (1). Pulmonary edema in the alveoli was possibly agonal in origin in 1 case. Changes in the liver were periportal hepatitis (1), cholangitis (1), purulent vasculitis of portal veins (1), necrosis of periportal hepatocytes (1), intrahepatic cholestasis (1), and generalized congestion (2). In 2 cases (1 foal and the horse with ataxia), the widespread pathology found throughout the body was attributed to Leptospira‐associated sepsis. Acute kidney injury was present in all cases.
4. DISCUSSION
Here we describe 11 cases of acute leptospirosis in horses with findings of renal, pulmonary, and hepatic disease. All 3 organs were affected in 6 of 11 horses. This study adds valuable insight into leptospirosis in horses because reports on systemic disease with respiratory and hepatic involvement are limited. 16 , 18 , 19 In our study, clinical signs of lung disease were epistaxis, tachypnea, abnormal lung sounds and coughing. Pulmonary hemorrhage was diagnosed on endoscopy or histopathology. In humans, severe pulmonary hemorrhagic leptospirosis is increasingly recognized and caries a poor prognosis with a high case fatality rate. 22 , 23 , 24 A similar severe manifestation of leptospirosis in dogs, leptospiral pulmonary hemorrhage syndrome (LPHS), is recognized. 25 , 26 , 27 Histopathologic findings in both humans and dogs consist of intra‐alveolar hemorrhage without inflammatory cell infiltrates or vasculitis, 24 , 25 , 27 which is similar to histopathology of lung tissue from the nonsurvivors in our study. The pathogenesis might involve direct injury to septal capillaries by Leptospira and leptospiral proteins by binding to endothelial cadherins which leads to loss of endothelial integrity, altered permeability, endothelial disruption, and rupture. 23 , 26 , 28 Overall case fatality rate in our study was 36% (4/11) with severe pulmonary hemorrhage evident in 3 nonsurvivors. Thus, it seems possible that a syndrome similar to LPHS in humans and dogs may exist in horses. Leptospira is the most common infectious cause of AKI in horses and AKI was present in all 11 cases in this study. It seems that AKI is a more common manifestation of acute leptospirosis in horses as it is in humans and dogs. 15 , 17 , 25 , 29 Renal failure, fever, hematuria, and pyuria without bacteriuria is suggestive of leptospirosis and should prompt diagnostic testing in this direction.
The liver is a common site of infection in humans and small animals with leptospirosis, 25 , 29 , 30 whereas reports on hepatic involvement in horses are sparse. 18 In our study, evidence of liver disease was found in 6/11 horses. Liver involvement was diagnosed on 1 or more of histopathology, values of serum activities of liver enzymes above the reference range, and concentration of bile acids above the reference range if available. Although bilirubin was markedly above the reference range in the 5/6 horses for which it was available, values for conjugated bilirubin were only available in 2/6 horses. We propose that hepatic involvement in cases of acute equine leptospirosis is more common than previously recognized.
Paraphimosis, which developed in the stallion in our study, occurs in cases of severe systemic diseases such as purpura hemorrhagica, equine herpesvirus type 1 infection and rabies but has not been reported in cases of leptospirosis.
Fever was a presenting clinical sign in 9/11 cases. Most cases (9/11) had been treated by the referring veterinarian (rDVM) before referral with treatments consisting of a variety of antimicrobials and NSAIDs, which could have masked pyrexia in the horses that were afebrile on presentation.
Systemic leptospirosis might be accompanied by hemolytic anemia either immune mediated or through the actions of bacterial virulence factors such as hemolysins. 31 , 32 , 33 Hemolysis might be both intravascular and extravascular. In our study, anemia was present in 6/11 cases of which none had signs of intravascular hemolysis. It is possible that extravascular hemolysis might have been a cause of anemia in some of the cases, although hyperbilirubinemia might also have been caused by concurrent liver disease. In horses with epistaxis and pulmonary hemorrhage, anemia might have resulted from blood loss. Thus, there was no clear evidence for the presence of extravascular hemolysis.
Regarding thrombocytopenia, which was present in 6/11 cases, the automated results were not reexamined manually. As platelets are prone to clumping, an automated result is seen as a minimum value in a given sample. In 2 nonsurvivors with Leptospira‐associated sepsis diagnosed at necropsy, thrombocytopenia was more severe. It is possible that disseminated intravascular coagulation could have caused the low numbers of circulating platelets. In the other 4 cases, thrombocytopenia was mild to moderate with no clear relation to clinical signs or survival.
Because the true degree of thrombocytopenia was not evaluated manually, the relevance of a calculated difference between groups should be weighed carefully. However, thrombocytopenia was moderate to severe in 2 nonsurvivors. The lower concentrations of albumin, sodium, and chloride in nonsurvivors likely reflect the degree of renal damage in this group.
The high case fatality rate among the foals included in our study (67%) contrasts with the results from a study in 4 foals with AKI because of Leptospira in which survival to discharge was 100%. 17 However, in another study case fatality rate was high (80%) in 5 foals with respiratory failure and AKI caused by Leptospira spp. 16 The 2 foals that died in our study both had anuria, mild to severe hyperkalemia and severe azotemia. Renal replacement therapy (RRT) might have been indicated in those cases although data on RRT in sick horses is limited with a successful outcome reported in only a few cases. 17 The dams of the 3 foals in our study were not tested for antibody against Leptospira spp. although insights into possible source of infection might have been gained from the results.
As leptospirosis is a potentially lethal zoonosis, a definitive diagnosis in suspect animal cases is of utmost importance. However, diagnosing leptospirosis in horses can be challenging because of the relatively high seroprevalence in the equine population, mild or subclinical disease in many cases and low sensitivity of some of the available diagnostic tests. In this case series, leptospirosis was diagnosed either by urinary PCR, MAT serology, or histopathology. Urinary PCR is a valuable tool for accurate diagnosis of leptospirosis in horses and other species. 34 A positive result of urine PCR is diagnostic for acute infection in a horse with appropriate clinical signs. However, a negative result does not rule out leptospirosis as urinary shedding might be intermittent and delayed in the disease course following the initial phase of leptospiremia. 25 Administration of a furosemide bolus and sampling the 2nd voided urine for PCR testing might increase test sensitivity as it does in cattle. 35 It is not known if this practice improves recovery of leptospires from equine urine. The presence of amplification inhibitors in clinical samples can cause false‐negative results, particularly in urine samples that might be contaminated with feces or subject to degradation because of delayed testing.
The MAT is considered the reference standard serologic test in the diagnosis of human leptospirosis and is also the most widely used test for diagnosing acute leptospirosis in animals. 11 , 13 , 22 , 25 Paired serum samples are recommended for diagnosis of equine, canine and human leptospirosis with a 4‐fold increase in MAT titer or conversion from a negative to a positive titer in the convalescent sample providing stronger evidence for an acute infection. 11 , 25 For optimization it is important to include a panel of serovars based on seroprevalence studies in the actual geographical region to minimize the risk of false‐negative results.
In our study sample, 2 horses were diagnosed based on the results of serologic testing alone. One horse had low antibody titers of 1 : 200 (Australis, Bratislava) which combined with clinical findings of high fever up to 40.4°C, epistaxis, severe pulmonary hemorrhage, and AKI was considered sufficient evidence. The horse had aborted 2 weeks before presentation, which could possibly further support the diagnosis although the aborted fetus and placenta were not tested for Leptospira. Ideally, a convalescent serum sample showing a 4‐fold or greater increase would have strengthened the diagnosis. The other horse had high antibody titers of 1 : 1600, 1 : 3200, and 1 : 6400 (Autumnalis, Australis, and Bratislava, respectively) which, in addition to clinical signs and laboratory findings consistent with systemic leptospirosis, was considered strong evidence for the diagnosis.
A study of seroprevalence of Leptospira spp. in 615 healthy horses in Switzerland used MAT serology to detect antibodies against 15 pathogenic serovars. The most prevalent serovars were Pyrogenes (22.6%), Canicola (22.1%), Australis (19.2%), Bratislava (15.9%), and Autumnalis (10.4%) with 79.7% of positive horses having titers 1 : 100 and 1 : 200. 6 In our study, serovars Australis, Autumnalis, and Bratislava accounted for the majority (86%) of all titers ≥1 : 800 and the 9 most prevalent serovars in the study by Blatti et al were included in the MAT serology used.
Leptospirosis is considered a seasonal disease with region specific seasonal patterns. In our study, most cases presented during summer and early fall which is in accordance to previous reports from similar geographic regions. 6 , 8
The main limitations of our study are the retrospective nature, and the relatively small study sample. Also, not all laboratory findings were available in all cases.
In conclusion, leptospirosis should be considered in horses with fever, lethargy, and azotemia, and respiratory and hepatic involvement should be investigated in suspect cases. Diagnostics should include qPCR of urine and MAT serology, ideally as paired samples as interpretation of a single titer may be challenging because of high seropositivity in the equine population. As urine PCR might be false negative, repeated PCR testing is indicated in cases where there is a strong clinical suspicion.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1. Supporting information.
Data S2. Supporting information.
Data S3. Supporting information.
ACKNOWLEDGMENT
No funding was received for this study.
Ramsay L, Eberhardt C, Schoster A. Acute leptospirosis in horses: A retrospective study of 11 cases (2015‐2023). J Vet Intern Med. 2024;38(5):2729‐2738. doi: 10.1111/jvim.17184
REFERENCES
- 1. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sykes JE, Hartmann K, Lunn KF, Moore GE, Stoddard RA, Goldstein RE. 2010 ACVIM Small Animal Consensus Statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med. 2011;25:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith AM, Stull JW, Moore GE. Potential drivers for the re‐emergence of canine leptospirosis in the United States and Canada. Trop Med Infect Dis. 2022;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Major A, Schweighauser A, Francey T. Increasing incidence of canine leptospirosis in Switzerland. Int J Environ Res Public Health. 2014;11:7242‐7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strand TM, Olsson Engvall E, Lahti E, Hjertqvist M, Lundkvist Å. Leptospira status in Sweden during the past century, neglected and re‐emerging? Microorganisms. 2023;11:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blatti S, Overesch G, Gerber V, Frey J, Hüssy D. Seroprevalence of Leptospira spp. in clinically healthy horses in Switzerland. Schweiz Arch Tierheilkd. 2011;153:449‐456. [DOI] [PubMed] [Google Scholar]
- 7. Fagre AC, Mayo CE, Pabilonia KL, Landolt GA. Seroprevalence of Leptospira spp. in Colorado equids and association with clinical disease. J Vet Diagn Investig. 2020;32:718‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Båverud V, Gunnarsson A, Engvall EO, Franzén P, Egenvall A. Leptospira seroprevalence and associations between seropositivity, clinical disease and host factors in horses. Acta Vet Scand. 2009;51:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trimble AC, Blevins CA, Beard LA, Deforno AR, Davis EG. Seroprevalence, frequency of leptospiuria, and associated risk factors in horses in Kansas, Missouri, and Nebraska from 2016‐2017. PLoS One. 2018;13:e0206639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wasiński B, Paschalis‐Trela K, Trela J, et al. Serological survey of Leptospira infection in Arabian horses in Poland. Pathogens (Basel, Switzerland). 2021;10:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma A, Stevenson B, Adler B. Leptospirosis in horses. Vet Microbiol. 2013;167:61‐66. [DOI] [PubMed] [Google Scholar]
- 12. Ellis WA. Animal leptospirosis. Curr Top Microbiol Immunol. 2015;387:99‐137. [DOI] [PubMed] [Google Scholar]
- 13. Divers TJ, Chang YF, Irby NL, Smith JL, Carter CN. Leptospirosis: an important infectious disease in North American horses. Equine Vet J. 2019;51:287‐292. [DOI] [PubMed] [Google Scholar]
- 14. Gerding JC, Gilger BC. Prognosis and impact of equine recurrent uveitis. Equine Vet J. 2016;48:290‐298. [DOI] [PubMed] [Google Scholar]
- 15. Frellstedt L, Slovis NM. Acute renal disease from Leptospira interrogans in three yearlings from the same farm. Equine Vet Educ. 2009;21:478‐484. [Google Scholar]
- 16. Broux B, Torfs S, Wegge B, Deprez P, van Loon G. Acute respiratory failure caused by Leptospira spp. in 5 foals. J Vet Intern Med. 2012;26:684‐687. [DOI] [PubMed] [Google Scholar]
- 17. Fouché N, Graubner C, Lanz S, Schweighauser A, Francey T, Gerber V. Acute kidney injury due to Leptospira interrogans in 4 foals and use of renal replacement therapy with intermittent hemodiafiltration in 1 foal. J Vet Intern Med. 2020;34:1007‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Ingh TS, Hartman EG, Bercovich Z. Clinical Leptospira interrogans serogroup Australis serovar lora infection in a stud farm in the Netherlands. Vet Q. 1989;11:175‐182. [DOI] [PubMed] [Google Scholar]
- 19. Hamond C, Martins G, Lilenbaum W. Pulmonary hemorrhage in horses seroreactive to leptospirosis in Rio de Janeiro, Brazil. J Vet Intern Med. 2012;26:1237; author reply 1238. [DOI] [PubMed] [Google Scholar]
- 20. Busoni V, De Busscher V, Lopez D, et al. Evaluation of a protocol for fast localised abdominal sonography of horses (FLASH) admitted for colic. Vet J. 2011;188:77‐82. [DOI] [PubMed] [Google Scholar]
- 21. Smythe LD, Smith IL, Smith GA, et al. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect Dis. 2002;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757‐771. [DOI] [PubMed] [Google Scholar]
- 23. Nicodemo AC, Duarte‐Neto AN. Pathogenesis of pulmonary hemorrhagic syndrome in human leptospirosis. Am J Trop Med Hyg. 2021;104:1970‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Truong KN, Coburn J. The emergence of severe pulmonary hemorrhagic leptospirosis: questions to consider. Front Cell Infect Microbiol. 2011;1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schuller S, Francey T, Hartmann K, et al. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract. 2015;56:159‐179. [DOI] [PubMed] [Google Scholar]
- 26. Sonderegger F, Nentwig A, Schweighauser A, et al. Association of markers of endothelial activation and dysfunction with occurrence and outcome of pulmonary hemorrhage in dogs with leptospirosis. J Vet Intern Med. 2021;35:1789‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klopfleisch R, Kohn B, Plog S, et al. An emerging pulmonary haemorrhagic syndrome in dogs: similar to the human leptospiral pulmonary haemorrhagic syndrome? Vet Med Int. 2010;2010:928541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evangelista K, Franco R, Schwab A, Coburn J. Leptospira interrogans binds to cadherins. PLoS Negl Trop Dis. 2014;8:e2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charles JC, Jayarajah U, Subasinghe D. Clinical characteristics and outcomes of patients with leptospirosis complicated with acute pancreatitis: a systematic review. J Int Med Res. 2023;51:3000605231197461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li D, Liang H, Yi R, et al. Clinical characteristics and prognosis of patient with leptospirosis: a multicenter retrospective analysis in south of China. Front Cell Infect Microbiol. 2022;12:1014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee SH, Kim S, Park SC, Kim MJ. Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore‐forming protein on mammalian cells. Infect Immun. 2002;70:315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furlanello T, Reale I. Leptospirosis and immune‐mediated hemolytic anemia: a lethal association. Vet Res Forum. 2019;10:261‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bovens C, Fews D, Cogan TA. Leptospirosis and immune‐mediated haemolytic anaemia in a dog. Vet Rec Case Rep. 2014;2:e000065. [Google Scholar]
- 34. Hamond C, Martins G, Loureiro AP, et al. Urinary PCR as an increasingly useful tool for an accurate diagnosis of leptospirosis in livestock. Vet Res Commun. 2014;38:81‐85. [DOI] [PubMed] [Google Scholar]
- 35. Nervig RM, Garrett LA. Use of furosemide to obtain bovine urine samples for leptospiral isolation. Am J Vet Res. 1979;40:1197‐1200. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data S2. Supporting information.
Data S3. Supporting information.


