Abstract
Atrial fibrillation (AF) is a rarely reported arrhythmia in otherwise healthy newborn foals, with a single case of cardioversion using procainamide administration described in the literature. Two neonatal Thoroughbred colts were presented to an equine hospital because of an irregularly irregular tachyarrhythmia and poor latching when trying to nurse. History, physical examination, and initial diagnostic testing including ECG and echocardiography confirmed AF without structural heart disease. The 1st foal converted into normal sinus rhythm after treatment with IV metoprolol and quinidine. The 2nd foal converted to normal sinus rhythm after a single IV dose of metoprolol, intended for rate control. Demeanor and nursing behavior improved markedly after conversion. The 2 foals had normal heart rates and sinus rhythm that persisted for 6 weeks until euthanasia in the 1st foal and for 2 years in the 2nd foal. Rate control and cardioversion should be considered as a treatment for persistent lone AF in neonatal foals.
Keywords: arrhythmia, cardioversion, electrocardiography, equine
Abbreviations
- AF
atrial fibrillation
- APCs
atrial premature complexes
- TTEC
transthoracic electrical cardioversion
- TVEC
transvenous electrical cardioversion
- VPCs
ventricular premature complexes
1. CASE 1
A 15‐hour‐old, 52 kg, Thoroughbred colt was presented for evaluation of tachycardia and arrhythmia. Gestational age was unknown, but the foal appeared to be to term and had an uneventful birth under direct observation. The multiparous 11‐year‐old dam had not experienced any complications during gestation. The foal was observed standing and nursing within 2 hours. An irregular tachyarrhythmia (180 beats per minute [bpm]) was noted on routine physical examination a few hours after birth. The foal was otherwise alert and active. The colt received 2 L of Rhodococcus equi hyperimmune plasma IV within 6 hours of birth. Complete blood count and IgG concentrations obtained after plasma administration were normal. No other treatments were administered before presentation. The foal was intended to be a racehorse.
On presentation, the foal was agitated, but responsive. The colt had a heart rate >200 bpm and an irregularly irregular heart rhythm. Peripheral pulse quality was irregular with normal strength. No murmurs, jugular distension or pulsation were noted. The colt had a good sucking reflex, but sustained latching was not established. The remainder of the physical examination was unremarkable.
Initial diagnostic evaluation included ECG, echocardiogram, serum cardiac troponin I concentration, stall‐side biochemistry analysis, arterial blood gas, and anaerobic and aerobic blood cultures. Stall‐side biochemistry analysis identified no major abnormalities apart from mild hypokalemia (2.5 mmol/L; normal, 2.7‐4.9 mmol/L), hyperglycemia (131 mg/dL; normal, 72‐114 mg/dL), increased serum creatinine concentration (2.1 mg/dL; normal, 0.6‐1.8 mg/dL), and hyperlactatemia (3.2 mmol/L; normal, <2 mmol/L). Arterial blood gas was performed with the foal in lateral recumbency and showed hypoxemia (PaO2, 56.6 mm Hg; normal, >80 mm Hg) with the remainder of the arterial blood gas results within normal limits.
The ECG confirmed atrial fibrillation (AF) with a ventricular rate of 190‐230 bpm and occasional wide QRS complexes (Figures 1 and 2). A 2‐dimensional, color Doppler and M‐mode echocardiographic evaluation identified normal cardiac chambers and major vessels (Tables S1 and S2). Global systolic function was adequate but mild dyskinesis of the left ventricle was observed, most likely secondary to the arrhythmia. Overall, no structural heart defects were identified that would cause the AF or preclude normal life expectancy or athletic future. Serum cardiac troponin I concentration was 2.67 ng/mL (Siemens Stratus CS, Tarrytown, New York, USA; normal, 0‐0.07 ng/mL in adult horses). An IV catheter was placed and telemetry was performed for continuous monitoring of the colt's heart rate and rhythm. Initial treatment was directed at rate control. Metoprolol 0.02 mg/kg (Almaject, Morristown, New Jersey) was administered IV without any effect on heart rate. Two more 0.01 mg/kg doses were given for a total dose of 0.04 mg/kg over 10 minutes. The heart rate subsequently decreased to 130 bpm. Three hours later, the heart rate approached 180‐200 bpm and a 2nd dose of 0.04 mg/kg metoprolol was administered, decreasing heart rate to 130‐150 bpm.
FIGURE 1.
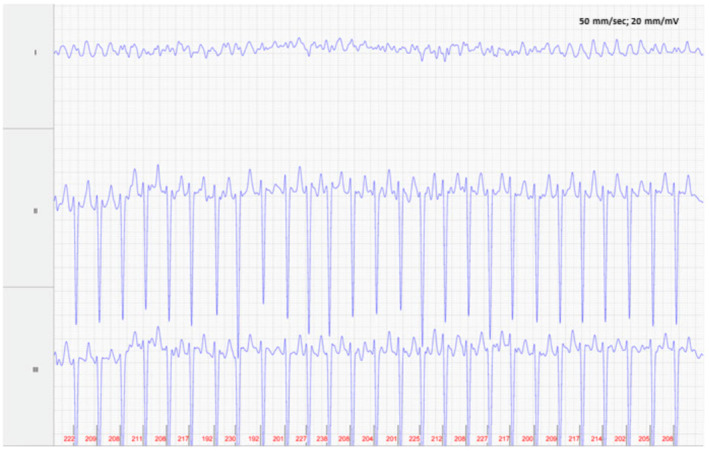
Foal 1, Admission ECG: Atrial fibrillation with normal QRS complexes and an average heart ventricular rate of 212 bpm. Lead II is in a base apex configuration. Lead I is a nontraditional lead and Lead III is calculated from Leads I and II. For all ECGs, the electrodes are placed around the girth area with the left leg electrode on the sternum, the left arm electrode on left side of the thorax slightly dorsal to level of the cardiac apex, the right arm electrode over the right thorax approximately 6 cm below the dorsal midline, and the right leg electrode over the left thorax approximately 6 cm below the dorsal midline.
FIGURE 2.
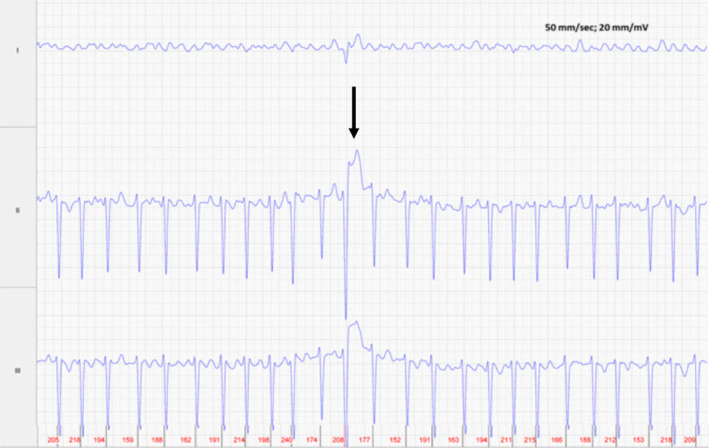
Foal 1, Day 1, before rate control: Atrial fibrillation with single tall, slightly wide QRS complex (arrow) (100 ms vs 88 ms) with elevated ST segment. Average ventricular rate of 190 bpm. Lead II is in a base apex configuration. Lead I is a nontraditional lead and Lead III is calculated from Leads I and II. For all ECGs, the electrodes are placed around the girth area with the left leg electrode on the sternum, the left arm electrode on left side of the thorax slightly dorsal to level of the cardiac apex, the right arm electrode over the right thorax approximately 6 cm below the dorsal midline, and the right leg electrode over the left thorax approximately 6 cm below the dorsal midline.
Supportive care consisted of antibiotics (5 mg/kg ceftiofur sodium IV q12h; Naxcel, Zoetis, Parsippany, New Jersey) to prevent secondary infections. The foal was assisted to stand and nurse every 3 hours and supplemented as needed. Because latching was not always established, mare's milk was fed via an indwelling nasoesophageal tube every 3 hours at 10% of body weight per day. Potassium phosphate was added to milk feedings to correct mild hypokalemia. The umbilicus was swabbed with tincture of iodine. Because the low PaO2 likely was associated with lateral recumbency and restraint, supplemental oxygen was not administered.
By 21 hours of age, the AF had not spontaneously resolved and continued rate control or cardioversion was considered necessary. A comprehensive echocardiogram had similar findings as the day before, but a patent foramen ovale with abnormal shunting from left to right also was discovered. Serum potassium concentration had returned to normal. Overall, the foal was considered a good candidate for cardioversion using quinidine gluconate.
Quinidine cardioversion was performed with the foal loose in the stall with its dam, free to nurse and move around, and monitored by continuous telemetric ECG. The foal was given 0.04 mg/kg metoprolol IV for heart rate control before starting the quinidine gluconate. Quinidine gluconate (Lilly USA LLC, Indianapolis, Indiana) then was administered IV every 10 minutes at 1‐2 mg/kg for a total of 12.5 mg/kg given over 85 minutes. The foal converted to sinus rhythm 6 minutes after the final dose. The treatment period was uneventful (Figure 3), and the foal behaved normally with no apparent adverse effects. The heart rate range during conversion was 140‐210 bpm (averaged over 30 RR intervals) with occasional wide QRS complexes or ventricular premature contractions (VPCs) seen. Intermittent periods of more organized atrial activity were observed, consistent with atrial flutter and tachycardia leading up to conversion. After cardioversion, the heart rate was 115‐152 bpm.
FIGURE 3.
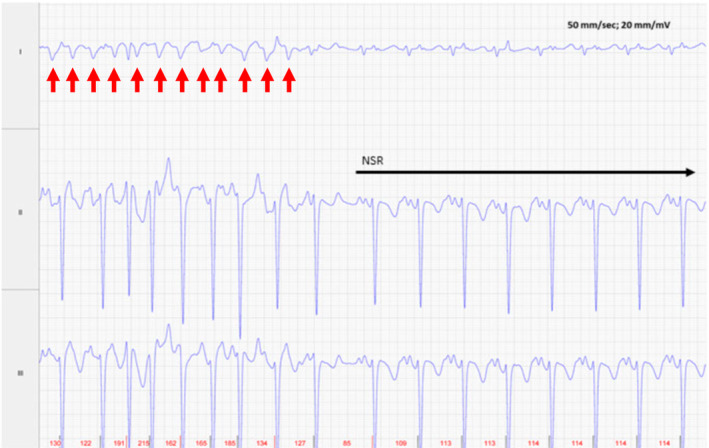
Foal 1, Day 2, at time of conversion to normal sinus rhythm (NSR). Before conversion to NSR, intermittent flutter or p waves preceding some of the QRS complexes are noted. The atrial rhythm remained irregular with a rate of 220‐254 bpm immediately before cardioversion (red arrows). Lead II is in a base apex configuration. Lead I is a nontraditional lead and Lead III is calculated from Leads I and II. For all ECGs, the electrodes are placed around the girth area with the left leg electrode on the sternum, the left arm electrode on left side of the thorax slightly dorsal to level of the cardiac apex, the right arm electrode over the right thorax approximately 6 cm below the dorsal midline, and the right leg electrode over the left thorax approximately 6 cm below the dorsal midline.
The foal was able to latch and nurse appropriately on Day 2. Ultrasonography of the umbilicus on Day 4 identified external omphalitis. Blood cultures yielded no growth. Serum biochemistry with serum electrolyte concentrations was normal on Day 4. The foal was monitored by continuous ECG for 4 days. Frequent atrial premature complexes (APCs; 0 to >100 per hour) were present in the 1st 24 hours after cardioversion (Figure 4), and consequently sotalol (Apotex Corp, Toronto, Ontario) was administered (2 mg/kg PO q12h). The foal's heart rate was 98‐120 bpm while on sotalol. Serum cardiac troponin I concentration decreased to 0.67 ng/mL at 24 hours after cardioversion and was normal (0.08 ng/mL) on the day of discharge (Day 5). Shunting across the foramen ovale also had ceased by the time of hospital discharge, making an atrial septal defect less likely. Only rare APCs were observed by 48 hours after conversion (1‐2 per hour on average). The foal was discharged on Day 5 of hospitalization. Medical treatment at discharge included ceftiofur sodium (5 mg/kg SC q12h for 5 days) and sotalol (2 mg/kg PO q12h for 5 days). Careful cardiac auscultation was strongly recommended at any time the foal was going to be examined before training, before sale or for other health reasons. The colt was thriving until presented to the hospital 3 weeks after discharge for Rhodococcal pneumonia. A Holter monitor was placed during hospitalization and a mean heart rate of 77 bpm and a normal sinus rhythm were observed. Two months later, the foal was euthanized for severe left hindlimb lameness. Necropsy examination identified Rhodococcal pneumonia, lymphadenitis, physitis, osteomyelitis, and arthritis. No heart lesions were noted.
FIGURE 4.
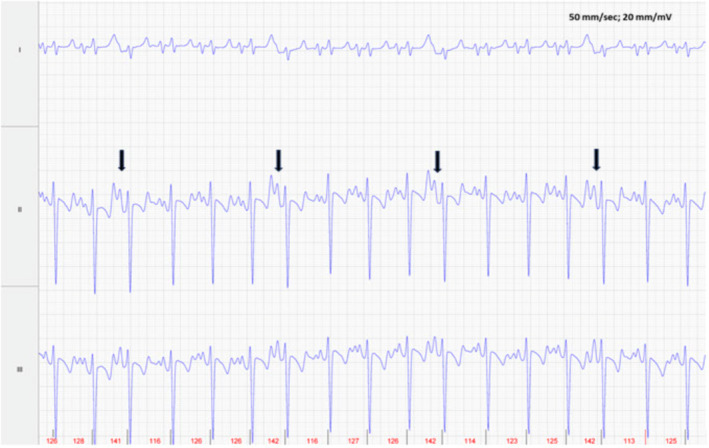
Foal 1, Day 3 post conversion ECG: Several APCs are evident (arrows) among the normal sinus rhythm. Lead II is in a base apex configuration. Lead I is a nontraditional lead and Lead III is calculated from Leads I and II. For all ECGs, the electrodes are placed around the girth area with the left leg electrode on the sternum, the left arm electrode on left side of the thorax slightly dorsal to level of the cardiac apex, the right arm electrode over the right thorax approximately 6 cm below the dorsal midline, and the right leg electrode over the left thorax approximately 6 cm below the dorsal midline.
2. CASE 2
A 24‐hour‐old, 47 kg, Thoroughbred colt was presented for evaluation of suspected neonatal encephalopathy. The colt was full term and had an uneventful birth but was not observed to stand and nurse without assistance. The multiparous 10‐year‐old dam had not experienced any complications during the gestational period but did have retained fetal membranes. No treatment had been administered before presentation. The colt was intended to be a racehorse.
On presentation, the foal was markedly agitated, but able to stand and walk with assistance. An irregularly irregular tachyarrhythmia was noted with a heart rate of 220 bpm. No murmurs were heard, and jugular distension was not identified. Peripheral pulse quality was irregular with normal strength. Mild bilateral forelimb contracture was noted. The colt had a strong sucking reflex but sustained latching was not observed. The remainder of the physical examination was within normal limits.
A CBC indicated mild neutrophilia (12.1 × 103/μL; reference interval, 5.0‐12.0 × 103/μL). A serum biochemistry panel showed increased serum creatinine concentration (3.7 mg/dL; reference interval, 0.6‐1.8 mg/dL) and hypoproteinemia (4.0 g/dL; reference interval, 4.6‐6.9 g/dL). Immunoglobulin G concentration was markedly decreased (2 mg/dL; reference interval, >800 mg/dL), confirming failure of transfer of passive immunity. Fibrinogen concentration was normal.
An ECG confirmed AF with a wide QRS complex. Serum cardiac troponin I concentration was 1.66 ng/mL (Siemens Stratus CS, Tarrytown, New York, USA). An IV catheter was placed and telemetry was performed for continuous ECG monitoring. Intravenous metoprolol (0.04 mg/kg) was administered as a single bolus for heart rate control. The heart rate subsequently decreased to 150 bpm followed shortly by conversion to sinus rhythm. No adverse effects were noted. The foal's heart rate ranged from 87 to 140 bpm depending on activity level. Twenty‐four hours after previous measurement, serum cardiac troponin I concentration had decreased to 0.60 ng/mL.
To prevent secondary infections, antibiotic treatment (5 mg/kg ceftiofur sodium IV q12h) was initiated. Two liters of HiGamm equine plasma (Lake Immunogenics, Ontario, New York, USA) were administered uneventfully. Recheck IgG concentration was adequate (805 mg/dL).
After cardioversion, repeat auscultation identified bilateral systolic murmurs (Grade 3 over the pulmonary region and Grade 2 over the tricuspid region). Two‐dimensional, color Doppler and M‐mode echocardiographic evaluation indicated no evidence of structural heart disease, and left ventricular systolic function was deemed adequate (Tables S1 and S2). The murmur was considered physiologic although it is possible a closing patent ductus arteriosus was present but missed on echocardiographic examination.
Continuous ECG monitoring for the next 48 hours identified multiform VPCs (total of 34) and APCs (maximum of 22 per hour) that progressively decreased in frequency over time. The foal remained in sinus rhythm throughout the remainder of its hospitalization, and the murmur disappeared. The foal was discharged on Day 4 of hospitalization without medications and with no audible murmur. At 2 years of age, the colt is reportedly thriving with a normal cardiac rhythm and in race training, but has not yet raced.
3. DISCUSSION
We report 2 Thoroughbred foals presented at 15 and 24 hours of age with lone rapid AF and clinical signs of poor cardiac output.
Atrial fibrillation in the neonatal period is very rarely reported in equine and human neonates. 1 , 2 Paroxysmal AF has been described in foals during the adaptive period from intra‐uterine to extra‐uterine life, occurring within the 1st 3 minutes after parturition and persisting up until 200 hours of age. 1 , 3 Excessive vagal tone, hypoxia and atrial stretch have been proposed as contributing factors. 4 Lone AF requiring cardioversion also has been described in a 3‐month‐old Thoroughbred filly. 5 In human neonates, infectious or inflammatory heart disease, cardiomyopathy, tumor, endocardial fibroelastosis, atrial lymphangiectasia, and idiopathic AF have been reported. 2 , 6 , 7
An arrhythmia induced by transition from intrauterine to extrauterine life (transitional arrhythmia) or myocardial injury or both were considered the most likely causes of AF in both foals of our report. Structural abnormalities were ruled out by echocardiographic examination. Sepsis was considered unlikely given the clinical course, clinicopathologic data and blood culture results. The rapid decrease in serum cardiac troponin I concentration supported myocardial injury either because of the rapid tachyarrhythmia or other single (non‐sustained) myocardial insult. The increase in serum cardiac troponin concentration shortly after birth with subsequent decrease may be normal for this age group because normal results for cardiac troponin I in neonatal foals have not been established for the troponin assay used in our study. Vitamin E and selenium deficiency could be a possible contributor to the myocardial injury, and it is unknown if the foals were supplemented at birth. Respiratory obstruction provoking a decrease in intrapleural pressure, sudden increase in venous return and secondary atrial stretch can cause a shortening of the refractory period in the atria, thereby predisposing to arrhythmias. 8 Although a transient respiratory obstruction could have gone unnoticed, the respiratory rate and effort were normal in both colts at the time of presentation. An accessory pathway with antidromic conduction was ruled out given the absence of pre‐excitation on the ECG after conversion.
In addition to the AF, occasional wide QRS complexes were noted in both foals before cardioversion. Differential diagnoses included VPCs and aberrant ventricular conduction secondary to changes in QRS cycle length that occur with AF or a combination thereof. 9 Aberrancies can occur in the absence of changes in cycle length or in conjunction with shortening or prolongation of the cycle length, such as Ashman's phenomena. 10 Ventricular premature contractions may have occurred secondary to the same mechanisms inducing AF or secondary to the rapid heart rate. With either VPCs or aberrancy, the goal is to treat the underlying cause, which in these cases meant rate control and cardioversion of the AF.
Rate control was considered the most urgent cardiospecific treatment in both foals. Although AF itself is not considered a life‐threatening arrhythmia at normal rates, rapid AF results in inadequate time for left ventricular filling and myocardial perfusion. Left untreated, it may lead to poor cardiac output with end organ effects and associated clinical signs. Choices for rate control include beta blockers, calcium channel blockers and digoxin and are discussed in Supporting Information.
Foal 2 spontaneously converted to normal sinus rhythm after a single 0.04 mg/kg IV bolus of metoprolol (Figure 5). Although metoprolol generally is not considered an antiarrhythmic drug, it does have mild class I effects and has been shown to prolong the atrial effective refractory period and slow the fibrillatory rate in a pig AF model. 11 The decision to convert Foal 1 on Day 2 of life rather than wait longer for potential spontaneous conversion was made because of persistence of clinical signs of poor cardiac output, continued poor latching and the need to administer the metoprolol every 3‐4 hours. Optimal PO dosing of metoprolol has not been described. Orally administered sotalol was considered at this time but was considered likely to have only a weak beta blocker effect. Normal sinus rhythm ultimately would be necessary for a good prognosis as a racehorse. Cardioversion is recommended in adult horses with AF persisting over 48 hours, because spontaneous conversion is considered less likely after this time. Progressive atrial remodeling also occurs with longer durations of AF, making the prognoses for conversion and for maintaining sinus rhythm worse the longer the AF persists. Ventricular arrhythmias, tachycardiomyopathy and heart failure, or sudden death are also potential outcomes of untreated rapid AF. 12
FIGURE 5.
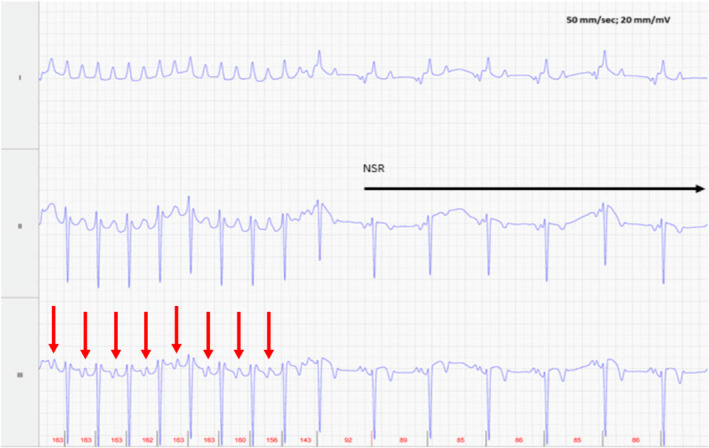
Foal 2, Day 1, at time of conversion to normal sinus rhythm (NSR). Lead II is in a base apex configuration. Lead I is a nontraditional lead and Lead III is calculated from Leads I and II. The atrial activity (red arrows) has become more organized before conversion with an atrial rate of 160 bpm just before conversion. For all ECGs, the electrodes are placed around the girth area with the left leg electrode on the sternum, the left arm electrode on left side of the thorax slightly dorsal to level of the cardiac apex, the right arm electrode over the right thorax approximately 6 cm below the dorsal midline, and the right leg electrode over the left thorax approximately 6 cm below the dorsal midline.
Both pharmacologic and nonpharmacologic options were considered for cardioversion. Quinidine, a class IA antiarrhythmic, is an effective treatment for AF of short duration and without heart disease (lone AF). 13 Intravenous quinidine salts are considered the most effective treatment of recent onset AF. It is preferred, when available, over PO quinidine for its rapid titration and elimination, making adverse effects from overdosing less likely.
Synchronized biphasic transthoracic electrical cardioversion (TTEC) also was considered for Foal 1. Successful TTEC of AF previously has been described in a 3‐month‐old Thoroughbred foal and in a 393 kg adult Arabian gelding. 5 , 14 Foals may be good candidates for conversion by TTEC because of their relatively small myocardial mass and transthoracic impedance. Because of the need for general anesthesia and additional personnel as well as clinician experience using quinidine gluconate in recent onset AF, pharmacologic cardioversion was chosen as the 1st‐line treatment after rate control in Foal 1.
Frequent APCs after cardioversion can lead to recurrence of AF. 5 , 14 , 15 , 16 , 17 Consequently, sotalol (a class III antiarrhythmic) was administered in attempt to decrease the frequency of APCs in Foal 1. The number of APCs had markedly decreased several hours after the initial dose. Whether this decrease was due to the medication or time after cardioversion is uncertain.
Our report describes cardioversion using quinidine or metoprolol or both in 2 colts with AF. Medical cardioversion may be a valid option with sustained lone AF in neonatal foals. The current lack of readily available quinidine gluconate will necessarily influence future choices for pharmacologic cardioversion.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Ceftiofur sodium 5 mg/kg IV BID used off‐label.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1: Supporting Information.
ACKNOWLEDGMENT
No funding was received for this study.
Leduc L, Abraham M, Slack J. Intravenous administration of quinidine and metoprolol for treatment of atrial fibrillation in 2 neonatal foals. J Vet Intern Med. 2024;38(5):2783‐2789. doi: 10.1111/jvim.17164
REFERENCES
- 1. Yamamoto K, Yasuda J, Too K. Arrhythmias in newborn Thoroughbred foals. Equine Vet J. 1992;24(3):169‐173. [DOI] [PubMed] [Google Scholar]
- 2. Li H, Wei X, Zheng F, Wen J, Sun G. Atrial fibrillation in preterm neonates: a case study. J Electrocardiol. 2021;65:66‐68. [DOI] [PubMed] [Google Scholar]
- 3. Machida N, Yasuda J, Too K. Three cases of paroxysmal atrial fibrillation in the Thoroughbred newborn foal. Equine Vet J. 1989;21(1):66‐68. [DOI] [PubMed] [Google Scholar]
- 4. Alessi R, Nusynowitz M, Abildskov JA, Moe GK. Nonuniform distribution of vagal effects on the atrial refractory period. Am J Physiol. 1958;194(2):406‐410. [DOI] [PubMed] [Google Scholar]
- 5. Potter BM, Scansen BA, Dunbar LK, Reed SM, Toribio RE. Transcutaneous direct current cardioversion in a foal with lone atrial fibrillation. J Vet Cardiol. 2017;19(1):99‐105. [DOI] [PubMed] [Google Scholar]
- 6. Bassareo PP, Marras AR, Marras M, Marras S, Mercuro G. Atrial fibrillation in a preterm newborn with structurally normal heart. Oxf Med Case Reports. 2017;2017(3):30‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ainger LE, Fitch CW, Lawyer NG. Atrial fibrillation in endocardial fibroelastosis: successful cardioversion with external countershock. Am J Dis Child. 1966;111(6):655‐660. [Google Scholar]
- 8. Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436‐1443. [DOI] [PubMed] [Google Scholar]
- 9. Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta‐analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol. 2013;112(8):1263‐1270. [DOI] [PubMed] [Google Scholar]
- 10. Singer DH, Ten Eick RE. Aberrancy: electrophysiologic aspects. Am J Cardiol. 1971;28(4):381‐401. [DOI] [PubMed] [Google Scholar]
- 11. Berg MPVD, Ven LLMVD, Witting W, et al. Effects of β‐blockade on atrial and atrioventricular nodal refractoriness, and atrial fibrillatory rate during atrial fibrillation in pigs. Jpn Heart J. 1997;38(6):841‐848. [DOI] [PubMed] [Google Scholar]
- 12. Lishmanov A, Chockalingam P, Senthilkumar A, Chockalingam A. Tachycardia‐induced cardiomyopathy: evaluation and therapeutic options. Congest Heart Fail Greenwich Conn. 2010;16(3):122‐126. [DOI] [PubMed] [Google Scholar]
- 13. Morris DD, Fregin GF. Atrial fibrillation in horses: factors associated with response to quinidine sulfate in 77 clinical cases. Cornell Vet. 1982;72(4):339‐349. [PubMed] [Google Scholar]
- 14. Frye MA, Selders CG, Mama KR, Wagner AE, Bright JM. Use of biphasic electrical cardioversion for treatment of idiopathic atrial fibrillation in two horses. J Am Vet Med Assoc. 2002;220(7):1039‐1045, 1007‐1045. [DOI] [PubMed] [Google Scholar]
- 15. Im SI, Park DH, Kim BJ, Cho KI, Kim HS, Heo JH. Clinical and electrocardiographic characteristics for prediction of new‐onset atrial fibrillation in asymptomatic patients with atrial premature complexes. Int J Cardiol Heart Vasc. 2018;19:70‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acharya T, Tringali S, Bhullar M, et al. Frequent atrial premature complexes and their association with risk of atrial fibrillation. Am J Cardiol. 2015;116(12):1852‐1857. [DOI] [PubMed] [Google Scholar]
- 17. Thong T, McNames J, Aboy M, Goldstein B. Prediction of paroxysmal atrial fibrillation by analysis of atrial premature complexes. IEEE Trans Biomed Eng. 2004;51(4):561‐569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information.


