Abstract
Background
To ameliorate anticipated or ongoing neurological deficits, dogs undergoing brain tumor irradiation often are prescribed lengthy courses of prednisone PO during and after radiotherapy (RT). This practice can contribute to unwanted corticosteroid‐associated morbidity and may be unnecessary.
Objective
Determine whether long‐term corticosteroid dependency can be minimized by use of succinct prednisone tapering.
Animals
Fifty‐five pet dogs undergoing brain tumor irradiation.
Methods
Nineteen dogs were treated using a “rapid‐taper” protocol wherein corticosteroid dose reduction began 0 to 20 days after completing RT. Outcomes were compared with a retrospectively studied control group (“slow‐taper”; N = 36 dogs) in which corticosteroids were tapered more slowly according to individual clinician recommendations.
Results
Patient demographics were similar between groups. Mean time to lowest prednisone dose was 41 days postirradiation in the rapid‐taper group and 117 days in the slow‐taper group (P = .003). In the rapid‐taper group, 15 of 19 dogs (84%) were completely tapered off prednisone, vs 18 of 36 (50%) in the slow‐taper group (P = .04). Rates at which corticosteroids had to be reinstituted later were similar for the 2 groups (approximately 1 in 3 dogs). Adverse effect rates were similar for the 2 groups. Although no comparable questionnaire‐derived data were available for the “slow‐taper” group, overall and neurologic quality of life remained stable after RT in the rapid‐taper group.
Conclusions and Clinical Importance
For many dogs, lengthy courses of PO prednisone are avoidable after intracranial RT. Future efforts should aim to identify which dogs benefit most from accelerated prednisone tapering.
Keywords: corticosteroids, glucocorticoids, neuroprotection, radiotherapy
Abbreviations
- ANOVA
analysis of variance
- CanBrainQOL
canine brain quality of life questionnaire
- CFRT
conventionally fractionated radiotherapy
- CT
computed tomography
- MRI
magnetic resonance imaging
- RT
radiotherapy
- SRS
stereotactic radiosurgery
- SRT
stereotactic radiotherapy
- VRTOG
Veterinary Radiation Therapy Oncology Group
1. INTRODUCTION
Commonly encountered intracranial neoplasms of the dog include meningioma, glial tumors, pituitary macrotumors, and secondary brain tumors (eg, nasal masses that extend into the calvarium and cancers metastatic from distant sites). For most of these diseases, an important therapeutic option is radiotherapy (RT). Definitive‐intent RT options often include conventionally fractionated radiotherapy (CFRT), stereotactic radiotherapy (SRT), and stereotactic radiosurgery (SRS). For CFRT, it is typical for 40 to 54 Gy total to be administered in 10 to 20 fractions. 1 , 2 , 3 , 4 For SRT, it is typical for a total dose of 15 to 35 Gy to be given in 2 to 5 consecutive daily fractions, 5 , 6 , 7 , 8 , 9 whereas SRS generally involves a single fraction of 14 Gy or more. 10 , 11 , 12
Although generally well‐tolerated, various forms of RT‐induced neurotoxicity can occur, including: (a) acute changes that occur during or shortly after completing a course of RT, and which are attributed to transient disruption of the blood‐brain‐barrier; (b) early‐delayed or subacute effects that occur anywhere from weeks up to about 5 months after RT, and which are thought to be a result of transient and generally reversible demyelination; or (c) late effects that occur many months to years after RT, and which are largely attributed to vascular damage or irreversible brain necrosis. 13
Some veterinary radiation oncologists (including ourselves) routinely prescribe glucocorticoids PO (eg, 0.5‐1.5 mg/kg daily of prednisone) to a majority of canine brain irradiation patients, with an intention of beginning to taper the corticosteroids 3 to 8 weeks after RT completion. 4 , 10 , 14 The rationale generally put forth is that corticosteroids could help decrease the risk, severity or both of acute and early‐delayed RT‐induced encephalopathies. However, no published research studies support this claim. Furthermore, prolonged corticosteroid use often creates unwanted adverse effects, such as polydipsia, polyuria, polyphagia, weight gain, and gastrointestinal tract ulceration and bleeding. 15
Beginning in 2021, a new standardized rapid‐tapering protocol was implemented at our 2 institutions to facilitate more succinct prednisone courses in dogs undergoing brain RT. The protocol (Table 1) categorizes dogs into 1 of 4 unique groups based on how long they will have been exposed to PO corticosteroids before a taper is attempted. The intention was that this protocol would provide general guidance, but that deviations from the protocol could be made at the discretion of the attending veterinarian, at any time and for any reason. At the same time this protocol was introduced, a prospective observational study was initiated to collect quality of life data in dogs treated using this new rapid‐tapering approach. Here, we report the outcomes of that study, and compare both prednisone usage and survival statistics with a historical comparator that includes dogs with brain tumors that were treated at our hospitals using RT and prednisone before institution of the new succinct taper (ie, they were treated using RT followed by a more traditional slow‐taper). The goals of this work were to better define: (1) how quickly dogs can be safely tapered off corticosteroids; (2) how often prednisone treatment needs to be reinstated after the taper is complete; and (3) whether a succinct taper has any measurably detrimental effects on either quality of life or overall survival.
TABLE 1.
Standardized rapid‐tapering oral prednisone protocol for dogs undergoing brain irradiation.
| During RT | At completion of RT | 1 week after RT | 2 weeks after RT | |
|---|---|---|---|---|
| Group 1: Dogs not on prednisone before RT (5 fractions or fewer); n = 7 dogs. | Prednisone (approximately 0.5 mg/kg PO) before each day's RT | No prednisone | No prednisone | |
| Group 2: Dogs not on prednisone before RT (6 fractions or greater); n = 0 dogs | Prednisone (approximately 0.5 mg/kg) before each day's RT | Decrease dose by 50% (ie, give approximately 0.25 mg/kg/d) for 1 week | If neurologically stable or improved, give prednisone (approximately 0.25 mg/kg) q48h for 3 doses, then stop | |
| Group 3: Dogs on prednisone for ≤1 month before starting RT; n = 9 dogs | Continue prednisone during RT (by end of RT, try to be at approximately 0.5 mg/kg/d) | Decrease prednisone dose by 50% (to approximately 0.25 mg/kg/d) | If neurologically stable/improved, give prednisone (approximately 0.25 mg/kg) q48h for 3 doses, then stop | |
| Group 4: Dogs on prednisone for >1 month before starting RT; n = 3 dogs | Continue prednisone during RT (by end of RT, try to be at approximately 0.5 mg/kg/d) | Decrease prednisone dose by 50% (to approximately 0.25 mg/kg/d) | Decrease prednisone dose by another 50% (approximately 0.125 mg/kg/d) | If neurologically stable/improved, give prednisone (approximately 0.125 mg/kg) q48h for 3 doses, then stop |
2. MATERIALS AND METHODS
Data were analyzed from dogs that underwent definitive‐intent RT (CFRT, SRT, or SRS) for an imaging‐diagnosed intracranial neoplasm between 2013 and 2023. A specific radiologist dedicated for this study was not used. Instead, the imaging diagnosis was based on the radiology report that was generated for each dog's diagnostic magnetic resonance imaging (MRI) study. In all cases, the report was approved by a veterinary diagnostic imaging specialist who was board certified by the American College of Veterinary Radiology. Dogs were divided into 2 groups.
The 1st group (“rapid‐taper”) included dogs treated using the new succinct prednisone tapering protocol (see Table 1), with quality‐of‐life data prospectively collected (with client consent and based on an observational study, institutional animal care and use committee protocol #21‐208), using a combination of recheck examinations and client questionnaires. The questionnaire was sent to owners by electronic mail at the start of RT, and then again at the time of recheck examinations performed at 2 and 4 weeks, and then 3 and 6 months after completing RT. The canine brain quality of life questionnaire (CanBrainQOL) was designed to evaluate health‐related quality of life in dogs with intracranial disease. 16 It includes 24 total questions, divided into 4 sections, including the pet's (1) physical well‐being (15 questions), (2) emotional well‐being and social interactions (4 questions), (3) brain‐specific concerns (5 questions), and (4) a visual assessment score (VAS) to reflect the family's overall assessment of their dog's quality of life in a single number (scored 1‐10). Aside from the VAS, all questions were structured using a Likert 1 to 5 rating scale. For all questions, an increasing score represents a worsening quality of life. As a frame of reference, the potential total score range is 24 to 120, and in the CanBrainQOL validation study, the average score of 52 healthy dogs was 29.4 ± 2.5, and the average score of 76 dogs with intracranial disease was 44.2 ± 9.5. 16
The 2nd group (“slow‐taper”) was a control group including dogs with brain tumors that were irradiated at a single institution before introduction of the new rapid‐taper protocol in 2021. The study was structured as a pilot study, without a formal power calculation to inform sample size. Therefore, this control group was constructed with the aim of including 2 control cases for every “rapid‐taper” case. A RT patient database was searched such that controls could be matched as closely as possible to cases with regard to sex, facial conformation (brachycephalic vs other), tumor type, and RT prescription.
For both groups, medical records were retrospectively reviewed, and the following data were recorded: age, sex, tumor type, RT prescription, prednisone taper schedule (starting dose of prednisone in mg/kg, ending dose of prednisone, time to start of prednisone taper after RT, and time to lowest dose of prednisone after starting RT), date RT began, and date of death. For purposes of the study, dogs were considered to have been completely tapered off prednisone if they were able to completely stop taking PO corticosteroids after RT, regardless of whether corticosteroids were restarted at a later time.
Radiation toxicities were retrospectively graded by a radiation oncology resident (JS) with oversight from an American College of Veterinary Radiology board‐certified specialist in radiation oncology (MWN) according to a recently updated version of the Veterinary Radiation Therapy Oncology Group criteria. 17 Acute adverse events were defined as instances occurring within 90 days of RT and late adverse events were defined as instances occurring at least 90 days after RT. 17 , 18 If there was no mention of adverse effects in the medical record, the patient was presumed to have had none.
Group demographics were compared using Mann‐Whitney U and Fisher's exact tests. Between the groups, starting vs ending prednisone doses were compared using 2‐way analysis of variance (ANOVA). Mean time to starting a prednisone taper was compared using t‐tests. Survival was characterized using Kaplan‐Meier statistics, and log‐rank testing. Overall survival time was measured as the time between the start of RT and death. Dogs that were alive at the end of the study were censored at the study's end date, and those lost to follow‐up were censored from at the date of last contact. Regarding the CanBrainQOL surveys, data were prospectively collected from dogs in the rapid‐taper group, and for analytical purposes, the dogs were subdivided based on whether they were treated with CFRT or SRT/SRS. Total CanBrainQOL scores, and subscores for each of the questionnaire domains (physical, social, brain, and visual assessment) then were compared using a mixed effects model (restricted maximum likelihood, with time and treatment type as fixed effects) to determine whether time postirradiation or radiation treatment approach had measurable impact. All statistical analyses were performed using commercial software (Prism 7.04; GraphPad Software, Inc); the threshold for statistical significance was set at P < .05.
3. RESULTS
A total of 55 dogs were included: 19 in the rapid‐taper group (treated in January 2021 to May 2023) and 36 in the slow‐taper group (treated in August 2013 to December 2020). The groups were similar in that the median age for the rapid‐taper group was 9 years (range, 5‐13), vs 10 years (range, 5‐15) in the slow‐taper group (Mann‐Whitney U‐test P = .1) and the median body weight was 26.1 kg for the rapid‐taper group (range, 6.1‐56.4 kg) and 22.2 kg (range, 2.9‐51.1 kg; Mann‐Whitney U‐test P = .1). The groups also were similar in terms of distribution of sex, breed (and facial and skeletal conformation), diagnoses, and treatment approach (Table 2). Total radiation doses were 20 to 54 Gy in the rapid‐taper group, and 16 to 54 Gy in the slow‐taper group.
TABLE 2.
Summary of demographics, diagnoses, and irradiation protocols.
| Descriptor | Rapid‐taper group (n = 19 dogs) | Slow‐taper group (n = 36 dogs) | Fisher's exact (P‐value) | |
|---|---|---|---|---|
| Sex | Male | 5 dogs (26%) | 18 dogs (50%) | .90 |
| Female | 14 (74%) | 18 (50%) | ||
| Breed | Brachycephalic | 5 (26%) | 6 (17%) | .39 |
| Nonbrachycephalic | 14 (74%) | 30 (83%) | ||
| Tumor type | Intra‐axial | 3 (16%) | 5 (14%) | .93 |
| Extra‐axial | 16 (84%) | 31 (86%) | ||
| Irradiation protocol | SRT (1‐5 fractions) | 12 (63%) | 27 (75%) | .36 |
| CFRT (10‐20 fractions) | 7 (37%) | 9 (25%) | ||
Abbreviations: CFRT, conventionally fractionated radiotherapy; SRT, stereotactic radiotherapy.
In the rapid‐taper group, there were 8 dogs of mixed breed, and the remaining were: boxer (3), French bulldog (2), Dachshund (1), German shepherd (1), golden retriever (1), Labrador retriever (1), treeing Walker coonhound (1), and West Highland white terrier (1). All brain tumors diagnoses were based on interpretations of MRI or computed tomography (CT) findings; none had biopsy confirmation. Four dogs were diagnosed with pituitary tumors, 3 with glioma, 5 with meningioma, 6 with trigeminal nerve sheath tumors, and 1 with neuroblastoma. The slow‐taper group included 3 dogs of mixed breed, and others included: Labrador retriever (5), boxer (3), Australian shepherd (2), French bulldog (2), golden retriever (2), Maltese (2), miniature schnauzer (2), dachshund (1), English bulldog (1), flat‐coated retriever (1), Australian cattle dog (1), American Staffordshire terrier (1), Doberman pinscher (1), Chihuahua (1), Norwich terrier (1), Gordon setter (1), border collie (1), bichon frise (1), labradoodle (1), Siberian husky (1), Scottish terrier (1), and Pomeranian (1). Twenty dogs were diagnosed with meningioma, 5 with glioma, 1 with histiocytic sarcoma, 1 with neuroblastoma, 3 with trigeminal nerve sheath tumors, and 6 with pituitary tumors; all were imaging diagnoses, and none had pathologic confirmation. Tumor location was skull base (ie, a tumor that was either near or including the pituitary gland or a cranial nerve) in 27 dogs (N = 11, rapid‐taper group; N = 16, slow‐taper group), forebrain in 22 dogs (N = 6, rapid‐taper group; N = 16, slow‐taper group), and brainstem in 6 dogs (N = 2, rapid‐taper group; N = 4, slow‐taper group).
The mean (±SD) starting dose of prednisone in dogs treated with a rapid‐taper was 0.55 (±0.22) mg/kg/d, vs 0.22 (±0.41) mg/kg/d at the end of the taper, representing a significant reduction in prednisone dose during the taper (2‐way ANOVA adjusted P = .001). In dogs treated using a slow‐taper, prednisone doses were similar (starting at 0.70 ± 0.25 mg/kg/d and ending at 0.26 ± 0.27 mg/kg/d; P < .0001). No significant differences were found in the starting and ending doses of prednisone between the 2 groups (P = .12 and .91, respectively). The mean time to starting a prednisone taper was significantly shorter in the rapid‐taper group (10.5 ± 10.3 days vs 55.0 ± 49.5 days after the start of RT, t‐test P = .0003). Likewise, the mean time to lowest prednisone dose also was shorter in the rapid‐taper group (41 ± 56.7 days vs 117 ± 97.1 days after the start of RT; t‐test P = .003).
In the rapid‐taper group, all 19 dogs were prescribed a taper per‐protocol (as indicated in the table). There were 7 dogs in Group 1 (mean duration of taper, 24.2 days; median, 0 days), 0 dogs in Group 2, 9 dogs in group 3 (mean duration of taper, 38.2 days; median, 13 days), and 3 dogs in Group 4 (mean duration of taper, 30.7 days; median, 26 days). Of those 19 dogs, 15 (84.2%) were able to be completely tapered off prednisone. The other 4 dogs in that group remained on corticosteroids long‐term (at a dose equal to or higher than their pre‐RT dose) because of worsening neurologic signs that were observed shortly after attempting to taper. In the slow‐taper group, 18 of 36 (50%) were able to be completely tapered off prednisone. Of the 18 that had incomplete tapers, 3 died before a taper was ever attempted, 1 dog remained on long‐term low dose prednisone for skin disease, and 15 dogs began to have worsening of their presenting neurologic symptoms shortly after starting the prednisone taper and thus remained on the initial or higher dose for the remainder of their lives. Overall, a significantly higher proportion of dogs could be completely tapered off corticosteroids in the rapid‐taper group, as compared with the slow‐taper group (P = .04).
In both groups, some dogs needed to be restarted on prednisone at some point after their initial complete taper. The reason was not always clear, and often prednisone was being used for management of recurrent or progressive neurologic signs that could have resulted from tumor progression or radiation toxicity. Here, we describe each of the dogs that restarted prednisone and also describe each of the cases for which a potential adverse event was recorded.
Of the 15 dogs that were completely tapered off corticosteroids in the rapid‐taper group, 4 (26.7%) had to be restarted on prednisone later. One of these dogs (with an imaging‐diagnosed glioma) was restarted on prednisone 3 weeks after finishing the initial taper because of lethargy, and the dog then was successfully retapered after 2 weeks and never again needed prednisone. Another (with an imaging‐diagnosed pituitary macroadenoma) restarted 5 weeks after finishing the initial taper because of recurrent neurologic signs, and the dog was successfully and completely tapered off corticosteroids after 4 months. The other 2 dogs (with glioma and pituitary macroadenoma) were restarted on prednisone for recurrent neurologic signs 8 months after the initial taper; both remained on prednisone for the remainder of their lives.
Of the 18 dogs in the slow‐taper group that completely were tapered off corticosteroids, 7 (38.9%) had to be restarted at some later point. One of these dogs (with an imaging‐diagnosed pituitary macroadenoma) had to be restarted on corticosteroids 21 days after the initial taper for unknown reasons; corticosteroids were then permanently discontinued 3.5 months later. Four were restarted on prednisone between 3 and 9 months after the initial taper because of recurrent or progressive neurologic signs. Each of these dogs then stayed on corticosteroids for the remainder of their lives (3 meningioma, 1 trigeminal nerve sheath tumor). Two dogs were restarted on prednisone, permanently because of unrelated disease. In 1 case (trigeminal nerve sheath tumor), prednisone was initiated 3.5 months after the initial taper to treat allergic rhinitis, and in the other case (trigeminal nerve sheath tumor), prednisone was restarted at an unknown time point to manage a cutaneous mast cell tumor. The percentage of dogs having to later be restarted on corticosteroids was not significantly different between the groups (Chi‐square P = .46).
In the rapid‐taper group, 74 CanBrainQOL questionnaires were distributed to the 19 pet owners and 65 responses were collected, for an overall 88% return rate. Notably, although 4 questionnaire timepoints initially had been planned, an error was made and the survey was not sent to the 1st half of subjects. Once that error was identified, it was decided to forgo the 3‐month timepoint entirely. Additionally, 2 scheduled surveys were not sent at the 6‐month time point because of earlier death of the pet.
At presentation to the radiation oncology clinics, the mean total CanBrainQOL score was 39 (±8) for the 7 dogs treated with CFRT, and 42 (±9) for the 12 dogs treated with SRT/SRS. At 2 weeks post‐RT, the mean total scores for the CFRT and SRT/SRS‐treated dogs were 34 (±5) and 40 (±9); at 4 weeks, they were 45 (±16) and 42 (±19), and at 6 months, they were 42 (±19) and 41 (±9). Neither significant time‐dependent changes from baseline in either group (eg, CFRT vs SRT/SRS) nor significant differences between those groups were identified at any of the individual time points. Also, no significant differences were detected when evaluating for potential time or irradiation protocol‐dependent changes in any of the individual subdomains (physical, social, and brain), or the visual assessment scores. CanBrainQOL scores and analyses are summarized in Figure 1 and Tables S1‐S5. Questionnaires were not distributed to owners of dogs in the slow‐taper group, and thus data are not available for direct comparison. However, in the rapid‐taper group, acute radiation adverse effects were recorded for 3 dogs. Two had Veterinary Radiation Therapy Oncology Group (VRTOG) grade 1 somnolence that occurred 2 weeks to 1 month after irradiation, and 1 dog was suspected to have developed VRTOG grade 3 adverse effects, manifested as seizures, ataxia, and dull mentation that was minimally responsive to corticosteroids. By contrast, no acute effects were recorded in the slow‐taper group, and no late effects were recorded in either group.
FIGURE 1.
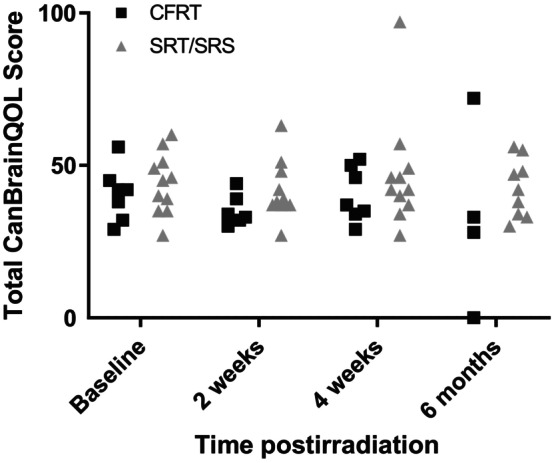
A caregiver reported quality of life assessment instrument (CanBrainQOL) was used to assess well‐being of dogs whose intracranial masses were treated with radiotherapy and prednisone on a rapid‐taper schedule. Total CanBrainQOL scores for individual dogs are shown in this graph; data from dogs treated with conventionally fractionated definitive‐intent radiotherapy are shown as solid black squares, whereas data from dogs treated with either stereotactic radiotherapy (SRT) or radiosurgery (SRS) are shown as solid gray triangles. There were neither significant differences in total scores when comparing treatment approach (CFRT vs SRT/SRS) nor were there significant differences over time.
Median overall survival time for the rapid‐taper group was 617 days vs 494 days for the slow‐taper group (log rank P = .36; Figure 2). In the rapid‐taper group, 12 dogs were alive at study completion and therefore censored, with a median follow‐up time of 398 days. In the slow‐taper group, 5 dogs were censored at a median of 723 days (2 were alive at study completion and 3 were lost to follow‐up).
FIGURE 2.
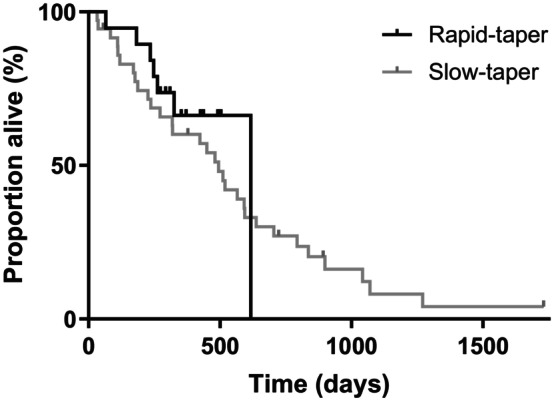
All‐cause mortality was analyzed using Kaplan‐Meier statistics. The median survival time for dogs in the rapid‐taper group was 617 days, and 494 days for the slow‐taper group; this numerical difference was not statistically significant (log rank P = .36).
4. DISCUSSION
Implementation of the rapid prednisone tapering protocol described here successfully decreased the amount of time over which dogs were given prednisone after a course of RT; as evidenced by the fact that dogs in the rapid‐taper group were able to achieve their lowest post‐RT dose of prednisone significantly sooner than dogs in the slow‐taper group. The rapid‐taper protocol also was associated with a significantly higher proportion of dogs being able to completely be tapered off corticosteroids after RT. Although some dogs later had to be restarted on prednisone, the rate at which this situation occurred was no different between the rapid‐ and slow‐taper groups. Three findings support the safety of the rapid‐tapering protocol: (1) the frequency of adverse events was similar between the rapid‐ and slow‐taper groups; (2) based on questionnaires complete by owners of dogs in the rapid‐taper group, both overall and neurologic quality of life remained stable after RT; and (3) the overall survival time was similar for the 2 groups.
As in veterinary medicine, dosages and duration of prescribed courses of corticosteroids in humans before and after treatment of intracranial tumors vary substantially among oncologists. 19 , 20 Toxicity and adverse effects from corticosteroids occur frequently and contribute to overall morbidity, mortality, and quality of life in cancer patients. 20 Data also suggest that lower doses of corticosteroids, shorter courses, or both may result in equally effective benefit with regard to neurological improvement. 21 , 22 A previous study evaluated the effect of a rapidly tapering dexamethasone dose during palliative‐intent RT for newly diagnosed brain metastases in humans. 22 Patients received 8 mg q12h for 4 days, then 4 mg q12h for 4 days, then 2 mg q12h until completion of RT (20‐58 Gy delivered in 5‐29 fractions). Only 1 patient of 20 needed to restart dexamethasone within 30 days of finishing RT because of a corticosteroid‐reversible neurologic deficit. This novel short course of corticosteroids was associated with good clinical outcomes and minimal corticosteroid‐associated morbidity. Although not specifically evaluated in our study, shortening the course of corticosteroids likely also will decrease the frequency and severity of corticosteroid‐associated morbidity in dogs with brain tumors. Another potential benefit is that decreased reliance on corticosteroids may improve prognosis for survival in certain disease states. Although corticosteroid use remains commonplace in management of human neurooncology patients, 20 , 21 , 23 this practice may be detrimental in patients with glioblastoma. Experiments in mice have shown that dexamethasone can have antiproliferative effects that protect against RT‐induced genotoxic stress. 23 The possibility that decreased corticosteroid usage could be advantageous with regard to overall survival of dogs undergoing brain tumor irradiation should be explored.
Our study had several limitations. First, a specific radiologist was not designated for the study, and none of the dogs had pathologic diagnoses of their intracranial tumors, which could have led to misdiagnosis in at least some of the cases. Similarly, none of the dogs underwent necropsy. As a result, it was impossible to know whether recurrent neurologic signs were caused by progressive tumor growth, radiation toxicity, or a combination of these. Importantly, this lack of pathology data means there is risk of undetected differences between the rapid‐ and slow‐taper groups regarding frequency or severity of subclinical radiation effects on brain parenchyma. Likewise, a relatively small sample size and short follow‐up time for the rapid‐taper group could have contributed to overestimation of overall survival time and underestimation of the risk for serious late effects. Quality and completeness of follow‐up data for the control cases was limited by the retrospective design for that group. In particular, CanBrainQOL scores were not available for the slow‐taper control group. Therefore, direct comparison of owners' perception of quality of life between the 2 tapering protocols was not possible. In designing future experiments and trials, efforts should be made to ensure that pet owners are properly trained to complete quality of life assessments. No standardized training was used in our study, which may have led to poor understanding or interpretation of the questionnaires, leading to bias and inconsistency in the results. Beyond inclusion of training efforts, future work also could benefit from utilization of objective measures of quality of life, such as neurologic assessments and behavioral observations, to complement the questionnaire‐based evaluations. Future work also should aim to control for potential confounding effects of tumor size and tumor location, which were not accounted for in the design of our study.
We compared quality of life between dogs treated with either CFRT or SRT/SRS because of anecdotal concern that neurologic adverse sequelae may be more likely with severe hypofractionation. We found no evidence that 1 approach was worse than the other, but the generalizability of our result is limited by small sample size. In our study population, the average total CanBrainQOL scores at baseline (41 for CFRT and 44 for SRT/SRS) were similar to those reported elsewhere (ie, in the initial validation study for this instrument, the mean total score for 76 dogs with intracranial disease was 44.2). 16 Although it is disappointing that the average score in our dogs did not significantly improve after irradiation, that result should be interpreted cautiously (eg, because of small sample size, measurement at relatively early time points postirradiation, and a study design not specifically optimized or intended for detecting a significant improvement over time).
In summary, most dogs in our study treated using the succinct tapering protocol could be completely tapered off prednisone (ie, 84.2% in the rapid‐taper group were able to be completely tapered off corticosteroids and the mean time to lowest prednisone dose in this group was 41 days vs 50% completely tapered and a mean of 117 days to lowest dose in the slow‐taper group), and this rapid‐tapering approach was achievable without any identifiable detriment to quality of life or prognosis for survival. These encouraging results suggest that in some dogs it may be possible to forgo corticosteroids entirely, which would be consistent with the observation that, in human brain irradiation patients, corticosteroids generally are only of substantial benefit when performance scores are low. 24 Thus a potentially valuable future direction would be to evaluate the safety of such an approach in the lowest risk population, which are those dogs that have not received corticosteroids at the time of presentation to the radiation oncology clinic. Given that a future goal should be to identify dogs that might benefit from rapid‐tapering (or no corticosteroids at all), results of our study might be used to identify potential associations among dogs that could not be tapered or that were required to be restarted on corticosteroids. For example, a reasonable clinical presumption would be that a dog might do well if it has a small supratentorial tumor with minimal perilesional edema and minimal to mild neurologic signs. However, considering the data together with the treating veterinarians, no obvious trends were identified, and some cases were symptomatic but still did well with RT and a succinct taper. It is beyond the scope of our study to make clear recommendations in this regard.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by North Carolina State University IACUC, number 21‐208.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGMENT
No funding was received for this study. The authors thank Mindy Quigley (Virginia Tech) for her technical assistance with this research.
Strasberg JR, Rossmeisl JH, Kelsey KL, Yoshikawa H, Gieger TL, Nolan MW. A prospective evaluation of succinct prednisone tapering after brain tumor irradiation in dogs. J Vet Intern Med. 2024;38(5):2571‐2577. doi: 10.1111/jvim.17163
REFERENCES
- 1. Schwarz P, Meier V, Soukup A, et al. Comparative evaluation of a novel, moderately hypofractionated radiation protocol in 56 dogs with symptomatic intracranial neoplasia. J Vet Intern Med. 2018;32(6):2013‐2020. doi: 10.1111/jvim.15324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Théon AP, Feldman EC. Megavoltage irradiation of pituitary macrotumors in dogs with neurologic signs. J Am Vet Med Assoc. 1998;213(2):225‐231. [PubMed] [Google Scholar]
- 3. Kent MS, Bommarito D, Feldman E, Theon AP. Survival, neurologic response, and prognostic factors in dogs with pituitary masses treated with radiation therapy and untreated dogs. J Vet Intern Med. 2007;21(5):1027‐1033. doi: 10.1892/0891-6640(2007)21[1027:snrapf]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 4. Staudinger C, Meier V, Beckmann K, Körner M, Rohrer BC. Treatment of intracranial neoplasia in dogs using higher doses: a randomized controlled trial comparing a boosted to a conventional radiation protocol. J Vet Intern Med. 2022;36(4):1353‐1364. doi: 10.1111/jvim.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter GL, Ogilvie GK, Mohammadian LA, Bergman PJ, Lee RP, Proulx DR. CyberKnife stereotactic radiotherapy for treatment of primary intracranial tumors in dogs. J Vet Intern Med. 2021;35(3):1480‐1486. doi: 10.1111/jvim.16086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dolera M, Malfassi L, Bianchi C, et al. Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet Comp Oncol. 2018;16(1):90‐101. doi: 10.1111/vco.12316 [DOI] [PubMed] [Google Scholar]
- 7. Griffin LR, Nolan MW, Selmic LE, Randall E, Custis J, LaRue S. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Vet Comp Oncol. 2016;14(4):e158‐e170. doi: 10.1111/vco.12129 [DOI] [PubMed] [Google Scholar]
- 8. Moirano SJ, Dewey CW, Haney S, Yang J. Efficacy of frameless stereotactic radiotherapy for the treatment of presumptive canine intracranial gliomas: a retrospective analysis (2014‐2017). Vet Comp Oncol. 2020;18(4):528‐537. doi: 10.1111/vco.12573 [DOI] [PubMed] [Google Scholar]
- 9. Hansen KS, Zwingenberger AL, Théon AP, Kent MS. Long‐term survival with stereotactic radiotherapy for imaging‐diagnosed pituitary tumors in dogs. Vet Radiol Ultrasound. 2019;60(2):219‐232. doi: 10.1111/vru.12708 [DOI] [PubMed] [Google Scholar]
- 10. Kelsey KL, Gieger TL, Nolan MW. Single fraction stereotactic radiation therapy (stereotactic radiosurgery) is a feasible method for treating intracranial meningiomas in dogs. Vet Radiol Ultrasound. 2018;59(5):632‐638. doi: 10.1111/vru.12636 [DOI] [PubMed] [Google Scholar]
- 11. Mariani CL, Schubert TA, House RA, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol. 2015;13(4):409‐423. doi: 10.1111/vco.12056 [DOI] [PubMed] [Google Scholar]
- 12. Gieger TL, Nolan MW. Treatment outcomes and target delineation utilizing CT and MRI in 13 dogs treated with a uniform stereotactic radiation therapy protocol (16 Gy single fraction) for pituitary masses: (2014‐2017). Vet Comp Oncol. 2021;19(1):17‐24. doi: 10.1111/vco.12627 [DOI] [PubMed] [Google Scholar]
- 13. Rossmeisl JH Jr, Garcia PA, Daniel GB, et al. Invited review—neuroimaging response assessment criteria for brain tumors in veterinary patients. Vet Radiol Ultrasound. 2014;55(2):115‐132. doi: 10.1111/vru.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Treggiari E, Maddox TW, Gonçalves R, Benoit J, Buchholz J, Blackwood L. Retrospective comparison of three‐dimensional conformal radiation therapy vs. prednisolone alone in 30 cases of canine infratentorial brain tumors. Vet Radiol Ultrasound. 2017;58(1):106‐116. doi: 10.1111/vru.12440 [DOI] [PubMed] [Google Scholar]
- 15. Elkholly DA, Brodbelt DC, Church DB, et al. Side effects to systemic glucocorticoid therapy in dogs under primary veterinary care in the UK. Front Vet Sci. 2020;7:515. doi: 10.3389/fvets.2020.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiske R, Sroufe M, Quigley M, Pancotto T, Werre S, Rossmeisl JH. Development and evaluation of a caregiver reported quality of life assessment instrument in dogs with intracranial disease. Front Vet Sci. 2020;7:537. doi: 10.3389/fvets.2020.00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poirier VJ, Keyerleber M, Gordon IK, et al. ACVR and ECVDI consensus statement: reporting elements for toxicity criteria of the veterinary radiation therapy oncology group v2.0. Vet Radiol Ultrasound. 2023;64(5):789‐797. doi: 10.1111/vru.13291 [DOI] [PubMed] [Google Scholar]
- 18. Van Asselt N, Christensen N, Meier V, et al. Definitive‐intent intensity‐modulated radiation therapy provides similar outcomes to those previously published for definitive‐intent three‐dimensional conformal radiation therapy in dogs with primary brain tumors: a multi‐institutional retrospective study. Vet Radiol Ultrasound. 2020;61(4):481‐489. doi: 10.1111/vru.12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sturdza A, Millar BA, Bana N, et al. The use and toxicity of steroids in the management of patients with brain metastases. Support Care Cancer. 2008;16(9):1041‐1048. doi: 10.1007/s00520-007-0395-8 [DOI] [PubMed] [Google Scholar]
- 20. Ryken TC, Kuo JS, Prabhu RS, Sherman JH, Kalkanis SN, Olson JJ. Congress of neurological surgeons systematic review and evidence‐based guidelines on the role of steroids in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84(3):E189‐E191. [DOI] [PubMed] [Google Scholar]
- 21. Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233‐242. doi: 10.1586/ecp.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weissman DE, Janjan NA, Erickson B, et al. Twice‐daily tapering dexamethasone treatment during cranial radiation for newly diagnosed brain metastases. J Neurooncol. 1991;11(3):235‐239. doi: 10.1007/bf00165531 [DOI] [PubMed] [Google Scholar]
- 23. Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458‐1471. doi: 10.1093/brain/aww046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marantidou A, Levy C, Duquesne A, et al. Steroid requirements during radiotherapy for malignant gliomas. J Neurooncol. 2010;100(1):89‐94. doi: 10.1007/s11060-010-0142-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


