Abstract
Emerging evidence suggests that females are less sensitive than males to the effects of kappa opioid receptor (KOR) ligands across multiple behavioral measures. The effects of the KOR agonist U50,488 and the KOR antagonist aticaprant were assessed on nest building behavior, an ethologically relevant indicator of overall well-being and affect, in adult male and female C57BL/6J mice. Females required a higher dose of U50,488 to suppress nesting, and a higher dose of aticaprant to restore U50,488-induced impairment of nesting. Females also required a higher dose of aticaprant to decrease immobility scores in the forced swim test. Pretreatment with the estrogen receptor modulator tamoxifen at a dose which blocked estrogen receptors, augmented the effect of U50,488 on nesting in female mice, suggesting that estrogen receptors play a key role in attenuating the effects of KOR ligands in female mice. Together, these results suggest that females are less sensitive to KOR mediation, requiring a higher dose to achieve comparable results to males. This behavioral sensitivity, as measured by nesting, may be mediated by estrogen receptors. Together these studies highlight the importance of comparing sex differences in response to KOR regulation on behaviors related to affective states.
Keywords: JNJ-67953964, Aticaprant, Kappa opioid receptors, Sex differences, Antidepressant, Nesting, Estrogen receptors
1. Introduction
Kappa opioid receptors (KORs) are expressed throughout the central and peripheral nervous system and regulate multiple physiological systems (Bruchas et al., 2010; Knoll and Carlezon, 2010; Lutz and Kieffer, 2013; Mansour et al., 1988; Van’t Veer and Carlezon, 2013). Pharmacological activation of KORs promotes dysphoria, aversion, and depression in humans (Pfeiffer et al., 1986; Ranganathan et al., 2012) and corresponding behaviors in rodents (Browne et al., 2020; Browne and Lucki, 2019; Bruchas et al., 2010; Jacobson et al., 2020; Knoll and Carlezon, 2010; Land et al., 2008; Shippenberg et al., 1993; Todtenkopf et al., 2004). In contrast, KOR antagonists produce beneficial effects in rodent tests of negative affect, anhedonia, and anxiety (Jacobson et al., 2020; Knoll and Carlezon, 2010; Van’t Veer and Carlezon, 2013). KOR antagonists also reverse behavioral deficits produced by a variety of stressors and consequently have been proposed as novel therapeutics for the treatment of stress-related disorders (Browne et al., 2020; Browne and Lucki, 2019; Jacobson et al., 2020; Knoll and Carlezon, 2010).
The majority of preclinical studies investigating the impact of KOR function on physiology and behavior have been conducted in males. Emerging evidence suggests that females are nonresponsive, or less sensitive, to the effects of KOR agonists across several different behavioral paradigms relevant to affect (Chartoff and Mavrikaki, 2015). Female rats were less sensitive to the decline in motivation to work for brain stimulation reward, as measured with intracranial self-stimulation (ICSS), following administration of the selective KOR agonist U50,488 relative to males (Conway et al., 2019; Russell et al., 2014). The suppressive effect of the selective KOR agonist U62,066 on social investigation was diminished in female rats relative to males (Varlinskaya et al., 2018). Similarly, infusions of U50,488 directly into the dorsal raphe nucleus increased anxiety-related behavior, illustrated by increased freezing in the resident intruder test, in male California mice but not in female mice (Wright et al., 2018). Consistent with this, female rats required higher doses to discriminate the selective KOR agonist U69,593 from vehicle relative to males (Craft et al., 1998). Additionally, male C57BL/6J mice developed a conditioned place aversion (CPA) to several doses of U50,488, that were ineffective in female mice (Liu et al., 2019). In contrast to this pattern, both male and female C57BL/6N mice produced aversion to the same dose of U50,488 (Abraham et al., 2018), and female California mice formed aversion to a lower dose of U50,488 relative to males (Laman-Maharg et al., 2017; Robles et al., 2014).
Few studies have directly compared the effects of selective KOR antagonists on behavioral measures related to affect between male and female subjects. The KOR antagonist nor-binaltorphimine (nor-BNI) did not reduce immobility in the forced swim test (FST) in stress naïve female California and C57BL/6J mice in contrast to male mice of these strains (Laman-Maharg et al., 2018). Conversely, the KOR antagonist JDTic effectively reversed the anxiogenic effects in the light dark box and the elevated immobility scores in the FST in male and female C57BL/6J mice following peripheral nerve injury (Liu et al., 2019). Additionally, treatment with the KOR antagonist AZ-MTAB blocked anhedonia measured using the sucrose preference test following social defeat in both male and female California mice (Williams et al., 2018). However, dose-response functions were not assessed in these latter studies (Liu et al., 2019; Williams et al., 2018).
The current set of experiments were conducted to determine (1) whether nest building behavior could be used as a measure for KOR activation and (2) whether male and female mice differed in sensitivity to KOR manipulation. Nesting is an innate, spontaneously occurring behavior observed across species (Deacon, 2006). Nesting is considered an ethologically relevant indicator of self-care and an animal’s overall well-being (Deacon, 2012; Deacon, 2006; Gaskill et al., 2013; Jirkof, 2014). As such, impairment in the quality of, or a delay in nest building, by a drug or environmental condition may serve as a viable measure of negative affect and impaired arousal, an important construct of the arousal and regulatory systems domain as defined by the Research Domain Criteria (RDoC) (Cuthbert and Insel, 2010).
It was proposed that female mice would require a higher dose of both the KOR agonist U50,488 and the KOR antagonist aticaprant to induce a comparable behavioral effect to that detected in male mice. Utilizing nesting as the primary endpoint, the effects of the selective KOR agonist U50,488 were assessed in adult male and female C57BL/6J mice. Second, the ability of the selective KOR antagonist aticaprant to block U50,488-induced nesting alterations was tested. As estrogen receptors may tonically affect KOR signaling, essentially protecting females against the actions of KOR manipulation, female mice were administered the estrogen receptor modulator tamoxifen prior to assessing U50,488-induced suppression of nesting to test this hypothesis. Specifically, it has previously been reported that behavioral effects of U50,488 were amplified after ovariectomy (Abraham et al., 2018). Thus, we hypothesized that tamoxifen would increase the effects of U50,488 in female mice by blocking estrogen receptors. Indeed, tamoxifen suppressed estrous behavior in female rodents (Etgen and Shamamian, 1986; McKenna et al., 1992) and acted as an estrogen receptor antagonist in the brain (Etgen and Shamamian, 1986; McKenna et al., 1992; Pareto et al., 2004; Sumner et al., 1999). Finally, to determine whether a sex difference in the sensitivity to the KOR antagonist aticaprant was specific to nesting, the behavioral effects of aticaprant were also screened in the FST and on locomotor activity. Our findings demonstrated that nesting is a useful test for measurement of KOR activation. Females required higher doses of KOR ligands than males to alter nesting and immobility in the FST. Critically, these studies suggest that estrogen receptors may protect females from the negative consequences of KOR activation but may also reduce the sensitivity of females to the effects of KOR antagonists. These studies highlight the importance of sex differences in the context and utilization of specific behavioral assays, and for incorporating these components into the programmatic development of new therapeutic treatments for stress-related disorders.
2. Methods and materials
2.1. Animals
Male and female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 7 weeks of age and allowed at least 1 week to adjust to the vivarium prior to any experiments. Mice were group housed (4–5 per cage) with standard enrichment of cotton nestlets (Ancare, Bellmore, NY) and huts (Bio-Serv, Flemington, NJ) under a 12-h-light/dark cycle (lights on at 6:00 AM) in temperature and humidity controlled rooms. Food and water were provided ad libitum. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Uniformed Services University Institutional Animal Care and Use Committee.
2.2. Drugs
Aticaprant (also known as JNJ-67953964 (Janssen, Piscataway, NJ), formerly LY-2456302 (Eli Lily, Indianapolis, IN) and CERC-501 (Cerecor, Rockville, MD) and (±)-trans-U-50,488 methanesulfonate salt (U50,488, Sigma-Aldrich, St. Louis, MO, D8040) were freshly prepared prior to use and injected intraperitoneally (i.p.) using a 10 mL/kg injection volume. Aticaprant was dissolved in vehicle (MilliQ water (Millipore, MA), 1% lactic acid (85%, Fisher Scientific, Waltham, MA, A162–500)), sonicated for 15 min and titrated to pH 5. Control mice were administered vehicle. U50,488 was dissolved in MilliQ water and control mice received the water vehicle. Tamoxifen (Sigma-Aldrich, T5648) was dissolved in sesame oil (Sigma-Aldrich, S3547) and was injected subcutaneously (s.c.). Control mice received sesame oil (100 μL).
2.3. Nest building assay
Nest building behavior was assessed in individual standard cages (Ancare N10). Prior to testing with drugs, mice were habituated to the test environment, their individual assessment cages, which remained the same throughout testing, and the nest building procedure for at least 2 consecutive days. The room temperature was 21±1°C. On each habituation day, mice were presented with one full 2.5 g pressed cotton 5 cm square nestlet identical to those given during weekly cage changes, for 5 h. Animals were returned to their normal housing conditions following each habituation session. Food and water were available during all sessions but no additional enrichment was provided. Only animals with final nest scores of at least 2.5 (Supplemental Materials) at all habituation sessions were included in the experiments to ensure those tested consistently built high-quality nests over time. Males always had 2 habituation sessions. In contrast, roughly 1 in 5 females did not manipulate the Nestlet material at all, despite several habituation sessions and were excluded from study. In several of the experiments including females, the number of habituation sessions were increased to determine whether females who did not nest initially would begin to nest following additional habituation sessions. However, nesting was consistent across sessions and scores were not dependent upon additional habituation exposures. Animals were then assigned to treatment groups that ensured habituation nest scores did not differ between groups.
Nests were photographed and visually inspected without disturbing the animal every 30 min. Scoring was performed by a rater that was blind to the treatment conditions as described previously (Deacon, 2006) but with several modifications (Supplemental Materials). We designed a 5-point rating scale of 1–5 with systematic increments of 0.25 (Figure 1a and Supplemental Table 1). To characterize the nest score, the amount of material torn up, the size and shape, wall height, and amount of nest material manipulated in a manner to produce “fluffy” pieces, were all considered. Higher scored nests were comprised of torn off Nestlet pieces that were more shredded and handled in a way to create soft and light pieces that with a “fluffier” appearance, ultimately increasing the volume and complexity of the nest.
Figure 1.
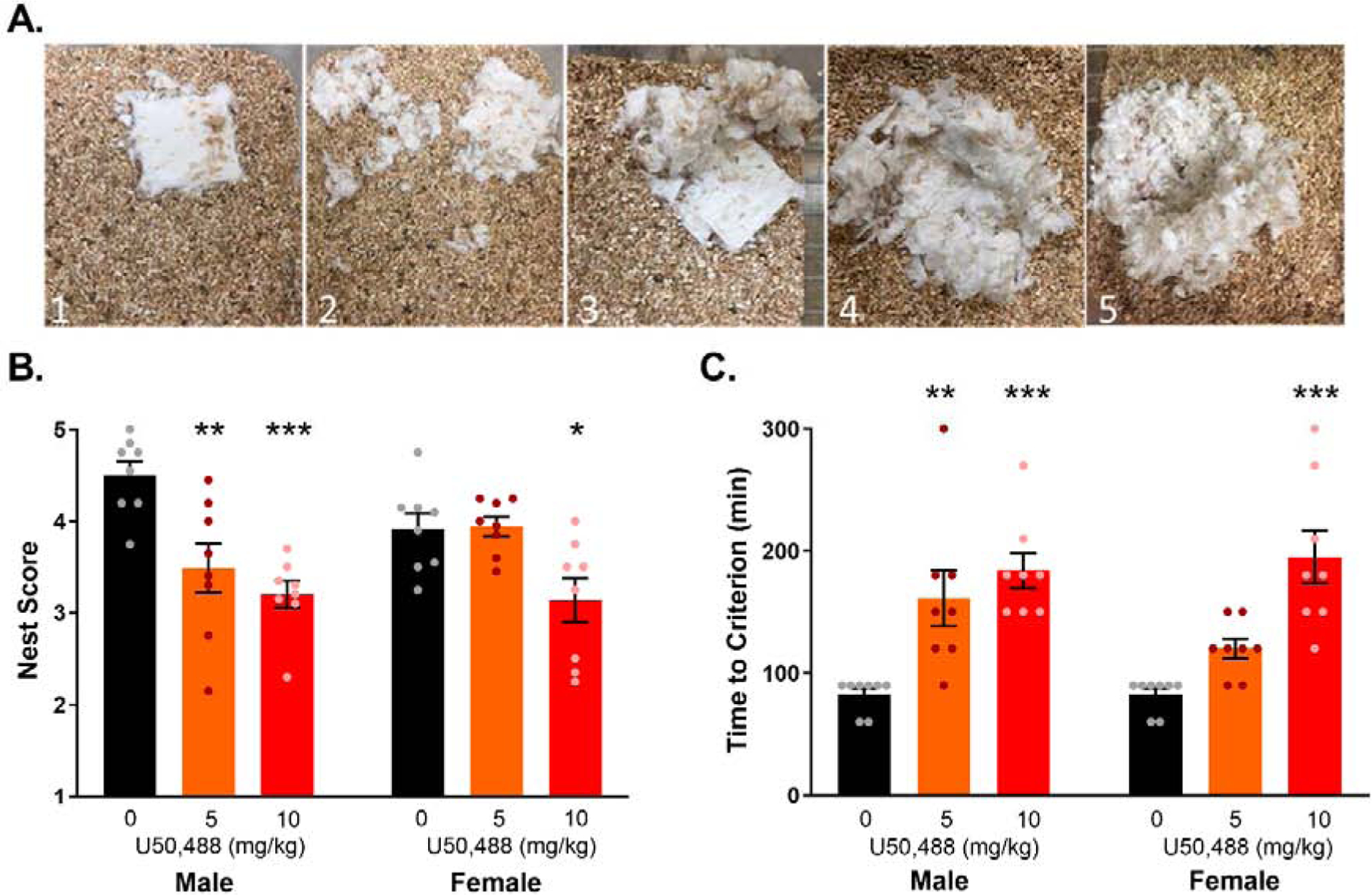
Females required a higher dose of the KOR agonist U50,488 to suppress nest building behavior. (A) Representative nest scores. A score of 1 is 0% torn up. A score of 2 consists of 20–50% torn up nestlet, material can be spread around the cage, or in one location but no clear nest is visible, and the material is not “fluffy”. A score of 3 consists of a nestlet 50% torn up with a clear nest location, but the nest is not dome-shaped and may not be “fluffy”. A score of 4 consists of >75% of the nestlet torn up with a clear dome-shaped nest location, some “fluffy” material, with walls partially formed, but below body height. A score of 5 is a perfect nest, where the nestlet is 100% torn up, with a dome shaped nest, 100% “fluffy” material, and >50% of the walls are higher than the height of the mouse. (B) Average final nest scores. The finalized nest scores were significantly lower in subjects exposed to U50,488, and a higher dose was required for suppression in females. (C) Time to a criterion score (in minutes). The time to reach the criterion score of 2.5 was significantly longer in subjects exposed to U50,488, and a higher dose was required to increase latency to criterion in female mice. n = 8 per group. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with vehicle (0 mg/kg) of the same sex.
The main dependent variables of nesting included the composite finalized nesting score and the time it took for mice to initiate nest building. The average nest score was calculated over the last 2 h of nesting to represent the finalized nest scores. The latency for nest building was defined as the time required for the nest to reach the criterion score of 2.5. At this score, subjects were fully engaged in the nest building process. If mice had not reached this score by 5 h, a value of 300 min was given.
2.4. Forced swim test (FST)
The FST was conducted as previously described (Browne et al., 2018; Lucki et al., 2001). Individual mice were gently placed in a cylinder of water (21 cm in diameter), filled with 15 cm of water (25 ± 1°C), for 6 min. Water was changed between each animal. Blind raters manually scored bouts of immobility during the full 6 min of the test from video recordings. Immobility was defined as the absence of movement, except that necessary to maintain the head above the water. The data are presented as immobility (s) during the final 4 min of the test.
2.5. Locomotor activity
Mice were monitored in an open field (40 × 40 cm) with opaque walls and an illumination of 7 lux. Each apparatus had an overhead camera connected to a computer with Any-Maze tracking software (Stoelting). Mice were individually placed into a corner of the apparatus to start, and the software recorded the total distance travelled (in m) for each animal.
2.6. Experimental procedures
Behavioral tests were conducted during the light cycle. Nest assessments began 4 h following commencement of the light cycle, when nesting behavior has been reported to be high (Heller et al., 2014; Jirkof et al., 2012; Jirkof et al., 2013). Individual cohorts of mice were utilized for each experiment, and mice received treatments with drugs only once. In the first cohort, mice were given U50,488 (0, 5, or 10 mg/kg) immediately prior to the nest building assay (n=8 per group). Then across two cohorts per sex, mice were given aticaprant (0, 1, 3, or 10 mg/kg) 24 h prior to treatment with U50,488 (0 or 10 mg/kg) and then nest building was immediately assessed (n=9–21 per group). Another cohort of female mice were injected with tamoxifen (400 μg in 100 μL sesame oil (equivalent to a 20 mg/kg dose in a 20 g mouse, subcutaneously (s.c.))) 1 h prior to treatment with U50,488 (0 or 5 mg/kg) on the nest building assay (n=8–11 per group). The average weight of each group was 20 g (average weight of all animals combined in the tamoxifen experiment was 20.11 g ± 0.15). The dose and timing of tamoxifen administration was chosen based on prior studies (Reid et al., 2014; Sthoeger et al., 1994; Sumner et al., 1999; Zou et al., 2002) with the peak plasma concentration estimated at 1–4 h following 20 mg/kg tamoxifen s.c. in female mice (Reid et al., 2014). Animals were habituated to s.c. injections for 3 d prior to the tamoxifen experiment. A separate cohort of male mice served as an internal control for the highest dose of aticaprant (10 mg/kg) on nesting (n=11–12 per group). Nesting was assessed for 5 h in all experiments.
Additional cohorts of mice were tested in the FST and locomotor activity. Aticaprant (0, 1, or 3 mg/kg) was administered 24 h prior to the FST (n=7–9 per group). Another cohort of mice were administered aticaprant (0, 1, 3 or 10 mg/kg) 24 h prior to a 30 min exploratory period in the open field (n=10 per group). Finally, a separate group of mice received U50,488 (0, 5, or 10 mg/kg) immediately prior to a 3 h exploratory period in the open field (n=8 per group).
2.7. Statistical analyses
Statistical analyses were performed using GraphPad Prism 7 for Windows (Graph Pad Prism, San Diego, CA) with alpha set at 0.05. Two-way ANOVAs were followed with Tukey’s test for multiple comparisons to analyze interactions between sex and drug treatment or tamoxifen pre-treatment and U50,488 post-treatment. Where appropriate, planned within sex comparisons with Tukey multiple comparisons were used to determine significant effects by dose. A three-way repeated ANOVA (time x sex x drug treatment) with individual ANOVAs for both time points was used to analyze open field data in response to U50,488. Effect sizes were reported as Cohen’s d. Values are presented as means ± SEM.
3. Results
3.1. Kappa opioid receptor activation impaired nesting behavior more in male than female mice
The general effect of KOR activation with U50,488 on nest building behavior was assessed over time in male and female mice. The finalized nest score measured over the last 2 h of nesting was dose-dependently suppressed by U50,488, with a significant treatment by sex interaction (F(2,42)=3.81, p=0.03) (Figure 1b). In male mice, exposure to either 5 mg/kg (p=0.005, d=1.6) and 10 mg/kg (p=0.0002, d=3.2) U50,488 suppressed nest scores. In female mice, exposure to 10 mg/kg (p=0.05, d=1.3) but not 5 mg/kg (p>0.99, d=.05) U50,488 reduced nest scores. Nest scores of vehicle-treated mice did not differ between sexes (p=0.26).
U50,488-exposed animals also took longer to initiate nesting as indicated by a delay in reaching the criterion score of 2.5 (Figure 1c). There was a significant effect of treatment (F(2,42)=26.44, p<0.0001), but no sex by treatment interaction (ns) or effect of sex alone (ns). Planned comparisons showed that male mice that received both 5 mg/kg (p=0.001, d=1.7) and 10 mg/kg (p<0.0001, d=3.3) U50,488 took longer to reach the criterion score relative to control mice. In females, treatment with 10 mg/kg (p<0.0001, d=2.5) increased latency to reach the criterion nest score compared to vehicle-treated controls. Criterion score latencies of female mice exposed to 5 mg/kg U50,488 did not differ from controls (p=0.17, d=2.0).
The time course of nest building behavior is shown in Supplemental Figures 1a and 1b. Across all experiments, nest scores plateaued in vehicle-treated subjects at 2–3 h (Supplemental Figures 1a–d). Nest scores were fully suppressed by exposure to U50,488 for the first 2 h, but increased gradually over the course of 5 h.
3.2. Aticaprant blocks nesting suppressed by U50,488
The 10 mg/kg dose of U50,488 was chosen for this experiment because it fully suppressed nesting in both sexes. As such, male and female mice were examined under conditions where KOR activation elicited a similar behavioral response to U50,488. The KOR antagonist aticaprant (0, 1, 3, or 10 mg/kg) was administered 24 h prior to U50,488 (0 or 10 mg/kg), after which animals were placed in their respective nesting cages for 5 h.
For the finalized nest scores, there was a significant effect of aticaprant treatment (F(4,146)=12.53, p<0.0001), but no treatment by sex interaction (ns) or effect of sex (ns) (Figure 2a). Planned within sex comparisons indicated that pretreatment with aticaprant 3 mg/kg (p=0.60, d=0.7) and 10 mg/kg (p>0.99, d=.07), but not 1 mg/kg (p=0.002, d=2.0) fully blocked U50–488 induced nest building suppression when compared to controls. Male mice pretreated with either 3 mg/kg (p=0.04, d=1.1) or 10 mg/kg (p=0.006, d=1.8) aticaprant had significantly higher nest scores relative to male mice given U50,488 alone. In contrast, only the highest dose of aticaprant (10 mg/kg) prevented U50,488-induced deficits in nest building behavior in female mice when compared to controls (p>0.99, d=0) and also differed significantly from female mice given U50,488 alone (p=0.02, d=1.3).
Figure 2.
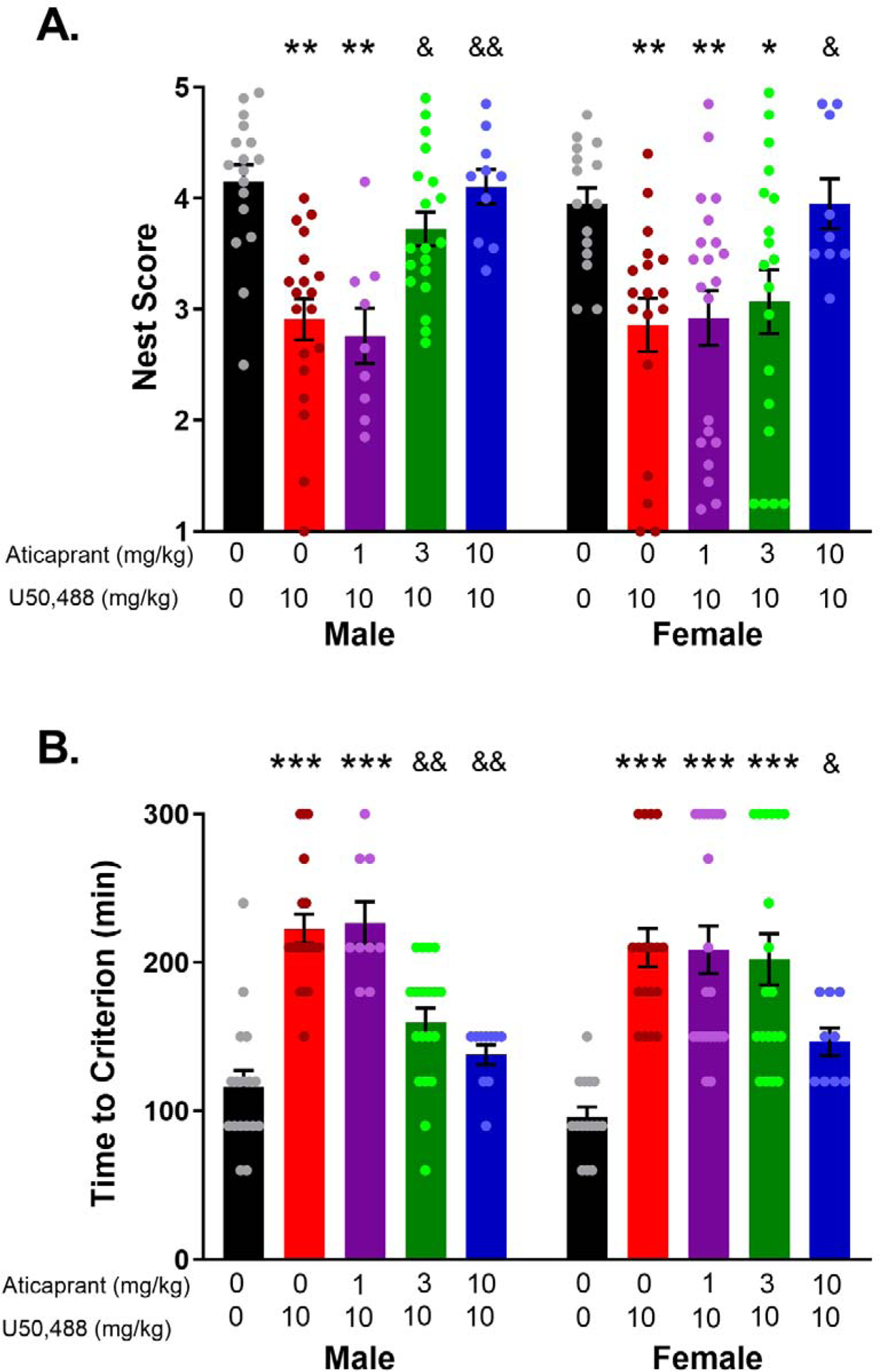
Prevention of U50,488-induced suppression of nesting by pretreatment with the KOR antagonist aticaprant 24 h prior to testing. (A) Finalized nest scores. Female mice required a higher dose of aticaprant to achieve a comparable blockade of the response to U50,488 as males. (B) Time to a criterion score (min). U50,488-induced increase in latency to reach a nest score of 2.5 was blocked by pretreatment with aticaprant. Female mice required a higher dose of aticaprant to achieve comparable results as males. The sample sizes were as follows, for males: vehicle/vehicle, n=17; vehicle/U50,488, n=19; aticaprant (1 mg/kg)/U50,488, n=9; aticaprant (3 mg/kg)/U50,488, n=19, aticaprant (10 mg/kg)/U50,488, n=10, and for females: vehicle/vehicle, n=15; vehicle/U50,488, n=18; aticaprant (1 mg/kg)/U50,488, n=21; aticaprant (3 mg/kg)/U50,488, n=19, aticaprant (10 mg/kg)/U50,488, n=9.*p < 0.05, **p < 0.01, ***p <0.0001 compared with vehicle of the same sex. &p < 0.05, &&p < 0.01 compared with animals pretreated with vehicle prior to U50,488 of the same sex.
For the latency to reach criterion score, there was a significant effect of treatment (F(4,146)=26.33, p<0.0001), a trend for a treatment by sex interaction (F(4,146)=2.29, p=0.06) and no effect of sex (ns) (Figure 2b). Male mice exposed to U50,488 took significantly longer to reach a nest score of 2.5 relative to the controls (p<0.0001, d=2.4). Pretreatment with 1 mg/kg aticaprant did not block the U50,488-induced delay to reach the criterion score relative to controls (p<0.0001, d=2.5), but pretreatment with either 3 mg/kg (p=0.10, d=1.0) or 10 mg/kg (p=0.84, d=0.6) aticaprant significantly blocked U50,488 effects on the criterion score. Additionally, male mice pretreated with 3 mg/kg (p=0.002, d=1.5) and 10 mg/kg (p=0.0005, d=2.5) aticaprant differed significantly from those given U50,488 alone. Female mice pretreated with 10 mg/kg aticaprant initiated nesting at a rate more equivalent to their controls based on the criterion score (p=0.14, d=1.9) and differed significantly from those administered U50,488 alone (p=0.03, d=1.5). No other dose of aticaprant altered the effects of U50,488 on the time to reach the criterion score in females.
Aticaprant (10 mg/kg) 24 h prior to a vehicle injection had no effect on nesting measures relative to controls not exposed to U50,488 (ns). (Supplemental Figure 2). Collectively, these data illustrated that a higher dose of aticaprant was necessary to fully block U50,488-induced nesting alterations in females relative to males.
3.3. Aticaprant reduced immobility in the FST
To assess whether there were sex differences in the effects of aticaprant on another behavioral test, a separate group of mice were tested in the FST (Figure 3). There were significant main effects for sex (F(1,41)=8.31, p=0.006), treatment (F(2,41)=11.84, p<0.0001) and a significant treatment by sex interaction (F(2,41)=7.48, p=0.002) on immobility scores. In males, treatment with 1 mg/kg (p=0.0009, d=2.1) and 3 mg/kg (p=0.008, d=2.3) aticaprant significantly reduced immobility relative to vehicles. In females, by contrast, only 3 mg/kg aticaprant significantly lowered immobility scores in the FST (p=0.03, d=1.6). Vehicle-treated females exhibited significantly lower immobility levels relative to vehicle-treated males (p=0.009).
Figure 3.
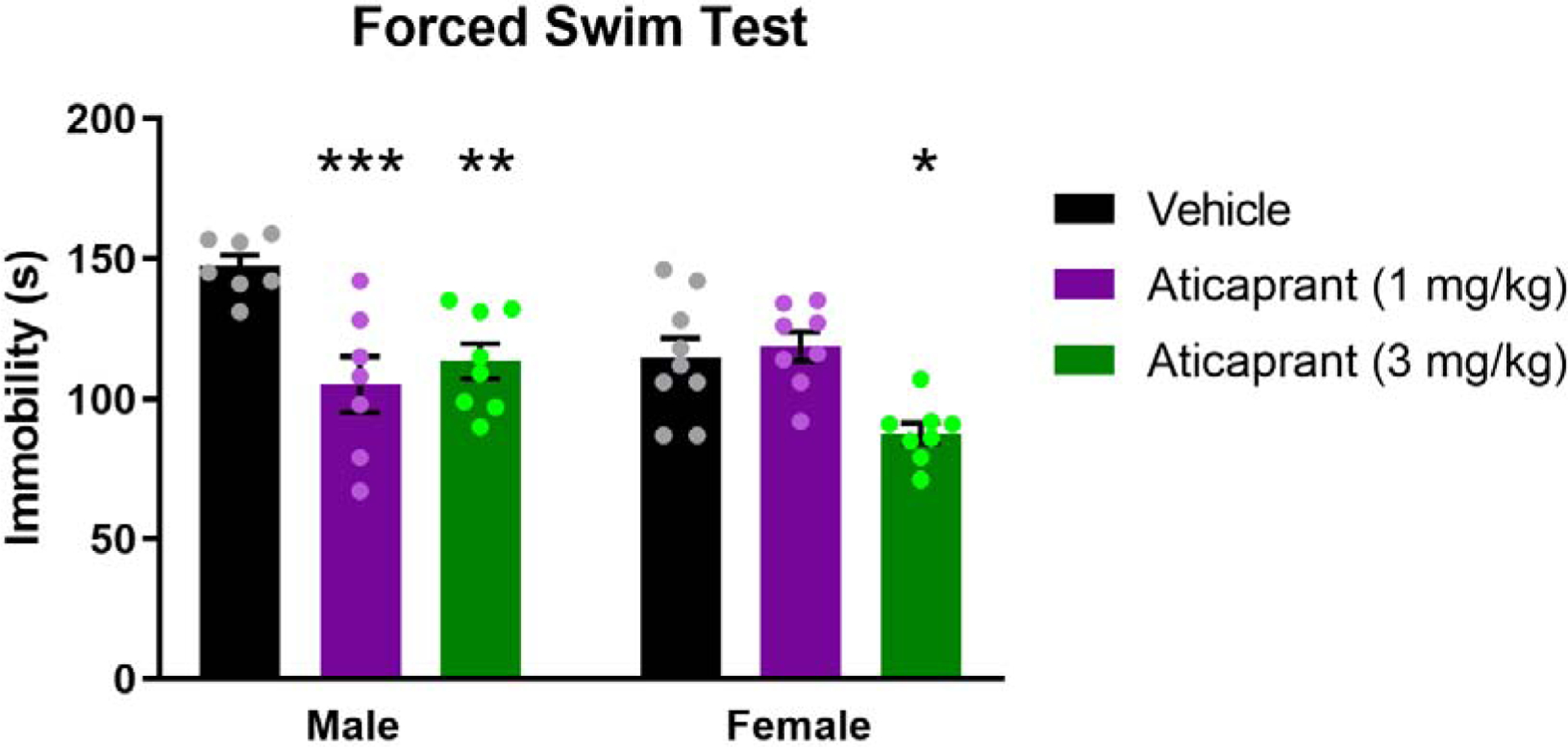
Effects of aticaprant on time spent immobile in the forced swim test (FST). Aticaprant pretreatment was given 24 h prior to testing. Aticaprant decreased the time spent immobile on the FST, and female mice required a higher dose to decrease immobility (in seconds). Female mice spent less time immobile than males. The sample sizes were as follows for males: vehicle, n=7; aticaprant (1 mg/kg), n=7; aticaprant (3 mg/kg), n=8, and for females: vehicle, n=9; aticaprant (1 mg/kg), n=8; aticaprant (3 mg/kg), n=8. **p < 0.01, ***p < 0.0001 compared with vehicle of each sex.
3.4. Effects of U50,488 or aticaprant on locomotor activity
Alterations in locomotor activity were assessed for 3 h after U50,488 (0, 5, or 10 mg/kg) administration (Supplemental Table 2). Data over the course of the 3 h session was divided into two 90 min components. There was a significant time and treatment interaction (F(2,42)=42.25, p<0.0001) and a significant effect of time (F(1,42)=26.50, p<0.0001). Specifically, U50,488 reduced locomotion (F(2,42)=38.75, p<0.0001) during the first 90 min component in both sexes. However, there was no significant suppression of locomotor activity by U50,488 during the second component of testing, from 90–180 min (F(2,42)=0.82, p=0.45). There were no interactions for either time component (ns), but there were significant effects of sex (p<0.0001), where females were more active overall than males.
To ensure that reductions in immobility in the FST and nest building behavior were not due to any changes in locomotion induced by aticaprant (0, 1, 3, or 10 mg/kg), a separate cohort of animals were tested in the open field for a 30 min duration starting 24 h post aticaprant injection. No significant effects of aticaprant treatment or a sex by treatment interaction were identified (ns) (Supplemental Table 3). However, there was a significant main effect of sex for total distance moved in the apparatus (F(1,72)=3.95, p=0.05). Overall, female mice were more active than male mice in the open field.
3.5. Suppression of nest building by U50,488 in females is estrogen sensitive
The hypothesis that estrogen receptors participated in limiting the effects of KOR activation was evaluated on the nesting response by assessing whether pretreatment with the estrogen receptor modulator tamoxifen induced responsiveness to a subthreshold dose of the KOR agonist U50,488. For the finalized nest score, there was a significant pre-treatment (tamoxifen) by post-treatment (U50,488) interaction (F(1,34)=5.59, p=0.02). (Figure 4). When tamoxifen was administered 1 h prior to U50,488, the KOR agonist reduced final nest scores relative to tamoxifen/vehicle-treated animals (p=0.0003, d=2.1). Replicating findings from the initial experiments above, female mice given vehicle/U50,488 (5 mg/kg) did not differ significantly from the vehicle/vehicle-treated group (p=0.76, d=0.5). Thus, blockade of estrogen receptors during KOR activation augmented the effects of U50,488 to fully suppress nesting in females at a dose that was previously ineffective, while having no effect on nesting when given alone.
Figure 4.
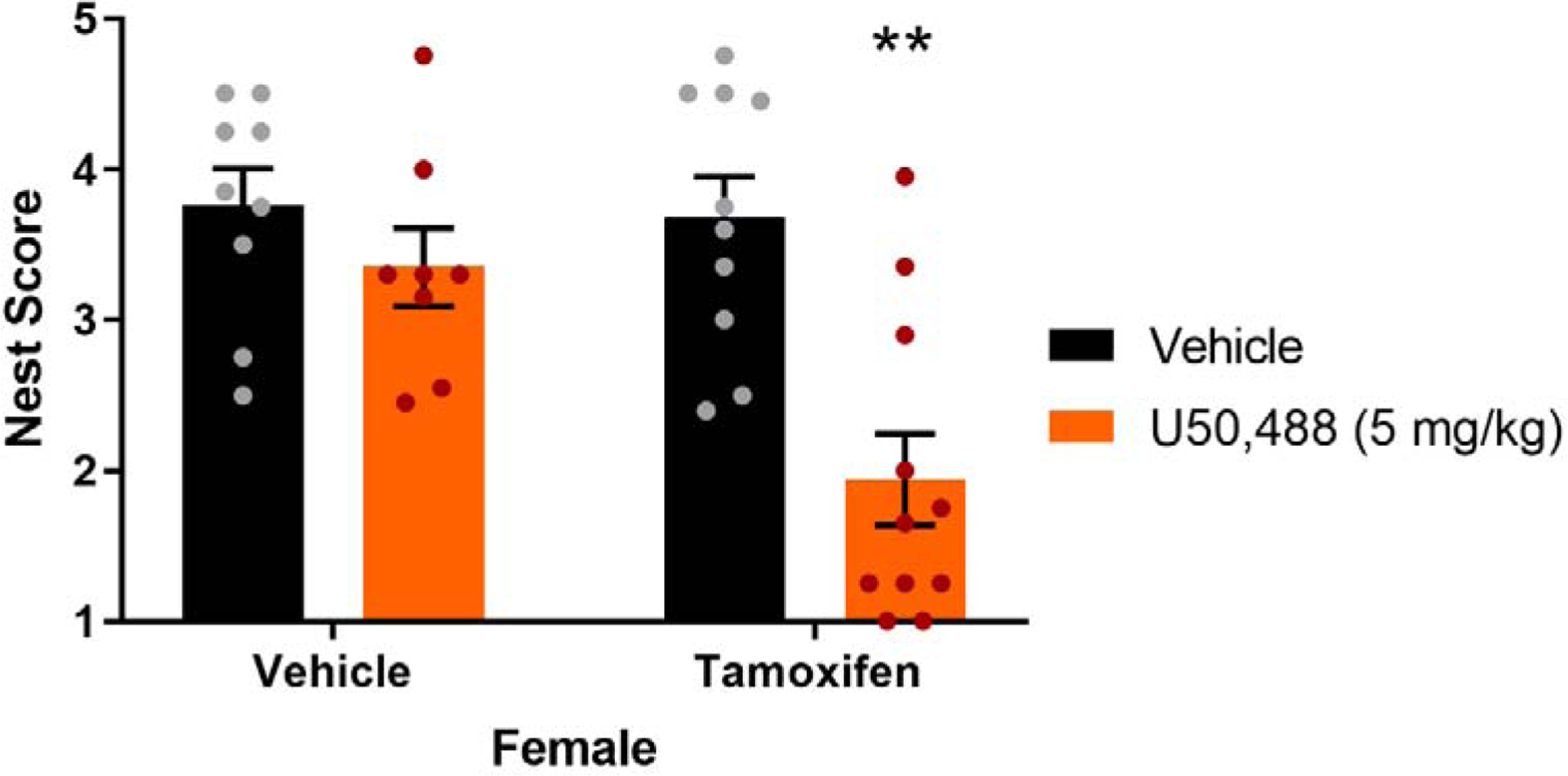
The estrogen receptor modulator tamoxifen (400 μg) suppressed nesting in female mice when given 1 h prior to U50,488 (5 mg/kg). In contrast, U50,488 was unable to reduce nesting when administered to vehicle-treated female mice. The sample sizes were as follows: vehicle/vehicle, n=9; vehicle/U50,488, n=10; tamoxifen/vehicle, n=8; tamoxifen/U50,488, n=11. ** p < 0.001 between tamoxifen and U50,488-treated groups.
4. Discussion
The current set of experiments examined nest building as a behavioral measure sensitive to KOR activation in male and female mice. Female mice were less sensitive to the effects of changes in KOR ligands relative to their male counterparts as they required higher doses of the KOR agonist U50,488 and the KOR antagonist aticaprant to produce comparable behavioral alterations in nesting and immobility scores in the FST. Blockade of estrogen receptors by tamoxifen increased the responsiveness of females to the effect of U50,488 on nesting. This is the first report to indicate that the sensitivity of females to the negative behavioral consequences of KOR activation is estrogen receptor dependent.
4.1. Nesting is sensitive to alterations in KOR signaling in both sexes, but females require higher doses
The disruptions of nesting behavior used to study alterations in response to KOR activation in both sexes may reflect disruptions of overall well-being. Nesting is a complex, naturally occurring, goal-directed behavior, which is ethologically conserved across species (Deacon, 2006; Gaskill et al., 2013; Jirkof, 2014) and closely resembles the “activities of daily living” of humans (Deacon, 2012). Nesting is a highly motivated process, and nesting material is greatly valued by rodents (Gaskill et al., 2012; Jansen et al., 1969; Roper, 1973; Van de Weerd et al., 1998). Indeed, rodents will work by lever pressing or lifting weighted barriers in order to gain access to nesting material (Manser et al., 1998; Roper, 1973). Furthermore, stress exposure impairs nesting in male (Dournes et al., 2013; Otabi et al., 2016, 2017; Woo et al., 2018) and female mice (Gjendal et al., 2019; Newman et al., 2019). Thus, disruptions of nesting reflected a failure to engage in a rewarding species-typical behavior and was used to measure the effects of KOR activation, consistent with reports that KOR agonists induce negative affective states.
In order to properly build nests, mice integrate a series of complex behaviors that rely on the functional integrity of sensorimotor systems. A novel standardized scoring scale measured the quality of the nest, that was derived, in part, from previously published methods (Deacon, 2006). The quality of the nest was scored by the amount of nestlet torn up, the nest size, shape, and height, and how much of the torn Nestlet material was manipulated to create soft and highly deconstructed “fluffy” pieces (Supplemental materials). The criterion score of 2.5 was obtained by mice that were fully engaged in the nest building process. Latency to criterion score and the finalized nest scores obtained after 5 h were the primary endpoints in this assay. Novel nest criteria were designed for these experiments because criteria in the literature did not account for the wide variations of behavior possible in nest building studies. For example, nests consisting of only 50% of the Nestlet torn up may be highly manipulated and elaborate with high walls all in the same location. This nest type is common but could be assigned a low score based on previous rubrics. Those rubrics typically require a majority of the Nestlet to be torn up but spread around the cage to reach a score of 3, which was a nest building behavior rarely observed. Thus, direct comparison with data from obtained with previous rubrics should be considered with these caveats in mind. The suppression of nesting by KOR activation has previously been reported in male mice, although a different KOR agonist (U69,593), time point (100 min), and endpoint (gathering and consolidation of nesting material previously separated into six zones) were used (Negus et al., 2015). Although U50,488 has locomotor-suppressing effects initially after injection (Supplemental Table 2 and (Robles et al., 2014), the reduction in nesting in this study was not due simply to motor impairment because decreased locomotor activity was not detected beyond 2 h post injection, whereas deficits in nest building persisted up to 5 h post U50,488 administration. Nest suppression due to KOR activation was largest in all subjects during the first few hours of the test (see Supplemental Figure 1), but the long-lasting suppression of finalized nest scores remained several hours following KOR activation. In the literature, tests employed to assess KOR activation usually interrogate behavioral changes within only 60 min following U50,488 administration. For example, it has been shown that analgesic responses induced by U50,488 are gone by 60 min (McLaughlin et al., 2006). Although most subjects eventually engaged in nest building behavior, the finished nest products following KOR activation remained lower than controls. Thus, a long-lasting U50,488-induced deficit in nest building is proposed as a measure of a KOR-induced negative affect and a resultant deficit in the arousal systems necessary to appropriately engage with environmental stimuli and partake in homeostatic driven goal-directed behavioral challenge.
Administration of the selective KOR agonist U50,488 increased the latency to criterion score and suppressed finalized nest scores in both male and female mice, but females required a higher dose of U50,488 in order to induce a deficit. To date, no direct sex comparisons on nesting behavior in response to KOR activation have been published. There are, however, other reports that indicated decreased sensitivity of KOR activation in females relative to males in several behavioral tests relevant to affective states. Female rats exhibited reduced sensitivity to U50,488-induced suppression of motivation to work for brain stimulation reward as measured with ICSS (Conway et al., 2019; Russell et al., 2014). Not only did females require a higher dose of U50,488 to induce a deficit with ICSS, but when there was a deficit, the magnitude of the effect was smaller than that of males (Conway et al., 2019; Russell et al., 2014). Similarly, failure to evoke deficits in social interaction have been reported in female rats treated with U62,066 (Varlinskaya et al., 2018) and in female mice treated with U50,488 using both social interaction and resident intruder tests (Wright et al., 2018) . In parallel to the present study, these publications (Conway et al., 2019; Russell et al., 2014; Varlinskaya et al., 2018; Wright et al., 2018) reported a reduced sensitivity of females to KOR-mediated disruptions of rewarding and motivated behaviors, or reductions of behaviors animals naturally partake in. A contrary pattern of sex differences were also reported where female California mice developed a CPA to a lower dose of U50,488 relative to males (Laman-Maharg et al., 2017; Robles et al., 2014). However, others have reported both male and female C57BL/6N mice form place aversion to a higher dose (Abraham et al., 2018) or that female C57BL/6J mice, the same strain used in these studies, did not form a CPA to several doses of the KOR agonist relative to males (Liu et al., 2019). Together these observations highlight the importance of the behavioral context, species, and strain investigated. Collectively, these data indicate that female rodents are less sensitive to the negative behavioral sequela of KOR activation.
4.2. Estrogen receptors may protect females from the negative consequences of KOR activation
Several underlying mechanisms that could drive sex differences in KOR function and behavior have been described (Chartoff and Mavrikaki, 2015; Rasakham and Liu-Chen, 2011). These include interactions with gonadal hormones (Abraham et al., 2018; Clemente-Napimoga et al., 2009; Lawson et al., 2010; Liu et al., 2011), alterations in KOR signaling (Abraham et al., 2018; Laman-Maharg et al., 2018; Martin et al., 2015; Rasakham et al., 2012; Robles et al., 2014; Russell et al., 2014; Wang et al., 2011), KOR and MOR heterodimerization (Chakrabarti et al., 2010; Liu et al., 2011), and dopamine neurotransmission (Conway et al., 2019; Liu et al., 2019; Russell et al., 2014). It is unlikely that the sex effects reported in this study are driven by differences in metabolism, or diffusion of KOR ligands into the brain, as others have reported no sex difference in the pharmacokinetics of KOR agonists (Craft et al., 1998; Russell et al., 2014), and KOR antagonists (Laman-Maharg et al., 2018) in the plasma and brain between males and females. There is substantial evidence for the role of gonadal hormones in mediation of KORs (Chartoff and Mavrikaki, 2015; Rasakham and Liu-Chen, 2011). Circulating estrogen affects KOR signaling (Dawson-Basoa and Gintzler, 1996; Gottsch et al., 2009; Spampinato et al., 1995). Estrogen receptors traffic to the plasma membrane where they associate with G-proteins to mediate activation of membrane signaling cascades associated with KORs. We tested the hypothesis that estrogen receptor modulation by tamoxifen, a selective estrogen receptor modulator (SERM), would sensitize females to the negative consequences of low dose U50,488 in intact, cycling female mice. Tamoxifen can act as an estrogen receptor agonist or antagonist depending on the tissue type, species, and dose employed. Based on prior evidence, we hypothesized that tamoxifen would block estrogen receptors in the brain at the dose we employed (Borgna and Rochefort, 1981; McKenna et al., 1992; Pareto et al., 2004; Sumner et al., 1999). When estrogen receptors were blocked, administration of a previously ineffective dose of U50,488 in females suppressed nesting. Previously, it has been reported that tamoxifen elevated prodynorphin mRNA in ovariectomized (OVX) females, suggesting that estrogen may suppress levels of dynorphin mRNA (Spampinato et al., 1995). In fact, estradiol administration has been shown to decrease immunoreactive dynorphin in OVX rats (Fullerton et al., 1988). This may be a possible mechanism underlying the effect reported in the present study. In line with the findings presented in this manuscript, females were less sensitive to the analgesic effects of KOR agonists compared with males (Rasakham and Liu-Chen, 2011), and these effects were modulated by estrogen (Abraham et al., 2018; Chakrabarti et al., 2010; Dawson-Basoa and Gintzler, 1996; Lawson et al., 2010; Mogil et al., 2003). Specifically, relative to males, females were unresponsive to U50-induced analgesia, but in OVX females, a previously inadequate dose became effective (Abraham et al., 2018). Thus, both analgesia and impaired nesting induced by KOR activation in females appear to be inhibited by estrogen. Estrogen stimulated G protein receptor kinase 2 (GRK2) to sequester Gβγ and block arrestin-independent signaling (Abraham et al., 2018). Although the molecular mechanisms underlying this effect were not investigated here, the signaling response investigated by Abraham & colleagues (2018) may be relevant to nesting as well. In summary, estrogen may be a protectant in females against the negative consequences of KOR activation. More research will be necessary to further build upon the initial results shown here and to build a more complete mechanistic understanding of estrogen receptor modulation of KOR signaling in females.
4.3. The KOR antagonist aticaprant induces antidepressant-like responses in both sexes, but females require a higher dose
Females are more likely to suffer from stress-related disorders including depression, as men (Kessler, 2003). Since depression is associated with endogenous opioid dysregulation, and selective KOR antagonists are currently being considered as novel therapeutics for treatment resistant depression (Browne et al., 2020; Browne and Lucki, 2019; Jacobson et al., 2020), it became valuable to determine if males and females respond similarly to treatment with KOR antagonists (Williams et al., 2018). In the studies presented in this manuscript, both male and female mice responded to the KOR antagonist aticaprant in the FST and exhibited blockade of KOR activation on nest building when aticaprant was administered 24 h prior to testing. It is important to note that this nesting experiment was conducted using the highest dose of U50,488, a condition under which the behavioral effects of U50,488 were not impacted by estrogen receptors. Even under these conditions, female mice required higher doses of aticaprant to exert a behavioral response comparable to males on both tasks. Doses of aticaprant were initially chosen based on previous research showing efficacious behavioral results at 24 h in male mice (Browne et al., 2018) and in vivo receptor occupancy, plasma and brain pharmacokinetics, and behavioral results in male rats and mice at 24 h (Rorick-Kehn et al., 2014). Overall, our results replicated previous findings of aticaprant on the FST in naïve male mice when tested at 24 h (Browne et al., 2018) and others illustrating behavioral effects in tests relevant to affect and motivation in male rodents (Domi et al., 2018; Jackson et al., 2015; Rorick-Kehn et al., 2014). Previous research suggested that female mice were resistant to the immobility-reducing effects of the KOR antagonist nor-BNI on the FST (Laman-Maharg et al., 2018). However, the KOR antagonists JDTic or AZ-MTAB successfully reversed the negative behavioral consequences produced by social defeat stress or peripheral nerve injury in both male and female mice, although only one dose was tested (Liu et al., 2019; Williams and Trainor, 2018). Aticaprant is currently under clinical investigation as a novel therapeutic for major depressive disorder in humans. In one study, male and female adults exhibiting anhedonia from numerous conditions were given daily aticaprant (10 mg/kg) for 8 weeks. Relative to placebo, aticaprant reduced anhedonia and increased nucleus accumbens activation during reward and loss anticipation in a probabilistic reward task (Krystal et al., 2018). In general, our preclinical findings agree with the early clinical research and suggest that aticaprant will ultimately have utility in both male and female subjects. However, careful dosing regimens for male and female subjects should be considered.
5. Conclusion
The results showed how nesting was a useful behavior to assess overall well-being and was sensitive to the behavioral effects of KOR activation in both male and female mice. Females required higher doses of the KOR agonist U50,488 and the KOR antagonist aticaprant to induce comparable behavioral alterations on the nest building assay and forced swim test. Reduced sensitivity to KOR activation on nesting in females may be mediated through an estrogen receptor dependent mechanism. Future studies should investigate mechanisms and neural networks that link delayed nest building and nest score suppression to negative affective states following KOR activation. Further, whether chronic stress impairs nesting as assessed in this manuscript, and if aticaprant restores this suppression, may be warranted as a translational study that bridges to affective disorders. A better understanding of the relationship between negative affect, KORs, and sex, could aid in the development for treatments of stress-related disorders and the progression of aticaprant as a therapeutic.
Supplementary Material
Highlights.
Nest building behavior in mice is useful to investigate overall well-being.
Kappa opioid receptor (KOR) activation impairs nesting in both sexes.
Female mice require higher doses to induce KOR-mediated changes.
Estrogen receptors diminish KOR-mediated alterations in females.
The KOR antagonist aticaprant is effective on the FST in both sexes.
Acknowledgements
This work was supported by US Public Health Service (USPHS) grant R01 MH105623. Aticaprant was a gift from Eli Lily. The authors gratefully acknowledge the valuable contributions of discussions with Drs. Linda Rorick-Kehn and Jeffrey Witkin. We thank Laura Drebushenko for her contribution to the FST and LMA data collection. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no competing financial interests.
References
- Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, Johnson SD, Land BB, Chavkin C, 2018. Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J Neurosci 38, 8031–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgna JL, Rochefort H, 1981. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem 256, 859–868. [PubMed] [Google Scholar]
- Browne CA, Falcon E, Robinson SA, Berton O, Lucki I, 2018. Reversal of Stress-Induced Social Interaction Deficits by Buprenorphine. Int J Neuropsychopharmacol 21, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Jacobson ML, Lucki I, 2020. Novel Targets to Treat Depression: Opioid-Based Therapeutics. Harv Rev Psychiatry 28, 40–59. [DOI] [PubMed] [Google Scholar]
- Browne CA, Lucki I, 2019. Targeting opioid dysregulation in depression for the development of novel therapeutics. Pharmacol Ther 201, 51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C, 2010. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Liu NJ, Gintzler AR, 2010. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A 107, 20115–20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Mavrikaki M, 2015. Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction. Front Neurosci 9, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Napimoga JT, Pellegrini-da-Silva A, Ferreira VH, Napimoga MH, Parada CA, Tambeli CH, 2009. Gonadal hormones decrease temporomandibular joint kappa-mediated antinociception through a down-regulation in the expression of kappa opioid receptors in the trigeminal ganglia. Eur J Pharmacol 617, 41–47. [DOI] [PubMed] [Google Scholar]
- Conway SM, Puttick D, Russell S, Potter D, Roitman MF, Chartoff EH, 2019. Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology 146, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Kruzich PJ, Boyer JS, Harding JW, Hanesworth JM, 1998. Sex differences in discriminative stimulus and diuretic effects of the k opioid agonist U69,593 in the rat Pharmacology Biochemistry and Behavior 61, 395–403. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2010. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull 36, 1061–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Basoa ME, Gintzler AR, 1996. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain 64, 169–177. [DOI] [PubMed] [Google Scholar]
- Deacon R, 2012. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp, e2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, 2006. Assessing nest building in mice. Nat Protoc 1, 1117–1119. [DOI] [PubMed] [Google Scholar]
- Domi E, Barbier E, Augier E, Augier G, Gehlert D, Barchiesi R, Thorsell A, Holm L, Heilig M, 2018. Preclinical evaluation of the kappa-opioid receptor antagonist CERC-501 as a candidate therapeutic for alcohol use disorders. Neuropsychopharmacology 43, 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dournes C, Beeske S, Belzung C, Griebel G, 2013. Deep brain stimulation in treatment-resistant depression in mice: comparison with the CRF1 antagonist, SSR125543. Prog Neuropsychopharmacol Biol Psychiatry 40, 213–220. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Shamamian P, 1986. Regulation of estrogen-stimulated lordosis behavior and hypothalamic progestin receptor induction by antiestrogens in female rats. Horm Behav 20, 166–180. [DOI] [PubMed] [Google Scholar]
- Fullerton MJ, Smith AI, Funder JW, 1988. Immunoreactive dynorphin is regulated by estrogen in the rat anterior pituitary. Neuroendocrinology 47, 1–6. [DOI] [PubMed] [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP, 2012. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One 7, e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR, 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp, 51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjendal K, Ottesen JL, Olsson IAS, Sorensen DB, 2019. Burrowing and nest building activity in mice after exposure to grid floor, isoflurane or ip injections. Physiol Behav 206, 59–66. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA, 2009. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29, 9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HC, Salehi A, Chuluun B, Das D, Lin B, Moghadam S, Garner CC, Colas D, 2014. Nest building is impaired in the Ts65Dn mouse model of Down syndrome and rescued by blocking 5HT2a receptors. Neurobiol Learn Mem 116, 162–171. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Jackson A, Carroll FI, Damaj MI, 2015. Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology 97, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ML, Browne CA, Lucki I, 2020. Kappa Opioid Receptor Antagonists as Potential Therapeutics for Stress-Related Disorders. Annu Rev Pharmacol Toxicol 60, 615–636. [DOI] [PubMed] [Google Scholar]
- Jansen PE, Goodman ED, Jowaisas D, Bunnell BN, 1969. Paper as a positive reinforcer for acquisition of a bar press response by the golden hamster. Psychon. Sci 16. [Google Scholar]
- Jirkof P, 2014. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234, 139–146. [DOI] [PubMed] [Google Scholar]
- Jirkof P, Cesarovic N, Rettich A, Fleischmann T, Arras M, 2012. Individual housing of female mice: influence on postsurgical behaviour and recovery. Lab Anim 46, 325–334. [DOI] [PubMed] [Google Scholar]
- Jirkof P, Fleischmann T, Cesarovic N, Rettich A, Vogel J, Arras M, 2013. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim 47, 153–161. [DOI] [PubMed] [Google Scholar]
- Kessler R, 2003. Epidemiology of women and depression. Journal of Affective Disorders 74, 5–13. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr., 2010. Dynorphin, stress, and depression. Brain Res 1314, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Mathew SJ, Sanacora G, Keefe R, Song A, Calabrese J, Goddard A, Goodman W, Lisanby SH, Smoski M, Weiner R, Iosifescu D, Nurnberger J Jr., Szabo S, Murrough J, Shekhar A, Potter W, 2018. The first implementation of the NIMH FAST-FAIL approach to psychiatric drug development. Nat Rev Drug Discov 18, 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg A, Williams AV, Zufelt MD, Minie VA, Ramos-Maciel S, Hao R, Ordones Sanchez E, Copeland T, Silverman JL, Leigh A, Snyder R, Carroll FI, Fennell TR, Trainor BC, 2018. Sex Differences in the Effects of a Kappa Opioid Receptor Antagonist in the Forced Swim Test. Front Pharmacol 9, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg AR, Copeland T, Sanchez EO, Campi KL, Trainor BC, 2017. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behav Brain Res 332, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C, 2008. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KP, Nag S, Thompson AD, Mokha SS, 2010. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain 151, 806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR, 2011. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal kappa- and mu-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J Neurosci 31, 11836–11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM, Cahill CM, 2019. Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J Neurosci 39, 4162–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ, 2001. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 155, 315–322. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL, 2013. Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser CE, Broom DM, Overend P, Morris TH, 1998. Operant studies to determine the strength of preference in laboratory rats for nest-boxes and nesting materials Laboratory Animals 32, 36–41. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ, 1988. Anatomy of CNS opioid receptors. Trends in Neurosciences 11, 308–314. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sexton T, Kim SA, Severino AL, Peters CM, Young LJ, Childers SR, 2015. Regional differences in mu and kappa opioid receptor G-protein activation in brain in male and female prairie voles. Neuroscience 311, 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna SE, Simon NG, Cologer-Clifford A, 1992. An assessment of agonist/antagonist effects of tamoxifen in the female mouse brain. Horm Behav 26, 536–544. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C, 2006. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology 31, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB, 2003. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans PNAS 100, 4867–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C. [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL, 2015. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 156, 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Covington HE 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF, Miczek KA, 2019. Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biol Psychiatry 86, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otabi H, Goto T, Okayama T, Kohari D, Toyoda A, 2016. Subchronic and mild social defeat stress alter mouse nest building behavior. Behav Processes 122, 21–25. [DOI] [PubMed] [Google Scholar]
- Otabi H, Goto T, Okayama T, Kohari D, Toyoda A, 2017. The acute social defeat stress and nest-building test paradigm: A potential new method to screen drugs for depressive-like symptoms. Behav Processes 135, 71–75. [DOI] [PubMed] [Google Scholar]
- Pareto D, Alvarado M, Hanrahan SM, Biegon A, 2004. In vivo occupancy of female rat brain estrogen receptors by 17beta-estradiol and tamoxifen. Neuroimage 23, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Branti V, Herz A, Emrich HM, 1986. Psychotomimesis mediated by k opiate receptors. Science 233. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC, 2012. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the kappa opioid agonist Salvinorin A in humans. Biol Psychiatry 72, 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, Liu-Chen LY, 2011. Sex differences in kappa opioid pharmacology. Life Sci 88, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, McGillivray KL, Liu-Chen LY, 2012. Sex differences in U50,488H-induced phosphorylation of p44/42 mitogen-activated protein kinase in the guinea pig brain. Neuroscience 223, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Goetz MP, Buhrow SA, Walden C, Safgren SL, Kuffel MJ, Reinicke KE, Suman V, Haluska P, Hou X, Ames MM, 2014. Pharmacokinetics of endoxifen and tamoxifen in female mice: implications for comparative in vivo activity studies. Cancer Chemother Pharmacol 74, 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles CF, McMackin MZ, Campi KL, Doig IE, Takahashi EY, Pride MC, Trainor BC, 2014. Effects of kappa opioid receptors on conditioned place aversion and social interaction in males and females. Behav Brain Res 262, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper TJ, 1973. Nesting material as a reinforcer for female mice. Animal Behaviour 21, 733–740. [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL, 2014. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 77, 131–144. [DOI] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH, 2014. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A, 1993. Examination of the neurochemical substrates mediating the motivational effects of opioids: Role of the mesolimbic dopamine system and D-1 Vs. D-2 dopamine receptors. The Journal of Pharmacology and Experimental Therapeutics 265, 53–59. [PubMed] [Google Scholar]
- Spampinato S, Canossa M, Campana G, Carboni L, Bachetti T, 1995. Estrogen regulation of prodynorphin gene expression in the rat adenohypophysis: Effect of the antiestrogen tamoxifen. Endocrinology 136, 1589–1594. [DOI] [PubMed] [Google Scholar]
- Sthoeger ZM, Bentwich Z, Zinger H, Mozes E, 1994. The beneficial effect of the estrogen antagonist, tamoxifen, on experimental systemic lupus erythematosus. J Rheumatol 21, 2231–2238. [PubMed] [Google Scholar]
- Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G, 1999. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res 73, 119–128. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr., 2004. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172, 463–470. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA Jr., 2013. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 229, 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Weerd HA, Van Loo PLP, Van Zutphen LFM, Koolhaas JM, Baumans V, 1998. Strength of preference for nesting material as environmental enrichment for laboratory mice. Applied Animal Behaviour Science 55, 369–382. [Google Scholar]
- Varlinskaya EI, Spear LP, Diaz MR, 2018. Stress alters social behavior and sensitivity to pharmacological activation of kappa opioid receptors in an age-specific manner in Sprague Dawley rats. Neurobiol Stress 9, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY, 2011. Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther 339, 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, Trainor BC, 2018. Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog Neuropsychopharmacol Biol Psychiatry 86, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Trainor BC, 2018. The impact of sex as a biological variable in the search for novel antidepressants. Front Neuroendocrinol 50, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H, Hong CJ, Jung S, Choe S, Yu SW, 2018. Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Mol Brain 11, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EC, Parks TV, Alexander JO, Supra R, Trainor BC, 2018. Activation of kappa opioid receptors in the dorsal raphe have sex dependent effects on social behavior in California mice. Behav Brain Res 351, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Steurer W, Klima G, Obrist P, Margreiter R, Brandacher G, 2002. Estradiol enhances murine cardiac allograft rejection under cyclosporin and can be antagonized by the antiestrogen tamoxifen. Transplantation 74, 354–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


