Abstract
Adoptive transfer of antigen-specific CD4+ and CD8+ T cells is one of the most efficient forms of cancer immunotherapy. However, the isolation of antigen-specific CD4+ T cells is limited because only few tumor-associated helper epitopes are identified. Here, we used T cell antigen receptor gene transfer to target CD4+ T cells against an MHC class I-presented epitope of a model tumor antigen. IFN-γ-producing CD4+ T cells were unable to expand in vivo and to provide help for tumor rejection. In contrast, CD4+ T cells producing high levels of IL-2 expanded in vivo, provided help for cytotoxic T lymphocyte-mediated tumor rejection, and developed T cell memory. The data demonstrate in vivo synergy between T cell antigen receptor-transduced CD4+ and CD8+ T cells specific for the same epitope resulting in long-term tumor protection.
Keywords: T cell antigen receptor gene transfer, tumor immunotherapy
The infusion of allogeneic T cells into patients with leukemia can provide long-term leukemia-free survival. Similarly, the adoptive transfer of Epstein–Barr virus-specific donor-derived T cells has been successfully used to treat Epstein–Barr virus-induced malignancies after allogeneic stem cell transplantation (1, 2) and solid organ transplants (3–5). More recently, it was demonstrated that adoptive T cell transfer into patients conditioned by lymphoablative chemotherapy resulted in strong antitumor effects in melanoma patients (6, 7).
Indirect evidence suggests that the success of the adoptive immunotherapy treatments described above may be related to the use of antigen-specific CD4+ and CD8+ T lymphocytes. For example, in melanoma the clinical response to infusion of CD8+ T cell clones was much less impressive than the effect of adoptive transfer of mixed CD4+ and CD8+ T cells, although a direct comparison in patients after lymphoablative conditioning has not yet been performed (6, 8). Administration of IL-2 can improve the antimelanoma efficacy of transferred CD8+ T cells (9), suggesting an important role of this cytokine that is typically produced by CD4+ T helper cells. Superiority of mixed CD4+ and CD8+ T cells was also suggested by adoptive cell transfer experiments in immunocompromised patients at risk of CMV disease. The cell dose of cloned CD8+ T cells used to control CMV was ≈1,000-fold higher than the dose of CMV-specific CD8+ T cells given together with CMV-specific CD4+ T cells (10, 11).
The generation of tumor antigen-specific CD4+ helper T cells is limited by the paucity of known MHC class II-binding tumor epitopes and the lack of expression of MHC class II molecules on most tumor cells. In contrast, a large number of CD8+ T cell-recognized tumor epitopes presented by MHC class I molecules has been identified, and in many cases cytotoxic T lymphocyte (CTL) clones against such epitopes were isolated. In this study, we explored in a murine model system whether the T cell antigen receptor (TCR) genes of tumor-specific CTL can be transferred to CD4+ T cells to generate MHC class I-restricted, tumor-specific T helper cells. We show that the TCR specific for an H2-Db-presented epitope of influenza nucleoprotein (NP) can be used to produce H2-Db-restricted, NP-specific CD4+ T cells. Adoptive transfer of CD4+ T cells producing high levels of IL-2 and low levels of IFN-γ provides help for CTL-mediated rejection of NP-expressing EL4 tumors, whereas adoptive transfer of helper T cells producing high levels of IFN-γ was ineffective.
Materials and Methods
Mice. C57BL/6 mice were purchased from Harlan (Harlan UK, Loughborough, United Kingdom) and maintained in the animal facility of Imperial College London. All procedures were carried out according to United Kingdom Home Office Regulations.
Peptides, Cells, Cell Lines, and Transfectants. The influenza virus A NP-derived peptide (ASNENDAM, pNP366), the control peptides pMDM100 (YAMIYRNL) derived from the murine double minute (MDM) 2 protein, and pSV9 (FAPGNYPAL) derived from the Sendai virus (12), were synthesized by ProImmune (Oxford). Murine splenocytes derived from C57BL/6 mice were cultured in RPMI medium 1640, 10% FCS/1% penicillin/1% streptomycin/2 mM l-glutamine. Murine bone marrow-derived dendritic cells (DCs) were isolated from C57BL/6 mice and cultured for 6–7 days in the presence of granulocyte/macrophage colony-stimulating factor. The murine lymphoma EL4 tumor cell line expresses H2-Db MHC class I molecules but not MHC class II molecules (13). The stably transfected EL4 NP tumor cells express the influenza virus A NP and were a kind gift from B. Stockinger (National Institute for Medical Research, London). Phoenix-Eco (PhEco) adherent packaging cells (Nolan Laboratory, Stanford University, Stanford, CA) were transiently transfected with retroviral vectors for the generation of cell supernatant containing the recombinant retrovirus required for infection of target cells.
Retroviral Transduction of Murine Splenocytes. The retroviral vector pMX-TCRα-IRES-TCRβ (pMX-F5) was a kind gift from T. Schumacher (Nederlands Kanker Instituut, Amsterdam). The α- and β-chain TCR genes were cloned from the NP-specific CD8+ CTL clone F5 (14). The F5 TCR recognizes the influenza virus A NP (NP366–379) peptide in the context of murine Db MHC class I. The PhEco packaging cell line was cotransfected by calcium phosphate precipitation with the pMX-F5 construct and the pCL-Eco construct. Splenocytes were harvested from C57BL/6 (H2-Db) female mice and activated with conconavalin A (2 μg/ml final concentration) and IL-7 (1 ng/ml final concentration) for 48–72 h. After 48 h, the PhEco supernatant was harvested and used to transduce activated splenocytes by coculture on fibronectin-treated tissue culture plates in the presence of IL-2 (100 units/ml). The murine CD8α gene [a kind gift from R. Zamoyska (National Institute for Medical Research, London)] was cloned into the pMP71-pre retroviral vector [received from W. Uckert (Humboldt University, Berlin) and originally made by C. Baum (Medical School Hannover, Hannover, Germany)].
Purification of TCR-Transduced (TCR-td) CD4+ Cells and TCR-td CD8+ Cells. Transduced splenocytes were stained with antimurine CD4, CD8, NP tetramers, and Vβ11 antibodies [antimurine CD4-FITC, antimurine CD8-allophycocyanin, and antimurine Vβ11-phycoerythrin (PE) antibodies were obtained from Pharmingen and used as directed]. The PE-labeled NP tetramer (Db/ASNENMDAM) was synthesized by Proimmune and was used as directed. Forty-eight hours after transduction cells were sorted into CD4+ and CD8+ populations using PE-labeled anti-CD4 antibodies and anti-PE beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Further enrichment for Vβ11-expressing cells was performed 72 h after transduction.
Functional Assays of TCR-td T Cells. For IFN-γ release assays, 106 DCs were incubated for 30 min in 200 μl of assay medium (RPMI medium 1640 with 5% heat-inactivated FCS) with synthetic peptides. TCR-td CD4+ or TCR-td CD8+ T cells (5 × 104) were incubated with 5 × 104 peptide loaded DCs (1:1 ratio) in duplicate or triplicate. After 24 h, the supernatant was harvested and tested in an IFN-γ ELISA assay by using anti-IFN-γ antibodies (Pharmingen). Further IFN-γ release assays used peptide-loaded EL4 cells or EL4NP cells as targets.
For proliferation assays, 106 stimulator cells were incubated for 1 h in 200 μl of assay medium (as above) with 100 μM synthetic peptide at 37°C. 5 × 104 TCR-td CD4+, or TCR-td CD8+ T cells were incubated with 5 × 104 peptide-loaded DCs (1:1 ratio) or tumor cells in duplicate or triplicate. After 6, 24, or 48 h, 0.5 μCi (1 Ci = 37 GBq) [3H]thymidine was added to each well. The cells were harvested by using a 96-well plate harvester (Amersham Pharmacia), and thymidine incorporation was measured by using a gamma counter (Wallace, Milton Keynes, United Kingdom).
IL-2 secretion of TCR-td T cells was measured by using IL-2-dependent CTLL cells. The stimulation phase of the IL-2 assay was performed exactly as the IFN-γ and proliferation assays, and after 24, 48, or 72 h, the supernatant was harvested. CTLL cells (5 × 103) were added to 50 μl of harvested supernatant and incubated for 24 h before [3H]thymidine pulsing and counting as above.
Adoptive Immunotherapy. C57BL/6 mice (Thy1.2) were conditioned with 600 rad on day –2. Twelve hours later (day –1), the mice were injected s.c. with a tumorigenic dose of EL4 NP cells (3 × 106 cells in 200 μl of PBS). After a further 24 h, mice were injected i.v. with Thy1.1+ TCR-td T cells, Thy1.1+ Mock-transduced (mocktd) T cells, or PBS alone (day 0). Mice were inspected daily and killed when tumor burden exceeded 1 cm2 or if tumor ulceration occurred regardless of tumor size. Representative surviving tumor-free mice, were rechallenged with tumor cells on day +90. These mice were injected in the right lower leg with 1 × 106 irradiated EL4 NP cells. After 5 days, mice were killed, and right and left inguinal and popliteal lymph nodes were stained with anti-Thy1.1 FITC, anti-CD4-allophycocyanin, and anti-Vβ11 PE antibodies before analysis by flow cytometry.
Results
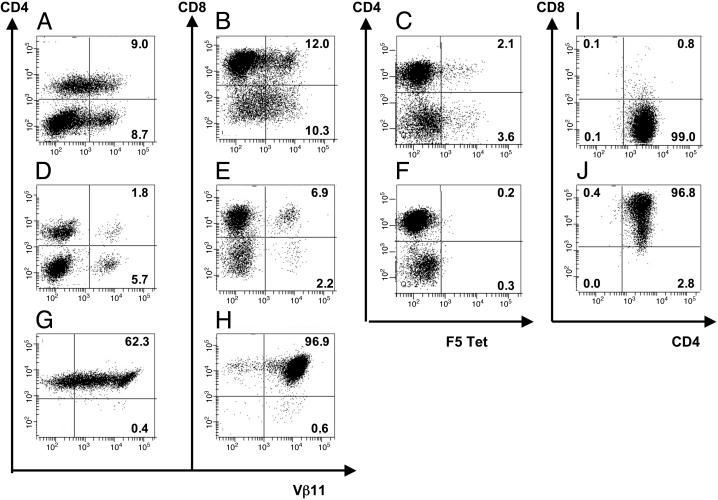
Generation of TCR-td CD4+, CD4+8+, and CD8+ T Cell Populations. Retroviral transduction of C57BL/6 murine splenocytes with the H2-Db-restricted, NP-specific F5 TCR resulted in successful introduction and surface expression of the TCR into CD8+ T cells and CD4+ T cells. Typically, after transduction, 6–12% of viable CD3+ T cells were Vβ11+ CD4+, and 10–15% were Vβ11+ CD8+, as determined by flow cytometry (Fig. 1 A and B). In each experiment, mock-td T cells were used to determine the percentage of CD4+ and CD8+ T cells expressing endogenous Vβ11 (Fig. 1 D and E). Tetramer analysis revealed that the F5 TCR-td splenocytes bound the NP (366–374) H2-Db tetramers, whereas the mock-td splenocytes did not (Fig. 1 C and F). Typically, only 40–50% of transduced T cells expressing the introduced Vβ11 chain were able to bind NP tetramers (Fig. 1, compare B, E, and C). TCR-td bulk T cells were sorted 48 h after transduction into CD4+ and CD8+ cells by using PE-labeled anti-CD4 antibody staining and anti-PE magnetic beads. After 24 h, the sorted populations were further enriched for Vβ11+ cells, again by using magnetic bead selection. The TCR-td CD4+ T cells used in all subsequent experiments contained >99% CD4+ T cells, always with <1% contaminating CD8+ T cells (Fig. 1G). After enrichment, >60% of the CD4+ T cells expressed the Vβ11. The TCR-td CD8+ T cell population was typically ≥95% pure (Fig. 1H). In some experiments, we introduced the CD8α gene together with the F5 TCR genes and purified CD4+ T cells expressing TCR and CD8. Most CD4+ T cells were found to coexpress CD8, as determined by flow cytometry (Fig. 1J). This expression pattern allowed us to assess the functional activity of the NP-specific, class I-restricted F5 TCR in CD4+ T cells and in CD4+ cells that also expressed CD8, referred to as CD4+8+ T cells. Each functional experiment was preceded by flow cytometric analysis to confirm purity of the TCR-td T cell populations.
Fig. 1.
Retroviral gene transfer of TCR chains, purification of TCR-expressing CD4+ and CD8+ T cells, and retroviral gene transfer of CD8α.(A–F) Flow cytometric analysis of viable CD3+ murine splenocytes 2 days after retroviral transfer with F5 TCRα and TCRβ genes (A–C) or mock transduction (D–F). Cells were stained with PE-anti-Vβ11, PE-labeled NP tetramer, FITC-anti-CD4, or allophycocyanin-anti-CD8. (G and H) Flow cytometric analysis of purified CD4+ and CD8+ T cells. Forty-eight hours after transduction, TCR-td bulk T cells were sorted into CD4+ and CD8+ T cells by using magnetic beads, followed by enrichment for Vβ11+ cells 1 day later. Cells were stained with PE-anti-Vβ11, FITC-anti-CD4, and/or allophycocyanin-anti-CD8, and quadrants were set based on anti-CD4 and anti-CD8 stained mock-td cells. (I and J) Flow cytometric analysis of purified TCR-td CD4+ T cells (I) and TCR-td CD4+8+ T cells (J) after retroviral cotransfer of F5 TCRα, TCRβ, and murine CD8α genes. After cotransfer of CD8α, >95% of CD4+ T cells coexpressed CD8α (TCR-td CD4+8+ T cells).
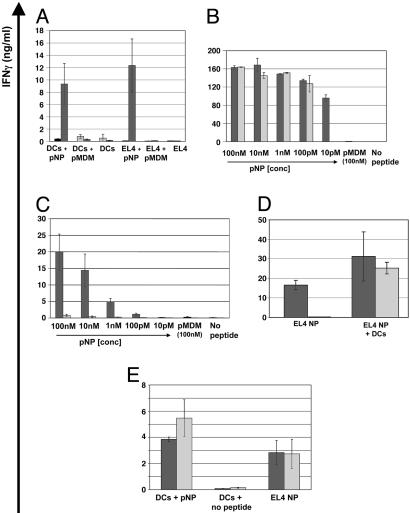
In Vitro Functional Activity of TCR-td CD4+ and CD8+ T Cells. TCR-td bulk T cells secrete IFN-γ in response to syngeneic bone marrow-derived DCs coated with NP peptide (pNP) but not in response to an irrelevant peptide (pMDM-100) or no peptide. Similarly, with peptide-coated EL4 tumor cells as stimulators, the TCR-td bulk T cells produced IFN-γ in response to pNP but not pMDM-100 or no peptide. Mock-td T cells did not secrete IFN-γ in response to any of the above targets. (Fig. 2A). Functional assays were repeated with purified TCR-td CD4+ T cells and TCR-td CD8+ T cells. Using peptide-coated syngeneic DCs as stimulator cells, 10 pM of pNP peptide was required to induce IFN-γ secretion by CD8+ T cells, whereas a 10-fold higher peptide concentration (100 pM) was required to trigger IFN-γ secretion by the CD4+ T cells (Fig. 2B). Once triggered, TCR-td CD4+ T cells secreted amounts of IFN-γ equivalent to the TCR-Td CD8+ T cells when stimulated with DC. However, the TCR-td CD4+ T cells produced little IFN-γ in response to peptide-coated EL4 tumor cells (Fig. 2C) or to EL4 cells transfected with NP (data not shown), whereas the purified TCR-td CD8+ T cells mounted an efficient IFN-γ response to peptide-coated EL4 cells and EL4-NP cells. We explored whether transcostimulation and/or the CD8 molecule could rescue the antitumor IFN-γ response of CD4+ T cells. The addition of syngeneic DCs to the stimulation cultures restored the peptide-specific IFN-γ secretion of CD4+ T cells to EL4-NP tumor cells (Fig. 2D). The same effect was observed with allogeneic DCs, indicating that cross-presentation was not involved, and as few as one DC per 1,000 tumor cells was sufficient (data not shown). The introduction of CD8 into TCR-td CD4+ T cells abolished the DC dependence of the IFN-γ response. Purified TCR-td CD4 +8+ T cells produced IFN-γ when stimulated with EL4-NP tumor cells in the absence of DC. (Fig. 2E).
Fig. 2.
In vitro functional analysis of TCR-td bulk T cells, CD8+ T cells, CD4+ T cells, and CD4+8+ T cells. (A) Peptide-specific IFN-γ secretion of TCR-td bulk T cells (black bars) in response to peptide-loaded DCs and MHC class-II negative peptide-loaded EL4 tumor cells. Mock-td bulk T cells (gray bars) did not secrete IFN-γ in response to any of the targets. (B) Purified populations of TCR-td CD8+ T cells (black bars) and TCR-td CD4+ T cells (gray bars) secreted IFN-γ in response to peptide-loaded syngeneic DCs. TCR-td CD8+ T cells recognized 10 pM peptide, whereas a 10-fold higher concentration of peptide (100 pM) was required to trigger IFN-γ secretion by the TCR-td CD4+ T cells. (C) Using peptide-loaded EL4 tumor cells as targets, TCR-td CD8+ T cells (black bars) mounted an efficient IFN-γ response, whereas the TCR-td CD4+ T cells (gray bars) did not. (D) Transcostimulation by means of the addition of syngeneic DCs rescued the peptide-specific IFN-γ response of TCR-td CD4+ T cells (gray bars) to MHC class II negative NP-expressing EL4 tumor cells (EL4 NP) compared with that observed with TCR-td CD8+ T cells (black bars). (E) Coexpression of murine CD8α abolished the DC dependence of the IFN-γ response. TCR-td CD4+8+ T cells (gray bars) and TCR-td CD8+ T cells (black bars) respond to antigen presented by DCs and EL4 NP.
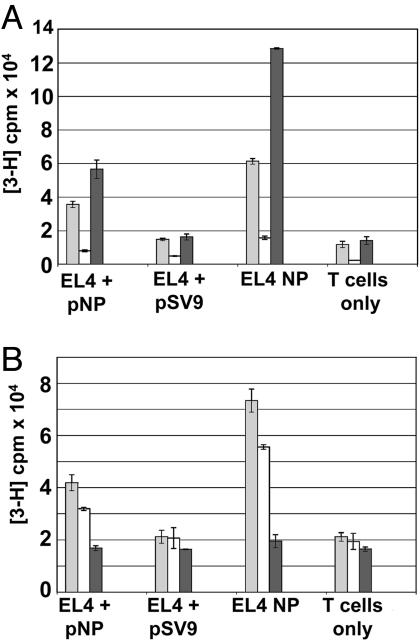
Surprisingly, we found that the ability of T cells to produce IFN-γ did not correlate with their ability to proliferate after stimulation with tumor cells. Despite the antitumor IFN-γ response of TCR-td CD4 + 8+ T cells, these cells proliferated poorly when stimulated with peptide presenting tumor cells (Fig. 3A). The opposite pattern was observed for TCR-td CD4+ T cells, which proliferated efficiently after stimulation with tumor cells, although they produced little IFN-γ (Figs. 2C and 3A). The TCR-td CD8+ T cells efficiently proliferated and produced IFN-γ after stimulation with tumor cells. There was a correlation between the proliferative potential of helper T cells and their IL-2 production. Stimulation with tumor cells triggered high levels of IL-2 production in CD4+ T cells and reduced levels in CD4+8+ T cells (Fig. 3B). However, the CD4+8+ T cells were not defective in IL-2 production, suggesting that the poor proliferation may be related to an antiproliferative effect of IFN-γ produced at high levels by these cells (15, 16).
Fig. 3.
In vitro proliferative potential and IL-2 production of TCR-td CD4+ T cells and TCR-td CD4+8+ T cells. (A) TCR-td CD4+8+ T cells proliferated poorly in response to peptide-presenting tumor cells (white bars) compared with TCR-td CD4+ T cells (gray bars) and TCR-td CD8+ T cells (black bars). The proliferative potential of the TCR-td T cell populations correlated well with IL-2 production. (B) TCR-td CD4+8+ T cells (white bars) secreted reduced levels of IL-2 in response to EL4 NP tumor cells as compared with TCR-td CD4+ T cells (gray bars). TCR-td CD8+ T cells (black bars) did not secrete IL-2 in response to any of the targets. Proliferation of the IL-2-dependent cell line CTLL was used to measure the IL-2 content in the supernatant taken from the stimulated T cells.
TCR-Td CD4+ T Cells Provide in Vivo Help for CD8-Mediated Tumor Rejection. We performed adoptive immunotherapy experiments in a syngeneic setting to determine whether CD4+ T cells with an IFN-γlow/proliferationhigh phenotype or CD4+8+ T cells with an IFN-γhigh/proliferationlow phenotype were more efficient in providing help for tumor rejection. Two groups of 5 C57BL/6 mice received a tumorigenic dose of EL4-NP cells followed by adoptive transfer of 106 purified TCR-td CD4+ or CD4+8+ helper T cells. To assess the helper function in vivo, a further group of five mice also received a small dose of 104 TCR-td CD8+ T cells, which was insufficient to cause tumor rejection in the absence of help. Tumor protection by large numbers of CTL (i.e., 106 TCR-td CD8+ T cells) was T helper cell-independent (data not shown).
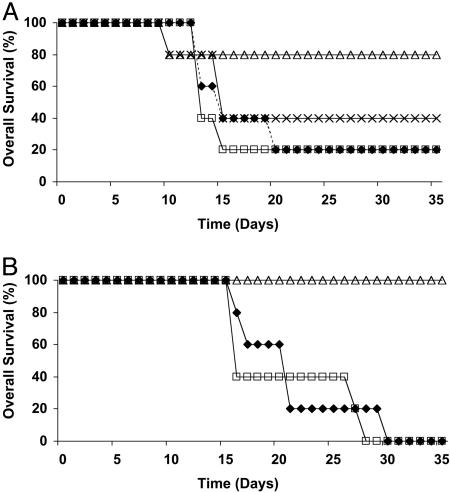
Fig. 4A shows poor survival of EL4-NP tumor-challenged mice that were treated with PBS (n = 5) or mock-td CD4+ T cells (n = 5). A small improvement was seen in the group of mice (n = 5) that was treated with 106 TCR-td CD4+8+ helper T cells together with 104 TCR-td CD8+ T cell. In contrast, substantial protection was seen in the five mice treated with 106 TCR-td CD4+ helper T cells in combination with 104 TCR-td CD8+ T cells. To explore whether the NP specificity of the CD4+ T cells was required, tumor-challenged mice (n = 5) were treated in a separate experiment with 106 mock-td CD4+ T cells combined with 104 TCR-td CD8+ T cells. This resulted in progressive tumor growth in all mice (Fig. 4B). Similarly, treatment of five more mice with TCR-td CD4+ T cells in the absence of CD8+ T cells was ineffective, indicating that protection required the combination of antigen-specific CD4+ T cells together with small numbers of TCR-td CD8+ T cells. Increasing the dose of TCR-td CD8+ T cells from 104 to 2 × 104 improved the level of tumor protection (Fig. 4, compare A with B).
Fig. 4.
TCR-td CD4+ T cells provide in vivo help for CD8 T cell-mediated tumor rejection. C57BL/6 mice received nonmyeloablative radiation (600 rad) and then 12 h later were injected s.c. with 3 × 106 EL4 NP tumor cells. (A) A further 24 h later, mice received 106 TCR-td CD4+ T cells with 104 TCR-td CD8+ T cells (open triangles), 106 TCR-td CD4+8+ T cells with 104 TCR-td CD8+ T cells (crosses), 106 mock-td bulk T cells (open squares), or PBS (diamonds). (B) In a separate tumor protection experiment, mice were conditioned and tumor-challenged as above, followed by transfer of 106 mock-td CD4+ T cells with 104 TCR-td CD8+ T cells (open squares), 106 TCR-td CD4+ T cells with anti-CD8-blocking antibody (filled diamonds), or 106 TCR-td CD4+ T cells with 2 × 104 TCR-td CD8+ T cells (open triangles).
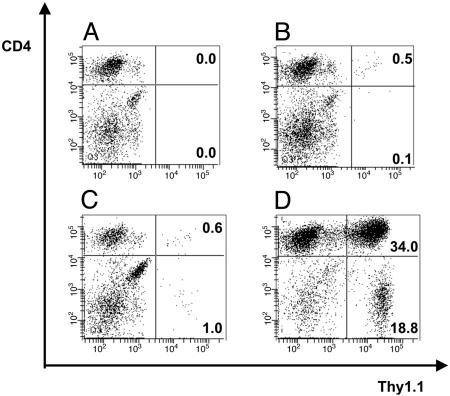
TCR-td CD4+ T Cells Develop Long-Term T Cell Memory. Spleens of all mice used in the tumor challenge experiments in Fig. 4A were analyzed by FACS to determine the number of adoptively transferred Thy1.1+/Vβ11+ TCR-td T cells at the time of antigen exposure in tumor-bearing mice and at later time points to analyze T cell persistence and recall memory responses in tumor-free mice. No Thy1.1+/Vβ11+ T cells were identified in the control PBS-treated mice (Fig. 5A). In tumor-bearing mice treated with mock-td CD4+ T cells, between 0.5% and 1.7% of the gated Vβ11+ cells were the transferred T cells (Fig. 5B). A similar proportion of transferred T cells, 0.5–3.3% of the gated Vβ11+ cells, was seen in three tumor-bearing mice treated with TCR-td CD4 + 8+ T cells, suggesting that there was no antigen-driven expansion of these cells compared with mock-td CD4+ T cells (Fig. 5C). Analysis of a tumor-bearing mouse treated with TCR-td CD4+ T cells showed a large expansion of transferred CD4+ cells (34% of gated Vβ11+ T cells) and an expansion of the cotransferred TCR-td CD8+ T cells (13.6% of Vβ11+ cells) (Fig. 5D).
Fig. 5.
Detection and in vivo persistence of TCR-td T cells in tumor-bearing mice. Shown is the analysis of splenocytes prepared from mice in Fig. 4A at the time they were killed because of tumor burden. Adoptively transferred T cells were identified by triple staining with antibodies specific for Thy1.1, Vβ11, and CD4. Representative examples are shown for each group of mice, and all FACS plots display viable lymphocytes gated on Vβ11+ T cells. (A) No Thy1.1+ Vβ11+ T cells were identified in four tumor-bearing PBS-treated control mice. (B) In four mice treated with mock-td CD4+ T cells, between 0.5% and 1.7% of the gated Vβ11+ cells were the CD4+ Thy1.1+ transferred T cells. (C) Similar numbers of TCR-td CD4+8+ cells were detected in three mice (0.5–3.3%) suggesting a lack of antigen-driven expansion of the TCR-td CD4+8+ as compared with the Mock-td CD4+ T cells. (D) Analysis of the spleen of a tumor-bearing animal that received TCR-td CD4+ T cells showed a large expansion of transferred Thy1.1+ CD4+ T cells (34.0% of gated Vβ11+), together with an expansion of the cotransferred Thy1.1+ CD8+ T cells (13.6% of gated Vβ11+). Staining of lymph node cells of mice in the four treatment groups showed similar levels of transferred Thy1.1+ Vβ11+ CD4+ T cells (data not shown).
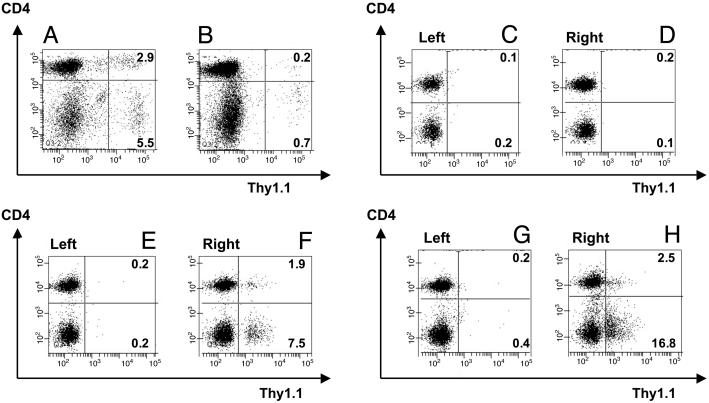
Analysis of tumor-free mice 35 and 90 days after adoptive T cell transfer showed persistence and memory development of TCR-td CD4+ T cells. On day 35, the transferred CD4+ T cells were present at a higher frequency than CD4+8+ cells (Fig. 6 A and B), and CD4+ but not CD4+8+ T cells responded to tumor rechallenge at day 90 (Fig. 6 C–H). The memory response of the TCR-td CD4+ T cells at day 90 was associated with a recall response of the TCR-td CD8+ T cells (Fig. 6 F and H).
Fig. 6.
TCR-td CD4+ T cells persist and develop long-term memory in vivo. Shown is the analysis of splenocytes and lymph node cells prepared from tumor-free mice in Fig. 4A on day 35 or day 95 after T cell transfer. Adoptively transferred T cells were identified by triple staining with antibodies specific for Thy1.1, Vβ11, and CD4, and all FACS plots display viable lymphocytes gated on Vβ11+ T cells. (A and B) A day-35 comparison of splenocytes of mice treated with TCR-td CD4+ (A) and CD4+8+ (B) cells. Similar frequencies of transferred Thy1.1+ CD4+ T cells were detected in lymph node samples (data not shown). (C–H) At day 90, three tumor-free mice were challenged s.c. with 1 × 106 irradiated EL4 NP tumor cells in the right lower leg. After 5 days, ex vivo FACS analysis was used to analyze T cell responses in the tumor-antigen-exposed right inguinal and popliteal lymph nodes, by using the nonexposed left inguinal and popliteal lymph nodes as a baseline control. The TCR-td CD4+8+ T cells did not respond to tumor rechallenge at day 90 (C and D), whereas both mice that received TCR-td CD4+ cells demonstrated an expansion of TCR-td CD4+ and CD8+ T cells in the lymph nodes of the tumor antigen exposed right side (E–H).
Discussion
Several groups have used retroviral TCR gene transfer to produce antigen-specific human and murine T cells (17–27). This strategy provides for the production of tumor-specific T cell populations for adoptive immunotherapy without the need for in vivo immunization and in vitro T cell expansion. In a previous study (20) and in this report T cells were transduced once on day 2–3 after polyclonal activation and subsequently used on days 5–7 for adoptive transfer without any further in vitro expansion. TCR gene transfer also provides a strategy to generate antigen-specific helper T cells for adoptive cancer immunotherapy. This strategy can be achieved by transfer of MHC class II-restricted TCRs into CD4+ cells (19, 27) or, as shown recently, by transfer of MHC class I-restricted TCRs into CD4+ T cells (28). One recent publication showed that TCRs isolated from CD8-independent CTL can be functionally active in CD4+ T cells, and another study showed that the cotransfer of CD8α and a TCR/CD3ζ hybrid construct can be used to produce HLA class I-restricted CD4+ T cells (29, 30). To date, a possible in vivo role of CD4+ T cells transduced with a class I-restricted TCR in tumor protection has not yet been explored.
Here, we have generated MHC class I-restricted helper T cells that respond to antigen-presentation by MHC class II-negative EL4-NP tumor cells. TCR-td CD4+ T cells proliferated and produced high levels of IL-2 but little IFN-γ, whereas CD4+ T cells transduced with the same TCR and CD8α produced high levels of IFN-γ and reduced levels of IL-2 and proliferated poorly when stimulated with tumor cells. The mechanism by which CD8α expression modifies the T cell response is currently unknown. Altered cytokine production might be due to the ability of CD8 to enhance the avidity of TCR/MHC peptide interaction. For example, a recent study demonstrated that high avidity TCR/MHC peptide interactions selectively impaired IL-2 production, which is similar to our observation with CD8-expressing helper T cells (31). Alternatively, the CD8 molecule may alter signal transduction in helper T cells because of its ability to recruit LAT, an adaptor molecule that preferentially associates with CD8 compared with CD4 (32, 33). It is possible that the observed functional changes of transduced CD4+ T cells reflect a feature of CD8α/α homodimers. For example, the expression of CD8α/α homodimers in TCR-td CD4+ cells may result in redirected homing to the gut, as is seen with CD8α/α positive intraepithelial lyphocytes, which might result in low numbers of T cells in lymphoid tissues. In fact, gene transfer into CD4+ T cells will provide an interesting model to dissect functional activities of CD8α/α homodimers and CD8α/β heterodimers in mature T cells.
We show that IL-2high/proliferationhigh CD4+ T cells effectively provide in vivo help for tumor rejection, whereas IFN-γhigh/proliferationlow CD4+ T cells are unable to do so despite having the same specificity. A similar in vivo protective effect has been described for IL-2high/proliferationhigh CD4+ T cells in HIV-infected patients. Large numbers of these CD4+ T cells were observed in patients controlling virus load, whereas the presence of IFN-γhigh/proliferationlow CD4+ T cells was associated with uncontrolled virus replication (34–37).
In addition to the efficient help for tumor rejection, we also showed that IL-2high/proliferationhigh CD4+ T cells persist and readily respond to tumor cell rechallenge 90 days after adoptive transfer. This finding is in line with a recent report showing that murine CD4+ T cells producing little IFN-γ were able to persist in vivo and develop into long-term memory cells, whereas CD4+ T cells producing high levels of IFN-γ survived poorly in vivo (38).
Our experiments suggest that an important mechanism by which proliferation competent helper T cells contribute to tumor immunity is by triggering the in vivo expansion of antigen-specific CD8+ T cells. Given separately, neither antigen-specific helper T cells nor CD8+ T cells were able to mediate protection in tumor-challenged mice. It is likely that antigen-specific IL-2 production by helper T cells at the site of tumor growth may enhance local proliferation and retention of CD8+ T cells, as described previously in a murine tumor model (39).
In our experiments, TCR-td CD4+ and TCR-td CD8+ T cells expanded and persisted at similar frequencies, even when 100-fold more CD4+ T cells were adoptively transferred into the mice. Although this result suggested a competitive advantage for CD8+ T cells, it is possible that some cross-regulation may determine the relative expansion rate of CD4+ and CD8+ cells. For example, the adoptive transfer of 106 TCR-td CD4+ cells along with 104 or 106 TCR-td CD8+ cells (i.e., 100:1 and 1:1 ratios) resulted in similar size CD8+ T cell populations 5 weeks after T cell transfer. A simple feedback loop may involve IL-2 that is provided by TCR-td CD4+ T cells and that controls the extent of expansion of TCR-td CD8+ T cells with the same specificity.
The observation that IFN-γhigh helper T cells were unable to contribute to tumor protection was surprising, considering the important role of this cytokine in preventing tumor growth by inhibition of tumor stroma (40). In addition, IFN-γ can lead to FAS ligand (CD178) up-regulation in T cells and FAS (CD95) up-regulation in target cells, thus enhancing CTL killing of target cells by means of the FAS pathway (41, 42). However, our data do not rule out a role of IFN-γ in tumor immunity. Rather, the data demonstrate that CD4+ T cells, which are unable to expand and persist in vivo, cannot exert antitumor effects even when they produce high levels of IFN-γ. It is possible that the tumor control, which is mediated by CD8+ T cells in our experiments, involves CD8+ T cell-derived IFN-γ.
The ability to produce CD4+ and CD8+ T cells specific for the same tumor antigen provides a powerful model to dissect the mechanisms of in vivo interaction between these T cell populations and provides an opportunity to design strategies to optimally exploit both populations for tumor immunotherapy. In the current experiments, T cells were directed against a model tumor antigen that is not expressed in normal tissues. In contrast, most human T cell-recognized tumor antigens are also expressed in normal cells. High-avidity CTL against such human tumor antigens have been isolated from melanoma patients (6, 43), or they can be isolated from the allogeneic T cell repertoire in cases in which autologous T cells are tolerant (44, 45). In principle, it is now possible to use TCRs isolated from high-avidity CTL to produce antigen-specific CD4+ and CD8+ T cells for adoptive therapy. It will be interesting to assess the antitumor effect of this combination therapy and to explore whether antigen-presentation by quiescent normal tissues poses an autoimmune risk, is ignored, or renders the transferred T cells unresponsive (46).
This study demonstrates that adoptive tumor immunotherapy with CD4+ and CD8+ T cells expressing the same TCR is an attractive concept to harness helper T cell functions and CTL effector functions for tumor eradication and long-term immunity.
Acknowledgments
This work was supported by the Leukaemia Research Fund, Medical Research Council, and Cancer Research UK.
Author contributions: E.C.M. and H.J.S. designed research; E.C.M. and A.T. performed research; G.M.B. and S.-a.X. contributed new reagents/analytic tools; E.C.M., A.T., and H.J.S. analyzed data; and E.C.M. and H.J.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TCR, T cell antigen receptor; TCR-td, TCR-transduced; mock-td, mock-transduced; CTL, cytotoxic T lymphocyte; NP, nucleoprotein; pNP, peptide NP; PE, phycoerythrin; DC, dendritic cell; PhEco, Phoenix-Eco; MDM, murine double minute.
References
- 1.Rooney, C. M., Smith, C. A., Ng, C. Y., Loftin, S., Li, C., Krance, R. A., Brenner, M. K. & Heslop, H. E. (1995) Lancet 345, 9–13. [DOI] [PubMed] [Google Scholar]
- 2.Rooney, C. M., Smith, C. A., Ng, C. Y., Loftin, S. K., Sixbey, J. W., Gan, Y., Srivastava, D. K., Bowman, L. C., Krance, R. A., Brenner, M. K. & Heslop, H. E. (1998) Blood 92, 1549–1555. [PubMed] [Google Scholar]
- 3.Khanna, R., Bell, S., Sherritt, M., Galbraith, A., Burrows, S. R., Rafter, L., Clarke, B., Slaughter, R., Falk, M. C., Douglass, J., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 10391–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque, T., Taylor, C., Wilkie, G. M., Murad, P., Amlot, P. L., Beath, S., McKiernan, P. J. & Crawford, D. H. (2001) Transplantation 72, 1399–1402. [DOI] [PubMed] [Google Scholar]
- 5.Haque, T., Wilkie, G. M., Taylor, C., Amlot, P. L., Murad, P., Iley, A., Dombagoda, D., Britton, K. M., Swerdlow, A. J. & Crawford, D. H. (2002) Lancet 360, 436–442. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg, S. A. & Dudley, M. E. (2004) Proc. Natl. Acad. Sci. USA 101, 14639–14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley, M. E., Wunderlich, J. R., Robbins, P. F., Yang, J. C., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., Sherry, R., Restifo, N. P., Hubicki, A. M., et al. (2002) Science 298, 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley, M. E., Wunderlich, J., Nishimura, M. I., Yu, D., Yang, J. C., Topalian, S. L., Schwartzentruber, D. J., Hwu, P., Marincola, F. M., Sherry, R., et al. (2001) J. Immunother. 24, 363–373. [DOI] [PubMed] [Google Scholar]
- 9.Yee, C., Thompson, J. A., Roche, P., Byrd, D. R., Lee, P. P., Piepkorn, M., Kenyon, K., Davis, M. M., Riddell, S. R. & Greenberg, P. D. (2000) J. Exp. Med. 192, 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter, E. A., Greenberg, P. D., Gilbert, M. J., Finch, R. J., Watanabe, K. S., Thomas, E. D. & Riddell, S. R. (1995) N. Engl. J. Med. 333, 1038–1044. [DOI] [PubMed] [Google Scholar]
- 11.Peggs, K. S., Verfuerth, S., Pizzey, A., Khan, N., Guiver, M., Moss, P. A. & Mackinnon, S. (2003) Lancet 362, 1375–1377. [DOI] [PubMed] [Google Scholar]
- 12.Dahl, A. M., Beverley, P. C. & Stauss, H. J. (1996) J. Immunol. 157, 239–246. [PubMed] [Google Scholar]
- 13.Gorer, P. A. (1950) Br. J. Cancer 4, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend, A. R., Gotch, F. M. & Davey, J. (1985) Cell 42, 457–467. [DOI] [PubMed] [Google Scholar]
- 15.Liu, Y. & Janeway, C. A., Jr. (1990) J. Exp. Med. 172, 1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu, C. Q., Wittmer, S. & Dalton, D. K. (2000) J. Exp. Med. 192, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clay, T. M., Custer, M. C., Sachs, J., Hwu, P., Rosenberg, S. A. & Nishimura, M. I. (1999) J. Immunol. 163, 507–513. [PubMed] [Google Scholar]
- 18.Cooper, L. J., Kalos, M., Lewinsohn, D. A., Riddell, S. R. & Greenberg, P. D. (2000) J. Virol. 74, 8207–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujio, K., Misaki, Y., Setoguchi, K., Morita, S., Kawahata, K., Kato, I., Nosaka, T., Yamamoto, K. & Kitamura, T. (2000) J. Immunol. 165, 528–532. [DOI] [PubMed] [Google Scholar]
- 20.Kessels, H. W., Wolkers, M. C., van den Boom, M. D., van der Valk, M. A. & Schumacher, T. N. (2001) Nat. Immunol. 2, 957–961. [DOI] [PubMed] [Google Scholar]
- 21.Stanislawski, T., Voss, R. H., Lotz, C., Sadovnikova, E., Willemsen, R. A., Kuball, J., Ruppert, T., Bolhuis, R. L., Melief, C. J., Huber, C., et al. (2001) Nat. Immunol. 2, 962–970. [DOI] [PubMed] [Google Scholar]
- 22.Heemskerk, M. H., Hoogeboom, M., de Paus, R. A., Kester, M. G., van der Hoorn, M. A., Goulmy, E., Willemze, R. & Falkenburg, J. H. (2003) Blood 102, 3530–3540. [DOI] [PubMed] [Google Scholar]
- 23.Schaft, N., Willemsen, R. A., de Vries, J., Lankiewicz, B., Essers, B. W., Gratama, J. W., Figdor, C. G., Bolhuis, R. L., Debets, R. & Adema, G. J. (2003) J. Immunol. 170, 2186–2194. [DOI] [PubMed] [Google Scholar]
- 24.Orentas, R. J., Bircher, L. A. & Roskopf, S. (2003) Scand. J. Immunol. 58, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahara, H., Fujio, K., Araki, Y., Setoguchi, K., Misaki, Y., Kitamura, T. & Yamamoto, K. (2003) J. Immunol. 171, 2154–2160. [DOI] [PubMed] [Google Scholar]
- 26.Heemskerk, M. H., Hoogeboom, M., Hagedoorn, R., Kester, M. G., Willemze, R. & Falkenburg, J. H. (2004) J. Exp. Med. 199, 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamoto, K., Tsuji, T., Funamoto, H., Kosaka, A., Matsuzaki, J., Sato, T., Abe, H., Fujio, K., Yamamoto, K., Kitamura, T., et al. (2004) Cancer Res. 64, 386–390. [DOI] [PubMed] [Google Scholar]
- 28.Morgan, R. A., Dudley, M. E., Yu, Y. Y., Zheng, Z., Robbins, P. F., Theoret, M. R., Wunderlich, J. R., Hughes, M. S., Restifo, N. P. & Rosenberg, S. A. (2003) J. Immunol. 171, 3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuball, J., Schmitz, F. W., Voss, R. H., Ferreira, E. A., Engel, R., Guillaume, P., Strand, S., Romero, P., Huber, C., Sherman, L. A. & Theobald, M. (2005) Immunity 22, 117–129. [DOI] [PubMed] [Google Scholar]
- 30.Willemsen, R., Ronteltap, C., Heuveling, M., Debets, R. & Bolhuis, R. (2005) Gene. Ther. 12, 140–146. [DOI] [PubMed] [Google Scholar]
- 31.La Gruta, N. L., Turner, S. J. & Doherty, P. C. (2004) J. Immunol. 172, 5553–5560. [DOI] [PubMed] [Google Scholar]
- 32.Bosselut, R., Zhang, W., Ashe, J. M., Kopacz, J. L., Samelson, L. E. & Singer, A. (1999) J. Exp. Med. 190, 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosselut, R., Kubo, S., Guinter, T., Kopacz, J. L., Altman, J. D., Feigenbaum, L. & Singer, A. (2000) Immunity 12, 409–418. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg, E. S., Billingsley, J. M., Caliendo, A. M., Boswell, S. L., Sax, P. E., Kalams, S. A. & Walker, B. D. (1997) Science 278, 1447–1450. [DOI] [PubMed] [Google Scholar]
- 35.Palmer, B. E., Boritz, E., Blyveis, N. & Wilson, C. C. (2002) J. Virol. 76, 5925–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younes, S. A., Yassine-Diab, B., Dumont, A. R., Boulassel, M. R., Grossman, Z., Routy, J. P. & Sekaly, R. P. (2003) J. Exp. Med. 198, 1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day, C. L. & Walker, B. D. (2003) J. Exp. Med. 198, 1773–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, C. Y., Kirman, J. R., Rotte, M. J., Davey, D. F., Perfetto, S. P., Rhee, E. G., Freidag, B. L., Hill, B. J., Douek, D. C. & Seder, R. A. (2002) Nat. Immunol. 3, 852–858. [DOI] [PubMed] [Google Scholar]
- 39.Shrikant, P. & Mescher, M. F. (1999) J. Immunol. 162, 2858–2866. [PubMed] [Google Scholar]
- 40.Qin, Z. & Blankenstein, T. (2000) Immunity 12, 677–686. [DOI] [PubMed] [Google Scholar]
- 41.Mullbacher, A., Lobigs, M., Hla, R. T., Tran, T., Stehle, T. & Simon, M. M. (2002) J. Immunol. 169, 145–150. [DOI] [PubMed] [Google Scholar]
- 42.Roth, E. & Pircher, H. (2004) J. Immunol. 172, 1588–1594. [DOI] [PubMed] [Google Scholar]
- 43.Yee, C., Thompson, J. A., Byrd, D., Riddell, S. R., Roche, P., Celis, E. & Greenberg, P. D. (2002) Proc. Natl. Acad. Sci. USA 99, 16168–16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stauss, H. J. (1999) Immunol. Today 20, 180–183. [DOI] [PubMed] [Google Scholar]
- 45.Gao, L., Bellantuono, I., Elsasser, A., Marley, S. B., Gordon, M. Y., Goldman, J. M. & Stauss, H. J. (2000) Blood 95, 2198–2203. [PubMed] [Google Scholar]
- 46.Bendle, G. M., Holler, A., Pang, L. K., Hsu, S., Krampera, M., Simpson, E. & Stauss, H. J. (2004) Cancer Res. 64, 8052–8056. [DOI] [PubMed] [Google Scholar]