Abstract
We have previously reported that intrauterine (i/u) administration of epidermal growth factor (EGF 500 ng) on day (d) 21 of pregnancy delayed 19.0 ± 0.6 h the onset of labor. Progesterone (P) is secreted by ovarian corpora lutea (CL) throughout gestation in the rat. Prepartum CL regression due to increased uterine cyclooxygenase I and prostaglandin F2α results in P withdrawal followed by labor. The aims of the present work were (i) to study whether EGF delayed-onset of labor was mediated by a mechanism that prevented CL regression; (ii) to determine amniotic fluid (AF) EGF in pregnant rats. Rats on d21 of pregnancy received i/u EGF (500 ng) and were killed 0, 4, 8, 12, 24, and 48 h later. Control AF from rats on d13 and 18–22 of pregnancy was obtained. EGF decreased uterine prostaglandin F2α synthesis 8 h after treatment. Twelve hours after EGF injection, P reached its highest serum level and uterine cyclooxygenase I expression was undetectable. CL from rats killed 8 and 12 h after EGF were similar to those from rats on d13 of pregnancy, when serum P is maximum. EGF in AF increased throughout gestation, reached a maximum on d21, and decreased before the onset of labor. We suggest that the effect of EGF on the onset of labor was mediated by an early effect on the uterus that prevented prepartum CL regression.
EGF isolated by Cohen (1), is present in large amounts in amniotic membranes (2). Receptors for EGF were also identified in placenta and fetal membranes (3, 4). This is consistent with the stimulatory action of EGF on amnion prostaglandins (PGs) biosynthesis, which has led to the suggestion of a role for EGF in parturition (5, 6). At the time of delivery in women, some cytokines and growth factors are higher in amniotic fluid (AF) (7, 8). Although EGF is sharply increased in AF near delivery, there is no effect of it on the timing of labor (9). We have shown that different doses of intrauterine (i/u) EGF administered on day 21 (d21) of pregnancy in the rat delayed the onset of parturition in a dose–response manner (10). EGF (500 ng) had the strongest effect to delay labor to 19 h.
Progesterone (P) is essential for uterine quiescence during gestation (11, 12). In the rat, the corpora lutea (CL) is responsible for P synthesis and secretion throughout gestation (13), even after complete development of the placenta. At term, CL regression, known as luteolysis, is followed by a decrease in serum P resulting in parturition (14). Near term, cyclooxygenase I (COX-I) expression is augmented in the rat uterus (15). It is thought that luteolysis is caused by this early increase in COX-I followed by an increase in prostaglandin F2α (PGF2α) secretion (16). When PGF2α reaches the ovary, it triggers luteolysis and causes a fall in serum P (17). Thus, we investigated whether EGF delayed the onset of labor through a mechanism that prevented luteolysis, a necessary event before labor in the rat.
Rat uteri showed positive staining for EGF (18), and two classes of binding sites were found in pregnant mice uteri (19). EGF is also present in maternal AF (20), and it increases with the progression of pregnancy (9). However, there is still some controversy regarding the content of EGF in human AF, and there have been no studies in the rat. Thus, our second aim was to determine the level of EGF in AF of the rat during gestation.
Materials and Methods
Animals. The experimental procedures were approved by the Animal Care Committee of Center for Pharmacological and Botanical Studies in accordance with the Declaration of Helsinki.
Wistar rats housed in group cages under controlled conditions of light (14 h light, 10 h dark) and temperature, received food and water ad libitum. Time-mated pregnant rats (200–300 g) were used. The morning the spermatozoa were observed in the vaginal fluid was defined as d1 of pregnancy. Spontaneous term labor occurs on d22 (8 p.m. ± 2 h).
Pregnant rats were randomly divided into five groups: (i) in the control group, rats were killed on d13 and 18–22 of pregnancy between 10 and 11 a.m.; (ii) in the EGF-treated group, rats on d21 of pregnancy received i/u EGF; (iii) rats on d21 of pregnancy received i/u EGF vehicle; (iv) in the EGF antibody-treated group, rats on d20, d21, or d22 of pregnancy received i/u EGF antibody; (v) rats on d20, d21, or d22 of pregnancy received i/u rat preimmune serum. Groups ii and iii were killed as described below. Groups iv and v were monitored until delivery.
Intrauterine Administration of EGF and EGF Antibody. Pregnant rats were treated as described (10). Briefly, rats on d21 of pregnancy received i/u EGF (500 ng, 250 μl). Rats on d20, d21, or d22 of pregnancy received an i/u injection of rabbit antiserum against rat EGF (150 ng, 250 μl). Sham animals received 250 μl of 0.9% NaCl or preimmune serum. The uterus was surgically exposed, and the injections were made directly within the uterine lumen. Once injected, rats were housed in separate cages and continuously monitored.
Rats receiving the anti- or preimmune serum remained in their cages until delivery. Day and time of parturition were registered when the first pup was expulsed. Values were expressed as the mean variation in the onset of labor (h) compared to sham rats. Consequences of antibody administration to the mother and the pups were grossly evaluated by observing activity, feeding, and general well being.
Rats receiving saline solution were killed 0, 4, 8, 12, and 24 h (d22) after the treatment. EGF-treated rats were killed 4, 8, 12, 24, and 48 h (d23) after EGF. Uterine horns, serum, and ovaries were extracted.
Western Blotting. Uterine horns were homogenized in 20 mM Tris (pH 7.4) with inhibitors (10). Homogenates were sonicated for 30 s, centrifuged at 1,500 × g for 5 min, and boiled for 5 min in sample buffer (10). One hundred micrograms of total protein was loaded in each lane. Protein concentration was determined by Bradford (21). Goat seminal vesicles homogenate was loaded as positive control. Samples were carried out on SDS/7.5% PAGE gel (0.03 A) and transferred to a nitrocellulose membrane (40 V, overnight, 4°C). Membranes were blocked for 1 h at room temperature in Tris/saline (50 mM Tris·HCl, pH 7.5/500 mM NaCl) containing 5% of milk powder and incubated overnight at 4°C with a primary polyclonal antibody against COX-I (1:1,000 in Tris/saline buffer). Then they were incubated for 1 h at room temperature with a polyclonal rabbit antibody against actin and used as loading control (1:10,000 in Tris/saline buffer), followed by a 1-h incubation at room temperature with a goat anti-rabbit IgG alkaline phosphatase (1:5,000 in Tris/saline buffer). After incubations, membranes were washed with Tris/saline buffer containing 0.2% Tween-20. The developing solution was nitroblue tetrazolium with 5-bromo-4-chloro-3-indol phosphate. Molecular mass standards were also run. Developed membranes were scanned, and the intensity of bands was determined by using the image j program (National Institutes of Health, Bethesda). Each point represents pooled material from four different animals. The experiment was repeated three times. Values were expressed as relative optical density COX-I/actin.
PG RIA. Uterine horns were incubated in Krebs–Ringer bicarbonate modified solution (10) at 37°C for 1 h in a 95% O2/5% CO2 atmosphere. Afterward, medium was acidified to pH 3 with 1 M HCl in ethyl acetate and extracted twice. Pooled ethyl acetate extracts were dried. PGF2α concentration was determined by RIA (22). PGF2α antiserum was highly specific for PGF2α and showed low crossreactivity. Sensitivity was 5–10 pg per tube and ka = 1.5 × 1010 liters/mol. Protein concentration was determined by Bradford (21). Values were expressed as pg of PGF2α per mg of protein per h.
P RIA. Blood was allowed to clot and centrifuged at 1,100 × g for 10 min. Serum was removed and frozen at –20°C. P was extracted twice with diethyl ether, and its concentration was determined by RIA (23). P antiserum was highly specific for P and showed low crossreactivity. Sensitivity was 5–10 pg per tube. Values were expressed as ng of P per ml of serum.
EGF RIA. EGF iodination was carried as described (24). Two micrograms of EGF were dissolved in 0.05 M phosphate buffer (pH 7.5) and mixed with 0.5 M phosphate buffer (pH 7.5) containing 0.4 mCi of Na125I (molar ratio EGF/Na125I, 2:1). Chloramine T (2 μg/μl) was added. The reaction was stopped after 25 s with 4 μg/μl sodium metabisulfite. [125I]EGF was separated in a Sephadex G-25 column washed with 0.05 M buffer phosphate (pH 7.5) containing 0.075 M NaCl. Albumin (0.1%) was added to the elutions and stored at –20°C. Specific activity of [125I]EGF was 120,000–145,000 cpm/ng.
AF samples were centrifuged at 5,000 × g for 10 min and incubated at 4°C for 72 h with the primary antibody against EGF (polyclonal antibody against rat EGF, 1:50,000) and [125I]EGF. The second antibody was added (1:50,000 in 8% polyethylenglycol) and incubated at 4°C for 2 h. The reaction mixture was centrifuged at 1,500 × g for 30 min at 4°C. Pellet radioactivity was measured. Method sensitivity was 50 pg/μl. Results were expressed as pg of EGF per ml of AF.
Histological Analysis of the Ovaries. Ovaries were cut in half and fixed in 4% paraformaldehyde for 18 h at 4°C. Fixed ovaries were dehydrated with 70–100% ethyl alcohol and embedded in paraffin. Microtome sections, stained with hematoxylin/eosin, were mounted with Permount. Sections were evaluated under microscope and photographed at ×31.25 and ×500. Cells of interest were counted in a ×40 field and normalized against a total number of 100 cells.
Preimmune Serum. Male rats were anesthetized by ether inhalation, and blood was extracted by cardiac puncture. Blood was allowed to clot and was incubated at 37°C for 30 min. Samples were centrifuged at 1,100 × g for 10 min. Supernatants were extracted, centrifuged again, and incubated at 56°C for 30 min. Serum was removed and frozen at –20°C. Samples were centrifuged at 20,000 × g for 5 min. Serum contained 8–12 mg of Ig per ml.
Statistics. Statistical analysis was performed by using prism 3.0 (GraphPad, San Diego). Comparisons between values of groups were performed by using one- and two-way ANOVA. Significance was determined by using Tukey's multiple comparison test for unequal replicates. Values represent means ± SEM. Differences between means were considered significant when P ≤ 0.05.
Drugs and Chemicals. EGF (murine submaxillar glands, culture grade) was from Calbiochem. [5,6,8,9,11,12,14,15(n)-3H]PGF2α (160 Ci/mmol), 17α-hydroxy[1,2,6,7-3H]-P (60 Ci/mmol), and 125I (0.25 mCi/μl) were from Amersham Pharmacia. The first antibody against EGF was from IgG Corporation, and the second was provided by Catholic University (Santiago, Chile). Rabbit polyclonal COX-I was from Cayman Chemical. PGF2α antiserum, chloramine T, sodium metabisulfite, Permount, goat anti-rabbit IgG alkaline phosphatase, actin, and other Western blot reagents were from Sigma. P antiserum was provided by G. D. Niswender (Colorado State University, Fort Collins). All other chemicals were analytical grade.
Results
Effect of i/u EGF on Uterine PGFα. EGF administrated on d21 delayed the onset of labor in a dose–response fashion (10). We assessed whether the dose that had the strongest effect (500 ng) was capable of inhibiting uterine PGF2α.
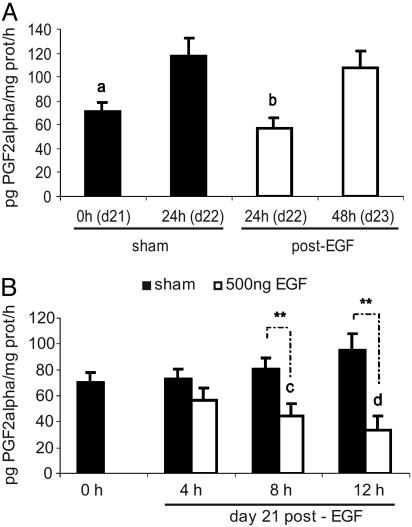
EGF decreased uterine PGF2α synthesis on d22 to the level observed on d21 in sham animals (Fig. 1A). In the EGF-treated group, PGF2α synthesis increased from d22 to d23 as it occurred in the sham rats before the onset of labor. Moreover, the EGF effect seemed to be time dependent. In the sham group, PGF2α increased gradually from 0 to 12 h, an effect that continued to d22 (data not shown). In contrast, PGF2α began to decrease 8 h after the EGF treatment and was minimal at 12 h (Fig. 1B).
Fig. 1.
EGF inhibited uterine PGF2α synthesis. (A) PGF2α in sham and EGF (500 ng)-treated rats. (B) PGF2α 0, 4, 8, and 12 h after treatment with vehicle (sham) or EGF (500 ng). Results represent mean ± SEM (n = 12). a, P < 0.001 vs. d22 sham; b, P < 0.001 vs. d22 sham and d23; c, P < 0.01 vs. 0h and 4h; d, P < 0.001 vs. the rest; **, P < 0.01 sham vs. EGF (500 ng).
Thus, the administration of EGF inverted the pattern observed for PGF2α synthesis in the sham group.
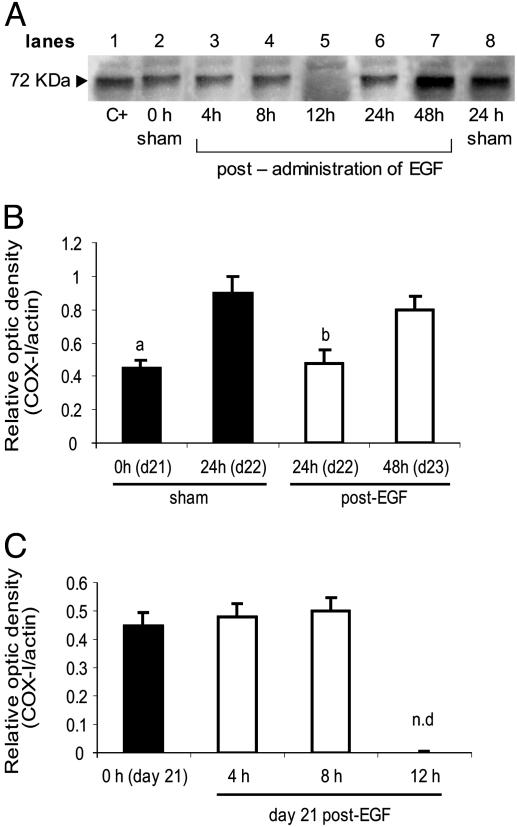
Effect of i/u EGF on Uterine COX-I. We analyzed whether EGF could regulate COX-I expression, the enzyme responsible for uterine PGF2α synthesis. The antibody against COX-I specifically reacted with a band of 72 kDa in the sham (lanes 1 and 8) and the EGF-treated groups (lanes 3–7) (Fig. 2A). On d22, COX-I expression in the EGF group was decreased compared to that in the sham animals on d22, but was similar to that in the sham group on d21 (Fig. 2B).
Fig. 2.
EGF decreased uterine COX-I expression. (A) Representative Western blot; C+, positive control. (B) Densitometric analysis of the bands obtained in sham and EGF (500 ng)-treated rats. (C) Densitometric analysis of the bands obtained 0, 4, 8, and 12 h after treatment. Results represent mean ± SEM (n = 3). a, P < 0.01 vs. d22 sham; b, P < 0.01 vs. d22 sham and d23; n.d., not detectable.
COX-I on d22 in the sham group and on d23 in the EGF-treated group were similar. Days 22 and 23 were the days of delivery in the sham and EGF-treated groups, respectively. The effect of EGF on COX-I expression was time dependent. COX-I was not detectable 12 h after the injection (Fig. 2C). Thus, the regulation of COX-I expression by EGF showed a similar pattern to that obtained for PGF2α synthesis.
Effect of i/u EGF on Serum P. Because we observed that EGF delayed the onset of labor (10), modulating uterine COX-I and PGF2α, we determined whether EGF could regulate serum P (25).
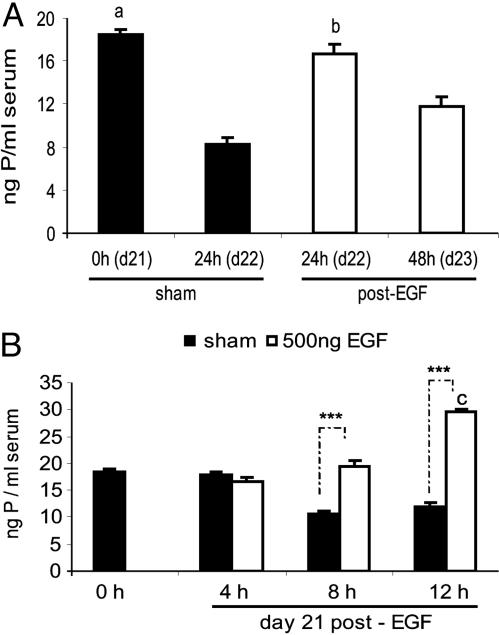
P on d22 in the EGF group was similar to P on d21 in the sham group. On d23 (the day of labor in the EGF-treated group), P diminished as occurred in d22 in the sham rats. In the sham group, P began to decrease at 8 h. This level was maintained until 12 h and continued at the level registered on d21 and d22. EGF administration elicited a time-dependent effect; first, it maintained P concentration (8 h), and then it increased it, being maximum 12 h after the treatment (Fig. 3B).
Fig. 3.
EGF increased serum P. (A) P in sham rats and in EGF (500 ng)-treated rats. (B) P 0, 4, 8, and 12 h after treatment with vehicle (sham) or EGF (500 ng). Results represent mean ± SEM (n = 12). a, P < 0.01 vs. d22 sham; b, P < 0.01 vs. d22 sham and d23; c, P < 0.001 vs. the rest; ***, P < 0.001 sham vs. EGF-treated rats.
The pattern of P increment was inverse compared to minimum uterine COX-I and PGF2α after EGF treatment.
Effect of i/u EGF on the CL. Functional luteolysis in rats is associated with morphologic features (26). Thus, the morphology of the CL reflects its capacity to synthesize and secrete P. Because the administration of EGF increased P, we wanted to discern whether the treatment with EGF could affect the number of cells from each population within the CL (26).
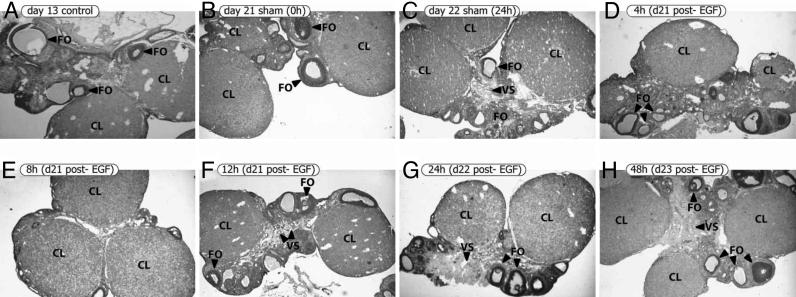
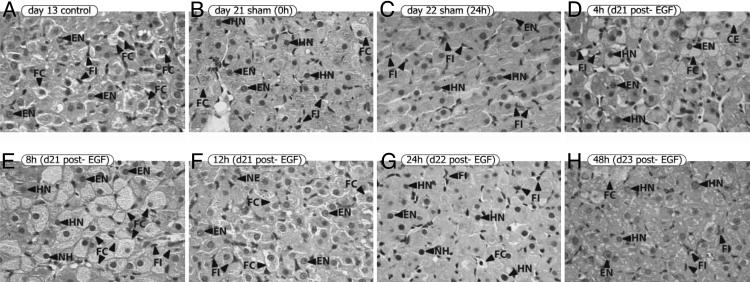
First, we analyzed ovaries from control, sham, and EGF-treated rats (Fig. 4). Control rats on d13 of pregnancy were included because we observed the highest P level at this time during gestation (15). Ovaries from control rats on d13 of pregnancy presented big CL, follicles in different developmental stages, and typical blood vessels (Fig. 4A). Spaces between the cells were occupied by a majority of connective tissue cells. Both sham and EGF-treated ovaries presented CL. Sham rats on d21 of pregnancy showed CL and follicles in different stages of development (Fig. 4B). CL from control rats on d13 and sham rats on d21 presented clear and well defined borders. No differences were detected between ovaries from sham animals on d21 and d22 (Fig. 4C) and EGF-treated animals (Fig. 4 D–H).
Fig. 4.
EGF did not affect the general appearance of the ovaries. Ovaries from control rats on d13 of pregnancy, sham rats on d21 and 22, and EGF-treated rats were obtained. Sections were stained with hematoxylin–eosin. Representative photographs of each group are shown. (Original magnification, ×31.25.) CL, corpora lutea; FO, follicles; VS, blood vessels.
The morphological characteristics and the number of cells belonging to different populations within the CL were analyzed (Fig. 5). CL from rats on d13 of pregnancy had a majority of luteal cells with a vast monomorphism and extremely foamy cytoplasms (Fig. 5A). This image corresponded to cytoplasms with a high concentration of lipid droplets, suggesting very active P synthesis (15). Their nuclei were eccentric and basophilic. The nuclear membranes appeared well defined, and the nucleoli were clearly evident. This is the typical image of a luteinized CL, with maximum P synthesis and secretion. In an early step of regression, the cytoplasms of sham rats on d21 of pregnancy were rather eosinophilic and less foamy, probably because of a lower lipid vesicle content (Fig. 5B). Their nuclei were basophilic and stained to an intense blue color. The nuclear membranes were well defined, and the nucleoli could not be distinguished. Between the eosinophilic luteal cells, there were still some cells that presented a clearer and foamy cytoplasm and some fibroblastic cellular elements. Luteal cells from sham rats on d22 presented a highly deteriorated organization and no continuity between them, showing an advanced step of degeneration of the CL (Fig. 5C). Images from sham rats on d21 and d22 corresponded to CL in process of luteolysis, as labor usually occurs on d22. In the EGF-treated group, the first detectable changes appeared 8 h after EGF administration. Four hours after EGF, luteal cells were similar to those observed on d21 (Fig. 5D). This CL displayed and initial step of luteolysis. However, 8 h after EGF treatment, luteolysis seemed to be stopped, and the CL looked as if they had been “reluteinized” (Fig. 5E). Luteal cells presented continuity and recovered their monomorphic and foamy aspect. Also, the number of fibroblasts was reduced. The nuclei maintained the blue and homogeneous staining, without evident nucleoli. Twelve hours after EGF, the majority of luteal cells lost their foamy cytoplasm, but showed evident nucleoli within the nuclei (Fig. 5F). Also, continuity between luteal cells disappeared. The appearance of the luteal cells 24 h after EGF injection was quite similar to that in sham animals on d21 (Fig. 5G), and the number of fibroblasts increased. On d23, the day of delivery in the EGF group, the appearance and number of the cells within the CL were similar to those described for the sham group on d22 (delivery day) (Fig. 5H). In an advanced stage of regression, a great cellular disorganization and pleyomorphism were observed. These results are shown in Table 1.
Fig. 5.
EGF seemed to stopped the process of luteolysis and “reluteinized” the CL. Ovaries from control rats on d13 of pregnancy, sham rats on d21 and 22, and EGF-treated rats were obtained. Sections were stained with hematoxylin–eosin. Representative photographs of each group are shown. (Original magnification, ×500.) FC, foamy cytoplasm; HN, homogeneous nucleus; EN, evident nucleolus; FI, fibroblasts.
Table 1. EGF effect on the number of cells from different populations within the CL.
| Foamy cells | Cells with evident nucleoli | Fibroblasts | |
|---|---|---|---|
| d13 control | 66 | 24 | 22 |
| d21 sham | 6 | 3 | 92 |
| d22 sham | 2 | 3 | 94 |
| 4 h after EGF | 21 | 1 | 78 |
| 8 h after EGF | 68 | 6 | 30 |
| 12 h after EGF | 13 | 17 | 51 |
| 24 h after EGF | 4 | 3 | 94 |
| d23 after EGF | 3 | 5 | 92 |
The number of cells from different populations within the CL was counted in four fields. Values are expressed as number of cells per 100 total cells.
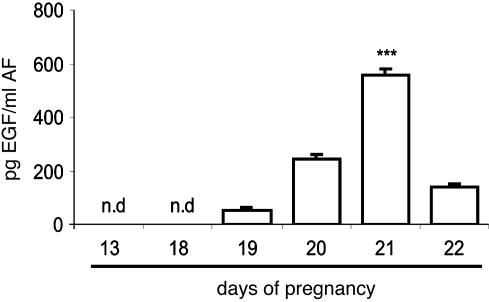
EGF Content in the AF. EGF concentration increased gradually during gestation, being highest on d21 and then declining on d22, before labor (Fig. 6).
Fig. 6.
EGF level in AF increased at term gestation. AF from rats on d13 and d18–22 of pregnancy were obtained. Control rats delivered during the night of d22. EGF concentration was determined by RIA. Results represent the mean ± SEM (n = 8); ***, P < 0.001 vs. the rest; n.d., not detectable.
Effect of i/u EGF Antibody in the Onset of Labor. We investigated whether the depletion of EGF within the uteri caused preterm labor. Based on the results about EGF content in AF, we knew that its highest level was ≈600 pg/ml. Thus, pregnant rats on d20, d21, or d22 received the EGF antibody (150 ng, i/u). Sham animals and rats receiving the EGF antibody on d20, d21, or d22 delivered during the night of d22, as did sham rats (Table 2).
Table 2. Effect of i/u administration of EGF antibody during term pregnancy.
| Day of administration | Time of administration | Sham rats | Treated rats |
|---|---|---|---|
| d20 | 10 a.m. | 2.0 ± 0.6 | 1.9 ± 0.5 |
| 5 p.m. | 2.8 ± 0.8 | 2.8 ± 0.4 | |
| 8 p.m. | 2.4 ± 0.6 | 2.6 ± 0.5 | |
| d21 | 10 a.m. | 2.6 ± 0.2 | 2.5 ± 0.5 |
| 5 p.m. | 1.9 ± 0.5 | 2.0 ± 0.6 | |
| 8 p.m. | 2.1 ± 0.8 | 2.3 ± 0.8 | |
| d22 | 10 a.m. | 2.8 ± 0.7 | 2.5 ± 0.3 |
| 5 p.m. | 1.8 ± 0.5 | 2.6 ± 0.6 |
Values are expressed as the mean variation (h) in the onset of labor compared with that of sham rats (250 μl of preimmune serum).
Discussion
Most of the studies on EGF have been focused on its stimulatory effect on cellular proliferation. However, its participation during pregnancy and labor has become more clear. We have reported that i/u administration of 120, 250, or 500 ng of EGF on d21 of pregnancy delayed the onset of labor in a dose–response fashion (10). The inflammatory cytokines have long been linked to i/u infection-driven preterm labor (7, 8, 27, 28), and there is accumulating evidence that they are also involved in normal term labor (29, 30). In fact, it has been proposed that human parturition represents an inflammatory process. Others have shown that EGF levels in AF increased sharply in late pregnancy near term (9). However, Varner et al. (9) did not observe an effect of chorioamnionitis of term or preterm labor on EGF levels in AF. Therefore, the role of EGF in the regulation of the parturition process is unclear.
Based on our previous results (10) and with regard to the crucial role of luteolysis in rat labor, we investigated whether the i/u administration of EGF on d21 of pregnancy could modulate the process of luteolysis. If the treatment with EGF prevented the onset of luteolysis, the CL would have remained functional and the withdrawal in serum P necessary for delivery beginning on d22 would not have taken place.
It is widely accepted that COX-I and PGF2α participate in the onset of luteolysis in the rat (17, 31, 32). At term gestation, and before the onset of labor, the expression of uterine COX-I augmented increasing the production of PGF2α. Gross et al. (16) reported that mice deficient in COX-I produced less PGF2α at the end of pregnancy and presented a delay in the time of delivery, whereas the administration of PGF2α restored it. Thus, COX-I was postulated as responsible for luteolytic PGF2α synthesis and for the decrease in serum P (33).
In the present work, we observed that EGF decreased COX-I and PGF2α in the uterus, being minimal at 12 h after EGF. Gardner et al. (34) observed that EGF produced uterine contractions in tissues removed from immature and adult rats. On the other hand, Tamada et al. (35) found that the intraluminal infusion of EGF into the uterine horns of the goat gradually reduced uterine activity.
We also observed that the time course for serum P after the treatment with EGF followed an inverse pattern compared to COX-I and PGF2α decrease, as P peaked 12 h after EGF. In the rat, uterine PGF2α avoids the systemic circulation by going from the uterine vein straight into the ovarian artery (36), stimulating the onset of luteolysis (36) and, consequently, the decrease in P. EGF stimulates P production by human granulosa cells in culture. Serta and Seibel (37) suggested that normal luteal function may require the early and continuous presence of EGF. Jones et al. (38) reported that EGF treatment of granulosa cells obtained from immature rats did not affect estrogen production but stimulated P in a dose-dependent manner.
The ability of the luteal cells to synthesize and secrete P is intimately associated with the CL functionality (26). CL structure and morphology reflects its functional state. Because PGF2α decreased and P increased after EGF treatment, we assessed whether any effect occurred with regard to CL morphology. Eight hours after EGF administration, the CL seemed to be reluteinized; their appearance was similar to that of CL obtained from control rats on d13 of pregnancy when P was maximum (15).
These results suggest that there is an early effect of EGF on uterine PGF2α production, 8 h after the treatment. This effect is correlated with the presence of a functional CL and the maintenance of serum P. P inhibits the increase in uterine COX-II expression (33), the enzyme responsible for the synthesis of the PGs necessary for the onset of uterine contractions during labor (27). Thus, this could be one of the ways by which EGF delayed labor. The results presented showed that EGF administration on d21 of pregnancy modified the mediators involved in the process of luteolysis maintaining the capacity of the CL to produce and secrete P. Thus, EGF protected the CL, delaying the onset of luteolysis and, therefore, the onset of labor, prolonging the duration of gestation. The modulation of the luteolytic process by EGF could be exerted in two ways: (i) a direct effect on the ovary: when EGF reaches the CL it regulates the synthesis and/or secretion of P; and/or (ii) an indirect effect on the ovary through the uterus: EGF inhibits uterine COX-I and PGF2α, subsequently increasing the secretion of P by the CL. The idea of a direct action on rat luteal cells is reinforced by the demonstration of high-affinity, low-capacity EGF-binding sites in these cells (38). On the other hand, EGF peptide is rapidly degraded after in vivo administration (39, 40). We demonstrated that EGF is exerting its effect through the second mechanism. However, we cannot rule out the first possibility, and more studies are necessary to elucidate it.
EGF is present in serum (20) and AF (41) from pregnant women. Uterine and fetoplacental human tissues have been shown to express EGF (18, 42). Despite all of these studies, there is still no consensus about regarding the levels of EGF in women AF, and there are no reports on this in rodents. We determined the concentration of EGF in the AF of control rats during gestation. The results showed that EGF levels in AF progressively increased during gestation, reaching a maximum on d21 and diminishing by d22, before labor. Romero et al. (43) suggested that there is an increase in the concentration of EGF/TGF-α in AF during labor; this led to the suggestion that EGF may play a part in the mechanism of parturition. Given our data and that described by Varner et al. (9), demonstrating no increase in EGF levels in AF during term labor, TGF-α may be increased. Alternatively, differences in methodology may account for the discrepancy.
The present data showed a gestation-related increase in EGF levels in the AF, suggesting that EGF may have an early role in the onset of labor. It has been reported that the expression of EGF in murine uterine epithelial cells is stimulated by estrogens (44). Besides, Trujillo et al. (45) found that, during gestation in the rat, estrogens increased on d21. Thus, we hypothesize that increased EGF in the AF on d21 of pregnancy could be due to increased levels of serum estrogen.
As we have mentioned before, in the rat, P maintains uterine quiescence during gestation and decreases at term, allowing for the increase in the synthesis of PGs involved in the onset of labor (15). The pattern of EGF in the AF showed an inverse correlation when compared with P. Thus, our results suggest that endogenous EGF could be responsible for a mechanism that maintains the function of the CL. We expected the rats to deliver preterm; however, no effect was observed and all of the animals delivered at term, as did the sham group. The targeting to appropriate cells and maintenance of adequate i/u levels becomes essential. We suspect that, because of the high molecular mass of the antibody, it was not able to get through the amniotic membranes and neutralize the EGF contained inside the amniotic sac.
EGF is known to promote fetal lung maturation (46–49), and therapies used to accelerate it can increase both EGF levels in AF (50) and pulmonary EGF receptors (51). The time of gestation when EGF levels in AF increase has led to the speculation that it may be a marker of fetal lung maturation. Because EGF may have a role in the onset of labor and is positively correlated with fetal lung maturation, the presence of EGF in gestational tissues and AF may be an excellent means to coordinate the onset of labor with fetal lung maturation, essential for the extrauterine surveillance. Our results showed that EGF had an effect on the length of pregnancy, suggesting that it could be an additional beneficial factor preventing preterm labor, one of the main problems in perinatology (52).
Acknowledgments
We thank R. Morales, A. Casella, and C. Llados for their technical support. We also thank Dr. C. M. Tellería for his assistance with the extraction of the CL and Dr. G. D. Niswender for P antiserum. This work was supported by Fondo Para la Investigación Científica y Tecnológica (PICT 10901) and PIP (97/814) (to A.M.F.) and by Fondo Nacional de Investigación Científica y Tecnológica Grant 1040804 (to M.V.).
Abbreviations: PG, prostaglandin, AF, amniotic fluid; i/u, intrauterine; dn, day n; P, progesterone; CL, corpora lutea; COX-I, cyclooxygenase I.
References
- 1.Cohen, S. (1962) J. Biol. Chem. 237, 1555–1562. [PubMed] [Google Scholar]
- 2.Scott, S. M., Buenaflor, G. G. & Orth, D. N. (1989) Biol. Neonate 56, 246–251. [DOI] [PubMed] [Google Scholar]
- 3.Carson, S. A., Chase, R., Ulep, E., Scommega, A. & Benveniste, R. (1983) Am. J. Obstet. Gynecol. 147, 932–939. [DOI] [PubMed] [Google Scholar]
- 4.Chegini, N. & Rao, C. V. (1983) J. Clin. Endocrinol. Metab. 61, 529–535. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell, M. D. (1987) Biochim. Biophys. Acta. 98, 240–242. [DOI] [PubMed] [Google Scholar]
- 6.Casey, M. L., Mitchell, M. D. & McDonald, P. C. (1987) Mol. Cell Endocrinol. 53, 169–176. [DOI] [PubMed] [Google Scholar]
- 7.Hsu, C. D., Meaddough, E., Aversa, K., Hong, S. F., Lu, L. C., Jones, C. D. & Copel, J. A. (1998) Am. J. Obstet. Gynecol. 179, 1267–1270. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Bosquet, E., Cerqueira, M. J., Dominguez, C., Gasser, I., Bermejo, B. & Cabero, L. (1999) J. Matern. Fetal. Neonatal Med. 8, 155–158. [DOI] [PubMed] [Google Scholar]
- 9.Varner, M., Dildy, A., Hunter, B., Dudley, D., Clark, S. & Mitchell, M. (1996) J. Soc. Gynecol. Investig. 3, 17–19. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro, M. L., Farina, M. & Franchi, A. (2003) Reproduction 126, 459–468. [DOI] [PubMed] [Google Scholar]
- 11.Siiteri, P. K., Febres, F., Clemens, L. E., Chang, R. J., Gondos, B. & Stites, D. (1977) Ann. N.Y. Acad. Sci. 286, 384–397. [DOI] [PubMed] [Google Scholar]
- 12.Niswender, G. D. & Nett, T. M. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 781.
- 13.Wright, K., Pang, C. Y. & Behrman, H. R. (1980) Endocrinology 106, 1333–1337. [DOI] [PubMed] [Google Scholar]
- 14.Soloff, M. F. (1989) in Biology of the Uterus, eds. Wynn, R. M. & Jollie, W. P. (Plenum, New York), pp. 559.
- 15.Farina, M., Ribeiro, M. L., Weissman, C., Estevez, A., Billi, S., Vercelli, C. & Franchi, A. (2004) J. Steroid Biochem. Mol. Biol. 91, 211–218. [DOI] [PubMed] [Google Scholar]
- 16.Gross, G. A., Imamura, T., Luedke, C., Vogt, S. K., Olson, L. M., Nelson, M. D., Sadosky, Y. & Muglia, L. J. (1998) Proc. Natl. Acad. Sci. USA 95, 11875–11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castracane, V. D. & Shaikh, A. A. (1976) J. Reprod. Fertil. 46, 101–104. [DOI] [PubMed] [Google Scholar]
- 18.Brown, M. N. & Lamartiniere, C. A. (2000) Cell Death Differ. 11, 255–260. [Google Scholar]
- 19.Das, S. K., Tsukamura, H., Paria, B. C., Andrews, G. K. & Dey, S. (1994) Endocrinology 134, 971–981. [DOI] [PubMed] [Google Scholar]
- 20.Barka, T., Vander Noen, H., Gresik, E. W. & Kerenyi, T. (1978) Mt. Sinai J. Med. 45, 679–684. [PubMed] [Google Scholar]
- 21.Bradford, M. (1976) Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 22.Campbell, W. B. & Ojeda, S. R. (1987) Methods Enzymol. 141, 323–341. [DOI] [PubMed] [Google Scholar]
- 23.Abraham, G. E., Swerdloff, R., Tulchinaky, D. & Odell, W. D. (1971) J. Clin. Endocrinol. 32, 619–624. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter, G. & Cohen, S. (1976) J. Cell. Biol. 71, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csapo, A. (1981) in Principles and Practice of Obstetrics and Perinatology, eds. Iffy, L. & Kaminetzky, H. (Wiley, New York), pp. 761.
- 26.Tellería, C. M., Goyeneche, A. A., Cavicchia, J. C., Stati, A. O. & Deis, R. P. (2001) Endocrine 15, 147–155. [DOI] [PubMed] [Google Scholar]
- 27.Hertelendy, F. & Zakar, T. (2004) Curr. Pharm. Des. 10, 2499–2517. [DOI] [PubMed] [Google Scholar]
- 28.Amory, J. H., Hitti, J., Lawler, R. & Eschenbach, D. A. (2001) Am. J. Obstet. Gynecol. 185, 1064–1067. [DOI] [PubMed] [Google Scholar]
- 29.Romero, R. & Mazor, M. (1988) Clin. Obstet. Gynecol. 31, 553–584. [DOI] [PubMed] [Google Scholar]
- 30.Kelly, R. W. (1996) Rev. Reprod. 1, 89–96. [DOI] [PubMed] [Google Scholar]
- 31.Arend, A., Masso, R., Masso, M. & Selstan, G. (2004) Prostaglandins Other Lipid Med. 74, 1–10. [DOI] [PubMed] [Google Scholar]
- 32.Cook, J. L., Shallow, M. C., Zaragoza, D. B., Anderson, K. I. & Olson, D. M. (2003) Biol. Reprod. 68, 579–587. [DOI] [PubMed] [Google Scholar]
- 33.Tsuboi, K., Iwane, A., Nakasawa, S., Sugimoto, Y. & Ichikawa, A. (2003) Biol. Reprod. 9, 195–201. [DOI] [PubMed] [Google Scholar]
- 34.Gardner, R. M., Lingham, R. B. & Stancel, G. M. (1987) FASEB J. 1, 224–228. [DOI] [PubMed] [Google Scholar]
- 35.Tamada, H., Yoh, C., Inaba, T., Takano, H., Kawate, N. & Sawada, T. (2000) Theriogenology 54, 159–169. [DOI] [PubMed] [Google Scholar]
- 36.Del Campo, C. H. & Ginther, O. J. (1972) Am. J. Vet. Res. 33, 2561–2578. [PubMed] [Google Scholar]
- 37.Serta, R. T. & Seibel, M. M. (1993) Hum. Reprod. 8, 1005–1010. [DOI] [PubMed] [Google Scholar]
- 38.Jones, P. B., Welsh, T. H., Jr., & Hsueh, A. J. (1982) J. Biol. Chem. 257, 11268–11273. [PubMed] [Google Scholar]
- 39.Kurihara, A., Deguchi, Y. & Pardridge, W. M. (1999) Bioconjug. Chem. 10, 502–511. [DOI] [PubMed] [Google Scholar]
- 40.Prats, P. A., Duconge, J., Valenzuela, C., Berlanga, J., Edrosa, C. R., Fernandez-Sanchez, E. (2002) Biopharm. Drug Dis. 23, 67–76. [DOI] [PubMed] [Google Scholar]
- 41.Ances, I. G. (1973) Am. J. Obstet. Gynecol. 115, 357–362. [DOI] [PubMed] [Google Scholar]
- 42.Faber, B., Metz, S. A. & Chegini, N. (1996) Obstet. Gynecol. 88, 174–179. [DOI] [PubMed] [Google Scholar]
- 43.Romero, R., Wu, Y. K., Oyarzun, E., Hobbins, J. C. & Mitchell, M. D. (1989) Eur. J. Obstet. Gynecol. Reprod. Biol. 33, 55–60. [DOI] [PubMed] [Google Scholar]
- 44.Huet-Hudson, Y. M., Chakraboty, C., De, S. K., Suzuki, Y., Andrews, G. K. & Dey, S. K. (1990) Mol. Endocrinol. 4, 510–523. [DOI] [PubMed] [Google Scholar]
- 45.Trujillo, M., Candenas, L., Cintado, C., Magraner, J., Fernandez, J., Martin, J. & Pinto, F. (2001) J. Pharmacol. Exp. Ther. 296, 841–848. [PubMed] [Google Scholar]
- 46.Catterton, W. Z., Escobedo, M. B., Sexson, W. R., Gray, M. E., Sundell, H. W. & Stahlman, M. T. (1979) Pediatr. Res. 13, 104–108. [DOI] [PubMed] [Google Scholar]
- 47.Gross, I., Dynia, D. W., Rooney, S. A., Smart, D. A., Warshaw, J. B., Sisson, J. F. & Hoath, S. B. (1986) Pediatr. Res. 20, 473–477. [DOI] [PubMed] [Google Scholar]
- 48.Plopper, C. G., St. George, J. A., Read, L. C., Nishio, S. J., Weir, A. J., Edwards, L., Tarantal, A. F., Pinkerton, K. E., Merrit, T. A. & Whitsett, J. A., et al. (1992) Am. J. Physiol. 262, 313–321. [DOI] [PubMed] [Google Scholar]
- 49.Wang, D., Cao, S. & Cui, L. (1998) Zhonhua Fu Chan Ke Za Zhi 33, 150–152. [PubMed] [Google Scholar]
- 50.Hofman, G. E., Romaguera, J., Williams, R. F. & Adamsons, K. (1993) Acta Obstet. Gynecol. Scand. 72, 252–257. [DOI] [PubMed] [Google Scholar]
- 51.Sadiq, H. F. & Devaskar, U. P. (1984) Biochem. Biophys. Res. Commun. 119, 408–414. [DOI] [PubMed] [Google Scholar]
- 52.Vohr, B. R. & Allen, M. (2005) N. Engl. J. Med. 352, 71–72. [DOI] [PubMed] [Google Scholar]