Type 1 (formerly “juvenile”) diabetes is believed to be an autoimmune disease in which host immune cells fail to recognize the insulin-producing beta cells contained within pancreatic islets of Langerhans as being “self” and inappropriately elect to destroy these critical insulin-producing cells. The traditional methods of treating type 1 diabetes are good, but not perfect. The studies reported by Sapir et al. (1) in this issue of PNAS suggest that new, more acceptable approaches to beta cell replacement may be feasible.
Treatment Obstacles in Type 1 Diabetes
In the current treatment of type 1 diabetes, insulin is given by injection or insulin pump. Patients must frequently check their blood glucose by finger stick to calculate and adjust their insulin doses. Undertreatment (hyperglycemia) and overtreatment (hypoglycemia) are common. The ideal therapy for diabetes would mimic the two essential features of normal beta cells: the ability to sense glucose continually coupled with intelligent and appropriate release of insulin in response to changes in blood glucose. One solution could be an implantable, glucose-sensing insulin delivery system. Attempts to develop such a computer-driven, “closed-loop” insulin delivery system have been aided substantially by the miniaturization of computer chips and insulin pumps. Important hurdles relate to development of reliable, long-term glucose sensors, miniaturization, replacement of batteries, and refillable reservoirs for the necessary insulin pumps.
Whole pancreas transplant is another option. Long-term graft function is excellent, but the procedure requires major surgery, is limited by the small numbers of pancreata available for transplant, and is accompanied by morbidity from immunosuppressive drugs used to prevent organ rejection (2). In 2000, a group in Edmonton, Canada, demonstrated the feasibility of pancreatic islet transplantation (3), which requires only trivial outpatient surgery. The Edmonton demonstration that long-term islet graft survival and function are possible has reinvigorated attempts at beta cell replacement therapy.
In broad terms, there are two formidable hurdles that must be addressed if pancreatic beta cell replacement therapy can be used on a wide scale. The first is immunological. Transplanted beta cells or their surrogates will need to evade two and possibly three levels of immune surveillance: alloimmunity, autoimmunity, and xenoimmunity. Alloimmunity encompasses the concept that beta cells from one person will be immunologically rejected as foreign invaders when implanted into another person. Autoimmunity refers to the disordered immunity in type 1 diabetes described above: the immune system of a type 1 diabetic will destroy any pancreatic beta cells it can find, whether they are derived from the host's body (e.g., one's own stem cells) or from an allograft donor. Xenoimmunity applies when beta cells from nonhuman species, such as pigs or nonhuman primates, are transplanted. Although the Edmonton Protocol (3) is a landmark study in demonstrating the feasibility of surmounting technical and immune hurdles to beta cell replacement, it also highlights the need for improved drugs for immunosuppression.
The second hurdle is transplanting an adequate mass of islet cells. Successful islet transplantation in humans requires up to four cadaver pancreas donors to correct a single type 1 diabetic. Because there are millions of type 1 diabetics, but only a few thousand human cadaver pancreases available each year, the impracticability of this approach is obvious. One approach to solving this problem has been to try to engineer or mimic the normal beta cell development process in vitro and in vivo by using stem cells (e.g., embryonic, bone marrow, and/or pancreatic ductal stem cells). Some progress has been made along these lines, but a fair assessment might be that the more we learn, the more difficult and complex this goal appears. Other approaches have included attempts to induce beta cells to replicate ex vivo or in vivo, to become “death-resistant” to overcome the massive beta cell death that accompanies pancreatic beta cell isolation and transplantation, and/or to become “super islets” that produce more insulin per beta cell than normal. Another approach has been to try to develop immortal, or at least long-lasting, lines of human pancreatic beta cells from human adult or fetal pancreatic islets. Yet another approach has been to substitute porcine or nonhuman primate islets, which in theory could be produced in unlimited quantities, for human islets. Each of these approaches has advantages and disadvantages, and each still has substantial practical, intellectual, regulatory, and, in some cases, ethical hurdles to overcome.
Transdifferentiating Liver Cells into Beta Cells
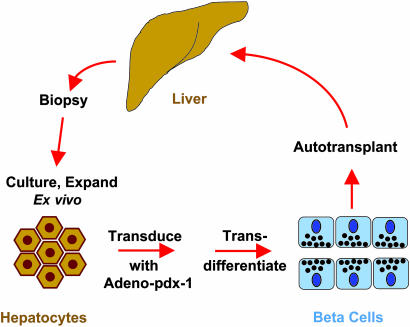
With this background, one can appreciate the report of Sapir et al. (1). Perhaps the most important observation is that by transducing liver cells (hepatocytes) with a single gene, Sapir et al. were able to induce them to transdifferentiate into human beta cells (Fig. 1). Importantly, they have used human adult liver cells as a starting point. This is an advantage, because it obviates allograft and xenograft issues, and because harvesting significant quantities of liver cells (hepatocytes) from children and adults with type 1 diabetes is technically relatively simple and currently possible. In addition, the cultures of human liver cells can be propagated for months, and the numbers of cells can be expanded substantially ex vivo. This is important, for it means that large numbers of hepatocytes or transdifferentiated beta cells could be harvested and stored from a single individual. This process would allow large numbers of beta cells to be transplanted initially, and others to be cryopreserved, if the “beta cell tank” needed to be refilled in the future.
Fig. 1.
The hepatocyte-to-beta cell transdifferentiation paradigm. Hepatocytes are obtained by liver biopsy from a donor or patient with diabetes, cultured and expanded ex vivo, transduced with a pdx-1 virus, transdifferentiated into functioning, insulin-producing beta cells, and then transplanted into a patient with diabetes.
What is the gene that confers these wonderful outcomes? The gene encodes a protein variously called Pdx-1, Stf-1, or Ipf-1. Pdx-1 stands for pancreatic and duodenal homeobox gene-1. Elegant work from the Edlund laboratory in Sweden (4) demonstrated that disruption of the pdx-1 gene in mice leads to failure of development of the entire pancreas, an absence of beta cells, and severe neonatal diabetes. Others have demonstrated that pdx-1 is a transcription factor that is required for normal beta cell development and function in mice (5, 6), that some humans with type 2 diabetes have heterozygous mutations in pdx-1, and that homozygous mutations in humans also lead to failure of pancreatic development and diabetes (7). Others have shown that pdx-1 is upstream of a complex family of transcription factors, whose careful, sequential orchestration of activation and inactivation are required for normal beta cell development and function.
Thus, it might not be surprising that overexpression of pdx-1 can lead to the development of insulin-producing cells. It is surprising, however, that overexpression of this single gene in hepatocytes would turn them into pancreatic beta cells so efficiently and effectively. First, most work to date has focused on pdx-1 as a regulator of “metabolic” genes in the islet (e.g., insulin, Glut-2, glucokinase, etc.), and less on structural genes required for the development of classical, authentic dense-core neuroendocrine, regulated secretory granules. Sapir et al. have converted a classical constitutively secreting cell type (the hepatocyte) into a classical neuroendocrine cell type (the beta cell): their new beta cells not only make insulin and other metabolic molecules (e.g., Glut-2 and glucokinase, which together comprise the glucose sensor) but also contain the key cellular components for regulated neuroendocrine secretion: secretory granules, secretory granule proteins, and neuroendocrine hormone processing enzymes.
A second surprise is that when others have introduced pdx-1 alone into liver cells in rodents, it triggers the development not only of islet cells, but also of the pancreatic exocrine (digestive enzyme-producing) cells that make destructive enzymes like amylase, trypsin, chymotrypsin, lipase, etc. (8). There is no evidence of “hepatitis” in the Sapir et al. transplanted beta cells, although exocrine differentiation was not rigorously sought.
A third surprise is that the transdifferentiation appears to be sustained in vivo for up to 60 days. One might have guessed that the transdifferentiation would last as long as the exogenous pdx-1 gene is expressed. Because the rat pdx-1 was delivered to the liver cells by using an adenovirus, and because adenovirus has a reputation in immunologically intact animals of being short-lived, one might have expected only a transient response and a need for sustained expression of pdx-1. So far, at least, this appears not to be true, perhaps because the endogenous human pdx-1 gene appears to be activated in the new islets.
Next Steps in the Transdifferentiation Paradigm
The studies need to be confirmed and extended to longer time points: 60 days may be a long time in a mouse with diabetes, but type 1 diabetes in humans is a multidecade disease. It is important to learn whether adenovirus persists in this model and whether such persistence is required. Safety studies need to be performed: what happens if the transduced islet grafts are followed for much longer periods of time? Will they continue to function? Will they develop tumors or inappropriate beta cell hyperplasia or induce hepatitis? Will they lead to dangerous hypoglycemia? And can the islet cells be delivered into the liver, as is done in humans? Will they dedifferentiate into hepatocytes if introduced into the liver? Because the beta cells that are created with this method produce relatively small quantities of insulin, can they be induced to produce even more insulin to mimic normal human islets or even become super islets? And, because the Sapir islet cells contain what appear to be secretory granules, can insulin really be secreted within 2 or 3 min of exposure to glucose, as occurs in normal beta cells?
Is Gene Therapy for Type 1 Diabetes Rational?
Finally, the gene therapy issue is a “hot button” topic. The pdx-1 gene was delivered by using a standard gene therapy vector: a replication-defective adenovirus. Adenovirus has acquired a bad reputation as a gene therapy vector because it induces host immune responses (anyphylaxis when given parenterally in large quantities, and rejection of transduced cells when used to deliver missing genes in cystic fibrosis and hypercholesterolemia) and because it is nonintegrating (does not incorporate into the host genome and is therefore lost from target cells over time). The Sapir paradigm is different, however, from other gene therapy strategies. Because the adenovirus is delivered ex vivo, and because transdifferentiation does not seem to require long-term expression of the adenoviral vector, adenovirus may be ideal. Moreover, immunologic issues that cloud adenoviral gene therapy are likely irrelevant in cell replacement therapy for type 1 diabetes, because such patients are already treated with immunosuppressants to combat alloimmunity and autoimmunity. Finally, results of human trials with integrating vectors have demonstrated that integration into the host genome runs the risk of inactivating or disrupting tumor suppressor genes (9). Perhaps nonintegration is not such a bad thing after all!
This is an exciting era in diabetes research, and progress is being made at an astonishing rate. The report by Sapir et al. represents one more intriguing step in the campaign against type 1 diabetes.
Acknowledgments
This work was supported by National Institutes of Health Grant R-33 DK 066127.
See companion article on page 7964.
References
- 1.Sapir, T., Shternhall, K., Meivar-Levy, I., Blumenfeld, T., Cohen, H., Skutelski, E., Eventov-Friedman, S., Barhack, I., Goldberg, I., Pri-Chen, S., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 7964–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen, J. (2004) Endocr. Rev. 25, 919–946. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro, A. M. J., Lakey, J. R. T., Ryan, E. A., Korbutt, G. S., Toth, E., Warnock, G. L., Kneteman, N. M. & Rajotte, R. V. (2000) N. Engl. J. Med. 343, 230–238. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson, J., Carlsson, L., Edlund, T. & Edlund, H. (1994) Nature 371, 606–609. [DOI] [PubMed] [Google Scholar]
- 5.Habener, J. F., Kemp, D. M. & Thomas, M. K. (2005) Endocrinology 146, 1025–1034. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni, R. N., Jhala, U. S., Winnay, J. N., Krajewski, S., Montminy, M. & Kahn, C. R. (2004) J. Clin. Invest. 114, 828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoffers, D. A., Zinkin, N. T., Stanojevic, V., Clarke, W. L. & Habener, J. F. (1997) Nat. Genet. 15, 106–110. [DOI] [PubMed] [Google Scholar]
- 8.Kojima, H., Fujimiya, M., Matsumura, K., Younan, P., Imaeda, H., Maeda, M. & Chan, L. (2003) Nat. Med. 9, 596–603. [DOI] [PubMed] [Google Scholar]
- 9.Williams, D. A. & Baum, C. (2003) Science 302, 400–401. [DOI] [PubMed] [Google Scholar]