Abstract
The tupaia herpesvirus (THV) was isolated from spontaneously degenerating tissue cultures of malignant lymphoma, lung, and spleen cell cultures of tree shrews (Tupaia spp.). The determination of the complete nucleotide sequence of the THV strain 2 genome resulted in a 195,857-bp-long, linear DNA molecule with a G+C content of 66.5%. The terminal regions of the THV genome and the loci of conserved viral genes were found to be G+C richer. Furthermore, no large repetitive DNA sequences could be identified. This is in agreement with the previous classification of THV as the prototype species of herpesvirus genome group F. The search for potential coding regions resulted in the identification of 158 open reading frames (ORFs) regularly distributed on both DNA strands. Seventy-six out of the 158 ORFs code for proteins that are significantly homologous to known herpesvirus proteins. The highest homologies found were to primate and rodent cytomegaloviruses. Biological properties, protein homologies, the arrangement of conserved viral genes, and phylogenetic analysis revealed that THV is a member of the subfamily Betaherpesvirinae. The evolutionary lineages of THV and the cytomegaloviruses seem to have branched off from a common ancestor. In addition, it was found that the arrangements of conserved genes of THV and murine cytomegalovirus strain Smith, both of which are not able to form genomic isomers, are colinear with two different human cytomegalovirus (HCMV) strain AD169 genomic isomers that differ from each other in the orientation of the long unique region. The biological properties and the high degree of relatedness of THV to the mammalian cytomegaloviruses allow the consideration of THV as a model system for investigation of HCMV pathogenicity.
The family Herpesviridae comprises more than 100 different virus species with a worldwide occurrence in all taxonomic groups of vertebrates. The supposed roots of this virus family are in very early evolutionary times, and a long period of development has resulted in the appearance of extremely well host-adapted virus species (47, 61, 62, 63), often more than one in a single host, for example, the eight different human herpesviruses HSV-1 (herpes simplex virus type 1), HSV-2, varizella-zoster virus, HCMV (human cytomegalovirus), EBV (Epstein-Barr virus), HHV-6 (human herpesvirus 6), HHV-7, and HHV-8, that are adapted to different cellular and molecular niches in the same host species (88).
A member of the large herpesvirus family is the Tupaia herpesvirus (THV) that infects tree shrews (Tupaia spp., family Tupaiidae), a group of primitive higher mammals (Proteutheria, Scandentia) that is supposed to have diverged at the base of the primate evolutionary tree (52, 69). Tree shrews were originally distributed in Southeast Asia and are used worldwide as laboratory animals in neurological and physiological research. THV was isolated by Mirkovic et al. in 1970 (68) from a spontaneously degenerating lung tissue culture of a tree shrew and subsequently classified as a herpesvirus by electron microscopic examination (57). From 1977 to 1985, six additional isolates were isolated from malignant lymphoma tissue cultures and degenerating spleen cell cultures of tree shrews (19, 20, 21, 22, 24, 49). The seven THV isolates were grouped into five strains (THV strains 1 to 5) according to their restriction endonuclease cleavage patterns. Molecular cloning and physical mapping of the genome of THV strain 2 was performed, and a complete genome library was established (49). THV strain 2 was isolated in 1979 from a spontaneously degenerating malignant lymphoma cell culture by Darai et al. (19).
THV particles show the classical morphology of herpesviruses (19, 61, 73) and contain a linear double-stranded DNA genome of about 200 kbp (21, 49). The detection of concatemeric viral DNA molecules in infected cells (50) corresponds to the rolling-circle model of herpesvirus genome replication. The herpesviruses have been classified into six genome groups (A to F) (72) according to the presence and arrangement of large repetitive DNA sequences. THV is the prototype species of genome group F, which is characterized by a unique DNA sequence without any extended repetitive DNA elements. The family Herpesviridae is subdivided into the subfamilies Alpha-, Beta-, and Gammaherpesvirinae according to the length of the replication cycle, speed of spreading in cell culture, host range, and location of latency, which is an important biological characteristic of herpesviruses (73). According to its biological properties (19, 20, 22, 24) and in agreement with recent data (5, 82), THV is supposed to be a member of the subfamily Betaherpesvirinae.
THV infections cause a remarkable variety of different clinical pictures in tree shrews, ranging from inapparent to deadly infections and the development of malignant lymphomas (19, 23, 41). Based on the evolutionary stage of tree shrews and the biological and genomic properties of THV, the elucidation of the viral coding strategy is of particular interest. The characterization of the primary structure of the whole genome of THV strain 2 isolated from a malignant lymphoma (19) is the subject of this report. This study allowed the determination of the final phylogenetic placement of THV within the family Herpesviridae.
MATERIALS AND METHODS
Viral DNA and genomic library.
Propagation of THV on tupaia baby fibroblasts and isolation of viral DNA were carried out as described previously (22). Recombinant plasmids harboring specific DNA sequences of the THV genome were obtained from a defined genome library as described elsewhere (49).
Strategy of determination of the THV genome nucleotide sequence.
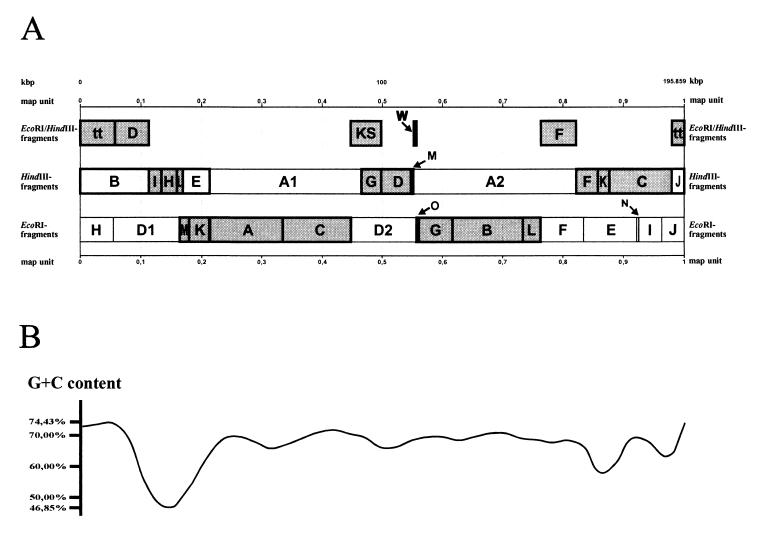
Determination of the complete nucleotide sequence of the THV genome was accomplished by analysis of the DNA nucleotide sequences of recombinant plasmids harboring specific EcoRI, HindIII, and EcoRI/HindIII fragments of the THV genome (Fig. 1) that form the entirety of the THV genome. The nucleotide sequences of the individual recombinant plasmids were determined by primer walking (86). The correctness of the physical map shown in Fig. 1 was confirmed by amplification and sequencing of the genome regions of original THV DNA around the endonuclease restriction sites, which allowed the assembly of the nucleotide sequences of the individual THV fragments resulting in the nucleotide sequence of the whole THV genome.
FIG. 1.
(A) Physical map of the THV strain 2 genome consisting of EcoRI, HindIII, and EcoRI/HindIII DNA fragments. The THV DNA fragments that are used to determine the nucleotide sequence of the whole THV genome are shaded and bordered in bold. Beneath the physical map, the G+C content over the course of the whole THV genome is shown (B). It varies between 46.85 and 74.43%.
Enzymes and DNA isolation.
The restriction endonucleases were purchased from Roche Diagnostics GmbH (Mannheim, Germany). Incubations were carried out according to standard procedures for each enzyme. The recombinant plasmids harboring the DNA sequences of the EcoRI, HindIII, and EcoRI/HindIII fragments that were used to determine the complete nucleotide sequence of the THV genome were purified using the Qiagen Plasmid Midi Prep (Qiagen GmbH, Hilden, Germany) procedure. The PCR products were purified using Micro-Spin S-300 HR columns (Pharmacia Biotech).
DNA sequencing.
The recombinant plasmids and the purified PCR products were sequenced using the DyeDeoxy Terminator Taq cycle sequencing technique (Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit; Applied Biosystems GmbH, Weiterstadt, Germany) and a 373A “Extended” DNA sequencer (Applied Biosystems) as described previously (86). The nucleotide sequence of the THV genome was determined by primer walking. The Sequence Navigator software (Version 1.01; Applied Biosystems) was used to assemble the nucleotide sequences obtained from individual sequencing reactions.
Computer-assisted analysis.
The strategies used to identify THV genes likely to encode were based on those used in the sequence analysis of other herpesviruses (71). The major criterion for identifying a coding sequence was the presence of an open reading frame (ORF) with a minimum length of 300 bp and less than 60% overlap with adjacent ORFs. The presence of ORFs with more than 60% overlap in Fig. 2 has its explanation in similar lengths and the absence of convincing identification criteria which distinguish between the corresponding ORFs. In addition, analysis of codon usage, the presence of consensus promoter sequences, and homology to known genes were used to support initial ORF selection. Gaps between ORFs were inspected for smaller ORFs and were included in the final ORF map (Fig. 2) if they satisfied the other criteria used for ORF selection. The identification of ORFs was performed with the program TRANSLATE of the the PC/GENE program, release 6.85 (Intelligenetics Inc., Mountain View, Calif.); the decisive criterion for the selection of an ORF was the presence of an ATG start codon and a stop codon (TAA, TGA, TAG) at the end. Searches of potential THV proteins for homology to known proteins were performed by applying BLAST (basic local alignment search tool) (3) and FSTPSCAN of PC/GENE to SWISSPROT database release 39. Protein alignments were carried out with the CLUSTAL program (39). Examination of the DNA sequence for transcription signals was performed by using the search features of the program EUKPROM of PC/GENE 6.85. Protein motif searches were performed with the program PROSITE of PC/GENE 6.85 and PROSITE release 16.25 on the ExPASy Molecular Biology Server (10, 40). The sequence was analyzed for tandem and inverted repeats by using the program REPEATS of PC/GENE 6.85. The phylogenetic trees were calculated using the personal computer programs ClustalW (version 1.64b) (85), Kitsch (version 10.0), Protdist (version 10.0), and Treeview (version 1.5.2).
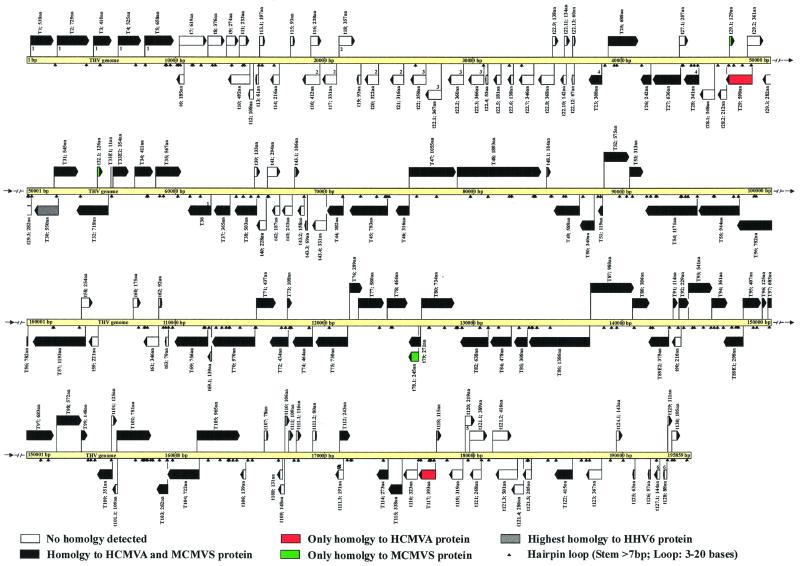
FIG. 2.
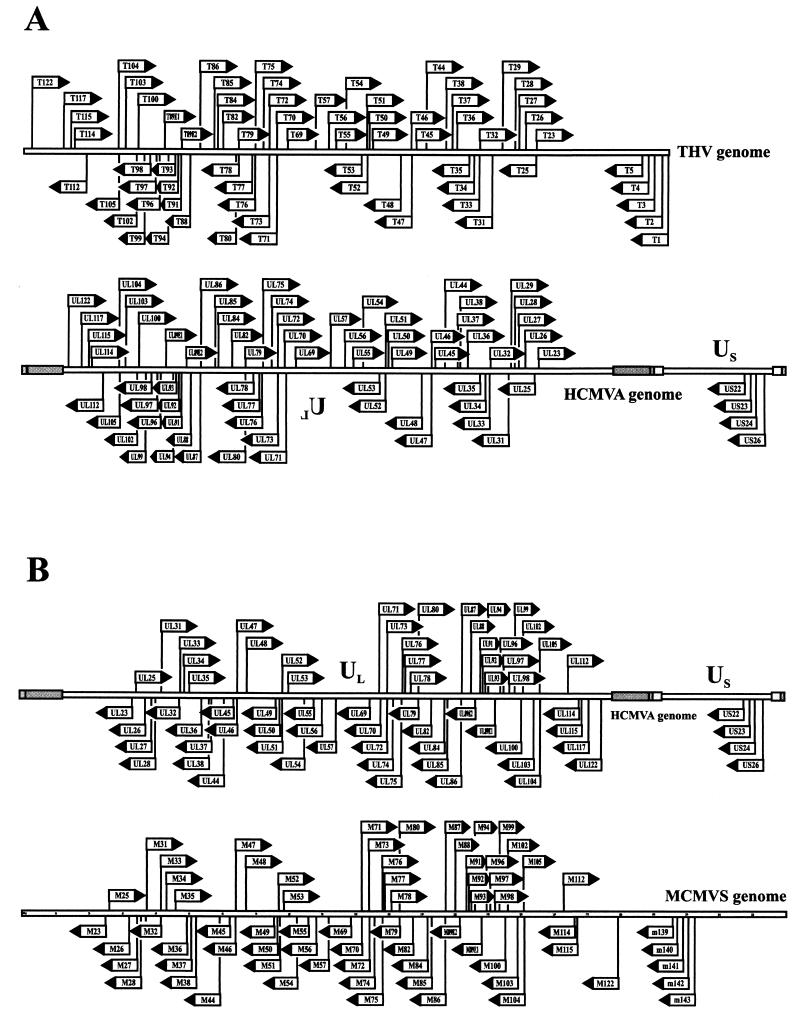
Coding strategy of the THV genome. ORFs that code for proteins with homologies to known HCMVA and MCMVS proteins are depicted as black arrows, and those with no detected homologies are shown as white arrows. ORFs that show the highest homology only to an HCMVA or an MCMVS protein are in red or green, respectively. The orientation of each arrow corresponds to the direction of transcription. Hairpin loops with a stem length of more than 7 bp and a loop of 3 to 20 bases are marked by little black arrows. ORFs with adjacent numbers are members of corresponding gene families defined by significant homology to one another.
Nomenclature.
All of the ORFs that are likely to be coding sequences are listed in Table 1. They are numbered in the order of their appearance when the genome sequence is analyzed from base pair 1 to base pair 195,859. ORFs that are oriented to the right are marked with the letter R, and ORFs that are oriented to the left are marked with the letter L. In addition, ORFs are marked with the letter T for THV and numbered according to homologous proteins of HCMV strain AD169 (HCMVA) and murine cytomegalovirus strain Smith (MCMVS). THV ORFs that are assumed to be coding sequences but have no homology to known proteins are marked with the letter t and labeled so as to fill the gaps between the homologous ORFs.
TABLE 1.
Supposed THV coding ORFsa
| THV strain 2 ORF | Nucleotide position | Length (aa) | Potential site and signature (position within protein sequence) | Most-homologous protein(s) (no. of aa); possible function | % I/S |
|---|---|---|---|---|---|
| T1 (R) | 258–1811 | 518 | Mitochondrial energy transfer protein signature (154–162); cereal trypsin–α-amylase inhibitor family signature (207–230) | HCMVA US23 (592), MCMVS M143 (557); transactivator | 23.0/34.8, 21.4/42.3 |
| T2 (R) | 2034–4220 | 729 | 4 bipartite nuclear targeting sequences (459–475; 460–476; 461–477; 462–478); leucine zipper pattern (244–265) | HCMVA US23 (592), MCMVS M143 (557); transactivator | 25.0/29.4, 21.5/33.2 |
| T3 (R) | 4486–5973 | 496 | Cell attachment sequence (372–374) | HCMVA US24 (500), MCMVS M141 (508); transactivator | 32.6/29.5, 25.6/29.5 |
| T4 (R) | 6111–7685 | 525 | HCMVA US23 (592), MCMVS M140 (484); transactivator | 32.6/32.1, 29.8/37.4 | |
| T5 (R) | 7917–9890 | 658 | Mitochondrial energy transfer protein signature (252–260) | HCMVA US22 (593), MCMVS M139 (644); transactivator | 29.4/32.7, 23.6/32.9 |
| t6 (L) | 9998–10552 | 185 | |||
| t7 (R) | 10231–12087 | 619 | |||
| t8 (R) | 12154–13281 | 376 | |||
| t9 (R) | 13387–14208 | 274 | Cell attachment sequence (227–229) | ||
| t10 (L) | 13488–14963 | 492 | Bipartite nuclear targeting sequence (426–442) | ||
| t11 (R) | 14252–14950 | 233 | Microbody C-terminal targeting signal (231–233) | ||
| t12 (L) | 14872–15195 | 108 | Bipartite nuclear targeting sequence (74–90) | ||
| t13 (R) | 15350–15532 | 61 | Bipartite nuclear targeting sequence (31–47) | ||
| t13.1 (R) | 15632–15952 | 107 | |||
| t14 (L) | 16283–16930 | 216 | |||
| t15 (R) | 17685–17963 | 93 | |||
| t16 (L) | 18440–19675 | 412 | Aminoacyl-transfer RNA synthetase class II signature (170–179) | ||
| t16.1 (R) | 19060–19773 | 238 | |||
| t17 (L) | 19807–20799 | 331 | |||
| t18 (R) | 20975–22045 | 357 | |||
| t19 (L) | 22166–22444 | 93 | |||
| t20 (L) | 22656–23621 | 322 | |||
| t21 (L) | 24376–25323 | 316 | |||
| t22 (L) | 25743–26816 | 358 | |||
| t22.1 (L) | 26749–27849 | 367 | |||
| t22.2 (L) | 28262–29341 | 360 | |||
| t22.3 (L) | 29622–30719 | 366 | |||
| t22.4 (L) | 30811–30969 | 53 | |||
| t22.5 (L) | 31313–31855 | 181 | |||
| t22.6 (L) | 32271–32684 | 138 | |||
| t22.7 (L) | 33015–34052 | 346 | |||
| t22.8 (L) | 34304–35407 | 368 | |||
| t22.9 (R) | 35289–35702 | 138 | Cell attachment sequence (51–53) | ||
| t22.10 (L) | 35795–36220 | 142 | |||
| t22.11 (R) | 36085–36486 | 134 | |||
| t22.12 (L) | 36591–36791 | 67 | |||
| t22.13 (R) | 36656–36835 | 60 | |||
| T23 (L) | 37706–38629 | 308 | HCMVA UL23 (342), MCMVS M23 (391); transactivator | 25.9/29.5, 24.6/31.7 | |
| T25 (R) | 39053–41116 | 688 | HCMVA UL25 (656), MCMVS M25 (932); tegument protein | 20.5/39.3, 17.8/35.6 | |
| T26 (L) | 41208–41933 | 242 | HCMVA UL26 (188), MCMVS M26 (192); virion protein | 27.3/33.1, 25.3/31.0 | |
| T27 (L) | 42066–43933 | 636 | Bipartite nuclear targeting sequence (266–282); multicopper oxidase signature 1 (164–184) | HCMVA UL27 (608), MCMVS M27 (682) | 28.2/34.4, 25.4/38.7 |
| T27.1 (R) | 43846–44466 | 207 | Bipartite nuclear targeting sequence (159–175); Cell attachment sequence (36–38) | ||
| T28 (L) | 44135–45157 | 341 | HCMVA UL29 (360), MCMVS M28 (430); transactivator | 17.9/35.1, 26.8/27.7 | |
| t28.1 (L) | 45174–46217 | 348 | Gram-positive coccus surface protein anchoring hexapeptide (162–167) | ||
| t28.2 (L) | 46395–47030 | 212 | Microbody C-terminal targeting signal (210–212) | ||
| T29 (L) | 46951–48744 | 598 | 3 bipartite nuclear targeting sequences (384–400; 385–401; 516–532) | HCMVA UL29 (360); transactivator | 21.7/22.1 |
| T29.1 (R) | 47212–47598 | 129 | Cell attachment sequence | MCMVS M106 (147) | 22.4/42.9 |
| t29.2 (R) | 48411–49493 | 361 | Bipartite nuclear targeting sequence (236–252) | ||
| t29.3 (L) | 49402–50247 | 282 | |||
| T30 (L) | 50424–52073 | 550 | HSV6 VU4 | 20.5/38.2 | |
| T31 (R) | 51785–53419 | 545 | HCMVA UL31 (694), MCMVS M31 (516) | 21.0/30.5, 14.6/40.9 | |
| T32 (L) | 53284–55437 | 718 | Leucine zipper pattern (193–214); pfkB family of carbohydrate kinase signature 1 (326–349) | HCMVA UL32 (1048), MCMVS M32 (718); major tegument protein | 18.3/32.1, 21.0/39.2 |
| T32.1 (R) | 54701–55078 | 126 | MCMVS M19 (147) | 20.4/28.3 | |
| T33 Exon 1 (R) Exon 2 (R) | 55618–55650 55754–56815 | 365 | GCR signature (119–135) | HCMVA UL33 (390), MCMVS M33 (377); GCR homologue | 39.2/30.3, 51.6/33.6 |
| T34 (R) | 57213–58445 | 411 | HCMVA UL34 (504), MCMVS M34 (854) | 21.5/36.4, 15.8/22.9 | |
| T35 (R) | 58631–60331 | 567 | HCMVA UL35 (640), MCMVS M35 (519) | 26.5/35.2, 30.9/38.3 | |
| T36 (L) | 60733–62329 | ? | ? | HCMVA UL36 (476), MCMVS M36 (507); immediate-early regulatory protein | ? |
| T37 (L) | 62518–63612 | 365 | HCMVA UL37 (932), MCMVS M37 (345); immediate early regulatory protein | 17.2/31.3, 22.8/39.9 | |
| T38 (L) | 63903–65411 | 503 | HCMVA UL38 (331), MCMVS M38 (497); immediate-early regulatory protein | 22.8/25.5, 22.6/40.7 | |
| t39 (R) | 65237–65635 | 133 | Microbody C-terminal targeting signal (131–133); bipartite nuclear targeting sequence (99–115) | ||
| t40 (L) | 65341–66024 | 228 | |||
| t41 (R) | 66103–66984 | 294 | Bipartite nuclear targeting sequence (15–31); cell attachment sequence (22–24) | ||
| t42 (L) | 66371–66931 | 187 | |||
| t43 (L) | 67053–67781 | 243 | |||
| t43.1 (R) | 67950–68267 | 106 | |||
| t43.2 (L) | 68122–68595 | 158 | |||
| t43.3 (L) | 68598–68804 | 69 | |||
| t43.4 (L) | 69102–70094 | 331 | |||
| T44 (L) | 70088–71233 | 382 | HCMVA UL44 (433), MCMVS M44 (411); polymerase accessory protein | 53.7/29.5, 54.1/30.4 | |
| T45 (L) | 71625–74729 | 1,035 | Bipartite nuclear targeting sequence (8–24) | HCMVA UL45 (906), MCMVS M45 (876); ribonucleotide reductase | 22.8/36.0, 26.0/37.4 |
| T46 (L) | 74745–75692 | 316 | HCMVA UL46 (290), MCMVS M46 (294); capsid assembly and maturation protein | 38.1/37.1, 39.5/32.6 | |
| T47 (R) | 75692–78856 | 1,055 | Leucine zipper pattern (672–693) | HCMVA UL47 (982); MCMVS M47 (1040); capsid assembly protein | 29.6/40.3, 24.9/40.2 |
| T48 (R) | 78881–85765 | 2,295 | Bipartite nuclear targeting sequence (2184–2200); cell attachment sequence (936–938); 3 leucine zipper patterns (431–452, 2260–2281, 2267–2288); sugar transport protein signature 1 (1610–1623) | HCMVA UL48 (2241), MCMVS M48 (2149); large tegument protein | 30.8/16.4, 29.4/16.6 |
| t48.1 (R) | 86101–86412 | 104 | Prenyl group binding site (CAAX box 101–104); Bowman-Birk serine protease inhibitor family signature (94–101) | ||
| T49 (L) | 86568–88331 | 588 | Bipartite nuclear targeting sequence (250–266); cell attachment sequence (122–124) | HCMVA UL49 (570), MCMVS M49 (536); viral protein | 40.3/29.6, 40.6/30.0 |
| T50 (L) | 88303–89322 | 340 | HCMVA UL50 (397), MCMVS M50 (316); viral protein | 40.5/31.2, 41.1/33.1 | |
| T51 (L) | 89539–89895 | 119 | HCMVA UL51 (157), MCMVS M51 (233); DNA cleavage and packaging protein | 38.9/27.4, 29.6/15.9 | |
| T52 (R) | 89985–91709 | 575 | HCMVA UL52 (668), MCMVS M52 (517); major envelope glycoprotein | 37.9/33.5, 45.1/32.2 | |
| T53 (R) | 91705–92643 | 313 | HCMVA UL53 (376), MCMVS M53 (333); Viral protein | 37.8/25.4, 43.7/35.4 | |
| T54 (L) | 92738–96250 | 1,171 | DNA polymerase family B signature (873–881) | RHCMV UL54, HCMVA UL54 (1,243), MCMVS M54 (1,097); DNA polymerase | 50.7/26.5, 43.5/25.5, 48.5/26.6 |
| T55 (L) | 96253–99084 | 944 | HCMVA UL55 (907), MCMVS M55 (937); glycoprotein B | 46.2/33.7, 41.6/35.8 | |
| T56 (L) | 98906–101251 | 782 | HCMVA UL56 (851), MCMVS M56 (798); probable processing and transport protein | 51.1/30.1, 54.1/32.0 | |
| T57 (L) | 101550–105128 | 1,193 | Leucine zipper pattern (497–518); aldehyde dehydrogenase cysteine active site (1087–1098); crystalline beta and gamma Greek key motif signature (809–824) | HCMVA UL57 (1,236), MCMVS M57 (1,191); major DNA-binding protein | 54.1/30.1, 52.7/33.7 |
| t58 (R) | 104869–105630 | 254 | 2 cell attachment sequences (212–214, 217–219) | ||
| t59 (L) | 105320–105982 | 221 | |||
| t60 (R) | 108366–108890 | 175 | 4 bipartite nuclear targeting sequences (120–136, 133–149, 134–150, 135–151); 7 leucine zipper pattern (44–65, 51–72, 58–79, 65–86, 72–93, 79–100, 86–107) | ||
| t61 (L) | 109040–110077 | 346 | |||
| t62 (R) | 110625–110900 | 92 | Bipartite nuclear targeting sequence (10–26) | ||
| t63 (L) | 110625–110861 | 79 | |||
| T69 (L) | 111240–113507 | 756 | HCMVA UL69 (744), MCMVS M69 (841); transcriptional regulator | 23.6/44.8; 22.8/36.4 | |
| t69.1 (L) | 113441–113857 | 139 | |||
| T70 (L) | 113758–116667 | 970 | Leucine zipper pattern (642–663) | HCMVA UL70 (1062), MCMVS M70 (964); helicase-primase complex protein | 38.3/27.9, 39.5/31.9 |
| T71 (R) | 116762–118072 | 437 | HCMVA UL71 (411), MCMVS M71 (299); viral protein | 27.0/32.0, 26.0/25.6 | |
| T72 (L) | 117603–118838 | 412 | Bipartite nuclear targeting sequence (389–405) | HCMVA UL72 (388), MCMVS M72 (382); dUTP-pyrophosphatase | 24.4/37.4, 20.0/35.5 |
| T73 (R) | 118838–119161 | 108 | HCMVA UL73 (138), MCMVS M73 (94); membrane protein | 27.5/34.1, 34.5/37.3 | |
| T74 (L) | 119153–120544 | 464 | HCMVA UL74 (466), MCMVS M74 (438); glycoprotein H-L complex component | 19.8/41.2, 16.9/39.6 | |
| Y75 (L) | 120745–122934 | 730 | Prenyl group binding site (CAAX box; 727–730) | HCMVA UL75 (743), MCMVS M75 (725); glycoprotein H | 30.3/39.8, 30.2/38.8 |
| T76 (R) | 123058–123924 | 289 | 3 bipartite nuclear targeting sequences (267–283, 268–284, 269–285) | HCMVA UL76 (325), MCMVS M76 (254); viral protein | 36.4/28.1, 37.7/25.7 |
| T77 (R) | 123662–125401 | 580 | Bipartite nuclear targeting sequence (29–45) | HCMVA UL77 (642), MCMVS M77 (628); DNA cleavage and packaging protein | 47.8/28.4, 45.1/31.1 |
| T78 (R) | 125593–126984 | 464 | HCMVA UL78 (431), MCMVS M78 (471); GCR homologue | 21.4/37.0, 16.4/42.4 | |
| T78.1 (L) | 126986–127720 | 245 | Bipartite nuclear targeting sequence (69–85) | MCMVS M59 (340) | 20.8/32.7 |
| T79 (L) | 127033–127845 | 271 | HCMVA UL79 (295), MCMVS M79 (258); viral protein | 45.9/28.7, 49.4/28.4 | |
| T80 (R) | 127913–130114 | 734 | HCMVA UL80 (708), MCMVS M80 (697); scaffolding protein: protease | 31.3/38.3, 33.1/38.4 | |
| T82 (L) | 130452–132365 | 638 | Microbody C-terminal targeting sequence (636–638); cell attachment sequence (86–88) | HCMVA UL82 (559), MCMVS M82 (598); tegument phophoprotein | 24.1/35.8, 18.5/40.3 |
| T84 (L) | 132502–133935 | 478 | Leucine zipper pattern (116–137, 123–144) | HCMVA UL84 (586), MCMVS M84 (587); DNA replication regulatory protein | 22.3/34.0, 17.3/36.5 |
| T85 (L) | 134070–134993 | 308 | HCMVA UL85 (306), MCMVS M85 (311); capsid protein | 58.3/27.2, 53.3/34.3 | |
| T86 (L) | 135032–139189 | 1,386 | Cell attachment sequence (1292–1294) | HCMVA UL86 (1,370), MCMVS M86 (1,353); major capsid protein | 57.9/15.2, 57.4/14.5 |
| T87 (R) | 139257–141196 | 980 | Cell attachment sequence (368–370) | HCMVA UL87 (941), MCMVS M87 (926); viral protein | 41.6/30.2, 45.5/28.7 |
| T88 (R) | 142066–143223 | 386 | Cell attachment sequence (158–160); leucine zipper pattern (333–354) | HCMVA UL88 (429), HCMVA M88 (426); virion protein | 29.0/34.9, 27.5/34.6 |
| T89E2 (L) | 143242–144366 | 375 | HCMVA UL89 (674), MCMVS M89 (671); DNA cleavage and packaging protein | 66.4/24.9, 68.9/23.8 | |
| t90 (L) | 144657–145304 | 216 | GCR signature (51–67) | ||
| T91 (R) | 144758–145099 | 114 | HCMVA UL91 (111), MCMVS M91 (134); viral protein | 33.3/47.4, 22.8/22.8 | |
| T92 (R) | 145145–145831 | 229 | Bipartite nuclear targeting sequence (149–165) | HCMVA UL92 (201), MCMVS M92 (230); viral protein | 48.5/28.8, 58.7/26.5 |
| T93 (R) | 145800–147422 | 541 | Cell attachment sequence (77–79) | HCMVA UL93 (594), MCMVS M93 (515); viral protein | 23.3/32.8, 28.1/37.8 |
| T94 (R) | 147368–148440 | 361 | HCMVA UL94 (345), MCMVS M94 (345); capsid-tegument protein | 33.5/35.1, 34.7/38.8 | |
| T89E1 (L) | 148569–149462 | 298 | HCMVA UL89 (674), MCMVS M89 (671); DNA cleavage and packaging protein | 66.4/24.9, 68.9/23.8 | |
| T96 (R) | 150689–151063 | 125 | HCMVA UL96 (115), MCMVS M96 (129); viral protein | 22.8/44.9, 26.8/41.3 | |
| T97 (R) | 151128–153176 | 683 | Tyrosine protein kinase-specific active-site signature (407–419) | HCMVA UL97 (707), MCMVS M97 (643); phosphotransferase | 32.2/36.3, 29.6/34.3 |
| T98 (R) | 153341–155056 | 572 | HCMVA UL98 (584), MCMVS M98 (561); alkaline exonuclease | 43.1/31.0, 33.2/34.1 | |
| T99 (R) | 154996–155439 | 148 | HCMVA UL99 (112), MCMVS M99 (190); tegument phosphoprotein | 18.6/43.8, 24.3/33.1 | |
| T100 (L) | 156038–157090 | 351 | HCMVA UL100 (372), MCMVS M100 (371); glycoprotein M | 49.5/30.4, 50.7/30.9 | |
| t101 (R) | 157036–157410 | 125 | Bipartite nuclear targeting sequence (50–66) | ||
| t101.1 (L) | 157060–157386 | 109 | Bipartite nuclear targeting sequence (13–29) | ||
| T102 (R) | 157386–159761 | 792 | Leucine zipper pattern (337–358) | HCMVA UL102 (798), MCMVS M102 (812); glycoprotein M | 26.3/34.9, 22.6/35.3 |
| T103 (L) | 160023–160808 | 262 | HCMVA UL103 (249), HCMVA M103 (317); viral protein | 34.3/38.5, 35.5/35.5 | |
| T104 (L) | 160762–162927 | 722 | Bipartite nuclear targeting sequence (687–703) | HCMVA UL104 (697), MCMVS M104 (704); DNA cleavage and packaging protein | 45.5/31.5, 40.4/35.4 |
| T105 (R) | 162774–165668 | 965 | ATP-GTP-binding site motif A (P loop) (176–183) | HCMVA UL105 (956), MCMVS M105 (948); (helicase) | 52.1/25.8, 47.5/25.4 |
| t106 (L) | 165625–166041 | 139 | |||
| t107 (R) | 167264–167497 | 78 | |||
| t108 (L) | 168103–168495 | 131 | |||
| t109 (L) | 168149–168592 | 148 | |||
| t110 (R) | 168666–168983 | 106 | |||
| t111 (R) | 168913–169239 | 109 | |||
| t111.1 (R) | 169430–169777 | 116 | |||
| t111.2 (R) | 170547–170786 | 80 | Cell attachment sequence (75–77) | ||
| t111.3 (L) | 172034–172606 | 191 | |||
| t112 (R) | 172345–173073 | 243 | HCMVA UL112 (268), MCMVS M112EI (264); (early phosphoprotein) | 33.5/33.5, 32.2/35.1 | |
| T114 (L) | 174794–175612 | 273 | HCMVA UL114 (250), MCMVS M114 (262); (uracil-DNA glycosylase) | 54.4/21.5, 50.9/23.7 | |
| T115 (L) | 175578–176591 | 338 | HCMVA UL115 (278), MCMVS M115 (274); (glycoprotein L) | 32.8/28.0, 33.9/32.4 | |
| t116 (L) | 176599–177567 | 323 | |||
| T117 (L) | 177626–178804 | 393 | HCMVA UL117 (424) | 20.9/45.3 | |
| t118 (R) | 178851–179195 | 115 | Leucine zipper pattern (29–50) | ||
| t119 (L) | 179674–180630 | 319 | ATP-dependent DNA ligase AMP-binding site (213–221) | ||
| t120 (R) | 180782–181438 | 219 | 2 bipartite nuclear targeting sequences (136–152, 137–153) | ||
| t121 (L) | 181046–181849 | 268 | |||
| t121.1 (R) | 181092–182258 | 389 | 2 bipartite nuclear targeting sequences (16–32, 373–389) | ||
| t121.2 (R) | 182632–183879 | 416 | 4 bipartite nuclear targeting sequences (313–329, 314–330, 320–336, 368–384); 2 cell attachment sequences (204–206, 214–216) | ||
| t121.3 (L) | 182778–184280 | 501 | 2 bipartite nuclear targeting sequences (99–115, 100–116); 2 cell attachment sequences (9–11, 388–390) | ||
| t121.4 (L) | 183833–184690 | 286 | Prenyl group binding site (CAAX box; 283–286) | ||
| t121.5 (L) | 184602–185216 | 205 | |||
| T122 (L) | 186729–187973 | 415 | HCMVA UL122 (411), MCMVS M122E5 (511); immediate-early regulatory protein | 33.2/44.0, 25.4/33.3 | |
| t123 (L) | 188849–189949 | 367 | |||
| t124 (R) | 190929–191357 | 143 | |||
| t125 (L) | 192003–192191 | 63 | |||
| t126 (L) | 193041–193211 | 57 | |||
| t127 (L) | 193423–193854 | 144 | 2 bipartite nuclear targeting sequences (33–49, | ||
| t128 (L) | 194052–194315 | 88 | |||
| t129 (R) | 194386–194718 | 111 | |||
| t130 (R) | 194655–195239 | 195 | Bipartite nuclear targeting sequence (82–98) |
aa, amino acids. %I/S percentage of amino acids identical (I) and similar (S) to the corresponding homologous proteins. RHCMV, rhesus cytomegalovirus. The homology values of T89 exons 1 and 2 are given for the complete corresponding proteins of HCMVA and MCMVS. Genetic analysis revealed that T36 is spliced, but it was not possible to prove this by RT-PCR experiments. For this reason, the length and homology values of T36 are replaced by question marks. The proteins that are most homologous to the individual potential THV proteins are underlined. The individual ORFs are numbered in the order in which they appear in the THV genome with the designation L for theoretical transcription to the left or R for theoretical transcription to the right. The sites and signatures found in the amino acid sequences of the potential THV proteins were determined by the program PROSITE of the PC/GENE software package and are putative. Shown are all ORFs that are longer than 300 bp and all ORFs that are longer than 150 bp if they are positioned between ORFs of more than 300 bp and that are supposed to code for viral proteins according to accepted rules for the choice of transcribed herpesvirus ORFs (see Materials and Methods). The THV ORFs are designated T (homologous to HCMVA and/or MCMVS proteins) or t (no homologous protein detected). The T THV ORFs are numerated according to the homologous proteins of HCMVA and MCMVS; the t THV ORFs are numbered to fill the gaps between the T THV ORFs.
RT PCR.
Tupaia baby fibroblasts were infected with THV strain 2. Total cellular RNA was isolated 14 h postinfection. RNA isolation was performed using the guanidinium-cesium chloride method as described previously (74). The reverse transcription step was carried out using the RNA LA PCR Kit, version 1.1 (Takara Shuzo Co., Shiga, Japan). PCR was performed using 0.5 fmol of the template DNA in 100-μl volumes containing 1.5 mM MgCl2, 12.5 nmol of each deoxynucleoside triphosphate, 50 pmol of each primer, and 2.5 U of ExTaq DNA polymerase (Takara Shuzo Co.). A total of 35 cycles were run in an automated temperature cycling reactor (Genius; Techne, Cambridge, United Kingdom) under cycling conditions of 96°C for 30 s, 60°C for 1 min, and 72°C for 2 min per cycle. Specific oligonucleotide primers were designed in order to amplify the coding region around the splice sites of THV genes T33 and T89. The following primers were used in the reverse transcriptase PCR (RT-PCR) experiments: 5′-CCATGGACGTCCTGCTGGCTC-3′ (primer 1) and 5′-CCCACGGTGCAGCTGGTGTAG-3′ (primer 2) for T33 and 5′-AAGCACGTTTCCCAGTTCGTCC-3′ (primer 3) and 5′-GAGTTTGGTCAGGAAGCAGGTG-3′ (primer 4) for T89. Primers 2 and 4 were used for first-strand synthesis of the cDNA by RT reaction.
Nucleotide sequence accession number.
The complete DNA sequence determined in this study has been submitted to the GenBank database and assigned accession number AF281817.
RESULTS
Features of the complete nucleotide sequence of the THV genome.
The nucleotide sequences of the accentuated THV fragments in Fig. 1 were determined by automated cycle sequencing and primer walking (86). The assembly of the nucleotide sequences of all of the THV fragments used according to the physical map of the viral genome (Fig. 1) resulted in determination of the complete DNA nucleotide sequence of the THV genome, comprising 195,859 bp. The DNA sequences of the genomic termini were defined by Albrecht et al. in 1985 (2) and were used to determine the left and the right ends of the genome in this study. Altogether, 1,473 sequencing reactions with a total of 684,259 determined bases were performed to obtain the nucleotide sequence of the complete THV genome. Both DNA strands were sequenced independently, and each nucleotide was determined with an average redundancy of 1.75. The average G+C content of the whole THV DNA molecule was found to be 66.5%. As shown in Fig. 1, the G+C distribution is not constant in the viral genome (46.85 to 74.43%). Analysis of the codon usage of THV revealed that the third base of codons of potential viral genes is almost exclusively a G or C.
Repetitive elements of the THV genome.
About 2,000 direct and 1,611 inverted repeats were identified. However, all of these repeats were no longer than 25 bp and occurred in short tandem arrangements. Some of the inverted repeats are supposed to form stable hairpin structures and are drawn in the ORF map of THV in Fig. 2. Furthermore, the analysis of the THV nucleotide sequence resulted in the identification of rare direct repeats between 25 and 105 bp in length. They are located between nucleotide positions 64165 and 64334, 108477 and 109442, 140381 and 140452, 186262 and 186305, and 193606 and 194265. The repetitive DNA sequences of the THV genome are restricted and are not comparable to the large repetitive elements of other herpesvirus genomes that are classified into genome groups A to E. This is in agreement with the previous nomination of THV as the prototype species of herpesvirus genome group F (49, 72).
ORFs of the THV genome.
THV genome analysis revealed 582 ORFs with a possible coding capacity of more than 40 amino acids. The distribution of the ORFs on the two DNA strands is very regular, with 286 oriented to the left and 296 oriented to the right. Altogether, 158 ORFs were selected to be actual coding sequences according to the criteria used to determine herpesvirus genes (see Materials and Methods) and are listed in Table 1. Potential sites and signatures of the hypothetical viral proteins and the highest homologies to known proteins are also given in Table 1. The 158 ORFs selected to encode viral proteins are depicted in the ORF map of the THV genome in Fig. 2. Those viral gene products that are homologous to known herpesvirus proteins are shown as black or colored arrows. It is evident that the homologous, conserved ORFs are accumulated in the center of the genome. t6-t22.13, t39-t43.4, t58-t63, and t106-t130 are genome regions with ORFs that encode THV-specific proteins with no known homologues. RT-PCR experiments revealed that T33 and T89 consist of two exons. Comparative amino acid analysis to known proteins showed that T36 could be spliced, but it was not possible to prove this by RT-PCR experiments.
Conserved THV proteins show the highest homologies to known proteins of HCMVA (13) and MCMVS (71) as representatives of the evolutionary lineages of primate and rodent cytomegaloviruses, respectively. The homology values of the THV proteins to the proteins of these two virus species and the pertinent potential functions are summarized in Table 1. The proteins with the highest homologies are underlined, and it is clear that they are distributed equally between HCMVA and MCMVS.
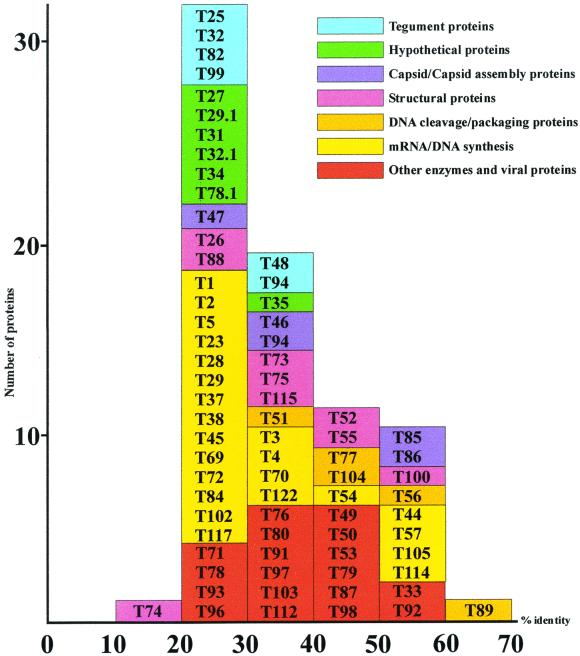
All of the homologous THV ORFs are classified into functional groups and plotted according to their highest values of identity to known herpesvirus proteins (Fig. 3). The number of proteins gets smaller when the identity values rise, with T89 (viral terminase) being the only protein with 60 to 70% identity to known herpesvirus proteins. None of the functional protein groups show an extraordinary distribution of identities or strikingly low or high conservation. As a rule, they are almost constantly distributed over the range of identity values, with a decreasing number of proteins when the values get higher.
FIG. 3.
Graphic representation of the homology value distribution of seven conserved THV protein groups that are classified according to possible functions. Individual groups are marked by distinct colors. The homology values that underlie this graphic are the highest detected homologies to known proteins regardless of the virus species the proteins belong to.
Comparison of the THV ORFs that are supposed to be coding sequences led to the identification of six gene families. The members of each of these six gene families are distinguished by significant identity and similarity to each other, raising the possibility that these gene groups in each case originated from one ancestral gene by duplication events. The gene families are numbered 1 to 6 and drawn in the ORF map in Fig. 2. A striking feature of the members of such gene groups is the fact that they are located in close proximity in the genome, often in a tandem-like arrangement, underlining the hypothesis that they were produced by gene duplication. A concentration of gene family members could be seen in the left part of the THV genome, especially in the nonconserved region between t6 and t22.13. A similar distribution of gene families is present in the genomes of HCMVA (13), MCMVS (71), rat cytomegalovirus (87), or HHV-6 (31), where duplicated genes are also concentrated in nonconserved regions at the left and right ends of the genomes. Significant homology between the members was the main criterion for the formation of the six THV gene families. Another possible way to relate genes to families is by the similarity of the functions of the encoded proteins. In HCMVA and MCMVS, homologues of G protein-coupled receptors (GCRs) form a family. For that reason, T33 and T78, both homologous to G protein-coupled receptors, could also be seen as members of a gene family. This GCR and members of the US22 gene family are conserved among THV, HCMVA, and MCMVS. Both members of the HCMVA UL25-UL35 gene family are conserved in the THV genome. However, homology between the two potential proteins is extremely low.
Gene arrangements of the THV genome.
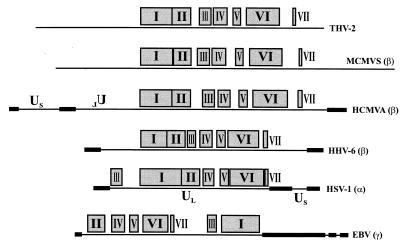
Homologous THV, HCMVA, and MCMVS proteins are summerized in Table 1. All together, 72 proteins are homologous between THV and HCMVA and 73 are homologous between THV and MCMVS. Both cytomegalvirus species are members of the subfamily Betaherpesvirinae. Table 2 shows these homology relationships extended by HHV-6 (Betaherpesvirinae) (31), EBV (Gammaherpesvirinae) (4), and HSV-1 (Alphaherpesvirinae) (58, 59, 60). HHV-6 shares 66, EBV shares 42, and HSV-1 shares 38 homologous proteins with THV. The most homologous proteins are found between THV and the mammalian cytomegaloviruses. The extent of homology between THV and alpha- or gammaherpesviruses is clearly less. Furthermore, Table 2 shows a group of almost 40 homologous proteins that could be found in every herpesvirus species of mammals and birds. These genes are termed core genes and are supposed to be part of the genome of the common ancestor of these herpesviruses. The core genes form seven conserved gene clusters whose arrangements are characteristic of the corresponding herpesvirus subfamily. A comparison of the gene block arrangements among THV, HCMVA, MCMVS, HHV-6, HSV-1, and EBV is shown in Fig. 4. In the genomes of THV, MCMVS, and HCMVA, the arrangement of the seven clusters is almost the same with regard to both the order of appearance and the spaces between the individual blocks. The same order of gene clusters could also be found in HHV-6, but they are more concentrated in the center of the genome. HCMVA, MCMVS, and HHV-6 are members of the subfamily Betaherpesvirinae. The gene cluster arrangement in the genome of HSV-1, and alphaherpesvirus, differs from those of the betaherpesviruses in the position of gene block III, which is localized at the left end of the UL region of the HSV-1 genome. The gammaherpesvirus EBV shows a further different arrangement that is characterized by the localization of gene blocks III and I at the right end of the long unique genome region of EBV. The colinear arrangement of conserved genes within the genomes of THV, HCMVA, and MCMVS is plotted in detail in Fig. 5. HCMVA is able to form four genomic isomers. The arrangement of genes in the prototype HCMVA isomer is colinear with that in the MCMVS genome, which is not able to form isomers. The arrangement and orientation of the homologous THV genes correspond to those of a different HCMVA genome isomer that is characterized by an inverted UL region compared to the prototype HCMVA isomer. The difference in total genome length between THV (195,857 bp) and HCMVA (229,354 bp) is caused mainly by the presence of the repetitive elements in the HCMVA genome. Without these elements, the lengths of the THV and HCMVA genomes are very similar.
TABLE 2.
HCMVA, MCMVS, HHV-6, EBV, and HSV-1 proteins homologous to potential THV proteinsa
| THV protein | HCMVA homologue | MCMVS homologue | HHV-6 homologue | EBV homologue | HSV-1 homologue |
|---|---|---|---|---|---|
| T1 (US22 family) | US23 | M143 | U16 | ||
| T2 (US22 family) | US23 | M143 | U16 | ||
| T3 (US22 family) | US24 | M141 | |||
| T4 (US22 family) | US23 | M140 | U16 | ||
| T5 (US22 family) | US22 | M139 | DR7 | ||
| T23 | UL23 | M23 | U2 | ||
| UL24 | M24 | U3 | |||
| T25 | UL25 | M25 | U14 | ||
| T26 | UL26 | M26 | |||
| T27 | UL27 | M27 | U4/U5 | ||
| T28 (US22 family) | UL29 | M28 | U8 | ||
| T29 (US22 family) | UL29 | U8 | |||
| T29.1 | M106 | ||||
| T30 | UL27 | M27 | U4/U5 | ||
| T31 | UL31 | M31 | U10 | ||
| T32 | UL32 | M32 | U11 | ||
| T32.1 | M19 | U12 | |||
| T33 | UL33 | M33 | EB11 | ||
| T34 | UL34 | M34 | |||
| T35 | UL35 | M35 | |||
| T36 (US22 family) | UL36 | M36 | |||
| T37 | UL37 | M37 | U18 | ||
| T38 | UL38 | M38 | U19 | ||
| UL43 | M43 | ||||
| T44 | UL44 | M44 | U27 | BMRF1 | UL42 |
| T45 | UL45 | M45 | U28 | BORF2 | UL39 |
| T46 | UL46 | M46 | U29 | BORF1 | UL38 |
| T47 | UL47 | M47 | U30 | BOLF1 | UL37 |
| T48 | UL48 | M48 | U31 | BPLF1 | UL36 |
| T49 | UL49 | M49 | U33 | BFRF2 | UL35 |
| T50 | UL50 | M50 | U34 | BFRF1 | UL34 |
| T51 | UL51 | M51 | U35 | UL33 | |
| T52 | UL52 | M52 | U36 | BFLF1 | UL32 |
| T53 | UL53 | M53 | U37 | BFLF2 | UL31 |
| T54 | UL54 | M54 | U38 | BALF5 | UL30 |
| T55 | UL55 | M55 | U39 | BALF4 | UL27 |
| T56 | UL56 | M56 | U40 | BALF3 | UL28 |
| T57 | UL57 | M57 | U41 | BALF2 | UL29 |
| T69 | UL69 | M69 | U42 | BMLF1 | UL54 |
| T70 | UL70 | M70 | U43 | BSLF1 | UL52 |
| T71 | UL71 | M71 | U44 | BSRF1 | UL51 |
| T72 | UL72 | M72 | U45 | BLLF2 | UL50 |
| T73 | UL73 | M73 | U46 | BLRF1 | UL49A |
| T74 | UL74 | M74 | U47 | ||
| T75 | UL75 | M75 | U48 | BXLF2 | UL22 |
| T76 | UL76 | M76 | U49 | BXRF1 | UL24 |
| T77 | UL77 | M77 | U50 | BVRF1 | UL25 |
| T78 | UL78 | M78 | U51 | ||
| T78.1 | M59 | ||||
| T79 | UL79 | M79 | U52 | BVRF1.5a/b | |
| T80 | UL80 | M80 | U53 | BVRF2 | UL26 |
| T82 | UL82 | M82 | U54 | ||
| UL83 | M83 | ||||
| T84 | UL84 | M84 | U55 | ||
| T85 | UL85 | M85 | U56 | BDLF1 | UL18 |
| T86 | UL86 | M86 | U57 | BcLF1 | UL19 |
| T87 | UL87 | M87 | U58 | BcRF1 | |
| T88 | UL88 | M88 | U59 | ||
| T89 | UL89 | M89 | U60/U66 | BDRF1/BGRF1 | UL15Ex2+1 |
| T91 | UL91 | M91 | U62 | ||
| T92 | UL92 | M92 | U63 | BDLF4 | |
| T93 | UL93 | M93 | U64 | BGLF1 | UL17 |
| T94 | UL94 | M94 | U65 | BGLF2 | UL16 |
| T95 | UL95 | M95 | U67 | BGLF3 | UL14 |
| T96 | UL96 | M96 | U68 | BGLF3.5 | |
| T97 | UL97 | M97 | U69 | BGLF4 | UL13 |
| T98 | UL98 | M98 | U70 | BGLF5 | UL12 |
| T99 | UL99 | M99 | |||
| T100 | UL100 | M100 | U72 | BBRF3 | UL10 |
| T102 | UL102 | M102 | U74 | BBLF3 | UL8 |
| T103 | UL103 | M103 | U75 | BBRF2 | UL7 |
| T104 | UL104 | M104 | U76 | BBRF1 | UL6 |
| T105 | UL105 | M105 | U77 | BBLF4 | UL5 |
| T112 | UL112 | M112E1 | U79 | ||
| T114 | UL114 | M114 | U81 | BKRF3 | UL2 |
| T115 | UL115 | M115 | U82 | BKRF2 | UL1 |
| UL116 | M116 | ||||
| T117 | UL117 | U84 | |||
| UL118 | M118 | ||||
| UL121 | M121 | ||||
| T122 | UL122 | M122E5 | U86 |
The data shown are from reference 31 and are complemented by THV and MCMVS protein data.
FIG. 4.
Arrangement of the seven conserved core gene blocks in herpesvirus genomes. The pattern of these seven clusters is characteristic for the three herpesvirus subfamilies. The assignment of the individual virus species to the subfamily Alpha-, Beta-, or Gammaherpesvirinae is given in parentheses after the particular virus name. Us (unique short region) and UL (unique long region) are the descriptions of the two parts of the herpesvirus genomes that are able to form isomers. The inverted spelling of UL in the genome of HCMVA characterizes a distinct isomer that differs from the prototype L-S isomer in the orientation of the UL region. The black boxes mark the positions of large repetitive elements. The diagram corresponds to the 1996 publication of Gompels et al. (31) complemented by data on the THV and MCMV genomes.
FIG. 5.
Comparative analysis of the arrangement of homologous ORFs among THV, HCMVA, and MCMVS. Each arrow shows the orientation of transcription. The length of each arrow is standardized and does not correspond to the actual length of the ORF. The vertical lines designate the start points of the individual ORFs. The shaded boxes of the HCMVA genome represent repetitive DNA elements that are, in part, responsible for genomic isomerization. These repetitive DNA elements divide the HCMVA genome into UL (unique long) and Us (unique short) regions. (A) Comparison of the arrangement of homologous ORFs between THV and HCMVA. The THV ORFs show colinearity with those of a distinct HCMVA genome isomer that is characterized by an inverted UL region compared to the prototype L-S isomer. (B) Comparison of the arrangement of homologous ORFs between HCMVA and MCMVS. The MCMVS ORFs show colinearity with those of the prototype HCMVA genome isomer.
Phylogenetic classification of THV.
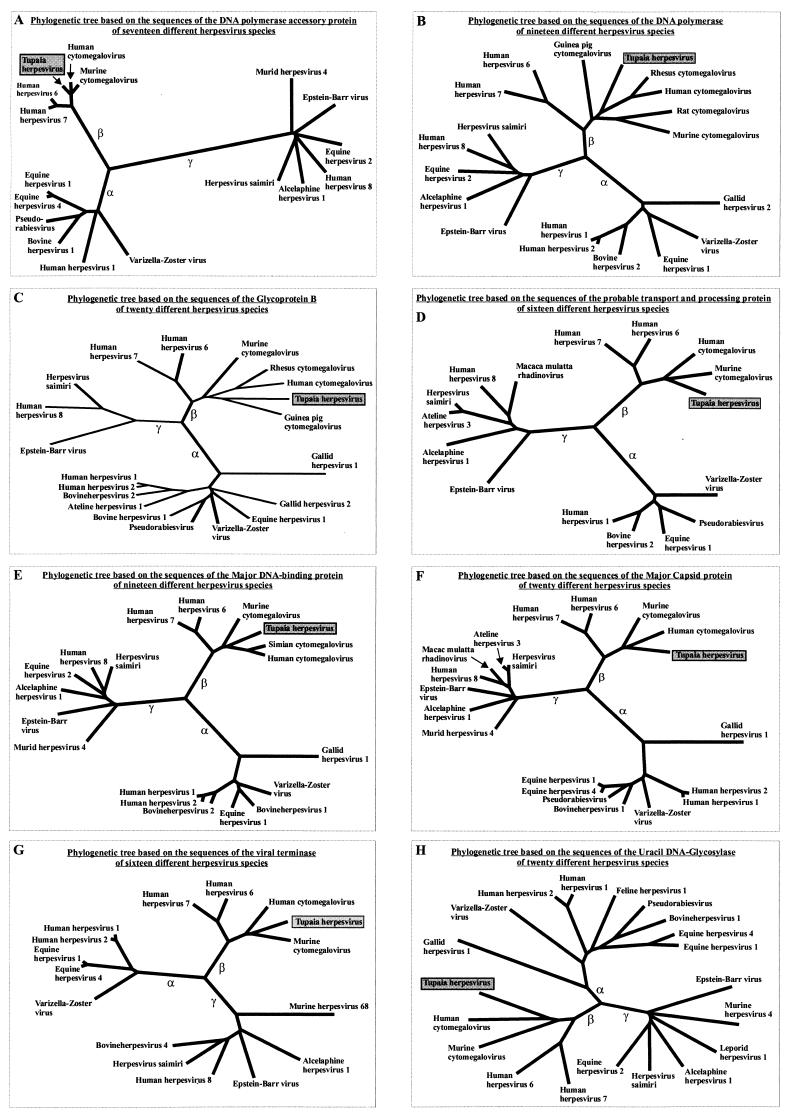
The phylogenetic trees derived from the comparison of the DNA polymerase, DNA polymerase accessory protein, glycoprotein B, probable transport and processing protein, major DNA-binding protein, major capsid protein, viral terminase, and uracil DNA-glycosylase amino acid sequences of different herpesviruses are shown in Fig. 6A to H. The selected proteins are those with the highest levels of homology between different members of the family Herpesviridae. The phylogenetic trees show a distinct subdivision into three main branches corresponding to the herpesvirus subfamilies Alpha-, Beta-, and Gammaherpesvirinae, which are groups of herpesvirus species with similar biological properties and phylogenetic relatedness. In all eight trees, THV is a member of the subfamily Betaherpesvirinae. In addition, the evolutionary lineage of this subfamily is divided into two branches that correspond to the genus Roseolovirus with HHV-6 and HHV-7, the so-called Beta2 herpesviruses, and the mammalian cytomegaloviruses, the so-called Beta1 herpesviruses. Within the subfamily Betaherpesvirinae, THV is most closely related to the mammalian cytomegaloviruses, with similar evolutionary distances to the phylogenetic lineages of primate and rodent cytomegaloviruses.
FIG. 6.
Eight phylogenetic trees derived by comparison of the DNA polymerase, DNA polymerase accessory protein, glycoprotein B, probable transport and processing protein, major DNA-binding protein, major capsid protein, viral terminase, and uracil DNA-glycosylase amino acid sequences of different herpesvirus species. The three main branches of the trees represent the evolutionary lineages of the herpesvirus subfamilies Alpha (α)-, Beta (β)-, and Gammaherpesvirinae (γ). The sequences of the individual proteins used to construct the phylogenetic trees were taken from the GenBank and SwissPort release 39 databases.
DISCUSSION
The complete nucleotide sequence (195,857 bp) and the coding capacity of the THV genome were determined. The position of THV as the prototype species of herpesvirus genome group F (49, 72) was confirmed. The G+C content (66.5%) of the THV genome varies over the course of the genome. The highest values were found at the termini of the viral genome and within the DNA sequences of the conserved genes. The mechanism for G+C accumulation is not known. It occurs in the genomes of different herpesvirus species (e.g., EBV [4] or HSV-1 [58, 59, 60]) regardless of phylogenetic relatedness and is supposed to be an adaptation to an unknown evolutionary pressure.
Seventy-six out of 158 potential gene products of THV were identified as significantly homologous to known herpesvirus proteins of primate and rodent cytomegaloviruses, mainly those of HCMVA and MCMVS. The thorough examination of many of these homologous proteins allows the assignment of functions to the potential THV proteins. T1 to T5, T28, T29, and T36 are homologues of the US22 gene family of HCMVA. The members of the US22 gene family are known to regulate gene expression (13). m139 to m143 are the corresponding MCMVS homologues to THV T1 to T5. They are supposed to play an essential role in pathogenicity in the natural host and seem to be important for genome replication (35). HCMVA UL122 and UL123 are the main components of the so-called major immediate-early region (32, 45, 83, 84). These genes correspond to M122, M123, and T122 of MCMVS (12, 65) and THV, respectively. The mRNAs of UL122, UL123, M122, and M123 consist of several exons composed by alternative splicing events. It is very probable that the corresponding THV genes have similar exon structures. However, the transcription of these viral genes will be the subject of future studies. HCMVA immediate-early genes play an essential role in the regulation of the expression of early and late viral genes and are indispensable for the correct course of the lytic replication cycle. THV T36 to T38 and T115 are homologous to HCMVA immediate-early proteins UL36 to UL38 and UL115 (14, 15). UL37 is an integral membrane protein (1) that is supposed to be located in mitochondria and to inhibit Fas-mediated apoptosis of the host cell (30).
Herpesvirus tegument proteins have structural functions in viral morphogenesis and are involved in the regulation of gene expression immediately after penetration of host cells. In addition, some of them are responsible for the activation of the cellular immune response (33). THV gene products T25, T32, T47, T48, T69, T82, and T99 are homologous to tegument proteins of HCMVA and MCMVS (6, 16, 17, 18, 37, 38, 64, 91, 94). HCMVA UL32, UL82, and UL83 are the main components of the viral tegument. UL32 was designated the major tegument phosphoprotein and makes up 15% of the total protein mass of a virion and plays an important role in viral morphogenesis (34, 66). HCMVA UL83 is essential for the viral life cycle in the natural host but not in cell culture (77). A homologue to UL83 is present in MCMVS (71) and rat cytomagalovirus (87) but absent in HHV-6 (31) and THV. HCMVA UL69, which is homologous to THV T69, has been supposed to be a transactivator (90) and plays a role in the G1 phase of the host cell cycle (36, 55).
THV T46 (minor capsid protein), T85, T86 (major capsid protein), and T94 are homologous to the corresponding capsid proteins of HCMVA and MCMVS (13, 29, 71, 89). The transcription unit of the smallest capsid protein of HCMVA is 225 bp in length and located between UL48 and UL49 (28). A homologous protein in THV could not be detected. However, a small ORF (t48.1) of 312 bp located between ORFs T48 and T49 has, moreover, a potential Bowman-Birk serine protease inhibitor family signature. THV T80 is the homologue of the viral protease, one of the most important herpesvirus enzymes, which is a scaffolding protein with essential functions in the morphogenesis of the viral capsid (7, 11, 54, 75, 92).
Eleven gene loci were identified within the HCMVA genome, which are essential for DNA replication. UL54 (DNA polymerase), UL44 (DNA polymerase accessory protein), UL57 (major DNA-binding protein), UL70 (primase), UL102 (primase-helicase complex-associated protein), and UL105 (helicase) (46, 70, 80, 81) are replication fork proteins and are also present in THV. T54, the DNA polymerase of THV, is highly conserved and possesses characteristic sites and signatures of the B family of DNA polymerases (82). T57 shows a similar strong conservation and possesses sequence motifs essential for DNA binding (5). Homologues of THV T36-38, T84, T112, and T122 are also essential for DNA replication. They are supposed to activate the expression of the replication fork genes (27, 76, 79).
In the course of herpesvirus DNA replication, which proceeds by the rolling-circle mechanism, concatemeric genomes are formed (50). HCMVA UL51, UL52, UL56, UL77, UL89, and UL104 were identified as the vital components of the cleavage and packaging processes that are essential for the formation of unit length viral genomes and correct packaging (51). THV possesses individual homologues to these six proteins.
THV homologues of nucleic acid metabolism are T45, T72 (dUTPase), and T114 (uracil DNA-glycosylase). T45 is the large chain of the ribonucleotide reductase. All betaherpesviruses, including THV, have no homologous ORF for the small chain of the ribonucleotide reductase that actually possesses the active site of the enzyme. It is not known how the betaherpesvirus ribonucleotide reductases retain their function. Like other G+C-rich herpesviruses like EBV (4) or HSV-1 (58, 59, 60), THV is missing important nucleic acid metabolism enzymes like thymidylate synthase or dihydrofolate reductase. In addition, THV possesses no thymidine kinase, the gene for which is absent from all betaherpesvirus genomes. An important putative THV gene product is T97, which is homologous to HCMVA UL97 (ganciclovir kinase). This protein is responsible for the ganciclovir effect caused by chemotherapy of HCMV infection (53). THV T98 is homologous to the herpesvirus alkaline exonuclease that plays a role in DNA processing and capsid transport from the nucleus to the cytoplasm (26, 78).
As far as viral glycoproteins are concerned, THV T37, T50, T55 (glycoprotein B), T73, T74 (glycoprotein O), T75 (glycoprotein H), T100 (glycoprotein M), and T115 (glycoprotein L) are homologous to HCMVA and MCMVS glycoproteins. HCMVA UL74, UL75, and UL115 form the gCIII glycoprotein complex (42, 43, 44, 48). The gCIII glycoprotein complex and glycoprotein B are the most important structural surface proteins of HCMV. HCMVA UL55 and UL75 are supposed to start pathways that lead to the activation of cellular transcription factors Sp1 and NFκB by binding to special cellular receptors (93). In the THV genome, 10 potential glycoproteins (t7, t11, t17, t22, t22.1, t22.2, t22.3, t22.5, t22.7, and t22.8) with no homologies to known proteins were identified according to characteristic sites and signatures.
THV T33 and T78 are GCR homologues and are supposed to be homologous to host proteins. It is presumed that the function of viral GCR homologues is to catch extracellular signals and block the pertinent intracellular pathways. HCMVA UL33 is a CC chemokine receptor (9) and is not essential for growth in cell culture (56). UL33 homologues are conserved only in betaherpesviruses (8, 25). The HHV-6 homologue of THV T78 was found to be a CC chemokine receptor in vitro (67). t90 is not homologous to any known protein but was found to hold a GCR signature. However, further work is necessary to determine if t90 is actually a GCR homologue. US27 and US28 of HCMVA are CC chemokine receptor homologues and also members of the GCR family (9, 95). However, no corresponding homologous proteins could be identified in the THV genome.
The THV genome possesses a number of ORFs that code for proteins with no homologies to known proteins. These genes seem to code for virus species-specific functions in the natural host. t16 was found to possess a potential aminoacyl-transfer RNA synthetase class II signature, and t119 holds an ATP-dependent DNA ligase AMP-binding site. However, it has to be verified whether these signatures are actually functional. Interestingly, the locations of the unconserved genes are almost in the same genomic areas within the genomes of the members of the subfamily Betaherpesvirinae. The majority of these ORFs code for glycoproteins and are members of gene families. HCMVA UL24, UL43, UL83, UL116, UL118, and UL121 have corresponding homologues in MCMVS but not in THV. The latter five seem to have functions that are specific to cytomegaloviruses.
The biological and genomic properties of THV correspond to the criteria prepared for the classification of herpesviruses in the subfamily Betaherpesvirinae (73). THV is evolutionarily placed within the group of mammalian cytomegaloviruses. There is good reason to suppose that the separation of the three evolutionary lineages which lead to THV and the primate and the rodent cytomegaloviruses has taken place in a very short evolutionary period. This assumption is in accordance with the phylogenetic tree of the hosts of these virus species and confirms the accepted hypothesis that herpesviruses follow the development of their hosts, a process known as coevolution (47, 61, 62, 63). The identification of the genetic structure of the viral ancestor of these three herpesviruses is very difficult. It is certain that the homologous genes of THV, HCMVA, and MCMVS were also present in the ancestral genome. However, it is not clear why the gene arrangements of THV and MCMVS correspond to two different HCMVA genome isomers. A simple explanation would be that the viral ancestor had repetitive DNA elements similar to those of HCMVA and the ability to form genome isomers. However, one can assume that the repetitive DNA elements disappeared in the MCMVS and THV phylogenetic lineages due to distinct evolutionary pressures. In view of the high degree of relatedness of THV to the mammalian cytomegaloviruses, THV can be considered a model system for the investigation of HCMV infection and pathogenesis.
ACKNOWLEDGMENTS
We thank the Deutsche Forschungsgemeinschaft, project Da-142/10-1-5, for providing the automatic DNA sequencing equipment (373 “Extended” DNA sequencer; Perkin-Elmer Corporation, Applied Biosystems).
We thank Michaela Handermann and Nurith J. Jakob for critical comments and helpful discussion.
REFERENCES
- 1.Al-Barazi H O, Colberg-Poley A M. The human cytomegalovirus UL37 immediate-early regulatory protein is an integral membrane N-glycoprotein which traffics through the endoplasmic reticulum and Golgi apparatus. J Virol. 1996;70:7198–7208. doi: 10.1128/jvi.70.10.7198-7208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht M, Darai G, Flügel R M. Analysis of the genomic termini of tupaia herpesvirus DNA by restriction mapping and nucleotide sequencing. J Virol. 1985;56:466–474. doi: 10.1128/jvi.56.2.466-474.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 5.Bahr U, Springfeld C, Tidona C A, Darai G. Structural organization of a conserved gene cluster of Tupaia herpesvirus encoding the DNA polymerase, glycoprotein B, a probable processing and transport protein, and the major DNA binding protein. Virus Res. 1999;60:123–136. doi: 10.1016/s0168-1702(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 6.Battista M C, Bergamini G, Boccuni M C, Campanini F, Ripalti A, Landini M P. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J Virol. 1999;73:3800–3809. doi: 10.1128/jvi.73.5.3800-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum E Z, Bebernitz G A, Hulmes J D, Muzithras V P, Jones T R, Gluzman Y. Expression and analysis of the human cytomegalovirus UL80-encoded protease: identification of autoproteolytic sites. J Virol. 1993;67:497–506. doi: 10.1128/jvi.67.1.497-506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besieger P S, Vink C, Van Dam J G, Grauls G, Vanherle S J, Bruggeman C A. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J Virol. 1998;72:2352–2363. doi: 10.1128/jvi.72.3.2352-2363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodaghi B, Jones T R, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier J L, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188:855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucher P, Bairoch A. A generalized profile syntax for biomolecular sequence motifs and its function in automatic sequence interpretation. In: Altman R, Brutlag D, Karp P, Lathrop R, Searls D, editors. In ISMB-94. Proceedings of the 2nd International Conference on Intelligent Systems for Molecular Biology. Menlo Park, Calif: AAAI Press; 1994. pp. 53–61. [PubMed] [Google Scholar]
- 11.Burck P J, Berg D H, Luk T P, Sassmannshausen L M, Walkulchik M, Smith D P, Hsiung H M, Becker G W, Gibson W, Villarreal E C. Human cytomegalovirus maturational proteinase: expression in Escherichia coli, purification, and enzymatic characterization by using peptide substrate mimics of natural cleavage sites. J Virol. 1994;68:2937–2946. doi: 10.1128/jvi.68.5.2937-2946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardin R D, Abenes G B, Stoddart C A, Mocarski E S. Murine cytomegalovirus IE2, an activator of gene expression, is dispensible for growth and latency in mice. Virology. 1995;209:236–241. doi: 10.1006/viro.1995.1249. [DOI] [PubMed] [Google Scholar]
- 13.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison III C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Colberg-Poley A M. Functional roles of immediate early proteins encoded by the human cytomegalovirus UL36–38, UL115–119, TRS1/IRS1 and US3 loci. Intervirology. 1996;39:350–360. doi: 10.1159/000150506. [DOI] [PubMed] [Google Scholar]
- 15.Colberg-Poley A M, Huang L, Soltero V E, Iskenderian A C, Schumacher R F, Anders D G. The acidic domain of pUL37 × 1 and gpUL37 plays a key role in transactivation of HCMV DNA replication gene promoter constructions. Virology. 1998;246:400–408. doi: 10.1006/viro.1998.9212. [DOI] [PubMed] [Google Scholar]
- 16.Cranmer L D, Clark C, Spector D H. Cloning, characterization, and expression of the murine cytomegalovirus homologue of the human cytomegalovirus 28-kDa matrix phosphoprotein (UL99) Virology. 1994;205:417–429. doi: 10.1006/viro.1994.1662. [DOI] [PubMed] [Google Scholar]
- 17.Cranmer L D, Clark C L, Morello C S, Farrell H E, Rawlinson W D, Spector D H. Identification, analysis, and evolutionary relationships of the putative murine cytomegalovirus homologs of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins. J Virol. 1996;70:7929–7939. doi: 10.1128/jvi.70.11.7929-7939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallas P B, Lyons P A, Hudson J B, Scalzo A A, Shellam G R. Identification and characterization of a murine cytomegalovirus gene with homology to the UL25 open reading frame of human cytomegalovirus. Virology. 1994;200:643–650. doi: 10.1006/viro.1994.1227. [DOI] [PubMed] [Google Scholar]
- 19.Darai G, Matz B, Schroeder C H, Flügel R M, Berger U, Munk K, Gelderblom H. Characterization of a tree shrew herpesvirus isolated from a lymphosarcoma. J Gen Virol. 1979;43:541–551. [Google Scholar]
- 20.Darai G, Zöller L, Matz B, Flügel R M, Hofmann W, Gelderblom H. Herpesvirus tupaia: isolation, characterization and oncogenicity. In: Lapin B, Yohn S, editors. Advances in leucaemia research, 1979. Moscow, Russia: USSR Academy of Medical Science, IEPT; 1980. pp. 141–148. [Google Scholar]
- 21.Darai G, Flügel R M, Matz B, Delius H. DNA of tupaia herpesviruses. In: Becker Y, editor. Herpes virus DNA. The Hague, The Netherlands: Martinus Nijhoff; 1981. pp. 345–361. [Google Scholar]
- 22.Darai G, Koch H G, Flügel R M, Gelderblom H. Tree shrew (Tupaia) herpesviruses. In: Bonneau M, Hennessen W, editors. Herpesvirus of man and animal. Developments in biological standardization. Basel, Switzerland: Karger; 1983. pp. 39–51. [PubMed] [Google Scholar]
- 23.Darai G, Rosen A, Scholz J, Gelderblom H. Induction of generalized and lethal herpesvirus infection in the tree shrew by intrahepatic transfection of herpes simplex virus DNA. J Virol Methods. 1983;7:305–314. doi: 10.1016/0166-0934(83)90083-6. [DOI] [PubMed] [Google Scholar]
- 24.Darai G, Koch H G. Tree shrew herpesvirus: pathogenicity and latency. In: Wittmann G, Gaskell R M, Rziha R J, editors. Latent herpesvirus infections in veterinary medicine. The Hague, The Netherlands: Martinus Nijhoff; 1984. pp. 1–102. [Google Scholar]
- 25.Davis-Poynter N J, Lynch D M, Vally H, Shellam G R, Rawlinson W D, Barrell B G, Farrell H E. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J Virol. 1997;71:1521–1529. doi: 10.1128/jvi.71.2.1521-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M, Robertson B J, McCann P J, O'Boyle D R, Weller S K, Newcomb W W, Brown J C, Weinheimer S P. Functional conservations of the alkaline nuclease of herpes simplex type 1 and human cytomegalovirus. Virology. 1998;249:460–470. doi: 10.1006/viro.1998.9344. [DOI] [PubMed] [Google Scholar]
- 27.Gebert S, Schmolke S, Sorg G, Floss S, Plachter B, Stamminger T. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J Virol. 1997;71:7048–7060. doi: 10.1128/jvi.71.9.7048-7060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson W, Clopper K S, Britt W J, Baxter M K. Human cytomegalovirus (HCMV) smallest capsid protein identified as product of short open reading frame located between HCMV UL48 and UL49. J Virol. 1996;70:5680–5683. doi: 10.1128/jvi.70.8.5680-5683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson W, Baxter M K, Clopper K S. Cytomegalovirus “missing” capsid protein identified as heat-aggregable product of human cytomegalovirus UL46. J Virol. 1996;70:7454–7461. doi: 10.1128/jvi.70.11.7454-7461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldmacher V S, Bartle L M, Skaletskaya A, Dionne C A, Kedersha N L, Vater C A, Han J W, Lutz R J, Watanabe S, McFarland E D, Kieff E D, Mocarski E S, Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E D, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 32.Greenaway P J, Wilkinson G W G. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirus strain AD169. Virus Res. 1987;7:17–31. doi: 10.1016/0168-1702(87)90055-4. [DOI] [PubMed] [Google Scholar]
- 33.Greijer A E, van de Crommert J M, Stevens S J, Middeldorp J M. Molecular fine-specificity analysis of antibody responses to human cytomegalovirus and design of novel synthetic-peptide-based serodiagnostic assays. J Clin Microbiol. 1999;37:179–188. doi: 10.1128/jcm.37.1.179-188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greis K D, Gibson W, Hart G W. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J Virol. 1994;68:8339–8349. doi: 10.1128/jvi.68.12.8339-8349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson L K, Dalton B L, Karabekian Z, Farell H E, Rawlinson W D, Stenberg R M, Campbell A E. Transcriptional analysis of the murine cytomegalovirus HindIII-I region: identification of a novel immediate-early gene region. Virology. 1999;260:156–164. doi: 10.1006/viro.1999.9796. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi M L, Blankenship C, Shenk T. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc Natl Acad Sci USA. 2000;97:2692–2696. doi: 10.1073/pnas.050587597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensel G, Meyer H, Gartner S, Brand G, Kern H F. Nuclear localization of the human cytomegalovirus tegument protein pp150 (ppUL32) J Gen Virol. 1995;76:1591–1601. doi: 10.1099/0022-1317-76-7-1591. [DOI] [PubMed] [Google Scholar]
- 38.Hensel G M, Meyer H H, Buchmann I, Pommerehne D, Schmolke S, Plachter B, Radsak K, Kern H F. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. J Gen Virol. 1996;7:3087–3097. doi: 10.1099/0022-1317-77-12-3087. [DOI] [PubMed] [Google Scholar]
- 39.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann K, Bucher P, Falquet L, Bairoch A. The PROSITE database, its status in 1999. Nucleic Acids Res. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann W, Moller P, Schwaier A, Flugel R M, Zoller L, Darai G. Malignant tumours in tupaia (tree shrew) J Med Primatol. 1981;10:155–163. doi: 10.1159/000460067. [DOI] [PubMed] [Google Scholar]
- 42.Huber M T, Compton T. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J Virol. 1997;71:5391–5398. doi: 10.1128/jvi.71.7.5391-5398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber M T, Compton T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J Virol. 1998;72:8191–8197. doi: 10.1128/jvi.72.10.8191-8197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber M T, Compton T. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J Virol. 1999;73:3886–3892. doi: 10.1128/jvi.73.5.3886-3892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchinson N I, Tocci M J. Characterization of a major early gene from the human cytomegalovirus long inverted repeat; predicted amino acid sequence of a 30-kDa protein encoded by the 1.2kb mRNA. Virology. 1986;155:172–182. doi: 10.1016/0042-6822(86)90177-7. [DOI] [PubMed] [Google Scholar]
- 46.Iskenderian A C, Huang L, Reilly A, Stenberg R M, Anders D G. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to active expression of replication genes. J Virol. 1996;70:383–392. doi: 10.1128/jvi.70.1.383-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlin S, Mocarski E S, Schachtel G A. Molecular evolution of herpesviruses: genomic and protein sequence comparisons. J Virol. 1994;68:1886–1902. doi: 10.1128/jvi.68.3.1886-1902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaye J F, Gompels U A, Minson A C. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J Gen Virol. 1992;73:2693–2698. doi: 10.1099/0022-1317-73-10-2693. [DOI] [PubMed] [Google Scholar]
- 49.Koch H G, Delius H, Matz B, Flugel R M, Clarke J, Darai G. Molecular cloning and physical mapping of the tupaia herpesvirus genome. J Virol. 1985;55:86–95. doi: 10.1128/jvi.55.1.86-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch H G, Flugel R M, Darai G. Concatemeric forms of intracellular tupaia herpesvirus DNA. Virology. 1986;151:211–221. doi: 10.1016/0042-6822(86)90043-7. [DOI] [PubMed] [Google Scholar]
- 51.Krosky P M, Underwood M R, Turk S R, Feng K W H, Jain R K, Ptak R G, Westerman A C, Biron K K, Townsend L B, Drach J C. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar S, Hedges S B. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 53.Littler E, Stuart A D, Chee M S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 54.Liu F, Roizman B. Differentiation of multiple domains in the herpes simplex virus 1 protease encoded by the UL26 gene. Proc Natl Acad Sci USA. 1992;89:2076–2080. doi: 10.1073/pnas.89.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu M, Shenk T. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J Virol. 1999;73:676–683. doi: 10.1128/jvi.73.1.676-683.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margulies B J, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCombs R M, Brunschwig J P, Mircovic R, Benyesh-Melnick M. Electron microscopic characterization of a herpes-like virus isolated from tree shrews. Virology. 1971;45:816–820. doi: 10.1016/0042-6822(71)90203-0. [DOI] [PubMed] [Google Scholar]
- 58.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type I. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 59.McGeoch D J, Dolan A, Donald S, Brauer D H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986;14:1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 61.McGeoch D J. The genomes of the human herpesviruses: contents, relationship and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- 62.McGeoch D J, Cook S. Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol. 1994;238:9–22. doi: 10.1006/jmbi.1994.1264. [DOI] [PubMed] [Google Scholar]
- 63.McGeoch D J, Cook S, Dolan A, Jamieson F E, Telford E A. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J Mol Biol. 1995;247:443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 64.McLauchlan J. The abundance of the herpes simplex virus type 1 UL37 tegument protein in virus particles is closely controlled. J Gen Virol. 1997;78:189–194. doi: 10.1099/0022-1317-78-1-189. [DOI] [PubMed] [Google Scholar]
- 65.Messerle M, Keil G M, Koszinowski U H. Structure and expression of murine cytomegalovirus immediate-early gene 2. J Virol. 1991;65:1638–1643. doi: 10.1128/jvi.65.3.1638-1643.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer H H, Ripalti A, Landini M P, Radsak K, Kern H F, Hensel G M. Human cytomegalovirus late-phase maturation is blocked by stably expressed UL32 antisense mRNA in astrocytoma cells. J Gen Virol. 1997;78:2621–2631. doi: 10.1099/0022-1317-78-10-2621. [DOI] [PubMed] [Google Scholar]
- 67.Milne R S B, Mattick C, Nicholson L, Devaray P, Alcami A, Gompels U. RANTES binding and down-regulation by a novel human herpesvirus-6β chemokine receptor. J Immunol. 2000;164:2396–2404. doi: 10.4049/jimmunol.164.5.2396. [DOI] [PubMed] [Google Scholar]
- 68.Mirkovic R, Voss W R, Beyesh-Melnick M. Proceedings of the 10th International Congress of Microbiology, Mexico City, Mexico. 1970. Characterization of a new herpes-type virus indigenous for tree shrews; pp. 181–189. [Google Scholar]
- 69.Novacek M J. Mammalian phylogeny: shaking the tree. Nature. 1992;356:121–125. doi: 10.1038/356121a0. [DOI] [PubMed] [Google Scholar]
- 70.Pari G S, Anders D G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J Virol. 1993;67:6979–6988. doi: 10.1128/jvi.67.12.6979-6988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roizman B, Carmichael L E, Deinhardt F, de-The G, Nahmias A J, Plowright W, Rapp F, Sheldrick P, Takahashi M, Wolf K. Herpesviridae: definition, provisional nomenclature, and taxonomy. Intervirology. 1981;16:201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- 73.Roizman B. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2221–2230. [Google Scholar]
- 74.Rösen-Wolff A, Ben-Hur T, Becker Y, Darai G. Comparative analysis of the transcripts mapped in the BamHI DNA fragment B of avirulent HSV-1 HFEM, virulent HSV-1 F, and their intratypic recombinant viruses. Virus Res. 1988;10:315–324. doi: 10.1016/0168-1702(88)90073-1. [DOI] [PubMed] [Google Scholar]
- 75.Sardana V V, Wolfgang J A, Veloski C A, Long W J, LeGrow K, Wolanski B, Emini E A, LaFemina R L. Peptide substrate cleavage specificity of the human cytomegalovirus protease. J Biol Chem. 1994;269:14337–14340. [PubMed] [Google Scholar]
- 76.Sarisky R T, Hayward G S. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J Virol. 1996;70:7398–7413. doi: 10.1128/jvi.70.11.7398-7413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmolke S, Kern H F, Drescher P, Jahn G, Plachter B. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J Virol. 1995;69:5959–5968. doi: 10.1128/jvi.69.10.5959-5968.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheaffer A K, Weinheimer S P, Tenney D J. The human cytomegalovirus UL98 gene encodes the conserved herpesvirus alkaline nuclease. J Gen Virol. 1997;78:2953–2961. doi: 10.1099/0022-1317-78-11-2953. [DOI] [PubMed] [Google Scholar]
- 79.Smith J A, Pari G S. Expression of human cytomegalovirus UL36 and UL37 genes is required for viral DNA replication. J Virol. 1995;69:1925–1931. doi: 10.1128/jvi.69.3.1925-1931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith J A, Pari G S. Human cytomegalovirus UL102 gene. J Virol. 1995;69:1734–1740. doi: 10.1128/jvi.69.3.1734-1740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith J A, Jairath S, Crute J J, Pari G S. Characterization of the human cytomegalovirus UL105 gene and identification of the putative helicase protein. Virology. 1996;220:251–255. doi: 10.1006/viro.1996.0310. [DOI] [PubMed] [Google Scholar]
- 82.Springfeld C, Tidona C A, Kehm R, Bahr U, Darai G. Identification and characterization of the tupaia herpesvirus DNA polymerase gene. J Gen Virol. 1998;79:3049–3053. doi: 10.1099/0022-1317-79-12-3049. [DOI] [PubMed] [Google Scholar]
- 83.Stenberg R M, Thomsen D R, Stinski M F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984;49:190–191. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stenberg R M, Witte P R, Stinski M F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985;56:665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tidona C A, Darai G. The complete DNA sequence of lymphocystis disease virus. Virology. 1997;230:207–216. doi: 10.1006/viro.1997.8456. [DOI] [PubMed] [Google Scholar]
- 87.Vink C, Beuken E, Bruggeman C A. Complete DNA sequence of the rat cytomegalovirus genome. J Virol. 2000;74:7656–7665. doi: 10.1128/jvi.74.16.7656-7665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weir J P. Genomic organization and evolution of the human herpesviruses. Virus Genes. 1998;16:85–93. doi: 10.1023/a:1007905910939. [DOI] [PubMed] [Google Scholar]
- 89.Wing B A, Lee G C, Huang E-S. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J Virol. 1996;70:3339–3345. doi: 10.1128/jvi.70.6.3339-3345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winkler M, Rice S A, Stamminger T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J Virol. 1994;68:3943–3954. doi: 10.1128/jvi.68.6.3943-3954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winkler M, Stamminger T. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J Virol. 1996;70:8984–8987. doi: 10.1128/jvi.70.12.8984-8987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood L J, Baxter M K, Plafker S M, Gibson W. Human cytomegalovirus capsid assembly protein precursor (pUL80.5) interacts with itself and with the major capsid protein (pUL86) through two different domains. J Virol. 1997;71:179–190. doi: 10.1128/jvi.71.1.179-190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yurochko A D, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zini N, Battista M C, Santi S, Riccio M, Bergamini G, Landini M P, Maraldi N M. The novel structural protein of human cytomegalovirus, pUL25, is localized in the viral tegument. J Virol. 1999;73:6073–6075. doi: 10.1128/jvi.73.7.6073-6075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zipeto D, Bodaghi B, Laurent L, Virelizier J L, Michelson S. Kinetics of transcription of human cytomegalovirus chemokine receptor US28 in different cell types. J Gen Virol. 1999;80:543–547. doi: 10.1099/0022-1317-80-3-543. [DOI] [PubMed] [Google Scholar]