Abstract
This study aims to analyze changes in health-related quality of life (HRQoL) and safety in patients with generalized anxiety disorder (GAD) prescribed a homogenous selection of cannabis-based medicinal products (CBMPs). Patients prescribed Adven CBMPs (Curaleaf International, UK) for GAD were identified from the UK Medical Cannabis Registry. Primary outcomes were changes in patient-reported outcome measures (PROMs) from baseline up to 12 months, including GAD-7, Single-Item Sleep Quality Scale (SQS), and EQ-5D-5L. Adverse events were recorded using CTCAE version 4.0. A total of 120 patients were identified for inclusion, of which 38 (31.67%), 52 (43.33%), and 30 (25.00%) were prescribed oils, dried flower, and both formulations of CBMP. Associated improvements in GAD-7, SQS, and EQ-5D-5L at 1, 3, 6, and 12 months were observed compared to baseline (P < 0.010). There were 24 (20.00%) patients who reported 442 (368.33%) adverse events, most of which were mild (n = 184, 41.63%) and moderate (n = 197, 44.57%). This study reports an association between initiation of a homogeneous CBMP therapy and improvements in anxiety severity and HRQoL in individuals with GAD. Moreover, therapy was well-tolerated at 12 months follow-up. Further investigation through randomized controlled trials will ultimately be required to determine causation.
Keywords: anxiety, cannabidiol, cannabinoids, cannabis, tetrahydrocannabinol
Background
Generalized anxiety disorder (GAD) is defined as disproportionate, persistent, and excessive anxiety for a minimum of 6 months (DeMartini et al., 2019). The prevalence of GAD in the adult English population is estimated to be 5.9% (NHS, 2014). GAD patients have an increased risk of impairment in mental health, social functioning, and overall well-being (Comer et al., 2011). GAD is consequently the most impairing anxiety disorder (Comer et al., 2011). With anxiety disorders estimated to have an annual direct cost of $42.3 billion globally (Remes et al., 2018), the health and economic burden of GAD on patients and wider society is evident.
Despite the pharmacotherapeutic options available, only 60–85% of patients with anxiety disorders experience at least a 50% improvement in symptoms (Garakani et al., 2020). Moreover, a study by the Harvard/Brown Anxiety Disorders Research Program reported over a 12-year follow-up period, there was a 0.45 probability of GAD recurrence in patients who had previously recovered (Bruce et al., 2005), demonstrating a need for novel therapeutic options.
Cannabis-based medicinal products (CBMPs) are derived from cannabis, of which (−)-trans-Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the major active pharmaceutical ingredients (Mechoulam, 2005). THC is a partial agonist of cannabinoid type 1 (CB1) receptors (Dutta et al., 2022). THC has demonstrated conflicting effects with respect to anxiety. Stimulation of CB1 receptors in the prefrontal cortex and ventral hippocampus of rats has been associated with anxiolytic effects (Rubino et al., 2008). However, stimulation in the basolateral amygdala results in anxiogenic behavior, even at low doses (Rubino et al., 2008). It has since been established in pre-clinical studies that CB1 agonists have a biphasic response on anxiety, whereby they are anxiolytic at low doses, but anxiogenic at high doses (Rey et al., 2012; Stoner, 2017). A 2020 review of pre-clinical and clinical studies on CBD treatment for anxiety stated that the pharmacology of CBD is not fully understood (Wright et al., 2020). However, CBD has been shown to have anxiolytic effects by interacting with serotonin 5-HT1A receptors, cannabinoid type 1 and 2 receptors, and transient receptor potential vanilloid 1 channels in the central and peripheral nervous system (Wright et al., 2020).
There have been two randomized controlled trials which have examined the outcomes in individuals prescribed CBD for social anxiety disorder (Bergamaschi et al., 2011; Crippa et al., 2011; Black et al., 2019). Participants were prescribed between 400-600 mg of CBD prior to anxiety-inducing event, with each study finding a benefit in reported outcomes (Bergamaschi et al., 2011; Crippa et al., 2011). This was not maintained on pooling of outcomes (Black et al., 2019). A systematic review investigating changes in anxiety with pharmaceutical THC treatment with or without CBD reported an associated improvement in anxiety-specific outcomes (Black et al., 2019). However, this data is obtained from studies where participants were primarily prescribed CBMPs for indications other than anxiety disorders, and the evidence quality was described as very low (Black et al., 2019). This may be attributed to the heterogeneity of study methodologies, with inconsistent administration and formulations of CBMPs (Häuser et al., 2018; Montero-Oleas et al., 2020). This results in inconsistent cannabinoid concentrations and makes drawing conclusions on CBMP effectiveness in the context of anxiety and GAD difficult (Banerjee et al., 2022). Moreover, existing studies frequently use isolate CBMPs (Black et al., 2019), despite over 60% of UK patients being treated with CBMPs receiving a full-spectrum dried flower product (Olsson et al., 2023). Whilst some studies have shown a relationship between recreational cannabis use and anxiety, this evidence is not strong, and some systematic review and meta-analyses even find no association between recreational cannabis use and anxiety (Crippa et al., 2009; Gobbi et al., 2019).
There is a complete absence of randomized controlled trials which have sought to evaluate the long-term effects of CBMPs in individuals with an anxiety disorder. Consequently, there has been a reliance on observational studies to advance current knowledge and clinical practice, whilst randomized controlled trials are awaited (Banerjee et al.,2022). The UK Medical Cannabis Registry was established in 2019 and is the largest CBMP-specific patient registry in the UK, with data published on autism spectrum disorder, post-traumatic stress disorder, depression, and attention-deficit/hyperactivity disorder, alongside chronic physical health conditions (Erridge et al., 2021, 2022, 2023a, 2023b; Kawka et al., 2021; Ergisi et al., 2022, 2023; Harris et al., 2022; Mangoo et al., 2022; Nimalan et al.,2022; Pillai et al., 2022; Bapir et al., 2023; Dalavaye et al., 2023; Ittiphakorn et al., 2023; Nicholas et al., 2023; Olsson et al., 2023; Rifkin-Zybutz et al., 2023; Tait et al., 2023b; Wang et al., 2023). Across most of these studies, improvements in generalized anxiety symptoms have been observed as either a primary or secondary outcome of treatment. Project Twenty21, another CBMP patient registry in the UK, similarly reported improved generalized anxiety symptoms in a cohort of patients followed up for three months (Lynskey et al., 2023). In the most recent analysis of patients prescribed CBMPs for GAD from the UK Medical Cannabis Registry 38.3% and 43.6% of participants reported a ≥ 50% reduction or minimal clinically important difference in generalized anxiety severity at 6 months (Rifkin-Zybutz et al., 2023). However, this data is affected by the heterogeneity of prescribed CBMPs, alongside other implicit biases. Therefore, the primary aim of this study is to analyze key changes in anxiety-specific and general HRQoL outcomes and safety in patients with GAD prescribed a homogenous selection of CBMPs, to reduce the associated biases that otherwise affect UK Medical Cannabis Registry data. Secondary aims are to report the incidence of adverse events during CBMP therapy for GAD.
Methods
Study design and participants
The UK Medical Cannabis Registry (UKMCR) is the first prospective registry to collect pseudonymised outcome data on patients prescribed CBMPs in the UK (Erridge et al., 2021), enrolling more than 15 000 patients.
Using the UKMCR, a prospective clinical case series on patients prescribed Adven (Curaleaf International Guernsey, UK) CBMPs for GAD was conducted, as these were identified as the most prescribed CBMPs in prior analyses (Olsson et al., 2023; Rifkin-Zybutz et al., 2023). The UKMCR has obtained ethical approval from the Central Bristol Research Ethics Committee (reference 22/SW/0145). Every patient provided formal, written consent prior to enrollment. Participants were enrolled consecutively. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (Vandenbroucke et al., 2007).
On initial consultation, a specialist clinician designated a primary indication for CBMP therapy. Patients were prescribed dried flower (Adven, Curaleaf International Guernsey, UK), medium-chain triglyceride oils (Adven, Curaleaf International, Guernsey, UK) or a combination of both. The medication prescribed was dictated by a consultant physician according primarily to individual patient characteristics incorporating patient preferences. Recording of CBMP prescription data included formulation, daily THC and CBD doses (mg/day) and strain.
Patients on the UKMCR who were enrolled for a minimum of 12 months on the UKMCR with a primary diagnosis of GAD and were only prescribed Adven CBMPs (Curaleaf International Guernsey, UK) were included in this study. Patients continued with pre-existing treatments for anxiety and any changes were recorded. Individuals who did not complete baseline patient-reported outcome measures (PROMs) were excluded from analysis.
Data collection
Baseline data collection included demographic data: age, sex, occupation, and BMI. Weekly alcohol consumption, tobacco use history, and prior cannabis usage were also recorded. Cannabis usage was quantified using ‘cannabis gram years’, a metric calculated by multiplying mean daily cannabis consumption (grams) by years of prior cannabis use (Erridge et al., 2021).
At initial assessment, clinicians also recorded comorbidity data. Comorbidity data was used to later calculate each patient’s Charlson comorbidity index, used to predict 10-year mortality of patients (Charlson et al., 2022).
Changes in PROMs were recorded by prompting participants to complete relevant surveys electronically at baseline and 1, 3, 6, and 12 months following CBMP therapy initiation. This method has been evaluated within a patient and public evaluation, which found the platform to be easy to use by most participants (Tait et al., 2023a). PROMs included in this study were the GAD-7, the Single-Item Sleep Quality Scale (SQS), EQ-5D-5L, and Patient Global Impression of Change (PGIC). GAD-7, SQS, EQ-5D-5L, and PGIC are all validated and reliable measurement tools for GAD, sleep quality, health-related quality of life (HRQoL), and patients’ perception of change, respectively (Löwe et al., 2008; Rampakakis et al., 2015; Snyder et al., 2018; Feng et al., 2021).
Generalized anxiety disorder-7
The GAD-7 is a seven-item tool that assesses severity of GAD. Each item describes a symptom of GAD and subjects are asked to choose which of the following four categories is most accurate to their experience: ‘not at all’, ‘several days’, ‘more than half the days,’ and ‘nearly every day’. Each option is scored 0, 1, 2, and 3 respectively and a total score out of 21 is calculated. Total scores greater than or equal to 5, 10, and 15 correspond to mild, moderate, and severe anxiety, respectively (Löwe et al., 2008). A minimal clinically important difference (MCID) is determined as a reduction of 4 points or more (Toussaint et al., 2020). The Cronbach’s alpha and intraclass correlation are 0.92 and 0.83, respectively, indicating good internal consistency and test-retest reliability (Spitzer et al., 2006).
Single-Item Sleep Quality Scale
The SQS is a single-item questionnaire in which subjects rate the quality of their last seven days of sleep on a scale of 0–10. A score of 0 is ‘terrible’, 1–3 ‘poor’, 4–6 ‘fair’, 7–9 ‘good’ and 10 ‘excellent’ (Snyder et al., 2018). A score of 3 or less is determined as sleep impaired (Snyder et al., 2018). The SQS has moderate test-retest reliability in patients with insomnia (Snyder et al., 2018).
EQ-5D-5L
The EQ-5D-5L consists of five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Subjects score each on a five-level scale from ‘no problems’ to ‘extreme problems’ which corresponds to a score of 1–5 (Herdman et al., 2011). These scores are used to generate a five-digit code which is mapped to an EQ-5D-5L index value valid for the UK population. The methodology used is detailed by Van Hout et al. (2012), the preferred method according to the NICE guidelines (NICE, 2019). An index value of 0 represents a HRQoL worse than death and an index value of 1 represents optimal HRQoL. The test-retest reliability of the index value has been consistently shown to be good (intraclass correlation ≥0.70) across multiple settings.
Patient global impression of change
The PGIC is a single-item questionnaire which asks patients to complete this statement: ‘Since beginning treatment at this clinic, how would you describe the change (if any) in activity limitations, symptoms, emotions, and overall quality of life-related to your condition?’ Patients select an answer on a scale of 1–7 corresponding where 1 represents ‘no change (or condition has got worse)’ and 7 represents ‘a great deal better, and a considerable improvement that has made all the difference’ (Rampakakis et al., 2015).
Missing data
On graphical assessment, data was adjudged to be missing not at random. To account for missing data, a baseline observation carried forward (BOCF) approach was utilized to provide a more conservative estimation of outcomes in the event of loss to follow-up (Haukoos and Newgard, 2007).
Adverse events
Adverse events were recorded via an online reporting platform (Tait et al., 2023a). Participants have the facility to log into the reporting platform and record an adverse event contemporaneously when an adverse event is experienced. Following the completion of each round of PROMs patients are also directed to the same form to report adverse events if appropriate. These are completed in free text to allow reporting in lay language, which is mapped to appropriate terminology (National Cancer Institute, 2021). Finally, if still unreported, clinicians could record adverse events through a clinician reporting portal during routine follow-up. Adverse events were graded in accordance with the Common Terminology Criteria for Adverse Events version 4.0 as ‘Mild’, ‘Moderate’, ‘Severe’, and ‘Life-threatening’ (National Cancer Institute, 2021).
Statistical analysis
Data relating to demographics, comorbidities, CBMP prescriptions, and adverse events was analyzed using descriptive statistics. Where appropriate, data was presented as mean (±SD), median (interquartile range) or frequency (%).
Statistical differences in PROMs data from baseline were analyzed using a repeated measures one-way analysis of variance (ANOVA) tests using the Greenhouse-Geiser correction. A post-hoc pairwise comparison was conducted for those variables with statistically significant findings on repeated measures ANOVA, to which Bonferroni correction was applied. A univariate and multivariate analysis was performed to identify variables with increased odds of clinically significant reductions in GAD-7. Statistical significance was defined as P-value<0.050.
Results
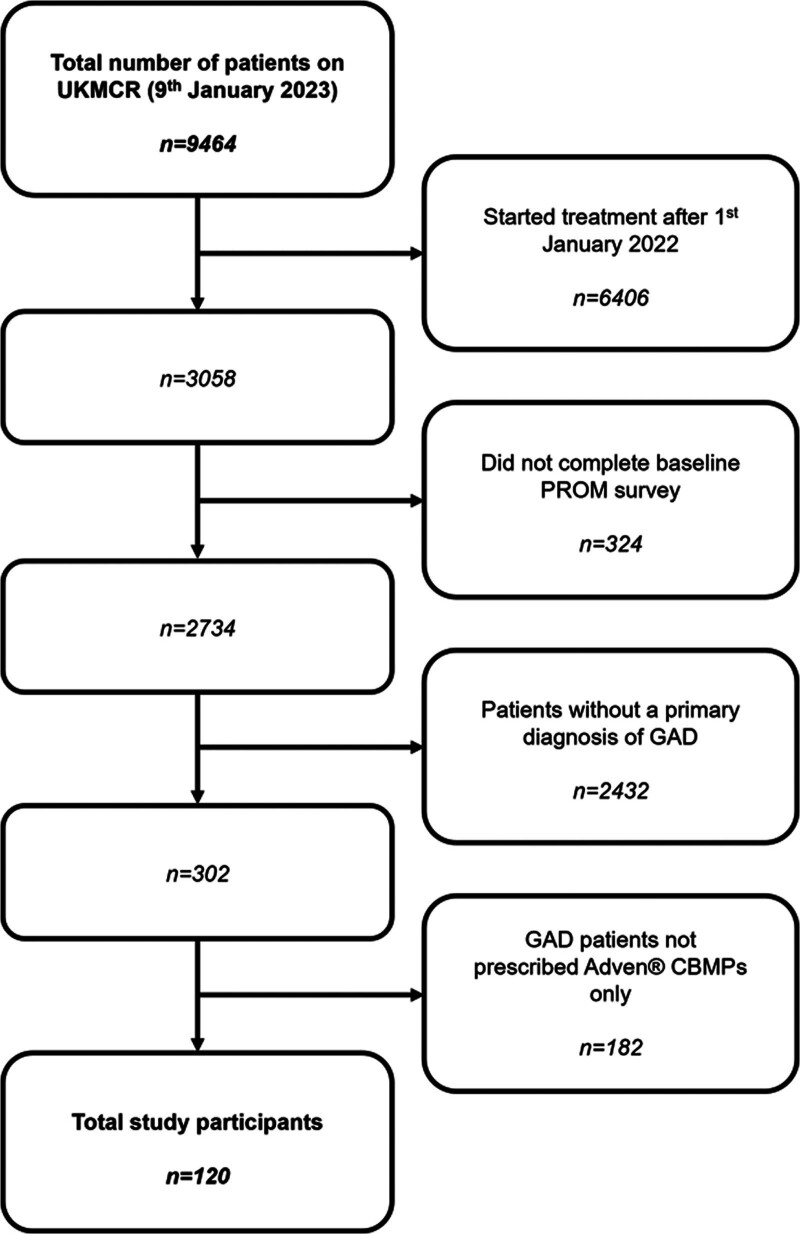
From 9464 patients’ data extracted from the UKMCR, 120 patients were included for final analysis (Fig. 1).
Fig. 1.
Flowchart detailing exclusion criteria and n numbers for each criterion. CBMPs, Cannabis-Based Medicinal Products; GAD, Generalized Anxiety Disorder; UKMCR, UK Medical Cannabis Registry.
Demographic data
Participant demographic data for study participants was analyzed (Table 1). The mean age was 39.38 (±12.48) years. Seventy-four (61.67%) participants were male and 46 (38.33%) participants were female. Mean BMI was 27.80 (±12.48) kg/m2. The most frequently recorded occupation was ‘unemployed’ (n = 28, 23.33%). There was no statistically significant difference between any demographic variables between those individuals with and without missing PROM data (P > 0.050).
Table 1.
Demographic data and tobacco, alcohol, and cannabis history collected at baseline (n = 120)
| Demographic category | n (%)/mean ± SD |
|---|---|
| Sex | |
| Male | 74 (61.67%) |
| Female | 46 (38.33%) |
| Age (years) | 39.38 ± 12.48 |
| BMI (kg/m2) | 27.80 ± 8.25 |
| Underweight | 3 (2.50%) |
| Healthy | 41 (34.17%) |
| Overweight | 37 (30.83%) |
| Obese | 28 (23.33%) |
| Unknown | 11 (9.17%) |
| Occupation | |
| Clerical support workers | 8 (6.67%) |
| Craft and related trades workers | 9 (7.50%) |
| Elementary occupation | 5 (4.17%) |
| Managers | 8 (6.67%) |
| Professional | 15 (12.5%) |
| Service and sales workers | 8 (6.67%) |
| Skilled agricultural, forestry, and fishery workers | 1 (0.83%) |
| Technicians and associate professionals | 5 (4.17%) |
| Unemployed | 28 (23.33%) |
| Other occupations | 18 (15.00%) |
| Unknown | 15 (12.50%) |
| Smoking, alcohol, and cannabis history | n (%)/median (IQR) |
|---|---|
| Smoking status | |
| Current smoker | 45 (37.50%) |
| Ex-smoker | 47 (39.17%) |
| Never smoked | 28 (23.33%) |
| Smoking pack years (current and ex-smokers) | 6.50 (3.00–19.50) |
| Weekly alcohol consumption (units) | 0.00 (0.00–6.75) |
| Recreational cannabis usage status | |
| Current user | 59 (49.17%) |
| Ex-user | 31 (25.83%) |
| Never used | 30 (25.00%) |
| Cannabis gram years (current and ex-users) | 5.00 (1.00–15.00) |
Tobacco, alcohol, and cannabis history data
Participant tobacco, alcohol, and cannabis history data were analyzed (Table 1). Ninety-two (76.67%) participants were current or ex-smokers with a median pack year history of 6.50 (3.00–19.50). Median weekly alcohol consumption was 0.00 (0.00–6.75) units. Ninety (75.00%) participants were current or ex-users of cannabis (n = 59, 49.2% and n = 31, 25.8% respectively). Median lifetime cannabis consumption for current and ex-users was 5.00 (1.00–15.00) grams per year. There was no difference in prior tobacco, alcohol, and cannabis use between those individuals with and without missing PROM data (P > 0.050).
Indications for CBMP prescription data
Indications for CBMP prescription were analyzed (see Table A, Supplemental digital content 1, http://links.lww.com/ICP/A128). As per inclusion criteria, all (100%) participants had a primary indication for treatment with CBMPs of GAD. The most common secondary indication was depression (n = 24, 20.00%) and the most common tertiary indications were depression and insomnia (both n = 4, 3.33%).
Comorbidity data
Participant comorbidity data was analyzed (see Table B, Supplemental digital content 1, http://links.lww.com/ICP/A128). Median Charlson Comorbidity Index score was 0.00 (0.00–1.00).
CBMP prescription data
Thirty-eight (31.67%) patients were prescribed oil-based CBMPs, 52 (43.33%) were prescribed dried flowers, and 30 (25.00%) were prescribed both. Most participants were prescribed a combination of CBD and THC (n = 104, 86.67%), 15 (12.50%) participants were prescribed THC only, and one (0.83%) participant was prescribed CBD only. Median CBD dose was 31.25 (5.00–55.00) mg and median THC dose was 100.00 (10.00–150.00) mg. There was no difference in CBD or THC dose in those individuals with and without missing PROM data (P > 0.050). The most prescribed dried flos was Adven EMT1 (Curaleaf International, UK). The most common medium-chain triglyceride oils were Adven 50 mg/ml CBD (Curaleaf International, UK) and Adven 20 mg/ml THC (Curaleaf International, UK).
Missing PROMs data
As per inclusion criteria, 100.00% (n = 120) of participants completed every PROM at baseline. At 1-month follow-up twenty-one (17.50%) participants failed to complete the GAD-7, SQS, and EQ-5D-5L. At 3 months of follow-up 35 (29.17%) participants, at 6 months of follow-up 50 (41.67%) participants and at 12 months of follow-up, 71 (59.17%) participants failed to complete the GAD-7, SQS, and EQ-5D-5L. As per the study protocol this data was treated with a BOCF method.
PROMs analysis
Analysis of longitudinal changes in HRQoL are presented in Table 2. There were improvements in GAD-7 and SQS at 1, 3, 6, and 12 months (P < 0.001). There were also improvements in EQ-5D-5L index value at 1, 3, 6 (P < 0.001), and 12 months (P = 0.006). Regarding the EQ-5D-5L domains, there were statistically significant improvements in usual activities at 1 month (P = 0.039) and 3 months (P = 0.007); pain and discomfort at 1 (P = 0.001), 3 (P = 0.015), and 6 months (P = 0.002) and anxiety and depression at 1, 3, 6 (P < 0.001) and 12 months (P = 0.007).
Table 2.
Mean baseline and follow-up GAD-7, SQS, and EQ-5D-5L at 1, 3, 6, and 12 months (n = 120)
| Patient-reported outcome measure | Follow-up (months) | Mean ± SD | P-value compared to previous follow-up period | P-value compared to baseline |
|---|---|---|---|---|
| GAD-7 | Baseline | 13.87 ± 0.50 | ||
| 1 | 9.51 ± 0.55 | <0.001 | ||
| 3 | 9.717 ± 0.57 | 1.000 | <0.001 | |
| 6 | 9.97 ± 0.60 | 1.000 | <0.001 | |
| 12 | 11.12 ± 0.60 | 0.129 | <0.001 | |
| SQS | Baseline | 3.77 ± 0.21 | ||
| 1 | 5.64 ± 0.23 | <0.001 | ||
| 3 | 5.50 ± 0.24 | 1.000 | <0.001 | |
| 6 | 4.99 ± 0.27 | 0.071 | <0.001 | |
| 12 | 4.73 ± 0.26 | 1.000 | <0.001 | |
| EQ-5D-5L mobility | Baseline | 1.50 ± 0.08 | ||
| 1 | 1.43 ± 0.08 | 1.000 | ||
| 3 | 1.46 ± 0.08 | 1.000 | 1.000 | |
| 6 | 1.52 ± 0.08 | 1.000 | 1.000 | |
| 12 | 1.50 ± 0.08 | 1.000 | 1.000 | |
| EQ-5D-5L self-care | Baseline | 1.51 ± 0.09 | ||
| 1 | 1.47 ± 0.09 | 1.000 | ||
| 3 | 1.45 ± 0.08 | 1.000 | 1.000 | |
| 6 | 1.54 ± 0.09 | 0.337 | 1.000 | |
| 12 | 1.53 ± 0.09 | 1.000 | 1.000 | |
| EQ-5D-5L usual activities | Baseline | 2.33 ± 0.12 | ||
| 1 | 2.06 ± 0.11 | 0.039 | ||
| 3 | 1.98 ± 0.11 | 1.000 | 0.007 | |
| 6 | 2.15 ± 0.11 | 0.194 | 0.399 | |
| 12 | 2.13 ± 0.11 | 1.000 | 0.141 | |
| EQ-5D-5L pain and discomfort | Baseline | 2.13 ± 0.11 | ||
| 1 | 1.83 ± 0.09 | 0.001 | ||
| 3 | 1.89 ± 0.10 | 1.000 | 0.015 | |
| 6 | 1.89 ± 0.10 | 1.000 | 0.002 | |
| 12 | 1.98 ± 0.10 | 0.858 | 0.074 | |
| EQ-5D-5L anxiety and depression | Baseline | 3.43 ± 0.10 | ||
| 1 | 2.89 ± 0.10 | <0.001 | ||
| 3 | 2.94 ± 0.11 | 1.000 | <0.001 | |
| 6 | 2.94 ± 0.11 | 1.000 | <0.001 | |
| 12 | 3.15 ± 0.10 | 0.180 | 0.007 | |
| EQ-5D-5L index value | Baseline | 0.53 ± 0.03 | ||
| 1 | 0.64 ± 0.03 | <0.001 | ||
| 3 | 0.63 ± 0.03 | 1.000 | <0.001 | |
| 6 | 0.61 ± 0.03 | 1.000 | <0.001 | |
| 12 | 0.58 ± 0.03 | 0.848 | 0.006 | |
| PGIC | 1 | 5.29 ± 0.17 | ||
| 3 | 5.55 ± 0.13 | 0.223 | - | |
| 6 | 5.42 ± 0.16 | 1.000 | - | |
| 12 | 5.59 ± 0.15 | 1.000 | - | |
PGIC data was collected at 1, 3, 6, and 12 months. Twenty-three (19.17%) participants had missing PGIC scores at 1 month and 12 months. Twenty-one (17.50%) participants had missing PGIC scores at 3 and 6 months. The median PGIC score was 6.00 (5.00–6.00) at each follow-up period. At 12 months follow-up, nine (7.50%) participants reported a PGIC of 1–3 (no noticeable change or worsening of condition), one (0.83%) participant reported a PGIC of 4 (better, but not made any real difference) and 87 (72.50%) reported a PGIC of 5–7 (better, with a noticeable improvement).
There was a clinically significant (4 points or greater) reduction in GAD-7 score for thirty-two (26.67%) participants at 12 months compared to baseline (Table 3). Twenty (32.79%) patients with severe baseline GAD-7 scores had clinically significant reductions in GAD-7 at 12 months. Seven (21.88%) participants with moderate baseline GAD-7 scores had clinically significant reductions in GAD-7 at 12 months. Five (27.78%) participants with mild baseline GAD-7 scores had a clinically significant reduction in GAD-7 score.
Table 3.
Two-way frequency table showing participants’ GAD-7 classification at baseline and 12 months (n = 120)
| GAD-7 score at 12 months | Total | |||||
|---|---|---|---|---|---|---|
| 0–4 (minimal) |
5-9 (mild) |
10–14 (moderate) |
15–21 (severe) |
|||
| GAD-7 score at baseline | 0–4 (minimal) | 8 | 1 | 0 | 0 | 9(7.50%) |
| 5–9 (mild) |
5 | 13 | 0 | 0 | 18(15.00%) | |
| 10–14 (moderate) |
4 | 3 | 22 | 3 | 32(26.67%) | |
| 15–21 (severe) |
8 | 9 | 3 | 41 | 61(50.83%) | |
| Total | 25(20.83%) | 26(21.67%) | 25(20.83%) | 44(36.67%) | 120 | |
Univariate logistic regression assessing the association between variables and the likelihood of experiencing a clinically significant benefit in GAD-7 at 12 months (Table C, Supplemental digital content 1, http://links.lww.com/ICP/A128) showed those taking a dried flower CBMP preparation and those with a daily THC dose greater than 100 mg to have increased odds (OR = 3.800, 95%CI = 1.269–11.381, P = 0.017 and OR = 2.714, 95%CI = 1.062–6.937, P = 0.037 respectively).
Multivariate logistic regression (Table 4) showed those aged 41–50 years old and those with a baseline GAD-7 score of 15–21 (severe) to have increased odds of clinically significant improvement in GAD-7 (OR = 6.721, 95%CI = 1.334–33.858, P = 0.021 and OR = 16.018, 95%CI = 2.157–118.693, P = 0.007 respectively).
Table 4.
Multivariate analysis assessing association between variables and the likelihood of experiencing a clinically significant benefit in GAD-7 at 12 months
| Variable | n | OR (95% CI) | P-value |
|---|---|---|---|
| Age | |||
| <30 | 28 | 1 | |
| 31–40 | 41 | 2.674 (0.621–11.506) | 0.187 |
| 41–50 | 23 | 6.721 (1.334–33.858) | 0.021* |
| 51–60 | 12 | 1.852 (0.280–12.240) | 0.523 |
| 60+ | 5 | 1.156 (0.057–23.424) | 0.925 |
| Gender | |||
| Male | 67 | 1 | |
| Female | 42 | 1.568 (0.467–5.261) | 0.466 |
| BMI (kg/m2) | |||
| <25 | 44 | 1 | |
| 25–30 | 37 | 0.435 (0.109–1.731) | 0.237 |
| 30–35 | 12 | 1.662 (0.280–9.868) | 0.576 |
| >35 | 16 | 3.911 (0.845–18.108) | 0.081 |
| Prior cannabis usage status | |||
| Current user | 53 | 1 | |
| Ex-user | 29 | 1.031 (0.299–3.551) | 0.961 |
| Never used | 27 | 0.958 (0.196–4.685) | 0.958 |
| CBMP prescription | |||
| Oils | 34 | 1 | |
| Dried flower | 48 | 21.964 (0.752–641.491) | 0.073 |
| Both | 27 | 11.563 (0.442–302.527) | 0.142 |
| CBD contents in CBMP | |||
| No CBD | 13 | 1 | |
| ≤ Median dose of cohort (≤31.25 mg/day) | 41 | 4.583 (0.625–33.605) | 0.134 |
| > Median dose of cohort (>31.25 mg/day) | 55 | 3.248 (0.417–25.316) | 0.261 |
| THC contents in CBMP | |||
| ≤ Median dose of cohort (≤100.00 mg/day) | 40 | 1 | |
| > Median dose of cohort (>100.00 mg/day) | 69 | 0.311 (0.021–4.680) | 0.398 |
| Baseline GAD-7 score | |||
| 0–9 (minimal or mild) | 23 | 1 | |
| 10–14 (moderate) | 30 | 4.537 (0.735–28.004) | 0.103 |
| 15–21 (severe) | 56 | 16.018 (2.157–118.963) | 0.007** |
| Baseline SQS score | |||
| ≤ 3 (sleep impaired) | 61 | 1 | |
| > 3 (non-sleep impaired) | 48 | 0.991 (0.320–3.066) | 0.987 |
Significant differences are denoted by asterisks (*P < 0.050,**P = 0.010, and ***P < 0.001).
Changes in medication
Seventy-seven (64.17%) of the cohort were being treated with antidepressants. Sixty-two (80.52%) patients had no change in their antidepressant medication over the 12 month period, four (5.19%) reduced dose, six (7.79%) stopped completely, one (1.30%) patients increased dose, and four (5.19%) started a new antidepressant (Table D, Supplemental digital content 1, http://links.lww.com/ICP/A128). Twenty-four (20.00%) patients were being treated with benzodiazepines. Nineteen (79.17%) patients had no change in benzodiazepine medication, one (4.17%) reduced dose, three (12.50%) stopped completely and one (4.17%) patient started a new benzodiazepine (Table D, Supplemental digital content 1, http://links.lww.com/ICP/A128). Nine (7.50%) patients were being treated with insomnia-related medications. Seven (77.78%) patients had no change in insomnia-related medication and two (22.22%) patients changed to a new insomnia-related medication (Table D, Supplemental digital content 1, http://links.lww.com/ICP/A128).
Adverse event data
Participants reported adverse events were analyzed (Table 5). Twenty-four (20.00%) patients reported a total of 442 (368.33%) adverse events. The most common adverse events were concentration impairment and dry mouth (both n = 35, 7.92%). One hundred and eighty-four (41.63%) adverse events reported were mild, 44.57% (n = 197) were moderate and 13.80% (n = 61) were severe. There were no life-threatening or disabling adverse event reports. Adverse event data was not suitable for logistic regression analysis due to limitations of sample size.
Table 5.
Adverse events reported by participants (n = 120)
| Adverse event | Mild | Moderate | Severe | Total (%) |
|---|---|---|---|---|
| Abdominal pain | 3 | 2 | 0 | 5 (1.13%) |
| Agitation | 0 | 1 | 0 | 1 (0.23%) |
| Akathisia | 0 | 1 | 0 | 1 (0.23%) |
| Amnesia | 4 | 16 | 6 | 26 (5.88%) |
| Anorexia | 2 | 3 | 2 | 7 (1.58%) |
| Anxiety | 3 | 4 | 3 | 10 (2.26%) |
| Ataxia | 5 | 5 | 0 | 10 (2.26%) |
| Blurred vision | 11 | 1 | 0 | 12 (2.71%) |
| Bruxism | 1 | 0 | 0 | 1 (0.23%) |
| Chest pain | 1 | 0 | 0 | 1 (0.23%) |
| Cognitive disturbance | 6 | 15 | 3 | 24 (5.43%) |
| Concentration impairment | 14 | 19 | 2 | 35 (7.92%) |
| Confusion | 7 | 3 | 2 | 12 (2.71%) |
| Constipation | 5 | 1 | 0 | 6 (1.36%) |
| Costochondritis | 1 | 0 | 0 | 1 (0.23%) |
| Delirium | 4 | 3 | 1 | 8 (1.81%) |
| Depression | 1 | 4 | 15 | 20 (4.52%) |
| Dissociation | 0 | 2 | 0 | 2 (0.45%) |
| Dizziness | 12 | 6 | 1 | 19 (4.30%) |
| Dry mouth | 20 | 15 | 0 | 35 (7.92%) |
| Dysgeusia | 7 | 5 | 2 | 14 (3.17%) |
| Dyspepsia | 4 | 0 | 1 | 5 (1.13%) |
| Fall | 1 | 0 | 0 | 1 (0.23%) |
| Fatigue | 8 | 11 | 3 | 22 (4.98%) |
| Fever | 1 | 0 | 0 | 1 (0.23%) |
| Generalized muscle weakness | 0 | 5 | 1 | 6 (1.36%) |
| Headache | 10 | 4 | 3 | 17 (3.85%) |
| Hypertension | 1 | 0 | 0 | 1 (0.23%) |
| Insomnia | 4 | 9 | 5 | 18 (4.07%) |
| Lethargy | 11 | 13 | 0 | 24 (5.43%) |
| Libido decreased | 1 | 0 | 0 | 1 (0.23%) |
| Nausea | 14 | 0 | 2 | 16 (3.62%) |
| Palpitations | 0 | 1 | 0 | 1 (0.23%) |
| Paranoia | 2 | 5 | 0 | 7 (1.58%) |
| Pharyngitis | 0 | 5 | 0 | 5 (1.13%) |
| Rash | 0 | 4 | 0 | 4 (0.90%) |
| Seizure | 0 | 0 | 2 | 2 (0.45%) |
| Sensory overload | 0 | 0 | 1 | 1 (0.23%) |
| Sinus pain | 1 | 0 | 0 | 1 (0.23%) |
| Sneezing | 1 | 0 | 0 | 1 (0.23%) |
| Somnolence | 0 | 25 | 6 | 31 (7.01%) |
| Toothache | 0 | 1 | 0 | 1 (0.23%) |
| Tremor | 3 | 1 | 0 | 4 (0.90%) |
| Upper respiratory infection | 0 | 2 | 0 | 2 (0.45%) |
| Urinary tract infection | 0 | 4 | 0 | 4 (0.90%) |
| Vertigo | 6 | 1 | 0 | 7 (1.58%) |
| Vomiting | 3 | 0 | 0 | 3 (0.38%) |
| Weight loss | 6 | 0 | 0 | 6 (1.36%) |
| Total | 184 (41.6%) | 197 (44.6%) | 61 (13.8%) | 442 (368.33%) |
Discussion
This study analyzed outcomes in GAD patients enrolled in the UKMCR. Improvements in GAD-7, SQS, EQ-5D-5L index, and EQ-5D-5L anxiety and depression scores up until 12 months follow-up demonstrate an association between CBMP treatment initiation and improvements in HRQoL measures in GAD patients. Additionally, one-quarter of patients reported a minimal clinically important difference in anxiety at 12 months. Only twenty-four (20.00%) patients reported adverse events, the majority of which were mild or moderate, suggesting that CBMPs were largely well-tolerated by participants in this study. However, these outcomes must be appreciated within the context of the limitations of the study design.
Improvements in GAD-7 score were observed at all follow-up periods in this study, with one-quarter reporting clinically significant reductions at 12 months. Stith et al. previously reported an association between self-directed cannabis flower use and improvements in anxiety severity (Stith et al., 2020). Whilst this corroborates the findings of this study, anxiolytic effects were not investigated longitudinally with symptoms monitored up to 4 h post-CBMP administration. When considering these findings with the findings of this study, a more complete understanding of CBMP therapy’s association with anxiety improvement, in the short-term and long-term, may be reached. Multivariate analysis of this study showed an association between patients on CBMPs with severe baseline GAD-7 scores and clinically significant improvements in GAD-7 compared to moderate, mild, and subclinical GAD-7 CBMP patients (P = 0.007). A 2021 review concluded that greater anxiety symptoms are linked with a poorer quality of life (Wilmer et al., 2021). Consequently, the findings of this study provide promise for a treatment which is potentially more effective for patients with severe anxiety. This is important considering 50% and 30% of patients with GAD will not respond to first-line therapies and multiple medications, respectively.
Anxiety was also reported as an adverse event during the present study, with an incidence of 2.26%. Although the adverse events are not assessed to determine if they are treatment-related, the potential anxiogenic effects of THC must also be considered (Rey et al., 2012; Stoner, 2017). This is particularly important, considering the median daily THC dose was 100.00 mg/day, which is high relative to studies which have evaluated anxiety as an outcome in the past (Bystritsky, 2006; Ansara, 2020; Black et al., 2019).
This study suggests an association between improvements in sleep quality and CBMP therapy. A 2018 study investigating cannabis flower’s effect on insomnia reported improvements in perceived insomnia (Vigil et al., 2018). Considering sleep disorders are one of the most common reasons cited for CBMP use (Hazekamp et al., 2013), these findings are particularly promising for future research. This study also reported that CBMP patients with baseline sleep impairment were not more likely to report clinically significant improvements in GAD-7 compared to patients with no sleep impairment at baseline on multivariate logistic regression. This suggests that sleep quality was not a confounding factor for improvement in GAD-7 in this cohort.
Observed improvements in HRQoL are supported by a previous clinical case series of GAD patients enrolled on the UKMCR. Statistically significant improvements in EQ-5D-5L were associated with CBMP therapy in GAD patients, concordant with the findings of this study (Ergisi et al., 2022). This illustrates a potentially reproducible associated improvement in HRQoL with CBMP therapy for GAD patients. This study adds to the limited evidence regarding CBMP therapy’s effect on HRQoL, suggesting an association between the two in GAD patients. However, further research would need to be conducted to further investigating CBMP’s effects on HRQoL in a broader patient population.
CBMP therapy appears to be tolerated well by the majority of this cohort. Due to the design of this study, adverse events due to CBMP therapy were unable to be distinguished from adverse events resulting from natural or coincidental causes. For example, the most frequently reported severe adverse event was depression, which GAD is a significant risk factor for (Hirschfeld, 2001). As a result, adverse event data should be interpreted with caution. Additionally, drug-drug interactions were not investigated as a part of this study, which also may potentially exacerbate adverse events. Despite this, these findings suggest CBMPs are well-tolerated in this population.
Limitations
Due to the observational nature of this study, no causative relationship can be established between CBMP therapy and improvements in GAD, sleep, and HRQoL outcomes. Internal validity was limited because of a lack of blinding and randomization. With no control arm to this trial, genuine CBMP treatment effects cannot be distinguished from potential confounding effects, such as regression to the mean. Another example was that many patients were former or current tobacco smokers, which is known to be associated with increased anxiety symptoms (Moylan et al., 2013). In addition, despite PROMs being the gold-standard assessment for subjective symptoms of anxiety, they remain subject to recall bias. PROMs are also affected by ceiling effects, which might be responsible for those individuals with the highest GAD-7 scores at baseline being the most likely to report a clinically significant improvement at 12 months. Additionally, the placebo effect of CBMPs may be exaggerated secondary to the expectancy bias shown to be associated through positive media reporting (Gedin et al., 2022). Moreover, paying for medications has been shown to increase perceived medication quality (Díaz-Lago et al., 2023). CBMPs are also associated with an exaggerated placebo effect due to the associated biological effects and aroma (Gertsch, 2018). Finally, 59 (49.17%) participants had previously acquired cannabis prior to enrollment, which may also lead to expectancy bias. However, these individuals may conversely have developed tolerance to the effects of CBMPs, which would bias the results towards a null finding. Therefore, the positive findings suggest that there may be supplementary benefits to sourcing CBMPs compared to illicit cannabis, such as consistent supply of pharmaceutical quality medication overseen by a specialist physician. Moreover, this may also lead to a reduction in anxiety through reducing engagement in illegal activity. However, it has been shown by our group that medical cannabis patients still perceive themselves to be subject to stigma (Troup et al., 2022). This is just one example of the effects of sampling bias, including a higher than anticipated proportion of male participants compared to the general population with GAD. Finally, it was not possible to screen for the development of cannabis use disorder, considering currently available screening tools have at best not been validated for clinical populations and at worse heavily utilize questions around frequency of cannabis use, which are inappropriate for populations who are prescribed CBMPs daily.
Conclusion
This study reports that initiation of Adven (Curaleaf International, Guernsey, UK) CBMP treatment is associated with significant improvement in HRQoL outcomes in this population of GAD patients. This suggests the therapeutic potential of CBMPs for GAD. Although limitations due to study design mean that a causative relationship cannot be established, these findings suggest that the benefits of CBMPs may be most marked in individuals with severe anxiety at baseline. The findings of this study can act as the basis for future controlled studies, with study designs that can infer causative relationships, and determine the optimal dosing strategies, and most appropriate populations who may benefit from this treatment.
Acknowledgements
Data that support the findings of this study are available from the UK Medical Cannabis Registry. Restrictions apply to the availability of these data. Data specifications and applications are available from the corresponding author.
All authors have contributed to and approved the final manuscript.
The authors confirm that the PI for this paper is Mikael H. Sodergren and that he had direct clinical responsibility for patients.
Ethical approval provided by Central Bristol Research Ethics Committee (Reference: 22/SW/0145).
Patient consent statement: All participants completed written, informed consent prior to enrollment in the registry.
Conflicts of interest
Adam Li is a medical student at Imperial College London. Adam Li has no shareholdings in pharmaceutical companies. Simon Erridge is a junior doctor and is the Head of Research at Sapphire Medical Clinics. Simon Erridge is an honorary clinical research fellow at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS. Simon Erridge has no shareholdings in pharmaceutical companies. Carl Holvey is Chief Clinical Pharmacist at Sapphire Medical Clinics. Carl Holvey has no shareholdings in pharmaceutical companies. Ross Coomber is a consultant orthopedic surgeon, Operations Director at Sapphire Medical Clinics and a consultant at St George’s Hospital, London. The views expressed are those of the author(s) and not necessarily those of the NHS. Ross Coomber has no shareholdings in pharmaceutical companies. Daniela Barros is a consultant psychiatrist at Sapphire Medical Clinics. Daniela Barros has no shareholdings in pharmaceutical companies. Urmila Bhoskar is a consultant psychiatrist at Sapphire Medical Clinics. Urmila Bhoskar has no shareholdings in pharmaceutical companies. Mathieu Crews is a consultant psychiatrist at Sapphire Medical Clinics. Mathieu Crews has no shareholdings in pharmaceutical companies. Lorna Donnelly is a consultant psychiatrist at Sapphire Medical Clinics. Lorna Donnelly has no shareholdings in pharmaceutical companies. Muhammad Imran is a consultant psychiatrist at Sapphire Medical Clinics. Muhammad Imran has no shareholdings in pharmaceutical companies. Laura Korb is a consultant psychiatrist at Sapphire Medical Clinics and North London Mental Health Partnership. Laura Korb has no shareholdings in pharmaceutical companies. The views expressed are those of the author(s) and not necessarily those of the NHS. Gracia Mwimba is a consultant psychiatrist at Sapphire Medical Clinics. Gracia Mwimba has no shareholdings in pharmaceutical companies. Simmi Sachdeva-Mohan is a consultant psychiatrist at Sapphire Medical Clinics. Simmi Sachdeva-Mohan has no shareholdings in pharmaceutical companies. James Rucker is a consultant psychiatrist and a former director at Sapphire Medical Clinics (London). James Rucker is an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King’s College London. James Rucker is funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. James Rucker has no shareholdings in pharmaceutical companies. James Rucker reviewed this article and made comments. Mikael Sodergren is a consultant hepatopancreatobiliary surgeon at Imperial College NHS Trust. He is the Chief Medical Officer at Curaleaf International. He is a senior clinical lecturer at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.intclinpsychopharm.com.
References
- Ansara ED. (2020). Management of treatment-resistant generalized anxiety disorder. Ment Health Clin. 10:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Erridge S, Salazar O, Mangal N, Couch D, Pacchetti B, et al. (2022). Real world evidence in medical cannabis research. Ther Innov Regul Sci. 56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapir L, Erridge S, Nicholas M, Pillai M, Dalavaye N, Holvey C, et al. (2023). Comparing the effects of medical cannabis for chronic pain patients with and without co-morbid anxiety: a cohort study. Expert Rev Neurother. 23:281–295. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RHC, Chagas MHN, De Oliveira DCG, De Martinis BS, Kapczinski F, et al. (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 36:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. (2019). Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 6:995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, et al. (2005). Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 162:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A. (2006). Treatment-resistant anxiety disorders. Mol Psychiatry. 11:805–814. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Carrozzino D, Guidi J, Patierno C. (2022). Charlson comorbidity index: a critical review of clinimetric properties. Psychother Psychosom. 91:8–35. [DOI] [PubMed] [Google Scholar]
- Comer JS, Blanco C, Hasin DS, Liu SM, Grant BF, Turner JB, et al. (2011). Health-related quality of life across the anxiety disorders. J Clin Psychiatry. 72:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martín‐Santos R, Bhattacharyya S, Atakan Z, McGuire P, et al. (2009). Cannabis and anxiety: a critical review of the evidence Human Psychopharmacology: clinical and experimental. Hum Psychopharmacol - Clin Exp. 24:515–523. [DOI] [PubMed] [Google Scholar]
- Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 25:121–130. [DOI] [PubMed] [Google Scholar]
- Dalavaye N, Erridge S, Nicholas M, Pillai M, Bapir L, Holvey C, et al. (2023). The effect of medical cannabis in inflammatory bowel disease: analysis from the UK Medical Cannabis Registry. Expert Rev Gastroenterol Hepatol. 17:85–98. [DOI] [PubMed] [Google Scholar]
- DeMartini J, Patel G, Fancher TL. (2019). Generalized anxiety disorder. Ann Intern Med. 170:ITC49–ITC64. [DOI] [PubMed] [Google Scholar]
- Díaz-Lago M, Blanco F, Matute H. (2023). Expensive seems better: the price of a non-effective drug modulates its perceived efficacy. Cogn Res Princ Implic. 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Selvam B, Das A, Shukla D. (2022). Mechanistic origin of partial agonism of tetrahydrocannabinol for cannabinoid receptors. J Biol Chem. 298:101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergisi M, Erridge S, Harris M, Kawka M, Nimalan D, Salazar O, et al. (2022). UK Medical Cannabis Registry: an analysis of clinical outcomes of medicinal cannabis therapy for generalized anxiety disorder. 10.1080/17512433.2022.2020640 [DOI] [PubMed]
- Ergisi M, Erridge S, Harris M, Kawka M, Nimalan D, Salazar O, et al. (2023). An updated analysis of clinical outcome measures across patients from the UK Medical Cannabis Registry. Cannabis Cannabinoid Res. 8:557–566. [DOI] [PubMed] [Google Scholar]
- Erridge S, Salazar O, Kawka M, Holvey C, Coomber R, Usmani A, et al. (2021). An initial analysis of the UK Medical Cannabis Registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 41:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge S, Kerr-Gaffney J, Holvey C, Coomber R, Barros DAR, Bhoskar U, et al. (2022). Clinical outcome analysis of patients with autism spectrum disorder: analysis from the UK Medical Cannabis Registry. Ther Adv Psychopharmacol. 12. doi:https://doi.org/10.1177%2F20451253221116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge S, Holvey C, Coomber R, Hoare J, Khan S, Platt MW, et al. 2023. a). Clinical outcome data of children treated with cannabis-based medicinal products for treatment resistant epilepsy-analysis from the UK Medical Cannabis Registry. Neuropediatrics. 54:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge S, Leung O, Holvey C, Coomber R, Beri S, Khan S, et al. 2023. b). An observational study of clinical outcome measures in patients treated with cannabis-based medicinal products on the UK Medical Cannabis Registry. Neuropsychopharmacol Rep. doi: 10.1002/npr2.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YS, Kohlmann T, Janssen MF, Buchholz I. (2021). Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 30:647–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, et al. (2020). Pharmacotherapy of anxiety disorders: current and emerging treatment options. Front Psychiatry. 11:1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedin F, Blomé S, Pontén M, Lalouni M, Fust J, Raquette A, et al. (2022). Placebo Response and media attention in randomized clinical trials assessing cannabis-based therapies for pain: a systematic review and meta-analysis. JAMA Network Open. 5:e2243848–e2243848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J. (2018). The intricate influence of the placebo effect on medical cannabis and cannabinoids. Med Cannabis Cannabinoids. 1:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. (2019). Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry. 76:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Erridge S, Ergisi M, Nimalan D, Kawka M, Salazar O, et al. (2022). UK Medical Cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for chronic pain conditions. Expert Rev Clin Pharmacol. 15:473–485. [DOI] [PubMed] [Google Scholar]
- Haukoos JS, Newgard CD. (2007). Advanced statistics: missing data in clinical research—part 1: an introduction and conceptual framework. Acad Emerg Med. 14:662–668. [DOI] [PubMed] [Google Scholar]
- Häuser W, Petzke F, Fitzcharles MA. (2018). Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management – an overview of systematic reviews. Eur J Pain. 22:455–470. [DOI] [PubMed] [Google Scholar]
- Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Grotenhermen F. (2013). The medicinal use of cannabis and cannabinoids—an International Cross-Sectional Survey on Administration Forms. J Psychoactive Drugs. 45:199–210. [DOI] [PubMed] [Google Scholar]
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 20:1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RMA. (2001). The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 3:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittiphakorn P, Erridge S, Holvey C, Coomber R, Rucker JJ, Sodergren MH. (2023). UK Medical Cannabis Registry: an analysis of clinical outcomes of medicinal cannabis therapy for attention‐deficit/hyperactivity disorder. Neuropsychopharmacol Rep. doi: 10.1002/npr2.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawka M, Erridge S, Holvey C, Coomber R, Usmani A, Sajad M, et al. (2021). Clinical outcome data of First cohort of chronic pain patients treated with cannabis‐based sublingual oils in the United Kingdom: analysis from the UK medical cannabis registry. J Clin Pharmacol. 61:1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. (2008). Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 46:266–274. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Schlag AK, Athanasiou-Fragkouli A, Badcock D, Nutt DJ. (2023). Characteristics of and 3-month health outcomes for people seeking treatment with prescribed cannabis: Real-world evidence from Project Twenty21. Drug Sci Policy Law. 9:9. [Google Scholar]
- Mangoo S, Erridge S, Holvey C, Coomber R, Barros DAR, Bhoskar U, et al. (2022). Assessment of clinical outcomes of medicinal cannabis therapy for depression: analysis from the UK Medical Cannabis Registry. Expert Rev Neurother. 22:995–1008. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. (2005). Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 146:913–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Oleas N, Arevalo-Rodriguez I, Nuñez-González S, Viteri-García A, Simancas-Racines D. (2020). Therapeutic use of cannabis and cannabinoids: evidence mapping and appraisal of systematic reviews. BMC Complement Med Ther. 20:12 10.1186/S12906-019-2803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Jacka F, Pasco J, Berk M. (2013). How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav. 3:302–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute . (2021). Criteria for adverse common terminology events. 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/
- NHS . (2014). Adult psychiatric morbidity survey: survey of mental health and wellbeing. https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014. [Accessed 9 May 2023]
- NICE . (2019). Position statement on use of the EQ-5D-5L value set for England. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l. [Accessed 7 May 2023]
- Nicholas M, Erridge S, Bapir L, Pillai M, Dalavaye N, Holvey C, et al. (2023). UK medical cannabis registry: assessment of clinical outcomes in patients with headache disorders. Expert Rev Neurother. 23:85–96. [DOI] [PubMed] [Google Scholar]
- Nimalan D, Kawka M, Erridge S, Ergisi M, Harris M, Salazar O, et al. (2022). UK Medical Cannabis Registry palliative care patient’s cohort: initial experience and outcomes. J Cannabis Res. 4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson F, Erridge S, Tait J, Holvey C, Coomber R, Beri S, et al. (2023). An observational study of safety and clinical outcome measures across patient groups in the United Kingdom Medical Cannabis Registry. Expert Rev Clin Pharmacol. 16:257–266. 10.1080/17512433.2023.2183841. [DOI] [PubMed] [Google Scholar]
- Pillai M, Erridge S, Bapir L, Nicholas M, Dalavaye N, Holvey C, et al. (2022). Assessment of clinical outcomes in patients with post-traumatic stress disorder: analysis from the UK Medical Cannabis Registry. Expert Rev Neurother. 22:1009–1018. [DOI] [PubMed] [Google Scholar]
- Rampakakis E, Ste-Marie PA, Sampalis JS, Karellis A, Shir Y, Fitzcharles MA. (2015). Extended report: Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open. 1:e000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes O, Wainwright N, Surtees P, Lafortune L, Khaw K-T, Brayne C. (2018). Generalised anxiety disorder and hospital admissions: findings from a large, population cohort study. BMJ Open. 8:18539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. (2012). Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 37:2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Zybutz R, Erridge S, Holvey C, Coomber R, Gaffney J, Lawn W, et al. (2023). Clinical outcome data of anxiety patients treated with cannabis-based medicinal products in the United Kingdom: a cohort study from the UK Medical Cannabis Registry. Psychopharmacology (Berl). 240:1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, et al. (2008). CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 54:151–160. [DOI] [PubMed] [Google Scholar]
- Snyder E, Cai B, DeMuro C, Morrison MF, Ball W. (2018). A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 14:1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Löwe B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 166:1092–1097. [DOI] [PubMed] [Google Scholar]
- Stith SS, Li X, Diviant JP, Brockelman FC, Keeling KS, Hall B, et al. (2020). The effectiveness of inhaled Cannabis flower for the treatment of agitation/irritability, anxiety, and common stress. J Cannabis Res. 2:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner SA. (2017). Effects of marijuana on mental health: anxiety disorders. Alcohol & Drug Abuse Institute, University of Washington. https://adai.uw.edu/pubs/pdf/2017mjanxiety.pdf [Google Scholar]
- Tait J, Erridge S, Sodergren MH. (2023. a). UK Medical Cannabis Registry: A Patient Evaluation. J Pain Palliat Care Pharmacother. 37:170–177. [DOI] [PubMed] [Google Scholar]
- Tait J, Erridge S, Holvey C, Coomber R, Usmani A, Sajad M, et al. 2023. b). Clinical outcome data of chronic pain patients treated with cannabis-based oils and dried flower from the UK Medical Cannabis Registry. Expert Rev Neurother. 23:413–423. [DOI] [PubMed] [Google Scholar]
- Toussaint A, Hüsing P, Gumz A, Wingenfeld K, Härter M, Schramm E, et al. (2020). Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J Affect Disord. 265:395–401. [DOI] [PubMed] [Google Scholar]
- Troup LJ, Erridge S, Ciesluk B, Sodergren MH. (2022). Perceived Stigma of Patients Undergoing Treatment with Cannabis-Based Medicinal Products. Int J Environ Res Public Health. 19:7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. (2007). Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 4:1628–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. (2012). Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 15:708–715. [DOI] [PubMed] [Google Scholar]
- Vigil J, Stith S, Diviant J, Brockelman F, Keeling K, Hall B. (2018). Effectiveness of raw, natural medical cannabis flower for treating insomnia under naturalistic conditions. Medicines. 5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Erridge S, Holvey C, Coomber R, Usmani A, Sajad M, et al. (2023). Assessment of clinical outcomes in patients with fibromyalgia: Analysis from the UK Medical Cannabis Registry. Brain Behav. 13. doi: 10.1002/brb3.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer MT, Anderson K, Reynolds M. (2021). Correlates of quality of life in anxiety disorders: review of recent research. Curr Psychiatry Rep. 23:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Di Ciano P, Brands B. (2020). Use of cannabidiol for the treatment of anxiety: a short synthesis of pre-clinical and clinical evidence. Cannabis Cannabinoid Res. 5:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.