Abstract
Borna disease virus (BDV) is a highly neurotropic virus that causes Borna disease, a virus-induced immune-mediated encephalomyelitis, in a variety of warm-blooded animals. Recent studies reported that BDV can be detected in patients with psychiatric disorders. BDV is noncytopathic, replicates in the nucleus of infected cells, and spreads intraaxonally in vivo. Upon infection of susceptible cultured cells, virus can be detected in foci. Little is known about the cellular components required for BDV replication. Here, we show that the cellular Raf/MEK/ERK signaling cascade is activated upon infection with BDV. In the presence of the MEK-specific inhibitor U0126, cells get infected with BDV; however, there is a block in virus spread to neighboring cells. The effect of the inhibitor on virus spread was still observed when the compound was added 2 h postinfection but not if treatment was initiated as late as 4 h after infection. Our results provide new insights into the BDV-host cell interaction and show that virus infection can be controlled with drugs interfering with a cellular signaling pathway. Since concentrations of the MEK inhibitor required to block BDV focus formation are not toxic for the host cells, our finding may be important with respect to antiviral drug development.
Mitogen-activated protein kinase (MAPK) cascades have been implicated in a variety of cellular functions ranging from regulation of the proliferative response to the control of apoptotic cell death. The prototype of the MAPK family of signaling pathways is the Raf/MEK/extracellular signal-regulated kinase (ERK) cascade, which plays a major role in the regulation of cell growth and differentiation. Growth factor-induced signals are transmitted by consecutive phosphorylation from the serine/threonine kinase Raf via the dual-specificity kinase MEK (MAPK kinase/ERK kinase) to ERK. Active ERK subsequently translocates to the nucleus to phosphorylate a variety of substrates and mediate changes in gene expression (33, 44). Specific and selective inhibitors have been used to elucidate the function of this kinase cascade in cellular regulation by extracellular stimuli. These inhibitors block activation of MEK by Raf and thus interfere with signaling at the bottleneck of the cascade (1, 14, 15). The MEK-specific inhibitor U0126 (1,4- diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene) was first described as a compound that partially blocks AP-1 transactivation (15) and T-cell proliferation (12). Inhibition of MEK is selective as U0126 shows little, if any, effect on the kinase activities of protein kinase C, Abl, Raf, MEKK, ERK, JNK, Cdk2, or Cdk4 and the MEK-related kinases MKK-3, MKK-4/SEK, and MKK-6 (15). Further, U0126 has an approximately 100-fold-higher affinity for active MEK than does the previously identified MEK inhibitor, PD98059 (15).
A variety of DNA and RNA viruses induce signaling via MAPK pathways in infected host cells, suggesting that these kinase cascades may play a functional role in virus replication (3, 7, 34). Borna disease virus (BDV), a noncytolytic single-stranded RNA virus, is the only known member of Bornaviridae in the order of Mononegavirales. BDV is highly neurotropic and cell associated. The 8.9-kb-size genome with negative polarity is replicated in the nucleus and encodes at least six different known viral proteins: the nucleoprotein (p40), the phosphoprotein (p24), the X protein (p10), and two glycosylated proteins, the matrixprotein (gp18) and the glycoprotein (gp94). Furthermore, an l-polymerase of 190 kDa has been described (18, 23, 26, 37, 39, 43, 45, 46, 48). The phosphoprotein p24 is phosphorylated at serine residues, suggesting that the function of this protein is controlled by cellular kinases (38, 43). A recent report by Walker et al. shows that the l-polymerase of BDV is also phosphorylated, making this protein a further candidate for BDV-host cell interactions (45). BDV induces Borna disease, a T-cell-mediated encephalomyelitis originally described in horses and sheep (24, 35). In recent years this viral infection of the central nervous system has been diagnosed in a wide variety of animals, including cattle, cats, dogs, and birds (reviewed in reference 42). Furthermore, BDV, nucleic acid, and antibodies were detected in blood of patients with psychiatric diseases (2, 5, 6, 22, 30, 31, 36), although no direct correlation between BDV as the causative agent and a particular mental disorder in humans has been demonstrated yet. To date, amantadine and ribavirin have been described as anti-BDV drugs. The effect of amantadine is controversial, and ribavirin reduces infectivity in vitro by only 1 log10 (4, 11, 16, 21, 27, 41).
Here we show that BDV infection of different cell lines leads to activation of the Raf/MEK/ERK signaling cascade. Activity of the cascade appears to be essential for BDV spread, since inhibition of the pathway using the potent MEK-specific inhibitor U0126 efficiently blocks infection of cells with progeny virus without being toxic for the host cell.
MATERIALS AND METHODS
Cell lines and virus.
The guinea pig cell line CRL 1405 was subcloned, and cells highly susceptible to BDV were used as a standard laboratory cell line for BDV infection (40). Furthermore, the human oligodendrocyte cell line OL (29), also highly susceptible to BDV infection, was used throughout this study. In addition, persistent BDV-infected and -uninfected F10 (rat astrocytes) (47), C6 (8), Vero (17), and 293T (human embryonal kidney cells, expressing SV40 large T antigen) cells were used. The cells were cultured with Iscove modified Dulbecco's medium (IMDM) supplemented with 5% fetal calf serum (FCS), 2 mM l-glutamin, and 100 U of gentamicin/ml.
The fourth rat passage of the Giessen strain He/80 was used for infection (28). In general, adherent cells were infected with a multiplicity of infection (MOI) of 1 or 0.01 focus-forming units in either 96-well or 6-well plates for 1 h in a volume of 25 μl (for 96-well plate) or 200 μl (for 6-well plate) of IMDM–2% FCS. For mock infection, 10% normal rat brain homogenate in IMDM–2% FCS was used. Thereafter, culture medium was added and cells were cultivated for 5 to 7 days.
Treatment of cells with the MEK inhibitor U0126.
MEK inhibitor U0126 (Promega, Heidelberg, Germany) was dissolved in dimethyl sulfoxide (DMSO) leading to a 50 mM U0126 stock solution. For experiments, U0126 was used at either 6, 12.5, 25, or 50 μM concentrations in medium. In parallel, control cells were treated with DMSO alone in the respective concentrations. Full activity of U0126 was still observed after 10 h of cell culture. Nevertheless, activity may decline after longer incubation.
Viability staining.
CRL and OL cells were treated with 50, 25, and 12.5 μM U0126 or with DMSO. One day and 6 days later the cells were trypsinized and washed twice with phosphate-buffered saline (PBS). Cells (5 × 105) were treated with 2 μl (50 μg/ml) of propidium iodide for 10 min, and viable cells were counted by flow cytometry (FACS-Scan; Becton Dickinson, Heidelberg, Germany).
Infectivity assay and viral antigen detection by immunocytochemistry.
Virus infectivity was determined on CRL 1405 cells by a standard immunocytochemistry assay (40). Briefly, 106 BDV-infected cells/ml were lysed by sonification and centrifuged, and titers of the supernatant were determined. Titrations were carried out in flat-bottomed 96-well microtiter plates. CRL 1405 cells were cultured for 7 days. Thereafter, the cells were fixed with 4% formaldehyde–PBS and treated with 1% Triton X-100–PBS and viral antigen was demonstrated in an immunocytochemical reaction using anti-BDV-specific mouse monoclonal antibodies directed against the nucleo- and phosphoprotein. Nonspecific binding of antibodies was blocked by incubation of plates with 10% FCS–PBS. The reaction of monoclonal antibodies with cells was detected by a secondary anti-species biotin-labeled antibody (Dianova, Hamburg, Germany) and by a streptavidin-peroxidase conjugate (Dianova). The reaction was visualized with 0.4% ortho-phenylendiamine and 0.5 μl of H2O2 (Sigma, Taufkirchen, Germany)/ml.
ERK2 immune-complex kinase assays.
Cells were lysed in Triton lysis buffer (TLB) (20 mM Tris HCl, pH 7.4, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2mM EDTA, 50 mM Na glycerolphosphate, 20 mM Na pyrophosphate, 5 μg of aprotinin ml−1, 5 μg of leupeptin ml−1, 1 mM Na vanadate, 5 mM benzamidin) on ice for 10 to 20 min. Cell lysates were then centrifuged at 13,000 × g for 10 min at 4°C, and supernatants were incubated with an ERK2-specific antiserum (Santa Cruz, Heidelberg, Germany) and protein A-agarose (Roche, Mannheim, Germany) for 2 h at 4°C. Immune complexes were used for in vitro kinase assays with myelin basic protein as a substrate for ERK as previously described (25). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were blotted onto polyvinylidene difluoride membranes. Phosphorylated substrates were detected by a BAS 2000 Bio Imaging Analyzer (Fuji, via Raytest, Staubenhardt, Germany) and by autoradiography. Equal loading of immunoprecipitated ERK2 was analyzed by Western blotting using an ERK2-specific antiserum (Santa Cruz) and peroxidase-coupled protein A followed by a standard enhanced chemiluminescence reaction (Amersham, Freiburg, Germany).
RESULTS
BDV infection results in activation of the classical MAPK ERK.
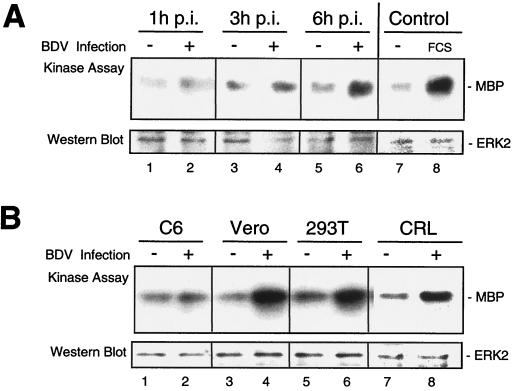
A variety of DNA and RNA viruses induce signaling via MAPK cascades in infected host cells (3, 7, 32, 34). However, it is largely unknown which intracellular signaling processes are induced by BDV to support viral replication. To assess whether BDV infection results in an activation of the classical MAPK pathway, the guinea pig cell line CRL 1405 was infected at an MOI of 1 and cell lysates were analyzed for ERK activity in immune-complex kinase assays at several time points postinfection (p.i.). As a control, mock-infected cells were assessed at the same time points. Figure 1A shows that BDV infection induces a detectable and sustained increase in ERK2 activation 1, 3, and 6 h p.i. The finding that activation of ERK2 by BDV is only marginal compared to mitogenic stimulation (20% FCS [Fig. 1A, lane 8]) may be due to the fact that only a small portion of cells are infected at an MOI of 1 and the measurable activity is diluted by the ERK activity of uninfected cells. Since it is not possible to prepare BDV stocks that allow acute infection with an MOI higher than 1, we analyzed different cell lines which are persistently infected with BDV, thus carrying virus in almost every cell. As shown in Fig. 1B, ERK2 activity is elevated in four different persistently infected cell lines. These findings clearly indicate that BDV induces a sustained activation of the classical MAPK cascade in infected cells.
FIG. 1.
BDV induces activation of ERK2 in acute and persistently infected cells. (A) CRL cells were either mock infected (lanes 1, 3, and 5) or infected with BDV at an MOI of 1 (lanes 2, 4, and 6) for 1, 3, or 6 h as indicated. In the control reaction, cells were either left untreated (lane 7) or treated with 20% FCS for 1 h as a mitogenic stimulus to activate ERK2 (lane 8). Cell lysates were subjected to ERK2 immune complex kinase assays as described in Materials and Methods by using myclin basic protein as a substrate. Phosphorylated substrates were visualized on X-ray films. ERK2 Western blots were analyzed to confirm equal loading of the kinases. ERK activation upon BDV infection is weak (2- to 4-fold) compared to the control sample stimulated with 20% FCS (13-fold). (B) Cell lysates of parental cells (lanes 1, 3, 5, and 7) or persistently BDV-infected C6 (lane 2), Vero (lane 4), 293T (lane 6), and CRL (lane 8) cells were analyzed for ERK2 activity as described above. ERK activity in persistently infected cell lines ranges roughly from two- to fourfold.
BDV spread is blocked upon specific inhibition of the Raf/MEK/ERK cascade.
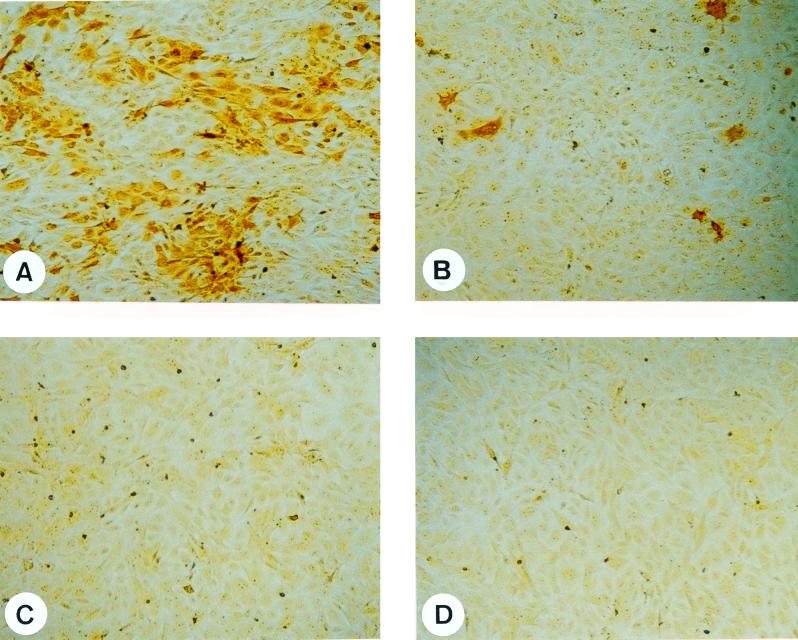
Infection of CRL cells with BDV leads to focus formation visible by immunocytochemistry within 5 to 7 days after infection (40). Since acute BDV infection leads to ERK2 activation of CRL cells, it was assessed whether BDV replication requires activity of the Raf/MEK/ERK pathway. CRL cells were pretreated 30 min prior to infection (MOI = 0.01) with a single dose of different concentrations of the MEK inhibitor U0126 ranging from 6 to 50 μM. Infected cells were examined daily for morphological alterations and were stained 7 days p.i. for the presence of viral proteins. At concentrations of 6 μM U0126, no difference in virus distribution was observed compared to cells treated with the solvent alone, whereas at 12.5 μM U0126 the size of foci was significantly reduced. In contrast to cells treated with DMSO (Fig. 2A) in the presence of 25 μM U0126 and above, no foci could be observed anymore and only single cells were found positive for viral proteins (Fig. 2B). Essentially the same inhibitory effects on BDV spread were obtained when human oligodendrocytes (OL cells) were used as host cells (Table 1).
FIG. 2.
U0126 inhibits BDV foci formation. BDV-infected (A and B) or mock-infected (C and D) CRL cells were treated with either DMSO (A and C) or 25 μM U0126 (B and D). Seven days after infection, cells were stained by a standard immunocytochemistry protocol. Magnification, ×60.
TABLE 1.
Inhibitory effect of U0126 treatment on BVD spread by the concentration and time of application
| Cell line | Inhibitory effect by:
|
||||||
|---|---|---|---|---|---|---|---|
| Concn of U0126 (μM)
|
Time of treatment before or after BDV infection (h)
|
||||||
| 50 | 25 | 12.5 | 6 | −0.5 | 2 | 4 | |
| CRL | +a | + | +/− | −b | + | + | − |
| OL | NDc | + | − | − | + | + | − |
+, inhibition of BDV spread; only single infected cells are visible.
−, no inhibition of BDV spread to neighboring cells.
ND, not done.
We further analyzed whether the inhibitory effect of U0126 on virus spread is dependent on the time of administration of the compound. Infected cells were treated with 25 μM U0126 starting 2 or 4 h after infection, and infectivity was analyzed 5 days after infection. While the inhibiting effect was still obvious if U0126 was added 2 h p.i., no reduction of focus formation could be observed if the reagent was given 4 h p.i. (Table 1).
These results show that the block of BDV spread is dependent on the concentration of U0126, which appears to target a crucial step in the replication cycle occurring between 2 and 4 h after infection. In contrast, no inhibitory effect of U0126 was observed when persistent BDV-infected CRL cells were treated with the MEK inhibitor (data not shown). This indicates that the spread of the virus from cell to cell rather than the initial infection or the intracellular expression of the nucleo- and phosphoprotein is affected by the drug.
BDV-infected cells harbor infective virus after U0126 treatment.
To analyze whether the presence of detectable amounts of viral antigen in cells after U0126 treatment still represents infectious virus, CRL and OL cells were treated with U0126 before BDV infection and were cultured for 5 days in the presence of the inhibitor. Thereafter, cell lysates were analyzed for infectious virus by a standard virus titration assay (33). In cells treated with DMSO, a virus titer of 4.7 log10 per 106 cells was detected, which is the regular level of virus production in these cells (40). In contrast, the virus titer in U0126-treated cells was drastically reduced to 2.3 log10 per 106 cells, representing a reduction in the virus titer of 99.5%; however, there were still infectious viral particles left. Essentially the same results were observed when OL cells were used as host cells for BDV infection (Tables 2 and 3). This indicates that the positive staining for viral proteins in the single stained cells (Fig. 2B) is not due to aberrantly produced viral proteins; infectious virus particles are formed but appear to be retained in primary infected cells in the presence of the inhibitor.
TABLE 2.
U0126 treatment reduces BDV infectivity
| Inhibitor used | Virus titer (log10)/% reduction for cell line:
|
|
|---|---|---|
| CRL | OL | |
| DMSO | 4.7 | 4.6 |
| U0126 | 2.3/99.5 | 2.6/99.0 |
TABLE 3.
BDV infectivity after culture periods in the presence or absence of U0126
| Cell line and inhibitor used | Virus titer (log10) after culture period:
|
|
|---|---|---|
| Firsta | Second | |
| CRL | ||
| U0126 | 2.3 | 2.9 |
| DMSO | 4.4 | |
| DMSO | 4.6 | 5.0 |
| OL | ||
| U0126 | 2.6 | 3.1 |
| DMSO | 4.5 | |
| DMSO | 4.6 | 4.9 |
Cells were cultured for 5 days (first culture period), one portion was used for virus titration, and the remaining portion was cultured for an additional 5 days (second culture period) in the presence or absence of U0126.
Next, we questioned if BDV spread is restrained if U0126 is removed from the culture medium. Therefore, CRL and OL cells were treated with 25 μM U0126 prior to BDV infection and were cultured for five days in the presence of the MEK inhibitor. Thereafter, cells were trypsinized; one part was used for virus titration and the other part was cultured either with or without U0126 for an additional 5 days before they were used for virus titration. As shown in Table 3, the virus titer increased when U0126 was removed from the medium and virus foci were visible (data not shown), indicating that BDV regains its ability to spread in cell culture when the inhibition of the Raf/MEK/ERK signaling cascade is omitted.
U0126 is not toxic for CRL and OL cells.
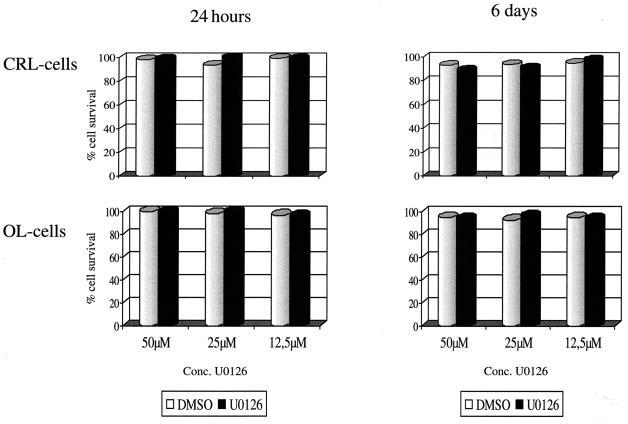
Since U0126 targets an essential signaling pathway for growth regulation, an extended presence of the inhibitor might be toxic for the cell. Confluent CRL or OL cells were incubated for 24 h or 6 days in the presence of different concentrations of U0126 (12.5, 25, and 50 μM) or the appropriate amount of the solvent alone. Morphological examination revealed no differences for cells cultured in either condition (Fig. 2C and D). Cells were subsequently stained with propidium iodide, and samples were evaluated by flow cytometry analysis (Fig. 3). Neither the presence of the inhibitor nor of the solvent alone resulted in a decrease of the total cell numbers or in an increased number of dead cells. This indicates that at the concentrations used, U0126 is not toxic for either CRL or OL cells.
FIG. 3.
CRL cells (upper panels) and OL cells (lower panels) were incubated with U0126 or with DMSO alone for either 1 day (left panels) or 6 days (right panels). Cells were treated with propidium iodide and were analyzed by flow cytometry. The percentage of viable cells after treatment with U0126 or DMSO was compared with the viability of untreated cells. Experiments were repeated twice with almost the same results.
DISCUSSION
The cellular factors and processes crucial for BDV replication are poorly defined. Here, we show that the Raf/MEK/ERK signaling pathway is activated upon BDV infection after acute infection. At 1 h after infection, ERK activation is already detected. This very early time point at which ERK activation is detected correlates well with the requirements of the pathway early during infection; however, it would suggest that gene expression is not involved in activation of the kinase cascade. The question remains as to what mechanisms specifically associated with BDV infection are responsible for this activation. In addition, we show that ERK is activated in different persistent BDV-infected cell lines to distinct levels. After this report was submitted, Hans et al. showed that BDV caused constitutive activation of the ERK1/2 pathway and that activated ERKs were not translocated to the nucleus efficiently in persistently infected PC12 cells. That might account for the absence of neuronal differentiation of BDV-infected PC12 cells treated with NGF (20).
Here, we show for the first time that inhibition of the cascade by the MEK inhibitor U0126 results in a block of BDV spread in cell culture which reduces virus yields up to 99%. Inhibition was observed in two different cell lines highly susceptible to BDV infection, showing that the effect is not restricted to a particular cell type. Moreover, the inhibitory concentrations of U0126 used in this study were not toxic for the host cells, indicating that inhibition of BDV spread is not an indirect effect due to a loss of cell viability. However, it is known that agents that interfere with cellular functions but are not fatal to the cell can alter cell physiology and, after virus infection, may affect virus replication. Thus, we cannot distinguish at the moment whether the kinase cascade leads to a direct modification of viral proteins or whether the effects are indirectly caused by an altered cell state. Nevertheless, there is an obvious requirement of the Raf/MEK/ERK pathway for the outcome of BDV infection.
Our results show that the Raf/MEK/ERK signaling pathway plays an important role in BDV spread from cell to cell. From our data, we conclude that virus entry into the cell and virus replication are not influenced by the inhibitor, since infectious virus is still detected and can be recovered from primarily infected cells treated with the inhibitor. After immunohistochemistry of U0126-treated and BDV-infected cells, it appears that most of the BDV antigen is concentrated in the nucleus. Therefore, one might speculate that viral proteins are located in the cytoplasm to a lesser extent. This could contribute to the inability of the virus to spread beyond the cell, as virus assembly should occur in the late phase of the replication cycle. Furthermore, treatment of persistently BDV-infected cells did not result in a reduction of viral titer, indicating that U0126 treatment does not affect viral replication but rather inhibits the spread of BDV from cell to cell.
Inhibition of virus spread was still achieved when the inhibitor was added to the medium 2 h p.i., indicating that the effect of the drug is not due to an impaired binding of virus to the putative virus receptor or inhibition of virus entry into the cell. Duchala and colleagues have shown that binding and entry of BDV can take up to 4 h (13). Therefore, it is tempting to speculate that virus entry could be limited by the MEK inhibitor. Consequently, this would reduce virus spread and focus formation. Nevertheless, from experiments using UV-inactivated influenza virus, we could show that ERK is not activated and thus virus binding and the uptake of viruses might not be the ERK activating principle (S. Ludwig, unpublished data). Therefore, one might speculate that an early step in the viral replication cycle is affected upon inhibition of the signaling cascade. Inhibition was no longer successful if U0126 was added 4 h p.i. Thus, between 2 and 4 h p.i. the Raf/MEK/ERK signaling pathway is required for secondary infection of neighboring cells. It is puzzling that inhibition of the Raf/MEK/ERK pathway does not result in a loss of infectious virus particle formation, which still can be recovered after 5 days p.i. from primary infected, U0126-treated cells. This observation could be explained by a model in which MEK inhibition results in alteration of a cellular or viral-mediated process which subsequently prevents BDV from spreading from cell to cell but does not interfere with infectivity once the virus is released by cell disruption. Furthermore, the inhibitory effect of U0126 on BDV spread is dependent on the concentration, since doses less than 25 μM still allow foci formation, demonstrating virus spread.
The efficiency of anti-RNA virus drugs targeting a viral factor is limited as applications of these compounds frequently result in the rapid selection of drug-resistant virus variants. So far, compounds are very rare that can be used in the antiviral treatment against BDV. Using the nucleoside analog ribavirin, BDV infection could be reduced by 90%, while the effect of amantadine treatment of BDV-infected cells is still controversial (4, 6, 11, 19, 21, 27, 41). As the MEK inhibitor targets a cellular component, it is unlikely that the virus can escape the selection pressure by resistance.
For DNA viruses and in particular for oncogenic viruses, much is known about how these viruses interact with the host cell (3, 7, 32, 34). For RNA viruses little information exists, although interaction of RNA viruses with the Raf/MEK/ERK signaling pathway has been reported. Infection of cells with respiratory syncytial virus results in an increased activity of ERK2. The MEK1 inhibitor PD98059, like U0126, blocks activation of MEK 1 and inhibits the increase in ERK 2 activity after respiratory syncytial virus infection by about 50% (10). Activation of the ERK MAPK pathway also plays a role in human immunodeficiency virus type 1 (HIV-1) replication by enhancing the infectivity of HIV-1 virions through Vif-dependent as well as Vif-independent mechanisms. Inhibition of HIV-1 infectivity could be observed after treatment with a MEK inhibitor (9, 49, 50). Although our present data clearly shows that signaling though MEK is essential for BDV spread, the exact molecular mechanism of how BDV interacts with the cellular Raf/MEK/ERK signaling pathway remains to be elucidated. Since the phosphoprotein p24 is phosphorylated via serine residues, one might speculate that this viral protein could play an important role in BDV-host cell interaction (39, 43).
Our results suggest that BDV-induced early signaling events through the Raf/MEK/ERK cascade are required for BDV spread in cell culture. Leaving important steps of replication to the host is an economic way of reducing the viral genome size and accelerating viral multiplication, but it also clearly creates dependencies that are crucial for the viral life cycle. Thus, the identification of cellular factors which are essential for BDV replication might become an important issue with regard to the understanding of viral biology and for therapeutic intervention.
ACKNOWLEDGMENTS
We thank L. Stitz for critical reading of the manuscript and helpful discussions, Katja Oesterle for expert technical assistance, and Manuela Flüss for providing BDV-293T cells.
The work was supported in part by grants from the Deutsche Forschungsgemeinschaft (to O.P. and S.L.).
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam J D, Winokur A, Dyson W, Herzog S, Gonzalez F, Rott R, Koprowski H. Borna disease virus. A possible etiologic factor in human affective disorders? Arch Gen Psychiatry. 1985;42:1093–1096. doi: 10.1001/archpsyc.1985.01790340077011. [DOI] [PubMed] [Google Scholar]
- 3.Benn J, Su F, Doria M, Schneider R J. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode L, Dietrich D E, Stoyloff R, Emrich H M, Ludwig H. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet. 1997;349:178–179. doi: 10.1016/S0140-6736(05)60979-8. [DOI] [PubMed] [Google Scholar]
- 5.Bode L, Riegel S, Ludwig H, Amsterdam J D, Lange W, Koprowski H. Borna disease virus-specific antibodies in patients with HIV infection and with mental disorders. Lancet. 1988;2:689. doi: 10.1016/s0140-6736(88)90505-3. [DOI] [PubMed] [Google Scholar]
- 6.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 7.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone K M, Rubin S A, Sierra-Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartier C, Deckert M, Grangeasse C, Trauger R, Jensen F, Bernard A, Cozzone A, Desgranges C, Boyer V. Association of ERK2 mitogen-activated protein kinase with human immunodeficiency virus particles. J Virol. 1997;71:4832–4837. doi: 10.1128/jvi.71.6.4832-4837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Monick M M, Carter A B, Hunninghake G W. Activation of ERK2 by respiratory syncytial virus in A549 cells is linked to the production of interleukin 8. Exp Lung Res. 2000;26:13–26. doi: 10.1080/019021400269934. [DOI] [PubMed] [Google Scholar]
- 11.Cubitt B, de la Torre J C. Amantadine does not have antiviral activity against Borna disease virus. Arch Virol. 1997;142:2035–2042. doi: 10.1007/s007050050220. [DOI] [PubMed] [Google Scholar]
- 12.DeSilva D R, Jones E A, Favata M F, Jaffee B D, Magolda R L, Trzaskos J M, Scherle P A. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- 13.Duchala C S, Carbone K M, Narayan O. Preliminary studies on the biology of Borna disease virus. J Gen Virol. 1989;70:3507–3511. doi: 10.1099/0022-1317-70-12-3507. [DOI] [PubMed] [Google Scholar]
- 14.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 16.Ferszt R, Kuhl K P, Bode L, Severus E W, Winzer B, Berghofer A, Beelitz G, Brodhun B, Muller-Oerlinghausen B, Ludwig H. Amantadine revisited: an open trial of amantadinesulfate treatment in chronically depressed patients with Borna disease virus infection. Pharmacopsychiatry. 1999;32:142–147. doi: 10.1055/s-2007-979220. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Dunia D, Cubitt B, de la Torre J C. Mechanism of Borna disease virus entry into cells. J Virol. 1998;72:783–788. doi: 10.1128/jvi.72.1.783-788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Dunia D, Cubitt B, Grasser F A, de la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallensleben W, Zocher M, Staeheli P. Borna disease virus is not sensitive to amantadine. Arch Virol. 1997;142:2043–2048. doi: 10.1007/s007050050221. [DOI] [PubMed] [Google Scholar]
- 20.Hans A, Syan S, Crosio C, Sassone-Corsi P, Brahic M, Gonzalez-Dunia D. Borna disease virus persistent infection activates mitogen-activated protein kinase and blocks neuronal differentiation of PC12 cells. J Biol Chem. 2001;276:7258–7265. doi: 10.1074/jbc.M005107200. [DOI] [PubMed] [Google Scholar]
- 21.Jordan I, Briese T, Averett D R, Lipkin W I. Inhibition of Borna disease virus replication by ribavirin. J Virol. 1999;73:7903–7906. doi: 10.1128/jvi.73.9.7903-7906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi M, Arimura Y, Ikuta K, Shoya Y, Lai P K, Kakinuma M. Sequence variability of Borna disease virus open reading frame II found in human peripheral blood mononuclear cells. J Virol. 1996;70:635–640. doi: 10.1128/jvi.70.1.635-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliche S, Briese T, Henschen A H, Stitz L, Lipkin W I. Characterization of a Borna disease virus glycoprotein, gp18. J Virol. 1994;68:6918–6923. doi: 10.1128/jvi.68.11.6918-6923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig H, Thein P. Demonstration of specific antibodies in the central nervous system of horses naturally infected with Borna disease virus. Med Microbiol Immunol. 1977;163:215–226. doi: 10.1007/BF02125505. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig S, Engel K, Hoffmeyer A, Sithanandam G, Neufeld B, Palm D, Gaestel M, Rapp U R. 3pK, a novel mitogen-activated protein (MAP) kinase-activated protein kinase, is targeted by three MAP kinase pathways. Mol Cell Biol. 1996;16:6687–6697. doi: 10.1128/mcb.16.12.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik T H, Kishi M, Lai P K. Characterization of the P protein-binding domain on the 10-kilodalton protein of Borna disease virus. J Virol. 2000;74:3413–3417. doi: 10.1128/jvi.74.7.3413-3417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizutani T, Inagaki H, Hayasaka D, Shuto S, Minakawa N, Matsuda A, Kariwa H, Takashima I. Transcriptional control of Borna disease virus (BDV) in persistently BDV-infected cells. Arch Virol. 1999;144:1937–1946. doi: 10.1007/s007050050716. [DOI] [PubMed] [Google Scholar]
- 28.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 29.Pauli G, Ludwig H. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 1985;2:29–33. doi: 10.1016/0168-1702(85)90057-7. [DOI] [PubMed] [Google Scholar]
- 30.Planz O, Rentzsch C, Batra A, Rziha H-J, Stitz L. Persistence of Borna disease virus-specific nucleic acid in the blood of a psychiatric patient. Lancet. 1998;352:623. doi: 10.1016/S0140-6736(05)79577-5. [DOI] [PubMed] [Google Scholar]
- 31.Planz O, Rentzsch C, Batra A, Winkler T, Buttner M, Rziha H J, Stitz L. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6256. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popik W, Hesselgesser J E, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 34.Rodems S M, Spector D H. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J Virol. 1998;72:9173–9180. doi: 10.1128/jvi.72.11.9173-9180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 36.Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 37.Schneider P A, Kim R, Lipkin W I. Evidence for translation of the Borna disease virus G protein by leaky ribosomal scanning and ribosomal reinitiation. J Virol. 1997;71:5614–5619. doi: 10.1128/jvi.71.7.5614-5619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwemmle M, De B, Shi L, Banerjee A, Lipkin W I. Borna disease virus P-protein is phosphorylated by protein kinase Cepsilon and casein kinase II. J Biol Chem. 1997;272:21818–21823. doi: 10.1074/jbc.272.35.21818. [DOI] [PubMed] [Google Scholar]
- 39.Schwemmle M, Salvatore M, Shi L, Richt J, Lee C H, Lipkin W I. Interactions of the borna disease virus P, N, and X proteins and their functional implications. J Biol Chem. 1998;273:9007–9012. doi: 10.1074/jbc.273.15.9007. [DOI] [PubMed] [Google Scholar]
- 40.Stitz L, Noske K, Planz O, Furrer E, Lipkin W I, Bilzer T. A functional role for neutralizing antibodies in Borna disease: influence on virus tropism outside the central nervous system. J Virol. 1998;72:8884–8892. doi: 10.1128/jvi.72.11.8884-8892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stitz L, Planz O, Bilzer T. Lack of antiviral effect of amantadine in Borna disease virus infection. Med Microbiol Immunol. 1998;186:195–200. doi: 10.1007/s004300050064. [DOI] [PubMed] [Google Scholar]
- 42.Stitz L, Rott R. Borna disease virus (Bornaviridae) In: Granoff A, Webster R G, editors. Encyclopedia of virology. New York, N.Y: Academic Press, Inc.; 1999. pp. 167–173. [Google Scholar]
- 43.Thiedemann N, Presek P, Rott R, Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992;73:1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]
- 44.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 45.Walker M P, Jordan I, Briese T, Fischer N, Lipkin W I. Expression and characterization of the Borna disease virus polymerase. J Virol. 2000;74:4425–4428. doi: 10.1128/jvi.74.9.4425-4428.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehner T, Ruppert A, Herden C, Frese K, Becht H, Richt J A. Detection of a novel Borna disease virus-encoded 10 kDa protein in infected cells and tissues. J Gen Virol. 1997;78:2459–2466. doi: 10.1099/0022-1317-78-10-2459. [DOI] [PubMed] [Google Scholar]
- 47.Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 48.Wolff T, Pfleger R, Wehner T, Reinhardt J, Richt J A. A short leucine-rich sequence in the Borna disease virus p10 protein mediates association with the viral phospho- and nucleoproteins. J Gen Virol. 2000;81:939–947. doi: 10.1099/0022-1317-81-4-939. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Gabuzda D. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J Biol Chem. 1998;273:29879–29887. doi: 10.1074/jbc.273.45.29879. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J Virol. 1999;73:3460–3466. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]