Abstract
Thyrotoxicosis has been associated with several cardiac conditions including atrial fibrillation, congestive heart failure due to left ventricular dysfunction, and cardiomyopathy. However, few cases of ventricular fibrillation as a complication of thyrotoxicosis have been reported. Our case described a 45-year-old male with a history of hypertension and Graves’ disease, who presented with 1 week of left-sided chest pain associated with shortness of breath on exertion and occasional palpitations. His workup revealed acute diastolic congestive heart failure secondary to thyrotoxicosis, causing pulmonary hypertension, which led to ventricular fibrillation and cardiac arrest. After being treated with methimazole and metoprolol, the patient’s symptoms improved. This case underscores the significance of assertive medical interventions alongside both invasive and non-invasive cardiac procedures. Addressing thyrotoxicosis and ventricular arrhythmia in hyperthyroid patients is crucial to prevent potentially life-threatening complications.
Keywords: Hyperthyroidism, Pulmonary hypertension, Ventricular fibrillation/ventricular tachycardia, Graves’ disease
Introduction
Graves’ disease is a known risk factor for pulmonary hypertension (PH) [1]. In a prospective study of patients with Graves’ disease, PH was present in 48% of patients who were hyperthyroid [1]. It is thought that enhanced vasoconstriction in the pulmonary vasculature may be a major cause. Proposed mechanisms of enhanced vasoconstriction include increased catecholamine secretion, increased degradation of pulmonary vasodilators, and decreased degradation of pulmonary vasoconstrictors [1-4].
Thyrotoxicosis has been associated with several cardiac complications including atrial fibrillation, congestive heart failure (CHF) and cardiomyopathy [5]. CHF is the initial clinical presentation in approximately 6% of patients with hyperthyroidism, and half of those have left ventricular systolic dysfunction (left ventricular ejection fraction ≤ 50%) [6].
CHF involving predominately the right heart is an unusual manifestation of thyrotoxicosis [7-10]. Development of right heart failure in hyperthyroidism is attributed to a combination of factors such as 1) pressure overload from PH; 2) tachycardia-mediated mechanism causing increased level of cytosolic calcium during diastole resulting in diastolic dysfunction; 3) volume overload with the development of secondary tricuspid regurgitation; and 4) direct effect of the excess circulating thyroid hormone on myocardium especially of the right ventricle [11-14].
Atrial fibrillation emerges as the predominant arrhythmia in thyrotoxicosis, affecting 5-15% of patients aged over 60 years, while ventricular arrhythmias are considered uncommon manifestations [15].
In this case report we present a patient with acute diastolic CHF with severe PH caused by untreated hyperthyroidism. This patient’s hospital course was complicated by intermittent non-sustained ventricular tachycardia (NSVT) and two episodes of ventricular fibrillation (VF). The patient suffered flash pulmonary edema and ventricular arrhythmia following coronary angiography, which was thought to be related to coronary vasospasm. This combination of symptoms is infrequently observed as a result of Graves’ disease.
Case Report
Investigations
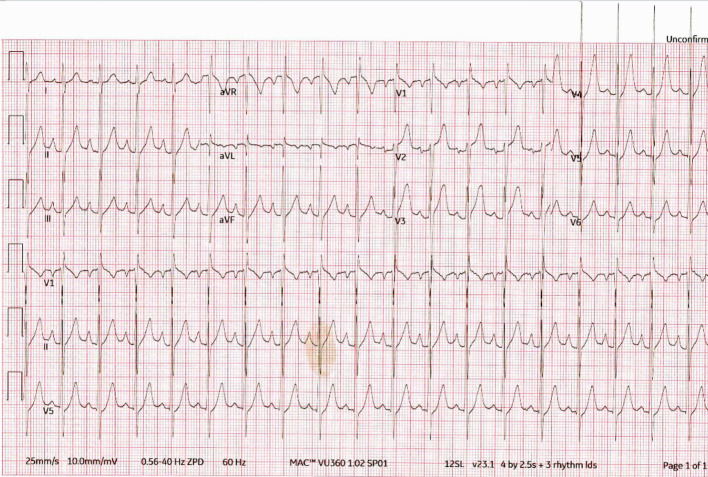
A 45-year-old male presented to the emergency department (ED) with complaints of left-sided chest pain of 1-week duration. He described the pain as burning, intermittent, and lasting for 20 min with radiation to the left arm. The pain was associated with diaphoresis, palpitations, and dyspnea on exertion. There was no fever, cough, or swelling. The rest of his review of systems was unremarkable. His medical history was notable for hypertension and Graves’ disease diagnosed 7 years prior to admission, but he was non-adherent with his prescribed atenolol 25 mg daily and methimazole 15 mg daily. He denied smoking, alcohol, and recreational drug use. In the ED, initial blood pressure was 140/78 mm Hg, heart rate117 beats/min, and oxygen saturation 97% on room air. His examination was notable for jugular venous distention, tachycardia, 3/6 systolic murmur in the pulmonary and tricuspid regions without radiation and an enlarged thyroid gland. Bilateral lung auscultation revealed clear breath sounds, and there was no edema observed in his extremities. Initial differential diagnosis included acute CHF, acute myocardial infarction (MI), acute pulmonary embolism (PE), and hyperthyroidism due to uncontrolled Graves’ disease. Electrocardiogram (ECG) revealed sinus tachycardia with biatrial enlargement and left ventricular hypertrophy (Fig. 1). While on telemetry monitoring, the patient remained in sinus tachycardia with short runs of intermittent NSVT. Laboratory results were significant for elevated troponin of 0.13 ng/mL (normal 0.00 - 0.03 ng/mL), low thyroid-stimulating hormone (TSH) < 0.05 µIU/mL (normal 0.27 - 4.20 µIU/mL), high free T4 > 6.99 ng/dL (normal 0.93 - 1.70 ng/dL), and high free T3 > 22.8 pg/mL (normal 2.77 - 5.27 pg/mL); urine toxicology was negative. Chest X-ray showed a superior mediastinal opacity consistent with a thyroid goiter (Fig. 2). Computed tomography pulmonary angiography (CTPA) was negative for PE.
Figure 1.
ECG: sinus tachycardia with biatrial enlargement and left ventricular hypertrophy. ECG: electrocardiogram.
Figure 2.
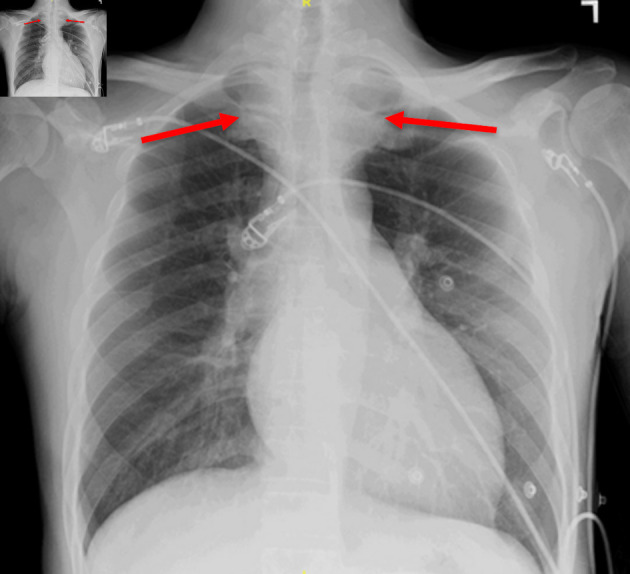
Chest X-ray showing a superior mediastinal opacity consistent with a thyroid goiter (red arrows).
Diagnosis
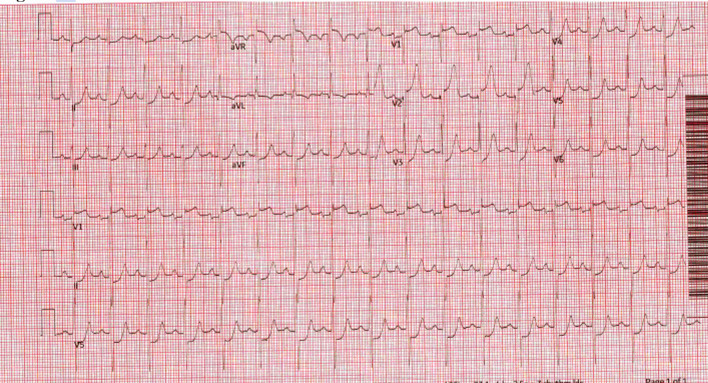
On hospital day 2, initial transthoracic echocardiography (TTE) showed left ventricular ejection fraction of 55-60%, grade two diastolic dysfunction, normal chamber sizes, PH with estimated pulmonary artery systolic pressure (PASP) of 66 mm Hg and elevated estimated right atrial (RA) pressure of 15 mm Hg. Left heart catheterization was performed because of elevated troponin, ongoing chest pain, and NSVT with intermittent ST elevation on telemetry. It revealed mild, non-obstructive coronary artery disease with elevated left ventricular end diastolic pressure (LVEDP) of 32 mm Hg. While in the holding area after cardiac catheterization, the patient developed severe chest pain, tachycardia, diffuse rales on auscultation, and hypoxemia with oxygen saturation dropping to 88% on room air. Subsequently, he became unresponsive and had VF on the monitor. Normal sinus rhythm was achieved with defibrillation. ECG post-cardiac arrest showed significant ST elevation in aVR, V1-V2 and severe ST depression across precordial and limb leads (Fig. 3). A repeat coronary angiogram was emergently performed, which documented unchanged coronary anatomy without evidence of spasm or dissection. During his stay in the cardiac catheterization laboratory (Cath Lab), the patient had another episode of ventricular tachycardia with progression to VF without provoking maneuvers such as wire or catheter manipulation and subsequent defibrillation. Shortly after defibrillation, the patients’ heart rate was normal with resolution of ST elevation suggestive of tachycardia-driven ischemia.
Figure 3.
ECG post-cardiac arrest showing significant ST elevation in aVR, V1-V2 and severe ST depression across precordial and limb leads. ECG: electrocardiogram.
Treatment
On day 1 of his hospital stay, the patient was initially treated with methimazole 30 mg twice daily, metoprolol 50 mg twice daily and intravenous (IV) heparin infusion. Defibrillation was used to manage VF and for the repeat episodes of ventricular tachycardia, and he was treated with an increased dose of metoprolol tartrate, furosemide and methimazole. During his hospital course, thyroid hormone levels decreased, and sinus tachycardia with ST segment elevation resolved. Repeat TTE on day 3 showed improvement in PH with estimated PASP of 37 mm Hg. Cardiac magnetic resonance imaging (MRI) showed biatrial enlargement, but was negative for tissue fibrosis, infiltration, or intracardiac shunt. He still had occasional NSVT and was given an option for implantable cardioverter defibrillator (ICD) placement, but he declined and opted for the LifeVest (Zoll, Pittsburgh, PA). He was discharged home in stable condition on metoprolol tartrate 50 mg twice daily and methimazole 30 mg twice daily.
Follow-up and outcomes
The patient was seen for follow-up 1 month after discharge, at which time he denied chest pain, shortness of breath, and palpitations. He was able to function normally without exertional shortness of breath. The patient was advised to continue with methimazole 30 mg twice daily, to discontinue atenolol 25 mg twice daily, and to start metoprolol tartrate 50 mg twice daily. On his follow-up at 8 months post hospitalization, his free T4 decreased to 1.48 (from 6.99 ng/dL), free T3 from > 22.8 to 6.30 pg/mL, while TSH remained < 0.05 µIU/mL. His blood pressure was 122/76 mm Hg, heart rate 91/min, his jugular venous pressure (JVP) resolved, and there were no murmurs present. He was asymptomatic and gained 26 lb since his initial admission and had no recurrence of arrhythmias. Clinical improvement correlated with improvement in hormone levels. Patient was lost to follow-up after 8 months.
The strength of the workup is a comprehensive diagnostic approach that revealed the most likely common denominator for the patient’s morbidity. However, it is limited by potential for adverse outcomes due to lethal arrhythmias, given LifeVest’s unequal efficacy and limited safety compared to ICD and non-compliance with long term follow-up.
Discussion
We report on a patient who presented with acute diastolic CHF and resultant severe PH and signs of right-sided heart failure caused by untreated hyperthyroidism, which led to PH and VF with cardiac arrest.
Thyrotoxicosis has been associated with several cardiac complications including atrial fibrillation, CHF and cardiomyopathy [7, 14]. CHF is the initial clinical presentation in approximately 6% of patients with hyperthyroidism, but only half of them had left ventricular systolic dysfunction [7]. CHF with mostly right heart failure is an unusual manifestation of thyrotoxicosis with very few reported cases in the literature [8-11]. Development of right heart failure in hyperthyroidism is attributed to a combination of factors such as: 1) pressure overload from PH; 2) tachycardia-mediated mechanism causing increased level of cytosolic calcium during diastole resulting in diastolic dysfunction; 3) volume overload with the development of secondary tricuspid regurgitation; and 4) direct effect of the excess circulating thyroid hormone on myocardium especially of the right ventricle [12-15].
Atrial fibrillation is the most common arrhythmia in thyrotoxicosis, occurring in 5-15% of patients over 60 years of age, whereas ventricular arrhythmia is an unusual manifestation [14]. There are two possible mechanisms for VF associated with thyrotoxicosis: coronary vasospasm and early repolarization. Endothelial dysfunction and primary hyperreactivity of vascular smooth muscle cells are responsible for coronary vasospasm. Thyroid hormones affect intracellular pathways that regulate the vascular resistance and cause hyperreactivity, causing spasm which leads to myocardial ischemia and possibly VF. Early repolarization caused by thyrotoxicosis may exaggerate the J-point elevation and induce VF [14, 15]. Our patient experienced flash pulmonary edema and ventricular arrhythmia following coronary angiography, which was suspected to be related to coronary vasospasm. His hospital course was complicated with intermittent NSVT and two episodes of VF. His symptoms improved with treatment of underlying Graves’ disease in addition to diuresis and beta-blockers.
Conclusions
Hyperthyroidism is commonly associated with PH, and if left untreated it can lead to acute diastolic CHF and complex ventricular arrhythmias. Every effort should be made to treat hyperthyroidism and avoid progression of disease, which can result in major cardiac comorbidities. Instilling the seriousness of possible cardiac complications related to medication noncompliance to the patient is of utmost importance. In the case of progression, aggressive medical treatment of hyperthyroidism and ventricular arrhythmias is mandated while in more severe cases external defibrillators or ICDs might be warranted.
Learning points
Untreated hyperthyroidism can lead to a combination of diastolic dysfunction and PH, as well as malignant ventricular arrhythmias. Aggressive treatment with methimazole, diuretics, and beta-blockers is needed to stabilize the patient. Instilling the importance of medication compliance to the patient is important for avoiding serious cardiac complications.
Acknowledgments
None to declare.
Funding Statement
None to declare.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Informed consent is not applicable to this case report because the patient was lost to follow-up and could not be contacted. His whereabouts and health status beyond the last follow-up exam are unknown.
Author Contributions
ARN participated in patient care, writing of the case report, revisions, and submission process. MA participated in patient care and revisions. SA participated in patient care and revisions. SPJ participated in patient care and revisions. AM participated in patient care and revisions. ZL participated in patient care, writing of the case report, revisions, and submission process.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Marvisi M, Balzarini L, Mancini C, Mouzakiti P. Thyroid gland and pulmonary hypertension. What's the link? Panminerva Med. 2013;55(1):93–97. [PubMed] [Google Scholar]
- 2.Patel S, Patel N. Pulmonary hypertension in hyperthyroidism: a reversible cause. Chest. 2016;150(4):1100A. doi: 10.1016/j.chest.2016.08.1207. [DOI] [Google Scholar]
- 3.Virani SS, Mendoza CE, Ferreira AC, de Marchena E. Graves' disease and pulmonary hypertension: report of 2 cases. Tex Heart Inst J. 2003;30(4):314–315. [PMC free article] [PubMed] [Google Scholar]
- 4.Rashidi F, Sate H, Faraji E, Tahsini Tekantapeh S. Thyrotoxicosis presenting as exertional dyspnea and pulmonary hypertension: Case report and review of literature. SAGE Open Med Case Rep. 2017;5:2050313X17715584. doi: 10.1177/2050313X17715584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu CW, Yeung CY, Lau CP, Kung AW, Tse HF. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93(4):483–487. doi: 10.1136/hrt.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin T, Lubina A, Levy Y, Shoenfeld Y. Graves' disease presenting as right heart failure. Isr Med Assoc J. 2006;8(3):217–218. [PubMed] [Google Scholar]
- 7.Cohen J, Schattner A. Right heart failure and hyperthyroidism: a neglected presentation. Am J Med. 2003;115(1):76–77. doi: 10.1016/s0002-9343(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 8.Lozano HF, Sharma CN. Reversible pulmonary hypertension, tricuspid regurgitation and right-sided heart failure associated with hyperthyroidism: case report and review of the literature. Cardiol Rev. 2004;12(6):299–305. doi: 10.1097/01.crd.0000137259.83169.e3. [DOI] [PubMed] [Google Scholar]
- 9.Ismail HM. Reversible pulmonary hypertension and isolated right-sided heart failure associated with hyperthyroidism. J Gen Intern Med. 2007;22(1):148–150. doi: 10.1007/s11606-006-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 11.Khan R, Sikanderkhel S, Gui J, Adeniyi AR, O'Dell K, Erickson M, Malpartida J. et al. Thyroid and cardiovascular disease: a focused review on the impact of hyperthyroidism in heart failure. Cardiol Res. 2020;11(2):68–75. doi: 10.14740/cr1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omotosho YB, Farooqi A, Bakar A. et al. Thyrotoxicosis: a primary cause of arrhythmias and acute heart failure. Journal of the Endocrine Society. 2021;5(Supplement_1):A967. doi: 10.1210/jendso/bvab048.1976. [DOI] [Google Scholar]
- 13.Anakwue RC, Onwubere BJ, Anisiuba BC, Ikeh VO, Mbah A, Ike SO. Congestive heart failure in subjects with thyrotoxicosis in a black community. Vasc Health Risk Manag. 2010;6:473–477. doi: 10.2147/vhrm.s9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Haketa A, Abe M, Tahira K, Hatanaka Y, Tanaka S, Ueno T. et al. Unusual manifestation of Graves' disease: ventricular fibrillation. Eur Thyroid J. 2015;4(3):207–212. doi: 10.1159/000437225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaetano A, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.