Abstract
Differentiation and polarization of epithelial cells depends on the formation of the apical junctional complex (AJC), which is composed of the tight junction (TJ) and the adherens junction (AJ). In this study, we investigated mechanisms of actin reorganization that drive the establishment of AJC. Using a calcium switch model, we observed that formation of the AJC in T84 intestinal epithelial cells began with the assembly of adherens-like junctions followed by the formation of TJs. Early adherens-like junctions and TJs readily incorporated exogenous G-actin and were disassembled by latrunculin B, thus indicating dependence on continuous actin polymerization. Both adherens-like junctions and TJs were enriched in actin-related protein 3 and neuronal Wiskott-Aldrich syndrome protein (N-WASP), and their assembly was prevented by the N-WASP inhibitor wiskostatin. In contrast, the formation of TJs, but not adherens-like junctions, was accompanied by recruitment of myosin II and was blocked by inhibition of myosin II with blebbistatin. In addition, blebbistatin inhibited the ability of epithelial cells to establish a columnar phenotype with proper apico-basal polarity. These findings suggest that actin polymerization directly mediates recruitment and maintenance of AJ/TJ proteins at intercellular contacts, whereas myosin II regulates cell polarization and correct positioning of the AJC within the plasma membrane.
INTRODUCTION
Two major functions of epithelia in multicellular organisms are protection from the external environment and preservation of the unique biochemical composition within different body compartments (Schock and Perrimon, 2002). These functions require restriction of the passage of solutes and macromolecules (Madara, 1998) that depends on three types of specialized plasma membrane structures termed tight junction (TJ), adherens junction (AJ), and desmosomes (Tsukita et al., 2001; Matter and Balda, 2003). TJs and AJs are closely positioned at the apical-most aspect of the lateral plasma membrane and are functionally coupled in the regulation of paracellular permeability. Thus, the TJ and AJ are collectively referred to as the apical junctional complex (AJC). Both TJs and AJs represent multiprotein complexes consisting of integral membrane proteins and peripheral proteins associated with the cytosolic side of the plasma membrane (Yap et al., 1997; Tsukita et al., 2001; Pokutta and Weis, 2002; Matter and Balda, 2003). It is generally thought that integral membrane proteins of TJs and AJs interact with partners on the opposing cell plasma membrane in a trans manner providing mechanical forces for cell-cell adhesion and creating a physical barrier that limits diffusion of solutes and macromolecules. The transmembrane proteins of TJs include occludin, members of the claudin protein family and two immunoglobulin (Ig)-like proteins, junctional adhesion molecule (JAM)-A and coxsackie adenovirus receptor (Tsukita et al., 2001; Gonzalez-Mariscal et al., 2003; Matter and Balda, 2003). The major integral membrane components of epithelial AJs are E-cadherin and Ig-like nectin proteins (Yap et al., 1997; Takai and Nakanishi, 2003). On the cytosolic side of the epithelial plasma membrane, TJs and AJs contain a great number of peripheral membrane proteins creating so-called cytosolic junctional plaques (Pokutta and Weis, 2002; Gonzalez-Mariscal et al., 2003). Such cytosolic plaques cluster and stabilize transmembrane components of junctions and form an organizing platform for variety of scaffolding, signaling, and vesicle-trafficking proteins.
It is well recognized that TJs and AJs contain a number of actin-binding proteins, including members of “zonula occludens” (ZO) family, afadin, α-catenin, and vinculin (Pokutta and Weis, 2002; Gonzalez-Mariscal et al., 2003). Furthermore, light and electron microscopic analyses demonstrated physical association of TJs and AJs with thick bundles of actin microfilaments, forming a characteristic perijunctional actin ring at the apical pole of differentiated epithelial cells (reviewed in Mooseker, 1985; Madara, 1998; Turner, 2000).
Several lines of evidence suggest that actin microfilaments are critically involved in AJC biogenesis and function. First, remodeling of apical junctions is accompanied by dramatic reorganization of the perijunctional F-actin ring (Ma et al., 2000; Vasioukhin et al., 2000; Ehrlich et al., 2002; Vaezi et al., 2002; Ivanov et al., 2004a). Second, disorganization of actin microfilaments by cell-permeable actin-binding drugs such as cytochalasin D or latrunculin A results in the disappearance of AJC proteins from areas of cell-cell contact and prevents AJC assembly (Stevenson and Begg, 1994; Adams et al., 1998; Vasioukhin et al., 2000; Yamada et al., 2004). In addition, similar disruption of apical junctions can be achieved by either inactivation or hyperactivation of Rho family GTPases, which are well known modulators of F-actin architecture (Walsh et al., 2001; Hopkins et al., 2003). Finally, genetic elimination of actin-binding proteins or overexpression of mutant junctional proteins lacking actin-binding sites impairs the formation of the epithelial AJC (reviewed in Vasioukhin and Fuchs, 2001; Bershadsky, 2004).
Given the critical role of the actin cytoskeleton in the biogenesis of the epithelial AJC, it is important to understand mechanisms of F-actin reorganization that drive the assembly of intercellular junctions. Generally, such mechanisms imply either dynamic turnover (polymerization/depolymerization) of F-actin (Pollard and Borisy, 2003; Lambrechts et al., 2004) or myosin II-dependent translocation of actin microfilaments (Maciver, 1996; Cramer, 1999). De novo actin polymerization has recently been implicated in the formation of nascent E-cadherin-based junctions in epidermal keratinocytes (Vasioukhin et al., 2000; Vaezi et al., 2002) and Madin-Darby canine kidney (MDCK) cells (Adams et al., 1998; Helwani et al., 2004; Verma et al., 2004). It is not known whether this mechanism regulates initial cell-cell adhesion in simple human epithelia. Furthermore, the role of actin polymerization in assembly of epithelial TJs remains poorly understood.
In addition to direct actin polymerization, another important regulator of F-actin reorganization in epithelial cells is myosin II (Mooseker, 1985; Turner, 2000). Myosin II has been shown to be enriched in the perijunctional F-actin ring (Mooseker, 1985; Castillo et al., 1998) and to physically interact with the TJ plaque protein cingulin (Cordenonsi et al., 1999). Furthermore, recent pharmacological analyses suggest that myosin II-driven contraction is involved in the transient opening of TJs after activation of Na+-glucose cotransport (Turner et al., 1997) and in the disassembly of the AJC induced by depletion of extracellular calcium (Ma et al., 2000; Ivanov et al., 2004a). However, the role of myosin II in the formation of epithelial apical junctions remains uninvestigated.
The present study was designed to investigate mechanisms by which the actin cytoskeleton regulates formation of the epithelial AJC. Using a classical calcium switch model in T84 intestinal epithelial cells, we determined that actin polymerization and myosin II activity are both involved in the formation of apical junctions, but these two mechanisms play different roles in junctional assembly. Whereas continuous actin polymerization is required for recruitment/retention of junctional proteins at the plasma membrane, myosin II regulates formation of apico-basal cell polarity and correct positioning of the AJC.
MATERIALS AND METHODS
Antibodies and Other Reagents
The following primary polyclonal (pAb) and monoclonal (mAb) antibodies were used to detect TJ, AJ, and cytoskeletal proteins by immunofluorescence labeling and Western blotting: anti-occludin, ZO-1, claudin-1 and JAM-A pAbs (Zymed Laboratories, South San Francisco, CA); anti-E-cadherin mAb (HECD-1; Zymed Laboratories); anti-β-catenin pAb (Sigma-Aldrich, St. Louis, MO), anti-neuronal Wiskott-Aldrich syndrome protein (N-WASP), and antiregulatory myosin light chain pAbs (Santa Cruz Biotechnology, Santa Cruz, CA); anti-mammalian nonmuscle myosin (MNMM) IIA pAb (Covance, Berkley, CA); and anti–mono- and -diphosphorylated regulatory myosin light chain (Cell Signaling Technology, Beverly, MA). Anti-actin-related protein (Arp) 3 pAb was generously provided by Dr. Matthew Welch (University of California, Berkeley, CA). Alexa-488 or Alexa-568 dyes conjugated G-actin, phalloidin, donkey anti-rabbit, and goat anti-mouse secondary antibodies were obtained from Molecular Probes (Eugene, OR); horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Latrunculin B, cytochalasin D, phalloidin, and saponin were obtained from Sigma-Aldrich; jasplakinolide and wiskostatin were purchased from Calbiochem (San Diego, CA); S-(–)-blebbistatin was obtained from Toronto Research Chemicals (North York, Ontario, Canada). Other reagents were of the highest analytical grade and were obtained from Sigma-Aldrich.
Cell Culture
T84 intestinal epithelial cells (American Type Culture Collection, Manassas, VA) were cultured in a 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 10 mM HEPES, 14 mM NaHCO3, 40 μg/ml penicillin, 100 μg/ml streptomycin, and 5% newborn calf serum, pH 7.4 (further designated as high calcium medium [HCM]). For all experiments, T84 cells were grown for 7–12 d on collagen-coated, permeable polycarbonate filters, 0.4-μm pore size (Costar, Cambridge, MA). Filters with a surface area of 0.33 and 5 cm2 were used for immunocytochemical and biochemical experiments, respectively.
Calcium Switch and Pharmacological Modulation of Apical Junction Reassembly
To disassemble the AJC, confluent T84 monolayers were incubated in a low-calcium medium (LCM; calcium-free Eagle's minimum essential medium for suspension culture [Sigma-Aldrich]) supplemented with 10 mM HEPES, 14 mM NaHCO3, 40 μg/ml penicillin, 100 μg/ml streptomycin, 10 μM CaCl2, and 5% dialyzed newborn calf serum, pH 7.4) overnight at 37°C. To induce reassembly of the AJC, the cells were returned to the HCM for indicated times at 37°C. For pharmacological modulation of AJC reassembly, T84 cells were subjected to overnight incubation in the LCM and then preincubated for 30 min with drugs in the LCM followed by transferring into HCM containing the same concentration of inhibitor. Stock solutions of water-insoluble inhibitors were prepared in dimethyl sulfoxide (DMSO) and diluted in cell culture media immediately before each experiment. The final concentration of DMSO was 0.1%, and the same concentration of vehicle was included in appropriate controls.
Immunofluorescence Labeling and Image Analysis
T84 monolayers were rinsed twice with ice-cold 10 mM HEPES-buffered Hank's balanced salt solution (HBSS), pH 7.4. Cells were fixed/permeabilized in absolute ethanol for 20 min at –20°C followed by blocking in HBSS containing 1% bovine serum albumin (BSA) (blocking buffer) for 60 min at room temperature and incubation for 60 min with primary antibodies in the blocking buffer. Cell monolayers were then washed, incubated for 60 min with Alexa dye-conjugated secondary antibodies followed by rinsing and mounting on slides with ProLong antifade reagent (Molecular Probes, Eugene, OR). For an immunolabeling of actin-nucleating proteins, T84 cells were preextracted with a cytoskeleton-stabilizing buffer (CSB; 0.5% Triton X-100 [TX-100], 10 mM 2-(N-morpholino)ethanesulfonic acid, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA, 0.32 M sucrose, 1 μg/ml phalloidin, pH 6.1) for 15 min at 4°C (Cramer et al., 2002). For double labeling of junctional proteins with F-actin, monolayers were fixed in 3.7% paraformaldehyde, permeabilized with 0.5% TX-100, and sequentially incubated with primary and Alexa dye-conjugated secondary antibodies, whereas F-actin was labeled with either Alexa-488 or Alexa-568 dye-conjugated phalloidin. Labeled monolayers were examined using a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY) with a Zeiss Axioplan 2e microscope and either 63× or 100× Pan-Apochromat oil lenses. Fluorescent dyes were imaged sequentially in frame-interlace mode to eliminate fluorescence bleed-over between channels. Images shown are representative of at least three experiments, with multiple images taken per slide. To quantify recruitment of TJ proteins to intercellular junctions, five random areas per experimental group were imaged at the focal level of TJ with the same contrast and the detector gain settings. Average pixel intensity of each area was measured using Axiovision software (release 4.3; Carl Zeiss).
Transmission Electron Microscopy
T84 monolayers were fixed in 4% buffered glutaraldehyde and sectioned into 1-μm strips by using a microtome. The sections were postfixed in 1% osmium tetroxide, sequentially dehydrated through graded alcohols and propylene oxide, and infiltrated with Embed-812 (Electron Microscopy Sciences, Ft. Washington, PA). Epithelial sections were then embedded in a cross-section orientation. Semithin (0.5-mm) sections were cut, stained with toluidine blue, and examined for adequacy. Ultrathin sections (900 Å) were cut with a diamond knife, stained with uranyl acetate and lead citrate, and examined with a Philips EM201 electron microscope.
Immunoblotting
Cells were homogenized in a PhosphoSafe extraction buffer (EMD Biosciences, Madison, WI) containing a proteinase inhibitor cocktail (1:100; Sigma-Aldrich) and 1 μM phenylmethylsulfonyl fluoride. Lysates were then cleared by centrifugation (20 min at 14,0000 × g) and immediately boiled in SDS sample buffer. PAGE and immunoblotting were performed by standard methods. Quantification of protein expression was performed by densitometric analysis of Western blot images on UN-SCAN-IT digitizing software (Silk Scientific, Orem, UT).
Fractionation of G-Actin and F-Actin and Incorporation of Exogenous G-Actin
Quantification of G-actin and F-actin was performed by TX-100 fractionation of intracellular actin as described previously (Cramer et al., 2002). Briefly, T84 monolayers were washed with HBSS and G-actin was extracted by gentle shaking for 5 min at room temperature in HBSS containing 1% TX-100, proteinase inhibitor cocktail and 1 μg/ml phalloidin to prevent filament disassembly. The TX-100–soluble G-actin fraction was mixed with an equal volume of SDS sample buffer and boiled. Filters were then briefly washed with HBSS and the TX-100–insoluble F-actin fraction was collected by scraping cells in two volumes of SDS sample buffer, and boiled. The amount of actin in each fraction was determined by gel electrophoresis, Western blotting, and densitometry as described above.
Introduction of exogenous G-actin into permeabilized cells was performed as described previously (Symons and Mitchison, 1991). Briefly, calcium-depleted T84 monolayers were transferred into HCM for either 0.5 or 3 h and then washed twice in a rinsing buffer (20 mM HEPES, 138 mM KCl, 4 mM MgCl2, pH 7.4). Cells were then incubated for 5 min at room temperature in a permeabilizing buffer (0.2 mg/ml saponin and 1 mM ATP in the rising buffer) containing 1 μM Alexa-488 G-actin or the same concentration of the fluorescently-labeled BSA. Thereafter, cells were washed with HBSS, fixed with paraformaldehyde, permeabilized with 0.5% TX-100, and counterstained for AJC proteins.
Statistics
Numerical values from individual experiments are pooled and expressed as mean ± SE. Values obtained for a calcium-depleted and calcium-repleted groups were compared by a single-tailed Student's t test, with statistical significance assumed at p < 0.05.
RESULTS
Calcium-dependent Reassembly of the AJC Occurs though Early Formation of Adherens-like Junctions and Subsequent Assembly of TJs
In the present study, a well established “calcium switch” model (Gumbiner et al., 1988) was used to investigate the assembly of the AJC in T84 epithelial cells. Mature intercellular junctions in T84 monolayers were disrupted by overnight incubation in LCM containing 10 μM calcium. The reassembly of junctions was initiated by switching from LCM to HCM (calcium concentration ∼1.8 mM). Because the calcium-induced establishment of the AJC in T84 cells has not been previously characterized in detail, we first investigated the dynamics of AJ and TJ reassembly and its relationship with the reorganization of the actin cytoskeleton.
Overnight incubation in LCM resulted in T84 cells rounding up and losing a majority contacts with neighboring cells, but they remained attached to the substratum (Figure 1C). In agreement with our previous study (Ivanov et al., 2004b), the majority of E-cadherin (Figure 1A), β-catenin, and p120 catenin (our unpublished observations) in calcium-depleted T84 monolayers were internalized and accumulated in a cytosolic compartment. TJ proteins ZO-1 (Figure 1B), occludin, and JAM-A (our unpublished observations) had mostly disappeared from the plasma membrane and were visualized as dot-like structures inside the cell or at the residual cell-cell contacts. F-actin labeling demonstrated a thick layer of cortical microfilaments at the periphery of calcium-depleted cells (Figure 1C).
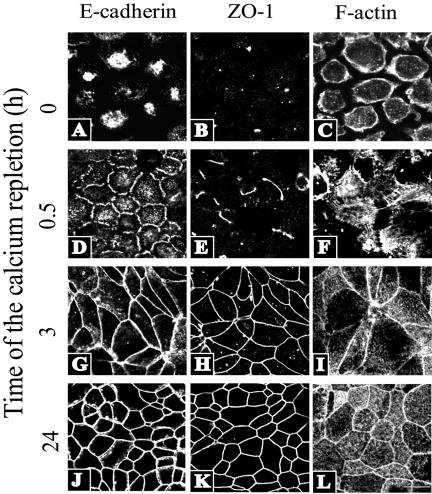
Figure 1.
Dynamics of calcium-induced reassembly of apical junctions and F-actin reorganization in intestinal epithelial cells. In calcium-depleted T84 cells, an AJ protein E-cadherin and a TJ protein ZO-1 are localized in intracellular aggregates (A and B), whereas F-actin is primarily distributed at the cell cortex (C). Restoration of normal extracellular calcium level results in recruitment of AJ/TJ proteins to areas of cell-cell contacts to form a characteristic “chicken wire” staining pattern (G, H, J, K). Note the faster dynamics of formation of adherens junctions (D, G, J) compared with TJs (E, H, and K). The assembly of apical junctions is accompanied by dramatic reorganization of actin cytoskeleton manifested by early formation of radiating F-actin cables (F) that are later transformed into a perijunctional F-actin belt (I and H). Bar, 20 μm.
Reintroduction of calcium to T84 monolayers induced a rapid reassembly of intercellular junctions. Within the first 0.5 h of the calcium repletion, a majority of cells acquired nascent E-cadherin–based junctions that seemed as either discontinuous dots or wavy ribbons at cell-cell contacts (Figure 1D). At that time, nascent junctions contained only occasional dot-like aggregates or short strands of TJ proteins such as ZO-1 (Figure 1E). After 3 h of calcium repletion, E-cadherin–based junctions became enriched in TJ proteins and transformed into continuous linear AJ and TJ ribbons between adjacent cells (Figure 1, G and H). At later times (3–24 h of repletion), circumferential AJs and TJs expanded throughout the entire T84 monolayer to form the AJC typical of fully differentiated epithelial cells (Figure 1, J and K). The assembly of nascent junctions coincided with the appearance of thick radiating F-actin cables between adjacent cells (Figure 1F), whereas the maturation of the initial cell-cell contacts into the AJC was paralleled by the assembly of a circumferential perijunctional F-actin belt (Figure 1, I and L).
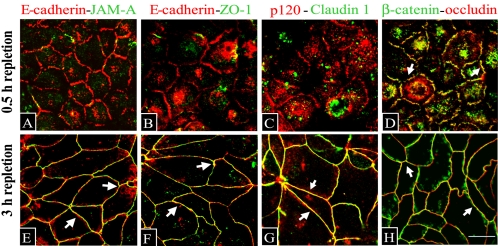
Because of seemingly different dynamics of accumulation of AJ and TJ proteins at the areas of cell contact (Figure 1), we next compared protein composition of nascent E-cadherin–based junctions and the fully formed AJC. To selectively visualize junction-associated proteins, we took advantage of their insolubility in TX-100–containing buffers due to either association with actin cytoskeleton (Nagafuchi et al., 1994) or localization within lipid raft membrane domains (Nusrat et al., 2000). Depolarized T84 cells were transferred to HCM for 0.5 or 3 h, extracted with TX-100–containing CSB before fixation, and double-immunolabeled for different AJ and TJ proteins. After 0.5 h of contact formation, nascent junctions were enriched in the AJ proteins E-cadherin, β-catenin, and p120 catenin (Figure 2, A–D). Of all TJ proteins studied, only occludin was incorporated in the E-cadherin–based junctions (Figure 2D), whereas no significant presence of JAM-A, ZO-1, and claudin-1 was detected (Figure 2, A–C). In contrast, after 3 h of calcium repletion, apical junctions contained an ensemble of major AJ and TJ proteins (Figure 2, E–H). Nascent adherens-like junctions of T84 cells seem to correspond to E-cadherin–rich spot-like junctions previously referred to as puncta in mammary and renal epithelial cells (Adams and Nelson, 1998; Vasioukhin and Fuchs, 2001; Bershadsky, 2004). However, these “classical” puncta were described as including TJ components such as ZO-1 and JAM-A (Takai and Nakanishi, 2003; Ebnet et al., 2004), whereas adherens-like junctions that we observed in T84 cells contained occludin but not other TJ proteins (Figure 2). These data suggest that whereas early accumulation of AJ proteins represents a common feature of all epithelial cell-cell adhesions, the dynamics of TJ protein recruitment may differ among various cell types.
Figure 2.
Nascent junctions and the mature AJC are composed of different sets of junctional proteins. Nascent junctions that are assembled after 0.5 h of calcium repletion, accumulate AJ proteins E-cadherin (A and B, red), p120 catenin (C, red), and β-catenin (D, green). Of all TJ proteins, only occludin (D, red) is present and colocalizes with β-catenin (D, arrows) in nascent junctions, whereas no significant accumulation JAM-A, ZO-1, and claudin-1 (A–C, green) is detected. In contrast, all of these TJ proteins colocalize with AJ components at AJC formed after 3 h of calcium repletion (E–H, arrows). Bar, 10 μm.
Assembly of Nascent Junctions and TJs Depends on Polymerization of Dynamic Actin Microfilaments
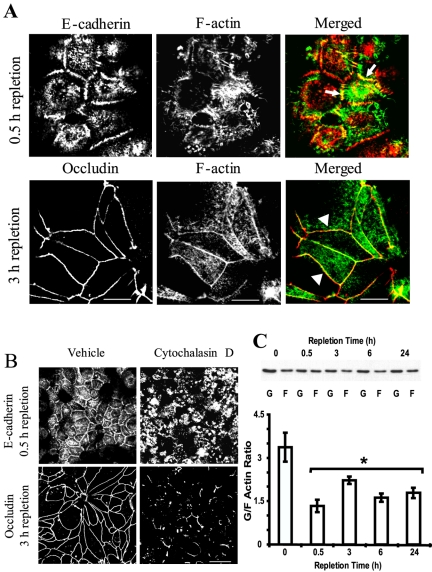
Identifying a stepwise assembly of AJC during calcium repletion of T84 cells allowed us to separately investigate mechanisms regulating early and late steps of intercellular adhesion. To do that, we used E-cadherin and occludin as marker proteins for respectively nascent AJ-like junctions and TJs. We first asked whether the assembly of nascent junctions and TJs is simply coincidental with reorganization of actin microfilaments or whether they are causally connected. To answer this question, we analyzed colocalization of F-actin with junctional proteins and investigated the effect of F-actin disorganization by cytochalasin D (Verkhovsky et al., 1997) on the formation of adherens-like junctions and TJs. In nascent junctions, E-cadherin showed significant colocalization with radiating F-actin cables (Figure 3A, arrows), whereas at TJs, occludin colocalized with the perijunctional F-actin belt (Figure 3A, arrowheads). Calcium repletion in the presence of cytochalasin D (10 μM for 1 or 3 h) caused aggregation of F-actin throughout the cell and prevented the formation of radiating F-actin cables and an F-actin belt at early and later steps of junctional reassembly (our unpublished observations). Furthermore, such F-actin disorganization blocked the accumulation of E-cadherin in nascent junctions and the formation of TJs (Figure 3B). Collectively, these experiments demonstrate that the reorganization of the actin cytoskeleton is a driving force in the formation of both adherens-like junctions and TJs during calcium repletion of T84 cells.
Figure 3.
Formation of nascent junctions and TJs depends on the integrity of the actin cytoskeleton and is accompanied by global increase in intracellular F-actin. (A) In nascent junctions, E-cadherin (red) colocalizes with F-actin (green) cables that radiate between adjacent cells (arrows), whereas in TJs, occludin (red) colocalizes with a linear perijunctional F-actin belt (arrowheads). Bar, 10 μm. (B) Control T84 cells subjected to the calcium switch for either 1 or 3 h show typical adherens-like junctions and TJs at early and late stages of junctional assembly, whereas cells that were calcium-repleted for the same times in the presence of F-actin–disorganizing drug cytochalasin D (10 μM) develop neither nascent junctions nor TJs. Bar, 20 μm. (C) Representative Western blot and densitometric quantification show a dramatic decrease of G/F actin ratio starting from the early time point during calcium repletion. Data are presented as mean ± SE (n = 3); *p < 0.05 compared with the calcium-depleted group.
We next investigated mechanisms by which F-actin reorganization establishes initial cell-cell contacts and the mature AJC. Two potential mechanisms can be envisioned: one involves de novo F-actin polymerization (Pollard and Borisy, 2003; Lambrechts et al., 2004) and the second involves translocation of preexisting actin microfilaments by myosin motors (Maciver, 1996; Cramer, 1999). To test the role of actin polymerization, we used several experimental approaches. First, we analyzed whether calcium repletion alters relative amounts of intracellular G- and F-actin. In calcium-depleted T84 cells, we determined that the majority of actin exists in monomeric form, which was manifested by a high G/F actin ratio (∼4; Figure 3C). Interestingly, after 0.5 h of calcium-induced assembly of cell-cell contacts, the G/F ratio significantly decreased (∼2.5-fold) compared with the calcium-depleted group (Figure 3C). This indicates a rapid and dramatic shift of G/F actin equilibrium toward polymerization of microfilaments that was sustained through 24 h of junctional reassembly (Figure 3C).
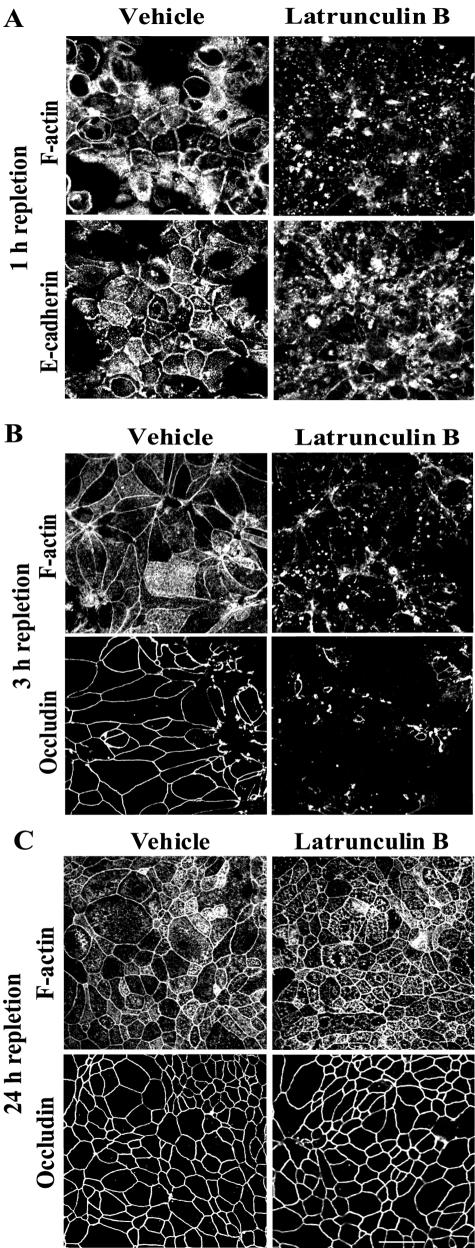
To test whether the formation of nascent junctions and TJs are dependent on continuous rapid turnover (depolymerization/repolymerization) of actin microfilaments, we treated T84 monolayers with latrunculin B. Latrunculins have been shown to selectively bind G-actin and to prevent its incorporation into microfilaments (Coue et al., 1987; Morton et al., 2000). Such sequestration of G-actin inhibits de novo F-actin polymerization and results in disassembly of unstable (e.g., rapidly turning over) microfilaments. We induced formation of junctions by either 0.5- or 3-h incubation in HCM followed by treatment of cells for additional 0.5 h with either 1 μM latrunculin B or vehicle. Latrunculin B treatment caused disassembly of F-actin bundles associated with both nascent junctions (Figure 4A) and TJs (Figure 4B). Furthermore, the sequestration of G-actin induced disappearance of E-cadherin from the nascent junctions (Figure 4A) and loss of occludin from TJs (Figure 4B). These results demonstrate a critical role for actin polymerization in the assembly of both types of junctions and also indicate a rapid turnover of actin microfilaments affiliated with the assembling junctions. In contrast to profound sensitivity of adherens-like junctions and newly formed TJs to latrunculin B-induced actin depolymerization, we observed that fully mature AJC became resistant to this treatment. Indeed, when T84 cells were incubated for 24 h in the HCM followed by standard treatment with 1 μM latrunculin B (for 0.5 h), the drug caused neither depolymerization of perijunctional F-actin belt nor dislocation of occludin (Figure 4C) or E-cadherin (our unpublished observation) from the intercellular junctions. These data suggest that continuously turning over actin filaments are selectively associated with newly formed junctions and that perijunctional F-actin becomes much less dynamic in the mature AJC.
Figure 4.
Formation of nascent junctions and TJs requires continuous polymerization of actin microfilaments, whereas ultimate maturation of the AJC decreases its dependence on actin polymerization. Calcium-depleted T84 cells were incubated in HCM for indicated times followed by additional 0.5-h incubation in HCM containing either G-actin–sequestering drug latrunculin B (1 μM) or vehicle. Latrunculin B treatment that prevents de novo actin polymerization causes rapid disassembly of preformed perijunctional actin bundles, and loss of junctional proteins from newly formed nascent junctions (A) and TJs (B), but it has no effect on the architecture of F-actin and occludin in mature AJC (C). Bar, 20 μm.
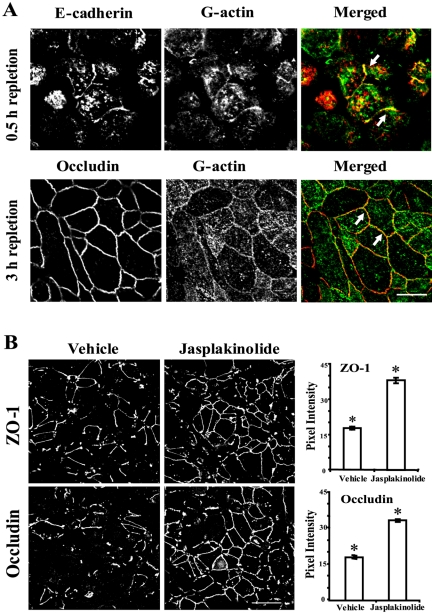
Given the dependence of cell-cell junctions on de novo actin polymerization, we sought to detect in situ polymerization of junction-associated actin microfilaments. Calcium-depleted monolayers were induced to form nascent AJ-like junctions and TJs during either 0.5 or 3 h of calcium repletion, followed by introduction of a fluorescently labeled G-actin or BSA after gentle cell permeabilization with saponin. We observed that exogenous G-actin rapidly (within 5 min) accumulated in areas of cell-cell contact and colocalized with E-cadherin in nascent junctions and with occludin in TJs (Figure 5A, arrows). No such accumulation was found after introduction of fluorescently labeled BSA (our unpublished observation), suggesting that this effect is specific for G-actin and that both junctions are areas of rapid actin polymerization.
Figure 5.
Nascent junctions and TJs represent the areas of active actin polymerization, and acceleration of F-actin polymerization promotes the assembly of TJs. (A) In calcium-repleted, saponin-permeabilized T84 cells, exogenous fluorescently labeled G-actin (green), is incorporated into nascent junctions and TJs where it colocalizes with, respectively, E-cadherin and occludin (arrows). Bar, 10 μm. (B) Jasplakinolide (1 μM) that stimulates F-actin polymerization accelerates recruitment of ZO-1 and occludin to the areas of cell-cell contact and promotes formation of circumferential TJs during calcium repletion. Data are presented as mean ± SE (n = 5); *p < 0.01 compared with the vehicle-treated group Bar, 20 μm.
If actin polymerization is indeed critical for the formation of epithelial junctions, then it could be expected that stimulation of actin polymerization would accelerate junctional assembly. We tested this hypothesis with jasplakinolide, a cell-permeable drug that promotes formation of actin microfilaments (Bubb et al., 1994). Given the rapid formation of adherens-like junctions, it was not feasible to detect an accelerating effect of jasplakinolide on their assembly. Hence, we investigated the effect of this drug on the establishment of TJs. Calcium-depleted T84 cells were incubated for 1 h in HCM containing either 1 μM jasplakinolide or vehicle followed by immunolabeling for occludin and ZO-1. As expected, jasplakinolide treatment caused about twofold increased accumulation of occludin and ZO-1 in areas of cell-cell contacts (Figure 5B). Collectively, the pharmacological modulation of F-actin assembly and perijunctional incorporation of G-actin strongly suggest that establishment of the nascent adherens-like junctions and TJs are dependent on continuous polymerization of dynamic F-actin microfilaments.
Inhibition of N-WASP-Arp2/3–dependent Nucleation of Actin Microfilaments Blocks Assembly of Nascent Junctions and TJs
Polymerization of F-actin occurs as sequential events involving nucleation followed by elongation of microfilaments (dos Remedios et al., 2003). Nucleation is the rate-limiting step for the entire process and is tightly regulated by a number of accessory proteins. Among them, the role of the Arp2/3 complex has been studied in great detail (Higgs and Pollard, 2001; May, 2001). This complex consists of seven different subunits, and it mediates Y-branching microfilament growth. To trigger actin nucleation, the Arp2/3 complex requires activation, usually by members of the WASP family. The Arp2/3 complex has recently been implicated in the formation of initial cell-cell contacts in E-cadherin–transfected fibroblasts and MDCK cells (Kovacs et al., 2002b; Helwani et al., 2004; Verma et al., 2004). Based on these observations, we investigated the role of Arp2/3-driven actin nucleation in the calcium-induced assembly of nascent junctions and TJs in T84 cells.
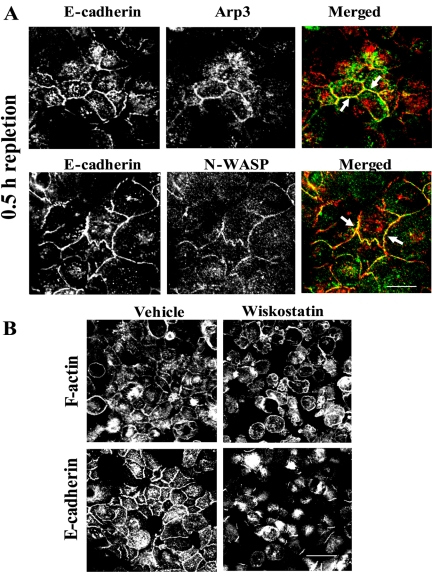
After calcium depletion in LCM, we subjected cells to short-term (0.5-h) calcium repletion. The cells were then preextracted with CSB, fixed, and double labeled for E-cadherin and either Arp3 or N-WASP, the most abundantly expressed member of the WASP family (Vartiainen and Machesky, 2004). We observed accumulation of Arp3 and N-WASP at nascent junctions where both proteins colocalized with E-cadherin (Figure 6A, arrows). To investigate the functional significance of the accumulation of Arp2/3 and N-WASP proteins at adherens-like junctions, we took advantage of the known allosteric regulation of N-WASP activity (Caron, 2002; Prehoda and Lim, 2002). In particular, under resting conditions, WASP has been shown to exist in a self-folded autoinhibited conformation. Activation results in conformational changes that allow interaction with Arp2/3 and actin (Caron, 2002; Prehoda and Lim, 2002). Recently, a cell-permeable chemical inhibitor of N-WASP, wiskostatin, was identified (Peterson et al., 2004). Wiskostatin blocks N-WASP activity by stabilizing its autoinhibited conformation, thus leading to a significant decrease in the rate of actin polymerization. We preincubated calcium-depleted cells for 0.5 h with 50 μM wiskostatin or vehicle followed by transferring cells for additional 1 h to HCM containing the same concentration of the inhibitor. To ensure specificity of the inhibitor, we used 50 μM wiskostatin, a concentration that is below of the reported apparent Kd values for its in vitro binding to WASP fragments (∼100 μM; Peterson et al., 2004). We found that wiskostatin treatment blocked reorganization of the actin cytoskeleton into junction-associated F-actin bundles (Figure 6B). Furthermore, inhibition of N-WASP activity dramatically attenuated the assembly of E-cadherin–based junctions (Figure 6B).
Figure 6.
N-WASP-Arp2/3–dependent actin nucleation is involved in formation of nascent junctions. (A) After 0.5 h of calcium repletion, nascent junctions are enriched in actin-nucleating proteins Arp3 and N-WASP (green) that colocalize with E-cadherin (arrows). Bar, 10 μm. (B) Control T84 cells calcium repleted for 1 h show typical assembly of F-actin bundles and accumulation of E-cadherin at nascent junctions, whereas cells that were calcium repleted in the presence of the N-WASP inhibitor wiskostatin (50 μM) demonstrate formation of neither F-actin–rich cell-cell contacts nor adherens-like junctions. Bar, 20 μm.
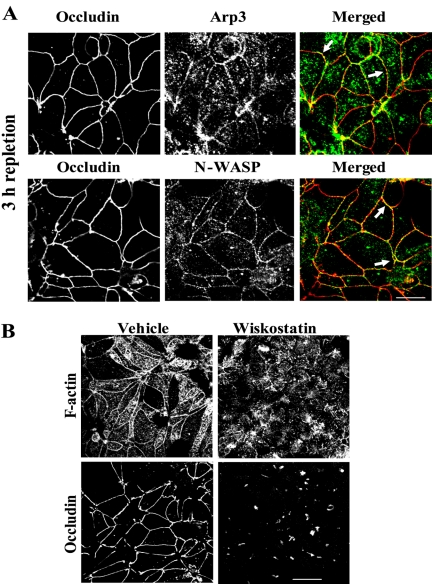
The same experimental approaches were used to analyze the role of N-WASP-Arp2/3–mediated actin nucleation in the formation of TJs. We incubated calcium-depleted cells in the HCM for 3 h, followed by their preextraction with CSB and double immunolabeling for the actin-nucleating proteins and occludin. Figure 7A shows significant colocalization of both Arp3 and N-WASP with occludin within TJ strands (arrows). To analyze the effect of wiskostatin specifically on the assembly of TJs, we preincubated calcium-depleted cells in HCM for 0.5 h to allow formation of nascent junctions before the addition 50 μM wiskostatin or vehicle for additional 2.5 h. Under these conditions, we observed that N-WASP inhibition completely prevented the assembly of perijunctional F-actin rings and the formation of TJs (Figure 7B). Interestingly, the wiskostatin-treated cells retained radiating F-actin cables (Figure 7B) and AJ proteins at intercellular contacts (our unpublished observation). However these cells never established linearized cell borders or became columnar shaped and seemed to not progress beyond the adherens-like junctions stage. Collectively, these data strongly implicate a role of N-WASP-Arp2/3–dependent actin polymerization in regulation of the assembly of both nascent junctions and TJs.
Figure 7.
N-WASP-Arp2/3–dependent actin nucleation is critical for TJ assembly. (A) Arp3 and N-WASP (green) colocalize with occludin (arrows) in TJs formed after 3 h of calcium repletion. Bar, 10 μm. (B) Control T84 cells calcium repleted for 3 h demonstrate extensive assembly of both the perijunctional F-actin belt and TJs, whereas wiskostatin-treated cells form neither apical F-actin belt nor occludin-rich TJs. Bar, 20 μm.
Inhibition of Myosin II Motor Prevents Assembly of TJs at the Cell Apex by Blocking Formation of Apico-basal Cell Polarity
Large-scale cytoskeletal reorganizations require not only F-actin polymerization but also transport and reorientation of actin filaments by a myosin II motor (Maciver, 1996; Cramer, 1999; Mandato and Bement, 2001). Thus, we performed experiments to determine whether myosin II activity is important for calcium-driven assembly of nascent junctions and TJs in T84 cells.
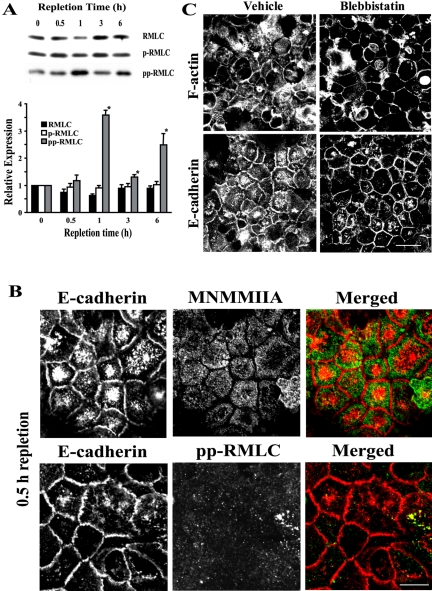
A major mechanism for myosin II activation involves phosphorylation of the regulatory myosin light chain (RMLC) on either one (Ser-19) or two (Thr-18/Ser-19) amino acid residues (Tan et al., 1992; Bresnick, 1999). We analyzed the level of RMLC phosphorylation at different times of calcium-induced junctional reassembly. Western blotting analyses of T84 cell lysates showed no significant differences in the level of total and monophosphorylated RMLC between calcium-depleted cells and cells at different times of calcium repletion (Figure 8A). In contrast, a significant increase (∼3.5-fold) in the level of diphosphorylated RMLC was observed after 1 h of junctional reassembly (Figure 8A).
Figure 8.
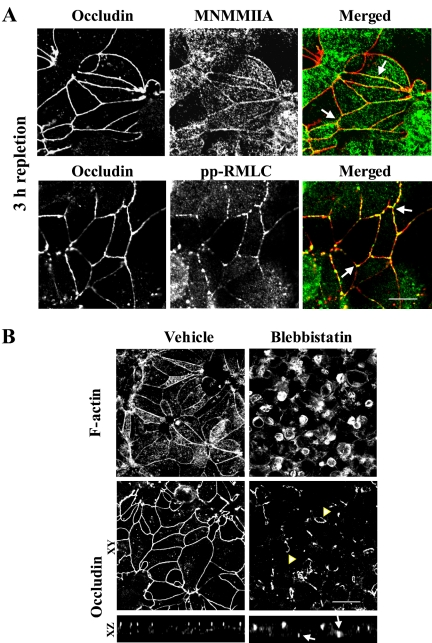
Myosin II is not essential for assembly of nascent AJ-like junctions. (A) Representative Western blots and densitometric quantification show an increase in the amount of diphosphorylated (pp) but not total or monophosphorylated (p) RMLC in T84 cell lysates during calcium repletion. Data are presented as mean ± SE (n = 4); *p < 0.05 compared with the calcium-depleted group. (B) Neither MNMMIIA heavy chain nor pp-RMLC (green) accumulated at E-cadherin–based junctions (red) after 0.5 h of calcium repletion. Bar, 10 μm. (C) A selective inhibitor of MNMM motor activity, blebbistatin (50 μM), does not affect the assembly of F-actin–rich adherens-like junctions after 1 h of calcium repletion. Bar, 20 μm.
To examine the involvement of myosin II in the formation of nascent junctions, we analyzed colocalization of this motor protein with E-cadherin and the effect of myosin II inhibition (Straight et al., 2003) on the assembly of initial cell-cell adhesions. For myosin II immunolabeling, we used two different antibodies, one recognizing the heavy chain of mammalian nonmuscle myosin IIA (MNMMIIA), which is the predominant myosin II isoform in T84 cells (Ivanov et al., 2004a); and another that reacts with RMLC phosphorylated at Thr-18 and Ser-19 (pp-RMLC). Neither MNMMIIA nor pp-RMLC colocalized with E-cadherin in nascent junctions after 0.5 h of calcium repletion (Figure 8B). For myosin II inhibition, calcium-depleted T84 cells were preincubated with a selective inhibitor of MNMMII motor activity, blebbistatin (50 μM for 0.5 h) followed by 1 h of calcium repletion in the presence of the inhibitor. This concentration of blebbistatin is close to the apparent Kd of its binding to myosin II–ATP complexes (24–35 μM; Kovacs et al., 2004), and it was shown to completely inhibit myosin II ATPase activity in vitro (Straight et al., 2003). We found that blebbistatin-treated cells still formed extensive cell-cell contacts that were enriched in F-actin and E-cadherin (Figure 8C). Collectively, these data suggest that myosin II is not essential for the formation of nascent E-cadherin based junctions in T84 monolayers.
Different results were obtained from experiments probing the role of myosin II in the assembly of TJs. After 3 h of calcium repletion, we found accumulation of MNMMIIA and pp-RMLC at the apical junctions where they colocalized with occludin (Figure 9A, arrows). Furthermore, when cells were subjected to 3 h of calcium repletion in the presence of blebbistatin, the reorganization of apical F-actin and the assembly of TJs were blocked (Figure 9B). Unlike vehicle-treated cells that formed typical perijunctional F-actin belts, apical actin filaments in blebbistatin-treated cells were organized in round patches. Likewise, these cells did not develop a continuous circumferential staining of occludin at the cell apex and accumulated this TJ protein in short ribbons and small rings (Figure 9B, arrowheads) that localized at different levels along the lateral plasma membrane (Figure 9B, arrows on xz images). These data suggest that inhibition of myosin II results in mislocalization of TJs within the plasma membrane.
Figure 9.
Myosin II is critical for assembly of apical TJs. (A) Both MNMMIIA heavy chain and pp-RMLC (green) colocalize with occludin (arrows) in TJs after 3 h of calcium repletion. Bar, 10 μm. (B) After 3 h of calcium repletion, the vehicle-treated T84 cells develop normal perijunctional F-actin belts and circumferential occludin strands, whereas the blebbistatin-treated cells demonstrate round F-actin–rich patches as well as small ring-like occludin staining (arrowheads). Bar, 20 μm.
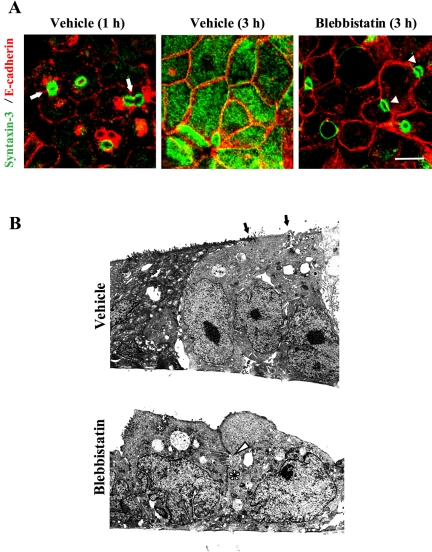
We hypothesized that such mislocalization of TJs in blebbistatin-treated cells is a consequence of global effects of myosin II inhibition on cell polarization. To test this possibility, we investigated formation of the apical plasma membrane domain in T84 cells by using a selective marker of the apical domain, syntaxin-3 (Low et al., 2000). During the assembly of adherens-like junctions (0.5–1 h of calcium repletion), syntaxin-3 was visualized in small rings that were frequently positioned laterally at areas of cell-cell contacts (Figure 10A, arrows). These data confirm recent observations in MDCK cells showing the formation of primordial apical plasma membrane domains at lateral intercellular adhesions (Cohen et al., 2004a,b). In control T84 cells, the lateral syntaxin-3–enriched rings redistributed to the apex at later times of calcium repletion (≥3 h), creating evenly dispersed apical syntaxin-3 labeling (Figure 10A). In contrast, blebbistatin-treated cells failed to form such uniformly stained apical surfaces and retained syntaxin-3–positive rings at the areas of cell-cell contacts even after 3–5 h of calcium repletion (Figure 10A, arrowheads). Similarly, a Rho kinase inhibitor Y-27632, which reportedly causes dephosphorylation and inhibition of myosin II (Bresnick, 1999), blocked the establishment of normal apical plasma membrane domain in calcium-repleted T84 cells (our unpublished observation).
Figure 10.
Inhibition of myosin II activity prevents formation of the apico-basal cell polarity. (A) Early during calcium repletion (1 h), control T84 cells form ring-like structures containing the apical membrane domain marker syntaxin-3 (green) that are frequently located at lateral E-cadherin–rich contacts (arrows). Later (3 h) the rings disappear and syntaxin-3 becomes distributed evenly at the apical surface. In contrast, blebbistatin-treated cells retain undeveloped apical plasma membrane in a form of syntaxin-3–positive rings at areas of cell-cell contacts (arrowheads). Bar, 10 μm. (B) Transmission electron micrograph shows that after 3 h of calcium repletion, the vehicle-treated T84 monolayer is composed of columnar-shaped cells that possess typical apical surfaces with microvilli and electrondense apical junctions (arrows). In contrast, blebbistatin-treated monolayers contain rounded cells that do not have an extended apical surface with microvilli. Instead, these cells possess microvilli containing vacuoli (arrowhead) and lateral junctions at areas of cell-cell contacts (asterisks).
The inhibitory effect of blebbistatin on cell polarization also was evident at the ultrastructural level. Indeed, an electron micrograph of control T84 monolayers subjected to 3 h of calcium repletion demonstrated columnar-shaped cells with electron-dense junctions at the most apical part of the lateral plasma membrane (Figure 10B, arrows) and numerous apical microvilli. By contrast, T84 cells incubated for the same amount of time with blebbistatin acquired neither columnar shape nor a normal apical surface. Instead, such cells possessed multiple vacuolar-like structures containing microvilli (Figure 10B, arrowhead). Such vacuoli were frequently located in areas of lateral cell-cell contact and were associated with lateral electron-dense junctions (Figure 10B, asterisk). We speculate that such microvilli-containing vacuoli correspond to the syntaxin-3–labeled rings visualized by confocal microscopy (Figure 10A) and may represent a primordial apical plasma membrane domain. Together, these data strongly suggest that inhibition of myosin II blocks normal development of the apical plasma membrane in T84 cells and may underlie the observed abnormal localization of TJ proteins in blebbistatin-treated cells.
DISCUSSION
The enormous complexity of epithelial apical junctions indicates that their assembly may occur in a stepwise manner. In the present study, we distinguished two major events in the establishment of AJCs in model intestinal epithelia (Figures 1 and 2). The first event involves formation of nascent junctions containing the majority of AJ proteins and a single TJ protein, occludin. The second event involves assembly of mature AJs and TJs at the cell apex (Figure 2). Although we did not intend to investigate the precise dynamics of recruitment of different junctional proteins to intercellular contacts, the separation of two major events during AJC assembly that was helpful in understanding mechanisms of F-actin reorganization involved in this process.
Our data suggest that actin cytoskeletal rearrangements mediate the formation of adherens-like junctions and TJs in T84 cells. This conclusion is supported by the observation that reorganization of perijunctional F-actin accompanied the assembly of adherens-like junctions and TJs (Figures 1 and 3) and by inhibition of junctional assembly with the F-actin–disorganizing drug cytochalasin D (Figure 3B).
To elucidate the driving forces of F-actin–mediated junctional assembly, we first investigated the role of actin polymerization. We found that this mechanism regulates the formation of both E-cadherin–based junctions and TJs. Rapid incorporation of exogenous G-actin within both junctions (Figure 5A) supports this conclusion. Furthermore, adherens-like junctions and newly formed TJs could be easily disrupted by the G-actin–sequestering agent latrunculin B (Figure 4, A and B). This suggests that continuous polymerization of actin microfilaments with a rapid turnover rate is critical for the integrity of immature AJ-like junctions and TJs. In support of this, we observed that stimulation of actin polymerization with jasplakinolide accelerated the assembly of TJs (Figure 5B). Interestingly, terminal maturation of apical junctions in T84 cells resulted in a drastic reduction in the turnover rate of perijunctional actin microfilaments manifested in a decreased sensitivity to latrunculin B (Figure 4C). Collectively, these data suggest different roles of dynamic and stable actin microfilaments, with the former involved in assembly of AJs and TJs and the latter responsible for maintenance of the mature AJC.
To understand the mechanisms involved in polymerization of perijunctional F-actin, we next investigated which protein machinery is involved in nucleation of actin microfilaments. Our results strongly suggest that the N-WASP–Arp2/3 pathway regulates reorganization of perijunctional F-actin and assembly of both nascent junctions and TJs (Figures 6 and 7). In previous reports, the Arp2/3 complex has been implicated in assembly of adherens-like junctions in E-cadherin–transfected fibroblasts and in MDCK cells (Kovacs et al., 2002b; Verma et al., 2004). This study is the first to localize the Arp2/3 complex and N-WASP to epithelial TJs and demonstrate their involvement in the TJ assembly.
Our findings provide a missing link between two critical events in intercellular adhesion, namely, activation of the Rho family of small GTPases and actin polymerization. Indeed, initial cell-cell adhesions have been shown to activate Rac1 and cdc42 at the plasma membrane (Kim et al., 2000; Nakagawa et al., 2001; Kovacs et al., 2002a). Such activation is required to trigger actin polymerization that results in formation of E-cadherin–based junctions (Ehrlich et al., 2002; Chu et al., 2004). Rac1 and cdc42 reportedly bind to the GTPase-binding domain of WASP proteins and destabilize the autoinhibited conformation (Rohatgi et al., 1999; Eden et al., 2002). Because our data demonstrate an obligate role of N-WASP in F-actin–mediated formation of intercellular junctions, it is likely that N-WASP transduces a signal from activated Rac1/cdc42 to regulate Arp2/3-mediated actin polymerization during the assembly of AJC.
F-actin polymerization seems not to be the sole mechanism controlling the formation of the AJC, because at least one step in junctional assembly required activity of a myosin II motor. Myosin II was previously implicated in lateral expansion of initial E-cadherin–based contacts in hepatocytes (Krendel and Bonder, 1999). However, our data do not support a role for such a “myosin II zipper” in the formation of adherens-like junctions. Indeed, adherens-like junctions in T84 cells did not accumulate myosin II, and inhibition of myosin II motor activity failed to prevent formation and extension of initial cell-cell contacts (Figure 8, B and C). In addition, we observed an increase in myosin II activation (phosphorylation) only after the assembly of a majority of E-cadherin–based junctions was completed (Figure 8A). Interestingly, recent studies demonstrated a lack of accumulation of myosin II within E-cadherin–based intercellular contacts (Vaezi et al., 2002) and a lack of attenuation of AJ assembly after inhibition of myosin II phosphorylation (Avizienyte et al., 2004). Collectively, these data argue that myosin II is not involved in the lateral extension of E-cadherin–based cell-cell contacts as has been suggested previously (Krendel and Bonder, 1999; Vasioukhin and Fuchs, 2001).
In contrast to the lack of myosin II involvement in formation of adherens-like junctions, we observed a critical role for this motor in the formation of TJs as demonstrated by significant colocalization of activated myosin II with TJ proteins (Figure 9A) and by prevention of the TJ assembly by selective inhibition of myosin II activity (Figure 9B). When this manuscript was under review, a similar attenuation of the TJ assembly by blebbistatin was reported in MDCK cells (Chen and Macara, 2005). Furthermore, our data clearly demonstrate that inhibition of myosin II blocks formation of epithelial apico-basal polarity (Figure 10). How does myosin II regulate apico-basal polarity in epithelial cells? According to a recently proposed model (Cohen et al., 2004a,b), polarization in MDCK cells after calcium switch occurs in two distinct stages. The first step involves formation of a so-called hepatic-like polarity characterized by the assembly of a primordial apical plasma membrane domain within a lateral lumen that is reminiscent of bile canaliculum in differentiated hepatocytes (Cohen et al., 2004a,b). In the second step, this lateral lumen then translocates to the cell apex to give rise to the apical plasma membrane. We observed that calcium-repleted T84 monolayers also transiently contain apical plasma membrane elements in vacuoli at areas of cell-cell contact (Figure 10). As the cells became columnar, these vacuoli disappeared, presumably by incorporation into the apical plasma membrane. Remarkably, we observed that inhibition of myosin II resulted in retention of apical plasma membrane-like elements in lateral vacuoli and blockage of cell columnarization (Figure 10). These data strongly suggest that myosin II regulates the transition from a hepatic-like stage to a mature polarized phenotype during calcium repletion of T84 monolayers.
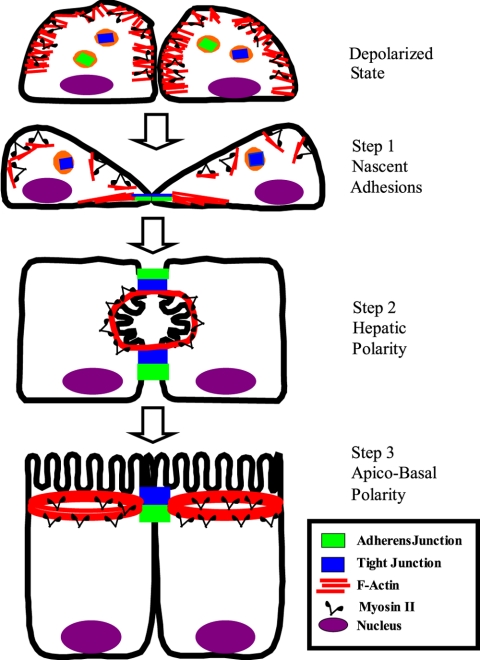
In conclusion, this study has revealed mechanisms of F-actin reorganization that regulate formation of the AJC in intestinal epithelial cells. Although involvement of both actin polymerization and myosin II-dependent microfilament transport were observed, their roles in junctional assembly are distinct. We summarize these findings in a three-step model that describes formation of the AJC and cell polarity in epithelia (Figure 11). The first step involves establishing nascent adherens-like junctions. The second step is assembly of TJs at lateral cell-cell contacts, resulting in hepatic-type polarity. The final step represents a transition from hepatic to apico-basal polarity and formation of the mature AJC. All three steps are regulated by N-WASP-Arp2/3–driven actin polymerization. In addition, the transformation from hepatic to apico-basal polarity is mediated by myosin II motor. These findings may describe a common mechanism underlying remodeling of epithelial barriers in normal embryonic morphogenesis or during epithelial repair.
Figure 11.
Three-step model of the formation of the apical junctional complex and apico-basal polarity in epithelial cells. Epithelial cells transiently lose columnar polarity and apical junctions after incubation at micromolar concentrations of extracellular calcium. Their TJ/AJ proteins are accumulated in the cytosol and F-actin is evenly distributed under the plasma membrane. Restoration of normal calcium level in cultural medium induces cell repolarization and reformation of the AJC in a process that can be divided into three major steps. The first step involves accumulation of F-actin bundles in areas of cell-cell contacts and the assembly of nascent adherens-like junctions. The second step results in the assembly of TJs encircling a primordial apical plasma membrane that is formed in a laterally located lumen with hepatic-type polarity. The final step is the transition from hepatic to apico-basal polarity resulting in formation of the apical plasma membrane domain and mature AJC.
Acknowledgments
We thank Dr. M. Welch for generous donation of antibodies, S. Voss and R. Santoianni for the excellent technical assistance, G. T. Brown for help in the manuscript preparation, and Dr. E. Vassilieva for valuable comments on the manuscript. This work was supported by National Institute of Health Grants DK-61379 and DK-72564 (to C.A.P.), DK-55679 and DK-59888 (to A. N.), a Digestive Diseases Minicenter Grant DK-64399 and a Senior Investigator Award from the Crohn's and Colitis Foundation (to A. N.), and by grant from the German research foundation (Deutsche Forschungsgemeinschaft UT 42/1-1) (to M. U.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0043) on March 30, 2005.
Abbreviations used: AJ, adherens junction; AJC, apical junctional complex; Arp2/3, actin-related proteins 2/3; CSB, cytoskeleton-stabilizing buffer; HBSS, HEPES-buffered Hank's balanced salt solution; HCM, high calcium medium; JAM, junctional adhesion molecule; LCM, low calcium medium; MNMM, mammalian nonmuscle myosin; N-WASP, neuronal Wiskott-Aldrich syndrome protein; RMLC, regulatory myosin light chain; TJ, tight junction; TX-100, Triton X-100.
References
- Adams, C. L., Chen, Y. T., Smith, S. J., and Nelson, W. J. (1998). Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 142, 1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, C. L., and Nelson, W. J. (1998). Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10, 572–577. [DOI] [PubMed] [Google Scholar]
- Avizienyte, E., Fincham, V. J., Brunton, V. G., and Frame, M. C. (2004). Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial-mesenchymal transition. Mol. Biol. Cell 15, 2794–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky, A. (2004). Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 14, 589–593. [DOI] [PubMed] [Google Scholar]
- Bresnick, A. R. (1999). Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26–33. [DOI] [PubMed] [Google Scholar]
- Bubb, M. R., Senderowicz, A. M., Sausville, E. A., Duncan, K. L., and Korn, E. D. (1994). Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269, 14869–14871. [PubMed] [Google Scholar]
- Caron, E. (2002). Regulation of Wiskott-Aldrich syndrome protein and related molecules. Curr. Opin. Cell Biol. 14, 82–87. [DOI] [PubMed] [Google Scholar]
- Castillo, A. M., Lagunes, R., Urban, M., Frixione, E., and Meza, I. (1998). Myosin II-actin interaction in MDCK cells: role in cell shape changes in response to Ca2+ variations. J. Muscle Res. Cell Motil. 19, 557–574. [DOI] [PubMed] [Google Scholar]
- Chen, X., and Macara, I. G. (2005). Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol. 7, 262–269. [DOI] [PubMed] [Google Scholar]
- Chu, Y. S., Thomas, W. A., Eder, O., Pincet, F., Perez, E., Thiery, J. P., and Dufour, S. (2004). Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 167, 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, D., Brennwald, P. J., Rodriguez-Boulan, E., and Musch, A. (2004a). Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 164, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, D., Rodriguez-Boulan, E., and Musch, A. (2004b). Par-1 promotes a hepatic mode of apical protein trafficking in MDCK cells. Proc. Natl. Acad. Sci. USA 101, 13792–13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi, M., D'Atri, F., Hammar, E., Parry, D. A., Kendrick-Jones, J., Shore, D., and Citi, S. (1999). Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J. Cell Biol. 147, 1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coue, M., Brenner, S. L., Spector, I., and Korn, E. D. (1987). Inhibition of actin polymerization by latrunculin A. FEBS Lett. 213, 316–318. [DOI] [PubMed] [Google Scholar]
- Cramer, L. P. (1999). Organization and polarity of actin filament networks in cells: implications for the mechanism of myosin-based cell motility. Biochem. Soc. Symp. 65, 173–205. [PubMed] [Google Scholar]
- Cramer, L. P., Briggs, L. J., and Dawe, H. R. (2002). Use of fluorescently labelled deoxyribonuclease I to spatially measure G-actin levels in migrating and non-migrating cells. Cell Motil. Cytoskeleton 51, 27–38. [DOI] [PubMed] [Google Scholar]
- dos Remedios, C. G., Chhabra, D., Kekic, M., Dedova, I. V., Tsubakihara, M., Berry, D. A., and Nosworthy, N. J. (2003). Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 83, 433–473. [DOI] [PubMed] [Google Scholar]
- Ebnet, K., Suzuki, A., Ohno, S., and Vestweber, D. (2004). Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 117, 19–29. [DOI] [PubMed] [Google Scholar]
- Eden, S., Rohatgi, R., Podtelejnikov, A. V., Mann, M., and Kirschner, M. W. (2002). Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790–793. [DOI] [PubMed] [Google Scholar]
- Ehrlich, J. S., Hansen, M. D., and Nelson, W. J. (2002). Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell 3, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal, L., Betanzos, A., Nava, P., and Jaramillo, B. E. (2003). Tight junction proteins. Prog. Biophys. Mol. Biol. 81, 1–44. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B., Stevenson, B., and Grimaldi, A. (1988). The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107, 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwani, F. M., Kovacs, E. M., Paterson, A. D., Verma, S., Ali, R. G., Fanning, A. S., Weed, S. A., and Yap, A. S. (2004). Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 164, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs, H. N., and Pollard, T. D. (2001). Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676. [DOI] [PubMed] [Google Scholar]
- Hopkins, A. M., Walsh, S. V., Verkade, P., Boquet, P., and Nusrat, A. (2003). Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 116, 725–742. [DOI] [PubMed] [Google Scholar]
- Ivanov, A. I., McCall, I. C., Parkos, C. A., and Nusrat, A. (2004a). Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell 15, 2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, A. I., Nusrat, A., and Parkos, C. A. (2004b). Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15, 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. H., Li, Z., and Sacks, D. B. (2000). E-Cadherin-mediated cell-cell attachment activates Cdc42. J. Biol. Chem. 275, 36999–37005. [DOI] [PubMed] [Google Scholar]
- Kovacs, E. M., Ali, R. G., McCormack, A. J., and Yap, A. S. (2002a). E-Cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277, 6708–6718. [DOI] [PubMed] [Google Scholar]
- Kovacs, E. M., Goodwin, M., Ali, R. G., Paterson, A. D., and Yap, A. S. (2002b). Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 12, 379–382. [DOI] [PubMed] [Google Scholar]
- Kovacs, M., Toth, J., Hetenyi, C., Malnasi-Csizmadia, A., and Sellers, J. R. (2004). Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279, 35557–35563. [DOI] [PubMed] [Google Scholar]
- Krendel, M. F., and Bonder, E. M. (1999). Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil. Cytoskeleton 43, 296–309. [DOI] [PubMed] [Google Scholar]
- Lambrechts, A., Van Troys, M., and Ampe, C. (2004). The actin cytoskeleton in normal and pathological cell motility. Int. J. Biochem. Cell Biol. 36, 1890–1909. [DOI] [PubMed] [Google Scholar]
- Low, S. H., Miura, M., Roche, P. A., Valdez, A. C., Mostov, K. E., and Weimbs, T. (2000). Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol. Biol. Cell 11, 3045–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T. Y., Tran, D., Hoa, N., Nguyen, D., Merryfield, M., and Tarnawski, A. (2000). Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc. Res. Tech. 51, 156–168. [DOI] [PubMed] [Google Scholar]
- Maciver, S. K. (1996). Myosin II function in non-muscle cells. Bioessays 18, 179–182. [DOI] [PubMed] [Google Scholar]
- Madara, J. L. (1998). Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60, 143–159. [DOI] [PubMed] [Google Scholar]
- Mandato, C. A., and Bement, W. M. (2001). Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J. Cell Biol. 154, 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter, K., and Balda, M. S. (2003). Signalling to and from tight junctions. Nat. Rev. Mol. Cell. Biol. 4, 225–236. [DOI] [PubMed] [Google Scholar]
- May, R. C. (2001). The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell Mol. Life Sci. 58, 1607–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker, M. S. (1985). Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu. Rev. Cell Biol. 1, 209–241. [DOI] [PubMed] [Google Scholar]
- Morton, W. M., Ayscough, K. R., and McLaughlin, P. J. (2000). Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2, 376–378. [DOI] [PubMed] [Google Scholar]
- Nagafuchi, A., Ishihara, S., and Tsukita, S. (1994). The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J. Cell Biol. 127, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, M., Fukata, M., Yamaga, M., Itoh, N., and Kaibuchi, K. (2001). Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 114, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Nusrat, A., Parkos, C. A., Verkade, P., Foley, C. S., Liang, T. W., Innis-Whitehouse, W., Eastburn, K. K., and Madara, J. L. (2000). Tight junctions are membrane microdomains. J. Cell Sci. 113, 1771–1781. [DOI] [PubMed] [Google Scholar]
- Peterson, J. R., Bickford, L. C., Morgan, D., Kim, A. S., Ouerfelli, O., Kirschner, M. W., and Rosen, M. K. (2004). Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat. Struct. Mol. Biol. 11, 747–755. [DOI] [PubMed] [Google Scholar]
- Pokutta, S., and Weis, W. I. (2002). The cytoplasmic face of cell contact sites. Curr. Opin. Struct. Biol. 12, 255–262. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D., and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465. [DOI] [PubMed] [Google Scholar]
- Prehoda, K. E., and Lim, W. A. (2002). How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2. Curr. Opin. Cell Biol. 14, 149–154. [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T., and Kirschner, M. W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231. [DOI] [PubMed] [Google Scholar]
- Schock, F., and Perrimon, N. (2002). Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 18, 463–493. [DOI] [PubMed] [Google Scholar]
- Stevenson, B. R., and Begg, D. A. (1994). Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J. Cell Sci. 107, 367–375. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., Cheung, A., Limouze, J., Chen, I., Westwood, N. J., Sellers, J. R., and Mitchison, T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743–1747. [DOI] [PubMed] [Google Scholar]
- Symons, M. H., and Mitchison, T. J. (1991). Control of actin polymerization in live and permeabilized fibroblasts. J. Cell Biol. 114, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai, Y., and Nakanishi, H. (2003). Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116, 17–27. [DOI] [PubMed] [Google Scholar]
- Tan, J. L., Ravid, S., and Spudich, J. A. (1992). Control of nonmuscle myosins by phosphorylation. Annu. Rev. Biochem. 61, 721–759. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., Furuse, M., and Itoh, M. (2001). Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell. Biol. 2, 285–293. [DOI] [PubMed] [Google Scholar]
- Turner, J. R. (2000). `Putting the squeeze' on the tight junction: understanding cytoskeletal regulation. Semin. Cell Dev. Biol. 11, 301–308. [DOI] [PubMed] [Google Scholar]
- Turner, J. R., Rill, B. K., Carlson, S. L., Carnes, D., Kerner, R., Mrsny, R. J., and Madara, J. L. (1997). Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273, C1378–C1385. [DOI] [PubMed] [Google Scholar]
- Vaezi, A., Bauer, C., Vasioukhin, V., and Fuchs, E. (2002). Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381. [DOI] [PubMed] [Google Scholar]
- Vartiainen, M. K., and Machesky, L. M. (2004). The WASP-Arp2/3 pathway: genetic insights. Curr. Opin. Cell Biol. 16, 174–181. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V., Bauer, C., Yin, M., and Fuchs, E. (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209–219. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V., and Fuchs, E. (2001). Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 13, 76–84. [DOI] [PubMed] [Google Scholar]
- Verkhovsky, A. B., Svitkina, T. M., and Borisy, G. G. (1997). Polarity sorting of actin filaments in cytochalasin-treated fibroblasts. J. Cell Sci. 110, 1693–1704. [DOI] [PubMed] [Google Scholar]
- Verma, S., Shewan, A. M., Scott, J. A., Helwani, F. M., den Elzen, N. R., Miki, H., Takenawa, T., and Yap, A. S. (2004). Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 279, 34062–34070. [DOI] [PubMed] [Google Scholar]
- Walsh, S. V., Hopkins, A. M., Chen, J., Narumiya, S., Parkos, C. A., and Nusrat, A. (2001). Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology 121, 566–579. [DOI] [PubMed] [Google Scholar]
- Yamada, A., Irie, K., Fukuhara, A., Ooshio, T., and Takai, Y. (2004). Requirement of the actin cytoskeleton for the association of nectins with other cell adhesion molecules at adherens and tight junctions in MDCK cells. Genes Cells 9, 843–855. [DOI] [PubMed] [Google Scholar]
- Yap, A. S., Brieher, W. M., and Gumbiner, B. M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119–146. [DOI] [PubMed] [Google Scholar]