Abstract
Background:
Proper nutrition is known to hasten healing, reduce treatment-related morbidity, and improve outcomes. Children with high-risk solid tumors often have gastrostomy tubes (GTs) placed for supplemental nutrition during cancer therapy. Gastrostomy tubes, however, are not without risks, and many patients develop erythema concerning for infection at the stoma site. Gastrostomy complications are described in the literature, but knowledge regarding this topic is limited.
Methods:
In this retrospective descriptive study, the authors reviewed 3 years of clinical data regarding pediatric patients with solid tumors who had GTs at a pediatric medical center. Descriptive statistics were used to describe the incidence of erythema concerning for infection, identify factors most likely to be associated with this complication, and understand how erythema impacts the completion of cancer therapy.
Results:
In a sample of 58 children with high-risk solid tumors who had GTs placed between 2018 and 2021, 53% developed erythema concerning for infection. More subjects who experienced episodes of GT erythema had neuroblastoma (48%), tubes placed after the start of cancer therapy (74%), and erythema during periods of neutropenia (71%). Only one subject experienced a treatment delay due to GT erythema.
Discussion:
Despite the rate of GT erythema among study subjects, most completed cancer therapy without delay related to this complication. Additionally, the incidence of stoma site erythema was notably less when tubes were placed prior to the start of cancer therapy. Therefore, the authors recommend GT placement prior to therapy start when possible and further attention be paid to this complication during cancer therapy.
Children with high-risk solid tumors who receive systemic chemotherapy or undergo radiation to the head and neck may experience excessive nausea and vomiting, altered taste, anorexia, and mucositis that will dramatically reduce oral intake. When these patients are undernourished, they are at an increased risk for infection, delayed wound healing, treatment toxicity, reduced chemotherapy tolerance, and poor outcomes (Trimpe et al., 2017; Fernandez-Pineda et al., 2016; Sacks et al., 2014; Tenardi et al., 2012). Thus, timely nutrition evaluations and support via gastrostomy tube (GT) are often necessary to optimize caloric intake in this population. Many patients with GTs, however, will experience stoma site erythema concerning for infection (Figure 1). In this retrospective, descriptive study, the authors sought to determine the incidence of GT erythema concerning for infection as well as identify factors that may increase the rate of GT erythema in pediatric patients with high-risk solid tumors. They also explored whether cancer therapy was delayed due to the presence of GT erythema and developed practice recommendations for the placement of GTs in this patient population.
Figure 1.
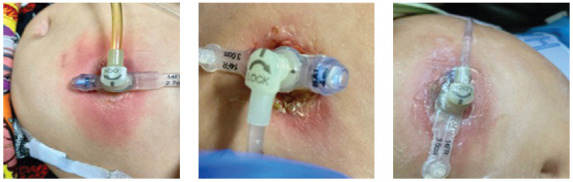
Images of gastrostomy tube erythema. Photographs shared with permission from patient/caregiver.
Patients with GTs may develop erythema concerning for infection in the days following placement or at any point thereafter because the tube is an indwelling foreign body. Unfortunately, the incidence of this complication is elusive because stoma site erythema is often pooled with other GT complications, reported irrespective of timing (post procedure vs. late) or combined with statistics from adult patients. For example, Fernandez-Pineda and colleagues (2016) report 60% of pediatric patients with solid tumors who have GTs experienced related problems, and they cite stoma site infections as the most common complication, occurring more frequently in younger children. Lucendo and colleagues (2014) report the incidence of GT complications in pediatric patients as between 3% and 6%, whereas Zopf and colleagues (2008) describe minor complications including stoma site erythema or infection in 2% to 30% of adult patients. Additionally, the degree of erythema surrounding the GT stoma site may vary and is often subjective, which makes gathering and interpreting data difficult. Given the inconsistent and limited data in the literature, the authors sought to compare the incidence of GT erythema in a large population of pediatric patients with these previous findings.
Certain factors might impact the development of GT erythema in pediatric patients with high-solid tumors including the type of GT. Placement of a Corpak tube, for instance, has been shown to be more cost effective, less invasive, and safer with less morbidity (Blumenstein et al., 2014). Alternatively, the Mic-Key button may be preferable given its convenience and the comfort of not having a long, stiff tube hanging from the abdomen. However, placement of the skin-level button is more invasive, requiring the stomach to be pulled and attached to the abdomen for placement, which takes longer to form a fistulous tract and may result in higher rates of complications (Blumenstein et al., 2014). Given that there is no consensus regarding the preferred GT type in the literature, the authors sought to investigate whether either of the most commonly used GTs increases the risk for stoma site erythema.
The timing of GT placement may also impact the development of stoma site erythema. Patients who undergo GT placement due to massive weight loss secondary to chemotherapy, for instance, endure more complications. This is likely secondary to a decrease in body mass index (BMI) and a decrease in serum albumin, which is necessary for wound healing (Lucendo et al., 2014). Preemptive placement prior to starting cancer therapy, therefore, may be beneficial as patients' immune systems are not yet impacted by myelosuppressive chemotherapy. Lucendo and colleagues (2014) support this theory as they report it takes 2 to 3 weeks for the stoma site to heal and a fistulous tract to form after GT placement. In pediatrics, GT placement is often planned in combination with port-a-cath placement or imaging under general anesthesia or sedation prior to starting cancer therapy. However, the procedure is occasionally delayed until after therapy begins due to scheduling logistics or patients' clinical status. Understanding whether this variable and others cited in the literature (including institutional factors, proceduralist experience, malnutrition, comorbidities, and GT size) will aid in the development of important practice recommendations that will positively impact patient care (Lucendo et al., 2014; Zopf et al., 2008).
METHODS
The authors reviewed 3 years of clinical data from the medical records of pediatric patients with high-risk solid tumors who had GTs placed at the Children's Hospital of Philadelphia (CHOP) between August 2018 and August 2021. CHOP is one of the largest free-standing pediatric medical centers in the world, with a cancer center that sees more than 650 newly diagnosed patients each year. The project was deemed exempt by the hospital institutional review board, so did not require formal review. A quality improvement specialist generated a list of patients with solid tumors who had a GT during the defined study period, which was used for data collection.
Patients were included in the study if they had a solid tumor (diagnosed or relapsed) between August 2018 and August 2021. As care at CHOP is siloed by cancer type, the authors chose to exclude patients with leukemia, lymphoma, and brain tumors to avoid confounding variables. Patients were considered to have a high-risk solid tumor if they had solid tumor malignancies that required myelosuppressive chemotherapy or radiation to the head and neck as treatment. Patients were included in the study if they received their cancer therapy at CHOP and had a GT placed at any point during the study period. Patients were excluded if their GT was placed at an outside institution as the authors would not have access to data regarding tube placement or any relevant complications prior to assuming care at CHOP. The authors confirmed eligibility by carefully examining patients' demographic data, problem lists, and treatment summaries in the electronic medical record.
The authors used REDCap, a secure platform for database development, to store data extracted from the electronic health record. Demographic data collected for the study included subjects' oncologic diagnosis, percentage weight loss at diagnosis or relapse, the timing of GT placement (at cancer diagnosis vs. after therapy start), GT placement location (operating room vs. interventional radiology), and the presence of erythema concerning for infection at the gastrostomy stoma site. If study participants developed erythema concerning for infection, further data were captured, including the timing of each episode of erythema (during a period of neutropenia or not), the number of episodes of erythema, whether erythema led to a delay in cancer therapy, and the use and type of antimicrobial prophylaxis. As there was no standard format for providers and nurses to document erythema concerning for infection at the gastrostomy stoma site, the authors determined the presence of this complication in a qualitative manner. The authors (seasoned nurse practitioners with experience in data extraction) retrieved these data manually from the electronic medical record, reading through study participants' problem lists, outpatient progress notes, hospital admission notes, nursing documentation, and discharge summaries. The authors used descriptive statistics to characterize the study sample.
RESULTS
Demographic Characteristics of the Sample
The authors identified 59 patients with GTs who were diagnosed with a solid tumor or experienced a cancer relapse during the 3-year study period. From this list of patients, 58 pediatric patients—a mix of children, adolescents, and young adults—met the inclusion criteria for diagnosis and GT placement location and timing. Those excluded from the study included one patient who had a GT placed either prior to the study period or at another institution. Of the 58 subjects included in the study, 24 subjects had neuroblastoma (41%) while 17 had osteosarcoma (29%), 9 had Ewing sarcoma (16%), 6 had rhabdomyosarcoma (10%), 1 had Wilms tumor (2%), and 1 subject had hepatoblastoma (2%). Twenty-five subjects in the study (43%) had a GT placed at cancer diagnosis while 33 subjects (57%) had a GT placed after the start of cancer therapy. Seven subjects (12%) had their GT placed in interventional radiology while 51 subjects (88%) had their GT placed in the operating room. The location for GT placement was generally based on availability or the need to combine GT placement with other procedures under the same sedation or anesthesia. Only 3 subjects (5%) had Corpak GTs while the remaining 55 subjects (95%) had Mic-Key button GTs placed during the study period. Gastrostomy tube type was chosen based on availability and proceduralist preference. Demographic information is summarized in Table 1.
Table 1. Breakdown of Patient Diagnoses Included in the Study.
| Diagnosis | Count |
|---|---|
| Neuroblastoma | 24 |
| Osteosarcoma | 17 |
| Ewing sarcoma | 9 |
| Rhabdomyosarcoma | 6 |
| Wilms tumor | 1 |
| Hepatoblastoma | 1 |
| Total | 58 |
Erythema Concerning for Infection
Thirty-one subjects (53%) experienced at least one episode of erythema concerning for infection at their gastrostomy stoma site during the study period. Most of the subjects who experienced episodes of erythema had neuroblastoma (48%), GTs placed after treatment start (74%), and Mic-Key buttons placed in the operating room. Most subjects developed erythema during periods of neutropenia (71%). While 87% of subjects who developed erythema concerning for infection at the gastrostomy stoma site were not given prophylactic antibiotics following the first episode, those who were given prophylactic antibiotics (13%) experienced more episodes of erythema and were most often given oral clindamycin as prophylaxis. Only 1 out of the 31 subjects with erythema concerning for infection experienced a cancer therapy delay due to the complication.
When broken down by diagnosis, the authors found that subjects with neuroblastoma experienced erythema concerning for infection at a rate of 63%. Eighty-eight percent of those who developed erythema had their GT placed after chemotherapy start. Alternatively, only 29% of subjects with osteosarcoma developed erythema concerning for infection and most of these subjects had their tubes placed prior to chemotherapy start. The one subject with osteosarcoma whose GT was placed after chemotherapy start developed erythema concerning for infection. Fifty-six percent of subjects with Ewing sarcoma developed GT erythema concerning for infection, approximately half of whom had their tubes placed after chemotherapy start. Subjects with rhabdomyosarcoma developed GT erythema at a rate of 83%, and most subjects had GTs placed after treatment start. The one subject with rhabdomyosarcoma who had their GT placed at diagnosis did not develop erythema. Finally, the one patient with Wilms tumor who developed erythema received higher risk therapy and had their GT placed after chemotherapy start. Figure 2 describes GT erythema by cancer diagnosis. Figure 3 further displays the difference in GT erythema based on timing of tube placement.
Figure 2.
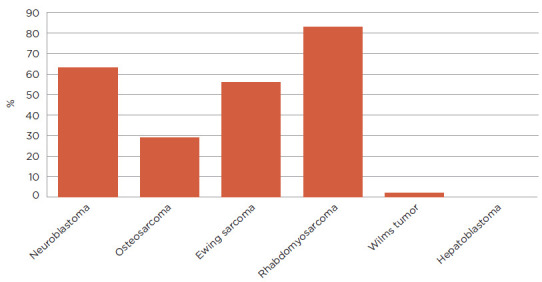
Rate of gastrostomy tube erythema by cancer diagnosis.
Figure 3.
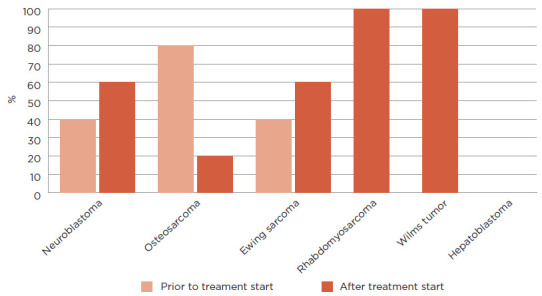
Percentage of patients with erythema based on timing of gastrostomy tube placement. A total of 53% (31/58) solid tumor patients developed erythema at their GT site. Fifteen patients with Neuroblastoma developed erythema with 40% (6/15) of these patients having the GT placed prior to treatment start and 60% (9/15) having it placed after treatment start. Five patients with osteosarcoma had erythema with 80% (4/5) of patients having their GT placed prior to treatment start and 20% (1/5) having it placed after treatment start. Five patients with erythema had Ewing sarcoma with 40% (2/5) having their GTs placed prior to start of treatment and 60% (3/5) having it placed after treatment start. Five patients with GT erythema had rhabdomyosarcoma with all 5 (100%) patients having their GT placed after treatment start. One patient with Wilms tumor had a GT placed after treatment start and developed erythema (100%). Lastly, no patients with hepatoblastoma experienced GT erythema.
DISCUSSION
The results from this study reveal that erythema concerning for infection is a relatively frequent complication for children with GTs present during cancer therapy. It does not appear to be a terribly morbid complication given only one study subject experienced a therapy delay due to concern for infection. Most subjects who developed erythema had their GTs placed after starting cancer therapy, which the authors hypothesize is related to the short interval between tube placement and myelosuppression from chemotherapy. Many subjects experienced these episodes during periods of neutropenia when they are already at higher risk for mucositis, stomatitis, and infection due to rapid cell turnover and a decrease or absence of infection-fighting white blood cells. Ultimately, the development of erythema appears more related to GT placement timing and the nature of cancer therapy than the oncologic diagnosis.
Most subjects in the study did not receive prophylactic antibiotics to prevent episodes of erythema at the GT site concerning for infection. Subjects who did receive prophylactic antibiotics experienced more episodes of erythema concerning for infection. The use of antibiotics may or may not have impacted outcomes for these subjects. Given the risk of antibiotic side effects, the chances of developing antimicrobial resistance, and the likelihood that most of these episodes are due to mucositis or stomatitis rather than infection, the use of prophylactic antibiotics is not recommended and should be avoided if possible.
It is difficult to quantify exactly how many patients may require or benefit from GT placement as providers define high-risk solid tumors and perceive the risks and benefits of enteral nutrition differently across institutions. However, nutrition assessment and early enteral feedings support patients' nutritional status and possibly bolster outcomes in children with high-risk solid tumors (Sacks et al., 2014; Schmitt et al., 2012). Therefore, the authors recommend nutrition evaluation at diagnosis and early GT placement to facilitate enteral feedings when feasible. Given the high rate of GT erythema concerning for infection among subjects in this study (especially among those who had GTs placed after the start of cancer therapy) the authors recommend the placement of GTs at cancer diagnosis, before patients' immune systems are suppressed by chemotherapy. Optimizing GT timing based on these study findings will facilitate surgical site and stoma healing as well as fistulous track formation with a goal of reducing the risk of GT erythema concerning for infection in pediatric patients with high-risk solid tumors.
STUDY LIMITATIONS
Several factors may have impacted the study results. First, there were several variables not captured during data collection that might have added to the interpretation of study results. For instance, variables including subject age, comorbidities, degree, and duration of neutropenia during episodes of erythema, GT size, proceduralist experience, and the interval between GT placement and onset of erythema were not extracted during data collection. Second, while most subjects had a nutrition evaluation at cancer diagnosis, it was difficult to find the percentage weight loss or degree of malnutrition in the documentation. Since nutritional status is related to healing and tolerance of chemotherapy, these data points may have helped to determine which subjects were at higher risk for erythema at the GT site. Third, the presence and degree of erythema were deemed and documented as such by providers in a subjective manner. Thus, the authors were not able to document the severity or determine the presence of variability in clinical presentation among subjects. Next, only two methods of GT placement and two GT brands (Corpak and Mic-Key) were reviewed due to proceduralist preference and hospital supply, so it may be difficult to generalize the results of this study to other institutions. Finally, there is a dearth of literature on this topic and many of the available articles are outdated. Thus, it is possible the procedure to place GTs, procedure location, type of GTs, postoperative management, and GT care have evolved since the publication of many of the articles.
IMPLICATIONS FOR ADVANCED PRACTICE PROVIDERS
Advanced practice providers (APPs) play a vital role in the nutritional assessment and care of pediatric patients with high-risk solid tumors, especially as advocates for nutrition optimization and education (Montgomery et al., 2016). For instance, APPs might prioritize obtaining accurate heights and weights at cancer diagnosis and throughout therapy as these measurements reflect patients' nutritional status. Advanced practice providers might also advocate for early nutrition evaluation and support clinical dieticians' recommendations for nutritional optimization and early GT placement (prior to treatment start) in patients with high-risk solid tumors. They should also educate members of the interprofessional team, patients, and families regarding the importance of nutrition in cancer therapy and seek to conduct large, multisite studies to further describe the use of GTs in pediatric patients with high-risk solid tumors, develop unbiased methods to assess GT erythema concerning for infection, and explore other GT complications in children with cancer.
CONCLUSION
There exist many benefits to providing early enteral nutrition to pediatric patients with high-risk solid tumors. Gastrostomy tube placement, however, is not without risk, including the development of erythema at the stoma site concerning for infection. The aim of this single site, retrospective descriptive study was to document the incidence and potential risk factors for the development of erythema concerning for infection in children with high-risk solid tumors. Based on the findings from this study, the authors recommend the interprofessional team prioritize GT placement prior to treatment start, avoid the use of prophylactic antibiotics when possible, and advocate for further study on this topic. Advanced practice providers play key roles in the care of these patients and should continue to partner with other members of the interprofessional team (including clinical dieticians) to advocate for nutritional optimization, educate team members and families regarding the importance of nutrition in cancer care, as well as use the results of this study to fuel further investigations to improve clinical care for children with cancer.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Blumenstein, I., Shastri, Y. M., & Stein, J. (2014). Gastroenteric tube feeding: Techniques, problems and solutions. World Journal of Gastroenterology, 20(26), 8505–8524. https://doi.org/10.3748%2Fwjg.v20.i26.8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pineda, I., Sandoval, J. A., Jones, R. M., Boateng, N., Wu, J., Rao, B. N.,…Shochat, S. J. (2016). Gastrostomy complications in pediatric cancer patients: A retrospective single-institution review. Pediatric Blood Cancer, 63(7), 1250–1253. 10.1002/pbc.25968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucendo, A. J., & Friginal-Ruiz, A. B. (2014). Percutaneous endoscopic gastrostomy: An update on its indications, management, complications, and care. Revista Espanola De Enfermedades Digestivas, 106(8), 529–539. 10.1097/SGA.0000000000000150 [DOI] [PubMed] [Google Scholar]
- Montgomery, K., Belongia, M., Schulta, C., Mulberry, M. H., Nugent, M. L., & Simpson, P. M. (2016). Health care providers' perceptions of nutrition support in pediatric oncology and hematopoietic stem cell transplant patients. Journal of Pediatric Oncology Nursing, 33(4), 265–272. 10.1177/1043454215616604 [DOI] [PubMed] [Google Scholar]
- Sacks, N., Hwang, W. T., Lange, B. J., Tan, K., Sandler, E. S., Rogers, P. C.,…Rheingold, S. R. (2014). Proactive enteral tube feeding in pediatric patients undergoing chemotherapy. Pediatric Blood & Cancer, 61(2), 281–285. 10.1002/pbc.24759 [DOI] [PubMed] [Google Scholar]
- Schmitt, F., Caldari, D., Corradini, N., Gicquel, P., Lutz, P., Leclair, M. D., & Podevin, G. (2012). Tolerance and efficacy of preventive gastrostomy feeding in pediatric oncology. Pediatric Blood & Cancer, 59(5), 874–880. 10.1002/pbc.24161 [DOI] [PubMed] [Google Scholar]
- Tenardi, R. D., Frühwald, M. C., Jürgens, H., Hertroijs, D., & Bauer, J. (2012). Nutritional status of children and young adults with Ewing sarcoma or osteosarcoma at diagnosis and during multimodality therapy. Pediatric Blood & Cancer, 59(4), 621–626. 10.1002/pbc.24001 [DOI] [PubMed] [Google Scholar]
- Trimpe, K., Shaw, M. R., Wilson, M., & Haberman, M. R. (2017). Review of the effectiveness of enteral feeding in pediatric oncology patients. Journal of Pediatric Oncology Nursing, 34(6), 439–445. 10.1177/1043454217712982 [DOI] [PubMed] [Google Scholar]
- Zopf, Y., Konturek, P., Nuernberger, A., Maiss, J., Zenk, J., Iro, H., Hahn, E. G., & Schwab, D. (2008). Local infection after placement of percutaneous endoscopic gastrostomy tubes: A prospective study evaluating risk factors. Canadian Journal of Gastroenterology, 22(12), 987–991. 10.1155/2008/530109 [DOI] [PMC free article] [PubMed] [Google Scholar]


