Abstract
Cold agglutinin disease (CAD) is a rare type of autoimmune hemolytic anemia (AIHA) distinct from warm antibody AIHA. One of the ways it is distinct is that CAD is usually not responsive to corticosteroids compared with warm antibody AIHA. Historically, CAD therapy has been limited to immunotherapy or chemoimmunotherapy with varying responses. Cold agglutinin disease also poses a risk for thrombosis and mortality. For patients, fatigue tends to be a common symptom of CAD. The hallmark of CAD is complement-mediated hemolysis, which makes complement inhibitors a critical therapeutic option for patients. Previously, eculizumab, a C5 inhibitor, had limited therapeutic effect for CAD. More recently, sutimlimab, a C1s inhibitor, was shown in two phase III studies to be an efficacious treatment for CAD, improving hemoglobin, hemolysis, and fatigue. However, there is a paucity of medical literature on CAD and on sutimlimab in particular that is geared toward advanced practice providers (APPs). This article aims to provide APPs with a background in CAD and a focus on sutimlimab, assisting these providers in caring for patients with CAD receiving this therapy.
Cold agglutinin disease (CAD) is a rare form of autoimmune hemolytic anemia (AIHA). It makes up 15% to 30% of AIHAs (Sokol et al., 1981). Its prevalence is 5 to 20 cases per million, and incidence is 2 cases per million per year (Moore & Arnall, 2023). Cold agglutinin disease is twice as common in females compared with males. It is unique because the antibody that causes red blood cell (RBC) hemolysis causes agglutination at colder temperatures than warm antibody AIHAs. Some cold agglutinins only bind at temperatures below body temperature and do not cause clinical hemolytic anemia. However, the cold agglutinins can bind to RBCs and induce hemolysis at body temperatures. A thermal amplitude test can be done to differentiate these, although usually, this is unnecessary as hemolysis markers can be done to look for clinical hemolytic anemia. While hemolysis in CAD has been thought to be more common during colder seasons and climates, it can also occur during warmer seasons, as hemolysis occurs at body temperatures in many cases (Berentsen et al., 2022). Additional clinical consequences of CAD include an increased risk of thrombotic events, mortality, and circulatory symptoms (acrocyanosis and Raynaud phenomenon). These circulatory symptoms are usually associated with cold environmental exposures (Broome et al., 2020; Bylsma et al., 2019).
In addition, CAD is diagnosed in the setting of hemolytic anemia with a monospecific direct antiglobulin test (DAT) strongly positive for complement C3d and negative or weakly positive for IgG and a cold agglutinin titer of 64 or greater at 4° Celsius (Berentsen & Barcellini, 2021). Cold agglutinin disease is usually associated with a CAD-associated lymphoproliferative disorder (Berentsen et al., 2020). The binding of these cold agglutinins to the surface of RBCs leads to agglutination and triggers complement-mediated hemolysis by activating the classical complement pathway (Figure 1), leading to additional burdens on quality of life, such as fatigue.
Figure 1.
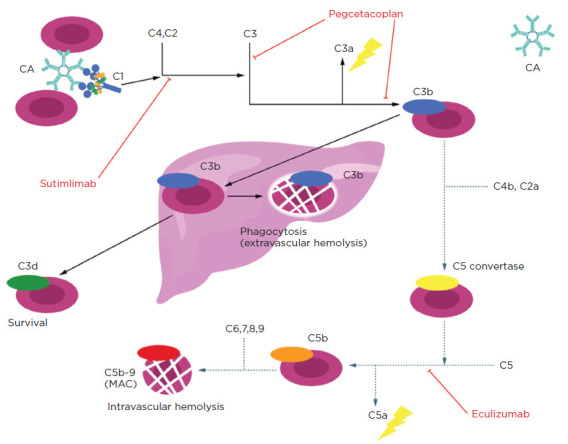
Complement pathway inhibition. Sutimlimab inhibits the pathway at C1s and can inhibit both the classical and alternate complement pathways, unlike eculizumab. Reproduced from Berentsen et al. (2022).
Another similar entity is cold agglutinin syndrome (CAS), which is, by definition, associated with another underlying disease, such as aggressive lymphoma or infection (Epstein-Barr virus or Mycoplasma pneumonia). Cold agglutinin syndrome is usually self-remitting within 4 to 6 weeks in cases of infection but can be problematic when associated with an overt malignancy. Therefore, it is imperative to rule out aggressive lymphoma and infection in patients suspected of having CAD to differentiate it from CAS.
Unlike other AIHAs, CAD does not tend to respond therapeutically to corticosteroids, with a low response rate of less than 20% (Table 1; Röth et al., 2021; Khellaf et al., 2014). However, rituximab (Rituxan), a CD20 monoclonal antibody, can be administered weekly for 4 to 8 weeks. Only 50% of patients who received monotherapy rituximab achieved partial remission (PR) for 6.5 to 11 months (Röth et al., 2021). Rituximab can take up to 6 weeks before any improvement is seen in hemolysis labs with a variable response. Rituximab also poses a major infection risk for older adults. In previous studies, the risk of infection with the use of rituximab for immune thrombocytopenia therapy is increased in populations over age 70, including the risk of severe infection and death. Patients with CAD are usually in this older adult age group. Therefore, infection prevention with rituximab is imperative (Khellaf et al., 2014). With its mechanism of action on B cells and resulting B-cell depletion, rituximab can be associated with impaired response to vaccination, hepatitis B reactivation, and progressive multifocal encephalopathy. In a real-world setting in the US, rituximab was predominantly (> 95%) given as monotherapy for CAD, with an average of 1.45 courses per patient. In this real-world analysis, the hemolysis markers such as hemoglobin (Hgb), bilirubin, and lactate dehydrogenase (LDH) level improvements were sustained for only a median of 44, 98, and 93 days, respectively (Piatek et al., 2023).
Table 1. Cold Agglutinin Disease Response Criteria.
Complete response
|
Partial response
|
Note. CAD = cold agglutinin disease; Hbg = hemoglobin. Treatment response criteria for CAD trials evaluating rituximab monotherapy and combination therapy with bendamustine. Information from Röth et al. (2021); Khellaf et al. (2014)
For a more sustained remission, medically fit patients can be treated with four (28-day) cycles of bendamustine plus rituximab (BR). Per the Nordic trial, BR can provide a response in 78% of patients (35 out of 45), with 53% achieving a complete remission (CR) and 24% a PR. An estimated 5-year remission was found to be at 77%. While other chemoimmunotherapy options have been studied, BR is the most effective in treating CAD (Berentsen et al., 2020). However, BR is not always viable for CAD patients because these patients, again, tend to be older and have comorbidities.
Complement inhibitors are another therapeutic option for patients with CAD. Eculizumab (Soliris) is a C5 complement inhibitor, but this does not inhibit C3b-mediated, extravascular hemolysis (Figure 1; Berentsen et al., 2022). The data is also limited to a small, open-label, phase II trial for C5 inhibition (Röth et al., 2018). Sutimlimab (Enjaymo), a C1s complement inhibitor, is first in its class and is designed to inhibit classical pathway activation and mediated hemolysis. Sutimlimab received US Food and Drug Administration (FDA) approval for the treatment of hemolysis in adults with CAD on February 4, 2022 (Sanofi, 2023).
SUTIMLIMAB
Sutimlimab is an intravenous, immunoglobulin G4 (IgG4), humanized monoclonal antibody that selectively inhibits C1s protein, which is responsible for activating the classical complement pathway (Figure 1; Berentsen et al., 2022). This inhibition prevents the opsonization (deposition of complement opsonins) of erythrocytes, leading to inhibition of cold agglutinin–induced hemolysis (Dhillon, 2022). Sutimlimab does not prevent circulatory symptoms such as acrocyanosis and Raynaud phenomenon. The dose is 6.5 g for a weight between 39 kg to 74 kg and 7.5 g for a weight of 75 kg or greater. The frequency of the sutimlimab infusion is weekly for the first two doses and then every 14 days after that. Following the first two doses, if the interval between subsequent doses exceeds 17 days, the patient would need to repeat the two weekly doses of sutimlimab before resuming every-14-day dosing (Sanofi, 2023).
Two significant studies (CARDINAL and CADENZA) led to the FDA approval of sutimlimab. The CARDINAL study was a 26-week, multicenter study to evaluate the safety and efficacy of sutimlimab in CAD patients with a recent RBC transfusion history. The 24 patients included in the trial were typical for a population with CAD, as 62% were women with a median age of 72. The patients' mean number of transfusions the year before enrolling in the study was 4.8 ± 6.2 (median 2, range 1 to 23). The mean baseline Hgb level was 8.6 ± 1.6 g/dL. According to the CARDINAL study, the classical complement pathway was completely inhibited within 1 week of starting sutimlimab. The composite primary endpoint was a normalization of the Hgb level to ≥ 12 g/dL or an increase of Hgb level of ≥ 2 g/dL from baseline in the absence of RBC transfusion or prohibited medications (Röth et al., 2021). In the CARDINAL study, 13 of 24 patients (54%; 95% confidence interval [CI] = 33–74) met the composite primary endpoint. Among 11 patients who did not meet the prespecified criteria for the primary endpoint, six had evidence of treatment response: four patients had an increase in the Hgb level of ≥ 1 g/dL over the study period; one patient had an increase of ≥ 2 g/dL and a level of ≥ 12 g/dL at the time of treatment assessment, and one patient had a change from 11 g/dL to 11.8 g/dL at the time of treatment assessment in the absence of transfusion. The bilirubin level had also normalized in four of these six patients during the treatment period. Overall, the mean increase in Hgb was 2.6 g/dL (95% CI = 0.7–4.5) at the time of treatment assessment. The mean Hgb level increased by 1.2 ± 1.3 g/dL within the first week of treatment and by 2.3 ± 1.5 g/dL by the third week. The mean Hgb level was maintained at more than 11 g/dL from week three until the end of the study. Hemolysis markers rapidly normalized in the sutimlimab cohort (Röth et al., 2021).
CADENZA was a phase III, randomized, double-blind, placebo-controlled study to assess the efficacy and safety of sutimlimab in patients with primary CAD without a recent (within 6 months) history of blood transfusion. The 42 patients in the trial had disease characteristics consistent with a CAD population. Seventy-nine percent were female, with a median age of 66 years. At baseline, the mean Hgb level was 9.2 g/dL and 9.3 g/dL for the sutimlimab and placebo arms, respectively. The composite primary endpoint was an increase in Hgb from a baseline of ≥ 1.5 g/dL in the absence of RBC transfusion and prohibited mediations (Röth et al., 2022). A total of 16 patients (73%) receiving sutimlimab compared with 3 patients (15%) receiving placebo met the composite primary endpoint (odds ratio [OR], 15.9; 95% CI = 29–88). Three patients in the sutimlimab arm did not meet the response criteria. Two of these patients had an increase of Hgb ≥ 1.5 g/dL over baseline on at least one occasion that was not associated with an RBC transfusion. All 16 patients treated with sutimlimab had increased Hgb levels ≥ 2 g/dL from baseline, compared with two patients on placebo. In this patient population that was not heavily RBC transfusion-dependent, RBC transfusions were still fewer in the sutimlimab group than in the placebo-treated patients. The Hgb increase occurred within 3 weeks of sutimlimab administration and was sustained over the 26-week treatment period. In comparison, no meaningful changes were reported in the placebo-treated patients. An increase in Hgb was 2.66 g/dL (95% CI = 2–3.22) and 0.09 g/dL (95% CI = –0.5–0.68) for the sutimlimab arm and the placebo group, respectively. After the first administration of sutimlimab, the Hgb increased from baseline by 1.2 g/dL within the first week and 2 g/dL by the third week. The mean Hgb level was maintained at more than 11 g/dL from week 3 until the end of the study in the sutimlimab arm. Like the CARDINAL study, hemolysis markers rapidly normalized in the sutimlimab cohort (Röth et al., 2022).
As fatigue is commonly reported as the primary complaint by patients with primary CAD, assessing the impact on this outcome is imperative. In the CARDINAL and CADENZA trials, fatigue was assessed by using the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-Fatigue) scale (Hill et al., 2021). The FACIT-Fatigue scale is a 13-item instrument that assesses fatigue and its impact on an individual's daily activities and functioning, with higher scores representing a better quality of life (Acaster et al., 2015). Notably, patient-reported fatigue was improved during sutimlimab treatment in the CARDINAL open-label study (mean score on the FACIT-Fatigue Scale at baseline, 32.5; mean score at the time of treatment assessment, 44.3). The reduction in fatigue was seen by the first week, with a mean difference of 7.2 points from baseline, a change in fatigue that was maintained throughout the study. The estimated mean increase in the score on the FACIT-Fatigue scale in all patients was 10.9 points (95% CI = 8.0–13.7) by the end of the CARDINAL study. Similarly, sutimlimab displayed improvements in FACIT-Fatigue scores by the end of the first week of treatment in the CADENZA trial. Patients treated with sutimlimab reported fatigue improvement by approximately 5 points by week 1, which is clinically meaningful compared with no change for the placebo cohort. This improvement in fatigue was maintained throughout the CADENZA study, with the overall score difference between the sutimlimab and placebo arms at the study endpoint being 8.9 points (95% CI = 4–13.85; Figure 2; Röth et al., 2022). A FACIT-Fatigue score increase of 5 points was estimated to be a clinically meaningful change in patients with CAD, which is consistent with estimates of between 2 and 10 points for other disease areas (e.g., rheumatoid arthritis, systemic lupus erythematosus, anemia related to cancer; Hill et al., 2021). Lastly, it is essential to note that the improvements in Hgb, hemolysis labs, and fatigue from sutimlimab last as long as the patient continues to receive this therapy.
Figure 2.
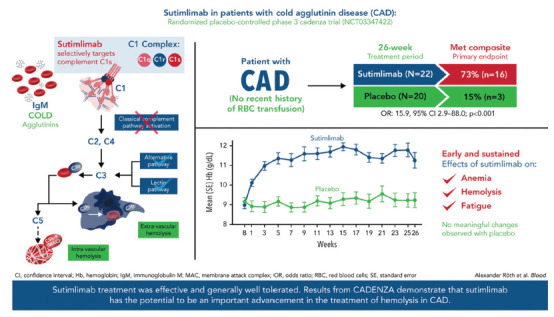
CADENZA trial results. Visual abstract demonstrating results from the CADENZA trial. The CADENZA trial was a 26-week randomized, placebo-controlled phase III study to assess safety and efficacy of sutimlimab in patients with CAD without recent (within 6 months prior to enrollment) transfusion history. Reproduced from Röth et al. (2022).
In the CARDINAL trial, the most frequent adverse events were infections and infestations, headache, dizziness, gastrointestinal disorders, fatigue, peripheral edema, pyrexia, arthralgia, hypertension, acrocyanosis, cough, and infusion-related reactions (Röth et al., 2021). The adverse events reported in the CADENZA study from the sutimlimab cohort included headache, hypertension, rhinitis, Raynaud phenomenon, and acrocyanosis (Röth et al., 2022). Three patients in the sutimlimab arm of the CADENZA trial experienced treatment-emergent serious adverse events, including febrile infection and increased blood IgM, Raynaud phenomenon, and cerebral venous thrombosis (Röth et al., 2022). The adverse events were consistent with an elderly and medically compromised patient population enrolled in CADENZA through randomization. No clinical evidence of systemic lupus erythematosus, autoimmune disease, or meningococcal infections was observed in patients after the administration of sutimlimab in either trial (Röth et al., 2021, 2022).
A recent combined safety analysis of the phase III CARDINAL and CADENZA studies demonstrated that sutimlimab was generally well tolerated, with the type and frequency of adverse events consistent with an older and medically complex population, and that COVID-19 vaccination response was not impaired, and there was no need to modify the sutimlimab dosing schedule (Broome et al., 2023; Fattizzo et al., 2024). While patients with CAD are at risk for a thromboembolic event (TE), a post-hoc analysis of the CARDINAL and CADENZA studies (with a small number of events) reported a nonsignificant 40% reduction in TE events after initiation of treatment with sutimlimab (Röth et al., 2023).
SUPPORTIVE CARE FOR SUTIMLIMAB
There are essential things to consider for the advanced practice provider (APP) who cares for a patient with CAD, particularly for a patient receiving sutimlimab. As sutimlimab blocks the complement cascade, these patients need to receive vaccinations for encapsulated bacteria (Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae) at least 2 weeks prior to starting this treatment per the Advisory Committee on Immunization Practices (ACIP) recommendations (Table 2; Moore & Arnall, 2023; Murthy et al., 2022; Kroger et al., 2022). Per the package insert, these vaccines do not eliminate but rather lessen the risk of encapsulated bacterial infections (Sanofi, 2023). These infections have not been seen in patients receiving sutimlimab, likely due to this preventative strategy based on earlier eculizumab experiences. It is also vital to ensure that the complete blood count lab specimens are handled with special procedures for CAD patients per your institution's laboratory to prevent ex vivo hemolysis and inaccurate laboratory results. Lastly, it is imperative to keep patients with CAD warm during the infusion visits and ensure that intravenous fluids and blood products are at least room temperature or warmer. The use of in-line infusion warmers is recommended, when available, to prevent agglutination and hemolysis.
Table 2. Prophylactic Vaccine Schedule.
| Time | Vaccine(s) |
|---|---|
| Day –14 |
|
| Day 14 |
|
| Day 42 |
|
Note. Advisory Committee on Immunization Practices recommendations in patients with persistent complement component deficiencies and patients treated with complement inhibitors. Information from Murthy et al. (2022); Kroger et al. (2022).
In the clinical setting at the James Cancer Hospital, patients receiving sutimlimab have reported mild fatigue, mild nausea, mild headaches, and mild brain fog the evening of their sutimlimab infusions. If these side effects occur, they usually resolve by the next morning or within 24 hours of the sutimlimab infusions and do not require any intervention. One patient who reported mild nausea following the sutimlimab treatment did not require any antiemetic therapy. The APP providing education about these side effects and their self-limiting nature can be helpful to patients and their families. Like the CARDINAL and CADENZA study findings, patients at the James Cancer Hospital report that their fatigue improves with sutimlimab. This improvement can occur even after the first infusion of sutimlimab. Unlike other monoclonal antibody treatments used at the James Cancer Hospital, infusion reactions have not been observed with sutimlimab. Overall, sutimlimab is a tolerable and effective treatment for CAD.
CONCLUSION
Cold agglutinin disease is a rare and complex form of AIHA. Sutimlimab is an important therapeutic option for CAD patients. Hopefully, further research will introduce additional efficacious complement inhibitor options for this patient population.
Footnotes
Mr. Reid has no conflicts of interest to disclose. Dr. Fedutes Henderson is an employee of Sanofi.
References
- Acaster, S., Dickerhoof, R., DeBusk, K., Bernard, K., Strauss, W., & Allen, L. F. (2015). Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health and Quality of Life Outcomes, 13, 60. 10.1186/s12955-015-0257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berentsen, S., & Barcellini, W. (2021). Autoimmune hemolytic anemias. New England Journal of Medicine, 385(1), 1407–1419. 10.1056/NEJMra2033982 [DOI] [PubMed] [Google Scholar]
- Berentsen, S., Barcellini, W., D'Souza, S., & Jilma, B. (2022). Sutimlimab for treatment of cold agglutinin disease: why, how and for whom? Immunotherapy, 14(15), 1191–1204. 10.2217/imt-2022-0085 [DOI] [PubMed] [Google Scholar]
- Berentsen, S., Barcellini, W., D'Sa, S., Broome, C., Jilma, B., Michel, M.,…Tjonnfirord, G. E. (2020). Cold agglutinin disease revisited: A multi-national, observational study of 232 patients. Blood, 136(4), 480–488. 10.1182/blood.2020005674 [DOI] [PubMed] [Google Scholar]
- Berentsen, S., D'Souza, S., Randan, U., Malecka, A., & Vos, J. (2022). Cold agglutinin disease: Improved understanding of pathogenesis helps define targets for therapy. Hemato, 3, 574-594. 10.3390/hemato3040040 [DOI] [Google Scholar]
- Broome, C., Barcellini, W., Ueda, Y., Khan, U., Patel, R., Wardęcki, M.,…Roeth, A. (2023). Combined safety data for sutimlimab in cold agglutinin disease: A post hoc analysis of the phase 3 CARDINAL and CADENZA studies. Blood, 142(Supplement 1), 3833. 10.1182/blood-2023-188686 [DOI] [Google Scholar]
- Broome, C., Cunningham, J., Mullins, M., Jiang, X., Bylsma, L., Fryzek, J., & Rosenthal, A. (2020). Increased risk of thrombotic events in cold agglutinin disease: A 10-year retrospective analysis. Research and Practice in Thrombosis and Haemostasis, 4(4), 628–635. 10.1002/rth2.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma, L., Gulbech Ording, A., Rosenthal, A., Öztürk, B., Fryzek, J., Morales Arias, J.,…Berentsen, S. (2019). Occurrence, thromboembolic risk, and mortality in Danish patients with cold agglutinin disease. Blood Advances, 3(20), 2980–2985. 10.1182/bloodadvances.2019000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon, S. (2022). Sutimlimab: First approval. Drugs, 82(7), 817–823. 10.1007/s40265-022-01711-5 [DOI] [PubMed] [Google Scholar]
- Fattizzo, B., Röth, A., Broome, C. M., Khan, U., Wardęcki, M., Cordoba, M., & Barcellini, W. (2024). COVID-19 vaccine safety and immunogenicity in patients with cold agglutinin disease receiving concomitant sutimlimab. American Journal of Hematology, 99, 789–791. 10.1002/ajh.27234 [DOI] [PubMed] [Google Scholar]
- Hill, Q. A., Röth, A., Jilma, B., Broome, C., Berentsen, S., Rizio, A.,…Cella, D. (2021). Fatigue score for patients with cold agglutinin disease: An analysis using the phase 3 CARDINAL and CADENZA studies. EHA Library. https://library.ehaweb.org/eha/2021/eha2021-virtualcongress/324900/quentin.a.hill.clinically.important.change.in.facit-fatigue.score.for.patients.html
- Khellaf, M., Charles-Nelson, A., Fain, O., Terriou, L., Viallard, J. F., Cheze, S.,…Godeau, B. (2014). Safety and efficacy of rituximab in adult immune thrombocytopenia: Results from a prospective registry including 248 patients. Blood, 124(22), 3228–3236. 10.1182/blood-2014-06-582346 [DOI] [PubMed] [Google Scholar]
- Kroger, A., Bahta, L., & Hunter, P. (2022). General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP): Altered Immunocompetence General Best Practice Guidelines for Immunization. CDC. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html.
- Moore, D., & Arnall, J. (2023). Sutimlimab: A complement C1s inhibitor for the management of cold agglutinin disease-associated hemolysis. Annals of Pharmacotherapy, 57(8), 970-977. 10.1177/10600280221138802 [DOI] [PubMed] [Google Scholar]
- Murthy, N., Wodi, A. P., Bernstein, H., & Ault, K. A. (2022). Recommended adult immunization schedule, United States. Annals of Internal Medicine, 175(3), 432–443. 10.7326/M22-0036 [DOI] [PubMed] [Google Scholar]
- Piatek, C., Murakhovskaya, I., Karaouni, A., Miles, G., Heller, C., Lucia, J.,…Gertz, M. (2023). A U.S. retrospective observational study of rituximab use in cold agglutinin disease. Blood, 142(Supplement 1), 3835. 10.1182/blood-2023-172563 [DOI] [Google Scholar]
- Röth, A., Barcellini, W., D'Sa, S., Miyakawa, Y., Broome, C. M., Michel, M.,…Berentsen, S. (2021). Sutimlimab in cold agglutinin disease. New England Journal of Medicine, 384(14), 1323–1334. 10.1056/NEJMoa2027760 [DOI] [PubMed] [Google Scholar]
- Röth, A., Berensten, S., Barcellini, W., D'Sa, S., Jilma, B., Michel, M.,…Broome, C. (2022). Sutimlimab in patients with cold agglutinin disease: Results of the randomized placebo-controlled phase 3 CADENZA trial. Blood, 140(9), 980–991. 10.1182/blood.2021014955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röth, A., Bommer, M., Hüttmann, A., Herich-Terhürne, D., Kuklik, N., Rekowski, J.,…Dührsen, U. (2018). Eculizumab in cold agglutinin disease (DECADE): An open-label, prospective, bicentric, nonrandomized phase 2 trial. Blood Advances, 2(19), 2543–2549. 10.1182/bloodadvances.2018024190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röth, A., Ueda, Y., McCrae, K., Khan, U., Kralova, K., Wardęcki, M.,…Broome, C. (2023). Thromboembolic events in cold agglutinin disease: A post hoc analysis pre- and on-sutimlimab treatment in the phase 3 CARDINAL and CADENZA studies. Hämostaseologie, 44, 17–18. 10.1055/s-0044-1779082 [DOI] [Google Scholar]
- Sanofi. (2023). Enjaymo (sutimlimab) package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761164s003lbl.pdf
- Sokol, R. J., Hewitt, S., & Stamps, B. K. (1981). Autoimmune hemolysis: An 18-year study of 865 cases referred to a regional transfusion centre. British Medical Journal (Clinical Research Edition), 282, 2023–2027. 10.1136/bmj.282.6281.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]


