Abstract
Neutrophil (polymorphonuclear leukocytes [PMN]) transepithelial migration during inflammatory episodes involves a complex series of adhesive interactions and signaling events. Previous studies have shown that key adhesive interactions between leukocyte CD11b/CD18 and basally expressed fucosylated glycoproteins followed by binding to desmosomal-associated JAM-C are key elements of the transmigration response. Here we provide the first evidence that PMN-expressed junctional adhesion molecule-like protein (JAML) regulates transmigration via binding interactions with epithelial coxsackie and adenovirus receptor (CAR). Experiments with a JAML fusion protein revealed specific binding of JAML to epithelial CAR expressed at tight junctions in T84 cell monolayers and normal human colonic mucosa. Furthermore, JAML-CAR binding is mediated via the membrane distal immunoglobulin (Ig) loop of CAR and the membrane proximal Ig loop of JAML. PMN bound to immobilized CAR but not JAML in a divalent cation-independent manner. Lastly, in assays of PMN transepithelial migration, JAML/CAR fusion proteins and their antibodies significantly inhibited transmigration in a specific manner. Taken together, these results indicate that JAML and CAR are a novel pair of adhesion molecules that play an important role in modulating PMN migration cross epithelial tight junctions. These findings add a new element to a multistep model of PMN transepithelial migration and may provide new targets for anti-inflammatory therapies.
INTRODUCTION
Polymorphonuclear leukocytes (PMN) are the first line of host defense against infection by bacterial pathogens and are rapidly recruited to sites of bacterial invasion. Because the majority of pathogens are encountered at mucosal surfaces, PMN must migrate out of the circulation, through the interstitium and across the epithelium to engage offending microbes. Although migration of PMN across the epithelium in this response is a terminal event, it is vitally important since elimination of pathogens and disease pathophysiology are direct consequences of PMN transepithelial migration. Despite the importance of this terminal event in the acute inflammatory response, many of the details regarding the regulation of PMN migration across mucosal surfaces remain undefined. Studies on this have revealed that migration of PMN across epithelial barriers involves a concerted series of cell-cell interactions between the PMN and epithelial cells (Zen and Parkos, 2003; Liu et al., 2004b). There is solid evidence that initial PMN-epithelial binding requires leukocyte β2 integrins, especially CD11b/CD18 (Parkos, 1997) and that the rate of PMN migration between epithelial cells is dependent on downstream signaling events from binding interactions between epithelial CD47 and PMN-expressed signal regulatory protein α (Liu et al., 2002). Recent studies have begun to shed light on the nature of additional receptor-ligand pairs that may regulate PMN transepithelial migration in an organ-specific manner that are distinct from processes regulating transendothelial migration.
Although the leukocyte β2 integrin CD11b/CD18 is a key adhesive element that regulates PMN transepithelial migration, there is evidence that additional adhesion molecules expressed on both PMN and epithelia must participate in PMN transepithelial migration, especially at the level of epithelial intercellular junctions. Recently, certain members of a growing family of proteins termed junctional adhesion molecules (JAMs) that are intercellular junction-associated, type-I Ig superfamily proteins (IgSFs) have been shown to serve as ligands for PMN and monocytes as they migrate across endothelial (Martin-Padura et al., 1998; Del Maschio et al., 1999; Johnson-Leger et al., 2002; Ostermann et al., 2002) and epithelial monolayers (Zen et al., 2004). Morphological studies have shown that certain JAMs localize to tight junctions (TJ) (Ebnet et al., 2000; Takekuni et al., 2003) or desmosomes (Zen et al., 2004). JAMs are differentially expressed on a variety of endothelia, epithelia, and leukocytes and, under specific conditions, have been shown to mediate homophilic or heterophilic binding interactions that are important in regulating epithelial/endothelial monolayer barrier function and leukocyte transmigration (Martin-Padura et al., 1998; Cunningham et al., 2000; Liu et al., 2000; Cohen et al., 2001; Johnson-Leger et al., 2002; Liang et al., 2002; Mandell et al., 2004). In addition to homophilic/heterophilic interactions among JAM proteins, two family members, JAM-A and JAM-C, have been recognized as ligands for the leukocyte adhesive integrins CD11a/CD18 (Ostermann et al., 2002) and CD11b/CD18 (Santoso et al., 2002; Zen et al., 2004), respectively. Furthermore, these binding interactions between these JAMs and leukocyte β2 integrins have been shown to play an important role in regulating leukocyte transmigration across endothelial (Chavakis et al., 2004b) and epithelial monolayers (Zen et al., 2004). With respect to PMN transepithelial migration, JAM-A does not appear to play a regulatory role (Liu et al., 2000; Zen and Parkos, 2003), whereas JAM-C mediates PMN migration across epithelial desmosomes (Zen et al., 2004). However, because blockage of JAM-C–mediated interactions was shown to result in only partial inhibition of PMN transepithelial migration, other ligands must be involved in regulating PMN migration across epithelial barriers, particularly at the level of the tight junction.
In the present study we sought to identify receptor ligand pairs that mediate PMN migration across epithelial tight junctions. Here we report that a JAM-like protein with expression largely restricted to granulocytes (Moog-Lutz et al., 2003) termed JAML that is identical to GenBank sequence ID AMICA (accession no. AY138965.1) and FLJ 003 protein (accession no. AK090409.1), plays a role in regulating PMN transepithelial migration. Through recombinant protein/cell-binding assays and cell-labeling experiments, we identified the epithelial counterreceptor for JAML as coxsackie and adenovirus receptor (CAR; Bergelson et al., 1997; Carson et al., 1997; Tomko et al., 1997), a TJ-associated IgSF protein in epithelial cells (Cohen et al., 2001; Ashbourne Excoffon et al., 2004). The importance of JAML interactions with CAR in regulation of mucosal inflammation is discussed.
MATERIALS AND METHODS
Cells
The human intestinal epithelial cell line T84 (passages 60–80) was grown in a 1:1 mixture of DMEM and Hams' F-12 medium supplemented with 15 mM HEPES buffer (pH 7.5), 14 mM NaHCO3, 40 μg/ml penicillin, 8 μg/ml ampicillin, 90 μg/ml streptomycin, and 6% newborn calf serum. For transmigration experiments, cells were grown on collagen-coated permeable filters (5-μm pore size; Costar, Cambridge, MA) as previously described in detail (Parkos et al., 1996b). PMN were isolated from whole blood of normal human volunteers using Ficoll/dextran sedimentation and resuspended in cold Hanks' balanced salt solution (HBSS) devoid of calcium or magnesium (HBSS–) as previously described (Parkos et al., 1996a).
Antibodies
Anti-CAR monoclonal antibody (mAb; clone RmcB) was a generous gift of Dr. Jeffrey Bergelson and used as previously described (Hsu et al., 1988). Inhibitory anti-CAR mAb (clone 2T6) was obtained from US Biological (Swampscott, MA). Inhibitory monoclonal anti-CD11b antibodies (CBRM1/29, OKM1) were used as previously described (Balsam et al., 1998). An inhibitory anti-CD11b mAb (CBRM1/29) and anti-JAM-A mAb (J10.3 or J10.4) were used as previously described (Balsam et al., 1998; Liu et al., 2000; Mandell et al., 2004). Mouse antiserum against JAML was generated by immunizing Balbc mice three times with an Fc chimeric fusion protein containing the putative extracellular domain of human JAML (AA 1–276; JAML-Fc) produced as detailed below. HRP-conjugated or Alexa Fluor 488 (495/519)- and Alexa Fluor 568 (578/603)-conjugated secondary antibodies were obtained from Molecular Probes (Eugene, OR).
Preparation of Recombinant Chimeric Proteins
Soluble recombinant proteins consisting of the extracellular domain of JAML and epithelial lateral membrane/TJ-associated JAM/CTX family members including JAM-A, JAM-C, CAR, nectin-2 (CD112), and human A33 antigen were prepared using methods previously described in detail (Liu et al., 2000). To produce JAML-Fc, cDNA encoding the extracellular domain of human JAML was amplified by PCR from a human leukocyte cDNA library (Clontech, Palo Alto, CA) using primers: 5′-ATATAAGCTTTTGAAAGTTGAGAGCATG-3′ and 5′-ATATGGATCCCACCAACTGATTACCACCCAA-3′. The cDNA product of JAML extracellular domain including a signal peptide was then fused to a to a cDNA encoding a modified region of rabbit IgG1 Fc. cDNA for JAML-Fc fusion protein was then cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA) followed by transient transfections in COS-7 cells. Secreted JAML-Fc was affinity-purified by Protein A-Sepharose resin (Sigma, St. Louis, MO) followed by concentration and dialysis (Liu et al., 2000). To produce GST chimeras of CAR, JAM-A, JAM-C, nectin-2 and A33, cDNA encoding the extracellular domain of each IgSF was amplified by PCR from a human colon Marathon cDNA library (Clontech). The amplified CTX protein extracellular domain encoding cDNA products were fused to GST fusion protein encoding region in pSj26(mod) (kindly provided by Dr. Axel Ullrich; Kharitonenkov et al., 1997). The pSj26(mod) cloning vector was designed for eukaryotic expression and secretion of recombinant GST fusion proteins and was derived from pCDNA3 (Invitrogen). Soluble proteins were produced after transfection of constructs into 293T cells and purified as previously described (Seiffert et al., 1999). To determine which Ig loop of CAR and JAML mediate binding interactions, GST or Fc chimeras of CAR, and JAML extracellular domains containing individual Ig loops were prepared in an analogous manner as above. As shown in Figure 2A, two CAR-GST recombinants (CAR1 + 2-GST: AA 1–240; CAR1-GST: AA 1–134) and three JAML-Fc recombinants (JAML1-Fc: AA 1–130; JAML2-Fc: AA 141–256; JAML1 + 2-Fc: AA 1–256) were prepared, accordingly. In the experiments, recombinant CD47 and SIRPα1 extracellular domain fusion proteins CD47-alkaline phosphatase (AP) and SIRPα-GST were produced as described previously (Seiffert et al., 1999; Liu et al., 2002, 2004a).
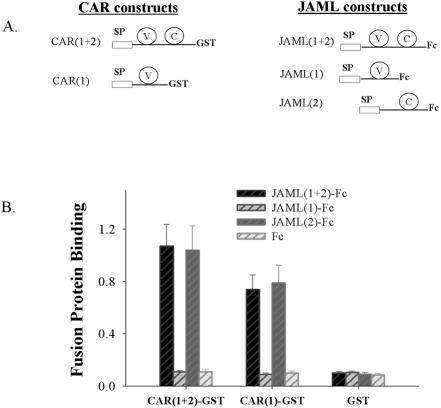
Figure 2.
Identification of the binding domains on JAML and CAR. (A) Diagram of GST or Fc constructs containing truncated versions of the extracellular domains of CAR and JAML. SP, signal peptide. C, IgC loop. V, IgV loop. (B) Binding of JAML-Fc fusion proteins to immobilized GST chimeras of CAR determined as in Figure 1. Data represent mean ± SE of three independent experiments performed in duplicate (p < 0.01).
Immunofluorescence Labeling and Flow Cytometry
Cell surface labeling with anti-JAML antiserum was performed by incubation of cells (5 × 106) with antiserum (1:200 dilution) for 1 h at 4°C in HBSS after blocking nonspecific binding with 1% bovine serum albumin (BSA). After washing, cells were fixed with 3.7% paraformaldehyde (PF) and then incubated with Alexa Fluor 488–conjugated goat anti-mouse secondary antibody (1:500 dilution in blocking solution) for 30 min at 4°C followed by three washes before analyzing by FACScalibre flow cytometer (Becton Dickinson, Oxford, United Kingdom). Fluorescence on individual cells was monitored with a total of 10,000 cells counted and compared with that obtained with preimmune mouse serum. To assay PMN surface JAML expression after fMLP stimulation, PMN in 2 ml of HBSS were allowed to settle in 24-well nontissue culture plates (Costar) at 20°C for 5 min. fMLP (10–7 M) was then added, followed by incubation at 37°C for 10 min. Cells were then chilled on ice and labeled as described above. Cells or monolayers were sequentially incubated with primary antibodies, followed by fluorescence conjugated secondary antibodies as previously described (Zen et al., 2004).
Binding of JAML-Fc to T84 Cells
T84 intestinal epithelial monolayers or 5-μm-thick frozen tissue sections of normal human colon were permeabilized with 0.03% Triton X-100 in HBSS (15 min, 4°C). After blocking nonspecific protein binding with blocking buffer (1% BSA in HBSS) for 30 min at 4°C, T84 monolayers or tissue sections were then incubated with JAML-Fc (10 μg/ml) in blocking buffer for 45–60 min at 37°C in the presence of cocktail of protease inhibitors (Sigma). Monolayers or tissue sections were washed three times and fixed with 3.7% PF (5 min, 20°C). After three washes, monolayers or tissue sections were then incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:500 dilution in blocking buffer) for 30 min at 20°C followed by three washes with HBSS. As a specificity control for JAML-Fc labeling, parallel samples of T84 monolayers or tissue sections were incubated with a purified recombinant rabbit Fc fragment derived from the same vector used to produce the fusion proteins (Fc only, 10 μg/ml). Monolayers/tissue sections were mounted in ProLong antifading embedding solution (Molecular Probes) and analyzed using a Zeiss LSM510 confocal microscope (Zeiss Microimaging, Thornwood, NY; Hopkins et al., 2003; Ivanov et al., 2004). Images shown are representative of at least three experiments, with multiple images taken per slide.
To identify JAML binding protein(s) in epithelial cells, JAML-Fc labeling experiments were performed as above except that incubation of T84 monolayers with JAML-Fc was done in the presence of soluble GST-chimeras (20 μg/ml) of other intercellular junction-associated IgSFs including JAM-A, JAM-C, CAR, nectin-2, and human A33 antigen.
Recombinant Protein-binding Assay
GST chimeras consisting of the extracellular domains of other intercellular junction-associated IgSFs including nectin-2-GST, JAM-A-GST, JAM-C-GST, CAR-GST, A33-GST, and JAML-GST were immobilized in 96-well microtiter plates (4°C, overnight) and blocked with 1% BSA. Purified recombinant GST was also immobilized in microtiter wells as a control. JAML-Fc or purified recombinant rabbit Fc fragment derived from the vector used to produce the fusion protein (Fc only; 10 μg/ml each) was added to wells and incubated for 1 h at 37°C. After three washes, binding of Fc chimeras to microtiter wells was detected by HRP-conjugated goat anti-rabbit Fc followed by addition of substrate and assessment of color development in a microtiter plate reader.
Cell Adhesion Assay
Adhesion of PMN to tissue culture wells coated with recombinant proteins was performed using previously described methods (Balsam et al., 1998; Zen et al., 2002). For these experiments, purified recombinant CAR-GST, JAML-GST, or GST alone was added to 24-well tissue culture plates (Costar) at a concentration of 10 μg/ml in HBSS and incubated overnight at 4°C for protein immobilization. Wells were then blocked with 1% BSA in HBSS for 1 h at 20°C. Freshly isolated PMN were added to fusion protein-coated wells (∼5 × 105 cells/well in a total volume of 300 μl) followed by stationary incubation (30 min, 37°C) in the presence or absence of inhibitors. After three washes, PMN adhesion was quantified by direct visualization using digitalized microscopy and by myeloperoxidase (MPO) assay (Parkos et al., 1996b; Liu et al., 2001).
PMN Transmigration Experiments
PMN transepithelial migration experiments were performed using confluent, high-resistance T84 cell monolayers cultured in an inverted manner on collagen-coated transwells (inverts) as previously described in detail (Parkos et al., 1996b; Liu et al., 2001). With this setup, fMLP-directed PMN migration was assessed in the physiologically relevant basolateral to apical (b-to-a) direction. Antibodies or recombinant proteins were added to the upper chamber or basolateral aspect of T84 monolayers and migration of PMN toward the lower reservoir containing 10–7 M fMLP was assayed as previously described (Liu et al., 2001). In some experiments, in order to allow antibodies against epithelial TJ components such as CAR and JAM-A to gain better access to antigen, T84 monolayers were pretreated with low Ca2+ (10 μM) Eagle's minimum essential medium (S-MEM, Sigma) for 30 min at 37°C to transiently open epithelial TJs (Ivanov et al., 2004; Zen et al., 2004).
SDS-PAGE and Western Blot
Cells (106 in each sample) were solubilized directly in 1 × SDS-containing sample buffer and boiled under reducing conditions. Equal amounts of proteins from different cell types were loaded on SDS-PAGE (10% acrylamide gel). Proteins were electrophoretically transferred onto nitrocellulose membranes followed by blocking with 10% nonfat milk in TTBS (1 h, 20°C). Membranes were then blotted with anti-JAML antiserum (1:1500 dilution) for 1 h. After three washes with TTBS, membranes were incubated with HRP-conjugated goat anti-mouse secondary antibody (1:10000 dilution in blocking solution) followed by ECL detection.
Statistics
Data are presented as the mean ± SE and were compared by Student's t test.
RESULTS
Identification of Epithelial CAR as a Binding Partner for JAML
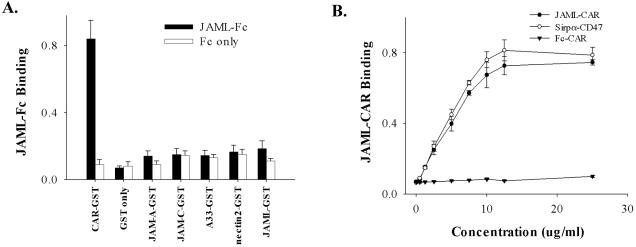
It is generally accepted that PMN migrate between epithelial cells within the paracellular space and that epithelial intercellular junction proteins are likely to play a key role in the regulation of this process. A number of studies have shown that several JAM-like or CTX family members are expressed at intercellular junctions including nectins (Bottino et al., 2003; Reymond et al., 2004), JAMs (Martin-Padura et al., 1998; Liu et al., 2000), A33 antigen (Johnstone et al., 2000), and CARs (Bergelson et al., 1997; Carson et al., 1997; Tomko et al., 1997). Given the known adhesion properties and tissue distribution profiles of CTX family members, we initiated studies to investigate the possibility of binding interactions between junctional CTX proteins and PMN in regulating PMN transepithelial migration. Recently a JAM-like protein expressed predominantly on leukocytes was described by Moog-Lutz (Moog-Lutz et al., 2003) and was proposed to mediate homotypic interactions between expressing cells. This protein, termed JAML, has a sequence identical to GenBank accessions termed AMICA or FLJ 003. Given the granulocyte-restricted lineage of JAML that was reported, we tested whether JAML/AMICA/FLJ 003 protein could bind to other CTX family members known to be expressed in intercellular junctions of epithelial cells. As detailed in Materials and Methods, GST chimeras consisting of the extracellular domains of nectin2, A33, JAM-A, JAM-C, JAML, and CAR were prepared and immobilized to 96-well microtiter wells. Purified GST was also immobilized as a control. GST chimera-coated wells were then incubated with JAML-Fc or Fc-only as controls. Bound JAML-Fc or Fc was detected by HRP-conjugated anti-Fc secondary. As shown in Figure 1A, significant binding activity was detected between JAML and CAR but not other junctional CTX proteins. Interestingly, only a low level of homophilic binding was detected for JAML. Specificity was confirmed by the lack of binding of the Fc only control. Affinity of the JAML-CAR binding interaction was further examined in assays using recombinant proteins consisting of the extracellular domains of these two structurally related molecules. As shown in Figure 1B, we observed that JAML-Fc bound to CAR-GST–coated surfaces in a saturable manner with maximum binding at 12.5 μg/ml (∼150 nM) and half maximal binding at ∼7.5 μg/ml (∼100 nM). In addition, the binding affinity of JAML to CAR was similar to that observed for CD47-SIRPα, another pair of Ig superfamily members implicated in the regulation of PMN transmigration (Liu et al., 2002). No significant binding of Fc alone (control) was detected.
Figure 1.
Identification and characterization of JAML-CAR binding. (A) GST chimeras consisting of the extracellular domains of known epithelial CTX proteins (JAM-A, -C, nectin-2, CAR, A33), JAML and GST were immobilized in individual wells of 96-well microtiter plates. After blocking with BSA, JAML-Fc or Fc only (10 μg/ml each) was added to the wells and incubated for 1 h at 37°C. After extensive washing, bound Fc was detected by HRP-conjugated goat anti-rabbit Fc followed by color development and OD measurement. (B) Dose dependence of JAML-Fc binding to CAR-GST. For comparison, the known binding of SIRPα-Fc to immobilized CD47 (Seiffert et al., 1999; Liu et al., 2002) served as a positive control. Data represent mean ± SE of three independent experiments performed in duplicate (p < 0.01).
Using soluble GST- and Fcfusion proteins consisting of the various Ig loops of CAR or JAML, assays were performed to further refine the binding domain of each molecule. As shown in Figure 2A, several different fusion proteins consisting of the different Ig loops of CAR or JAML were prepared: CAR(1)-GST, CAR(1 + 2)-GST and JAML(1)-Fc, JAML(2)-Fc, and JAML(1 + 2)-Fc. We then coated microtiter wells with CAR-GST fusion proteins and tested binding of JAML-Fc to the CAR-GST–coated wells. Fc alone served as a control in these experiments. As shown in Figure 2B, similar binding of JAML-Fc was found in both CAR(1)-GST– and CAR(1 + 2)-GST–coated wells, suggesting that the first Ig loop (membrane distal) of CAR mediates binding to JAML. However, we found that the membrane distal Ig loop of JAM-L (JAML(1)-Fc) did not bind to any of the CAR-GST chimeras. Interestingly, both JAML(2)-Fc and JAML(1 + 2)-Fc strongly bound to CAR(1)-GST and CAR(1 + 2)-GST, indicating that the second Ig loop (membrane proximal) of JAML is the major functional domain that mediates binding to CAR. Taken together, these binding results suggest that the binding of JAML to CAR is mediated by the membrane distal Ig loop of CAR and the membrane proximal Ig loop of JAML.
JAML Binds to CAR Expressed at TJs in Epithelial Cells and Intestinal Mucosa
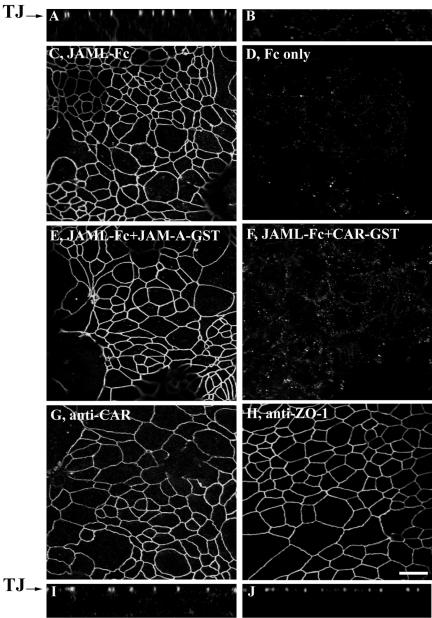
We further tested whether soluble JAML-Fc can bind to CAR expressed in epithelial cells and intestinal mucosa. Because CAR is specifically localized at epithelial tight junctions and access by soluble antibody or binding chimeras is restricted in intact cells, confluent T84 intestinal epithelial monolayers were gently permeabilized using 0.03% Triton X-100, followed by blocking with normal goat serum in phosphate-buffered saline. The nonfixed monolayers were subsequently incubated with JAML-Fc (10 μg/ml) for 1 h at 37°C in the presence of protease inhibitors. Fc only served as a control in these experiments. After thorough washing, monolayers were briefly fixed with paraformaldehyde and incubated with FITC-conjugated goat anti-rabbit Fc. As shown in Figure 3, incubation of T84 monolayers with JAML-Fc chimera resulted in a chicken-wire pattern of staining identical to that of TJs. The TJ staining pattern after JAML-Fc labeling is clearly shown in the X-Z image (Figure 3A). Specificity is confirmed by the lack of labeling after incubation with Fc alone (Figure 3, B and D). Although this result demonstrates a specific interaction between recombinant JAML and epithelial cells and suggests the existence of an epithelial TJ counterreceptor for JAML, additional labeling experiments were necessary to confirm that JAM-L binds to CAR at the level of the epithelial TJ. As shown in Figure 3, the TJ-labeling pattern of JAML-Fc was completely abolished by addition of soluble CAR-GST (panel F) but not by soluble JAM-A-GST (panel E), another related TJ-associated CTX family protein. JAML-Fc labeling experiments were also performed in the presence of soluble constructs of other junctional JAM/CTX members including JAM-C, nectin-2, and A33 antigen, which all failed to inhibit the TJ staining pattern of JAML-Fc (unpublished data). For reference, Figure 3, G–J, shows typical TJ staining patterns in T84 cells labeled with anti-CAR antibody and anti-ZO-1 antibody, respectively. In aggregate, these results suggest that soluble JAML specifically binds to epithelial CAR expressed at epithelial TJs.
Figure 3.
Soluble JAML binds to CAR in T84 intestinal epithelial monolayers. As detailed in Materials and Methods, T84 monolayers were gently permeabilized with 0.03% Triton X-100 followed by blocking with BSA and incubated with JAML-Fc (10 μg/ml) in blocking solution containing protease inhibitors for 1 h at 37°C. Monolayers were immediately fixed with 3.7% PF followed by incubation with Alexa Fluo 488–conjugated goat anti-rabbit Fc. Monolayers incubated with Fc only served as control. Binding of JAML-Fc to T84 monolayers was also performed in the presence of GST chimeras of various epithelial CTX proteins (20 μg/ml). In the X-Z image in A and en-face X-Y image in C, incubation of T84 cells with JAML-Fc resulted in characteristic TJ staining, whereas no labeling with was observed after incubation of T84 monolayers with Fc only (X-Z image in B and X-Y image in D). The TJ-labeling pattern of JAML-Fc was blocked by coincubation with CAR-GST (F) but not by coincubation with JAM-A-GST (E). For reference, G and I represent staining of T84 monolayers with anti-CAR mAb RmcB, whereas H and J represents staining of tight junctions in T84 monolayers with anti-ZO-1 mAb. Bars, 20 μm.
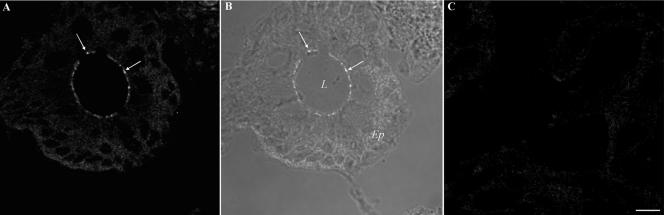
In addition to results obtained from labeling experiments using intestinal epithelial monolayers, the same TJ staining pattern for JAML-Fc was observed in normal human colon tissue sections. As can be seen in Figure 4, JAML-Fc labeling of a colonic crypt is observed at the level of epithelial TJs (panel A, arrows). For orientation, Figure 4B shows the bright-field image merged with the fluorescence image of the same colonic crypt section in panel A. No specific staining was observed after incubation with Fc alone (panel C). These findings indicate that the results obtained in T84 cells are applicable to normal human intestinal epithelium.
Figure 4.
Soluble JAML binds to TJs in human colonic mucosa. As detailed in Figure 3, frozen sections of normal human colonic mucosa were gently permeabilized with 0.03% Triton X-100, blocked, and incubated with JAML-Fc (10 μg/ml, A) or Fc only (10 μg/ml, C) followed by fixation and incubation with Alexa Fluo 488–conjugated goat anti-rabbit Fc. (B) The bright field image merged with the fluorescence image of the same colonic crypt section shown in A. Arrows highlight TJ staining. L, lumen; Ep, epithelium. Bar, 20 μm.
JAML Mediates Human PMN Adhesion to CAR and Transepithelial Migration
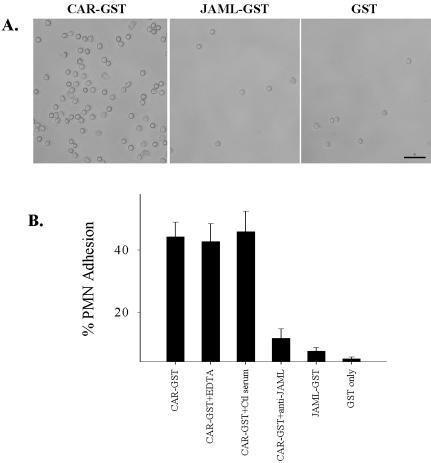
Because JAML is expressed on granulocytes, we tested whether JAML could mediate PMN adhesion to CAR. In these experiments, CAR-GST was immobilized on 24-well plastic plates. After blocking with BSA, freshly isolated PMN were added and incubated for 30 min at 37°C to allow for cell binding. GST only and JAML-GST–coated wells served as controls. As shown in Figure 5, there was abundant PMN adhesion to CAR-GST–coated plates but not to wells coated with JAML-GST or GST only. Control serum and chelation of divalent cations with 5 mM EDTA had no effect on PMN adhesion to immobilized CAR-GST. However, PMN adhesion to immobilized CAR-GST was significantly blocked by anti-JAML antiserum. These results demonstrate that PMN bind to immobilized CAR-GST and that such binding is most likely mediated by JAML. Furthermore, because no significant binding to immobilized JAML was observed, these findings fail to demonstrate a role of JAML in homophilic adhesive interactions in PMN.
Figure 5.
PMN specifically adhere to immobilized CAR. As detailed in Materials and Methods, 24-well nontissue culture plates were coated with CAR-GST, JAML-GST, and GST alone, respectively. After blocking with BSA, PMN (5 × 105) were added to wells and allowed to adhere for 30 min at 37°C. Adhesion was also tested in the presence of anti-JAML or control serum. (A) PMN are strongly adherent to CAR-GST coated wells but not JAML-GST– or GST-coated wells and binding was blocked by anti-JAML. Bar, 50 μm. (B) Quantitative analysis of PMN adhesion observed in A. Data represent mean ± SE of three independent experiments performed in duplicate (p < 0.01).
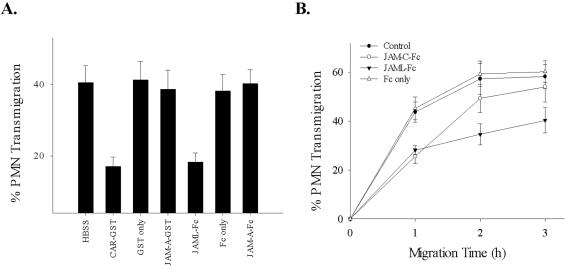
Having demonstrated specific binding interactions between fusion proteins of CAR and JAML, we next tested whether these reagents would inhibit PMN transepithelial migration. In these experiments, PMN transepithelial migration was assessed in the physiologically relevant basolateral to apical direction across T84 monolayers using fMLP gradients (Liu et al., 2001, 2002). As shown in Figure 6A, soluble CAR and JAML recombinant proteins both significantly reduced PMN transmigration after 1 h of transmigration (49.3 ± 3.8% inhibition and 44.2 ± 5.1% inhibition for CAR-GST and JAML-Fc, respectively). No inhibition was observed by incubating with GST or Fc only, JAM-A-GST and JAM-A-Fc at the same concentration (25 μg/ml). Time course assays of PMN transepithelial migration were performed to evaluate the effect of JAML-Fc on the kinetics of migration (Figure 6B). Interestingly, compared with the previously observed delayed migration in the presence of JAM-C-Fc (Zen et al., 2004), inclusion of JAML-Fc resulted in a decrease in total PMN migration after 2–3 h.
Figure 6.
Inhibition of PMN transepithelial migration by soluble JAML and CAR recombinant proteins. As detailed in Materials and Methods, soluble CAR and JAML recombinant proteins (20 μg/ml) were added to the upper chamber of inverted T84 cell monolayers followed by the addition of PMN. Migration in the presence of JAM-A fusion protein, GST or Fc only (25 μg/ml) and no fusion protein (HBSS) served as controls. PMN transmigration was initiated by adding fMLP (1 μM) to the lower chamber and migration into the lower chamber quantified by MPO assay. (A) PMN migration after 1 h; (B) a time course PMN transepithelial migration over 3 h in the presence or absence of the specified fusion proteins. Data represent mean ± SE of three independent experiments performed in duplicate (p < 0.01).
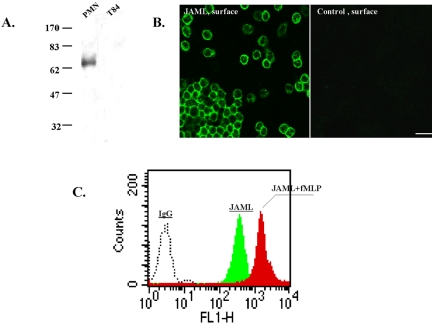
To further characterize JAML and its role in PMN transepithelial migration, we generated an anti-human JAML antiserum by immunizing mice with JAML-Fc. Mice developed reasonably high titers of antibody against the antigen after three immunizations and antiserum was obtained. Using PMN, monocytes and T84 cells, we performed Western blots and immunolabeling experiments using JAML-Fc antiserum. As shown in Figure 7A, Western blots probed with anti-JAM-L revealed a 70-kDa protein in PMN, and monocytes (unpublished data), which is consistent with that reported by Moog-Lutz et al. (2003). Furthermore, no protein was detected in Western blots of T84 cells. Cell surface labeling with this antiserum demonstrated bright labeling of PMN (Figure 7B) that increased after stimulation with fMLP, suggesting upregulation of JAML to the cell surface after stimulation (Figure 7C).
Figure 7.
Expression of JAML in PMN. (A) Western blot of PMN and T84 epithelial cells probed with anti-JAML antiserum. Molecular weight standards are shown to the left. (B) Surface labeling of freshly isolated, nonpermeabilized, PMN with anti-JAML antiserum or normal mouse serum (control). After incubation with primary antiserum, PMN were fixed, labeled with FITC goat anti-mouse IgG, and viewed with a fluorescence microscope. Bar, 20 μm. (C) Up-regulation of JAML on the PMN cell surface after fLMP stimulation. PMN were stimulated with/without fMLP (10–7 M; 10 min, 37°C) followed by cooling on ice and incubation with anti-JAML antiserum followed by wash, fixation, incubation with fluorescence-conjugated secondary antibody and flow cytometry. As a control (fluorescence baseline), PMN were treated with normal mouse IgG.
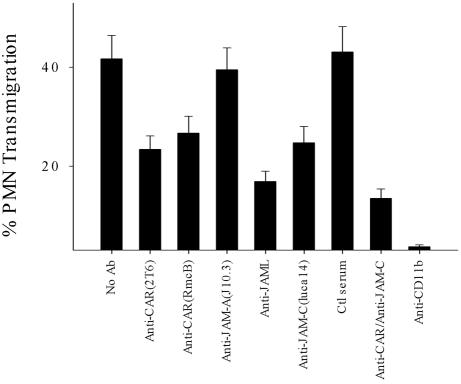
Lastly, we tested the ability of antibodies against CAR and JAML to inhibit PMN transepithelial migration. In these experiments, we used anti-CAR mAbs (RcmB and clone 2T6) and our anti-JAML antiserum. T84 monolayers were preincubated for 30 min in a low Ca2+ solution (S-MEM, Sigma) before addition of the antibodies to allow for better penetration intercellular junctions. As shown in Figure 8, addition of anti-CAR and anti-JAML resulted in significant inhibition of PMN transmigration. After 1 h, PMN transmigration was inhibited by 52.1 ± 4.7% by JAML antiserum (1:100 dilution), whereas preimmune mouse serum had no effect. Similarly, anti-CAR mAbs 2T6 and RcmB (20 μg/ml each) also reduced PMN transmigration by 30–40%. Interestingly, transmigration assays in the presence of mixtures of antibodies against epithelial CAR and JAM-C resulted in enhanced inhibition that was greater than either antibody alone. No inhibition was observed in the presence of mAb J10.3, a well-characterized antibody against JAM-A.
Figure 8.
Inhibition of PMN transepithelial migration by antibodies against CAR or JAML. As detailed in the methods inverted T84 monolayers were washed with HBSS– and incubated with S-MEM (Sigma) for 30 min at 37°C. Solutions of anti-CAR mAbs (RmcB and Clone 2T6, 20 μg/ml each) or anti-JAML antiserum (1:100 dilution) in HBSS were added to the upper chambers followed by addition of PMN 20 min later. Anti-JAM-C (LUCA14), anti-JAM-A (J10.3), and normal mouse serum at the same concentration were used as inhibitory or noninhibitory controls, respectively. Combination of anti-CAR (RmcB) and anti JAM-C (each at 20 μg/ml) was also evaluated for additive inhibitory effects. PMN transmigration was initiated by adding 1 μM fMLP to the lower chamber and transmigration and migration into the lower chamber quantified by MPO assay after 1 h (37°C). Data represent mean ± SE of three independent experiments performed in duplicate (p < 0.01).
DISCUSSION
JAML Is a Newly Described CTX Family Member Expressed on Granulocytes and Functions as an Adhesion Molecule
JAML was originally discovered as a protein induced by retinoic acid during differentiation of myeloid leukemia cells (Moog-Lutz et al., 2003). The sequence encoding JAML reported by these authors is identical to at least two other GenBank accessions: AMICA (accession no. AY358362.1) and FLJ 003 (accession no. AK090409.1). In this report, we confirm that JAML is strongly expressed on the cell surface of granulocytes. Furthermore, we report that JAML, like the β2 integrin CD11b/CD18, is up-regulated on cell surface after stimulation with the chemoattractant fMLP.
Structurally, JAML shares certain common features with other CTX molecules involved in adhesion, such as JAM-A, -B, and -C. Because human JAML has a motif (KID58 flanked by Y72) similar to the dimerization motif shown to be essential for dimer formation in JAM-A (Kostrewa et al., 2001), it was suggested that JAML may mediate cell-cell adhesion through homophilic interactions (Moog-Lutz et al., 2003). However, our binding/adhesion data in Figures 2 and 5 fails to demonstrate homophilic interactions with JAML in PMN. Instead, our results demonstrate functionally significant heterophilic adhesive interactions between JAML and the epithelial TJ-associated protein CAR.
An important distinction between JAML and other JAM proteins is in the lack of a traditional PDZ-binding motif at its C-terminus. Because the PDZ-binding motif of related proteins such as JAM-A mediates affiliation with scaffolding proteins such as ZO-1 (Ebnet et al., 2000), PAR3 (Itoh et al., 2001), and AF6 (Ebnet et al., 2000) and appears to be critical for targeting to specific sites such as intercellular junctions, the absence of this motif in JAML raises the likelihood of differences in function(s) and targeting patterns from those of other CTX proteins.
Identification of Epithelial CAR as a Cellular Ligand for JAML
CAR, the receptor shared between coxsackie B and adeno-viruses (Bergelson et al., 1997; Carson et al., 1997; Tomko et al., 1997), is a member of the CTX subfamily of IgSFs and is characterized by an extracellular domain containing one V- and one C-type Ig domain, a single membrane-spanning region, and an intracellular tail with a PDZ-binding motif (Coyne et al., 2004). Although it is best known for its role as a virus receptor (Bewley et al., 1999; Freimuth et al., 1999; Wang and Bergelson, 1999; Kirby et al., 2000; Walters et al., 2002), CAR is expressed in polarized epithelia at the TJ, where it colocalizes with ZO-1, MAGI-1b, and multi-PDZ domain protein-1 (MUPP-1; Cohen et al., 2001; Ashbourne Excoffon et al., 2004; Coyne et al., 2004), suggesting a role in regulation of barrier or polarity. Others have suggested that CAR may function as an adhesion molecule like other CTX family member proteins such as JAMS, and CAR has been shown to mediate homophilic interactions between cells (Honda et al., 2000; Cohen et al., 2001).
Here we show that CAR serves as a cellular adhesion receptor for JAML and that such heterophilic adhesive interactions can regulate PMN migration across epithelial tight junctions. Using soluble fusion proteins consisting of regions of the extracellular domains of JAML and CAR, we demonstrate that JAML specifically binds to CAR in a saturable manner (Figure 1B) and that such binding is mediated by the membrane distal Ig domain (Ig1) of CAR-interacting with the membrane proximal Ig domain (Ig2) of JAML (Figure 2). Furthermore, our results indicate that endogenous JAML expressed on PMN can bind to CAR. Conversely, endogenous CAR expressed at epithelial TJs can bind to JAML.
PMN Transepithelial Is a Multistep Event and Involves JAML-CAR–binding Interactions at the Level of the TJ
A number of previous studies indicate that PMN transepithelial migration is a multistep event regulated by a series of unique adhesive interactions and signaling events (Zen and Parkos, 2003; Liu et al., 2004b). It is well established that the leukocyte β2 integrin CD11b/CD18 plays a central role in regulating initial adhesive events during the PMN transmigration response. However, the current literature suggests that other proteins and adhesion molecules on both PMN and epithelial cells play important roles in the regulation of PMN transepithelial migration at distinct points along the migration pathway. Such molecules include CD47, which serves to facilitate migration of PMN after initial adhesion, and CD55 (Lawrence et al., 2003), which is critical for PMN detachment at late stages of transmigration.
As has been described for transendothelial migration (reviewed in Muller, 2001), PMN migrate across epithelia by passing between epithelial cells at cell-cell borders. After initial adhesive interactions with the basal aspect of epithelial membranes, PMN enter the lateral paracellular space and migrate to epithelial intercellular junctions. At this point, sequential adhesive interactions can be envisioned because the length of the lateral epithelial membrane is considerable and multiple structures including desmosomes, adherens, and tight junctions are present. Indeed, we recently reported that another member of the JAM family of proteins termed JAM-C is localized to epithelial desmosomes and, in agreement with others, demonstrated that JAM-C served as an adhesive ligand for migrating PMN via interacting with leukocyte β2 integrin CD11b/CD18 (Santoso et al., 2002, Chavakis etal., 2004a; Zen et al., 2004). However, our study also clearly demonstrated that other epithelial ligands for migrating PMN exist because blockage of JAM-C did not result in complete inhibition of transepithelial migration. Despite this observation, the identification of other ligands, particularly at the level of the TJ, has remained elusive.
Here, we identified JAML and CAR as a novel receptor-ligand pair that plays a significant role in regulating PMN migration across epithelial TJs in a physiologically relevant manner. As shown in Figures 6 and 8, fusion proteins containing JAML and CAR extracellular domains and antibodies against JAML and CAR all significantly inhibited PMN transepithelial migration. Interestingly, we observed that the inhibition of PMN transmigration by CAR and JAML reagents was incomplete. Explanations for this include inaccessibility of the TJ to permeation by antibodies and fusion proteins and the possibility of other binding partners. Concerning other binding partners, one possibility that has been shown to mediate leukocyte/lymphocyte interactions with endothelia in other systems is JAM-A (Martin-Padura et al., 1998; Del Maschio et al., 1999; Ostermann et al., 2002). However, we and others have not been able to demonstrate a direct role for JAM-A in in vitro assays of human PMN transmigration (Liu et al., 2000; Shaw et al., 2001; Zen et al., 2004). Additional studies will help to answer these questions.
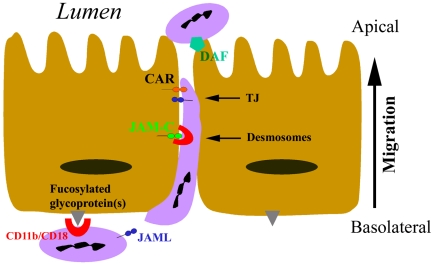
On the basis of our results, we propose a revised model of PMN transepithelial migration to include JAML-CAR interactions at the level of the TJ (Figure 9). In this model, PMN transepithelial migration begins with adhesion to the basal membrane through CD11b/CD18-mediated binding to presently uncharacterized epithelial fucosylated glycoproteins(s) (Zen et al., 2002). Subsequently, PMN migrate between epithelial cells, where sequential CD11b/CD18-mediated binding to JAM-C at desmosomes is followed by JAML binding to CAR as PMN cross the TJ. Once at the apical surface of the epithelium, membrane proteins such as DAF (CD55; Lawrence et al., 2003) mediate PMN detachment. Studies aimed at identification of new adhesion molecules that regulate PMN transepithelial migration such as JAML and CAR may provide new targets for anti-inflammatory therapies.
Figure 9.
Model of JAML-CAR regulation of PMN transepithelial migration.
Acknowledgments
We acknowledge Susan Voss and Dirk Hunt for expert cell culture and imaging assistance and Dr. Jeffrey Bergelson (Division of Infectious Diseases, Children's Hospital of Philadelphia, PA) for providing anti-CAR mAb RmcB. This work was supported, in part, by a National Institutes of Health (NIH) Digestive Diseases Research Development Center grant (tissue culture and morphology, DK64399) and by NIH grants DK72564, DK61379, HL72124 (C.A.P.), DK62894 (Y.L.), and DK59888 (A.N.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0036) on March 30, 2005.
Abbreviations used: PMN, polymorphonuclear neutrophil; JAM, junctional adhesion molecule; JAML, junctional adhesion molecule-like protein; CTX, cortical thymocyte marker in Xenopus; IgSF, Ig superfamily; CAR, coxsackie and adenovirus receptor; MPO, myeloperoxidase; PF, paraformaldehyde; HBSS–, Hanks' balanced salt buffer devoid of Ca2+ and Mg2+; fMLP, formylmethionylleucylphenylalanine.
References
- Ashbourne Excoffon, K. J., Hruska-Hageman, A., Klotz, M., Traver, G. L., and Zabner, J. (2004). A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J. Cell Sci. 117, 4401–4409. [DOI] [PubMed] [Google Scholar]
- Balsam, L. B., Liang, T. W., and Parkos, C. A. (1998). Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J. Immunol. 160, 5058–5065. [PubMed] [Google Scholar]
- Bergelson, J. M., Cunningham, J. A., Droguett, G., Kurt-Jones, E. A., Krithivas, A., Hong, J. S., Horwitz, M. S., Crowell, R. L., and Finberg, R. W. (1997). Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323. [DOI] [PubMed] [Google Scholar]
- Bewley, M. C., Springer, K., Zhang, Y. B., Freimuth, P., and Flanagan, J. M. (1999). Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286, 1579–1583. [DOI] [PubMed] [Google Scholar]
- Bottino, C. et al. (2003). Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 198, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, S. D., Chapman, N. N., and Tracy, S. M. (1997). Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem. Biophys. Res. Commun. 233, 325–328. [DOI] [PubMed] [Google Scholar]
- Chavakis, T., Keiper, T., Matz-Westphal, R., Hersemeyer, K., Sachs, U. J., Nawroth, P. P., Preissner, K. T., and Santoso, S. (2004a). The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 279, 55602–55608. [DOI] [PubMed] [Google Scholar]
- Chavakis, T., Keiper, T., Matz-Westphal, R., Hersemeyer, K., Sachs, U. J., Nawroth, P. P., Preissner, K. T., and Santoso, S. (2004b). The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 279, 55602–55608. [DOI] [PubMed] [Google Scholar]
- Cohen, C. J., Shieh, J. T., Pickles, R. J., Okegawa, T., Hsieh, J. T., and Bergelson, J. M. (2001). The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98, 15191–15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, C. B., Voelker, T., Pichla, S. L., and Bergelson, J. M. (2004). The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J. Biol. Chem. 279, 48079–48084. [DOI] [PubMed] [Google Scholar]
- Cunningham, S. A., Arrate, M. P., Rodriguez, J. M., Bjercke, R. J., Vanderslice, P., Morris, A. P., and Brock, T. A. (2000). A novel protein with homology to the junctional adhesion molecule. Characterization of leukocyte interactions. J. Biol. Chem. 275, 34750–34756. [DOI] [PubMed] [Google Scholar]
- Del Maschio, A. et al. (1999). Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM). J. Exp. Med. 190, 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet, K., Schulz, C. U., Meyer Zu Brickwedde, M. K., Pendl, G. G., and Vestweber, D. (2000). Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275, 27979–27988. [DOI] [PubMed] [Google Scholar]
- Freimuth, P., Springer, K., Berard, C., Hainfeld, J., Bewley, M., and Flanagan, J. (1999). Coxsackievirus and adenovirus receptor amino-terminal Ig V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J. Virol. 73, 1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, T. et al. (2000). The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res. Mol. Brain Res. 77, 19–28. [DOI] [PubMed] [Google Scholar]
- Hopkins, A. M., Walsh, S. V., Verkade, P., Boquet, P., and Nusrat, A. (2003). Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 116, 725–742. [DOI] [PubMed] [Google Scholar]
- Hsu, K. H., Lonberg-Holm, K., Alstein, B., and Crowell, R. L. (1988). A mAb specific for the cellular receptor for the group B coxsackieviruses. J. Virol. 62, 1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, M., Sasaki, H., Furuse, M., Ozaki, H., Kita, T., and Tsukita, S. (2001). Junctional adhesion molecule (JAM) binds to PAR-3, a possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 154, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, A. I., Nusrat, A., and Parkos, C. A. (2004). Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15, 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Leger, C. A., Aurrand-Lions, M., Beltraminelli, N., Fasel, N., and Imhof, B. A. (2002). Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood 100, 2479–2486. [DOI] [PubMed] [Google Scholar]
- Johnstone, C. N. et al. (2000). Characterization of mouse A33 antigen, a definitive marker for basolateral surfaces of intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G500–G510. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov, A., Chen, Z., Sures, I., Wang, H., Schilling, J., and Ullrich, A. (1997). A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186. [DOI] [PubMed] [Google Scholar]
- Kirby, I., Davison, E., Beavil, A. J., Soh, C.P., Wickham, T. J., Roelvink, P. W., Kovesdi, I., Sutton, B. J., and Santis, G. (2000). Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J. Virol. 74, 2804–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrewa, D. et al. (2001). X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 20, 4391–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, D. W., Bruyninckx, W. J., Louis, N. A., Lublin, D. M., Stahl, G. L., Parkos, C. A., and Colgan, S. P. (2003). Antiadhesive role of apical decayaccelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J. Exp. Med. 198, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, T. W. et al. (2002). Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J. Immunol. 168, 1618–1626. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Buhring, H. J., Zen, K., Burst, S. L., Schnell, F. J., Williams, I. R., and Parkos, C. A. (2002). Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem. 277, 10028–10036. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Merlin, D., Burst, S. L., Pochet, M., Madara, J. L., and Parkos, C.A. (2001). The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J. Biol. Chem. 276, 40156–40166. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Nusrat, A., Schnell, F. J., Reaves, T. A., Walsh, S., Pochet, M., and Parkos, C. A. (2000). Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113(Pt 13), 2363–2374. [DOI] [PubMed] [Google Scholar]
- Liu, Y., O'Connor, M. B., Mandell, K. J., Zen, K., Ullrich, A., Buhring, H. J., and Parkos, C. A. (2004a). Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha. J. Immunol. 172, 2578–2585. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Shaw, S. K., Ma, S., Yang, L., Luscinskas, F. W., and Parkos, C. A. (2004b). Regulation of leukocyte transmigration: cell surface interactions and signaling events. J. Immunol. 172, 7–13. [DOI] [PubMed] [Google Scholar]
- Mandell, K. J., McCall, I. C., and Parkos, C. A. (2004). Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J. Biol. Chem. 279, 16254–16262. [DOI] [PubMed] [Google Scholar]
- Martin-Padura, I. et al. (1998). Junctional adhesion molecule, a novel member of the Ig superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog-Lutz, C., Cave-Riant, F., Guibal, F. C., Breau, M. A., Di Gioia, Y., Couraud, P. O., Cayre, Y. E., Bourdoulous, S., and Lutz, P. G. (2003). JAML, a novel protein with characteristics of a Junctional Adhesion Molecule, is induced during differentiation of myeloid leukemia cells. Blood. 102, 3371–3378. [DOI] [PubMed] [Google Scholar]
- Muller, W. A. (2001). Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation 8, 181–193. [DOI] [PubMed] [Google Scholar]
- Ostermann, G., Weber, K. S., Zernecke, A., Schroder, A., and Weber, C. (2002). JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3, 151–158. [DOI] [PubMed] [Google Scholar]
- Parkos, C. A. (1997). Molecular events in neutrophil transepithelial migration. Bioessays 19, 865–873. [DOI] [PubMed] [Google Scholar]
- Parkos, C. A., Colgan, S. P., Diamond, M. S., Nusrat, A., Liang, T. W., Springer, T. A., and Madara, J. L. (1996a). Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol. Med. 2, 489–505. [PMC free article] [PubMed] [Google Scholar]
- Parkos, C. A., Colgan, S. P., Liang, T. W., Nusrat, A., Bacarra, A. E., Carnes, D. K., and Madara, J. L. (1996b). CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J. Cell Biol. 132, 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond, N., Imbert, A. M., Devilard, E., Fabre, S., Chabannon, C., Xerri, L., Farnarier, C., Cantoni, C., Bottino, C., Moretta, A., Dubreuil, P., and Lopez, M. (2004). DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J. Exp. Med. 199, 1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso, S., Sachs, U. J., Kroll, H., Linder, M., Ruf, A., Preissner, K. T., and Chavakis, T. (2002). The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 196, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert, M., Cant, C., Chen, Z., Rappold, I., Brugger, W., Kanz, L., Brown, E. J., Ullrich, A., and Buhring, H. J. (1999). Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 94, 3633–3643. [PubMed] [Google Scholar]
- Shaw, S. K., Perkins, B. N., Lim, Y. C., Liu, Y., Nusrat, A., Schnell, F. J., Parkos, C. A., and Luscinskas, F. W. (2001). Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-alpha plus interferon-gamma Does not reduce leukocyte transmigration under flow. Am. J. Pathol. 159, 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekuni, K., Ikeda, W., Fujito, T., Morimoto, K., Takeuchi, M., Monden, M., and Takai, Y. (2003). Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J. Biol. Chem. 278, 5497–5500. [DOI] [PubMed] [Google Scholar]
- Tomko, R. P., Xu, R., and Philipson, L. (1997). HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94, 3352–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, R. W., Freimuth, P., Moninger, T. O., Ganske, I., Zabner, J., and Welsh, M. J. (2002). Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110, 789–799. [DOI] [PubMed] [Google Scholar]
- Wang, X., and Bergelson, J. M. (1999). Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73, 2559–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen, K., Babbin, B. A., Liu, Y., Whelan, J. B., Nusrat, A., and Parkos, C. A. (2004). JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol. Biol. Cell 15, 3926–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen, K., Liu, Y., Cairo, D., and Parkos, C. A. (2002). CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J. Immunol. 169, 5270–5278. [DOI] [PubMed] [Google Scholar]
- Zen, K., and Parkos, C. A. (2003). Leukocyte-epithelial interactions. Curr. Opin. Cell Biol. 15, 557–564. [DOI] [PubMed] [Google Scholar]