Abstract
Membrane microdomains, the so-called lipid rafts, function as platforms to concentrate receptors and assemble the signal transduction machinery. Internalization, in most cases, is carried out by different specialized structures, the clathrin-coated pits. Here, we show that several endocytic proteins are efficiently recruited to morphologically identified plasma membrane lipid rafts, upon activation of the epidermal growth factor (EGF) receptor (EGFR), a receptor tyrosine kinase. Analysis of detergent-resistant membrane fractions revealed that the EGF-dependent association of endocytic proteins with rafts is as efficient as that of signaling effector molecules, such as Grb2 or Shc. Finally, the EGFR, but not the nonsignaling transferrin receptor, could be localized in nascent coated pits that almost invariably contained raft membranes. Thus, specialized membrane microdomains have the ability to assemble both the molecular machineries necessary for intracellular propagation of EGFR effector signals and for receptor internalization.
INTRODUCTION
On engagement of receptor tyrosine kinases (RTKs) by their cognate ligands, their intrinsic kinase activity is stimulated with ensuing receptor autophosphorylation and recruitment/activation of the signal transduction machinery, in turn responsible for several effector functions. Concurrently, activated receptors trigger their own endocytosis, whose ultimate goal is to extinguish signaling through removal of receptors from the cell surface (Carpenter, 2000). In the case of the epidermal growth factor (EGF) receptor (EGFR) the localization of the two processes is well characterized (Jorissen et al., 2003). Signaling occurs within specialized membrane microdomains, lipid rafts (Simons and Toomre, 2000; Maxfield, 2002), whereas endocytosis occurs mostly through the clathrin-coated pits (CCPs; Conner and Schmid, 2003).
Membrane rafts are cholesterol- and sphingolipid-rich membrane regions characterized by higher order and lower buoyant density than bulk plasma membrane (Simons and Toomre, 2000; Sprong et al., 2001; Kusumi et al., 2004). These structures are also characterized by their insolubility in some detergents at 4°C (DRM, detergent resistant membranes; Brown and Rose, 1992). Several transmembrane receptors have been reported to associate with membrane rafts (Cheng et al., 1999; Krauss and Altevogt, 1999; Mineo et al., 1999; Lamaze et al., 2001; Giurisato et al., 2003), including the EGFR (Mineo et al., 1999). The association of receptors with lipid rafts is thought to be functional to the activation of signaling cascades (Cheng et al., 1999; Waugh et al., 1999; Drevot et al., 2002; Matveev and Smart, 2002; Pierce, 2002; Stoddart et al., 2002; del Pozo et al., 2004). Accordingly, specific signaling (Gingras et al., 1998; Iwabuchi et al., 1998; Michaely et al., 1999; Kindzelskii et al., 2004) and adaptor proteins (e.g., shc and grb2; Biedi et al., 2003; Ridyard and Robbins, 2003; Yang et al., 2004) have been found associated to rafts. However, lipid rafts are rather small, possibly containing only few molecules (Prior et al., 2003), to function as stable signaling platforms (Harder and Engelhardt, 2004). Nevertheless, they are dynamic, and may diffuse (Pralle et al., 2000; Sprong et al., 2001) and coalesce into larger and more stable structures, in response to signaling, forming larger “signal transducing platforms” (Simons and Toomre, 2000; Kusumi et al., 2004; Mayor and Rao, 2004). These larger rafts may contribute to both signal amplification or attenuation (e.g., by synergistic engagement of protein kinases or phosphatases with cognate substrates; Jacobson and Dietrich, 1999; Kurzchalia and Parton, 1999a; Simons and Toomre, 2000; Anderson and Jacobson, 2002; Miljan and Bremer, 2002).
Raft associated receptors have been reported to follow either a non–clathrin- or a clathrin-dependent endocytic pathway (Nichols and Lippincott-Schwartz, 2001; Johannes and Lamaze, 2002; Conner and Schmid, 2003; Di Guglielmo et al., 2003; Felberbaum-Corti et al., 2003). In particular, EGFR relocates into CCPs, upon EGF binding, by promoting the recruitment of endocytic proteins involved in the assembly of CCP (e.g., eps15, AP2, and clathrin; Slepnev and De Camilli, 2000; Brodsky et al., 2001; Smythe, 2002). Although some evidence has been provided that these proteins are recruited to the plasma membrane (Mineo et al., 1999; Pike and Casey, 2002; Yamabhai and Anderson, 2002), the relationship between signaling- and internalization-competent compartments is far from being clear. We, therefore, endeavored to investigate this relationship, in the EGFR system (Johannes and Lamaze, 2002; Ringerike et al., 2002; Sandvig and van Deurs, 2002; Stoddart et al., 2002; Abrami et al., 2003).
Our results show that specialized membrane rafts have the ability to assemble the molecular machineries necessary for both intracellular propagation of EGFR effector signals, and for receptor internalization, and suggest that EGFR–internalizing CCPs can assemble within lipid raft platforms.
MATERIALS AND METHODS
Antibodies and Cells
Antibodies used were: rabbit anti-eps15 (577), rabbit anti-horseradish peroxidase (HRP; Sigma, St. Louis, MO), mouse anti-AP2 (Sigma), mouse anti-TfR (Zymed, San Francisco, CA), mouse anti-TfR (extracellular domain; 5E9C11 clone, ATCC, Rockville, MD), mouse anti-EGFR (extracellular domain; AB-5, Oncogene, San Diego, CA, and 13A9, Genentech, San Francisco, CA), rabbit anti-EGFR (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-EGFR, (Cell Signaling Technology, Beverly, MA), rabbit anti-calnexin (Santa Cruz Biotechnology), rabbit anti-β1 integrin (kindly provided by F. Giancotti), mouse anti-clathrin HC (BD Biosciences, San Jose, CA), rabbit anti-mouse (DAKO, Glostrup, Denmark), rabbit anti-PLAP (Rockland, Gilbertsville, PA), HRP-coupled secondary antibodies for ECL (DAKO), rabbit anti-Caveolin-1 (Santa Cruz Biotechnology or BD Biosciences). ProteinA/gold was from Dr. J. W. Slot (Utrecht University, Utrecht, The Netherlands), rabbit anti-Grb2 (Santa Cruz Biotechnology), rabbit anti-Shc (Santa Cruz Biotechnology). Secondary fluorochrome-labeled antibodies were from Jackson ImmunoResearch Laboratory (West Grove, PA).
For EGF treatment, cells were starved in serum-free DMEM for 18 h and incubated in the presence or in the absence of EGF, (100 ng/ml, unless otherwise indicated; Upstate Biotechnology, Lake Placid, NY). EGF stimulation was performed at 37°C or on ice, as indicated. In the latter case, the cells were prechilled at 0°C, in the starvation medium, for 30 min before stimulation. When indicated, the cells were subsequently shifted at 37°C for different lengths of time. For CT-B/HRP (Sigma) labeling, HeLa cells were starved in serum-free medium for 18 h and then incubated with CT-B/HRP (Sigma; 4 μg/ml), in the presence or in the absence of EGF, for 1 h, on ice.
For methyl-β-cyclodextrin (MβCD) treatment, HeLa cells were starved in serum-free medium for 18 h, and during the last hour of starvation, incubated with (or without) a mixture of 10 mM MβCD (Sigma, St. Louis, MO), 0.2% bovine serum albumin, 20 mM HEPES, in serum-free medium. Residual cholesterol was measured by lysing the cells with 0.5% SDS in phosphate-buffered saline (PBS). Cells were then scraped off the dish and passed through a syringe with a 25-gauge needle, to shear DNA. The enzymatic-colorimetric “Colesterolo SL” kit (Real Time Diagnostic System, Viterbo, Italy), was used to estimate the amount of cholesterol present in the cell lysate, according to the manufacturer's instructions.
Electron Microscopy
Ruthenium Red/Glutaraldehyde Fixation Cells were prepared for ruthenium red/glutaraldehyde fixation according to previously described protocols (Damke et al., 1994). Briefly, HeLa cells were fixed with 66 mM cacodylate buffer, pH 7.2, containing 2% glutaraldehyde, and 0.5 mg/ml Ruthenium red (Fluka, Buchs, Switzerland) at room temperature for 1 h. After washing with 150 mM cacodylate buffer, pH 7.2, the cells were postfixed with 33 mM cacodylate buffer, containing 1% OsO4, and 0.5 mg/ml Ruthenium red, at room temperature for 3 h. The cells were then washed with 150 mM cacodylate buffer, and processed for Epon embedding (Polybed 812, Polysciences, Warrington, PA).
Pre-embedding CT-B/HRP Labeling HeLa cells were labeled with CT-B/HRP as described, fixed with 2% paraformaldehyde/0.2% glutaraldehyde in PBS. The peroxidase reaction was developed with 0.2 mg/ml diaminobenzidine, 0.01% H2O2, in PBS. Cells were washed with PBS containing 0.02 M glycine, scraped off the dish, centrifuged, and embedded in 12% gelatin in PBS. Small blocks of embedded cells were incubated overnight with 2.3 M sucrose at 4°C, mounted on aluminum pins, and frozen in liquid nitrogen. Ultrathin cryo-sections of 100 nm were cut at –107°C, and picked up with 1% methylcellulose in 1.15 M sucrose. The larger thickness of the sections, compared with the standard 60 nm, was necessary to preserve the immunoperoxidase reaction product.
For double or triple immunoperoxidase/immunogold labeling, after incubation with gelatin 2% in PBS, cryosections were immunogold labeled according to previously described protocols (Confalonieri et al., 2000).
Epon Pre-embedding HeLa cells were fixed with 4% paraformaldehyde in PBS for 30 min, quenched with a solution of 0.02% glycine in PBS, and labeled with rabbit anti-PLAP followed by protein A gold, 10 nm, for 30 min each step. Cells were then fixed in 2.5% glutaraldehyde in cacodylate buffer, 0.1 M, pH 7.2, and processed for embedding in Polybed 812 (Polysciences).
Morphometry The morphometric analysis of immunogold and immunoperoxidase labeling was performed at the microscope at 12,000×, on at least 20 randomly selected cell profiles, or on systematically sampled micrographs, depending on the target. Each experiment was repeated at least three times. For immunogold labeling, the optimal concentration of the antibodies was tested by defining the background-to-signal ratio (Rabouille, 1999). In addition, the ratio between specific (labeling of compartments known to associate with the given antigen), and nonspecific gold staining (labeling of compartments known not to associate with the given antigen), was considered acceptable if >5.
The darkness (but not the size or the contrast) of the gold particles, displayed in the micrographs shown in this article, has been increased by digital processing, in order to facilitate the observation in the rather low magnifications used.
Immunofluorescence
HeLa cells were incubated for 3 h in serum-free medium, placed on ice, and washed briefly with prechilled serum free medium (SFM, DMEM containing 20 mM HEPES, and 1% bovine serum albumin). Cells were then incubated with anti-EGFR (13A9 antibody) in SFM, in the presence or in the absence of EGF (100 ng/ml), 60 min at 0°C on a rocker. Cells were then washed, fixed, and stained with a second-step fluorochrome-labeled anti-mouse antibody. Subsequently, cells were permeabilized with 0.1% Triton X-100 in PBS and stained with anti-AP2 β-subunit (Santa Cruz Biotechnology), revealed by fluorochrome-labeled antibody. Confocal microscopy was performed using a Leica DM IRE2 HC Fluo TCS inverted microscope (Deerfield, IL). For the quantization of EGFR/AP2 colocalization (shown in Figure 1C), we used the “colocalization finder” plug-in of ImageJ free image analysis software (W. Rasband, National Institutes of Health, Bethesda; http://rsb.info.nih.gov/ij/). Images were acquired in order to display the entire plasma membrane surface adhering to the culture dish. To maximize the chance of evaluating only the plasma membrane, excluding all intracellular staining, we performed the analysis on a restricted region of the images extending 15 pixels from the edge of the cell, toward the cytoplasm. A pixel-by-pixel correlation diagram for the channel red and green of the same image was obtained after subtracting the background noise. For AP2 dot counting, images were taken as above, after subtracting the background noise, AP2 dots were counted on the whole area of the cell. The surface was measured in pixels with ImageJ “Analyze” command after highlighting the edge of the cells with the “Wand (tracing) tool.” The area was subsequently converted into μm2 based on the pixel size. The number of dots was expressed as density of dots on the cell surface (dots/μm2 of surface).
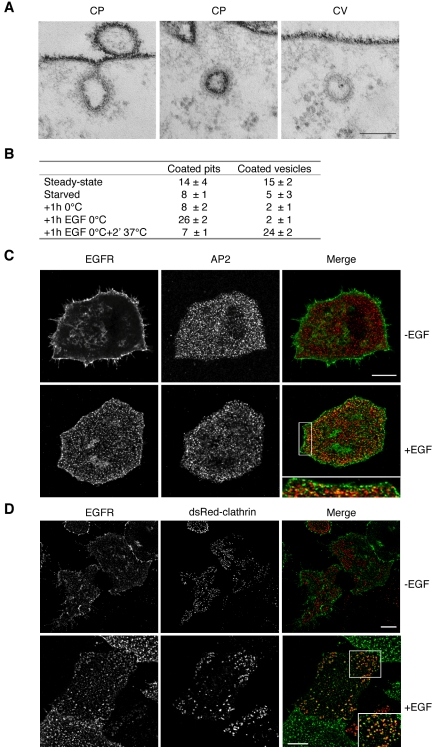
Figure 1.
EGF stimulation induces CCPs assembly at 0°C, but not CCV budding. (A) EM images HeLa cells fixed in the presence of Ruthenium red (RR). Dark staining indicates connection with the outer surface. Examples are shown of RR-positive clathrin-coated pits (CP), and RR-negative clathrin-coated vesicles (CV). Bar, 0.06 μm. (B) Morphometric analysis of cells processed as in A. HeLa cells in logarithmic growth (“Steady state” in B) were starved in serum-free medium for 18 h at 37°C (“starved” in B), and then transferred at 0°C for 30 min, to allow the chilling of the plate and cells. The medium was then removed and cells were incubated for 1 h with fresh medium, pre-chilled on ice, in the absence (“1 h 0°C” in B), or in the presence (“1 h EGF 0°C” in B) of EGF. A parallel dish of cells, treated with EGF at 0°C as above, was then transferred for 2 min at 37°C, in the absence of EGF, to allow internalization of preformed clathrin-coated pits (“1 h EGF 0°C + 2′ 37°C” in B). Morphometry performed on 6 series of 10 cell profiles. Data represent the average number of CPs or CVs in each series, ± SD. The CP category includes: (i) all RR-positive invaginated regions of the plasma membrane, displaying a clear morphologically identifiable clathrin coat associated to the inner surface; (ii) clathrin-coated vesicle-like structures displaying a clear RR staining. (C) Double immunofluorescence showing the localization of EGFR (EGFR) and of the β subunit of the AP2 complex (AP2), in starved HeLa stimulated with EGF (+EGF) for 1 h, at 0°C, or mock-treated at the same temperature (–EGF). The right panel (merge) shows the merged images. The boxed area is displayed at higher magnification in the inset placed at the bottom of the merge panel. Bar: (bottom-right panel) 7.6 μm; (inset) 3.9 μm; (all other panels) 12.5 μm. (D) Double immunofluorescence showing the localization of EGFR (EGFR) and clathrin (dsRed-clathrin), in starved HeLa cells transfected with RFP-clathrin and stimulated with EGF (+EGF) for 1 h, at 0°C, or mock treated at the same temperature (–EGF). The right panel (merge) shows the merged images. The boxed area is displayed at higher magnification in the inset placed at the bottom of the merge panel. Bars: 12.5 μm (–EGF, all panels); 12.5 μm (+EGF); 3.9 μm (inset).
For the quantitation of EGFR/clathrin colocalization and the number of CCPs, HeLa cells were transfected with dsRed-clathrin (Rappoport et al., 2003) using lipofectAMINE reagent (Invitrogen, Carlsbad, CA), incubated for 16 h in SFM, placed on ice, and washed with prechilled SFM. Cells were then incubated with anti-EGFR (13A9 antibody) in SFM, in the presence or in the absence of EGF (100 ng/ml), 60 min at 0°C on a rocker, washed, fixed, and stained with an anti-mouse fluorochrome-labeled antibody. The quantitative analysis of EGFR/clathrin colocalization and the density of clathrin dots/μm2 of surface were performed as for AP2. A total series of 26–30 confocal planes (obtained at 244-nm steps for axial scanning, according to Nyquist sampling) from the ventral surface to the top of the cell, were analyzed for each cell.
Preparation of DRMs
HeLa cells were serum starved (16–18 h) and treated ± EGF, 0°C for 30 min. Cells were lysed on ice in TNE buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, pH 8.5, 1%) containing 1% Triton X-100 (Pierce, Rockford, IL), protease and phosphatase inhibitors (Roche, Mannheim, Germany), and homogenized with of a tightly fitting Dounce homogenizer (40 strokes), and through a 25-gauge needle. Lysates were brought to 40% sucrose and over-layered on a step sucrose gradient (5–35% in TNE), followed by centrifugation at 190,000 × g, 4°C, 18 h in a SW41 rotor (Beckman, Fullerton, CA). One-milliliter fractions were harvested from the top of the gradient. Two microliters of fractions 2–11 were analyzed by dot-blot, where indicated. Nine hundred microliters of each floating fraction (2–6) were diluted in TNE buffer and concentrated by centrifugation at 100,000 × g, 4°C, 30 min in a MLA130 rotor (Beckman). Pellets were entirely resuspended in sample buffer and analyzed by Western blot. Forty microliters of each nonfloating fraction (7–11) were resuspended in sample buffer and analyzed by Western blot.
RESULTS
EGF Stimulation at Low Temperature Promotes CCP Assembly, but not Clathrin-coated Vesicle Budding
To explore the relationships between the signaling-competent and the internalization-competent compartments involved in EGFR activity and in order to better define the early events following EGFR activation, we sought for experimental conditions that allow dissociating the formation of CCPs from their internalization as clathrin-coated vesicles (CCV).
Several previous reports have suggested that CCPs can form normally at 0°C in the presence of EGF, but are not internalized (Moore et al., 1987; Beck et al., 1992; Brown and Petersen, 1998; Jiang et al., 2003; Huang et al., 2004). This contention is supported by the observations that under these conditions the lateral mobility of receptors is reduced, but not abolished (Hillman and Schlessinger, 1982), and that the reduction of the lateral diffusion of occupied receptors has been shown not to be a rate-limiting step for receptor clustering or internalization, neither at 37 nor at 4°C (Schlessinger et al., 1983). We directly tested the possibility to use these conditions for our studies. By electron microscopy (EM), we analyzed cells fixed in the presence of Ruthenium Red (RR). RR stains the cell surface and plasma membrane invaginations connected to the cell surface, but not intracellular membranes, distinguishing CCPs from CCVs (Figure 1A). HeLa cells were starved for 18 h, prechilled on ice, and then treated with EGF, or mock-treated, at 0°C. We found that EGF stimulation at 0°C induced an approximately threefold increase in the number of CCPs (compare the “1h 0°C” and “1h EGF 0°C” conditions in Figure 1B), whereas the number of CCVs remained unaltered (Figure 1B). Thus, under our conditions, the molecular machinery responsible for CCP formation is functional and can be triggered by EGF stimulation. Furthermore, when the EGF-stimulated cells were transferred at 37°C for 2 min, the number of CCVs increased dramatically (Figure 1B), indicating that coated pits formed at 0°C are functional and can bud into coated vesicles.
Further support for the assembly of CCPs upon EGF stimulation at 0°C, came from double immunofluorescence experiments. In a first series of experiments, starved HeLa cells, were stimulated with EGF for 60 min, at 0°C, and stained with antibodies to AP2 and EGFR (Figure 1C). Image analysis (see Materials and Methods) was performed to estimate the number of AP2 dots/μm2 of cell surface and the percent of EGFR pixels colocalizing with AP2 pixels. The analysis was performed on the plasma membrane region of the cell facing the plastic dish, in order to reduce the possible influence of changes in the 3D cell shape due to EGF treatment. The number of AP2 dots/μm2 of cell surface was found to increase 2.1-fold after EGF stimulation, compared with control unstimulated cells (0.59 ± 0.03 vs. 0.28 ± 0.03 dots/μm2, respectively). Similarly, we found a 1.9-fold increase in the percent of EGFR pixels colocalizing with AP2 pixels, upon EGF stimulation, compared with unstimulated cells (21.4 ± 4.0 vs. 11.2 ± 3.2%, respectively). These results are in line with those obtained by EM, and suggest that, at 0°C, the newly formed CCPs assemble in regions of EGFR clustering. In a second series of experiments we transfected HeLa cells with a clathrin-RFP cDNA (Rappoport et al., 2003), and performed immunofluorescence staining with antibodies to the extracellular portion of EGFR on unfixed, unpermeabilized cells, at 4°C (Figure 1D). Under these conditions only the surface associated EGFR was labeled, and we found a 1.7-fold increase (from 15.6 ± 2.1 to 26.3 ± 5.9%) in the percentage of EGFRs colocalizing with clathrin. Finally, we also performed a 3D analysis, counting the number of clathrin dots present on the entire cell surface, before and after EGF treatment. The analysis was performed on series of 26–30 confocal planes reconstructing each cell. The number of clathrin dots/μm2 of cell surface was found to increase from 0.14 ± 0.09 to 0.63 ± 0.32 dots/μm2, after EGF stimulation.
EGF Stimulation at Low Temperature Reveals a Simultaneous Recruitment of Signaling and Endocytic Proteins to DRMs
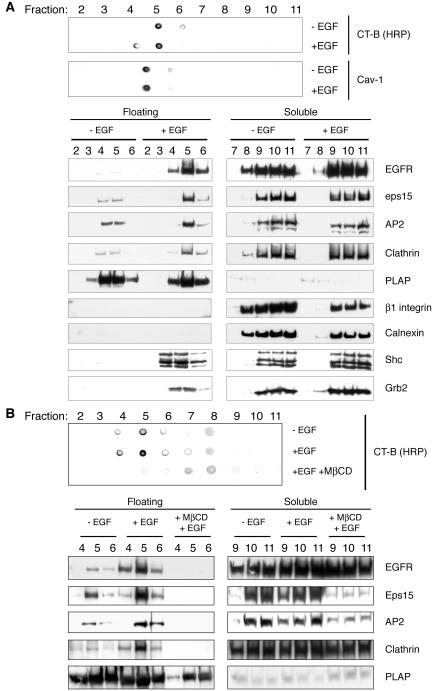
We next evaluated whether EGF stimulation, under conditions in which CCP formation can be dissociated from their internalization (0°C), could induce the recruitment of EGFR, and of two signaling adaptor proteins, i.e., Shc and Grb2, to DRMs. Shc and Grb2 are known to be actively recruited to rafts after ligand engagement of several receptors, including EGFR (Biedi et al., 2003; Ridyard and Robbins, 2003; Yang et al., 2004). Starved HeLa cells were cultured in the presence or in the absence of EGF, for 30 min at 0°C. Stimulated and control cells, were lysed with 1% Triton X-100, at 4°C, and fractionated on a 5–40% step sucrose gradient (11 fractions; Figure 2A). The DRM-containing floating fractions were identified by dot-blot analysis analyzing two raft markers, an HRP conjugate of the B subunit of the cholera toxin (CT-B/HRP), that specifically binds to GM1, an abundant lipid component of membrane rafts (Parton, 1994; Mobius et al., 1999), or antibodies to caveolin 1. Fractions 4–6 were found positive for one or both markers (Figure 2A, top panel). In these same fractions, by Western blot, we could readily detect a significant increase in the levels of EGFR, Grb2, and Shc upon EGF treatment (Figure 2A, bottom panel), indicating that the recruitment of receptor and adaptor proteins in raft signaling platforms is not impaired at 0°C. The specificity of the fractionization method was further validated by analyzing the distribution of two plasma membrane proteins, Placental Alkaline Phosphatase (PLAP), a GPI anchor protein known to exclusively associate with DRMs (Harder et al., 1998; Lipardi et al., 2000), and β1 integrin, a transmembrane protein reported to partition in non-raft regions of the plasma membrane (Cunningham et al., 2003). As an additional control we also tested the distribution of calnexin, a transmembrane protein reported to partition in non-raft regions of endoplasmic reticulum (Schuck et al., 2003). As expected, we found PLAP almost exclusively associated to fractions 4–6 and β1 integrin subunit and calnexin associated to the non-raft fractions 7–11 (Figure 2A). More importantly, neither of these proteins showed any change in distribution upon EGF stimulation (Figure 2A).
Figure 2.
EGF-dependent association of effector and endocytic proteins to DRMs. (A) Starved HeLa cells were stimulated at 0°C with EGF (+EGF) or mock-treated (–EGF) and processed for isolation of DRMs, as described in Materials and Methods, followed by analysis by dot-blot (top panel) or immunoblot with the indicated antibodies (bottom panel). Immunoblot, 1/500 of each fraction was spotted for dot-blot detection. Dot blot, 9/10 of each floating-fractions (2–6) and 1/25 of each soluble fractions (7–11), respectively, were loaded onto SDS-PAGE gels for subsequent immunoblot detection of EGFR, endocytic (Eps15, AP2, Clathrin), signaling effector (Shc, Grb2), and control proteins (PLAP, β1 integrin, Calnexin). Floating and soluble fractions were run on parallel SDS-PAGE gels and then stained under identical conditions. (B) Starved HeLa cells were either treated with MβCD (+MβCD) or mock-treated, transferred at 0°C and stimulated with EGF (+EGF), or mock-treated (–EGF). Cells were then processed for isolation of DRMs, followed by immunoblot with the indicated antibodies (bottom panel), or dot-blot (top panel). Loading of lanes was as in B.
We then evaluated whether a number of endocytic proteins, including clathrin, the adaptor complex AP2 (Smythe, 2002), and Eps15, are recruited to DRM upon EGF stimulation. In particular, Eps15 is known to redistribute to the plasma membrane, upon EGF stimulation (Tebar et al., 1996; van Delft et al., 1997; Torrisi et al., 1999; Confalonieri et al., 2000). In addition, there is evidence that supports the hypothesis that eps15 recruitment might be one of the first events in the assembly of a pit (van Delft et al., 1997), possibly due to its projected role in the docking of AP2 onto the plasma membrane (Benmerah et al., 1996; Tebar et al., 1996; Benmerah et al., 1999; Torrisi et al., 1999). Thus, eps15 represents a good marker of early events in the dynamics of coated pit formation. As shown in Figure 2A, we detected a significant increase in the levels of the three proteins in fractions 4–6, and most notably in fraction 5, upon EGF stimulation (see also Figure 2B and Supplemental Figure 1 for a quantitative analysis). Of note the ratio between DRM and soluble-fraction partition, upon EGF stimulation, was in the same order of magnitude for both endocytic and effector proteins (Figure 2A).
As a further control, we used the cholesterol-extracting drug MβCD, to disrupt rafts. Under the conditions used in our studies, the treatment with MβCD led to a cholesterol extraction of ∼60%, as determined by a colorimetric assay to measure the residual cholesterol (unpublished data; see Materials and Methods for details). We evaluated the effects of MβCD treatment on EGFR, Eps15, AP2, and clathrin recruitment to floating fractions. Starved HeLa cells, treated with MβCD, were cultured in the presence or in the absence of EGF, at 0°C for 60 min, and compared with untreated cells by a flotation assay. In the presence of MβCD, there was no recruitment of EGFR, AP2, clathrin, and Eps15 to floating fractions (Figure 2B). As a control for the effectiveness of the MβCD treatment, we analyzed the distribution of a known raft-associated protein, PLAP. In the MβCD-treated cells the amount of PLAP associated to rafts was reduced compared with controls (Figure 2B), consistently with previous reports (Lipardi et al., 2000).
The sum of all the above results indicates that DRMs are a site of recruitment of endocytic machinery, raising the possibility that they also constitute a site of formation of CCPs.
Morphological Identification of GM1-rich Membrane Domains by Electron Microscopy
The observation that active EGFR is recruited to DRM together with endocytic and signaling proteins prompted us to identify and characterize morphologically these specific sites. As a marker to identify membrane rafts by EM, we used CT-B/HRP. The specificity of the peroxidase staining for the membrane raft regions, upon labeling with CT-B/HRP, was proved through a series of tests.
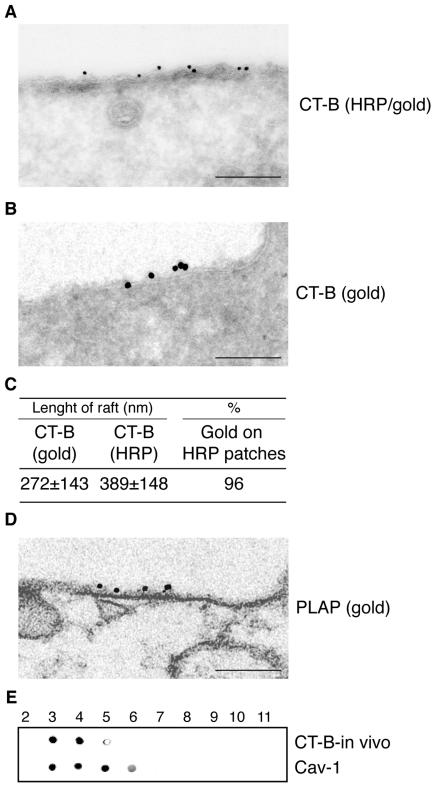
Starved HeLa cells were cultured at 0°C for 1 h in the presence of CT-B/HRP and EGF. The samples were then either processed (Figure 3A) or not processed (Figure 3B) to develop the HRP staining. Ultrathin cryosections were immunogold labeled with anti-HRP antibodies (Figure 3, A and B). No difference in gold distribution between the two conditions was found (Figure 3, A and B), demonstrating that the HRP reaction product did not interfere with the immunogold labeling. In addition, by morphometry, we found 96% of the HRP/gold particles associated to the electron-dense staining of the HRP reaction product (Figure 3, A–C), demonstrating that HRP staining was restricted to CT-B/HRP–labeled regions. We then determined the average length of the GM-rich regions detected by either peroxidase or immunogold. We found an average value of 389 and 272 nm, respectively, for the two conditions (Figure 3C), whereas the average ratio between the sizes of individual costained rafts, measured by the two methods on the same raft, was 1.3 ± 0.2 (n = 31 evaluated rafts). Thus, the spreading of the HRP product overestimated the dimension of a raft of only ∼15–20%, on each of the end of the sectioned patch, compared with immunogold.
Figure 3.
Specificity of CT-B/HRP to identify GM1-rich membrane domains by electron microscopy. Starved HeLa cells cultured at 0°C for 1 h in the presence of CT-B/HRP and epidermal growth factor (A–D). (A) Cells were processed to develop the HRP staining (electron-dense staining underneath the plasma membrane), and immunogold labeled with anti-HRP antibodies on ultrathin sections. Bar, 0.22 μm. (B) Cells were not processed to develop the HRP staining and immunogold labeled with anti-HRP antibodies. Bar, 0.15 μm. (C) Morphometry of the experiment shown in A (n = 40 cell profiles). [Length of rafts], average size of GM1-rich regions identified by CT-B/HRP labeling, and measured in two ways: [CT-B(gold)], distance between the first and last gold particle in cluster labeling HRP; [CT-B(HRP)], distance between the two edges of the electron dense staining of the HRP reaction product revealing HRP. Values are expressed in nanometers ± SD. [%] indicates the percent of the total gold particles identifying HRP present on the electron-dense staining of the HRP reaction product revealing HRP, respect to the total gold present on the plasma membrane. (D) Preembedding immunogold labeling of HeLa cells, stimulated with epidermal growth factor, at 0°C for 1 h, with anti-PLAP antibodies. Morphometry of the PLAP/gold patches associated to the plasma membrane reveals lengths ranging from 91 to 431 nm (mean = 212 ± 102; n = 25). Bar: (left) 0.16 μm. (E) Starved HeLa cells cultured at 0°C for 1 h in the presence of CT-B/HRP and epidermal growth factor. Cells were processed for the isolation of DRMs, as described in Materials and Methods, followed by dot-blot analysis of the fractions. The HRP reaction was developed to reveal the in vivo bound CT-B (CT-B-in vivo), and, after stripping, immunostained with antibodies to caveolin 1 (Cav-1).
As a further control, we performed detection of membrane rafts by immunogold localization of PLAP, under the same culture conditions (Figure 3D). By morphometry we measured the PLAP/gold patches associated to the plasma membrane, and found values ranging from 91 to 431 nm (mean = 212 ± 102 nm; n = 25), comparable to those obtained by measuring the size of the GM1-rich plasma membrane regions identified by CT-B in vivo (shown in Figure 3, A–C). These values are compatible with those described for clustered rafts (Kusumi et al., 2004; Mayor and Rao, 2004; and see also the Discussion of this article). Finally, we tested the specificity of the CT-B/HRP staining by a biochemical method. Starved HeLa cells were cultured at 0°C for 1 h, in the presence of CT-B/HRP and EGF, lysed, and processed for the isolation of DRMs, as described above. The collected fractions were analyzed by dot-blot. The HRP reaction was developed to reveal the in vivo bound CT-B (CT-B-in vivo, Figure 3E). As shown, CT-B provided to the cells in vivo, under the same conditions used for EM analysis, was found exclusively bound to DRM fractions (fractions 4–6; Figure 3E). To further demonstrate that fractions 4–6 are indeed DRMs, we stripped the nitrocellulose and immunostained the membrane with antibodies to caveolin 1 (Cav-1, Figure 3E), which reacted with floating fractions.
EGFR and Endocytic Proteins Are Recruited to GM1-rich Membrane Domains upon EGFR Stimulation
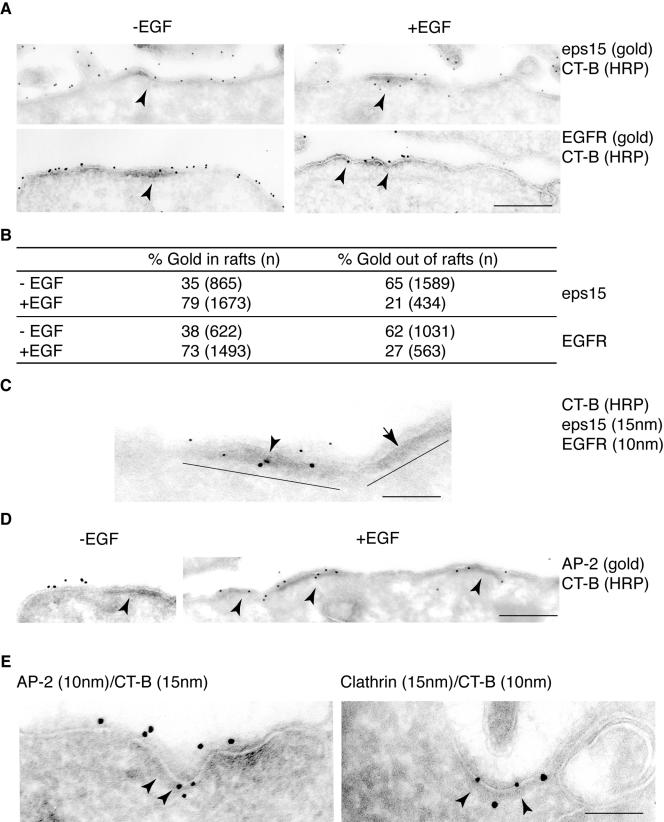
To evaluate whether endocytic proteins are recruited to the membrane raft platforms identified by CT-B/HRP labeling, we started our analysis with eps15. We first used immunogold to colocalize either eps15 or EGFR, on ultrathin cryo-sections of HeLa cells, with GM1-rich domains stained by CT-B/HRP. Comparison of experiments performed in the presence and in the absence of EGF stimulation (Figure 4A), revealed that in unstimulated cells (Figure 4A, left), both eps15 and EGFR were about equally distributed within and without GM1-rich regions. Conversely, after EGF treatment (Figure 4A, right), there was an approximately twofold enrichment of both eps15 and EGFR in lipid rafts (Figure 4B). Noteworthy, observation at low magnification, to obtain a comprehensive view of the total cell profile (unpublished data), revealed that only a minor fraction of the total plasma membrane profile was labeled by CT-B/HRP. Thus, we evaluated the percent of the total perimeter of a cell profile labeled by CT-B/HRP, and found this value to be, on average ∼19% (n = 10 cell profiles). This result strengthens the significance of the twofold increase of EGFR and Eps15 observed in raft regions of the plasma membrane upon EGF stimulation.
Figure 4.
Eps15 and EGFR localize in GM1-rich regions of the plasma membrane. (A) HeLa cells were treated with CT-B-HRP [CT-B (HRP)], either in the absence (–EGF, left panels) or presence (+EGF, right panels) of epidermal growth factor. CT-B-HRP labeling was followed by fixation and development of the peroxidase reaction. Ultrathin cryosections were then reacted with the indicated antibodies (gold), to identify eps15 or EGFR. Arrowheads point to raft regions. Bar, 0.56 μm. (B) Morphometric analysis of the experiment shown in A. Data are expressed as % of gold particles associated to CT-B (HRP) electron-dense patches, counted in 30 cell profiles under conditions of ± epidermal growth factor treatment. In parentheses the number of gold particles counted in 30 cell profiles, for each treatment, is indicated. Data are representative of three independent experiments. (C) Starved HeLa cells were treated at 0°C with CT-B-HRP, in the presence of epidermal growth factor. Ultrathin cryosections were reacted with the indicated antibodies (gold). The electron-dense patches, highlighted by thick solid bars identify GM1-rich regions [CT-B (HRP)]. Eps15, EGFR, and CT-B colocalize in one of the two rafts (arrowhead). The adjacent membrane raft, devoid of EGFR (arrow), does not associate with eps15, demonstrating the specificity of the event. Bar, 0.22 μm. (D) Starved HeLa cells were treated at 0°C with CT-B-HRP [CT-B (HRP)], either in the absence (–EGF) or presence (+EGF) of epidermal growth factor. GM1-rich regions are indicated by arrowheads. Gold particles identify AP2. Bar: (–EGF) 0.56 μm; (+EGF) 0.70 μm. (E) AP2 and clathrin are recruited to GM1-rich regions in epidermal growth factor–treated cells. Starved HeLa cells were treated at 0°C with CT-B/HRP, in the presence of epidermal growth factor. Ultrathin cryosections were then reacted with the indicated antibodies (gold) to HRP in combination with antibodies to either AP2 or Clathrin. Bar: (left) 0.20 μm; (right) 0.13 μm.
By comparing data obtained with immuno-EM (Figure 4, A and B) to those obtained on DRMs (Figure 2), it is obvious that the two methods showed considerable variations in assessing the magnitude of EGFR (and eps15 recruitment) to raft regions. By DRM analysis, a minute fraction of all EGFR was recruited to the floating fractions, upon EGF stimulation. Scanning of the blots in Figure 2 revealed that ∼ 2% of the EGFR pool was in DRMs after EGF treatment. Conversely, more than 70% of the EGFR was found in GM1-rich regions, by immuno-EM (Figure 4, A and B). It is of note, however, that in the case of DRMs, we analyzed the entire cellular pool of EGFR, whereas in the case of immuno-EM, only plasma membrane-associated EGFR was evaluated. To resolve, at least in part, this discrepancy, we measured which fraction of the EGFR is on the plasma membrane compared with other cellular compartments. To do this, we performed a surface biotinylation approach (see Supplemental Figure 2 for details) and found that around one-third of the total EGFR pool is present on the plasma membrane (Supplemental Figure 2). This result indicates that the recruitment of EGFR to fractions 4–6 of DRMs, once corrected for the fraction of EGFR present on the cell membrane, is even more significant (∼6%) than what appears from the Western blots. In addition, in the experiments on DRMs, the floating fractions (2–6) were further concentrated by a step of ultracentrifugation before loading, whereas the soluble fractions could be loaded directly. From a series of measurements (unpublished data), this procedure results in a variable loss of material, and in general of a factor of at least 2. Although these measurements render data obtained with the two methodologies (DRMs and immuno-EM) more internally consistent, a roughly fivefold difference, between the two detection methods, still persists. This is consistent with the possibility that the two methods explore regions of the plasma membrane that are not completely overlapping. Possibly, the DRM approach is more stringent, and, under conditions of lysis with Triton X-100, part of the GM1-rich regions of the plasma membrane is solubilized. Whatever the case, our data stress the need to comparatively use the two methodologies to acquire a comprehensive picture.
To define whether EGFR and eps15 were recruited to the same rafts, we performed a double immunogold staining, and found that, upon EGF stimulation, CT-B/HRP-positive membrane domains colocalized with both proteins (Figure 4C). We also evaluated the recruitment of other endocytic proteins to GM1-rich membranes upon EGF stimulation. By immunogold we found that AP2 is recruited to CT-B/HRP-positive membrane domains upon EGF stimulation (Figure 4D). This result was further reinforced by double immunogold labeling of CT-B/HRP and either AP2 (Figure 4E, left) or clathrin (Figure 4E, right). By this approach, we found that these structural endocytic proteins could be detected in nascent pits, which incorporated raft membranes detected by CT-B/HRP-gold, further supporting the hypothesis of their formation within membrane rafts (Figure 4E).
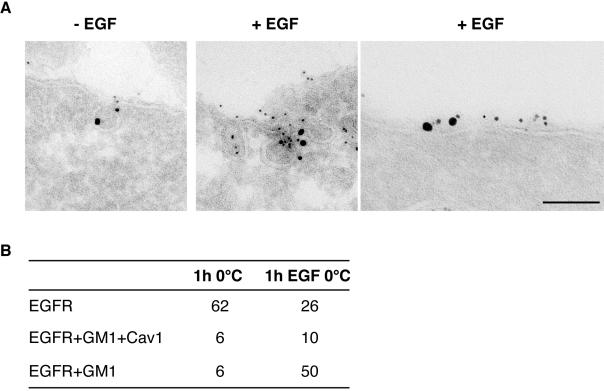
As EGFR has been suggested to localize also in caveolae, which constitute a specialized subpopulation of membrane rafts (Parton and Simons, 1995; Kurzchalia and Parton, 1999b; Nabi and Le, 2003) marked by the presence of caveolin (Smart et al., 1999), independently of EGF stimulation (Mineo et al., 1999), we checked whether, under our experimental conditions, the EGFR was recruited to caveolae, or to other raft regions of the plasma membrane (enriched or not in caveolin). A triple immunogold localization, to simultaneously detect EGFR, caveolin and GM1 (CT-B), was performed on cells cultured in the presence or the absence of EGF at 0°C. As shown in Figure 5, A and B, there was no significant difference in the extent of colocalization of EGFR with caveolin and GM1, in the two conditions. Of note, in all cases in which colocalization of the three proteins was detected, the staining was also associated to morphologically identifiable caveolae. However, there was also substantial colocalization of EGFR and GM1, in the absence of detectable caveolin, in flat regions of the plasma membrane (Figure 5A), which increased dramatically upon EGF stimulation (Figure 5B).
Figure 5.
EGFR is recruited to noncaveolar, GM1-rich regions of the plasma membrane. Triple immunogold labeling of ultrathin cryosections of starved HeLa cells, labeled with CT-B/HRP, and incubated in the presence (+EGF), or in the absence of epidermal growth factor (–EGF). (A) Cryosections were triple-labeled with antibody to HRP to localize GM-1 (5 nm), anti-caveolin (10 nm), and EGFR (15 nm). The first two panels on the left show colocalization of the three antigens in caveolae. The rightmost panel shows colocalizaton of EGFR and GM1 in a flat region of the plasma membrane Bar: (left) 0.16 μm; (middle) 0.15 μm; (right) 0.1 μm. (B) Morphometric analysis of the experiment shown in A. Results show the number of 15-nm gold particles (identifying EGFR) present on the plasma membrane, colocalizing with caveolin and GM1 (EGFR+GM1+Cav1), only with GM1 (EGFR+GM1), or with neither one of the other two antigens (EGFR). Data are referred to 10 cell profiles. Of note, the triple colocalization of EGFR with GM1 and caveolin 1 was found almost exclusively within morphologically identifiable caveolae, whereas the EGFR colocalized with GM1, but not with caveolin 1, in flat regions of the plasma membrane.
Altogether these results indicate that, upon EGF stimulation, the EGFR (and, by inference, the endocytic machinery) are recruited to plasma membrane raft regions clearly distinct from caveolae.
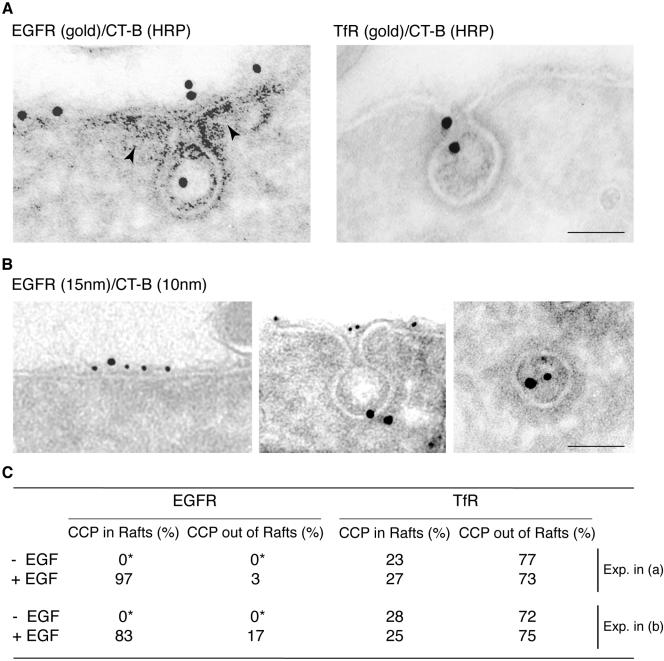
EGFR-containing CCPs Bud from GM1-rich Membrane Domains
The recruitment in GM1-rich membrane regions of EGFR and of the endocytic machinery necessary for CCP assembly, strongly suggested that these structures can assemble starting from lipid rafts, upon EGF stimulation. We sought for direct experimental confirmation of this hypothesis. HeLa cells, mock-treated or EGF-treated, were labeled with CT-B/HRP and gold-labeled with either anti-EGFR or anti-TfR antibodies (Figure 6A). Strikingly, almost all of the EGFR-containing pits were associated to CT-B/HRP-labeled membrane regions identified by the HRP reaction product (Figure 6A). Similar results were obtained by double immunogold labeling of CT-B/HRP and either TFR (unpublished data) or EGFR (Figure 6B). Of note, in cells transferred for 2 min at 37°C, after stimulation at 0°C, EGFR and HRP/gold colocalized within CCVs. Finally, morphometry (Figure 6C) performed on these samples revealed that most of the EGFR-containing coated pits, in the presence of EGF, formed in regions labeled by CT-B (97 and 83% in the experiment in Figure 6, A and B, respectively). Conversely, TfR-containing coated pits were randomly distributed in rafts and nonrafts regions (Figure 6C), independently from EGF stimulation. Thus EGFR-containing CCPs almost invariably include raft regions.
Figure 6.
EGFR-containing coated pits form within membrane rafts. (A) Starved HeLa cells were treated at 0°C with epidermal growth factor (or without epidermal growth factor, unpublished data) in the presence of CT-B-HRP, followed by fixation and development of the peroxidase reaction [CT-B-(HRP)]. Ultrathin cryosections were then reacted with the indicated antibodies (gold). Left, EGFR (gold) is internalized via a coated pit stemming from a GM1-rich region identified by CT-B (peroxidase) staining (arrowheads); right, TfR (gold), is internalized via a coated pit stemming from a non–GM1-rich region. Bar, 0.1 μm. (B) HeLa cells were treated as in A and then fixed (left and center) or treated as in A and transferred at 37°C for 2 min before fixation (right). Ultrathin cryosections were immunogold labeled for EGFR (15 nm) and HRP (10 nm). In cells stimulated at 0°C the two antigens colocalize within discrete flat regions of the plasma membrane (right), and budding clathrin-coated pits (center). In cells transferred for 2 min at 37°C the two antigens colocalize within clathrin-coated vesicles (left). Bar: (left) 0.11 μm; (middle) 0.13 μm; (right) 0.07 μm. (C) Morphometric analysis of the experiment shown in A and B. Data are expressed as the % of either EGFR-containing or TfR-containing coated pits (n = 60 coated pits/condition) found associated to patches of CT-B-HRP revealed by either immunoperoxidase or immunogold. In the –EGF samples, indicated by an asterisk, no EGFR-containing pits were detectable, due to the 18 h of starvation. The total number of CCPs labeled with TfR was similar in epidermal growth factor stimulated and nonstimulated cells, 30 and 35, respectively (n = 40 cell profiles). Data are representative of three experiments.
Recruitment of EGFR and Endocytic Proteins to DRMs Is Not Influenced by Dose and Temperature of EGF Stimulation
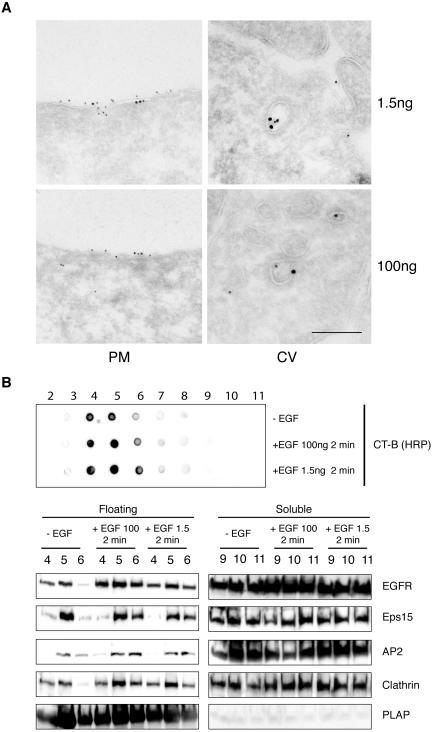
All our experiments were performed by EGF stimulation at 0°C, in order to work under conditions in which the formation of coated pits was dissociated from their budding, to allow an easier visualization of the events and their morphometric quantitation. In addition, we used high concentrations of EGF (100 ng/ml), to elicit maximal response. A possible caveat is that the low temperature impairs the exit of EGFR from membrane rafts, forcing their internalization from these regions. Moreover, one might ask whether the observed effect is a “nonphysiological” artifact of the high EGF concentration. Thus, we performed experiments at 37°C, comparing high (100 ng/ml) to low (1.5 ng/ml) doses of EGF.
In a first series of experiments, we labeled starved HeLa cells with CT-B/HRP, and stimulated them with either 1.5 or 100 ng/ml EGF, for 2 min, at 37°C. Cryo-ultrathin sections were double gold-labeled with anti-EGFR and anti-HRP antibodies (Figure 7A). The results showed EGFR and CT-B colocalized within discrete regions of the plasma membrane or in morphologically identified CCVs, in both conditions (Figure 7A).
Figure 7.
EGFR recruitment and clathrin-coated pit assembly in membrane rafts are not dependent on dose and temperature of epidermal growth factor treatment. (A) Double immunogold labeling of HeLa cells treated with CT-B-HRP for 20′ at 0°C and stimulated with epidermal growth factor at 37°C for 2 min (top panels, epidermal growth factor 1.5 ng/ml; bottom panels epidermal growth factor 100 ng/ml). CT-B-HRP (10 nm) and anti-EGFR (15 nm) colocalize within discrete regions of the plasma membrane (PM), or within clathrin coated vesicles (CV). Bar: (right panels) 0.21 μm: (top left) 0.37 μm; (bottom left) 0.43 μm. (B) Starved HeLa cells were stimulated for 2 min at 37°C with epidermal growth factor at 100 ng/ml (+EGF 100), or 1.5 ng/ml (+EGF 1.5), or mock-treated (–EGF) and processed for isolation of DRMs, as described in Materials and Methods, followed by analysis by immunoblot with the indicated antibodies (bottom panel), or dot-blot (top panel) with CT-B/HRP to identify GM1. Loading of lanes was as in Figure 1.
We then evaluated the recruitment of EGFR, AP2, clathrin, and Eps15 to floating fractions, upon stimulation of starved HeLa cells, at 37°C for 2 min, with 1.5 or 100 ng/ml EGF. The cells were lysed and processed for the isolation of DRMs, as described above. The DRM-containing floating fractions were identified by dot-blot analysis using CT-B/HRP. As shown in Figure 7B, all the proteins analyzed were recruited to floating fractions at 100 ng/ml EGF, demonstrating that this event is induced by EGF stimulation independently of the temperature conditions. A quantitative assessment of EGFR, Eps15, AP2, and clathrin in the floating fractions, normalized for the amount of PLAP, as a marker for membrane rafts (see Supplemental Figure 1), confirmed this result. However, the magnitude of the recruitment was lower with respect to the 0°C condition (Supplemental Figure 1). The difference between the two conditions is most likely due to the fact that at 37°C internalization rapidly removes the receptor and the associated endocytic machinery from the plasma membrane.
Finally, also under conditions of stimulation at 37°C with low amounts of EGF (1.5 ng/ml), EGFR, AP2, and clathrin were enriched in the DRM fractions (Figure 7B). As expected, the extent of this recruitment was lower compared with the condition of stimulation with 100 ng/ml EGF (Supplemental Figure 1), because of the lower extent of EGFR activation.
DISCUSSION
The role of lipid rafts in signaling is extensively documented, as they function as physical platforms to concentrate and assemble the signal transduction machinery (Carpenter, 2000; Pralle et al., 2000; McPherson et al., 2001). In the case of RTKs, e.g., EGFR, ligand-induced receptor internalization represents, in itself, the outcome of a signaling mechanism, which requires the intrinsic kinase activity of the receptor (Carpenter, 2000). Thus, in principle, nothing rules out the possibility that lipid rafts, just as they assemble the transduction machinery needed for the downstream propagation of the signal from RTK receptors, also serve as platforms to recruit the necessary machinery for their endocytosis. Nonsignaling receptors internalized through CCPs, such as the TfR and the low-density lipoprotein receptor (LDLR), are not enriched in lipid rafts (Simons and Ikonen, 1997; Brown and Petersen, 1998). This finding has led to the obvious conclusion that CCPs assembly must occur within the bulk of the plasma membrane. However, a number of observations are at odd with this view, pointing to a more complex situation. First, the B-cell antigen receptor (BCR) a non-RTK, multisubunit receptor, activates both tyrosine phosphorylation of downstream targets and recruitment of clathrin on membrane rafts (Stoddart et al., 2002). Second, cholera and shiga toxins interact with sphingolipids that are contained in lipid rafts, yet they are internalized through CCPs (Sandvig and van Deurs, 2002). Third, the anthrax toxin receptor partitions into rafts before internalization via CCPs (Abrami et al., 2003). Fourth, disruption of lipid rafts by cholesterol depletion, inhibits both clathrin and nonclathrin endocytosis (Johannes and Lamaze, 2002). Finally, the exit of the EGFR from the rafts, upon EGF stimulation, has been recently questioned (Ringerike et al., 2002). Therefore, the possibility exists that signaling receptors associated to membrane rafts, e.g., RTKs, may specifically recruit both signaling and endocytic proteins to these membrane platforms.
Our present results demonstrate that, in the case of the EGFR, the lipid rafts are competent for recruitment of both effector signaling molecules and endocytic machinery. Our findings are in line with those of Stoddart et al. (2002), who demonstrated a similar situation for CCP-mediated internalization of the BCR. Although, we do not provide evidence in our present study that the endocytic machinery recruited to CCPs is “active,” Stoddart et al. (2002) could show preferential recruitment of tyrosine phosphorylated clathrin to membrane rafts. In addition, these authors showed that src-dependent tyrosine phosphorylation of clathrin is a crucial event for the internalization of BCR (Stoddart et al., 2002). Similarly, phosphorylation of clathrin has been shown to be important in the internalization of EGFR (Wilde et al., 1999). Therefore, it is likely that the recruitment of the internalization machinery shown in our study may involve “active” elements, such as phosphorylated clathrin. In addition, we show here that EGFR-internalizing CCPs form preferentially from GM1-rich regions of the plasma membrane, whereas the nonsignaling TfR is randomly internalized through CCPs that form within or without GM1-rich regions. Thus, our results are compatible with a scenario in which a plasma membrane receptor is simply internalized from the plasma membrane location (be it a raft or not) in which it shows competence for interaction with the endocytic machinery. Nonsignaling receptors that are constitutively internalized, such as the TfR or the LDLR, constantly expose endocytic signals and are therefore internalized simply as a function of this property. On the other hand, EGFR, which is internalized in a ligand-dependent manner, triggers the assembly of the endocytic machinery, and the formation of a coated pit, in the same location in which it is activated, i.e., in a lipid raft.
Membrane rafts are dynamic entities, which can diffuse (Pralle et al., 2000; Sprong et al., 2001) and coalesce into larger and more stable structures in response to signaling (Simons and Toomre, 2000; Kusumi et al., 2004; Mayor and Rao, 2004). Current thinking holds that in resting cells rafts are small (possibly as small as 40 nm in diameter or even less, Prior et al., 2003) and unstable (or “reserve raft”; Simons and Toomre, 2000; Kusumi et al., 2004; Mayor and Rao, 2004). On stimulation, these small unstable rafts are clustered, inducing larger and more stable rafts, which can in turn function as effective signaling platform (or “receptor-cluster rafts”; Simons and Toomre, 2000; Kusumi et al., 2004; Mayor and Rao, 2004). Of note, it is possible that many controversies, present in the literature about the “real size” of rafts, could be due to “unintentional” cross-linking of small “reserve rafts” in resting cells, which can be caused by a wide variety of experimental manipulation (Simons and Toomre, 2000; Kusumi et al., 2004; Mayor and Rao, 2004).
A similar situation might apply to our present studies, as we detected rafts of similar size in both resting cells (without EGF) and in EGF-stimulated cells. Especially in unstimulated cells the size of rafts, as detected in our experiments, exceeded that of the “reserve rafts.” The explanation for this likely resides in the use of fixatives (for EM procedures) or of low temperature (used to uncouple CCP formation from their internalization in many experiments), in that both conditions are known to cause coalescence of rafts (Kusumi et al., 2004). Thus, most likely, our morphological analysis was performed in an “artifactual” situation, in which the size of rafts (especially in the absence of physiological clustering agents, such as EGF) was overestimated. We note, however, that, far from being a drawback, this actually represents a “useful artifact.” Indeed, it would be difficult to determine exactly whether or not a protein is enriched (or more stably associated) with rafts, at the morphological level, if rafts were to be detected as entities of 40 nm in diameter. Conversely, our data clearly show that EGFR and endocytic proteins are enriched in rafts, upon EGF stimulation, and not in other regions of the plasma membrane, and that rafts are incorporated into EGFR-containing nascent-coated pits. Of note these morphological results are precisely confirmed by independent biochemical approaches on DRMs. The above considerations, and the acceptance of the concept of a clear distinction between “reserve rafts” and “receptor-cluster rafts,” should also help in rationalizing the outstanding issue of whether there is sufficient membrane in a raft to form a CCP. In a small “reserve raft” this would certainly not be the case (a CCP of 90 nm of diameter would require ∼2.5 × 104 nm2 of membrane, vs. ∼1.2 × 103 nm2 available in a 40-nm-diameter raft). However, a larger stabilized “receptor-cluster raft” would contain enough plasma membrane to form a pit (∼5.7 × 104 nm2 in a raft of 270-nm diameter, such as those that we measured). We note incidentally, that the coalescence of rafts has also been invoked as the mechanism for the formation of caveolae (Pralle et al., 2000), a canonical raft-based internalization organelle: a caveola has an average diameter of 60 nm, corresponding to a surface of 1.1 × 104 nm2, about 10 times more then a single “reserve raft” could provide.
In summary, our results show that an RTK can be internalized from membrane rafts, via CCPs, supporting the notion that the signaling- and the internalization-competent domains on the plasma membrane are overlapping. This conclusion explains the lack of evidence identifying an “exit-raft-into-pit” signal for EGFR, at variance with the well-characterized “move-into-raft” signal (Yamabhai and Anderson, 2002). Consequently, we propose a scenario in which EGFR endocytosis is purely a signaling event, triggered by the kinase activity of the receptor, and as such takes place starting from the signaling-competent domain on the plasma membrane. It has not escaped our attention that if EGFR-containing CCPs were indeed to form starting from rafts, this might, in principle, blur the currently accepted distinction between the clathrin-dependent, and the clathrin-independent/raft-dependent internalization pathways. However, a clear distinction between “topology” and “mechanism” must be made. Our present results allow to draw conclusions only on topology, i.e., that there is only one plasma membrane compartment in which different molecular machineries are recruited, to execute diverse EGFR-activated functions. These machineries, from a combined analysis of our results and of existing literature, are i) the “effector” machinery, needed to propagate the signal down-stream, ii) the CCP machinery, and iii) a still ill-defined series of endocytic effectors needed for the nonclathrin raft-dependent pathway. Our results, therefore, do not implicate that rafts are mechanistically “needed” to execute the clathrin pathway. Actually, this possibility is disfavored by the fact that the TfR, which is not enriched in rafts, is internalized through coated pits (this study). In addition, cholesterol-blocking agents, such as nystatin or filipin, although disrupting nonclathrin raft-dependent internalization, do not interfere with clathrin endocytosis (Rothberg et al., 1992; Lisanti et al., 1993; Schnitzer et al., 1994), also in the case of the EGFR (Sigismund et al., 2005).
Although further investigation is inevitably warranted, our results clearly indicate that the focus should now be moved from a topological to a functional issue. In other words, the definition of what directs the EGFR to a clathrin or to a nonclathrin raft-dependent pathway should not rely on the understanding of what retains the receptor in rafts (for the nonclathrin pathway), or of what causes the receptor to exit a raft and to move into a pit, but rather on the elucidation of which endocytic machinery is recruited to the raft-resident receptor, and under which conditions, to allow internalization through one or the other pathway. In this contention, we have recently demonstrated that receptor ubiquitination couples the EGFR preferentially to the non-clathrin raft pathway (Sigismund et al., 2005). In addition, we showed that the EGFR undergoes distinct posttranslational modifications and becomes committed to different internalization routes, as a function of ligand dose and receptor activation. At low EGF concentrations, the EGFR is tyrosine phosphorylated, fully competent for signaling, not ubiquitinated and its is internalized through CCPs (Sigismund et al., 2005). At high EGF concentrations, the EGFR becomes ubiquitinated and shows substantial endocytosis through the nonclathrin raft pathway (Sigismund et al., 2005). It is thus conceivable that different posttranslational modifications, tyrosine phosphorylation (together with ensuing unmasking of in-cis endocytic signals) on the one hand, and ubiquitination on the other, might direct the raft-resident receptor respectively to the clathrin or the non-clathrin raft pathway.
Supplementary Material
Acknowledgments
We thank Dr. Tom Kirchhausen for critically reviewing the manuscript and Drs. Alberto Diaspro, Mario Faretta, Giuseppe Vicidomini, and Paolo Bianchini for help with the confocal analysis. This work was supported by grants from AIRC (Italian Association for Cancer Research), Telethon Foundation, European Community (VI Framework), MIUR (Italian Ministry for University and Research) and Human Science Frontier Program, to P.P.D.F., and from AIRC, FIRC (Italian Foundation for Cancer Research), MIUR, and Telethon Foundation (GTF03001) to C.T.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–07–0596) on March 16, 2005.
Abbreviations used: RTK, receptor tyrosine kinases; EGFR, epidermal growth factor receptor; CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; DRM, detergent-resistant membrane; GPI, glycosylphosphatidyl-inositol; HPR, horseradish peroxidase; CT-B/HRP, HRP-labeled cholera toxin B subunit; MβCD, methyl-β-cyclodextrin; PLAP, placental alkaline phosphatase; Tf, transferrin; TfR, transferrin receptor.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Abrami, L., Liu, S., Cosson, P., Leppla, S. H., and Van Der Goot, F. G. (2003). Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, R. G., and Jacobson, K. (2002). A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825. [DOI] [PubMed] [Google Scholar]
- Beck, K. A., Chang, M., Brodsky, F. M., and Keen, J. H. (1992). Clathrin assembly protein AP-2 induces aggregation of membrane vesicles: a possible role for AP-2 in endosome formation. J. Cell Biol. 119, 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303–1311. [DOI] [PubMed] [Google Scholar]
- Benmerah, A., Begue, B., Dautry-Varsat, A., and Cerf-Bensussan, N. (1996). The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 271, 12111–12116. [DOI] [PubMed] [Google Scholar]
- Biedi, C., Panetta, D., Segat, D., Cordera, R., and Maggi, D. (2003). Specificity of insulin-like growth factor I and insulin on Shc phosphorylation and Grb2 recruitment in caveolae. Endocrinology 144, 5497–5503. [DOI] [PubMed] [Google Scholar]
- Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C., and Wakeham, D. E. (2001). Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568. [DOI] [PubMed] [Google Scholar]
- Brown, C. M., and Petersen, N. O. (1998). An image correlation analysis of the distribution of clathrin associated adaptor protein (AP-2) at the plasma membrane. J. Cell Sci. 111(Pt 2), 271–281. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and Rose, J. K. (1992). Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68, 533–544. [DOI] [PubMed] [Google Scholar]
- Carpenter, G. (2000). The EGF receptor: a nexus for trafficking and signaling. Bioessays 22, 697–707. [DOI] [PubMed] [Google Scholar]
- Cheng, P. C., Dykstra, M. L., Mitchell, R. N., and Pierce, S. K. (1999). A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190, 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri, S., Salcini, A. E., Puri, C., Tacchetti, C., and Di Fiore, P. P. (2000). Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J. Cell Biol. 150, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, S. D., and Schmid, S. L. (2003). Regulated portals of entry into the cell. Nature 422, 37–44. [DOI] [PubMed] [Google Scholar]
- Cunningham, O., Andolfo, A., Santovito, M. L., Iuzzolino, L., Blasi, F., and Sidenius, N. (2003). Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. EMBO J. 22, 5994–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke, H., Baba, T., Warnock, D. E., and Schmid, S. L. (1994). Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo, M. A., Alderson, N. B., Kiosses, W. B., Chiang, H. H., Anderson, R. G., and Schwartz, M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839–842. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F., and Wrana, J. L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410–421. [DOI] [PubMed] [Google Scholar]
- Drevot, P., Langlet, C., Guo, X. J., Bernard, A. M., Colard, O., Chauvin, J. P., Lasserre, R., and He, H. T. (2002). TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 21, 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felberbaum-Corti, M., Van Der Goot, F. G., and Gruenberg, J. (2003). Sliding doors: clathrin-coated pits or caveolae? Nat. Cell Biol. 5, 382–384. [DOI] [PubMed] [Google Scholar]
- Gingras, D., Gauthier, F., Lamy, S., Desrosiers, R. R., and Beliveau, R. (1998). Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem Biophys. Res. Commun. 247, 888–893. [DOI] [PubMed] [Google Scholar]
- Giurisato, E., McIntosh, D. P., Tassi, M., Gamberucci, A., and Benedetti, A. (2003). T cell receptor can be recruited to a subset of plasma membrane rafts, independently of cell signaling and attendantly to raft clustering. J. Biol. Chem. 278, 6771–6778. [DOI] [PubMed] [Google Scholar]
- Harder, T., and Engelhardt, K. R. (2004). Membrane domains in lymphocytes—from lipid rafts to protein scaffolds. Traffic 5, 265–275. [DOI] [PubMed] [Google Scholar]
- Harder, T., Scheiffele, P., Verkade, P., and Simons, K. (1998). Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman, G. M., and Schlessinger, J. (1982). Lateral diffusion of EGF complexed to its surface receptors does not account for the thermal sensitivity of patch formation and endocytosis. Biochemistry 21, 1667–1672. [DOI] [PubMed] [Google Scholar]
- Huang, F., Khvorova, A., Marshall, W., and Sorkin, A. (2004). Analysis of clathrin-mediated endocytosis of EGF receptor by RNA interference. J. Biol. Chem. 279, 16657–16661. [DOI] [PubMed] [Google Scholar]
- Iwabuchi, K., Handa, K., and Hakomori, S. (1998). Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J. Biol. Chem. 273, 33766–33773. [DOI] [PubMed] [Google Scholar]
- Jacobson, K., and Dietrich, C. (1999). Looking at lipid rafts? Trends Cell Biol. 9, 87–91. [DOI] [PubMed] [Google Scholar]
- Jiang, X., Huang, F., Marusyk, A., and Sorkin, A. (2003). Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol. Biol. Cell 14, 858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes, L., and Lamaze, C. (2002). Clathrin-dependent or not: is it still the question? Traffic 3, 443–451. [DOI] [PubMed] [Google Scholar]
- Jorissen, R. N., Walker, F., Pouliot, N., Garrett, T. P., Ward, C. W., and Burgess, A. W. (2003). EGF receptor: mechanisms of activation and signalling. Exp. Cell Res. 284, 31–53. [DOI] [PubMed] [Google Scholar]
- Kindzelskii, A. L., Sitrin, R. G., and Petty, H. R. (2004). Cutting edge: optical microspectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of neutrophils: apparent role in calcium signaling. J. Immunol. 172, 4681–4685. [DOI] [PubMed] [Google Scholar]
- Krauss, K., and Altevogt, P. (1999). Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J. Biol. Chem. 274, 36921–36927. [DOI] [PubMed] [Google Scholar]
- Kurzchalia, T. V., and Parton, R. G. (1999a). Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11, 424–431. [DOI] [PubMed] [Google Scholar]
- Kurzchalia, T. V., and Parton, R. G. (1999b). Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11, 424–431. [DOI] [PubMed] [Google Scholar]
- Kusumi, A., Koyama-Honda, I., and Suzuki, K. (2004). Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 5, 213–230. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Dujeancourt, A., Baba, T., Lo, C. G., Benmerah, A., and Dautry-Varsat, A. (2001). Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7, 661–671. [DOI] [PubMed] [Google Scholar]
- Lipardi, C., Nitsch, L., and Zurzolo, C. (2000). Detergent-insoluble GPI-anchored proteins are apically sorted in fischer rat thyroid cells, but interference with cholesterol or sphingolipids differentially affects detergent insolubility and apical sorting. Mol. Biol. Cell 11, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti, M. P., Tang, Z. L., and Sargiacomo, M. (1993). Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J. Cell Biol. 123, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev, S. V., and Smart, E. J. (2002). Heterologous desensitization of EGF receptors and platelet-derived growth factor receptors by sequestration in caveolae. Am. J. Physiol. Cell Physiol. 282, C935–C946. [DOI] [PubMed] [Google Scholar]
- Maxfield, F. R. (2002). Plasma membrane microdomains. Curr. Opin. Cell Biol. 14, 483–487. [DOI] [PubMed] [Google Scholar]
- Mayor, S., and Rao, M. (2004). Rafts: scale-dependent, active lipid organization at the cell surface. Traffic 5, 231–240. [DOI] [PubMed] [Google Scholar]
- McPherson, P. S., Kay, B. K., and Hussain, N. K. (2001). Signaling on the endocytic pathway. Traffic 2, 375–384. [DOI] [PubMed] [Google Scholar]
- Michaely, P. A., Mineo, C., Ying, Y. S., and Anderson, R. G. (1999). Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J. Biol. Chem. 274, 21430–21436. [DOI] [PubMed] [Google Scholar]
- Miljan, E. A., and Bremer, E. G. (2002). Regulation of growth factor receptors by gangliosides. Sci. STKE 2002, RE15. [DOI] [PubMed] [Google Scholar]
- Mineo, C., Gill, G. N., and Anderson, R. G. (1999). Regulated migration of EGF receptor from caveolae. J. Biol. Chem. 274, 30636–30643. [DOI] [PubMed] [Google Scholar]
- Mobius, W., Herzog, V., Sandhoff, K., and Schwarzmann, G. (1999). Intracellular distribution of a biotin-labeled ganglioside, GM1, by immunoelectron microscopy after endocytosis in fibroblasts. J. Histochem. Cytochem. 47, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Moore, M. S., Mahaffey, D. T., Brodsky, F. M., and Anderson, R. G. (1987). Assembly of clathrin-coated pits onto purified plasma membranes. Science 236, 558–563. [DOI] [PubMed] [Google Scholar]
- Nabi, I. R., and Le, P. U. (2003). Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B. J., and Lippincott-Schwartz, J. (2001). Endocytosis without clathrin coats. Trends Cell Biol. 11, 406–412. [DOI] [PubMed] [Google Scholar]
- Parton, R. G. (1994). Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42, 155–166. [DOI] [PubMed] [Google Scholar]
- Parton, R. G., and Simons, K. (1995). Digging into caveolae. Science 269, 1398–1399. [DOI] [PubMed] [Google Scholar]
- Pierce, S. K. (2002). Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2, 96–105. [DOI] [PubMed] [Google Scholar]
- Pike, L. J., and Casey, L. (2002). Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry 41, 10315–10322. [DOI] [PubMed] [Google Scholar]
- Pralle, A., Keller, P., Florin, E. L., Simons, K., and Horber, J. K. (2000). Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior, I. A., Muncke, C., Parton, R. G., and Hancock, J. F. (2003). Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 160, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille, C. (1999). Quantitative aspects of immunogold labeling in embedded and nonembedded sections. Methods Mol. Biol. 117, 125–144. [DOI] [PubMed] [Google Scholar]
- Rappoport, J. Z., Taha, B. W., Lemeer, S., Benmerah, A., and Simon, S. M. (2003). The AP-2 complex is excluded from the dynamic population of plasma membrane-associated clathrin. J. Biol. Chem. 278, 47357–47360. [DOI] [PubMed] [Google Scholar]
- Ridyard, M. S., and Robbins, S. M. (2003). Fibroblast growth factor-2-induced signaling through lipid raft-associated fibroblast growth factor receptor substrate 2 (FRS2). J. Biol. Chem. 278, 13803–13809. [DOI] [PubMed] [Google Scholar]
- Ringerike, T., Blystad, F. D., Levy, F. O., Madshus, I. H., and Stang, E. (2002). Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J. Cell Sci. 115, 1331–1340. [DOI] [PubMed] [Google Scholar]
- Rothberg, K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R., and Anderson, R. G. (1992). Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002). Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18, 1–24. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J., Schreiber, A. B., Levi, A., Lax, I., Libermann, T., and Yarden, Y. (1983). Regulation of cell proliferation by EGF. CRC Crit. Rev. Biochem. 14, 93–111. [DOI] [PubMed] [Google Scholar]
- Schnitzer, J. E., Oh, P., Pinney, E., and Allard, J. (1994). Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck, S., Honsho, M., Ekroos, K., Shevchenko, A., and Simons, K. (2003). Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA 100, 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund, S., Voelk, T., Puri, C., Maspero, E., Tacchetti, C., Transidico, P., Di Fiore, P. P., and Polo, S. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, 2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and Ikonen, E. (1997). Functional rafts in cell membranes. Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Slepnev, V. I., and De Camilli, P. (2000). Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 1, 161–172. [DOI] [PubMed] [Google Scholar]
- Smart, E. J., Graf, G. A., McNiven, M. A., Sessa, W. C., Engelman, J. A., Scherer, P. E., Okamoto, T., and Lisanti, M. P. (1999). Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19, 7289–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe, E. (2002). Regulating the clathrin-coated vesicle cycle by AP2 subunit phosphorylation. Trends Cell Biol. 12, 352–354. [DOI] [PubMed] [Google Scholar]
- Sprong, H., van der Sluijs, P., and van Meer, G. (2001). How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell. Biol. 2, 504–513. [DOI] [PubMed] [Google Scholar]
- Stoddart, A., Dykstra, M. L., Brown, B. K., Song, W., Pierce, S. K., and Brodsky, F. M. (2002). Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462. [DOI] [PubMed] [Google Scholar]
- Tebar, F., Sorkina, T., Sorkin, A., Ericsson, M., and Kirchhausen, T. (1996). Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271, 28727–28730. [DOI] [PubMed] [Google Scholar]
- Torrisi, M. R., Lotti, L. V., Belleudi, F., Gradini, R., Salcini, A. E., Confalonieri, S., Pelicci, P. G., and Di Fiore, P. P. (1999). Eps15 is recruited to the plasma membrane upon EGF receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell 10, 417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft, S., Schumacher, C., Hage, W., Verkleij, A. J., and van Bergen en Henegouwen, P. M. (1997). Association and colocalization of Eps15 with adaptor protein-2 and clathrin [published erratum appears in J. Cell Biol. 1997 Apr 7;137(1):259]. J. Cell Biol. 136, 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh, M. G., Lawson, D., and Hsuan, J. J. (1999). EGF receptor activation is localized within low-buoyant density, non-caveolar membrane domains. Biochem. J. 337, 591–597. [PMC free article] [PubMed] [Google Scholar]
- Wilde, A., Beattie, E. C., Lem, L., Riethof, D. A., Liu, S. H., Mobley, W. C., Soriano, P., and Brodsky, F. M. (1999). EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 96, 677–687. [DOI] [PubMed] [Google Scholar]
- Yamabhai, M., and Anderson, R. G. (2002). Second cysteine-rich region of epidermal growth; Factor receptor contains targeting information for caveolae/rafts. J. Biol. Chem. 277, 24843–24846. [DOI] [PubMed] [Google Scholar]
- Yang, N., Huang, Y., Jiang, J., and Frank, S. J. (2004). Caveolar and lipid raft localization of the growth hormone receptor and its signaling elements: impact on growth hormone signaling. J. Biol. Chem. 279, 20898–20905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.