Abstract
We sought to examine the effects of varicella-zoster virus (VZV) infection on the expression of major histocompatibility complex class I (MHC I) molecules by human fibroblasts and T lymphocytes. By flow cytometry, VZV infection reduced the cell surface expression of MHC I molecules on fibroblasts significantly, yet the expression of transferrin receptor was not affected. Importantly, when human fetal thymus/liver implants in SCID-hu mice were inoculated with VZV, cell surface MHC I expression was downregulated specifically on VZV-infected human CD3+ T lymphocytes, a prominent target that sustains VZV viremia. The stage in the MHC I assembly process that was disrupted by VZV in fibroblasts was examined in pulse-chase and immunoprecipitation experiments in the presence of endoglycosidase H. MHC I complexes continued to be assembled in VZV-infected cells and were not retained in the endoplasmic reticulum. In contrast, immunofluorescence and confocal microscopy showed that VZV infection resulted in an accumulation of MHC I molecules which colocalized to the Golgi compartment. Inhibition of late viral gene expression by treatment of infected fibroblasts with phosphonoacetic acid did not influence the modulation of MHC I expression, nor did transfection of cells with plasmids expressing immediate early viral proteins. However, cells transfected with a plasmid carrying the early gene ORF66 did result in a significant downregulation of MHC I expression, suggesting that this gene encodes a protein with an immunomodulatory function. Thus, VZV downregulates MHC I expression by impairing the transport of MHC I molecules from the Golgi compartment to the cell surface; this effect may enable the virus to evade CD8+ T-cell immune recognition during VZV pathogenesis, including the critical phase of T-lymphocyte-associated viremia.
Varicella-zoster virus (VZV) is a human herpesvirus that causes varicella (chicken pox) as the primary infection in susceptible individuals, establishes latency in sensory nerve ganglia, and may reactivate as herpes zoster (shingles) (5, 6, 14). In healthy individuals, primary VZV infection induces both innate and antigen-specific immune responses. Innate immunity may limit the initial spread of VZV within the host, but VZV-specific adaptive immunity plays a crucial role in recovery from varicella and in the preservation of latency. Modulation of major histocompatibility complex class I (MHC I) expression may promote viral infection and persistence in the host by enabling infected cells to evade the CD8+ T-lymphocyte-mediated antiviral immune response. In this respect, several viruses, including adenovirus, human immmunodeficiency virus (HIV), murine and human cytomegalovirus (MCMV and HCMV, respectively), Epstein-Barr virus, bovine herpesvirus, pseudorabies virus, and herpes simplex virus (HSV), as well as VZV, have been found to downregulate cell surface MHC I expression (10, 15, 28, 29, 30, 31, 35, 42, 47, 49, 51). These viruses have evolved various strategies for disrupting the MHC I antigen presentation pathway, and some have been shown to coordinate multiple mechanisms of interference (reviewed in references 19 and 44).
MHC I molecules are heterodimers consisting of a membrane-bound heavy chain (αC) and a light chain β2 microglobulin (β2m) which present peptides derived from cytosolic proteins to CD8+ T lymphocytes. Antigenic peptides generated by cytosolic proteases are transported into the endoplasmic reticulum (ER) by the ATP-dependent transporter associated with antigen processing (TAP), where they associate with MHC I heterodimers. The resulting trimolecular complex is transported from the ER through the Golgi compartment to the cell surface where it presents the peptide to cytotoxic T lymphocytes (34). Each step in the MHC I biosynthesis and assembly pathway has been shown to be a potential target for viral interference and the subsequent modulation of MHC I expression on cell surfaces.
During primary VZV infection, both MHC I-restricted, CD8+ T lymphocytes and MHC II-restricted, CD4+ T lymphocytes are sensitized to VZV antigens (46). T-cell recognition of infected cells is essential for terminating primary infection (7). Interestingly, VZV appears to evade host recognition by T lymphocytes during a prolonged, 10- to 21-day incubation period following initial infection (6). Viral genes encoding immunomodulatory proteins may allow VZV to escape immune surveillance during this interval, permitting the virus to move from mucosal sites of inoculation and to disseminate to skin via T lymphocytes.
The purpose of this study was to examine the effect of VZV infection on cell surface expression of MHC I molecules by human fibroblasts and T lymphocytes. We used flow cytometry to demonstrate that VZV reduced cell surface MHC I expression on infected human fibroblasts and T lymphocytes. We applied a combination of biochemical analyses and confocal microscopy to show that VZV interferes with the transport of MHC I molecules from the Golgi compartment to the cell surface. Phosphonoacetic acid (PAA), an inhibitor of late viral gene synthesis, did not alter the ability of the virus to downregulate MHC I expression, suggesting that this function is not encoded by a late viral gene product(s). Cells transfected with plasmids expressing immediate early (IE) genes were not altered in the ability to express MHC I molecules, but a plasmid carrying the early gene ORF66 did significantly downregulate MHC I expression on transfected cells.
MATERIALS AND METHODS
Cells.
The following cell lines were grown in tissue culture medium (TCM) (Dulbecco's modified Eagle's medium; Gibco, Gaithersburg, Md.) supplemented with heat-inactivated fetal calf serum, 2 mM l-glutamine (Gibco), 50 IU of penicillin, 50 mg of streptomycin (Pen/Strep; ICN Biomedicals, Inc., Irvine, Calif.), and 0.5 mg of amphotericin B (Fungizone, Flow Laboratories, McLean, Va.): human fetal lung fibroblasts, MRC-5 (ATCC CCL 171), in passages 17 to 30; human foreskin fibroblasts (HFFs), in passages 5 to 25; human melanoma (MeWo) cells; and Vero (African green monkey) cells.
Antibodies.
The monoclonal antibodies (MAbs) used were specific for human MHC I (clone Tu149 recognizing complexed αC/β2m) (purified and R-phycoerythrin [PE] conjugated); human transferrin receptor (CD71; clone T56/14) (purified and PE conjugated); human CD3 (clone S4.1; tricolor conjugated); human CD4 (clone S3.5; PE conjugated); human CD8 (clone 3B5; fluorescein isothiocyanate [FITC] conjugated); goat anti-mouse immunoglobulin G (IgG) F(ab′)2 fragments, PE-conjugated goat anti-human IgG, and FITC-conjugated goat anti-human IgG (CalTag Laboratories, South San Francisco, Calif.); and human HLA-A,B,C (clone G46-2.6) and human transferrin receptor (clone M-A712) (Pharmingen, San Diego, Calif.). MAb W6/32 and rabbit antiserum specific for the human MHC I heavy chain were provided by H. Ploegh (Harvard University, Boston, Mass.) and were used in immunoprecipitation experiments. VZV-immune or nonimmune polyclonal human serum (IgG purified) was used to identify VZV-infected cells.
SCID-hu mice.
Male homozygous C.B-17 scid/scid mice were bred and maintained at SyStemix, Inc., Palo Alto, Calif. At 8 weeks of age, coimplants of human fetal thymus and liver (thymus/liver) tissue from 18- to 23-week fetuses were introduced under the kidney capsule as a cojoint implant (41). Human fetal tissues were obtained with informed consent according to federal and state regulations and were screened for HIV. The general care of these animals was done in accordance with the guidelines of the Administrative Panel on Laboratory Animal Care of Stanford University.
Viruses.
The VZV strain used in these studies was a low-passage clinical isolate designated strain Schenke. For cell infections, fibroblasts or melanoma cells were mixed with VZV-infected cells (3+ to 4+ cytopathic effect), at a ratio of one infected cell to five uninfected cells. Animal inoculations were done with VZV-infected MRC-5 cells at 3+ to 4+ cytopathic effect. The VZV-infected monolayers were trypsinized and washed once with phosphate-buffered saline (PBS), and the cells were counted and resuspended in TCM. Cells were briefly stored on ice until they were injected into the SCID-hu thymus/liver implants. Mock-infected implants were injected with an equal number of uninfected MRC-5 cells prepared by the same method. The virus inoculum was determined by plaque assay on Vero cell monolayers. The Oka vaccine strain and a glycoprotein C (gC)-negative mutant of the Oka strain were also tested for effects on MHC I expression (39).
Infection of thymus/liver implants.
Mice were anesthetized by intraperitoneal injection with a solution of 5% ketamine (Aveco Co., Fort Dodge, Iowa) and 2.5% xylazine (LyphoMed Inc., Rosemont, Ill.) in PBS. The left kidney was surgically exposed, and the thymus/liver implant was injected using a 27-gauge needle with approximately 20 to 50 μl of cells. The peritoneal incision was sutured, and the skin was stapled closed. Seven days after inoculation the implants were removed and disrupted between ground glass slides. Cell suspensions were filtered through a sterile nylon mesh to remove large debris, and the released cells were washed, counted, and used for fluorescence-activated cell sorting (FACS) analysis and virus titration.
FACS analysis.
Aliquots of approximately 106 cells obtained from uninfected and VZV-infected thymus/liver implants, uninfected and VZV-infected MRC-5 cells, and HFF or MeWo cells or transfected HFF cells were washed and resuspended in 100 μl of FACS staining buffer (PBS with 1% fetal calf serum and 0.2% sodium azide). The primary antibodies, VZV-immune or nonimmune polyclonal IgG, were diluted 1:40. Secondary mouse MAbs anti-MHC I, anti-CD71, anti-CD3, anti-CD4, anti-CD8, and anti-CD45 were diluted 1:50 with goat anti-human FITC-conjugated F(ab′)2 fragments (diluted 1:100). As a negative control cells were incubated with the appropriate isotype control antibodies. All antisera were diluted in FACS staining buffer, and all reactions were done in the dark on ice for 30 min. The cells were washed between each antibody step by adding 2 ml of FACS staining buffer, centrifuging, and aspirating the supernatant. After the final wash cells were resuspended in orthofixative (PBS with 1% electron microscopy grade formaldehyde), and cell suspensions were analyzed with a Becton Dickinson FACScan apparatus.
Immunofluorescence and confocal microscopy.
Approximately 104 cells per well were seeded in four-chamber slides (Lab-Tek, Inc., Naperville, Ill.). Adherent cells were infected with VZV strain Schenke at a ratio of one infected cell to three uninfected cells for 2 h at 37°C. The infected monolayers were washed three times with PBS and incubated with TCM for 24 h. In certain experiments, cells were fixed and permeabilized with acetone at 4°C for 15 min and air dried for 1 h. Slides were washed once in PBS and incubated with blocking buffer (1% bovine serum albumin [BSA] in PBS) at room temperature for 15 min, washed three times with PBS, and incubated with the primary antibodies diluted in blocking buffer for 1 h at 37°C. The primary antibodies used were mouse anti-human HLA-A,B,C (PharMingen) and a VZV-immune polyclonal IgG fraction, at a dilution of 1:10 and 1:100, respectively. An isotype control antibody was used to control for nonspecific binding. Slides were washed three times in the blocking solution, and the secondary antibodies were added for 30 min in the dark at 37°C. Secondary antibodies included Texas red-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.) (1:100) and FITC-conjugated goat anti-mouse IgG (CalTag Laboratories) (1:100) diluted in blocking solution. After three washes in PBS, slides were mounted with Vectashield (Vector Laboratories, Inc., Burlingame, Calif.) and examined using a Molecular Dynamics MultiProbe 2010 laser scanning confocal microscope. The images were transferred to graphics software (Adobe Photoshop, version 3.0) and printed with a Tektronix Phaser 440 dye sublimation printer.
BODIPY-Texas red-ceramide {N-[[4-[4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-yl]phenoxy]acetyl]sphingosine} (Molecular Probes, Inc., Eugene, Oreg.) is a fluorescent probe which localizes to the membranes of the Golgi complex (16, 37). BODIPY-Texas red-ceramide was reconstituted following the manufacturer's directions, diluted to a concentration of 10 nmol/ml in filter-sterilized RPMI 1640 (Gibco BRL, Grand Island, N.Y.) with 0.68 mg of fatty acid-free BSA (Sigma, St. Louis, Mo.) per ml, and subsequently sonicated for 30 s. The cell monolayers were labeled with 375 μl of the ceramide solution per well for 25 min in the dark at 4°C. Cells were washed once with BSA-free RPMI and then chased with TCM in the dark for 50 min at 37°C. Following ceramide staining, the monolayers were fixed and permeabilized with 2% paraformaldehyde–0.05% Triton X-100 in 0.2 M Na2HPO4 for 1 h. After five washes with PBS for 5 min each, the slides were blocked with 5% normal goat serum in PBS for 1 h at room temperature. The primary antibody against HLA-A,B,C (PharMingen), diluted 1:10, was incubated with the monolayers overnight at 4°C. Binding of primary antibody was detected using goat anti-mouse IgG FITC-conjugated antibody (Caltag) at a dilution of 1:100 for 1 h at 37°C. All antisera were diluted in PBS containing 1% normal goat serum, with a 10-min wash in PBS between each step. After the final wash, slides were mounted with Vectashield for confocal microscopy analysis as described above.
Concanavalin A (ConA) is a lectin that localizes to the ER. Upon reconstitution according to the manufacturer's recommendations, a Texas red conjugate (Molecular Probes) was used at a final concentration of 50 μg/ml. The probe was incubated with antibodies following the same immunofluorescence and confocal microscopy protocol as described for BODIPY-Texas red-ceramide.
Biochemical analysis and gel electrophoresis.
Cells were incubated for 45 min in methionine- and cysteine-free TCM and labeled with [35S]methionine-cysteine (NEN, Boston, Mass.) for 30 min for pulse-chase experiments and for 1 h for coimmunoprecipitation experiments. If indicated, cells were chased for various times in regular TCM supplemented with nonradioactive methionine and cysteine to a final concentration of 1 mM. Aliquots of cells were spun down at each chase point, and the cell pellets were frozen before lysis and immunoprecipitation. Cell pellets were lysed in NP-40 lysis buffer (0.5% NP-40, 50 mM Tris-HCl [pH 7.4], 5 mM MgCl2) containing 1 mM phenylmethylsulfonyl fluoride for 15 min on ice. Total cell lysates were cleared of debris by centrifugation, and supernatants were precleared twice with protein A (70 μl), 3 μl of normal rabbit serum/ml, and 3 μl of normal mouse serum/ml and then immunoprecipitated with either W6/32 (3 μl/ml) or rabbit anti-MHC I heavy chain (3 μl/ml). Immunoprecipitated products were washed four times with ice-cold NET buffer (0.5% NP-40, 50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA). Samples were boiled in sample buffer (62.5 mM Tris-HCl [pH 6.8], 5% 2-mercaptoethanol, 10% glycerol, 4% sodium dodecyl sulfate [SDS], and bromophenol blue) for 10 min before SDS-polyacrylamide gel electrophoresis (PAGE). In those experiments in which endoglycosidase H (endo H; New England Biolabs, Beverly, Mass.) was used, washed protein A-bound immune complexes were processed as follows. Immune complexes were boiled for 10 min in 30 μl of denaturation buffer (0.5% SDS, 1% 2-mercaptoethanol) and then incubated with endo H (1 μl) in 50 mM sodium citrate (pH 5.5) at 37°C for 1 h. Samples were then mixed in sample buffer and boiled for 10 min before SDS-PAGE. Samples were separated on SDS–12.5% PAGE gels, fixed, and washed prior to drying and autoradiography. Unlabeled samples prepared for coimmunoprecipitation experiments were prepared as described above, with the gel being Coomassie stained to reveal total protein before excision of the protein band of interest.
PAA experiments.
Cells were pretreated for 1 h in the presence of 300 μg of PAA (Sigma)/ml and then infected with VZV-infected cells. Following 2 h of adsorption of VZV-infected cells, cells were washed three times with PBS and then incubated with TCM containing PAA for a further 24 h. Cells were then harvested and analyzed by flow cytometry as described above, and cell lysates were tested by Western blot analysis for inhibition of late viral gene expression.
Western blot analysis.
Cells were washed once in PBS, sonicated for 1 min in cell extract buffer containing protease inhibitors (50 mM Tris [pH 7.4], 240 mM NaCl, 0.5% NP-40, 10% glycerol, 0.1 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride), and centrifuged (14,000 × g), and the supernatants were collected. The cell supernatants (5 × 104 cells/lane) were separated on SDS–7% PAGE gels, followed by electrotransfer to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). Membranes were stained with amido black (1% amido black [naptho, blue black], 45% methanol, 10% acetic acid) to reveal total protein before Western blot analysis. Membranes were incubated in blocking solution (5% nonfat milk in PBS) for 1 h. gC proteins were detected with a rabbit polyclonal antibody diluted 1:500 in blocking solution. Secondary goat anti-rabbit IgG-horseradish peroxidase conjugate (Amersham, Buckinghamshire, England) was diluted 1:2,000 and used for enhanced chemiluminescence detection of bound antibodies according to the manufacturer's protocol (Amersham). Molecular masses of proteins were determined using protein reference standards (Bio-Rad, Richmond, Calif.).
Plasmid DNA and transient transfection.
Plasmid pMS62 contains the IE62 coding sequence under the control of the HCMV IE promoter (43) and was kindly provided by J. Hay, State University of New York at Buffalo. Plasmid pON2345 contains the HCMV IE promoter without any VZV genes and was kindly provided by E. Mocarski, Stanford University. Plasmids encoding VZV ORF4 (pCMV4), ORF10 (pCMV10), ORF61 (pCMV61), ORF47 (pCMV47), ORF63 (pCMV63), and ORF66 (pCMV66) under the control of the HCMV IE promoter were kindly provided by P. Kinchington, University of Pittsburgh. These plasmids were transiently transfected into HFF cells using a calcium phosphate transfection protocol (12). Cells were harvested 48 h after transfection, stained for MHC I expression, and analyzed by flow cytometry.
RESULTS
VZV downregulates cell surface MHC I expression in primary and transformed human cells.
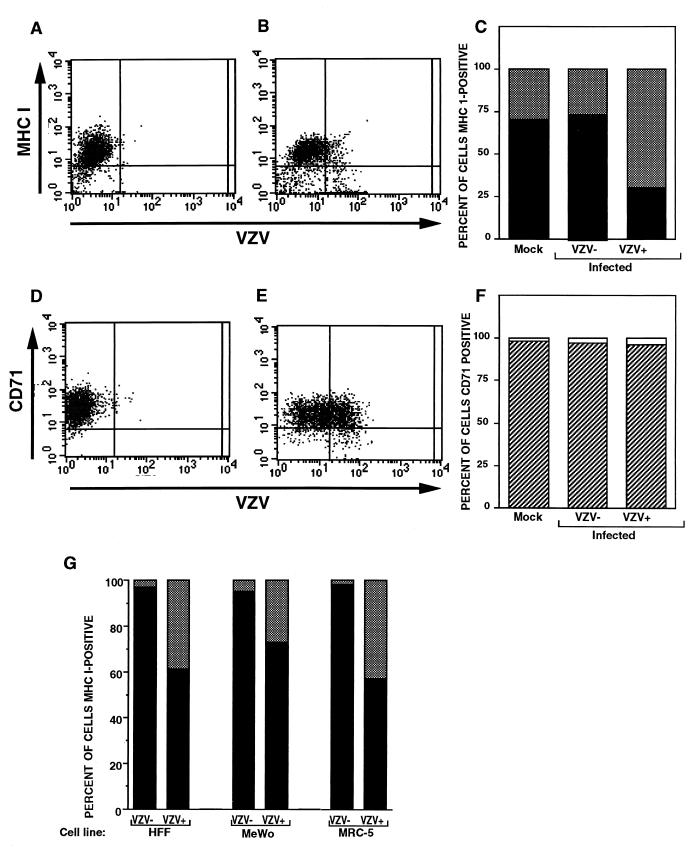
Human fibroblasts and melanoma (MeWo) cells were used to analyze the effects of VZV infection on MHC I expression because these cells are optimal for VZV replication in vitro (5). Due to the highly cell-associated nature of VZV, HFFs were infected with VZV strain Schenke by mixing VZV-infected and uninfected HFF cells at a ratio of 1:5. At 24 h postinfection, cells were stained for VZV and MHC I expression using polyclonal VZV-immune serum and a mouse MAb to MHC I antigens, respectively, and were analyzed by flow cytometry (Fig. 1A and B). Negative controls included mock-infected cells and incubation of both mock- and VZV-infected cells with isotype control antibodies. At 24 h postinfection, 20% of the cells were VZV+ and 80% of the cells remained VZV− as determined by flow cytometry. Of the VZV− cell population, 90% were MHC I+. In contrast, only 25% of the VZV+ cells were MHC I+ (Fig. 1C). To determine whether VZV downregulated MHC I expression in a selective manner and did not have a general effect on host cell surface molecules, these cells were also assessed by flow cytometry for transferrin receptor (CD71) expression using an anti-CD71 MAb (Fig. 1D and E). At 24 h postinfection, more than 98% of both VZV+ and VZV− cell populations expressed transferrin receptor (Fig. 1F). Taken together, these data show that VZV selectively downregulated cell surface MHC I expression on human fibroblasts. In a further four replicate experiments, specific downregulation of MHC I expression was also observed.
FIG. 1.
FACS analysis of MHC I molecules, transferrin receptor (CD71), and VZV proteins on VZV-infected cells. HFFs were either infected with VZV for 24 h (B and E) or mock infected (A and D), and cell preparations were stained with antibodies and fluorescent conjugates to MHC I and VZV proteins (A and B) or to transferrin receptor and VZV proteins (D and E). The percentages of VZV+ and VZV− cell populations expressing cell surface MHC I molecules (C) and transferrin receptor (F) are shown. (G) Percentage of VZV+ and VZV− cell populations expressing cell surface MHC I molecules. HFF, MeWo, and MRC-5 cells were infected with VZV for 24 h and stained with antibodies and fluorescent conjugates to MHC I and VZV proteins and analyzed by flow cytometry.
To determine whether VZV infection can downregulate cell surface MHC I expression on other cell types, primary human fetal lung fibroblast (MRC-5) cells and transformed human melanoma (MeWo) cells were also evaluated at 24 h postinfection for cell surface MHC I expression. The level of MHC I downregulation in VZV-infected cells was similar to that observed using HFFs (Fig. 1G). In all cell types tested, VZV infection did not suppress transferrin receptor expression (data not shown). Cells infected with the recombinant Oka vaccine strain of VZV had the same pattern of MHC I downregulation; 46% of VZV+ cells had MHC I expression versus 86% of VZV− cells. These data indicate that VZV isolates, whether fresh clinical isolates or tissue culture-passaged virus, specifically downregulate MHC I expression in primary and transformed human cells.
MHC I downregulation in human T lymphocytes infected with VZV.
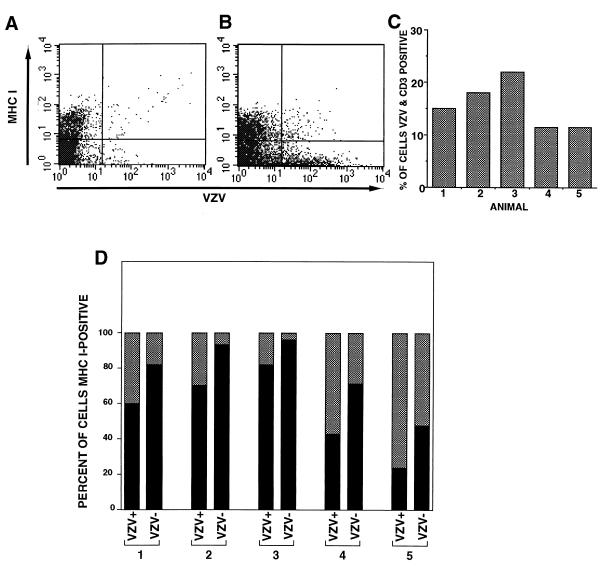
VZV causes a mononuclear cell-associated viremia and tropism for T lymphocytes, which is a critical event for VZV pathogenesis during primary infection (5, 38). We therefore sought to determine whether VZV infection altered cell surface MHC I expression on human T lymphocytes using the SCID-hu thymus/liver mouse model (38). Human fetal thymus/liver implanted under the kidney capsules of five SCID-hu mice was inoculated with HFF cells infected with VZV strain Schenke. Mock-infected implants were injected with an equal number of uninfected cells. Seven days after inoculation, total cells from the implants were collected and stained with antibodies to CD3 (a T-cell marker) and MHC I and VZV proteins. Flow cytometry was performed whereby CD3+ T lymphocytes were gated and analyzed for MHC I and VZV antigen expression (Fig. 2A and B). In all five mice inoculated with VZV-infected cells, VZV+ cells were readily detectable (11.5 to 23%) (Fig. 2C). There was a significant decrease in the percentage of MHC I+ cells in the CD3+ VZV+ population when compared to the CD3+ VZV− population in all animals (Fig. 2D). These data indicate that VZV infection of human CD3+ T lymphocytes modulates cell surface MHC I expression.
FIG. 2.
FACS analysis of MHC I and VZV proteins on VZV-infected human T lymphocytes. SCID-hu thymus/liver implants were inoculated with VZV- or mock-infected cells, and 7 days later cell preparations were stained with antibodies and fluorescent conjugates to MHC I and VZV proteins and CD3. CD3+ T lymphocytes were gated and analyzed for MHC I and VZV antigen expression. A typical FACS plot of MHC I and VZV antigens from mock- (A) and VZV-infected (B) thymus/liver implants is shown. The percentage of CD3+/VZV+ cells was determined for each mouse (C). The percentage of VZV+ and VZV− human CD3+ T-cell populations expressing cell surface MHC I molecules is shown (D).
Using the SCID-hu model, we have shown that VZV is lymphotropic and infects all T-cell subpopulations equally (38). To determine whether VZV infection could downregulate cell surface expression of MHC I molecules on T-cell subpopulations, cells from infected and uninfected implants were stained with antibodies to CD4, CD8, MHC I molecules, and VZV proteins and were analyzed by flow cytometry. Similar decreases in the levels of expression of MHC I molecules were observed on CD8+, CD4+, and immature CD4+ CD8+ T-cell populations. Furthermore, VZV infection did not alter the expression of CD4 and CD8 when detection of these T-cell surface markers was compared between VZV+ and VZV− cell populations (data not shown). These data indicate that VZV infection specifically downregulated cell surface MHC I expression on human T lymphocytes.
Retention of MHC I molecules in VZV-infected cells.
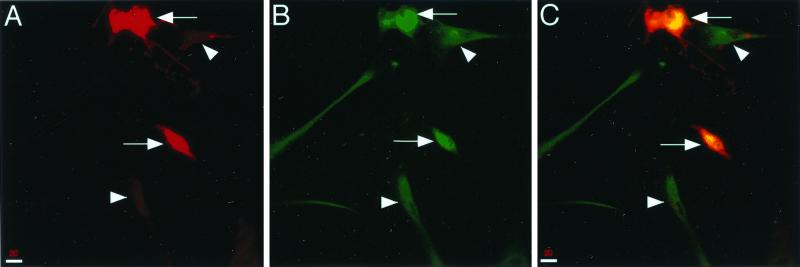
To further examine VZV-mediated downregulation of MHC I expression, we evaluated the intracellular localization of MHC I molecules by immunofluorescence and confocal microscopy on uninfected and VZV-infected cells. HFFs were infected with VZV strain Schenke by mixing infected and uninfected HFFs at a ratio of 1:3. At 24 h postinfection, cells were fixed and permeabilized with acetone and incubated with antibodies against MHC I and VZV antigens, followed by secondary antibodies conjugated with either FITC or Texas red. Negative controls included mock-infected cells and staining of VZV- and mock-infected cells with isotype control antibodies. VZV+ cells were readily detected in infected cell populations (Fig. 3A). In contrast to VZV− cells, which displayed diffuse cytoplasmic MHC I staining, VZV+ cells exhibited a significant accumulation of MHC I molecules in the perinuclear region (Fig. 3B and C). No staining was detected in VZV- or mock-infected cell populations when isotype control antibodies were used. These data suggest that MHC I molecules were retained within VZV-infected cells.
FIG. 3.
Immunofluorescent staining of intracellular MHC I and VZV antigens in VZV-infected cells. VZV-infected cells were fixed, permeabilized, and incubated with VZV-immune human serum and an anti-MHC I MAb, which were detected using Texas red-conjugated anti-human IgG and FITC-conjugated anti-mouse IgG antibodies. VZV antigen staining was detected in the majority of VZV-infected cells (A). Intense perinuclear MHC I staining was detected in VZV-infected cells (B, arrow), whereas diffuse cytoplasmic MHC I staining was detected in VZV-negative cells (B, arrowhead). The yellow color indicates colocalization of VZV and MHC I proteins in VZV-infected cells (C).
MHC I molecules are not retained in the ER of VZV-infected cells.
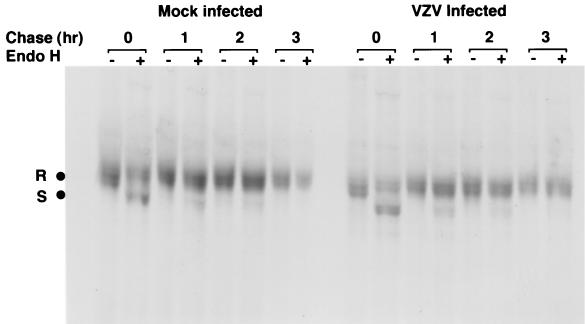
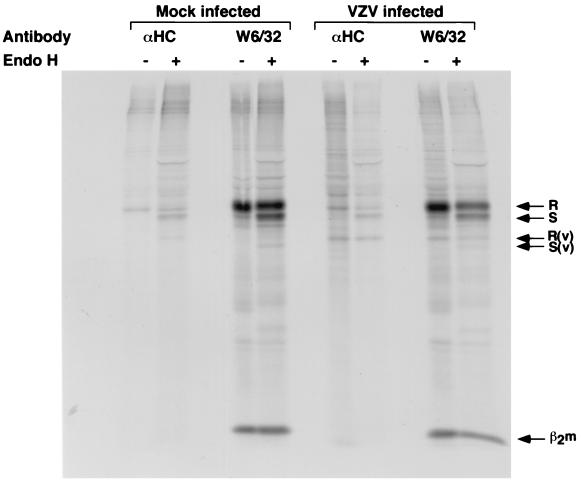
To determine the stage in the MHC I assembly process that was disrupted by VZV in fibroblasts, we used pulse-chase and immunoprecipitation experiments in the presence of endo H to examine the synthesis and cell surface transport of MHC I complexes. HFFs were infected with VZV strain Schenke by mixing VZV-infected and uninfected cells at a ratio of 1:2. These cells and mock-infected cells were labeled at 24 h postinfection with [35S]methionine-cysteine for 30 min, chased for 1, 2, and 3 h before immunoprecipitation of MHC I molecules using W6/32 MAb, and digested with endo H. Correctly assembled MHC I molecules enter the medial Golgi compartment, where they acquire endo H resistance, whereas unassembled or ER-retained MHC I molecules remain endo H sensitive. In mock-infected cells, MHC I molecules acquired endo H resistance by the 3-h chase period. Similarly, in VZV-infected cells the MHC I molecules became resistant to endo H at the 3-h chase period (Fig. 4). Furthermore, the transferrin receptor was efficiently converted to endo H-resistant forms during the pulse-chase period in infected and uninfected cells (data not shown). These data demonstrate that MHC I protein synthesis is unaltered in infected cells and that MHC I complexes are not retained in the ER of VZV-infected cells.
FIG. 4.
Biochemical analysis of the synthesis and transport of MHC I molecules in VZV-infected cells. VZV- and mock-infected cells were labeled with [35S]methionine-cysteine and then chased for 0, 1, 2, and 3 h. Total cell lysates were immunoprecipitated with W6/32, and immune complexes were treated either with (+) or without (−) endo H. The MHC I endo H-resistant (R) and -sensitive (S) forms are indicated.
Localization of MHC I molecules in the Golgi compartment of VZV-infected cells.
To confirm a post-ER retention of MHC I molecules and to demonstrate the intracellular localization of MHC I molecules in VZV-infected cells, intracellular probes for the ER or the Golgi compartment were used in conjunction with MHC I- and VZV-specific antibodies in immunofluorescence and confocal microscopy. HFF cells were infected with VZV and incubated with either BODIPY-Texas red-ceramide to label the Golgi compartment or with Texas red-conjugated ConA to label the ER 24 h after infection. Fixed and permeabilized cells were then stained with an antibody to MHC I antigen which was detected with a secondary antibody conjugated to FITC. Negative controls included mock-infected cells and staining of VZV- and mock-infected cells with isotype control antibodies. The individual scans of ConA-derived fluorescence (Fig. 5A) and MHC I antibody stains (Fig. 5B) on VZV-infected cells were electronically merged into one image (Fig. 5C). The dual-color image did not reveal any colocalization of these molecules in VZV-infected cells. The images of ceramide labeling of the Golgi complex (Fig. 5D) and the MHC I staining (Fig. 5E) of VZV-infected cells were similarly superimposed into a dual fluorescence image (Fig. 5F). The yellow color designates colocalization of the ceramide and MHC I molecules and was found only in VZV-infected cells. The colocalization of MHC I molecules with ceramide labeling in virally infected cells demonstrates the retention of MHC I molecules in the Golgi complex of VZV-infected cells.
FIG. 5.
ConA, ceramide, and MHC I immunofluorescent staining of VZV-infected cells. VZV-infected cells were labeled with Texas red-conjugated ConA (A to C) or Texas red-conjugated BODIPY-ceramide (D to F), fixed, permeabilized, and stained with anti-MHC I MAb and FITC-conjugated anti-mouse IgG antibody. Fluorescent images were overlayed to assess colocalization (C and F). The yellow color represents colocalization of MHC I molecules and the fluorescent marker of the Golgi compartment (F). No colocalization was detected with the fluorescent ER marker (C).
Immunoprecipitation of proteins from VZV-infected cells with MHC I antibodies.
To determine whether a viral protein specifically interacts with a component of the MHC I complex, thereby causing retention of MHC I molecules, we used immunoprecipitation with MHC I antibodies to assess whether any coprecipitating proteins could be detected in VZV-infected cell lysates. HFFs were infected with VZV strain Schenke by mixing VZV-infected and uninfected cells at a ratio of 1:2. These cells and mock-infected cells were labeled at 24 h postinfection with [35S]methionine-cysteine for 1 h before immunoprecipitation using W6/32 MAb and rabbit anti-heavy chain antibody and digestion with endo H. In mock- and VZV-infected cells, MHC I heavy chain and light chain (β2 microglobulin) were detected and showed similar endo H-resistant and -sensitive forms (Fig. 6). An ∼40-kDa protein that coprecipitated with MHC I molecules was detected in VZV-infected cell samples but not in mock-infected cell lysates. This protein was detected in four separate experiments and was detected only in VZV-infected cells. In order to identify this protein, immunoprecipitation was done in which half of the VZV-infected cell sample was radiolabeled and the other half was not; cell lysates were then immunoprecipitated with W6/32 and separated by SDS–12.5% PAGE. The ∼40-kDa protein was detected in the radiolabeled samples; the gel containing unlabeled sample was Coomassie stained, and the ∼40-kDa protein band was excised, trypsin digested, and analyzed by mass spectrometry. By sequence analysis, the ∼40-kDa protein belonged to MHC I as HLA class I alpha chain (data not shown), suggesting that VZV-infected cells have an unusual cleaved form of MHC I protein.
FIG. 6.
Biochemical analysis of the synthesis of MHC I molecules in VZV-infected cells. VZV- and mock-infected cells were labeled with [35S]methionine-cysteine and then chased for 1 h. Total cell lysates were immunoprecipitated with rabbit anti-heavy chain (αHC) or W6/32 antibody, and immune complexes were treated either with (+) or without (−) endo H. The MHC I endo H-resistant (R) and -sensitive (S) forms are indicated. β2m and the coimmunoprecipitating protein seen in VZV-infected cells are indicated.
Late viral gene products do not affect cell surface MHC I expression.
During productive VZV infection, viral genes are likely to be expressed in an ordered cascade which can be divided into three categories, the IE, early, and late genes characteristic of other herpesviruses (26). To identify the phase of viral gene expression responsible for MHC I modulation in VZV-infected cells, PAA, an inhibitor of viral DNA replication and thus late gene expression, was added to infected and mock-infected cells, and the cells were assessed for cell surface MHC I expression by flow cytometry.
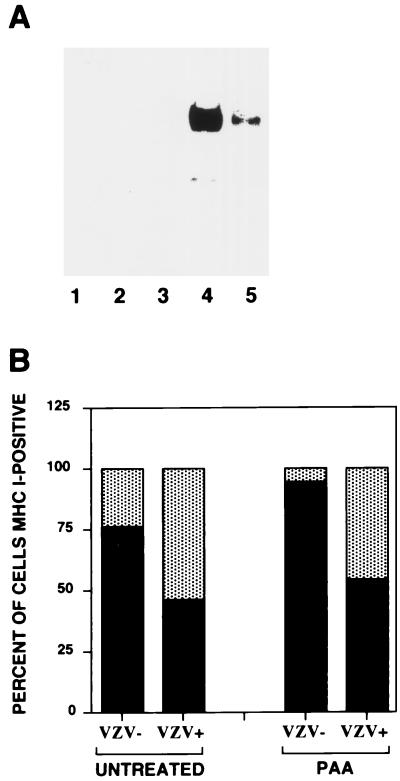
HFFs were pretreated with 300 μg of PAA/ml for 1 h prior to infection and then infected by mixing VZV-infected cells with uninfected, PAA-treated cells at a ratio of 1:5 and incubated in the presence of PAA for a further 24 h. Cells were then harvested and stained with antibodies to MHC I and VZV for flow cytometric analysis or prepared for Western blot analysis. As predicted, the expression of the late gene gC was reduced more than 50-fold after the addition of PAA compared to untreated cells (Fig. 7A). Treatment with PAA did not change the reduction in MHC I expression. In the absence of PAA, 46% of VZV+ cells expressed cell surface MHC I molecules. In the presence of PAA, 54% of VZV+ cells expressed cell surface MHC I molecules (Fig. 7B). The observation that gC expression did not influence MHC I expression was confirmed using a VZV gC-negative mutant strain to infect fibroblasts (39); the percentage of cells that were VZV+ and MHC I+ was 50%, compared to 80% for VZV− cells. Constitutive expression of VZV gE or gK in melanoma cells also had no effect on cell surface MHC I expression (data not shown). These data suggest that a VZV IE or early gene product(s) or a virion component(s) is involved in reducing cell surface MHC I expression on infected cells.
FIG. 7.
Analysis of MHC I downregulation in VZV- and mock-infected cells treated with the viral DNA inhibitor PAA. (A) Western blot of viral gC was performed on total cell lysates from mock-infected cells (lane 1), mock-infected cells with PAA (lane 2), VZV-infected cells at the time of inoculation onto uninfected cells (lane 3), and VZV-infected cells in the absence (lane 4) or presence (lane 5) of PAA at 24 h postinfection. (B) The percentage of VZV+ and VZV− cell populations expressing cell surface MHC I molecules. Cells were infected with VZV in the absence or presence of PAA for 24 h and stained with antibodies and fluorescent conjugates to MHC I and VZV proteins and analyzed by flow cytometry.
Effects of VZV IE and early genes on MHC I expression.
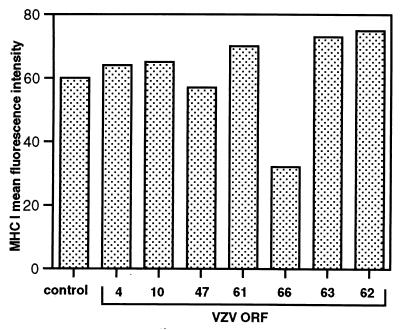
VZV encodes several genes which have been reported to be expressed under IE conditions (32). To test whether these IE proteins might mediate MHC I downregulation, human fibroblasts were transfected with the plasmids expressing ORF62, ORF63, ORF4, and ORF61 (pMS62, pCMV63, pCMV4, and pCMV61, respectively). In addition, we also tested plasmids encoding the early gene products ORF10, ORF47, and ORF66 (pCMV10, pCMV47, and pCMV66, respectively). After 48 h, transfected cells were stained for cell surface MHC I expression and analyzed by flow cytometry. Negative controls included cells transfected with the parental plasmid (pON2345) and incubation of transfected cells with an isotype control antibody. The mean fluorescence intensity of cell surface MHC I staining was determined for each transfected population of HFF cells (Fig. 8). Compared to the parental plasmid, those expressing ORF4, -10, -47, -61, -62, and -63 did not significantly alter cell surface MHC I expression. In contrast, the mean fluorescence intensity of cell surface MHC I staining was significantly decreased for cultures transfected with the ORF66-expressing plasmid. Similar results were obtained in two replicate experiments, indicating that ORF66 plays a role in downregulating MHC I.
FIG. 8.
Analysis of MHC I downregulation in cells transfected with plasmids expressing VZV proteins. HFFs were transiently transfected with plasmids expressing VZV ORF4, ORF10, ORF47, ORF61, ORF62, ORF63, and ORF66 or a parental control plasmid (control). At 48 h posttransfection cell preparations were stained for MHC I expression. Data are shown as the mean fluorescence intensities of specific cell surface MHC I staining.
DISCUSSION
These experiments demonstrate that VZV expresses a protein(s) with an immunomodulatory function that interferes with the transport of MHC I molecules through the Golgi complex of infected cells and thereby inhibits the cell surface expression of MHC I molecules. VZV pathogenesis is characterized by cellular tropisms for T lymphocytes, skin, and dorsal root ganglia (5, 14). The capacity of VZV to regulate cell surface MHC I expression on human fibroblasts and, importantly, on human T lymphocytes provides a mechanism by which the virus can limit the consequences of immunosurveillance by CD8+ T lymphocytes. Impaired CD8+ T-cell recognition of VZV-infected cells, which involves the T-cell receptor recognition of viral peptides complexed with MHC I molecules, may allow infected T lymphocytes to harbor VZV during the viremic phases of pathogenesis. VZV cell-associated viremia is necessary for viral dissemination to mucocutaneous sites, and infection at these sites is required to achieve VZV transmission to susceptible contacts and its persistence in the human population. During latency, VZV differs from HSV in its ability to persist in satellite cells as well as in neurons and in the synthesis of viral gene transcripts and proteins in these latently infected cells (14). While neurons do not usually express MHC I molecules, interference with MHC I expression may help the virus to survive in nonneuronal cells within the ganglia. It is interesting that MHC I expression was downregulated by the recombinant Oka vaccine strain, which has been passaged in guinea pig embryo fibroblasts and in tissue culture cells and is attenuated for replication in skin (39), as well as by a low-passage clinical isolate.
In investigations of the mechanism of cell surface MHC I downregulation, we found that VZV infection does not alter MHC I protein synthesis and that complexes are not retained in the ER compartment. However, MHC I molecules accumulate in the Golgi complex of VZV-infected cells. These observations indicate that the pathway by which VZV infection reduces MHC I cell surface expression is novel, differing from the effects of the other human herpesviruses, HSV and HCMV (24). HSV has been shown to induce the retention of MHC I molecules in the ER through a specific interaction of a viral protein (ICP47) with the TAP complex, resulting in the prevention of TAP-mediated antigen transport (4, 19, 21, 25, 48, 50). In contrast, HCMV has been demonstrated to use several viral immunomodulatory proteins that affect various stages of the MHC I assembly pathway, including prevention of TAP-mediated antigen transport, retention of MHC I molecules in the ER, and dislocation of retained complexes to the cytosol for degradation (2, 3, 22, 23, 29, 30, 36, 49). Interestingly, MCMV encodes a protein that disrupts the transport of MHC I molecules past the ERGIC/cis-Golgi compartment (51). Therefore, the human herpesviruses, VZV, HSV, and HCMV, all employ unique strategies to downmodulate cell surface MHC I expression. VZV resembles MCMV in that both viruses cause the retention of MHC I molecules in the post-ER compartment.
Despite the sequence similarities between the genomes of VZV and HSV, VZV does not encode an ICP47 homolog. In addition, VZV does not contain any identifiable homologs to the gene products of the other herpesviruses known to alter cell surface MHC I expression. Nonetheless, experiments using PAA to inhibit viral DNA replication suggest that an IE or early VZV gene product(s) is involved in the downmodulation of cell surface MHC I molecules, whereas late genes were not required. Expression of gC was not required for MHC I modulation, and melanoma cell lines that expressed gE or gK had no alteration in cell surface MHC I expression, which was consistent with the results of the PAA experiments. Further, the transient transfection experiments described in the present study provide evidence that IE gene products do not modulate MHC I expression but that an early gene product encoded by ORF66 is able to downregulate MHC I expression on HFF cells. ORF66 is a putative serine/threonine protein kinase which is dispensable for viral replication in tissue culture. However, ORF66 is required for T-cell infectivity, since deletion of ORF66 was associated with decreased virus replication in thymus/liver implants in the SCID-hu mouse (40). Additional genetic studies using recombinant viruses will further define the function(s) of ORF66 during VZV infection. It is also possible that other viral genes may encode proteins with the ability to downregulate MHC I expression, as another herpesvirus, HCMV, encodes no fewer than four viral genes which function to downregulate MHC I expression (20).
The intracellular localization of MHC I molecules to the Golgi complex in VZV-infected cells suggests that a viral protein interacts specifically with a component of the MHC I complex, thereby preventing efficient MHC I transport to the cell surface. In this respect, other herpesviruses, including HSV, HCMV, and MCMV, have been shown to encode immunomodulatory proteins that directly associate with components of the MHC I biosynthesis pathway (2, 19, 21, 25, 29, 33, 36, 45, 48, 49, 50). Immunoprecipitation of radiolabeled MHC I molecules in VZV- and mock-infected cells did not reveal binding of a viral protein, but a modified, ∼40-kDa MHC I molecule was detected consistently in VZV-infected cells, which may reflect accelerated degradation of MHC I molecules retained in the Golgi complex.
VZV and other viruses that modulate cell surface MHC I expression may evade cytotoxic T-lymphocyte (CTL) recognition, but by reducing the overall surface levels of MHC I expression, these cells may also become more sensitive to natural killer (NK) cell lysis. HCMV and MCMV have mechanisms to combat NK cell as well as CTL recognition (8, 9, 11, 17, 18, 33). Although an MHC I homolog has yet to be identified in the VZV genome, it is possible that VZV does encode a gene product to avoid NK cell lysis while maintaining immune evasion of CTL. Alternatively, as shown in HIV-infected cells, decreasing the cell surface concentration of certain MHC I alleles and not others may result in evasion of NK cell lysis (13). Our preliminary results suggest that VZV may also cause an allele-specific downmodulation of MHC I molecules (unpublished data).
In order to cause primary infection and to establish latency, VZV must be able to evade both MHC I- and MHC II-restricted immune responses with some efficiency. VZV memory immunity is characterized by the presence of equivalent numbers of antigen-specific CD4+ and CD8+ T lymphocytes (6). Our previous studies have shown that VZV has evolved a mechanism to minimize recognition of infected cells by CD4+ T lymphocytes by inhibiting gamma interferon-stimulated expression of MHC II molecules (1). Delayed induction of CD4+ T-lymphocyte responses to VZV antigens is accompanied by a longer period of formation of new skin lesions, which provide a reservoir for VZV spread to susceptible individuals (7). In addition, VZV reactivation and replication in skin cause herpes zoster, despite the presence of memory T lymphocytes that can produce high concentrations of gamma interferon when exposed to VZV antigens (27), as well as memory CD8+ CTL that recognize viral glycoproteins and structural and regulatory proteins. Thus, immunomodulatory mechanisms that limit the initial presentation of VZV peptides by MHC I or MHC II presentation pathways are likely to provide the virus with a transient advantage during primary and recurrent VZV infections.
In summary, we have demonstrated that VZV infection causes downregulation of MHC I molecules in human fibroblasts and T lymphocytes. The analysis of VZV-infected human T lymphocytes provides a novel demonstration of MHC I downregulation on a human cell type that is instrumental for viral pathogenesis. These experiments indicate that VZV encodes a protein(s) with an immunomodulatory function which does not affect biosynthesis of MHC I molecules but interferes with the transport of MHC I molecules through the cell, causing retention in the Golgi compartment. Immunomodulatory mechanisms that enhance the transport of VZV to skin and a period of viral replication before immunologic clearance in individuals with varicella or herpes zoster ensure opportunities for transmission to susceptible individuals and have allowed the virus to persist in the human population for millions of years.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, AI20459.
We thank Jennifer Moffat for help with the SCID-hu experiments, Hideto Kaneshima, SyStemix, Inc., for supplying the SCID-hu mice, Chengjun Mo in our laboratory for providing the gE- and gK-expressing melanoma cells, and Frank Masiarz, Chiron Corporation, Emeryville, Calif., for the mass spectrometry analysis.
REFERENCES
- 1.Abendroth A, Slobedman B, Lee E, Wallace M, Mellins E, Arvin A. Modulation of major histocompatibility complex class II expression by varicella-zoster virus. J Virol. 2000;74:1900–1907. doi: 10.1128/jvi.74.4.1900-1907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn K, Angulo A, Ghazal P, Peterson P A, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J, Ploegh H L, Peterson P A, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 4.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 5.Arvin A. Varicella-zoster virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphian, Pa: Lippincott-Raven Publishers; 1996. pp. 2547–2586. [Google Scholar]
- 6.Arvin A. Varicella-zoster virus: virologic and immunologic aspects of persistent infection. In: Ahmed R, Chen I, editors. Persistent viral infections. New York, N.Y: John Wiley & Sons Ltd.; 1998. pp. 183–208. [Google Scholar]
- 7.Arvin A M, Koropchak C M, Williams B R, Grumet F C, Foung S K. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 8.Beck S, Barrell B. Human cytomegalovirus encodes a glycoprotein homologous to MHC class I-antigens. Nature. 1988;331:269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- 9.Browne H, Smith G, Beck S, Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990;347:770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- 10.Burgert H G, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985;41:987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- 11.Chapman T, Bjorkman P. Characterization of a murine cytomegalovirus class I major histocompatibility complex (MHC) homolog: comparison to MHC molecules and to the human cytomegalovirus MHC homolog. J Virol. 1998;72:460–466. doi: 10.1128/jvi.72.1.460-466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2742–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen G, Gandhi R, Davis D, Mandelboin O, Chen B, Strominger J, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J, Straus S. Varicella-zoster virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2525–2546. [Google Scholar]
- 15.Cohen J I. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;177:1390–1393. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
- 16.Duus K, Grose C. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J Virol. 1996;70:8961–8971. doi: 10.1128/jvi.70.12.8961-8971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahenstock M, Johnson J, Feldman R, Neveu J, Lane W, Bjorkman P. The MHC class I homolog encoded by human cytomegalovirus binds endogenous peptides. Immunity. 1995;3:583–590. doi: 10.1016/1074-7613(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 18.Farrell H, Vally H, Lynch D, Fleming P, Shellam G, Scalzo A, Davis P. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 19.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 20.Fruh K, Gruhler A, Krishna R, Schoenhals G. A comparison of viral immune escape strategies targeting the MHC class I assembly pathway. Immunol Rev. 1999;168:157–166. doi: 10.1111/j.1600-065x.1999.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 21.Galocha B, Hill A, Barnett B, Dolan A, Raimondi A, Cook R, Brunner J, McGeoch D, Ploegh H. The active site of ICP47, a herpes simplex virus-encoded inhibitor of major histocompatibility complex (MHC)-encoded peptide transport associated with antigen processing (TAP), maps to the NH2-terminal 35 residues. J Exp Med. 1997;185:1565–1572. doi: 10.1084/jem.185.9.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengel H, Flohr T, Hammerling G J, Koszinowski U H, Momburg F. Human cytomegalovirus inhibits peptide translocation into the endoplasmic reticulum for MHC class I assembly. J Gen Virol. 1996;77:2287–2296. doi: 10.1099/0022-1317-77-9-2287. [DOI] [PubMed] [Google Scholar]
- 23.Hengel H, Koopmann J O, Flohr T, Muranyi W, Goulmy E, Hammerling G J, Koszinowski U H, Momburg F. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 1997;6:623–632. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 24.Hengel H, Koszinowski U. Interference with antigen processing by viruses. Curr Opin Immunol. 1997;9:470–476. doi: 10.1016/s0952-7915(97)80097-0. [DOI] [PubMed] [Google Scholar]
- 25.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 26.Honess R, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins D E, Redman R L, Lam E M, Liu C, Lin I, Arvin A M. Interleukin (IL)-10, IL-12, and interferon-gamma production in primary and memory immune responses to varicella-zoster virus. J Infect Dis. 1998;178:940–948. doi: 10.1086/515702. [DOI] [PubMed] [Google Scholar]
- 28.Jones T, Hanson L, Sun L, Slater J, Stenberg R, Campbell A. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69:4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones T, Sun L. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J Virol. 1997;71:2970–2979. doi: 10.1128/jvi.71.4.2970-2979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones T R, Wiertz E J, Sun L, Fish K N, Nelson J A, Ploegh H L. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerkau T, Bacik I, Bennik J R, Yewdell J W, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinchington, P. R., and J. I. Cohen. Varicella-zoster virus: viral proteins, p. 74-104. In A. Arvin and A. Gershon (ed.), Varicella zoster virus: virology and clinical management. Cambridge University Press, Cambridge, England.
- 33.Kleijnen M, Huppa J, Lucin P, Mukherjee S, Farrell H, Campbell A, Koszinowski U. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 1997;16:685–694. doi: 10.1093/emboj/16.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehner P, Trowsdale J. Antigen presentation: coming out gracefully. Curr Biol. 1998;8:R605–R608. doi: 10.1016/s0960-9822(98)70387-2. [DOI] [PubMed] [Google Scholar]
- 35.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci M. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen. Proc Natl Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machold R, Wiertz E, Jones T, Ploegh H. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallory S, Sommer M, Arvin A. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moffat J F, Stein M D, Kaneshima H, Arvin A M. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moffat J F, Zerboni L, Kinchington P R, Grose C, Kaneshima H, Arvin A M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffat J F, Zerboni L, Sommer M H, Heineman T C, Cohen J I, Kaneshima H, Arvin A M. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci USA. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namikawa R, Weilbaecher K, Kaneshima H, Yee E, McCune J. Long term human haematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nataraj C, Eidmann S, Hariharan M, Sur J, Perry G, Srikumaran S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997;10:21–34. doi: 10.1089/vim.1997.10.21. [DOI] [PubMed] [Google Scholar]
- 43.Perera L P, Mosca J D, Sadeghi-Zadeh M, Ruyechan W T, Hay J. The varicella-zoster virus immediate early protein, IE62, can positively regulate its cognate promoter. Virology. 1992;191:346–354. doi: 10.1016/0042-6822(92)90197-w. [DOI] [PubMed] [Google Scholar]
- 44.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 45.Reusch U, Muranyi W, Lucin P, Burgert H, Hengel H, Koszinowski U. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18:1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharp M, Terada K, Wilson A, Nader S, Kinchington P E, Ruyechan W T, Hay J, Arvin A M. Kinetics and viral protein specificity of the cytotoxic T lymphocyte response in healthy adults immunized with live attenuated varicella vaccine. J Infect Dis. 1992;165:852–858. doi: 10.1093/infdis/165.5.852. [DOI] [PubMed] [Google Scholar]
- 47.Sparks-Thissen R, Enquist L. Differential regulation of Dk and Kk major histocompatibility complex class I proteins on the cell surface after infection of murine cells by pseudorabies virus. J Virol. 1999;73:5748–5756. doi: 10.1128/jvi.73.7.5748-5756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 49.Wiertz E J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 50.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski U H. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]