Abstract
The widespread LIS1-proteins were originally identified as the target for sporadic mutations causing lissencephaly in humans. Dictyostelium LIS1 (DdLIS1) is a microtubule-associated protein exhibiting 53% identity to human LIS1. It colocalizes with dynein at isolated, microtubule-free centrosomes, suggesting that both are integral centrosomal components. Replacement of the DdLIS1 gene by the hypomorphic D327H allele or overexpression of an MBP-DdLIS1 fusion disrupted various dynein-associated functions. Microtubules lost contact with the cell cortex and were dragged behind an unusually motile centrosome. Previously, this phenotype was observed in cells overexpressing fragments of dynein or the XMAP215-homologue DdCP224. DdLIS1 was coprecipitated with DdCP224, suggesting that both act together in dynein-mediated cortical attachment of microtubules. Furthermore, DdLIS1-D327H mutants showed Golgi dispersal and reduced centrosome/nucleus association. Defects in DdLIS1 function also altered actin dynamics characterized by traveling waves of actin polymerization correlated with a reduced F-actin content. DdLIS1 could be involved in actin dynamics through Rho-GTPases, because DdLIS1 interacted directly with Rac1A in vitro. Our results show that DdLIS1 is required for maintenance of the microtubule cytoskeleton, Golgi apparatus and nucleus/centrosome association, and they suggest that LIS1-dependent alterations of actin dynamics could also contribute to defects in neuronal migration in lissencephaly patients.
INTRODUCTION
The LIS1 gene was originally identified as the target for sporadic mutations resulting in haploinsufficiency and a severe brain developmental disease called type I lissencephaly in human infants. Lissencephaly (Greek lissos = smooth) is characterized by a smooth appearance of the neocortical surface due to the absence of gyri and sulci (Reiner et al., 1993). This is believed to be the consequence of impaired migration of neuronal precursors from the paraventricular area, where they divide, to the cerebral cortex during development. The LIS1 protein has a calculated molecular mass of ∼45 kDa and is characterized by seven WD40-repeats, which are thought to form a β-propeller fold as in structurally similar β-subunits of heterotrimeric G-proteins. Indeed, LIS1 could be identified as a subunit of a brain-specific isoform of the G-protein-like platelet-activating factor acetylhydrolase. Yet, the first clues for the molecular function of LIS1 in neuronal migration came from a filamentous fungus. The Aspergillus nidulans LIS1 homologue, NUDF, was identified in a screen for nuclear distribution mutants (Xiang et al., 1995). Further nud mutants include nudA, encoding the cytoplasmic dynein heavy chain, and nudE. Mutations in nudA, nudE, and nudF caused similar defects in nuclear migration during hyphal stalk formation (Morris et al., 1998). Nuclear migration is an important factor in neuronal cell migration as well (reviewed by Gupta et al., 2002), and it is achieved through the activity of dynein/dynactin localized at the cell cortex. The microtubule minus end-directed pulling forces exerted by dynein are transmitted to the nucleus through microtubules emanating from the nucleus-associated centrosome (reviewed by Dujardin and Vallee, 2002). Because mammalian LIS1 could be immunoprecipitated with both dynein and dynactin subunits (Faulkner et al., 2000; Smith et al., 2000), it was hypothesized that defects in LIS1 disrupt dynein function, which in turn causes the neuronal migration disorder observed in lissencephaly. Interestingly, both NUDF and NUDE as well as their mammalian homologues LIS1 and NUDEL (=NUDE-like) directly interact with the dynein heavy chain (Sasaki et al., 2000). Recent data suggest that LIS1 and NUDEL form a complex with dynein and have a synergistic effect on the promotion of dynein function. Complex formation appears to be positively regulated by phosphorylation of NUDEL through CDK5/p53, whereas the ser/thr-phosphate-binding protein 14–3-3ε protects NUDEL from dephosphorylation by PP2A (Toyo-Oka et al., 2003). In addition to the LIS1 interactors mentioned above, there are several further binding partners such as CLIP170 or doublecortin (Caspi et al., 2000; Coquelle et al., 2002; Schaar et al., 2004), which may assist in the capture of microtubule plus ends by dynein/dynactin at the cell cortex.
The essential role of LIS1 for neuronal migration appears to be mediated not only through its interaction with dynein. Recently Kholmanskikh and coworkers showed that LIS1 haploinsufficiency resulted in a reduced F-actin content at the leading edge of migrating cerebellar granule cells (Kholmanskikh et al., 2003). Interestingly, this effect was accompanied by altered activity of small GTPases regulating cortical actin dynamics. Although Rac1 and Cdc42 activities were down-regulated, the antagonizing GTPase RhoA was up-regulated under these conditions. However, no binding of LIS1 to one of these GTPases or their regulators could be shown. Thus, the relationship between cellular LIS1 levels and GTPase activities remained unclear.
In addition to its role in actin dynamics and dynein function at the cell cortex, LIS1 is also a regulator of microtubule dynamics. LIS1 binds to microtubules in vivo and in vitro and promotes microtubule elongation by reducing the catastrophe rate (Sapir et al., 1997). Recently we have shown in Dictyostelium amoebae that DdCP224, a member of the ubiquitous XMAP215-family of microtubule-associated proteins (Ohkura et al., 2001), is also involved both in dynein-dependent microtubule interactions with the cell cortex and in the promotion of microtubule growth (Gräf et al., 2003; Hestermann and Gräf, 2004). Because of the similarity of LIS1 protein function in mammalian and fungal cells and the roles of DdCP224 in Dictyostelium cells, we suspected a functional relationship between LIS1 and XMAP215-family proteins and performed a functional analysis of DdLIS1, the Dictyostelium LIS1 homologue. We show that DdLIS1 is a microtubule-associated protein that also constitutes an integral component of the centrosome. Disruption of DdLIS1 function affects microtubule tip interactions with the cell cortex, cell morphology, and actin dynamics. Furthermore, we demonstrate for the first time an association of a LIS1 protein with a member of the XMAP215-family of microtubule-associated proteins and direct binding to an actin-regulating small GTPase. The elucidation of these interactions may help to explain the central role of DdLIS1 in the dynamics of both the microtubule and actin cytoskeleton.
MATERIALS AND METHODS
Construction of a Complete DdLIS1 cDNA
Clone SLE307 of the Dictyostelium cDNA project (Morio et al., 1998) was kindly provided by T. Morio (University of Tsukuba, Japan). It is based on pSPORT1 (Invitrogen, Karlsruhe, Germany) and encoded a partial DdLIS1 sequence. The missing 5′-cDNA sequence including 216 base pairs of additional coding sequence was amplified by PCR from a Dictyostelium cDNA library in λZAP (Gräf et al., 2000b) using the T3-primer and two nested DdLIS1-specific primers. The resulting 662-base pair fragment included an EcoRI site derived from the λ-vector and an NsiI site located within the DdLIS1 coding sequence. These restriction sites were used to construct a complete DdLIS1 cDNA clone in SLE307 (EMBL database accession no. AJ512794).
Vector Construction, Protein Expression, and Antibodies
To express DdLIS1 as a maltose-binding fusion protein in Escherichia coli, a PCR-fragment including the complete DdLIS coding sequence flanked by EcoRI and XbaI restriction sites was cloned into pMALc2. The fusion protein was expressed and purified by amylose affinity chromatography as described by the manufacturer (New England Biolabs, Frankfurt, Germany) and used for the custom immunization of a rabbit (Dr. J. Pineda Antikörperservice, Berlin, Germany).
Likewise, a His-DdLIS1 vector based on pQE30 (Qiagen, Hilden, Germany) was constructed using the PCR product obtained after amplification of the DdLIS1 coding sequence with an upstream BamHI linker primer and a downstream SalI linker primer. His-DdLIS1 was expressed and purified by NiNTA affinity chromatography as described by the manufacturer (Qiagen).
For expression of MBP-DdLIS1 in Dictyostelium, the MBP-DdLIS1/pMALc2 vector was used as a template to amplify the sequence encoding the fusion protein with a suitable upstream MBP-KpnI linker primer and a downstream DdLIS1-BamHI linker primer. The obtained MBP-DdLIS1 PCR fragment was subsequently cloned into p1ABsr8 (Gräf et al., 2000b) and transformed into Dictyostelium cells.
The gene replacement vector for generation of the DdLIS1-D327H point mutant was based on pLPBLP (Faix et al., 2004). The final plasmid contained the 5′ untranslated sequence and the complete coding sequence including the point mutation upstream from the blasticidin S resistance cassette and 887 base pairs of 3′ untranslated sequence of the gene downstream from the resistance cassette. A genomic DdLIS-fragment containing the point mutation was generated by PCR in a two-step approach using two mutagenesis primers that were used to introduce a new SalI site, resulting in the desired single amino acid exchange. Thus, two PCR fragments with 1466 and 1261 base pairs, respectively, were generated using genomic DNA as a template. For the first fragment, an upstream BamHI linker primer binding to the 3′ untranslated region of the gene and a downstream mutagenesis primer GTCGACTACCAGTAGCTAAATAACCACATT were used, and for the second fragment an upstream mutagenesis primer GTCGACATAAAACTATTAAAATTTGGG and a downstream PstI linker primer binding to the 5′ end of the coding sequence. Both PCR fragments were cleaved with BamHI/SalI and SalI/PstI, respectively, and were both cloned into pLPBLP in a single step using the BamHI and PstI sites of the vector. The resulting plasmid was cleaved with HindIII and KpnI and used to clone 887 base pairs of 3′ untranslated region of the gene, which was generated by PCR using genomic DNA as a template and an upstream HindIII linker primer directed to the beginning of the 3′ untranslated region and a downstream primer (named LIS1–14) binding immediately downstream from the endogenous KpnI site. Before transformation of Dictyostelium cells, the final gene replacement vector was linearized by cleavage with BamHI and KpnI, and the desired DdLIS1/blasticidin cassette was isolated from an agarose gel. After blasticidin selection, genomic DNA was isolated using the HighPure PCR template preparation kit (Roche, Mannheim, Germany) and positive clones were identified by a genotyping PCR using the LIS1–14 downstream primer and a GTGGTTATTTAGCTACTGGTAGTC upstream primer. The latter primer is specific for the point mutation because its last four bases fit only to the mutated but not to the original genomic sequence and the binding site for LIS1–14 is not located within the linearized transformation construct. Therefore amplification of a specific 2.6-kb PCR fragment from genomic DNA is only possible in clones, where gene replacement of endogenous DdLIS1 by the artificial construct through homologous recombination has occurred and which carry the D327H point mutation.
The vector for DICΔC overexpression corresponds to the DICΔC construct published previously (Ma et al., 1999). In brief the coding sequence encoding the N-terminal fragment of the Dictyostelium dynein IC (DIC; 279 amino acids) was amplified by PCR using linker primers and cDNA as a template. The PCR product was cloned into a derivative of pA15GFPSSEB2 using NheI and BamHI. This cloning vector is identical to pDiscGFPSSEB2 (Daunderer and Gräf, 2002) except that the discoidin promoter is replaced by the actin15 promoter.
Light Microscopy
Light microscopy was essentially performed as described previously (Gräf et al., 1998). All images and movies were acquired on a Zeiss Axiovert 200M/510META laser scanning system (Oberkochen, Germany) equipped with a 63×/1.4 lens. Live cells were viewed in glass bottom dishes (MatTek, Ashland, MA) either in phosphate buffer or under agar overlay (Fukui et al., 1987) at low laser intensity. GFP-actin cell lines were cultivated overnight in coculture with Klebsiella aerogenes in 17 mM Na-K-phosphate buffer (pH 6.0) in order to increase their resistance to phototoxic effects. For reflection interference contrast microscopy (RICM) a 633-nm HeNe-laser was used and the reflection image was recorded without any filter. In case of 4D live cell imaging, three to five z-slices per time point were acquired at a frame rate of two per second and a resolution of up to 512 × 512 pixels. Z-stacks were projected onto one plane using a brightest point algorithm and ImageJ software (National Institutes of Health, Bethesda, MD). All images from fixed cells were processed through the Huygens Essential Deconvolution software (SVI, Hilversum, Netherlands) using the maximum likelihood estimation method.
Estimation of the Actin Content
Cells cultivated overnight in shaking culture (density approx. 3 × 106/ml) were spread onto 5-cm Petri dishes to ∼50% confluency and allowed to settle for 10 min. Subsequently, medium was exchanged for phosphate buffer with or without 0.2 μM latrunculin A (Sigma, Deisenhofen, Germany) and cells were incubated for 1 h at room temperature. Cells lysis occurred on ice by rinsing the attached cells with 2 ml of ice cold lysis buffer (80 mM Na-PIPES, pH 6.8, 5 mM EGTA, 5 mM MgCl2, 1 mM dithiothreitol, 1% Triton X-100, 25% glycerol, protease inhibitors; Gräf et al., 1998). Two hundred fifty microliters of the lysate were ultracentrifuged with 80.000 rpm for 40 min at 4°C using a Beckman TLA100 rotor (Krefeld, Germany). The pellet containing the F-actin fraction was resuspended in 250 μl of lysis buffer. Both F- and G-actin (supernatant) fractions were supplemented with SDS-gel loading buffer and separated by SDS gel electrophoresis and Western blotting. The blot was stained using anti–Dictyostelium-actin antibodies. Actin bands were visualized using an enhanced chemiluminescence kit (Applichem, Darmstadt, Germany).
Other Methods
Dictyostelium cells (strain AX2) were cultivated and transformed as reported earlier (Gräf et al., 1998, 2000b). SDS gel electrophoresis and Western blotting were carried out according to Gräf et al. (1998). Centrosomes were isolated as described previously (Gräf, 2001a). Preparation of cell extracts of Dictyostelium cells was reported by Daunderer and Gräf (2002). Immunoprecipitation was performed according to Hestermann and Gräf (2004) and GST pull-down assays were carried out as described by Faix et al. (1998).
Antibodies
Primary antibodies: rabbit polyclonal anti-DdLIS1 (this work), mouse monoclonal anti-DdCP224 (Gräf et al., 1999), rabbit polyclonal anti-DdCP224 (Hestermann and Gräf, 2004), rabbit polyclonal antidynein heavy chain Y7 and 695 (Koonce and Samso, 1996), rat monoclonal anti-α-tubulin YL1/2 (Chemicon, Hofheim, Germany), mouse monoclonal anticomitin (Weiner et al., 1993), rabbit polyclonal anti-DdSpc97 (Daunderer and Gräf, 2002), rabbit polyclonal anti-MBP (Gräf, 2001b), monoclonal anti-Dictyostelium actin 224-236-1 (Westphal et al., 1997).
Secondary antibodies were from Dianova (Hamburg, Germany; all cy3 and peroxidase conjugates), Sigma (alkaline phosphatase conjugates); and Molecular Probes (Hilversum, Netherlands; all AlexaFluor 488 conjugates).
RESULTS
DdLIS1 Is Closely Related to Human LIS1
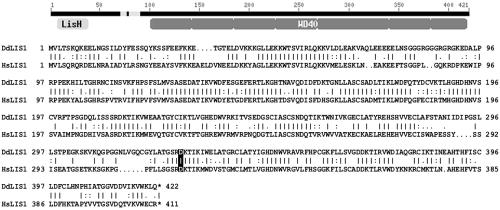
A partial cDNA sequence encoding a Dictyostelium homologue of LIS1 (DdLIS1) was identified on clone SLE307 in the data library of the Dictyostelium cDNA project (University of Tsukuba, Japan; Morio et al., 1998). Using this clone and a PCR-fragment containing the missing 5′-cDNA sequence we have constructed a complete DdLIS1 cDNA clone (EMBL database accession no. AJ512794; see Materials and Methods). Among all nonanimal LIS1-homologues, DdLIS1 is most closely related to human LIS1. Both proteins share 55% of identical amino acids (72% similarity). Moreover, with a calculated molecular mass of 47 kDa and an N-terminal LIS1-homology domain followed by a short coiled-coil domain and seven WD40 repeats, DdLIS1 displays the same domain structure and almost the same size as its human counterpart (Figure 1).
Figure 1.
Domain structure of DdLIS1 and alignment of the DdLIS1 and human LIS1 amino acid sequences. Like other LIS1 homologues, DdLIS1 is segmented into a LIS1-homology domain (LisH), a short coiled coil region (CC) and seven WD40 repeats. The alignment was performed with GAP-alignment (GCG package). The position of the D327H point mutation is highlighted by a black box.
DdLIS1 Is a Microtubule-associated Protein and an Integral Centrosomal Component together with Dynein
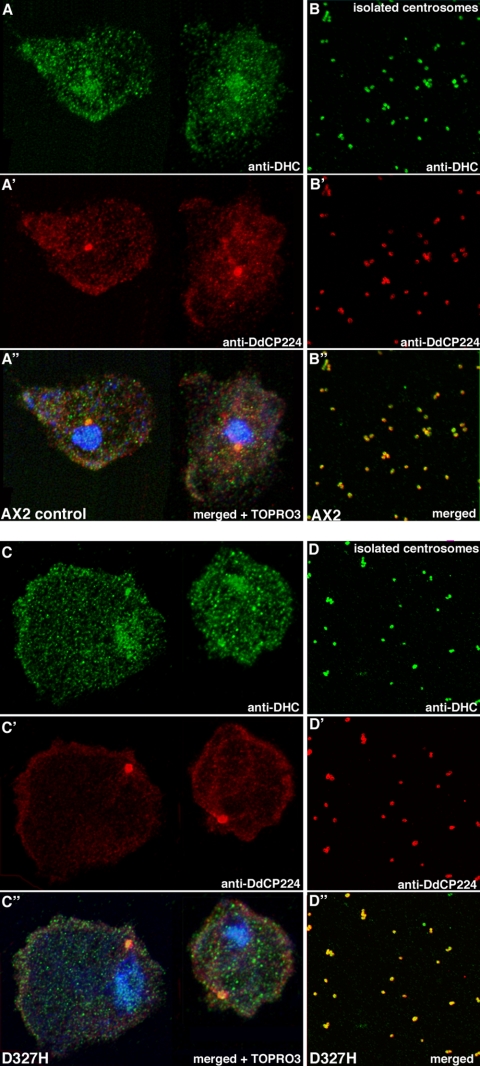
The subcellular localization of DdLIS1 was investigated using polyclonal antibodies raised against the recombinant protein expressed in E. coli (Figure 2A). DdLIS1 was localized along microtubules and at the centrosome throughout the entire cell cycle (Figure 2, B–D). In contrast to human LIS1 (Faulkner et al., 2000), microtubule labeling by anti-DdLIS1 antibodies was not biased toward the microtubule plus ends either at the peripheral microtubule tips in interphase or at kinetochores during mitosis. To test whether the centrosomal localization of DdLIS1 is microtubule-dependent, we analyzed the presence of DdLIS1 at isolated centrosomes. This assay was used because treatment with microtubule-depolymerizing drugs such as thiabendazole or nocodazole is rather ineffective in Dictyostelium and leads to only partial depolymerization of microtubules (Kitanishi et al., 1984), whereas isolated Dictyostelium centrosomes are completely devoid of microtubules (Gräf et al., 1998). The Dictyostelium centrosome is a cytosolic, nucleus-associated body that lacks centrioles. Instead it consists of a three-layered core structure surrounded by the corona, which is the functional equivalent of the pericentriolar matrix of centriole-containing centrosomes (Euteneuer et al., 1998; Gräf et al., 2000a). Confocal images of isolated centrosomes labeled with anti-DdLIS1 antibodies clearly revealed that DdLIS1 is part of the centrosomal corona (Figure 2D). When labeled with antibodies against known components such as DdCP224 or γ-tubulin (Gräf et al., 2000b), the corona displays a ring-like appearance in confocal images. In deconvolved confocal images, the diameter of the anti-DdCP224-labeled ring was always larger than that observed upon labeling with anti-DdLIS1 antibodies, suggesting that DdLIS is localized closer to the unlabeled centrosomal core structure than DdCP224 (Figure 2D). Therefore, DdLIS1 is a genuine centrosomal component. This was unexpected, because centrosomal LIS1 localization was thought to be mediated through its binding to the dynein heavy chain (Sasaki et al., 2000), which one would expect to require microtubules to localize it to the centrosome through its microtubule minus end-directed motor activity. However, our confocal analysis of isolated centrosomes revealed that the dynein heavy chain, which precisely colocalizes with DdCP224 at the centrosomal corona, is an integral component of the Dictyostelium centrosome as well (Figure 2E).
Figure 2.
DdLIS1 localizes to microtubules, the mitotic spindle, and isolated centrosomes. DdLIS1 was stained using affinity-purified anti-DdLIS1 antibodies. The specificity of affinity-purified anti-DdLIS1 antibodies used for immunofluorescence microscopy is shown on a Western blot (A) of a Dictyostelium cell extract where only a single band at ∼50 kDa is stained. Subcellular localization of DdLIS1 during interphase (B) and mitosis (metaphase; C) is shown. Counterstainings were performed with anti-α-tubulin and the anti-DdCP224 mAb 2/165 (C′), which mainly stains spindle poles and kinetochores during this mitotic stage (Gräf et al., 1999, 2000b). The merged images (B″ and C″) show DdLIS1 in green and α-tubulin in red. DdLIS1 and the dynein heavy chain are also present at isolated centrosomes, which are devoid of microtubules (D and E). The control stainings against DdCP224, which is an established component of the centrosomal corona, demonstrates colocalization of these proteins at the corona (D′, D″, E′, E″). The merged images (D″, E″) show DdCP224 in red and DdLIS1 and dynein, respectively in green. (D‴) and (E‴) show respective tracings of fluorescence intensity along a line through the center of the centrosome (white line in D and E). The positions of the maxima of fluorescence intensity reveal exact colocalization of the dynein heavy chain and DdCP224, whereas DdLIS1-labeling is concentrated closer to the centrosomal core structure than DdCP224. All specimens were fixed with methanol. Bar, 2 μm.
The DdLIS1 D327H Point Mutation Causes Defects in Microtubule/Cortex Interactions, Golgi Positioning, and Centrosome/Nucleus Association
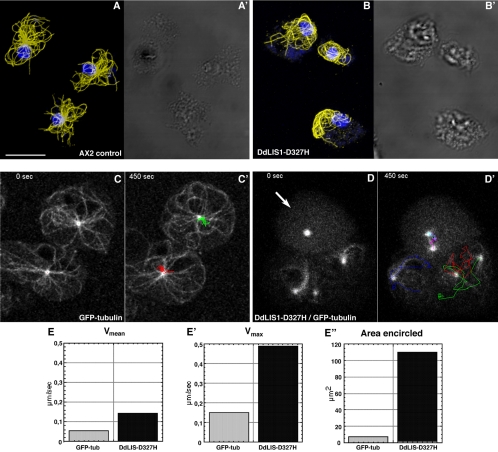
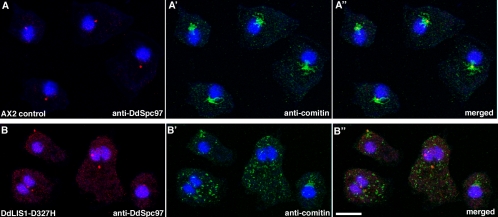
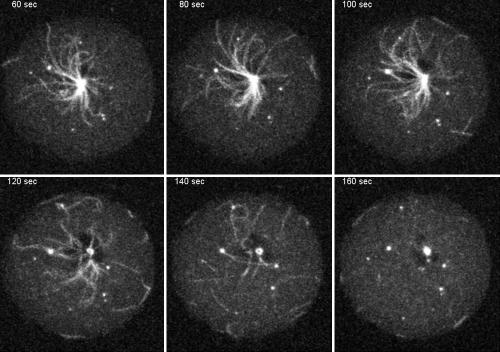
To shed light on DdLIS1 function we have created a loss-of-function mutant. Because a complete knockout of the gene encoding DdLIS1 (lis1) was unsuccessful, we replaced lis1 by a hypomorphic allele. The D317H point mutation in human LIS1 found in several lissencephaly patients causes partial misfolding of the LIS1 protein, which results in a mild form of lissencephaly characterized by pachygyria (Pilz et al., 1999; Sapir et al., 1999; Caspi et al., 2003). The corresponding D327H mutation in Dictyostelium was generated through homologous recombination by replacement of lis1 with a point-mutated copy. Although the subcellular localization of DdLIS1-D327H was indistinguishable from wild-type DdLIS1 (our own unpublished data), cells expressing only point-mutated DdLIS1 displayed a clear mutant phenotype. During interphase, the vast majority of cells expressing DdLIS1-D327H showed a striking disruption of their microtubule cytoskeleton. The usually radial interphase microtubule arrays were collapsed in a way that the microtubules appeared bundled and whorled around the nucleus (Figure 3, A and B). Four-dimensional confocal microscopy of DdLIS1-D327H cells expressing GFP-α-tubulin revealed an extraordinary motility of these microtubule arrays (Figure 3, C and D; see supplementary videos 1 and 2). The centrosome circulated rapidly and continuously, often close to the cell cortex, with most of the microtubules dragged behind like a comet tail (Figure 3D′). In GFP-α-tubulin–expressing control cells, the centrosome always stayed close to the cell center and moved only over short distances (Figure 3C′). Compared with GFP-α-tubulin cells where the centrosome moved with an average speed of 0.054 μm/s (n = 5; SD = 0.007) and a maximal speed of 0.15 μm/s, centrosomes in DdLIS1-D327H cells moved three times faster with an average speed of 0.142 μm/s (n = 21; SD = 0.022) and a maximum of even 0.5 μm/s (Figure 3E). Within 7 min, the area encircled by the moving centrosomes was 15-fold larger in DdLIS1-D327H cells than in GFP-α-tubulin cells (110 μm2 vs. 7.2 μm2; Figure 3E). This microtubule/centrosome phenotype was strikingly similar to Dictyostelium cells overexpressing the motor domain of the dynein heavy chain (Koonce and Samso, 1996; Koonce et al., 1999), fragments of the dynein IC (Ma et al., 1999), or an N-terminal fragment of DdCP224 (Hestermann and Gräf, 2004). In all these strains, including our DdLIS1-D327H cells, the microtubule phenotype was also accompanied by a dispersal of the Golgi apparatus (Figure 4). This remarkable phenotype was explained by a loss of dynein/dynactin function, which is required both for pericentrosomal localization of the Golgi apparatus and for the maintenance of radial microtubule arrays through cortically bound dynein pulling at microtubule ends. The existence of such cortical microtubule pulling forces is illustrated by Supplementary Movie3 (Figure 5), which shows the mitosis of a cell with GFP-fluorescent microtubules. As a normal feature of the Dictyostelium centrosome duplication cycle (Ueda et al., 1999), microtubules are severed from the centrosome at the G2/M transition before centrosome splitting (starting at time point 110 s in Supplementary Movie3). Subsequently the shrinking microtubules appear to be quickly pulled toward the cell cortex.
Figure 3.
The DdLIS1-D327H point mutation causes a collapse of interphase microtubule arrays and unusual centrosome motility. Immunofluorescence labeling of control cells (A) and DdLIS1-D327H cells (B) with anti-α-tubulin (yellow) and the DNA staining dye TOPRO3 (blue; Molecular Probes). Cells were fixed with glutaraldehyde. Live analysis of microtubule and centrosome behavior was performed with GFP-α-tubulin control cells (C, see Supplementary Movie1) and DdLIS1-D327H cells expressing GFP-α-tubulin (D, see Supplementary Movie2). Each image represents a brightest point z-projection of five confocal slices with a distance of 1 μm each. C′ and D′ display the movements of each centrosome within a time of 450 s by colored lines. The arrow points on a mitotic cell (prophase at time point 0 s). Average speed (E), maximum speed (E′), and the area enclosed by the centrosome movements (E″) within 450 s of control cells (gray columns) and DdLIS1-D327H cells (black columns) were evaluated using the manual tracking plugin for ImageJ. Bar, 5 μm.
Figure 4.
DdLIS1-D327H cells show Golgi dispersal. Immunofluorescence labeling of control cells (A) and DdLIS1-D327H cells (B) with anti-DdSpc97 as a centrosome marker (red; A and B) and anticomitin as a Golgi marker (green; A′ and B′). Nuclei were stained with TOPRO3 (blue). These stainings were merged in A″ and B″. Cells were fixed with methanol. Bar, 5 μm.
Figure 5.
Live observation of GFP-labeled microtubules during mitosis suggests the existence of cortical microtubule pulling forces. Supplementary Movie3. shows abscission of the radial microtubules from the centrosome in prophase (time point 120 s) and movement of the free microtubules toward the cell cortex. Each image represents a brightest point z-projection of five confocal slices with a distance of 1.6 μm each.
A further phenotype frequently observed in DdLIS1-D327H cells was an unusually large distance between the centrosome and the nucleus. In control cells, the centrosome was always tightly linked to the nucleus, with a distance to the nucleus rarely exceeding its own diameter (Figure 6A). However, in DdLIS1-D327H cells the centrosome was often located more than 2 μm away from the nucleus (Figure 6B), which, interestingly, can also be weakly labeled with antidynein heavy chain antibodies (Figure 7).
Figure 6.
The DdLIS1-D327H point mutation weakens the nucleus/centrosome association. Immunofluorescence labeling of control cells (A) and DdLIS1-D327H cells (B) with anti-α-tubulin antibodies (green; A and B) and polyclonal anti-DdCP224 as a centrosome marker (red; A′ and B′). Nuclei were stained with TOPRO3 (blue). These stainings were merged in A″ and B″. Cells were fixed with glutaraldehyde. In DdLIS-D327H cells the distance between centrosomes and the DNA is greatly enhanced. Bar, 2 μm.
Figure 7.
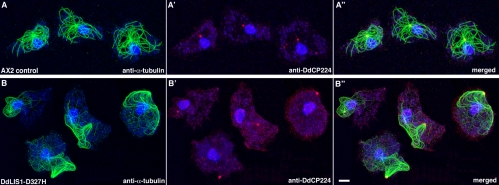
Subcellular localizations of dynein and DdCP224 are not significantly altered in DdLIS1-D327H cells. Immunofluorescence labeling of whole cells (A–A″ and C–C″) or isolated centrosomes (B–B″ and D–D″) with the antidynein heavy chain antibody 695 (green; A–D) and the monoclonal anti-DdCP224 antibody 2/165 (red; A′,B′,C′,D′). Nuclei were stained with TOPRO3 (blue; A″ and C″). Stainings were merged in A″–D″. AX2 control cells were used in (A–B″) and DdLIS1-D327H cells in (C–D″). Cells were fixed with formaldehyde/acetone and centrosomes with methanol.
Despite of its effects on the microtubule array and centrosome/nucleus association, the D327H mutation caused only a moderate reduction in growth rate (our own unpublished data) but had no striking influence on mitosis. The representative mitotic cell in Figure 3D (arrow) shows normal mitotic progression in terms of total duration, centrosome duplication, and spindle formation. The failure of cytokinesis in this example can be attributed to the imaging conditions under agar overlay, where proper cytokinesis is a rare event. Because multinucleated cells are not more frequent in the DdLIS-D327H strain than in control cells, neither in adherent nor in shaking culture, a cytokinesis defect can be excluded.
Taken together, the cellular defects observed in DdLIS1-D327H cells suggest that DdLIS1 is required for Golgi integrity, nucleus/centrosome association, and dynein/dynactin-mediated interactions of microtubule tips with the cell cortex.
DdLIS1 Interacts with Dynein and DdCP224
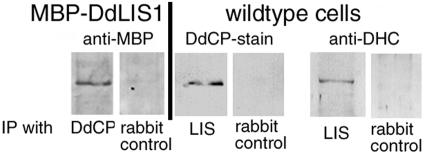
The similarity of the DdLIS1-D327H phenotype and the dynein and DdCP224 mutants mentioned above raised the question whether DdLIS1 could interact not only with dynein but also with DdCP224. Thus, we performed coimmunoprecipitations of these proteins from cytosolic Dictyostelium extracts using specific polyclonal antibodies and protein G–coated beads. Because the similar size of DdLIS1 and antibody heavy chains complicated the identification of DdLIS1 in Western blots of immunoprecipitates, we have overexpressed DdLIS1 as a fusion protein with the E. coli maltose-binding protein (MBP) in Dictyostelium (Gräf, 2001b). MBP-DdLIS1 was overexpressed ∼10-fold as judged from Western blots stained with anti-DdLIS1 antibodies. Using cytosolic extracts from these cells, the ∼90-kDa MBP-DdLIS1 fusion protein clearly coprecipitated with DdCP224 using anti-DdCP224 antibodies (Figure 8). Conversely, DdCP224 was coprecipitated with DdLIS1 when anti-DdLIS1 antibodies were used for coimmunoprecipitation (Figure 8). As expected for a LIS1-family protein, DdLIS1 also coprecipitated with the dynein heavy chain (Figure 8).
Figure 8.
Coprecipitation of DdLIS1 with DdCP224 with dynein. Experiments were performed using cytosolic extracts from MBP-DdLIS1 cells or wild-type cells (strain AX2). The respective antibodies used staining of the immunoblots and for immunoprecipitation are indicated above and below the blots, respectively. Abbreviations and antibodies: LIS, anti-DdLIS1; DdCP, anti-DdCP224 mAb 2/165 for immunoblot staining and polyclonal anti-DdCP224 for immunoprecipitation; DHC, antidynein heavy chain Y7; control, anti-rabbit preimmune serum.
We wondered whether disruption of DdLIS1 function affects subcellular localization of Dynein and DdCP224; however, we observed no displacement of the dynein heavy chain or DdCP224 in DdLIS1-D327H mutants, either at the cell cortex nor at isolated centrosomes or, in case of the dynein heavy chain, at the nucleus (Figure 7). Nevertheless, the coimmunoprecipitation of these proteins from cytosolic extracts, their colocalization and the similarity of phenotypes in corresponding mutants indicate that dynein, DdLIS1 and DdCP224 act together in the cortical attachment of microtubules.
DdLIS1-D327H cells and MBP-DdLIS1 Overexpressors Exhibit Altered Cell Shape
Overexpression of MBP-DdLIS1 frequently disrupted radial microtubule arrays in a similar manner as described for DdLIS1-D327H cells, suggesting a dominant-negative effect of MBP-DdLIS1 overexpression (Figure 9). However, the microtubule disruption phenotype upon MBP-DdLIS1 overexpression was less frequent (∼25% of cells in freshly transformed cultures) and tended to disappear in older cultures with reduced MBP-DdLIS1 expression.
Figure 9.
MBP-DdLIS1 overexpression disrupts radial microtubules arrays. Immunofluorescence labeling of MBP-DdLIS1 overexpressing cells with anti-α-tubulin antibodies (green; A) and anti-MBP antibodies (red; A′ and B′). Nuclei were stained with TOPRO3 (blue). In many cells microtubules show no point-symmetric radial arrangement. Like endogenous DdLIS1 (Figure 2), MBP-DdLIS1 colocalizes with microtubules as revealed by the merged image (C). Cells were fixed with glutaraldehyde. Bar, 5 μm.
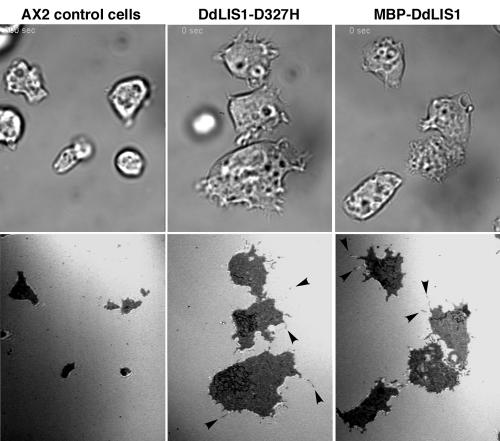
Because impaired LIS1 function strongly affects the motile behavior of neuronal precursors, we suspected that the DdLIS1-D327H point mutation or MBP-DdLIS1 overexpression may interfere with Dictyostelium cell motility. In neither case we could detect any defect in random cell motility or chemotaxis (our own unpublished data), but both MBP-DdLIS1 overexpressors and DdLIS1-D327H mutants were altered in cell shape and appeared much flatter than untransformed cells. This became obvious when both cell lines were viewed by reflection interference contrast microscopy (RICM) in comparison with control cells. This technique, which can be applied at conventional confocal microscopes, visualizes the contact surface of a cell to the substratum in a reflection image (Weber, 1999). In both MBP-DdLIS1 overexpressors and DdLIS1-D327H mutants the cell contact surface to the coverslip was greatly enlarged (Figure 10; see supplementary videos 4–6). Furthermore, these flat cells appeared to be more active in filopodia formation (arrowheads in Figure 10).
Figure 10.
DdLIS1-D327H cells and MBP-DdLIS1 overexpressors are flatter than usual and display an increase contact surface to the substrate. Living AX2 control cells (Supplementary Movie4), DdLIS-D327H cells (Supplementary Movie5), and MBP-DdLIS1 overexpressors (Supplementary Movie6) were viewed by RICM. RICM images are shown in the lower panel and corresponding bright-field images in the top panel. The dark area in the RICM channel corresponds to the cell's contact surface to the glass substrate. Only a single time point of each movie is shown here.
DdLIS1 Is Involved in the Regulation of the Cellular F-actin Content
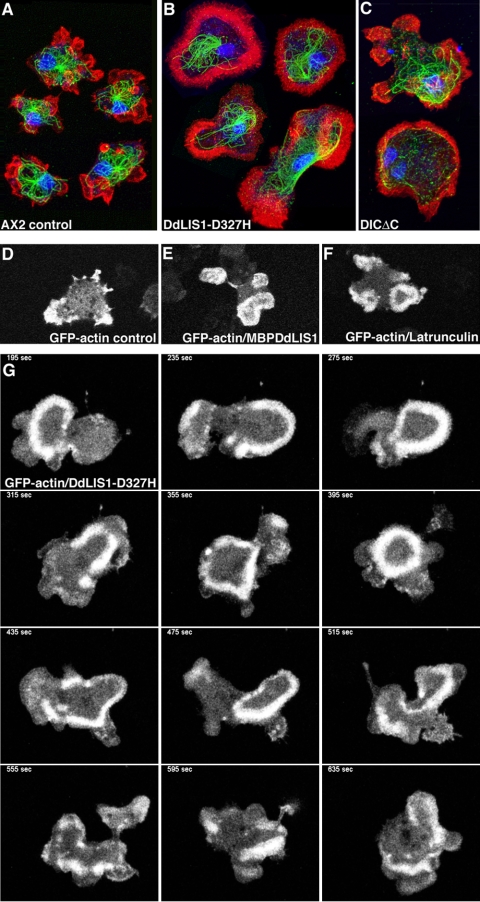
Alterations in cell shape usually involve the actin cytoskeleton; thus, we looked for changes in actin dynamics under these conditions. Indeed, phalloidin staining of DdLIS1-D327H cells revealed unusual distributions of F-actin, especially in very flat cells. Here F-actin was distributed in broad ring- or crescent-like arrangements close to the lower cell surface (Figure 11, A and B). To investigate these actin structures in living cells, we expressed GFP-actin (Westphal et al., 1997) in both DdLIS1-D327H mutants and MBP-DdLIS1 overexpressors and compared actin dynamics with GFP-actin control cells. When these control cells were migrating on a glass surface, actin was concentrated at the cell cortex, predominantly in protruding filopodia and lamellipodia (Figure 11D, see supplementary video 7; Bretschneider et al., 2004). By contrast, in DdLIS1-D327H cells and upon overexpression of MBP-DdLIS1, we frequently observed characteristic traveling waves of dense F-actin assemblies propagating along the substrate attached cell surface. This pattern was usually persistent throughout the entire observation period of more than 20 min (Figure 11, E–G; see supplementary videos 8–10). This dynamic actin organization seems to reflect changes in size and shape of cell/substrate contact sites. In contrast to DdLIS1-D327H mutants and MBP-DdLIS1 overexpressors, such actin waves were only rarely encountered in untreated GFP-actin cells. However, they were frequently observed in cells where the actin cytoskeleton was partially disrupted by treatment with a low concentration of latrunculin A (0.2 μM; Figure 11F). Latrunculin A causes F-actin depolymerization through sequestering of G-actin into nonpolymerizing complexes (Spector et al., 1989). At high latrunculin A concentrations of 2 μM, Dictyostelium cells round up completely and lose contact with the substratum (our own unpublished data). Because latrunculin A treatment reduces the cell's F-actin content, the similarity of altered actin dynamics upon treatment with low concentrations of latrunculin A, and our DdLIS1 mutants suggested that compromised DdLIS1 function may cause partial actin depolymerization. Thus, we compared the F- and G-actin content of these cells at the biochemical level. After cell lysis with Triton X-100 followed by ultracentrifugation, relative amounts of F-actin (pellet) and G-actin fractions (supernatant) were evaluated on Western blots stained with an antiactin monoclonal antibody (mAb). In untreated control cells, the amounts of F- and G-actin were approximately equal, whereas treatment with low concentrations of latrunculin A, as used to induce the actin waves, caused a prevalence of G-over F-actin (Figure 12). The same strong prevalence of G-actin was observed in DdLIS1-D327H cells and upon overexpression of MBP-DdLIS1. Taken together, these results indicate that impaired DdLIS1 function reduces the cellular F-actin content.
Figure 11.
DdLIS1-D327H cells, MBP-DdLIS1 overexpressors, and DICΔC overexpressors often show altered F-actin distribution and alterations in actin dynamics similar to control cells treated with low concentrations of latrunculin A. Immunofluorescence labeling of control cells (A), DdLIS1-D327H cells (B), and DICΔC overexpressors (C) with anti-α-tubulin (green) and phalloidin-AlexaFluor568 (Molecular Probes) as an F-actin labeling probe (red). Nuclei were stained with TOPRO3 (blue). Note the broad F-actin distribution at the bottom of the cells along with disrupted microtubule cytoskeletons in (B and C). Cells were fixed with glutaraldehyde. Bar, 5 μm. Note that phalloidin-labeling intensities in these images do not illustrate the F-actin content, because all images were acquired at microscope settings allowing coverage of the full dynamic range of the 8-bit scale. Live analysis of actin dynamics was performed with GFP-actin control cells (D, Supplementary Movie7), GFP-actin/MBP-DdLIS1 overexpressors (E, Supplementary Movie8), GFP-actin cells treated with 0.2 μM latrunculin A (F, Supplementary Movie9) and GFP-actin cells carrying DdLIS1-D327H mutation (G, Supplementary Movie10). Each image represents a brightest point z-projection of three confocal slices with a distance of 0.8 μm each. One a single, representative time frame of each movie is shown in D–F, whereas G represents a time sequence.
Figure 12.
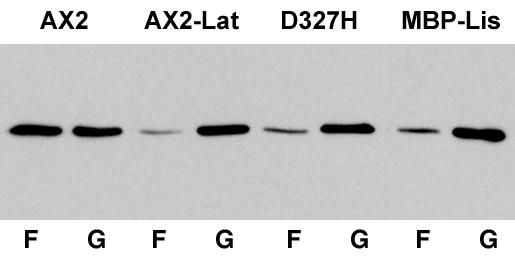
The F-actin content in DdLIS1-D327H cells and MBP-DdLIS1 overexpressors is reduced to a similar extent as upon treatment with low concentrations of latrunculin A. The Western blot containing equivalent amounts of F- and G-actin fractions (lanes F and G) from AX2 control cells, AX2 cells treated with 0.2 μM latrunculin A, DdLIS1-D327H cells and MBP-DdLIS1 overexpressors was stained with antiactin antibodies, and enhanced chemiluminescence.
Because LIS1 usually cooperates with dynein in its functions, we wanted to analyze whether the actin phenotype in DdLIS1-D327H cells could be dynein dependent. Therefore, we analyzed subcellular F-actin distribution in cells overexpressing an N-terminal fragment of the dynein IC (DICΔC), a treatment that is known to disrupt dynein function (Ma et al., 1999). As upon disruption of DdLIS1 function or latrunculin treatment, F-actin was spread in broad ring- or crescent-like arrangements close to the substratum (Figure 11C). Because of the instability of DICΔC overexpressors, we were unable to perform similar live cell studies as in case of the other cell lines or to obtain sufficient amounts of cells to evaluate the F-/G-actin ratio. Nevertheless, our data support a dynein-dependent role of DdLIS1 in actin dynamics.
DdLIS1 May Alter Actin Dynamics through Its Interaction with the Small GTPase Rac1A
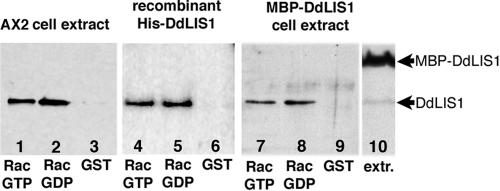
We wondered how DdLIS1 could participate in the regulation of actin dynamics. Because DdLIS1 showed no clear colocalization with actin, we suspected that DdLIS1 might interact with regulators of actin dynamics such as small GTPases. This assumption was also supported by the observation that both DdLIS1-D327H mutants and MBP-DdLIS1 overexpressors were more active in filopodia formation, a process regulated by Rac1 GTPases (Dumontier et al., 2000). Therefore, we investigated whether DdLIS1 was able to interact with Rac1A (Bush et al., 1993), the Dictyostelium Rac1 homologue (Dumontier et al., 2000). Because the available antibodies against Rac1A were not suitable for immunoprecipitations, we performed a GST-Rac1A pull-down experiment. GST-Rac1A preloaded with either nonhydrolyzable GTPγS or GDP was incubated with a cytosolic Dictyostelium extract (strain AX2). In both cases endogenous DdLIS1 clearly coprecipitated with GST-Rac1A (Figure 13, lanes 1–3). The interaction between DdLIS1 and Rac1A was specific as we detected no coprecipitation when the cytosolic extracts were incubated with GST alone. GST-Rac1A also specifically brought down purified, recombinant, 6xHis-tagged DdLIS1 (His-DdLIS1; Figure 13, lanes 4–6). This proves that DdLIS1 and Rac1A interact directly with each other. Again, the interaction was not dependent on the guanosine nucleotide, GTPγS or GDP that was bound to GST-Rac1A. When the GST-Rac1A pull-down assay was performed with cytosolic extracts from MBP-DdLIS1 overexpressing cells (Figure 13, lane 7–9), the MBP-DdLIS1 fusion protein surprisingly showed no coprecipitation with GST-Rac1A, although it was overexpressed ∼18-fold (as calculated from Western blot band intensities; Figure 13, lane 10). This suggests that the bulky N-terminal MBP-tag interfered with the ability of DdLIS1 to bind Rac1A resulting in a dominant-negative effect on DdLIS1 function.
Figure 13.
DdLIS1 but not MBP-DdLIS1 interacts with Rac1A. Western blots containing equivalent amounts of protein coprecipitated with GST-Rac1A-GTPγS, GST-Rac1A-GDP, or GST alone (control) from a wild-type cell extract (AX2, lanes 1–3), a purified His-tagged DdLIS1 protein solution (lanes 4–6), and an MBP-DdLIS1 cell extract (lanes 7–9) are shown. Blots were stained with anti-DdLIS1 antibodies and enhanced chemiluminescence. Lane 10 shows a sample of the same MPB-DdLIS1 cell extract as in lanes 7–9 and shows the extent of overexpression of MBP-DdLIS1 compared with the endogenous protein. Note that MBP-DdLIS1 bands are missing in lanes 7–9.
DISCUSSION
In vertebrate cells, the centrosomal localization of LIS1 is microtubule-dependent (Tanaka et al., 2004). It is thought to be mediated by binding of LIS1 to dynein, which accumulates at the centrosome through its microtubule minus end-directed motor activity. Thus, dynein itself is also considered a temporal guest at the centrosome. However, in Dictyostelium, the centrosomal presence of DdLIS1 and dynein was completely independent of microtubules, because both were integral components of isolated, microtubule-free centrosomes.
DdLIS1 was also strongly localized along microtubules, but there was no bias of its distribution toward microtubule plus ends as observed in mammalian cells, where LIS1 seems to cooperate with CLIP-170 and dynein/dynactin in the interaction of microtubule tips with capturing sites at the cell cortex (Coquelle et al., 2002). This suggests that DdLIS1 might not be necessary for microtubule capture itself, however, our data demonstrate that it is certainly required for maintenance of radial microtubule arrays and centrosome positioning near the centroid of the cell (Figure 3). This process is apparently based on microtubule pulling forces provided by dynein that is evenly distributed at the Dictyostelium cell cortex (Koonce and Khodjakov, 2002). The first experimental evidence for the necessity of cortical dynein/dynactin for centrosome positioning and maintenance of microtubule arrays came from Koonce and coworkers (Koonce and Samso, 1996; Koonce et al., 1999), who overexpressed the dynein motor domain in Dictyostelium. As a consequence, microtubules frequently lost contact with the cell cortex and trailed behind an extraordinarily motile centrosome that circulated around, often close to the cell cortex. This phenotype could also be elicited by overexpression of fragments of the dynein IC (Ma et al., 1999), an N-terminal fragment of the Dictyostelium XMAP215-homologue DdCP224 (DdCP224ΔC; Hestermann and Gräf, 2004) or, as described in this work, by interfering with DdLIS1 function through the D327H point mutation or overexpression of dominant-negative MBP-tagged DdLIS1. The simplest explanation for this phenotype is that all these manipulations disrupt dynein/dynactin-mediated linkage of membrane structures with microtubules. The observation of Golgi dispersal in all Dictyostelium cell lines mentioned above strongly supports this view, because dynein/dynactin-mediated coupling of Golgi vesicles and microtubules is crucial for Golgi positioning in the centrosomal vicinity (Burkhardt et al., 1997). Consequently, disrupted dynein/dynactin-mediated linkage of membrane structures and microtubules causes dissociation of Golgi vesicles from microtubules and, in a similar manner, loss of cortical tethering of microtubule ends. However, remaining, still functional cortical dynein/dynactin complexes could exert asymmetric pulling forces on single microtubules, resulting in rapid displacement of the centrosome with all the untethered microtubules dragged behind (reviewed in Gräf et al., 2004). In this work we have provided live cell data supporting the existence of such cortical pulling forces. Supplementary Movie3 reveals that microtubules are not just depolymerized once they lose their connection to the centrosome in early prophase, but that they move toward and along the cell cortex. It is conceivable that these microtubule movements are driven by cortical dynein.
The problems in dynein/dynactin-dependent linkage of membrane structures and microtubules could arise either from an inability of the dynein/dynactin complex to bind to microtubules, from dissociation of dynactin from membrane structures or, as it seems to occur in dynein IC mutants and DdCP224ΔC cells (Ma et al., 1999; Hestermann and Gräf, 2004), from dissociation of dynein from the dynactin complex. In case of our DdLIS1 mutants it appears more likely that the mutant phenotypes are based on defective binding of membrane associated dynein/dynactin to microtubules, because we found no indications for reduced presence of the dynein heavy chain at any of its localizations.
Because both DdCP224 (Hestermann and Gräf, 2004) and DdLIS1 interact with dynein and each other in the cytosol, it is likely that these proteins cooperate in Golgi positioning and the interaction of microtubule ends with the cell cortex. We cannot judge whether DdLIS1 and DdCP224 and dynein interact directly or indirectly, because so far it has been impossible to express functional, recombinant DdCP224 or dynein for in vitro binding assays. The interaction between a LIS1 and an XMAP215-family protein is shown here for the first time. However, because of the functional and structural conservation of these proteins, it is likely that it will also be found in higher cells where it could also be involved in microtubule/cortex interactions and centrosome positioning. The requirement of cortical dynein/dynactin for centrosome positioning in mammalian cells has been demonstrated in wound-healing experiments (Dujardin et al., 2003). In fibroblast monolayers, cells at the wound edge reorient their centrosomes toward the direction of migration during the healing process (Schliwa et al., 1999). Centrosome reorientation between the leading edge of the cell and the nucleus is blocked by inhibition of cytoplasmic dynein and dynactin and regulated through the small GTPase Cdc42 and protein kinase Cζ (Etienne-Manneville and Hall, 2001; Palazzo et al., 2001). This suggests that cortical dynein/dynactin is required for capturing of microtubules extending into cortical regions of a freshly formed pseudopod. An involvement of LIS1 in this process has not been demonstrated yet; however, it could explain at least part of the neuronal migration defect in lissencephaly.
Cortical anchorage of microtubule ends is a prerequisite for nucleokinesis, a major aspect of neuronal cell motility. The term nucleokinesis describes the “reeling in” of the nucleus into the freshly extended leading process of the migrating neuron. At the leading process, cortically anchored dynein is thought to exert a force on microtubules, which extend from the nucleus-associated centrosome to the leading edge. In this process, LIS1 appears to be not only required for cortical dynein function but also for the linkage of the centrosome to the nucleus. Recently, (Tanaka et al., 2004) could show in migrating cerebellar granule neurons that the distance between the centrosome and the nucleus was significantly larger in case of LIS1 haploinsufficiency. According to a model established in Caenorhabditis elegans, centrosome/nucleus attachment is mediated by centrosomally bound microtubules together with dynein immobilized at the nuclear surface and it is maintained through SUN and Hook-family proteins (Malone et al., 2003). It is conceivable that the role of LIS1 in this pathway is also mediated by dynein as in case of almost all other known LIS1 functions. This view is supported by our observation of a small fraction of the dynein heavy chain at the nucleus in immunofluorescence specimens in Dictyostelium. Thus, our data clearly support a dynein-dependent role of LIS1 in centrosome/nucleus association, even in cells with different centrosomal structure and closed mitosis such as Dictyostelium amoebae.
Until recently, the severe defects in neuronal cell migration observed upon compromised LIS1 function were mainly attributed to the role of LIS1 in microtubule-dependent dynamic processes through the regulation of dynein (Gupta et al., 2002). Yet, Kholmanskikh et al. (2003) have just shown that the actin cytoskeleton also contributes to the migration defect in LIS1-deficient neurons. Haploinsufficiency of LIS1 caused a reduced F-actin content at the leading edge of migrating neurons, which was accompanied by dysregulation of Rho-GTPases. Although RhoA was up-regulated under these conditions, the activities of the antagonistic GTPases Rac1 and Cdc42 were decreased. The motility defect of LIS1 deficient neurons was rescued when Rac1 and Cdc42 activity could be restored by inhibition of the RhoA effector p160ROCK. These data suggested that LIS1 promotes actin polymerization through the regulation of Rho-GTPase activities. However, no direct interaction of LIS1 with Rho-GTPases or any of their effectors or modulators could be shown. Because microtubule growth has been shown to stimulate Rac1 activity and thereby to promote actin polymerization at the leading edge (Waterman-Storer et al., 1999), LIS1 could also increase Rac1 activity indirectly through its role as a suppressor of microtubule catastrophes and promoter of increased MT length (Sapir et al., 1997).
In this article we have demonstrated for the first time a direct interaction of a LIS1-family protein with a Rac1-homologue (Dictyostelium Rac1A) by GST-pull down assays. This suggests that LIS1 could regulate actin dynamics directly through Rho-family GTPases. Because MBP-tagged DdLIS1 was essentially unable to bind Rac1A although it displayed the same subcellular localization as endogenous DdLIS1, we concluded that its overexpression exerts a dominant-negative effect, possibly through replacement of endogenous DdLIS1. This explains why MBP-DdLIS1 overexpression in Dictyostelium caused phenotypes, which were rather similar to those observed in DdLIS1-D327H cells and in LIS1+/– cerebellar granule cells with reduced LIS1 activity (Kholmanskikh et al., 2003). In all these cases, the F-actin content was reduced.
Furthermore, the microtubule-related phenotypes suggested a dominant-negative effect as well, because MBP-DdLIS1 overexpression caused Golgi dispersal and indicated reduced cortical pulling forces. By contrast, in mammalian cells LIS1 overexpression resulted in Golgi compaction and disordered microtubule arrays, which are indicative for increased cortical microtubule pulling forces (Faulkner et al., 2000; Smith et al., 2000). There are two likely explanations for the dominant-negative effect of MBP-DdLIS1 overexpression on dynein function. First, overexpressed MBP-DdLIS1 could sequester other dynein/dynactin-regulating binding partners, which would then be missing at the cell cortex or Golgi apparatus where they are required for proper dynein function. Second, the failure of MBP-DdLIS1 to associate with other binding partners such as Rac1A could be responsible for the disruption of dynein function.
How could DdLIS1 be involved in Rac1A-dependent actin dynamics? Rac1A regulates the cortical actin cytoskeleton through the actin-bundling proteins cortexillin I and II. Both are required for cortical stiffness and are directed to cortical sites with activated Rac1A through their Rac1A effector DGAP1 (Faix, 2002). It is unlikely that DdLIS1 acts as a Rac1A effector in this pathway, because it binds both Rac1A-GDP and Rac1A-GTPγS. Yet, it is more likely that DdLIS1 is required for Rac1A activation or for delivery of Rac1A to its site of action. This view is supported by the fact that MBP-tagged DdLIS1 localizes similar to endogenous DdLIS1 but does not bind to Rac1A. Replacement of endogenous DdLIS1 by overexpressed, nonfunctional MBP-DdLIS1 would lead to reduced amounts of active Rac1A at the cortex.
Traveling waves of actin polymerization appear to recruit Arp2/3-rich actin foci that are distributed all over the substrate attached surface of the cell (Bretschneider et al., 2004). Such foci are also visible in Supplementary Movie8 (for example, see time points 308–350 s). Although traveling actin waves have been described in untransformed Dictyostelium cells (Vicker, 2002; Bretschneider et al., 2004), we have only rarely encountered them in our AX2 wild-type control cells. Wave appearance seemed to depend on the cell shape, because traveling actin waves could only be detected in flattened cells. Yet, such cells were rare in AX2 cultures but were rather frequently observed after latrunculin treatment and upon disruption of DdLIS1 function because of the D327H mutation or MBP-DdLIS1 overexpression. This suggests that both the appearance of flattened cells and traveling actin waves were based on a reduced cortical F-actin content. Dynein has not yet been recognized as a player in actin dynamics and, thus, it was an interesting question whether disturbed dynein function could result in similar alterations of actin dynamics as observed in our DdLIS1 mutants. Although our dynein IC overexpression cell line was very unstable, we could reproducibly observe similarly altered F-actin distribution is these cells as detected upon disruption of DdLIS1 function. This strongly suggests that the role of DdLIS1 in actin dynamics also involves dynein just like all other LIS1 functions known so far.
Bearing in mind the effects of partial loss of DdLIS1 function on both the tubulin and the actin cytoskeleton it is surprising that our mutants showed no defects in cell migration as they were observed in migrating neurons. However, our own unpublished results with nocodazole clearly showed that Dictyostelium cells do not require microtubules for directed cell migration. Even the partial disassembly of the actin cytoskeleton by treatment with low dosages of latrunculin A, did not prevent chemotactic cell migration of Dictyostelium amoebae. Thus, Dictyostelium cells are more reminiscent of nonneuronal cells, which are also not strongly affected by partial loss of LIS1 function. However, like LIS1 in mammals, DdLIS1 is certainly an essential protein because it was not possible to knock out the DdLIS1 gene completely or to replace it by a stronger hypomorphic allele such as H149R.
Taken together, in this work we have shown that DdLIS1 is not only a microtubule-associated protein but also a genuine centrosomal component. The D327H point mutation of DdLIS1 as well as overexpression of MBP-tagged DdLIS1 disrupted DdLIS1 function and resulted in a palette of mutant phenotypes including Golgi dispersal, decreased centrosome/nucleus association, disturbed microtubule tip/cell cortex interactions, and altered actin dynamics. All these phenotypes appeared to be dynein-dependent. Furthermore, we have demonstrated for the first time an association between LIS1 and XMAP215-family proteins in a cytosolic complex and direct binding of a LIS1-family protein to an actin regulating small GTPase, suggesting a contribution of these proteins in LIS1 function and, thus, the pathogenesis of lissencephaly.
Supplementary Material
Acknowledgments
We thank Mike Koonce, Rex Chisholm, and Michael Schleicher for providing antibodies against the dynein heavy chain, dynein IC and comitin, respectively. We also would like to thank Manfred Schliwa for his continuous support and Alexandra Lepier for critical comments. This work was supported by the Deutsche Forschungsgemeinschaft (SFB413 and GR1642/2-1).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0069) on March 30, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bretschneider, T., Diez, S., Anderson, K., Heuser, J., Clarke, M., Müller-Taubenberger, A., Köhler, J., and Gerisch, G. (2004). Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14, 1–10. [DOI] [PubMed] [Google Scholar]
- Burkhardt, J. K., Echeverri, C. J., Nilsson, T., and Vallee, R. B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, J., Franek, K., and Cardelli, J. (1993). Cloning and characterization of seven novel Dictyostelium discoideum rac-related genes belonging to the rho family of GTPases. Gene 136, 61–68. [DOI] [PubMed] [Google Scholar]
- Caspi, M., Atlas, R., Kantor, A., Sapir, T., and Reiner, O. (2000). Interaction between LIS1 and doublecortin, two lissencephaly gene products. Hum. Mol. Genet. 9, 2205–2213. [DOI] [PubMed] [Google Scholar]
- Caspi, M., Coquelle, F. M., Koifman, C., Levy, T., Arai, H., Aoki, J., De Mey, J. R., and Reiner, O. (2003). LIS1 missense mutations: variable phenotypes result from unpredictable alterations in biochemical and cellular properties. J. Biol. Chem. 278, 38740–38748. [DOI] [PubMed] [Google Scholar]
- Coquelle, F. M. et al. (2002). LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol. Cell. Biol. 22, 3089–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunderer, C., and Gräf, R. (2002). Molecular analysis of the cytosolic Dictyostelium gamma-tubulin complex. Eur. J. Cell Biol. 81, 175–184. [DOI] [PubMed] [Google Scholar]
- Dujardin, D. L., Barnhart, L. E., Stehman, S. A., Gomes, E. R., Gundersen, G. G., and Vallee, R. B. (2003). A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 163, 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin, D. L., and Vallee, R. B. (2002). Dynein at the cortex. Curr. Opin. Cell Biol. 14, 44–49. [DOI] [PubMed] [Google Scholar]
- Dumontier, M., Hocht, P., Mintert, U., and Faix, J. (2000). Rac1 GTPases control filopodia formation, cell motility, endocytosis, cytokinesis and development in Dictyostelium. J. Cell Sci. 113(Pt 12), 2253–2265. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2001). Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106, 489–498. [DOI] [PubMed] [Google Scholar]
- Euteneuer, U., Gräf, R., Kube-Granderath, E., and Schliwa, M. (1998). Dictyostelium gamma-tubulin: molecular characterization and ultrastructural localization. J. Cell Sci. 111, 405–412. [DOI] [PubMed] [Google Scholar]
- Faix, J. (2002). The actin-bundling protein cortexillin is the downstream target of a Rac1-signaling pathway required for cytokinesis. J. Muscle Res. Cell Motil. 23, 765–772. [DOI] [PubMed] [Google Scholar]
- Faix, J., Clougherty, C., Konzok, A., Mintert, U., Murphy, J., Albrecht, R., Mühlbauer, B., and Kuhlmann, J. (1998). The IQGAP-related protein DGAP1 interacts with Rac and is involved in the modulation of the F-actin cytoskeleton and control of cell motility. J. Cell Sci. 111, 3059–3071. [DOI] [PubMed] [Google Scholar]
- Faix, J., Kreppel, L., Shaulsky, G., Schleicher, M., and Kimmel, A. R. (2004). A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32, e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner, N. E., Dujardin, D. L., Tai, C. Y., Vaughan, K. T., O'Connell, C. B., Wang, Y., and Vallee, R. B. (2000). A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2, 784–791. [DOI] [PubMed] [Google Scholar]
- Fukui, Y., Yumura, S., and Yumura, T. K. (1987). Agar-overlay immunofluorescence: high resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 28, 347–356. [DOI] [PubMed] [Google Scholar]
- Gräf, R. (2001a). Isolation of centrosomes from Dictyostelium. Methods Cell Biol. 67, 337–357. [DOI] [PubMed] [Google Scholar]
- Gräf, R. (2001b). Maltose-binding protein as a fusion tag for the localization and purification of cloned proteins in Dictyostelium. Anal. Biochem. 289, 297–300. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Brusis, N., Daunderer, C., Euteneuer, U., Hestermann, A., Schliwa, M., and Ueda, M. (2000a). Comparative structural, molecular and functional aspects of the Dictyostelium discoideum centrosome. Curr. Top. Dev. Biol. 49, 161–185. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Daunderer, C., and Schliwa, M. (1999). Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol. Cell 91, 471–477. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Daunderer, C., and Schliwa, M. (2000b). Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J. Cell Sci. 113, 1747–1758. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Daunderer, C., and Schulz, I. (2004). Molecular and functional analysis of the Dictyostelium centrosome. Int. Rev. Cytol. 241, 155–202. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Euteneuer, U., Ho, T. H., and Rehberg, M. (2003). Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 14, 4067–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf, R., Euteneuer, U., Ueda, M., and Schliwa, M. (1998). Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur. J. Cell Biol. 76, 167–175. [DOI] [PubMed] [Google Scholar]
- Gupta, A., Tsai, L. H., and Wynshaw-Boris, A. (2002). Life is a journey: a genetic look at neocortical development. Nat. Rev. Genet. 3, 342–355. [DOI] [PubMed] [Google Scholar]
- Hestermann, A., and Gräf, R. (2004). The XMAP215-family protein DdCP224 is required for cortical interactions of microtubules. BMC Cell Biol. 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholmanskikh, S. S., Dobrin, J. S., Wynshaw-Boris, A., Letourneau, P. C., and Ross, M. E. (2003). Disregulated RhoGTPases and actin cytoskeleton contribute to the migration defect in Lis1-deficient neurons. J. Neurosci. 23, 8673–8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanishi, T., Shibaoka, H., and Fukui, Y. (1984). Disruption of microtubules and retardation of development of Dictyostelium with ethyl N-phenylcarbamate and thiabendazole. Protoplasma 120, 185–196. [Google Scholar]
- Koonce, M. P., and Khodjakov, A. (2002). Dynamic microtubules in Dictyostelium. J. Muscle Res. Cell Motil. 23, 613–619. [DOI] [PubMed] [Google Scholar]
- Koonce, M. P., Kohler, J., Neujahr, R., Schwartz, J. M., Tikhonenko, I., and Gerisch, G. (1999). Dynein motor regulation stabilizes interphase microtubule arrays and determines centrosome position. EMBO J. 18, 6786–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce, M. P., and Samso, M. (1996). Overexpression of cytoplasmic dynein's globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol. Biol. Cell 7, 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S., Triviños Lagos, L., Gräf, R., and Chisholm, R. L. (1999). Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, C. J., Misner, L., Le Bot, N., Tsai, M. C., Campbell, J. M., Ahringer, J., and White, J. G. (2003). The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell 115, 825–836. [DOI] [PubMed] [Google Scholar]
- Morio, T. et al. (1998). The Dictyostelium developmental cDNA project: generation and analysis of expressed sequence tags from the first-finger stage of development. DNA Res. 5, 335–340. [DOI] [PubMed] [Google Scholar]
- Morris, N. R., Efimov, V. P., and Xiang, X. (1998). Nuclear migration, nucleokinesis and lissencephaly. Trends Cell Biol. 8, 467–470. [DOI] [PubMed] [Google Scholar]
- Ohkura, H., Garcia, M. A., and Toda, T. (2001). Dis1/TOG universal microtubule adaptors—one MAP for all? J. Cell Sci. 114, 3805–3812. [DOI] [PubMed] [Google Scholar]
- Palazzo, A. F., Joseph, H. L., Chen, Y. J., Dujardin, D. L., Alberts, A. S., Pfister, K. K., Vallee, R. B., and Gundersen, G. G. (2001). Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Pilz, D. T., Kuc, J., Matsumoto, N., Bodurtha, J., Bernadi, B., Tassinari, C. A., Dobyns, W. B., and Ledbetter, D. H. (1999). Subcortical band heterotopia in rare affected males can be caused by missense mutations in DCX (XLIS) or LIS1. Hum. Mol. Genet. 8, 1757–1760. [DOI] [PubMed] [Google Scholar]
- Reiner, O., Carrozzo, R., Shen, Y., Wehnert, M., Faustinella, F., Dobyns, W. B., Caskey, C. T., and Ledbetter, D. H. (1993). Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature 364, 717–721. [DOI] [PubMed] [Google Scholar]
- Sapir, T., Eisenstein, M., Burgess, H. A., Horesh, D., Cahana, A., Aoki, J., Hattori, M., Arai, H., Inoue, K., and Reiner, O. (1999). Analysis of lissencephaly-causing LIS1 mutations. Eur. J. Biochem. 266, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Sapir, T., Elbaum, M., and Reiner, O. (1997). Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO J. 16, 6977–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, S., Shionoya, A., Ishida, M., Gambello, M. J., Yingling, J., Wynshaw-Boris, A., and Hirotsune, S. (2000). A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 28, 681–696. [DOI] [PubMed] [Google Scholar]
- Schaar, B. T., Kinoshita, K., and McConnell, S. K. (2004). Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron 41, 203–213. [DOI] [PubMed] [Google Scholar]
- Schliwa, M., Euteneuer, U., Gräf, R., and Ueda, M. (1999). Centrosomes, microtubules and cell migration. Biochem. Soc. Symp. 65, 223–231. [PubMed] [Google Scholar]
- Smith, D. S., Niethammer, M., Ayala, R., Zhou, Y., Gambello, M. J., Wynshaw-Boris, A., and Tsai, L. H. (2000). Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2, 767–775. [DOI] [PubMed] [Google Scholar]
- Spector, I., Shochet, N. R., Blasberger, D., and Kashman, Y. (1989). Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 13, 127–144. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., Serneo, F. F., Higgins, C., Gambello, M. J., Wynshaw-Boris, A., and Gleeson, J. G. (2004). Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 165, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-Oka, K. et al. (2003). 14–3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat. Genet. 34, 274–285. [DOI] [PubMed] [Google Scholar]
- Ueda, M., Schliwa, M., and Euteneuer, U. (1999). Unusual centrosome cycle in Dictyostelium: correlation of dynamic behavior and structural changes. Mol. Biol. Cell 10, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicker, M. G. (2002). F-actin assembly in Dictyostelium cell locomotion and shape oscillations propagates as a self-organized reaction-diffusion wave. FEBS Lett. 510, 5–9. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer, C. M., Worthylake, R. A., Liu, B. P., Burridge, K., and Salmon, E. D. (1999). Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1, 45–50. [DOI] [PubMed] [Google Scholar]
- Weber, I. (1999). Computer-assisted morphometry of cell-substratum contacts. Croat. Med. J. 40, 334–339. [PubMed] [Google Scholar]
- Weiner, O. H., Murphy, J., Griffiths, G., Schleicher, M., and Noegel, A. A. (1993). The actin-binding protein comitin (p24) is a component of the golgi apparatus. J. Cell Biol. 123, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal, M., Jungbluth, A., Heidecker, M., Mühlbauer, B., Heizer, C., Schwartz, J. M., Marriott, G., and Gerisch, G. (1997). Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion protein. Curr. Biol. 7, 176–183. [DOI] [PubMed] [Google Scholar]
- Xiang, X., Osmani, A. H., Osmani, S. A., Xin, M., and Morris, N. R. (1995). NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol. Biol. Cell 6, 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.