Abstract
The function of the prion protein (PrPc), implicated in transmissible spongiform encephalopathies (TSEs), is largely unknown. We examined the possible influence of PrPc on Ca2+ homeostasis, by analyzing local Ca2+ fluctuations in cells transfected with PrPc and Ca2+-sensitive aequorin chimeras targeted to defined subcellular compartments. In agonist-stimulated cells, the presence of PrPc sharply increases the Ca2+ concentration of subplasma membrane Ca2+ domains, a feature that may explain the impairment of Ca2+-dependent neuronal excitability observed in TSEs. PrPc also limits Ca2+ release from the endoplasmic reticulum and Ca2+ uptake by mitochondria, thus rendering unlikely the triggering of cell death pathways. Instead, cells expressing Doppel, a PrPc paralogue, display opposite effects, which, however, are abolished by the coexpression of PrPc. These findings are consistent with the functional interplay and antagonistic role attributed to the proteins, whereby PrPc protects, and Doppel sensitizes, cells toward stress conditions.
INTRODUCTION
The cellular prion protein (PrPc) is a highly conserved cell surface glycoprotein, particularly expressed in the CNS with a still unrecognized function. A conformationally modified isoform (PrPSc) of PrPc is the major component of prions, the etiological agent at the basis of fatal neurodegenerative disorders, called transmissible spongiform encephalopathies (TSEs). TSEs present as sporadic, genetic, and infectious illnesses, and include Creutzfeldt-Jakob disease (CJD) in humans, and bovine spongiform encephalopathy in cattle (Prusiner, 1998).
The mechanism of PrPc conversion into PrPSc is not yet elucidated, nor it is clear if the disease progression relates to PrPSc toxic effect, or to the deprivation of PrPc functionality. The most prominent evidence in support of the former hypothesis is that most PrP-knockout mice remain viable, and do not develop spontaneous neurodegeneration (Bueler et al., 1992; Manson et al., 1994), even if the PrPc gene is postnatal deleted (Mallucci et al., 2002). An essential role of PrPc in cell survival comes, however, from the finding that wild-type PrP transgenes abrogate the cerebellar degeneration and late-onset ataxia developed by some PrP-knockout lines over expressing a protein, named Doppel (Dpl; Sakaguchi et al., 1996; Li et al., 2000; Moore et al., 1999, 2001; Rossi et al., 2001). Dpl is normally absent in the CNS of adult animals and resembles truncated PrPc; it lacks the copper-binding N-terminus (Brown et al., 1997) and is structurally and biochemically similar to PrPc carboxyl end (Silverman et al., 2000; Mo et al., 2001). Also the role of Dpl in the CNS is obscure, but the capacity of PrPc to restore the normal phenotype of Dpl-over expressing mice has suggested that PrPc and Dpl have antagonistic functions and that PrPc suppresses a death signal triggered by Dpl.
Comparative studies using cells from wild-type or PrP-knockout animals or infected by prions also argue in favor of “the-loss-of-function hypothesis,” suggesting that PrPc protects cells by controlling the copper metabolism governing the cell resistance to oxidative stress (Brown et al., 2002) or by playing an antiapoptotic role (reviewed in Hetz et al., 2003a). Importantly, other data from electrophysiologic studies, and/or cell Ca2+ measurements, converge in supporting that PrPc absence, or its recruitment into prions, leads to a compromised Ca2+ homeostasis and that such a defect ultimately impinges on a few Ca2+-dependent neurophysiologic functions, such as plasma membrane K+ currents, after-hyperpolarization potential (AHP) and depolarizing after-potential (DAP) events (Jefferys et al., 1994; Colling et al., 1996; Johnston et al., 1998; Barrow et al., 1999; Herms et al., 2000, 2001; Mallucci et al., 2002).
Yet, if PrPc is involved in the correct handling of cell Ca2+, the above-reported findings are hardly surprising, given that Ca2+ is an ubiquitous intracellular messenger regulating a large amount of cell functions and that subtle alterations of endoplasmic reticulum (ER) and mitochondrial Ca2+ pools control initiation of cell death pathways. For example, both overload and depletion of the ER Ca2+ concentration ([Ca2+]) can impinge on correctly folded protein levels, thereby inducing (ER–) stress responses that may determine apoptosis (Orrenius et al., 2003). Accordingly, perturbed ER Ca2+ regulation contributes to neuronal degeneration (reviewed in Mattson et al., 2000; Paschen, 2001), including Alzheimer's disease and TSEs (Nakagawa et al., 2000; Hetz et al., 2003b). Also mitochondrial Ca2+ fluxes are integrated modulators of the cell Ca2+ signaling and intimately govern the cell fate. Indeed, mitochondria respond to high [Ca2+] microdomains that form in proximity of plasma membrane, and/or sarco/endoplasmic reticulum, Ca2+ channels upon cell stimulation (Brini, 2003). By activating the production of ATP, such a relayed signal satisfies immediate physiological energy demands (Jouaville et al., 1999). However, it can also turn into a death signal, given that excessive Ca2+ accumulation triggers abnormal mitochondrial membrane permeability (Bernardi, 1999) and release of proapoptotic factors to the cytoplasm (Li et al., 1997).
In view of the many implications related to the fine tuning of local Ca2+ changes, we have investigated on the involvement of PrPc in Ca2+ homeostasis, using CHO cells transiently transfected with PrPc and the recombinant Ca2+-sensitive photoprotein aequorin (AEQ) targeted to specific cell compartments. Furthermore, within the proposed functional antagonism of PrPc and Dpl, we also monitored the Ca2+ metabolism in AEQ-transfected cells over expressing Dpl, either alone or together with PrPc.
MATERIALS AND METHODS
Expression Plasmids
PrPc and Dpl isoforms used in all experiments were the murine (m) and human (h) ones, respectively. The expression vector for eukaryotic cells containing the coding sequence for hDpl was constructed as in Massimino et al. (2004), whereas that coding for mPrPc was constructed using a mouse genomic DNA as template, and by Prnp amplification by PCR with primers 5′ GCGCTAGCATGGCGAACCTTGGCTACTGGCTGC 3′; and 5′ CGGAATTCATCCCACGATCAGGAAGATGAG 3′. Amplified products were cut at NheI and EcoRI sites and cloned directly in the phEGFP plasmid (Clontech, Palo Alto, CA) between the same restriction sites to obtain plasmid pcDNAm-PrP. To express mPrPc carrying the epitope specific for monoclonal antibody (mAb) 3F4, L108M, and V111M point mutations were introduced in pcDNAmPrP by inverse PCR, using primers 5′ CATATGGCAGGGGCTGCGGCAGCTGGGC 3′ and 5′ CTTCATGAAGGTTTTTGGTTTGCTGGGCT 3′. The amplified product was itself ligated after T4 polynucleotide kinase treatment, to yield plasmid pmPrP3F4. The expression plasmid for the fusion protein between the green fluorescent protein (GFP) and the glycosylphosphatidylinositol (GPI) attachment signal of bovine PrPc (GFP-GPIPrP) was as described in Cereghetti et al. (2004), whereas plasmids for the photoprotein AEQ targeted to the various cell compartments were obtained according to Brini et al. (1995) for cytAEQ; Montero et al. (1995, 2000) for erAEQ; Marsault et al. (1997) for pmAEQ; and Rizzuto et al. (1992) for mtAEQ. Recombinant (rec) mPrP (23–230; containing the mAb 3F4 epitope) was generated in, and purified from Escherichia coli, following the method described in Negro et al. (2000) for the bovine PrP isoform, whereas hDplrec (28–152) was obtained as in Cereghetti et al. (2004).
Cell Cultures and Transfection
Experiments were carried out with CHO cells, cultured in 75-cm2 flasks (37°C, 5% CO2 atmosphere), using Ham's F12 medium (EuroClone, Devon, United Kingdom) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), and streptomycin (100 μg/ml). The cells, seeded onto glass coverslips at 4.5 × 104 and 3.5 × 105 cells/cm2 for fura-2 and aequorin measurements, respectively, were transiently transfected preferentially with the Lipofectamine Plus reagent (Invitrogen, Milano, Italy), following manufacturer's instructions (in double and triple transfection experiments the quantity of each added plasmid was proportionally reduced). To optimize PrPc and Dpl expression, 6 h after transfection the medium was changed, and cells were kept at 30°C for 48 h before fura-2-, or aequorin-based, Ca2+ measurements, or immunolabeling assays. Importantly, identical results were obtained when cells were transfected using the Ca2+ phosphate-based procedure (Rizzuto et al., 1995) and a total amount of 3 μg of plasmid DNA (1.5:1.5 μg in the case of double transfection). To generate CHO cells stably expressing mPrPc, the method was as described in Massimino et al. (2004).
Immunocytochemistry, Western Blotting, and Densitometric Analysis
Immunocytochemistry and image analysis of intact cells, immunoblotting, and densitometric analyses were carried out as in Negro et al. (2001) and Massimino et al. (2004), except that 20 μg of cell lysates were loaded in each gel lane.
Antibodies
For immunocytochemistry and immunoblotting of PrPc and Dpl, the following antibodies were used (dilutions are given in parenthesis): anti-PrP mAb 8H4, raised against the human PrP 173–185 sequence (a kind gift of Dr. M.S. Sy, Case Western University, Cleveland, OH; 1/300 for immunolocalization; 1/8000 for immunoblotting), or mAb 3F4 (DAKO, Glostrup, Denmark; 1/100 for immunolocalization; 1/1000 for immunoblotting), which gave the same results as with mAb 8H4; and anti-Dpl mAb Dpl-79 (kindly provided by Dr. J. Grassi, Commissariat a l'Energie Atomique, Saclay, France) raised against hDplrec (28–152; 1/100 for immunolocalization; 1/1000 for immunoblotting). The immunoblotting of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and β-actin was carried out using commercially available mAbs against the ubiquitous isoform (SERCA2; clone IID8, Affinity Bioreagents, Golden, CO; 1/1000), and β-actin (Sigma, St. Louis, MO; 1/4000), respectively, and anti-calreticulin polyclonal antibody (Affinity Bioreagents; 1/1000) was used for calreticulin immunolabelings.
Ca2+ Measurements: Aequorin
Although the cytAEQ plasmid coded for the wild-type AEQ, to reduce the affinity for Ca2+, erAEQ, mtAEQ, and pmAEQ plasmids expressed aequorins carrying a mutation in one Ca2+ binding site (Montero et al., 1995). Functional erAEQ was reconstituted with a modified prosthetic group (coelenterazine n, Molecular Probes, Eugene, OR), after depleting ER Ca2+ (Montero et al., 1995; Barrero et al., 1997). To this end, cells were incubated (1 h, 4°C) in KRB (Krebs-Ringer modified buffer: 125 mM NaCl, 5 mM KCl, 1 mM Na3PO4, 1 mM MgSO4, 5.5 mM glucose, 20 mM HEPES, pH 7.4) supplemented with ionomycin (5 μM), EGTA (600 μM), and coelenterazine n (5 μM). After extensive washings with KRB containing 2% bovine serum albumin and 1 mM EGTA, the coverslip with the cells was transferred to the thermostatted (37°C) chamber of a purpose-built luminometer. Conversely, transfected cytAEQ, mtAEQ, and pmAEQ were reconstituted by incubating cells (1–3 h, 37°C, 5% CO2 atmosphere) with wild-type coelenterazine (5 μM, Molecular Probes) in DMEM containing 1% FCS. Measurements started by perfusing cells with KRB supplemented with 1 mM CaCl2. Alternatively, pmAEQ was reconstituted in cells incubated with coelenterazine in KRB containing EGTA (100 μM). In this case, experiments started with cells in the EGTA-supplemented KRB, which was then replaced by CaCl2 (1 mM)-containing KRB. Other additions were as specified in the figures. Experiments ended by lysing cells with digitonin (100 μM) in a hypotonic Ca2+-rich solution (10 mM CaCl2 in H2O), to discharge the remaining aequorin pool. The light signal was collected and stored in an IBM-compatible computer for further analyses. Aequorin luminescence data were calibrated off-line into [Ca2+] values, using a computer algorithm based on the Ca2+ response curve of wild-type and mutant aequorins, as previously described (Brini et al., 1995).
Fura-2
Cells, cotransfected with the phEGFP plasmid (Clontech) and either PrPc or Dpl, were loaded with fura-2 AM (5 μM, Molecular Probes) as in Malgaroli et al. (1987), and the coverslip was then placed on the stage of a Zeiss Axiovert 100 epifluorescence microscope (Göttingen, Germany), equipped with a 16-bit digital CCD videocamera (Micromax, Princeton Instruments, Trenton, NJ). Samples were alternately illuminated at 340 and 380 nm, and the emitted light (filtered with an interference filter centered at 510 nm) was collected by the camera. Images were acquired using the Metafluor software (Universal Imaging, West Chester, PA). The ratio values (1 ratio image/s) were calculated off-line, after background subtraction from each single image.
Statistical Analysis
Data are reported as means ± SD; n indicates the number of experiments. Statistical differences were evaluated by Student's two-tailed t test for impaired samples, with a p value lower than 0.05 being considered statistically significant.
RESULTS
Localization and Expression of Transiently Transfected PrPc or Dpl
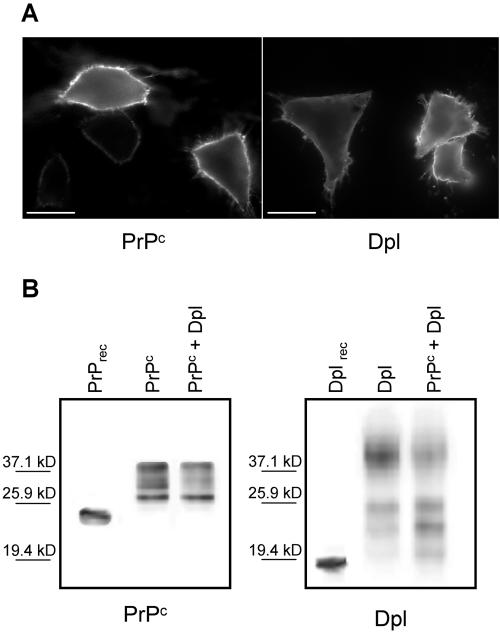
Before analyzing whether PrPc, or Dpl, affects Ca2+ homeostasis, we ascertained their correct cell distribution by immunostaining CHO cells transiently transfected with either PrPc or Dpl. As evident from the uniform labeling of the plasma membrane (Figure 1A), obtained by adding (4°C) anti-PrP-, or anti-Dpl-, mAbs to intact cells, PrPc and Dpl are exposed to the exoplasmic space, in accord with the presence of a GPI anchor in both proteins, and with similar data reported previously (Massimino et al., 2004). Using dual-labeling immunocytochemistry, an identical localization of the proteins to the cell surface was observed in cells expressing PrPc, or Dpl, together with the AEQ probes, and in those cotransfected with both PrPc and Dpl (see also Massimino et al., 2004; unpublished data). Figure 1B (second lanes), reporting the immunoblots of PrPc and Dpl in cells housing each protein alone, shows that the two cell types express similar protein amounts. Importantly, by applying immunocytochemistry and Western blot techniques to untransfected CHO cells, we found that no endogenous PrPc, or Dpl, was detectable over the entire experimental time period.
Figure 1.
Localization (A) and expression level (B) of PrPc and Dpl in transiently transfected CHO cells. (A) Distribution of PrPc and Dpl in intact cells expressing each protein alone, after treatment (4°C) with anti-PrP mAb 8H4 (left), or anti-Dpl mAb Dpl-79 (right), followed by fluorescein isothiocyanate-conjugated secondary antibody. Both proteins are present on the cell surface, correctly exposed to the exoplasmic space. Bar, 20 μm. (B) Western blots of PrPc (left) and Dpl (right) of cells expressing each protein alone (second lanes), or together (third lanes), show that both proteins exhibit high mass diffuse bands typical of their complex N-linked glycosylation (Massimino et al., 2004). Cell lysate SDS-PAGE separated proteins were immunoblotted with the above-described mAbs, and immunoreactive bands were then subjected to densitometric analysis. Comparison between the density of PrPc- and Dpl-containing bands and the band density elicited by a known quantity of PrPrec or Dplrec (0.5 ng, first lane of the left and right panel, respectively) yielded a value of 3.8 and 4.0 ng (in singly transfected cells, second lanes of both panels), and 2.1 and 2.3 ng (in doubly transfected cell, third lanes of both panels) for PrPc and Dpl amounts, respectively.
ER Ca2+ in PrPc- or Dpl-expressing Cells
The ER is the main Ca2+ storage of the cell as it houses Ca2+-binding proteins and SERCA pumps that transport Ca2+ against a high concentration gradient. To measure the ER luminal [Ca2+] ([Ca2+]er), we used erAEQ that targets exclusively to this compartment (Montero et al., 1995). To function as a Ca2+ probe, recombinant AEQ needs to be reconstituted in the active complex with its prosthetic group, coelenterazine; added to cells, coelenterazine permeates the cell membranes and combines efficiently with AEQ. However, to avoid the instantaneous consumption of the holophotoprotein by the high luminal [Ca2+]er, erAEQ-containing cells had to be first reduced in the ER Ca2+ content (by adding the Ca2+ ionophore ionomycin in the absence of external Ca2+) before reconstitution (in a Ca2+-free medium) with coelenterazine n, which is modified so as to reduce the Ca2+ affinity of AEQ (Barrero et al., 1997).
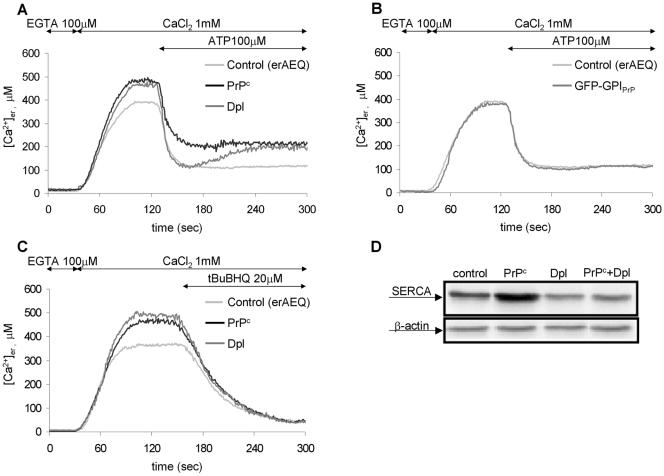
Figure 2A reports ER Ca2+ measurements in parallel batches of cells transiently expressing erAEQ alone (control cells, light gray trace) or together with PrPc (black trace) or Dpl (dark gray trace). In agreement with previous reports (Brini et al., 2000), ∼1 min after adding CaCl2 (1 mM), the [Ca2+]er of control cells reached a steady state level close to 400 μM (390 ± 20 μM, n = 15). This value, however, is ∼15% lower than that observed in the presence of PrPc, or Dpl, which was 455 ± 20 μM (n = 15) and 460 ± 20 μM (n = 15), respectively. Importantly, unless an ER Ca2+-discharging stimulus was applied, in all cases the shown Ca2+ plateau levels were stably maintained (unpublished data) and thus well represent the uppermost free Ca2+ amount that the three cell types can accumulate in the ER.
Figure 2.
Effects of the presence of PrPc or Dpl (A), and of GFP-GPIPrP (B), on the Ca2+ filling of the ER lumen and on the agonist-stimulated ER Ca2+ discharge, and effects of the presence of PrPc, or/and Dpl, on the ER membrane Ca2+ passive efflux (C), and SERCA expression (D). (A–C) CHO cells, transiently expressing erAEQ alone (control, light gray trace), or together with PrPc (black trace) or Dpl (dark gray trace; A and C), or GFP-GPIPrP (dark gray trace; B), were first depleted of Ca2+ (see Materials and Methods and text) and then incubated with 5 μM coelenterazine n (in EGTA-supplemented KRB), and finally, after extensive washings, transferred to the thermostatted chamber of the luminometer and perfused with EGTA-containing KRB. As monitored by erAEQ, EGTA replacement by CaCl2(1 mM) at the indicated time points induced the Ca2+ accumulation into the ER lumen, whereas addition of InsP3-generating agonist ATP (100 μM; A and B), or of the SERCA inhibitor, tBuBHQ (20 μM; C), stimulated the ion discharge. Note that, although cells with either PrPc or Dpl maintained a similar resting [Ca2+]er (by ∼15% higher than in controls; A and C), ATP-induced Ca2+ efflux from the ER was more extensive in Dpl-containing cells (A), followed by a partial refilling of ER stores. Conversely, the levels of resting and discharged [Ca2+]er in cells with GFP-GPIPrP were as in the control (B). When cells were challenged with tBuBHQ, no difference was observed in the ER membrane passive permeability of the three cell types (C). Presented data are typical of at least seven independent experiments, which yielded equivalent results. (D) Western blots of the endogenous SERCA and β-actin in controls (first lane) or in cells expressing PrPc (second lane) and Dpl (third lane) separately, or together (fourth lane), using mAbs against the ubiquitous SERCA 2 isoform and β-actin, respectively. By normalizing the density of each SERCA band to the corresponding β-actin band, cells with PrPc resulted to express the highest, and those expressing Dpl (alone or together with PrPc) the lowest, quantity of SERCA.
Next, we examined cells for the capacity to release Ca2+ after the addition of ATP (100 μM) at the indicated time point (Figure 2A). By activating plasma membrane P2Y receptors coupled to Gq proteins and phospholipase C, ATP induces the generation of inositol-1,4,5-triphosphate (InsP3), which promotes the discharge of the ER Ca2+ stores through the channel activity of InsP3 receptors (InsP3Rs; Streb et al., 1983). Obtained results clearly demonstrate that the dynamics of Ca2+ efflux differs in the three cell types. The largest Ca2+ discharge occurs in the presence of Dpl (dark gray trace), so that the remaining [Ca2+]er (130 ± 20 μM, n = 9) is less than in PrPc-containing cells (black trace; 215 ± 20 μM, n = 7), whereas it equals that of controls (light gray trace; 110 ± 20 μM, n = 9; note, however, the lower starting [Ca2+]er in the latter cells). Yet differences regard also the kinetics of the process, in that the fastest value is exhibited by Dpl-expressing cells, whereas the rate rapidly slows down in cells with PrPc (velocity at half maximal Ca2+ discharge [V1/2]: 27 ± 3 μM/s, n = 5, in cells with Dpl; 18 ± 1 μM/s, n = 5, in cells with PrPc; 20 ± 2 μM/s, n = 5, in controls). Intriguingly, however, after ∼30–40 s from the maximal Ca2+ depletion and in the continuous presence of the agonist, a partial refilling of ER stores occurred in Dpl-containing cells, whereby the final [Ca2+]er plateau settled to a value (210 ± 30 μM, n = 9), closely approximating that observed with PrPc.
Notably, we could exclude that the peculiar ER Ca2+ handling in cells with PrPc, or Dpl, is the consequence of the double transfection protocol (i.e., PrPc, or Dpl, associated with erAEQ), given that cells coexpressing erAEQ and a non-natural GPI-linked protein (GFP-GPIPrP, made of GFP fused to the GPI-attachment signal of PrPc), have the same behavior of controls (Figure 2B). Nor did PrPc, or Dpl, alter the passive permeability of the ER membrane, at least judging from the similar response (in terms of both kinetics and amplitude) in PrPc- and Dpl-containing cells to the addition of 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBuBHQ), an inhibitor of the ER Ca2+ pump (Kass et al., 1989; Figure 2C). After the demonstration that IP3-sensitive stores are fully depleted by inhibiting ER Ca2+ ATPases (Vanoevelen et al., 2004), these data also suggest that the larger Ca2+ amounts accumulated by cells with PrPc (or Dpl), and the smaller IP3-induced Ca2+ efflux by cells expressing PrPc (shown in Figure 2A), are not due to the recruitment of IP3-insensitive Ca2+ compartments. This conclusion is further corroborated by the finding that, when pretreating control, and PrPc-expressing, cells with thapsigargin (an irreversible SERCA inhibitor; Thastrup et al., 1990), in both cases the uptake of Ca2+ in the ER was <10% of the maximal quantity observed in thapsigargin-untreated controls (unpublished data; see also Brini et al., 2000). We could also exclude that data with PrPc are biased by the mode of transfection, or the protein quantity, in that the effects of the protein on the ER Ca2+ handling are reproduced in PrPc-stably transfected CHO cells, which express the protein in lower amounts (unpublished data). Unfortunately, analysis of stably expressed Dpl molecules could not be carried out, given the impossibility, under our conditions, to establish viable clones with CHO cells.
We then examined whether the presence of PrPc and Dpl affected the expression of ER proteins such as SERCA or calreticulin. The latter protein is not only crucial for the ER Ca2+-buffering capacity (Krause and Michalak, 1997), but can also regulate InsP3Rs (Arnaudeau et al., 2002). We could not attribute to calreticulin the different modes of Ca2+ efflux from the ER, because the three cell types expressed the protein at similar levels (unpublished data). However, with respect to controls, we found higher and lower SERCA amounts in the presence of PrPc and Dpl, respectively (Figure 2D, lanes 1–3). Possibly, PrPc and Dpl trigger a signal that interferes with the expression of the protein in an opposed way.
Cytosolic Ca2+ in PrPc- or Dpl-expressing Cells
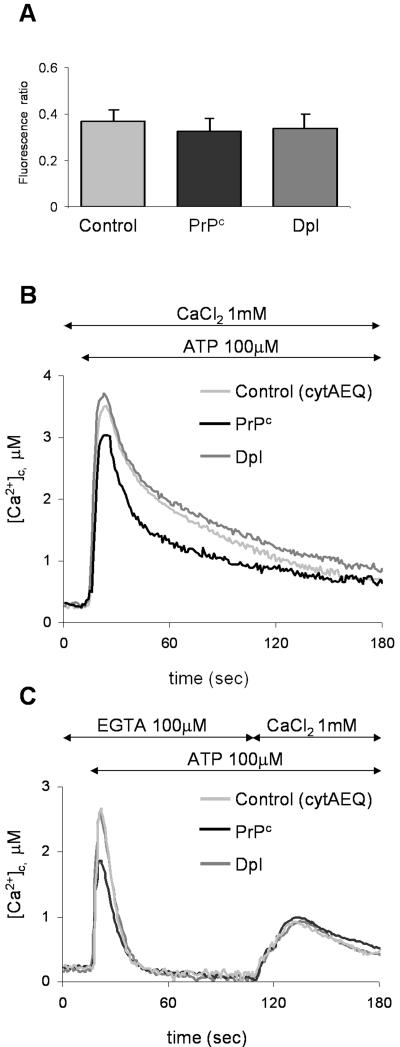
Having found that the presence of PrPc, or Dpl, affects the Ca2+ handling of the main intracellular Ca2+ store, we then set out to evaluate if and how the proteins influenced cytosolic Ca2+ concentration ([Ca2+]c) under resting conditions, or after cell stimulation. AEQ steep response curve precludes the accurate estimation of [Ca2+] lower than 200–300 nM (Brini et al., 1995), a concentration typical of the cytosol of resting cells. This parameter was therefore determined through the image analysis of single cells loaded with the fluorescent Ca2+ indicator fura-2, after identifying successfully transfected cells by the coexpression of GFP (Brini et al., 1995). We found that fura-2 signals (given by the ratio of fluorescence emitted by illuminating cells at 340 and 380 nm) detected in PrPc- or Dpl-expressing cells have a magnitude similar to that of the control (Figure 3A), suggesting that no appreciable alteration in basal [Ca2+]c is attributable to these proteins.
Figure 3.
Resting [Ca2+]c (A) and ATP-induced effects on [Ca2+]c (B and C), in CHO cells transiently expressing cytAEQ alone (control), or together with PrPc, or Dpl. (A) Resting [Ca2+]c, monitored by the Ca2+-indicator fura-2, is expressed as the ratio of the fluorescence emitted by fura-2 after cell excitation at 340 and 380 nm (see Materials and Methods). No statistically significant difference was found in the three cell types. (B) [Ca2+]c transients, after ATP (100 μM) addition at the indicated time point, were monitored with cytAEQ reconstituted with wild-type coelenterazine (5 μM; see Materials and Methods). Although a similar peak was found in controls (light gray trace) and Dpl-containing cells (dark gray trace), cells with PrPc (black trace) gave rise to a transient of smaller magnitude. (C) Using the above described cytAEQ, a protocol was applied so as to evaluate the contribution to the ATP-induced [Ca2+]c movements shown in B, first of the InsP3-induced Ca2+ mobilization (first peak, ATP added in the presence of 100 μM EGTA, i.e., with no external Ca2+) and then of the Ca2+ influx from the external medium (second peak, in the presence of 1 mM CaCl2). On Ca2+ release from the ER (first peak), a transient of smaller magnitude was again observed in cells with PrPc, whereas no difference in the second peak was evident in the three cell types. Presented data are typical of at least seven independent experiments, which yielded equivalent results.
Conversely, cytAEQ was used to monitor changes in [Ca2+]c after the cell stimulation by ATP. As shown (Figure 3B), addition of the agonist to cells induced a similar rise of [Ca2+]c in both control cells (light gray trace; 3.9 ± 0.4 μM, n = 19) and in those expressing Dpl (dark gray trace; 3.9 ± 0.3 μM, n = 17), whereas in cells with PrPc a statistically significant reduction of the Ca2+ transient was observed (black trace; 3.4 ± 0.4 μM, n = 18). It is to be noted, however, that under the used conditions two processes contribute to elevating [Ca2+]c; one is the InsP3-induced discharge of ER Ca2+ stores; the other is the Ca2+ influx from the extracellular space through the so-called capacitative Ca2+ entry (CCE), which becomes activated by a retrograde signal arising from the ER Ca2+ depletion (Putney et al., 2001). Hence, to dissect the contribution of each individual process, cells were subjected to a time-based protocol. First, adding ATP to cells placed in a Ca2+-free medium induced Ca2+ efflux from the ER (Figure 3C). As shown, we found an identical peak transient in control cells (light gray trace; 2.7 ± 0.6 μM, n = 12) and in those with Dpl (dark gray trace; 2.7 ± 0.3 μM, n = 14), whereas a reduced value was again observed in cells with PrPc (black trace; 2.1 ± 0.3 μM, n = 12). After the transients subsided, cells were exposed to a 1 mM CaCl2-containing medium. This caused a second peak, as expected from CCE through activated store-operated Ca2+ channels (SOCC). In this case, however, no statistically significant difference was evident in the three cell types (0.92 ± 0.13 μM, n = 10 in control cells; 0.80 ± 0.09 μM, n = 7, in cells with Dpl; 0.89 ± 0.14 μM, n = 8, in cells with PrPc). Similar results were obtained with fura-2 (unpublished data).
Subplasma Membrane Ca2+ Pools in PrPc- or Dpl-expressing Cells
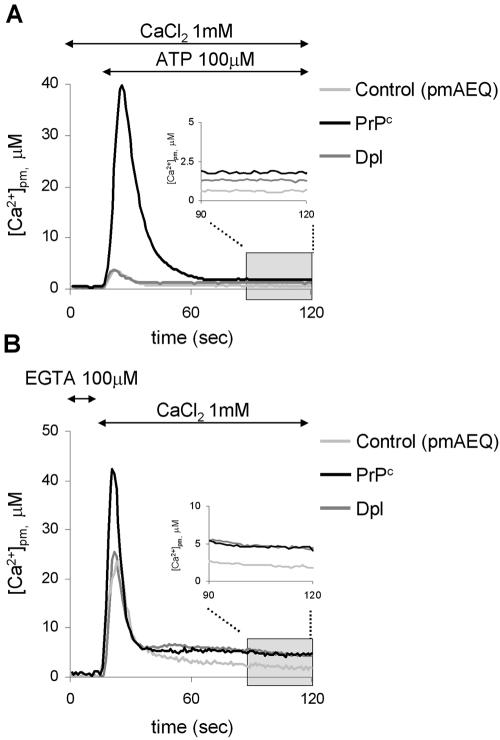
pmAEQ, which targets to the cytosolic rim of the plasma membrane (Marsault et al., 1997), was used to monitoring the Ca2+ concentration in these restricted cytosolic domains ([Ca2+]pm; Figure 4). ATP addition to cells in the presence of 1 mM CaCl (panel A) resulted in transient [Ca2+]2 pm rises of similar magnitude in controls (light gray trace; 4.7 ± 0.8 μM, n = 12) and in Dpl-expressing cells (dark gray trace; 5.3 ± 1.5 μM, n = 9), in contrast to the almost eightfold higher [Ca2+]pm peak found in those housing PrPc (black trace; 42 ± 12 μM, n = 7). After the reestablishment of resting conditions, cells with PrPc continued to maintain a statistically significant enhanced [Ca2+]pm value (1.9 ± 0.6 μM, n = 6) than in controls (0.90 ± 0.20 μM, n = 10), although also in the presence of Dpl the basal [Ca2+]pm remained at a higher level (1.5 ± 0.4 μM, n = 7; see inset to the figure). As reported in panel B, a similar influence of PrPc and Dpl on [Ca2+]pm was evident when CCE was alternatively activated by adding CaCl2 (1 mM) to Ca2+-depleted cells (peak and steady state values were, respectively, 40 ± 12 μM, n = 11 and 6.2 ± 2.7 μM, n = 7, in cells with PrPc; 24 ± 7 μM, n = 11 and 4.5 ± 0.9 μM, n = 8, in cells with Dpl; and 22 ± 8 μM, n = 7 and 2.9 ± 0.7 μM, n = 6, in controls). Altogether, these results suggest that the recombinant expression of PrPc strongly increases Ca2+ entry from the extracellular space, whereas the attenuated effect by Dpl becomes evident only under steady state conditions.
Figure 4.
Monitoring the subplasma membrane Ca2+ concentration with pmAEQ. (A) Response to ATP (100 μM) of CHO cells transiently expressing pmAEQ alone (control, light gray trace), or together with PrPc (black trace) or Dpl (dark gray trace), and maintained in 1 mM CaCl2. pmAEQ was reconstituted by incubating cells with wild-type coelenterazine (5 μM; see Materials and Methods). Addition of ATP provoked a [Ca2+]pm transient that in cells with PrPc was by far more elevated than in the other two cell types. With respect to the control, however, the resting [Ca2+] level remained significantly higher in the presence of both PrPc pmand Dpl (in the inset, resting [Ca2+]pm are reported on an expanded scale). (B) Response to CaCl2 addition (1 mM) in the same cell types described in A, but maintained in (Ca2+-free) KRB supplemented with EGTA (100 μM). After pmAEQ reconstitution, addition of 1 mM CaCl2 induced a response that mimicked that shown in A with respect to both peak and resting [Ca2+]pm levels. The latter are also shown in the inset on an expanded scale. Presented data are typical of at least six independent experiments, which yielded equivalent results.
Mitochondrial Ca2+ in PrPc- or Dpl-expressing Cells
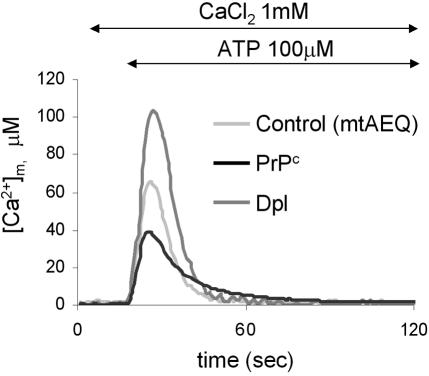
Compelling evidence indicates that in several cell types, including CHO cells, mitochondria behave as reporters of localized Ca2+ changes originating from the stimulation of the InsP3Rs, in that the Ca2+ rise at the channels' mouth triggers the activity of the low-affinity Ca2+ uniporter of mitochondria juxtaposed to InsP3Rs (Rizzuto et al., 1998). Such a spatial link between the two organelles, which according to recent data may also involve stable physical interactions (Filippin et al., 2003), ensures that mitochondria receive ER signals with maximal efficiency. To examine the influence of PrPc and Dpl on such mechanism, we used mtAEQ targeted to the mitochondrial matrix (Rizzuto et al., 1992; Montero et al., 2000). Intriguingly, ATP addition resulted in a mitochondrial Ca2+ signal ([Ca2+]m) that was specific for each case (Figure 5); cells with PrPc showed the lowest value (black trace; 42 ± 11 μM, n = 9), the control ones the intermediate value (light gray trace; 62 ± 15 μM, n = 7), whereas the highest Ca2+ accumulation was detected in the presence of Dpl (dark gray trace; 110 ± 15 μM, n = 10). In the latter case, the average [Ca2+]m value was more than twice that occurring with PrPc. Therefore, in line with the close coupling between quantity of Ca2+ released from the ER and Ca2+ amounts accumulated by mitochondria, these data emphasize the findings on the ER Ca2+ discharge monitored by erAEQ (see Figure 2A).
Figure 5.
ATP-induced effects on [Ca2+]m in CHO cells transiently expressing mtAEQ alone (control, light gray trace), or together with PrPc (black trace) or Dpl (dark gray trace). mtAEQ was reconstituted by incubating cells with wild-type coelenterazine (5 μM; see Materials and Methods). The addition of ATP (100 μM) induced a rise in the [Ca2+]m that was higher in Dpl-expressing cells than in the other two cell types. Presented data are typical of at least seven independent experiments, which yielded equivalent results.
Coexpression of PrPc and Dpl Abolishes the Divergent Effects on Ca2+ Signaling Observed in Cells Expressing PrPc, or Dpl, Alone
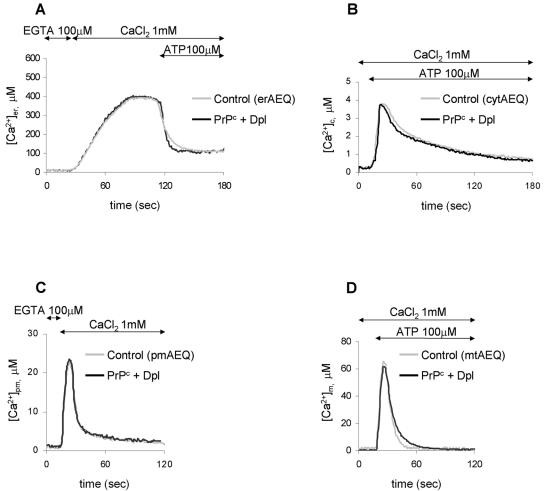
In view of the opposite effects on local Ca2+ fluctuations elicited by PrPc and Dpl reported here and of the functional interplay of the two proteins suggested by genetic approaches (Kuwahara et al., 1999; Moore et al., 2001; Rossi et al., 2001; Yamaguchi et al., 2004), one expects that the Ca2+ signals observed in cells expressing PrPc and Dpl separately will be modified by the copresence of the proteins in the same cell. To verify this assumption, we monitored Ca2+ homeostasis in the various compartments of cells transiently cotransfected with PrPc and Dpl, after controlling that the two proteins are expressed at levels comparable to those found in singly transfected cells (Figure 1B, third lanes; note that, irrespective of the used plasmid batch(es), the transfection efficiency was generally ∼35%). Indeed, we found that under this condition local Ca2+ signals are extremely close to those of control cells (Figure 6), but for the ER Ca2+ efflux rate (panel A) that after PrPc and Dpl cotransfection is faster than in controls by around 30% (V1/2 being 33 ± 3 μM/s, n = 6, in cotransfected cells; 23 ± 4 μM/s, n = 5, in controls). It is interesting to note that a similar percentage difference is also found when comparing the corresponding V1/2 values of controls and Dpl-expressing cells (Figure 2A, and text), and that cells transfected with Dpl, either alone or together with PrPc, present the lowest amounts of SERCA (third and fourth lanes of Figure 2D).
Figure 6.
Monitoring [Ca2+]er (A), [Ca2+]c (B), [Ca2+]pm (C), and [Ca2+]m (D) in CHO cells transiently expressing the corresponding AEQ alone (control, light gray trace), or together with both PrPc and Dpl (black trace). Experimental conditions were as reported in the legend to Figures 2A, 3B, 4B, and 5, respectively. Clearly, the copresence of PrPc and Dpl abolished the divergent Ca2+ signaling observed in the various compartments of cells expressing PrPc, or Dpl, alone. Presented data are typical of at least nine independent experiments, which yielded equivalent results.
DISCUSSION
Several reports indicate that reduction of PrPc functionality causes perturbations in Ca2+ signaling and that the provoked alteration in some plasma membrane currents may explain aspects of TSEs, including a few neurologic symptoms (Cathala and Baron, 1987) and loss of neurons (Gray et al., 1999). On this background, we examined whether PrPc takes part in the complex mechanism that controls Ca2+ homeostasis by analyzing, for the first time, Ca2+ fluctuations in different compartments of cells transfected with PrPc and recombinant targeted aequorins. We found that the way cells with PrPc handle compartmentalized Ca2+ favors the cell protective role attributed to PrPc, in contrast with the effects arising from the presence of Dpl. Such a divergent activity, and the capacity of PrPc—cotransfected with Dpl—to suppress the effects elicited by Dpl alone, highlights the protective function of PrPc N-domain (absent from Dpl), as also deduced from experiments with cells challenged by serum deprivation (Sakudo et al., 2003) or over expressing toxic PrP fragments (Shmerling et al., 1998), proapoptotic Bax (Bounhar et al., 2001), or Dpl itself (Atarashi et al., 2003).
The most remarkable features observed in PrPc-containing cells regard [Ca2+]pm pools and the ER-mitochondria Ca2+ coupling. In considering the highest [Ca2+]pm found in the presence of PrPc (Figure 4), the simplest explanation is that PrPc activates SOCCs. This is important, in view of the suggestion that the capacitative entry of Ca2+ in neuronal cells may serve in signaling pathways, not only in the refilling of Ca2+ stores observed in nonexcitable cells, and that subplasma membrane Ca2+-rich domains may trigger key physiological cell events (Putney, 2003). For example, SOCCs can be functionally coupled with adenylyl cyclase (Fagan et al., 2000); PrPc-elicited [Ca2+]pm rises may thus represent the so-far missing intermediate linking the activation of cytosolic cAMP-dependent protein kinase A by cell surface-attached PrPcs, which was shown to inhibit apoptosis in retinal explants (Chiarini et al., 2002). Also, in analogy with SOCCs modulation of transmitter release and synaptic plasticity in certain neurons (Emptage et al., 2001), PrPc-elicited Ca2+ hotspots may recruit effectors in the immediate vicinity, e.g., Ca2+-activated K+ channels, thereby explaining why impairment of (Ca2+-dependent) K+ currents in PrP-null cells could not be attributed to specific alterations of voltage-gated Ca2+ channels (Herms et al., 2000; but see Whatley et al., 1995). In the light of all these considerations, it is thus tempting to speculate that, as observed in the used cell model system, PrPc affects SOCC-dependent subplasma membrane Ca2+ pools also in neurons. If this were the case, impairment of SOCCs could play a major role in TSE neurologic manifestations (Cathala and Baron, 1987).
Equally important is the way by which PrPc-expressing cells control ER and mitochondrial Ca2+ homeostases, either of which is crucial in cell survival. Indeed, [Ca2+]er variations may trigger stress responses, whereas substantial ER Ca2+ discharges may increase mitochondrial Ca2+ levels that, by activating caspase-9, a member of the death-specific caspase family (Cryns and Yuan, 1998), determine the pathway leading to apoptotic death (Li et al., 1997). Hence, despite the potentially dangerous elevated [Ca2+]er (Pinton et al., 2000, 2001), our demonstration that the presence of PrPc limits the agonist-stimulated ER Ca2+ release (Figures 2A and 3C) and, most importantly, the ion accumulation by mitochondria (Figure 5), is not only consistent with a PrPc protective role, but it also predicts an increased cell vulnerability upon reducing PrPc molecules, and/or expression of TSE-associated PrP mutants that accumulate in the ER (Singh et al., 1997; Zanusso et al., 1999; Jin et al., 2000; Negro et al., 2001). Accordingly, one of the first events observed in neuronal cells exposed to purified PrPSc is a substantial ER Ca2+ discharge, followed by up-regulation of stress proteins and activation of the ER-resident caspase-12 that acts on other effector caspases to produce apoptosis (Hetz et al., 2003b).
Conversely, stimulation of Dpl-expressing cells leads to a more pronounced and faster efflux of ER Ca2+ (Figure 2A) and to a large rise in [Ca2+]m (Figure 5). These results, collected in the absence of apoptotic stimuli, disclose a possible mechanism by which Dpl sensitizes cells to environmental insults, whereas the difficulty in obtaining neuroblastoma (Massimino et al., 2004), or CHO (this article), clones with Dpl suggests that the intrinsic toxicity of Dpl may manifest in cells other than Purkinje cells, the prime target of Dpl pathogenicity (Sakaguchi et al., 1996). Interestingly, biochemical analyses of Dpl-over-expressing brain tissues have proposed that the protein toxicity relates to an increased oxidative damage, possibly linked to impaired copper metabolism (Wong et al., 2001). These data well agree with the demonstration that, despite the capacity of Dpl to bind copper like the related PrPc C-terminus, different structural and functional roles pertain to Dpl- and PrPc-bound coppers (Cereghetti et al., 2004; see also Sakudo et al., 2004). However, it seems also consistent with the influence of Dpl on mitochondrial Ca2+ handling reported here, given that deregulation of the organelle Ca2+ homeostasis can lead to the overproduction of toxic reactive oxygen species (Toescu and Verkhratsky, 2003).
As for the divergent ER Ca2+ handling, we found that cells with PrPc and Dpl express higher and lower amounts of SERCA, respectively, than control cells (Figure 2D). This data may explain why, irrespective of an identical Ca2+ load, the balance between InsP3R-mediated Ca2+ efflux from, and SERCA-mediated Ca2+ (re-)loading in, the ER results in a less pronounced net Ca2+ discharge with PrPc than with Dpl (Figure 2A). ER Ca2+ load, however, depends on both the amount and activity of the SERCA pumps. We have shown that in cells with Dpl CCE generates steady state [Ca2+]pm levels higher than in controls (Figure 4). It seems therefore possible to hypothesize that, in line with other observations (Mogami et al., 1997), [Ca2+]pm in Dpl-expressing cells stimulates the SERCA activity, thus explaining why, despite lower SERCA amounts, also these cells maintain a higher resting [Ca2+]er than in controls (Figure 2A). Likewise, [Ca2+]pm may also account for the slow refilling of ER stores occurring after the agonist-induced maximal Ca2+ depletion (Figure 2A). An important support to the key role of [Ca2+]pm magnitude in regulating ER Ca2+ handling comes from cells housing PrPc and Dpl together; they display low SERCA levels as well as low [Ca2+]pm and are unable to exhibit the same [Ca2+]er features observed in cells with Dpl alone (Figure 6).
In accord with the suggested functional interrelationship of the proteins, PrPc and Dpl copresence abolishes Dpl effects on local Ca2+ movements (Figure 6). Several hypotheses have been suggested to explain PrPc protection against Dpl (Behrens and Aguzzi, 2002): that PrPc and Dpl compete for a common ligand (in the absence of PrPc, the binding of Dpl to the ligand would elicit an apoptotic signal); that PrPc antagonizes an independent action of Dpl that sensitizes neurons to apoptosis (PrP-null cells would no longer control oxidative stress and Dpl deleterious function would predominate); and that PrPc interferes with unselective multimeric pores formed by Dpl in the ER and/or plasma membrane. With respect to the latter hypothesis, the similar passive ER Ca2+ efflux in PrPc- or Dpl-expressing cells (Figure 2C) implies that Dpl is unlikely to form (Ca2+) leak pathways in the ER membrane. On the other hand, the Ca2+ handling in cells expressing PrPc and Dpl together closely matches that of control cells (Figure 6), not that observed in the presence of PrPc alone. A possible interpretation for such a balanced effect entails that, at least in CHO cells, PrPc and Dpl have independent, opposite activities, rather than the capacity to competing for a common ligand.
In conclusion, we have shown that the recombinantly expressed PrPc governs the cell Ca2+ metabolism in a cellprotective manner, by limiting the Ca2+ accumulation into mitochondria. Yet, PrPc consistently increases [Ca2+]pm, suggesting that these elevated Ca2+ pools may contribute to regulating the (Ca2+-dependent) cell excitability and/or PrPc-mediated signaling, either of which is critical for neuronal survival.
Acknowledgments
M.B. acknowledges funding from the Ministero dell'Istruzione, dell'Università e della Ricerca (MUIR) (PRIN 2003), and M.C.S. from the European Union (BioMed.2 BMH4-CT98–6050), Telethon Onlus (E.0945), MIUR (PRIN 2004 and RBNE01S29H), and the Italian Ministry of Health (1AA/F, 2001).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–10–0915) on March 23, 2005.
Abbreviations used: AEQ, aequorin; cytAEQ, erAEQ, mtAEQ, and pmAEQ, aequorin targeted to the cytosol, the endoplasmic reticulum, the mitochondrial matrix and the plasma membrane, respectively; tBuBHQ, 2,5-di-(tert-butyl)-1,4-benzohydroquinone; CCE, capacitative Ca2+ entry; Dpl, Doppel; Dplrec, recombinant Doppel; ER, endoplasmic reticulum; GFP, green fluorescent protein; GPI, glycosylphosphatidylinositol; InsP3, inositol-1,4,5-triphosphate; mAb, monoclonal antibody; PrPc, cellular prion protein; PrPSc, pathological isoform of prion protein; PrPrec, recombinant prion protein; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; SOCC, store-operated Ca2+ channels; TSE, transmissible spongiform encephalopathy.
References
- Arnaudeau, S., Frieden, M., Nakamura, K., Castelbou, C., Michalak, M., and Demaurex, N. (2002). Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J. Biol. Chem. 277, 46696–46705. [DOI] [PubMed] [Google Scholar]
- Atarashi, R., Nishida, N., Shigematsu, K., Goto, S., Kondo, T., Sakaguchi, S., and Katamine, S. (2003). Deletion of N-terminal residues 23–88 from prion protein (PrP) abrogates the potential to rescue PrP-deficient mice from PrP-like protein/doppel-induced neurodegeneration. J. Biol. Chem. 278, 28944–28949. [DOI] [PubMed] [Google Scholar]
- Barrero, M. J., Montero, M., and Alvarez, J. (1997). Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells: a comparative study. J. Biol. Chem. 272, 27694–27699. [DOI] [PubMed] [Google Scholar]
- Barrow, P. A., Holmgren, C. D., Tapper, A. J., and Jefferys, J. G. (1999). Intrinsic physiological and morphological properties of principal cells of the hippocampus and neocortex in hamsters infected with scrapie. Neurobiol. Dis. 6, 406–423. [DOI] [PubMed] [Google Scholar]
- Behrens, A., and Aguzzi, A. (2002). Small is not beautiful: antagonizing functions for the prion protein PrP(C) and its homologue Dpl. Trends Neurosci. 25, 150–154. [DOI] [PubMed] [Google Scholar]
- Bernardi, P. (1999). Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155. [DOI] [PubMed] [Google Scholar]
- Bounhar, Y., Zhang, Y., Goodyer, C. G., and LeBlanc, A. (2001). Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem. 276, 39145–39149. [DOI] [PubMed] [Google Scholar]
- Brini, M. (2003). Ca2+ signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium 34, 399–405. [DOI] [PubMed] [Google Scholar]
- Brini, M., Bano, D., Manni, S., Rizzuto, R., and Carafoli, E. (2000). Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca2+ signalling. EMBO J 19, 4926–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini, M., Marsault, R., Bastianutto, C., Alvarez, J., Pozzan, T., and Rizzuto, R. (1995). Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c): a critical evaluation. J. Biol. Chem. 270, 9896–9903. [DOI] [PubMed] [Google Scholar]
- Brown, D. R., Nicholas, R. S., and Canevari, L. (2002). Lack of prion protein expression results in a neuronal phenotype sensitive to stress. J. Neurosci. Res. 67, 211–224. [DOI] [PubMed] [Google Scholar]
- Brown, D. R. et al. (1997). The cellular prion protein binds copper in vivo. Nature 390, 684–687. [DOI] [PubMed] [Google Scholar]
- Bueler, H., Fischer, M., Lang, Y., Bluethmann, H., Lipp, H. P., DeArmond, S. J., Prusiner, S. B., Aguet, M., and Weissmann, C. (1992). Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356, 577–582. [DOI] [PubMed] [Google Scholar]
- Cathala, F., and Baron, H. (1987). Clinical aspects of Creutzfeldt–Jakob disease. In: Prions: Novel Infectious Pathogens Causing Scrapie and Creutzfeldt-Jakob Disease, ed. S. B. Prusiner and M. P. McKinley, San Diego: Academic Press, 467–509.
- Cereghetti, G. M., Negro, A., Vinck, E., Massimino, M. L., Sorgato, M. C., and Van Doorslaer, S. (2004). Copper(II) binding to the human Doppel protein may mark its functional diversity from the prion protein. J. Biol. Chem. 279, 36497–36503. [DOI] [PubMed] [Google Scholar]
- Chiarini, L. B., Freitas, A. R., Zanata, S. M., Brentani, R. R., Martins, V. R., and Linden, R. (2002). Cellular prion protein transduces neuroprotective signals. EMBO J. 21, 3317–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colling, S. B., Collinge, J., and Jefferys, J. G. (1996). Hippocampal slices from prion protein null mice: disrupted Ca2+-activated K+ currents. Neurosci. Lett. 209, 49–52. [DOI] [PubMed] [Google Scholar]
- Cryns, V., and Yuan, J. (1998). Proteases to die for. Genes Dev. 12, 1551–1570. [DOI] [PubMed] [Google Scholar]
- Emptage, N. J., Reid, C. A., and Fine, A. (2001). Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron 29, 197–208. [DOI] [PubMed] [Google Scholar]
- Fagan, K. A., Graf, R. A., Tolman, S., Schaack, J., and Cooper, D. M. (2000). Regulation of a Ca2+-sensitive adenylyl cyclase in an excitable cell. Role of voltage-gated versus capacitative Ca2+ entry. J. Biol. Chem. 275, 40187–40194. [DOI] [PubMed] [Google Scholar]
- Filippin, L., Magalhaes, P. J., Di Benedetto, G., Colella, M., and Pozzan, T. (2003). Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J. Biol. Chem. 278, 39224–39234. [DOI] [PubMed] [Google Scholar]
- Gray, F., Chretien, F., Adle-Biassette, H., Dorandeu, A., Ereau, T., Delisle, M. B., Kopp, N., Ironside, J. W., and Vital, C. (1999). Neuronal apoptosis in Creutzfeldt-Jakob disease. J. Neuropathol. Exp. Neurol. 58, 321–328. [DOI] [PubMed] [Google Scholar]
- Herms, J. W., Tings, T., Dunker, S., and Kretzschmar, H. A. (2001). Prion protein affects Ca2+-activated K+ currents in cerebellar Purkinje cells. Neurobiol. Dis. 8, 324–330. [DOI] [PubMed] [Google Scholar]
- Herms, J. W., Korte, S., Gall, S., Schneider, I., Dunker, S., and Kretzschmar, H. A. (2000). Altered intracellular calcium homeostasis in cerebellar granule cells of prion protein-deficient mice. J. Neurochem. 75, 1487–1492. [DOI] [PubMed] [Google Scholar]
- Hetz, C., Maundrell, K., and Soto, C. (2003a). Is loss of function of the prion protein the cause of prion disorders? Trends Mol. Med. 9, 237–243. [DOI] [PubMed] [Google Scholar]
- Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J., and Soto, C. (2003b). Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys, J. G., Empson, R. M., Whittington, M. A., and Prusiner, S. B. (1994). Scrapie infection of transgenic mice leads to network and intrinsic dysfunction of cortical and hippocampal neurones. Neurobiol. Dis. 1, 25–30. [DOI] [PubMed] [Google Scholar]
- Jin, T., Gu, Y., Zanusso, G., Sy, M., Kumar, A., Cohen, M., Gambetti, P., and Singh, N. (2000). The chaperone protein BiP binds to a mutant prion protein and mediates its degradation by the proteasome. J. Biol. Chem. 275, 38699–38704. [DOI] [PubMed] [Google Scholar]
- Johnston, A. R., Fraser, J. R., Jeffrey, M., and MacLeod, N. (1998). Synaptic plasticity in the CA1 area of the hippocampus of scrapie-infected mice. Neurobiol. Dis. 5, 188–195. [DOI] [PubMed] [Google Scholar]
- Jouaville, L. S., Pinton, P., Bastianutto, C., Rutter, G. A., and Rizzuto, R. (1999). Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 96, 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, G.E.N., Dudd, S. K., Moore, G. A., and Orrenius, S. A. (1989). 2,5-Di(tertbutyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J. Biol. Chem. 264, 15192–15198. [PubMed] [Google Scholar]
- Krause, K. H., and Michalak, M. (1997). Calreticulin. Cell 88, 439–443. [DOI] [PubMed] [Google Scholar]
- Kuwahara, C. et al. (1999). Prions prevent neuronal cell-line death. Nature 400, 225–226. [DOI] [PubMed] [Google Scholar]
- Li, A., Sakaguchi, S., Shigematsu, K., Atarashi, R., Roy, B. C., Nakaoke, R., Arima, K., Okimura, N., Kopacek, J., and Katamine, S. (2000). Physiological expression of the gene for PrP-like protein, PrPLP/Dpl, by brain endothelial cells and its ectopic expression in neurons of PrP-deficient mice ataxic due to Purkinje cell degeneration. Am. J. Pathol. 157, 1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S., and Wang, X. (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Malgaroli, A., Milani, D., Meldolesi, J., and Pozzan, T. (1987). Fura-2 measurements of cytosolic free Ca2+ monolayers and suspensions of various cell types of animal cells. J. Cell Biol. 105, 2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci, G. R., Ratte, S., Asante, E. A., Linehan, J., Gowland, I., Jefferys, J. G., and Collinge, J. (2002). Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 21, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson, J. C., Clarke, A. R., Hooper, M. L., Aitchison, L., McConnell, I., and Hope, J. (1994). 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 8, 121–127. [DOI] [PubMed] [Google Scholar]
- Marsault, R., Murgia, M., Pozzan, T., and Rizzuto, R. (1997). Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 16, 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimino, M. L., Ballarin, C., Bertoli, A., Casonato, S., Genovesi, S., Negro, A., and Sorgato, M. C. (2004). Human Doppel and prion protein share common membrane microdomains and internalization pathways. Int. J. Biochem. Cell Biol. 36, 2016–2031. [DOI] [PubMed] [Google Scholar]
- Mattson, M. P., LaFerla, F. M., Chan, S. L., Leissring, M. A., Shepel, P. N., and Geiger, J. D. (2000). Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 23, 222–229. [DOI] [PubMed] [Google Scholar]
- Mo, H., Moore, R. C., Cohen, F. E., Westaway, D., Prusiner, S. B., Wright, P. E., and Dyson, H. J. (2001). Two different neurodegenerative diseases caused by proteins with similar structures. Proc. Natl. Acad. Sci. USA 98, 2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami, H., Nakano, K., Tepikin, A. V., and Petersen, O. H. (1997). Ca2+ flow via tunnels in polarized cells: recharging of apical stores by focal Ca2+ entry through basal membrane patch. Cell 88, 49–55. [DOI] [PubMed] [Google Scholar]
- Montero, M., Brini, M., Marsault, R., Alvarez, J., Sitia, R., Pozzan, T., and Rizzuto, R. (1995). Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 14, 5467–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero, M., Alonso, M. T., Carnicero, E., Cuchillo-Ibanez, I., Albillos, A., Garcia, A. C., Garcia-Sancho, J,. and Alvarez, J. (2000). Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell. Biol. 2, 57–61. [DOI] [PubMed] [Google Scholar]
- Moore, R. C., Mastrangelo, P., Bouzamondo, E., Heinrich, C., Legname, G., Prusiner, S. B., Hood, L., Westaway, D., DeArmond, S. J., and Tremblay, P. (2001). Doppel-induced cerebellar degeneration in transgenic mice. Proc. Natl. Acad. Sci. USA 98, 15288–15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, R. C. et al. (1999). Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 292, 797–817. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T., Zhu, H., Morishima, N., Li, E., Xu, J., Yankner, B. A., and Yuan, J. (2000). Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403, 98–103. [DOI] [PubMed] [Google Scholar]
- Negro, A., Ballarin, C., Bertoli, A., Massimino, M. L., and Sorgato, M. C. (2001). The metabolism and imaging in live cells of the bovine prion protein in its native form or carrying single amino acid substitutions. Mol. Cell Neurosci. 17, 521–538. [DOI] [PubMed] [Google Scholar]
- Negro, A., Meggio, F., Bertoli, A., Battistutta, R., Sorgato, M. C., and Pinna, L. A. (2000). Susceptibility of the prion protein to enzymic phosphorylation. Biochem. Biophys. Res. Commun. 271, 337–341. [DOI] [PubMed] [Google Scholar]
- Orrenius, S., Zhivotovsky, B., and Nicotera, P. (2003). Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell. Biol. 4, 552–565. [DOI] [PubMed] [Google Scholar]
- Paschen, W. (2001). Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium 29, 1–11. [DOI] [PubMed] [Google Scholar]
- Pinton, P., Ferrari, D., Rapizzi, E., Di Virgilio, F., Pozzan, T., and Rizzuto, R. (2001). The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton, P., Ferrari, D., Magalhaes, P., Schulze-Osthoff, K., Di Virgilio, F., Pozzan, T., and Rizzuto, R. (2000). Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 148, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S. B. (1998). Prions. Proc. Natl. Acad. Sci. USA 95, 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney, J. W., Jr. (2003). Capacitative calcium entry in the nervous system. Cell Calcium 34, 339–344. [DOI] [PubMed] [Google Scholar]
- Putney, J. W., Jr., Broad, L. M., Braun, F. J., Lievremont, J. P., and Bird, G. S. (2001). Mechanisms of capacitative calcium entry. J. Cell Sci. 114, 2223–2229. [DOI] [PubMed] [Google Scholar]
- Rizzuto, R., Simpson, A.W.M., Brini, M., and Pozzan, T. (1992). Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358, 325–328. [DOI] [PubMed] [Google Scholar]
- Rizzuto, R., Brini, M., Bastianutto, C., Marsault, R., and Pozzan, T. (1995). Photoprotein-mediated measurement of calcium ion concentration in mitochondria of living cells. Methods Enzymol. 260, 417–428. [DOI] [PubMed] [Google Scholar]
- Rizzuto, R., Pinton, P., Carrington, W., Fay, F. S., Fogarty, K. E., Lifshitz, L. M., Tuft, R. A., and Pozzan, T. (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Rossi, D., Cozzio, A., Flechsig, E., Klein, M. A., Rulicke, T., Aguzzi, A., and Weissmann, C. (2001). Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 20, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, S. et al. (1996). Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 380, 528–531. [DOI] [PubMed] [Google Scholar]
- Sakudo, A., Lee, D. C., Saeki, K., Nakamura, Y., Inoue, K., Matsumoto, Y., Itohara, S., and Onodera, T. (2003). Impairment of superoxide dismutase activation by N-terminally truncated prion protein (PrP) in PrP-deficient neuronal cell line. Biochem. Biophys. Res. Commun. 308, 660–667. [DOI] [PubMed] [Google Scholar]
- Sakudo, A. et al. (2004). Prion protein suppresses perturbation of cellular copper homeostasis under oxidative conditions. Biochem. Biophys. Res. Commun. 313, 850–855. [DOI] [PubMed] [Google Scholar]
- Shmerling, D. et al. (1998). Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93, 203–214. [DOI] [PubMed] [Google Scholar]
- Silverman, G. L., Qin, K., Moore, R. C., Yang, Y., Mastrangelo, P., Tremblay, P., Prusiner, S. B., Cohen, F. E., and Westaway, D. (2000). Doppel is an N-glycosylated, glycosylphosphatidylinositol-anchored protein. Expression in testis and ectopic production in the brains of Prnp0/0 mice predisposed to Purkinje cell loss. J. Biol. Chem. 275, 26834–26842. [DOI] [PubMed] [Google Scholar]
- Singh, N., Zanusso, G., Chen, S. G., Fujioka, H., Richardson, S., Gambetti, P., and Petersen, R. B. (1997). Prion protein aggregation reverted by low temperature in transfected cells carrying a prion protein gene mutation. J. Biol. Chem. 272, 28461–28470. [DOI] [PubMed] [Google Scholar]
- Streb, H., Irvine, R. F., Berridge, M. J., and Schulz, I. (1983). Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69. [DOI] [PubMed] [Google Scholar]
- Thastrup, O., Cullen, P. J., Drobak, B. J., Hanley, M. R., and Dawson, A. P. (1990). Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 87, 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu, E. C., and Verkhratsky, A. (2003). Neuronal ageing from an intraneuronal perspective: roles of endoplasmic reticulum and mitochondria. Cell Calcium 34, 311–323. [DOI] [PubMed] [Google Scholar]
- Vanoevelen, J., Raeymaekers, L., Parys, J. B., De Smedt, H., Van Baelen, K., Callewaert, G., Wuytack, F., and Missiaen, L. (2004). Inositol trisphosphate producing agonists do not mobilize the thapsigargin-insensitive part of the endoplasmic-reticulum and Golgi Ca2+ store. Cell Calcium 35, 115–121. [DOI] [PubMed] [Google Scholar]
- Whatley, S. A., Powell, J. F., Politopoulou, G., Campbell, I. C., Brammer, M. J., and Percy, N. S. (1995). Regulation of intracellular free calcium levels by the cellular prion protein. Neuroreport 6, 2333–2337. [DOI] [PubMed] [Google Scholar]
- Wong, B. S. et al. (2001). Induction of HO-1 and NOS in doppel-expressing mice devoid of PrP: implications for doppel function. Mol. Cell. Neurosci. 17, 768–775. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, N., Sakaguchi, S., Shigematsu, K., Okimura, N., and Katamine, S. (2004). Doppel-induced Purkinje cell death is stoichiometrically abrogated by prion protein. Biochem. Biophys. Res. Commun. 319, 1247–1252. [DOI] [PubMed] [Google Scholar]
- Zanusso, G., Petersen, R. B., Jin, T., Jing, Y., Kanoush, R., Ferrari, S., Gambetti, P., and Singh, N. (1999). Proteasomal degradation and N-terminal protease resistance of the codon 145 mutant prion protein. J. Biol. Chem. 274, 23396–23404. [DOI] [PubMed] [Google Scholar]