Abstract
Porcine reproductive and respiratory syndrome (PRRS) consistently elevates the frequency of disease and mortality in young pigs. Many different secondary bacterial diseases occur in PRRS virus (PRRSV)-infected pigs. However, to date, establishing a reproducible experimental model of PRRSV infection in weaned pigs, with subsequent clinical disease following secondary bacterial challenge, has been difficult. PRRSV is frequently isolated during outbreaks from weak-born piglets affected by secondary bacterial diseases. This study was performed to investigate the potential role of intrauterine PRRSV infection on piglet susceptibility to secondary bacterial infection. PRRSV-free pregnant sows were intranasally infected at 98 days of gestation with PRRSV strain SD 23983. All piglets born to the PRRSV-infected sows were viremic. Piglets were removed from the sows at birth and deprived of colostrum. Piglets from PRRSV-infected and noninfected sows were randomly assigned to Streptococcus suis challenge or control subgroups. At 5 days of age, piglets were challenged intranasally with strain MN 87555 of S. suis type II. Total and differential leukocyte counts were performed on blood samples collected at 3 days of age. The numbers of leukocytes, lymphocytes, and monocytes were significantly reduced in the PRRSV-infected piglets. Lesions were observed in bone marrow, brain, lung, heart, spleen, lymph node, tonsil, and thymus of PRRSV-infected piglets. Thymus/body weight ratios of in utero PRRSV-infected piglets were significantly reduced compared to those of non-PRRSV-infected piglets, and thymic lesions were characterized by severe cortical depletion of thymocytes. Lesions were not observed in piglets born to PRRSV-free sows. Overall, 20 out of 22 piglets in the PRRSV-S. suis dual-infection group died within 1 week after challenge with S. suis (10 of 11 in each of two trials). This contrasts with 1 of 18 piglets in the PRRSV-infection-only group and 5 of 23 piglets in the S. suis-challenge-only group (1 of 12 in trial 1 and 4 of 11 in trial 2). No piglets died in the uninfected control groups. Most of the piglets in the PRRSV-S. suis dual-infection group developed suppurative meningitis. S. suis type II was recovered from their brains and joints. These results indicate that in utero infection by PRRSV makes piglets more susceptible to infection and disease following challenge by S. suis type II. In utero infection by PRRSV may provide a useful model to study the interaction between PRRSV and bacterial coinfections in piglets.
Porcine reproductive and respiratory syndrome virus (PRRSV) is a member of the genus Arterivirus, family Arteriviridae, order Nidovirales (5), together with Equine arteritis virus, Lactate dehydrogenase-elevating virus, and Simian hemorrhagic fever virus (17). As is characteristic of arteriviruses, PRRSV is a small enveloped RNA virus which replicates primarily in macrophages. The genome of PRRSV is a single-stranded polyadenylated RNA 15 kb in length which contains eight open reading frames (ORFs). ORF 1 encodes the RNA replicase, the only nonstructural protein of PRRSV that has been identified. ORFs 2 to 7 are thought to encode structural proteins. The genome organization and the replication of arteriviruses, which takes place via a 3′-coterminal nested set of subgenomic mRNAs, are similar to those of coronaviruses (2, 5, 20).
Sows affected with PRRS often have late-term abortions, excessive stillbirths, and mummified fetuses (9, 15, 29). Live-born piglets can be viremic at birth, are thin and often weak, and display respiratory distress or clinical signs of secondary bacterial infections (9, 10, 15, 27). PRRS in weaned pigs is usually associated with secondary bacterial diseases and can continue after the reproductive disease outbreak has ended (27). PRRSV antigen has been demonstrated previously within macrophages of pig lung, liver, thymus, and lymph nodes (14, 21, 31). PRRSV can induce apoptosis (26) and alter the function of infected porcine alveolar macrophages (PAM) in vitro (18, 25, 30). It has been presumed from these clinical and experimental observations that PRRSV causes immunosuppression of infected pigs, resulting in increased susceptibility to secondary bacterial diseases.
However, attempts to recreate an immunosuppressed condition with PRRSV have been difficult. Galina et al. (11) reported that PRRSV infection predisposed pigs to more severe disease and mortality following infection with a virulent strain of Streptococcus suis (MN 87555) compared with those infected with either PRRSV or S. suis alone. Wills et al. (34) demonstrated an increase in severity of disease when PRRSV challenge followed Salmonella choleraesuis infection and dexamethasone treatment. Also, Reeth et al. (22) reported a positive association of PRRSV with porcine respiratory coronavirus or swine influenza virus. However, Cooper et al. (7) reported that infection with the NEB-1 strain of PRRSV did not exacerbate disease when infection was followed 2 or 7 days later with Haemophilus parasuis, S. suis, S. choleraesuis, or Pasteurella multocida infection. Other investigators have also reported failed attempts to recreate secondary H. parasuis, Actinobacillus pleuropneumoniae, or P. multocida infection following infection with either the ATCC VR 2332 strain or the Lelystad strain of PRRSV (4, 21, 26).
In this paper, we explore a novel model to recreate the secondary bacterial disease complex observed in PRRSV-infected animals. We based this model on the observation that piglets born to PRRSV-infected sows can be viremic at birth and display clinical signs resulting from secondary bacterial infections (6, 10, 31). We hypothesized that in utero infection of piglets with PRRSV was causing extreme susceptibility to secondary bacterial infection and disease and that we might recreate this susceptibility experimentally. Our experiments with in utero-infected piglets, subsequently challenged with S. suis type II, suggest that this hypothesis was correct.
MATERIALS AND METHODS
Animals.
PRRSV-free pregnant gilts were purchased from a geographically isolated herd that had not been vaccinated for PRRSV, was free of clinical signs of PRRS, and had remained seronegative for PRRSV for the past 2 years. Eight gilts were randomly assigned to PRRSV-infected versus noninfected control groups for each trial of the experiment. All gilts were allowed to farrow naturally. Piglets were colostrum deprived and removed from their dam at birth. They were fed a 50% mixture of frozen cow colostrum and artificial milk replacer for 24 h and milk replacer only thereafter. Piglets were weighed and placed individually into cages for housing and feeding as described previously (12). Protective clothing was changed between treatment groups.
Virus inoculum preparation and challenge.
The SD 23983 strain of PRRSV was used in this study (gift of David Benfield, South Dakota State University). The field isolate SD 23983 was selected because it resulted in a higher number of viremic piglets born and surviving during studies of viral persistence than did the PRRSV reference strain 2332 (3). Viral inoculum was prepared in cultures of MARC-145 cells, which is a PRRSV-permissive cell line subcloned from MA-104 cells (16). Confluent monolayers of MARC-145 cells were inoculated with plaque-purified virus. Infected cultures were frozen and thawed three times after 60 to 80% cytopathic effect was observed. Virus was titrated on MARC-145 cell culture and diluted to a final inoculation dose of 103.5 50% tissue culture infective doses. Control inoculum consisted of mock-infected MARC-145 cell cultures that were processed identically to virus inoculum preparations.
For each trial, five gilts were housed in isolation and infected intranasally (1 ml of virus solution per nares) at 98 days of gestation with 103.5 50% tissue culture infective doses of the SD 23983 strain of PRRSV. The other three gilts in each trial were inoculated with control inoculum. These animals were housed in a separate building and remained uninfected.
Experiment design.
Piglets of each litter were allocated into birth weight-matched pairs (either both PRRSV infected or both PRRSV free). A piglet from each pair was then randomly assigned to either S. suis-challenged or nonchallenged control treatments. Piglets from non-PRRSV-infected gilts were allotted to group 1 and group 2, and piglets from PRRSV-infected gilts were assigned to group 3 and group 4. The resulting treatment groups were group 1 (trial 1, 12 piglets; trial 2, 8 piglets), negative controls; group 2 (trial 1, 12 piglets; trial 2, 11 piglets), S. suis challenged only; group 3 (trial 1, nine piglets; trial 2, nine piglets), PRRSV infected only; and group 4 (trial 1, 11 piglets; trial 2, 11 piglets), PRRSV infected and S. suis challenged. Piglets were bled within 24 h after birth to confirm in utero PRRSV infection. Surviving piglets were bled and euthanatized at 12 days of age.
S. suis propagation and challenge.
S. suis type II strain MN 87555 (11) was used to challenge piglets intranasally. To ensure the virulence of this laboratory-propagated reference strain, we passaged MN 87555 through two 3-week-old piglets. Piglets were euthanatized once central nervous system signs were observed. Their brains were collected aseptically, and 40 ml of nutrient broth yeast extract was added, homogenized, filtered, and stored in 1-ml aliquots with 10% dimethyl sulfoxide at −80oC. For challenge inoculum, an aliquot was streaked onto a blood agar plate and allowed to grow at 37°C overnight. A single isolated colony was then used to inoculate nutrient broth yeast extract with 10% fetal calf serum and was grown for 48 h at 38°C. Cultures were centrifuged and resuspended in sterile phosphate-buffered saline to approximately 107.0 CFU/ml for each trial. Piglets were held in dorsal recumbency, and 1 ml of bacterial suspension was injected into nares at 5 days of age. Back titration of the challenge dose for the first trial was 4 × 107.0 CFU of S. suis MN 87555/ml and for the second trial was 6 × 106.0 CFU/ml for piglets. Piglets were returned following challenge to their individual cages for feeding and observation.
Clinical observation.
Temperatures were taken, and piglets were observed at 12-h intervals beginning the day of streptococcal challenge. Liquid diet intake was recorded for each feeding. Any piglets that did not consume 75% or more of the dispensed diet for four feedings in a row and were showing clinical signs of lameness, fever (>40°C), or central nervous system disease were euthanatized by injection with 1 ml of Beuthanasia solution (Schering-Plough Animal Health Corp., Kenilworth, N.J.). All remaining piglets were euthanatized at 7 days postchallenge.
Postmortem examination.
All piglets were necropsied, and tissues were examined microscopically by the same pathologist. Swabs for bacterial isolation were aseptically collected from the foramen magnum, submeninges, and fourth ventricle of the brains and from one elbow and one knee joint from each piglet. Selected tissues were fixed in 10% buffered neutral formalin for 24 to 48 h. Fixed tissues were dehydrated, embedded in paraffin, sectioned at 4 to 6 μm, and stained by hematoxylin-eosin.
Total and differential leukocyte counts.
Blood samples of 3-day-old piglets were collected in tubes containing EDTA. Total peripheral blood leukocytes were counted using an automated cell counter (SeronoBaker 9010+; Allentown, Pa.). Differential cell counts were performed on blood smears stained with Wright-Giemsa dyes.
Virus isolation.
Virus isolation from serum samples was performed as previously described (24), except that PAM cultures were used. Cultures not displaying cytopathic effect after two passages were considered negative. PRRSV-induced cytopathic effect on PAM cultures was confirmed by immunofluorescent staining using fluorescein-conjugated SDOW 17 monoclonal antibody (MAb) (a gift from E. Nelson, South Dakota State University) (19) against PRRSV nucleoprotein.
Bacterial isolation and identification.
Samples for bacterial isolation were plated onto Columbia agar with 5% sheep blood. S. suis was tentatively identified by colony morphology and hemolysis patterns. A representative colony was selected from both a brain isolate and a joint isolate from each culture-positive pig and tested using the API 20 Strep identification system (BioMerieux Vitek, Inc., Hazelwood, Mo.). The biochemical pattern for each isolate was compared to that of strain MN 87555.
Immunohistochemistry, histopathology, and immunohistopathology.
Immunohistochemistry was performed using a modification of a previously described technique (13) using the MAb SDOW 17 directed to the 15-kDa nucleocapsid protein of PRRSV (19). After removal of paraffin and dehydration, endogenous peroxidase was blocked by three 10-min changes of 3% hydrogen peroxide. Next, sections were washed in Tris-buffered saline (TBS; pH 7.4), digested by immersion in 0.1% protease XIV (Sigma Chemical Co., St. Louis, Mo.) for 10 min at 37°C, washed again in TBS, and blocked with 10% normal goat serum for 20 min at room temperature (RT). The sections were then incubated with primary SDOW 17 MAb (diluted 1:500 in TBS) overnight at 4oC in a humidified chamber. After primary antibody incubation and a subsequent wash in TBS, the slides were incubated with biotinylated goat anti-mouse antibody (Biogenex, San Ramon, Calif.) for 20 min at RT. The sections were washed in TBS and treated with streptavidin-labeled peroxidase for 20 min at RT. The sections were washed in TBS and incubated with chromogen for 2 min at RT. Sections were counterstained with hematoxylin. Immunohistochemical controls consisted of sections treated with normal mouse serum diluted in TBS instead of the primary antibody. Non-PRRSV-infected piglet tissues also served as negative controls.
Statistical analysis.
Statistical analysis was carried out using the Student t test or chi-square analysis on results combined from both trials to avoid any cells with expected values of <5.0.
RESULTS
Reproductive effects.
Many of the piglets from PRRSV-infected sows were born dead, 57% in trial 1 and 29% in trial 2 (Table 1). Most of the stillborn fetuses were autolytic as evidenced by extensive subcutaneous edema and gray-brown discoloration of the skin in the abdominal and thoracic regions. Some stillborn fetuses had areas of umbilical cord edema and hemorrhage. Piglets born live to PRRSV-infected sows were smaller than control piglets. They often had rough hair coats and occasional subcutaneous edema of eyelids or foreheads. Some of these piglets were weak at birth. PRRSV was isolated from serum collected the first day after birth from all but one of the piglets born live to PRRSV-infected sows (Table 2). That piglet was viremic on the third day of age. No PRRSV was isolated in sera collected from piglets born to PRRSV-free sows collected at birth, on the third day of life, or at the end of the experiment (12 days of age) during either trial.
TABLE 1.
Effect of in utero infection by PRRSV at 98 days of gestation on fetuses
| Trial no. | PRRSV status of sow | No. of sows | Total no. (%) of piglets
|
||
|---|---|---|---|---|---|
| Live born | Stillborn | Mummified fetuses | |||
| 1 | Infected | 5 | 25 (43) | 25 (43) | 8 (18) |
| Control | 3 | 25 (93) | 1 (4) | 1 (4) | |
| 2 | Infected | 5 | 24 (58) | 8 (24) | 2 (6) |
| Control | 3 | 22 (96) | 0 (0) | 1 (5) | |
TABLE 2.
Rates of piglet viremia, fever, and mortality observed following intranasal challenge with S. suis type II at approximately 5 days of age
| Trial no. | Treatment groupa | Rate of viremiab | Feverc | Mortality rated |
|---|---|---|---|---|
| 1 | 1 | 0/12 (0) | 0/12 (0) | 0/12 (0) |
| 2 | 0/12 (0) | 2/12 (17) | 1/12 (8) | |
| 3 | 9/9 (100) | 5/9 (56) | 0/9 (0) | |
| 4 | 10/11 (90.9) | 9/11 (82) | 10/11 (91) | |
| 2 | 1 | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 2 | 0/11 (0) | 3/11 (27) | 4/11 (36) | |
| 3 | 9/9 (100) | 4/9 (44) | 1/9 (11) | |
| 4 | 11/11 (100) | 5/11 (45) | 10/11 (91) |
Group 1, negative controls; group 2, S. suis challenged only; group 3, PRRSV infected only; group 4, PRRSV infected and S. suis challenged.
Number of piglets (percent) from which PRRSV was isolated over number of piglets in the group. Virus isolation was performed on serum samples collected on the day of birth.
Number of piglets (percent) from that group that were febrile (≥40°C). Piglets' temperatures were taken at 12-h intervals beginning on the day of S. suis challenge.
Number of piglets (percent) that had to be euthanatized or that died over number of piglets in the group.
Piglet clinical signs.
Mortality was extensive for the PRRSV- S. suis dual-infection group—four piglets in both trials (Table 2). Of the 22 total piglets in group 4, 20 piglets died after S. suis challenge (10 of 11 for both trials). Group 2 S. suis-challenge-only piglets had low mortality in trial 1 (1 of 12) and modest mortality in the second trial (4 of 11). Mortality was low in unchallenged PRRSV-infected piglets (zero of nine in trial 1 and one of nine in trial 2), and no piglet from the noninfected control group died. There was a significant difference in the mortality observed between the dual-infection group and other groups (chi-square = 51.97, P < 0.0001).
Clinical signs of bacterial disease before death after S. suis challenge for PRRSV-infected or non-PRRSV-infected piglets were similar. Piglets initially displayed rough hair coats, swollen leg joints, individual or multiple leg lameness, tremors, incoordination, labored breathing, circling, rear leg paresis or paralysis, loss of righting reflex, depression, lateral recumbency, nystagmus, opisthotonus, paddling, and convulsions. Anorexia was usually observed to begin only within hours of death. Many of the central nervous system signs were initially periodic and increased in duration and frequency as the clinical syndrome progressed. The onset of clinical signs varied greatly between treatment groups. Occurrence of clinical signs correlated closely with isolation of bacteria from joints and brain. There was a significant difference in the rates of isolation of strain MN 87555 of S. suis from brains or joints between the dual-infection group and other groups (chi-square = 45.50, P < 0.0001) (see Table 4).
TABLE 4.
Rates of isolation of S. suis bacteria from brain or joints of piglets challenged intranasally with S. suis type II strain MN 87555
| Trial no. | Treatment groupa | Brain isolatesb
|
Joint isolatesc
|
Strain MN 87555 by API Strep 20 from brain and/or jointsd | |||
|---|---|---|---|---|---|---|---|
| FM | Sbmng | 4th V | Elbow | Stifle | |||
| 1 | 1 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 (0) |
| 2 | 2/12 | 2/12 | 2/12 | 1/12 | 1/12 | 2/12 (17) | |
| 3 | 2/9 | 2/9 | 2/9 | 0/9 | 0/9 | 0/9 (0) | |
| 4 | 9/11 | 8/11 | 9/11 | 9/11 | 8/11 | 10/11 (91) | |
| 2 | 1 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 (0) |
| 2 | 5/11 | 5/11 | 5/11 | 4/11 | 3/11 | 4/11 (36) | |
| 3 | 0/9 | 1/9 | 1/9 | 0/9 | 0/9 | 0/9 (0) | |
| 4 | 8/11 | 7/11 | 7/11 | 7/11 | 6/11 | 8/11 (73) | |
Group 1, negative controls; group 2, S. suis challenged only; group 3, PRRSV infected only; group 4, PRRSV infected and S. suis challenged.
Swabs from foramen magnum (FM), submeninges (Sbmng), or fourth ventricle (4th V) were plated on blood agar. The numbers indicate the numbers of plates (piglets) per group with medium or high growth of streptococcus-type colonies over the total numbers of piglets in the treatment groups.
Swabs from one elbow and one stifle joint per pig were plated on the blood agar. The numbers indicate the numbers of plates (piglets) per group with medium or high growth of streptococcus-type colonies over the total numbers of piglets in the treatment groups.
A representative streptococcus-type colony was selected from both a brain swab plate and a joint swab plate for each pig that had medium or high growth of streptococcus-type colonies. The selected colonies were grown and tested using the API 20 Strep identification system according to the manufacturer's recommendations. The numbers indicate the numbers (percents) of piglets per group with the MN 87555 isolate of S. suis type II over the total numbers of piglets in the treatment groups.
The frequency of fever was highest in the combined-infection group 4 from trial 1 (Table 2). Of 11 piglets in this group, 9 had fever. The frequency of fever observed for this treatment group in trial 2 was lower (5 of 11). Piglets in group 4 showed either no fever or only one febrile observation before a rapid death in the second trial. Unchallenged PRRSV-infected group 3 piglets had the second highest rate of fever observed in both trials (five of nine in trial 1 and four of nine in trial 2). The S. suis-challenge-only groups had relatively low rates of fever observed in both trials, even though a modest rate of mortality was observed among these pigs in the second trial. The total number of temperature observations taken between treatments varied widely as a result of different times of death and different mortality rates experienced by different groups.
Virus isolation.
PRRSV was isolated from sera of all sows challenged with PRRSV and not from any control sows 7 days after challenge (data not shown). PRRSV was also isolated from all piglets in the PRRSV-only group (group 3) and nearly all in the PRRSV-S. suis dual-infection group (group 4) (Table 2). Virus was not isolated from one pig in group 4 at birth but was isolated at 3 days of age. PRRSV infection of these piglets was also confirmed by immunohistochemistry tests on lung tissues (data not shown). All piglets in the control or S. suis-only groups remained PRRSV negative throughout both trials (Table 2).
Effects of PRRSV on total leukocytes and differential leukocyte counts.
The PRRSV-infected piglets had a significant decrease in the number of peripheral blood leukocytes compared to that for non-PRRSV-infected piglets at 3 days after birth (P < 0.05 [Table 3]). There was significant lymphocytopenia and monocytopenia observed in the PRRSV-infected piglets compared to non-PRRSV-infected piglets (P < 0.05). There was no significant difference in the number of neutrophils observed between PRRSV-infected and non-PRRSV-infected piglets.
TABLE 3.
Total and differential peripheral blood leukocyte counts at 3 days of age from piglets infected in utero with PRRSV at 98 days of gestation and uninfected piglets
| Piglet treatment (n) | No. of cells (103/mm3 of blood)a
|
|||
|---|---|---|---|---|
| Total leukocytes | Lymphocytes | Monocytes | Neutrophils | |
| Infected (19) | 11.18 ± 3.73b | 1.83 ± 0.7b | 0.45 ± 0.23b | 8.53 ± 3.50 |
| Control (19) | 14.77 ± 6.25 | 3.02 ± 1.4 | 1.29 ± 0.58 | 10.13 ± 2.75 |
Data are expressed as mean cell numbers ± standard deviations.
Statistically significant (P < 0.05) compared with controls using the t test.
Bacterial isolation.
Streptococcus-like bacteria were isolated at high rates from the dual-infection group 4 piglets in both trials (Table 4). S. suis type II was recovered from 18 of 22 piglets in the S. suis-PRRSV dual-infection group, 6 of 23 piglets in the S. suis-only group, and none in either the PRRSV-only or the control group. Most of the colony types identified as having streptococcus-like morphology and hemolysis from both trials displayed a biochemical reaction pattern identical to that of S. suis type II strain MN 87555 when they were analyzed using the API Strep 20 system. This biochemical reaction pattern is expected to occur with only 1% of all S. suis isolates (API manual). In the first trial, nonstreptococcal bacteria were isolated from the brains of two piglets from the PRRSV-only infection group. In the second trial, moderate to high numbers of nonstreptococcal bacteria were isolated from three piglets in the dual-infection group. S. suis type II strain MN 87555 appeared to be isolated from the brains and joints of one group 2 piglet that died during the first trial and all four group 2 piglets that died during the second trial.
Lesions.
Microscopic lesions are summarized in Table 5. Lung lesions were observed in approximately half of piglets in the PRRSV-only and PRRSV-plus-S. suis treatment groups. The interstitial pneumonia was primarily characterized by septal thickening resulting from infiltration by lymphocytes and macrophages. Alveolar exudate, which consisted primarily of macrophages, necrotic debris, and occasional multinucleated cells, was also observed. There was type 2 pneumocyte hypertrophy and hyperplasia (Fig. 1A). The types and severities of pneumonia in PRRSV-only piglets and PRRSV-plus-S. suis piglets were similar. There were no lung lesions observed in either the control or the S. suis-only piglets.
TABLE 5.
Microscopic lesions observed in lung, brain, thymus, and middle iliac lymph nodea
| Treatment groupb | Interstitial pneumonia | Meningitis | Thymic involution | Polykaryocytes in lymph nodes |
|---|---|---|---|---|
| 1 | 0/20 | 0/20 | 0/20 | 0/20 |
| 2 | 0/23 | 2/23 | 0/23 | 0/20 |
| 3 | 10/18 | 6/18 | 15/18 | 8/18 |
| 4 | 11/22 | 15/22 | 19/22 | 13/22 |
Data are expressed as the numbers of piglets that microscopically had interstitial pneumonia, meningitis, thymic involution, or polykaryocytes over the total numbers of piglets in the treatment groups. The lesions listed in the table were scored microscopically as either present or absent.
Group 1, negative controls; group 2, S. suis challenged only; group 3, PRRSV infected only; group 4, PRRSV infected and S. suis challenged.
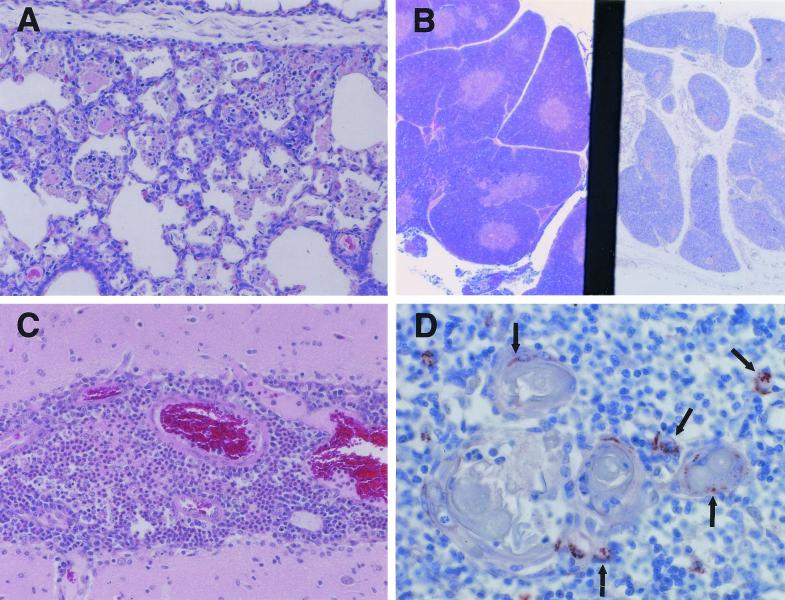
FIG. 1.
Pathology of the pig lung, thymus, and brain. (A) Interstitial pneumonia in piglet (12 days old) infected in utero with PRRSV (hematoxylin and eosin). Magnification, ×150. (B) Thymus: right section from piglet infected in utero with PRRSV 12 days after birth, showing cortical involution and loss of corticomedullary definition; left section from age-matched control piglet, showing normal structure of thymus (hematoxylin and eosin stain). Magnification, ×70. (C) Meningitis in a piglet infected in utero with PRRSV and subsequently challenged with S. suis 5 days after birth (hematoxylin and eosin stain). Magnification, ×150. (D) Thymus cells positive for PRRSV antigen by immunohistochemistry using SDOW 17 MAb. Arrows indicate some of the PRRSV-positive cells. Magnification, ×300.
The thymus was also severely affected in PRRSV-infected animals. Histologically, PRRSV-infected piglets had thymic lesions of cortical involution, causing a poor demarcation between the cortical and medullary zones (Fig. 1B). Nearly complete disappearance of cortical thymocytes was observed in some PRRSV-infected piglets. The acellularity of the thymus was reflected in a 52% reduction in thymic weights. The reduced cellularity of the thymus also caused a significant (P < 0.05, t test) shift of thymus/body weight ratios from 1.277 ± 0.503 (n = 15, trial 2) in control animals to 0.778 ± 0.453 (n = 9, trial 2) in PRRSV-infected animals. PRRSV antigen was consistently detected in thymic medullar areas of PRRSV-infected piglets by immunohistochemistry (Fig. 1D) and less often in thymic cortical areas. PRRSV antigen was also identified in interlobular regions. The positive cells probably were macrophages or dendritic cells. No thymic lesions were observed in piglets of either the control or the S. suis-only group.
Lymph node lesions were similar in all PRRSV-infected piglets. The lesions were found in multiple sites and were characterized by germinal center hypertrophy and hyperplasia, lymphocyte necrosis and apoptosis, and numerous polykaryocytes. Severe lymphocyte depletion was observed in splenic periarterial lymphoid sheaths in PRRSV-infected pigs. These lesions were not observed in piglets of the control or S. suis group.
Meningitis was occasionally observed in piglets in the S. suis-only group (2 out of 23). Six of 18 piglets in the PRRSV-only group and 15 of 22 piglets in the PRRSV-plus-S. suis group had meningitis. The meningitis observed in piglets in the PRRSV-plus-S. suis group was much more severe than that in piglets in S. suis-only and PRRSV-only groups. The meningitis in the piglets in the PRRSV-plus-S. suis group was characterized by a marked meningeal infiltration of macrophages, lymphocytes, and neutrophils (Fig. 1C). Lesions were minimal and randomly scattered in the other tissues collected and evaluated.
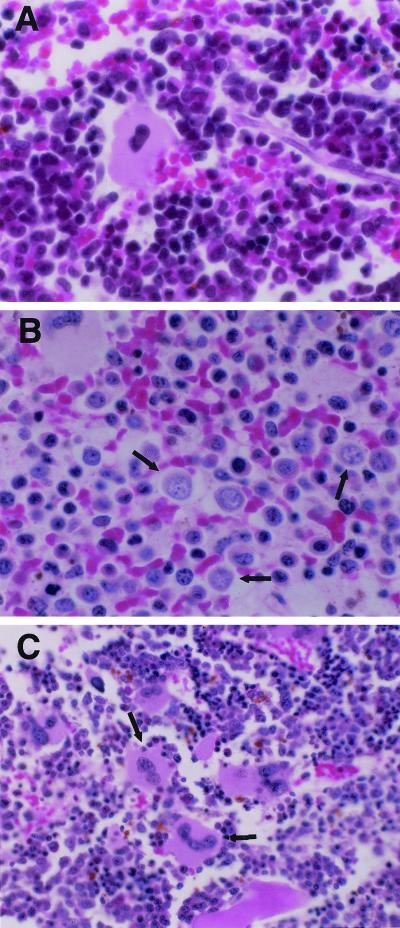
The bone marrow from 15 piglets infected with PRRSV only was examined. In 11 of the piglets, there was mild to severe bone marrow hypoplasia characterized by an absence of normal myeloid and erythroid precursors (Fig. 2). The hypoplastic marrow spaces were focally to diffusely filled with loosely arranged small to medium-sized mononuclear cells with round, vesicular nuclei and moderate amounts of cytoplasm (Fig. 2B). Megakaryocytes were numerous (Fig. 2C). In some marrow spaces, small islands of nucleated erythrocyte precursors were scattered among the regenerating marrow progenitor cells. These were not observed in uninfected piglets.
FIG. 2.
Pathology of the pig bone marrow (hematoxylin and eosin stain). (A) Bone marrow from a noninfected control piglet (12 days old), showing the normal structure of bone marrow. Magnification, ×600. (B) Bone marrow from a PRRSV-infected piglet (12 days old), showing the reduced cellularity and increased number of mononuclear cells (arrows). Magnification, ×600. (C) Bone marrow from a PRRSV-infected piglet (12 days old), showing increased numbers of megakaryocytes (arrows). Magnification, ×396.
DISCUSSION
In this report, we have attempted to develop a model that recreates the immunosuppression and increased susceptibility to bacterial infection commonly observed in PRRSV-infected pigs. Piglets infected in utero with PRRSV and challenged with S. suis type II at 5 days of age displayed significantly higher rates of mortality and infections of joints and brains than did piglets in either the bacterium-only or the virus-only group. The experiment was repeated, with similar results each time. Our findings indicate that late-gestation infection of pig fetuses by PRRSV causes immune system lesions in surviving newborn piglets that may enhance piglet susceptibility to microbial infections by agents such as S. suis.
In our study, we found an extraordinary level of mortality following infection with S. suis (91%) compared to the 50% mortality rates reported by Galina et al. (11), who also used strain MN 87555 for bacterial challenge. In a third study, Cooper et al. (7) failed to show any increased susceptibility to S. suis in PRRSV-infected piglets. A different strain of PRRSV was used in each of these studies, which may account for the different results. We used a strain of PRRSV from South Dakota which was selected because it caused high rates of in utero infection and birth of viremic piglets that would survive past weaning. Galina et al. (11) used strain ATCC VR 2332, and Cooper et al. (7) used strain NEB-1. Alternatively, the extreme susceptibility to S. suis that we have observed may stem from the age of the pig at the time of PRRSV infection and the route of infection. We infected sows late in pregnancy, and virtually 100% of their piglets were born PRRSV infected. In contrast, Galina et al. (11) and Cooper et al. (7) infected pigs intranasally at 3 to 4 weeks of age, following weaning. It is possible that the early delivery of PRRSV to the fetal pig results in more profound effects on the immune system and that this results in extreme susceptibility to S. suis. Further studies will be necessary to discriminate between these possibilities.
Whatever the reason for the elevated susceptibility, our data strongly support the hypothesis that PRRSV enhances susceptibility to secondary bacterial infection by negatively affecting the immune system. When pigs were necropsied, peripheral lymph node hypertrophy, hyperplasia, necrosis, and apoptosis were all observed. These lesions were similar to those induced by strain ATCC VR 2332 in 1-, 4-, or 10-week-old pigs (24). However, we also observed significant changes in the thymus and bone marrow which were not seen in pigs infected after weaning with strain ATCC VR 2332 (23, 24). Most striking were the effects on the thymus, which was almost completely depleted of thymocytes and significantly decreased in size and weight. The exact mechanism underlying these changes is not known. One possibility is that the ORF 5 of PRRSV caused thymocyte depletion by inducing apoptosis, an effect which has been demonstrated previously in vitro (28). Alternatively, depletion of thymocytes may occur through an indirect mechanism. Infected thymic macrophages and dendritic cells could secrete proapoptotic cytokines or deliver improper apoptotic signals to thymocytes undergoing selection for self-recognition. Stress can also cause thymic involution (1), and it is possible that the thymic involution observed in these animals was caused by early separation from their sows. This is not likely, however, as the control groups were also separated from their sows at birth. It is also possible that infection with another, inapparent organism resulted in thymic involution. We cannot rule out this possibility, since the animals were not housed under gnotobiotic conditions. Lastly, the thymic involution that we noted may stem from changes in patterns of lymphocyte trafficking. Effects on the bone marrow were noted, and it is possible that PRRSV-infected animals fail to produce a sufficient number of prethymocytes or that the prethymocytes which are produced fail to properly target themselves to the thymus. We did note a decreased number of lymphocytes in the blood and an increase in the size of peripheral lymph nodes, suggesting that levels and patterns of lymphocyte trafficking may be abnormal.
The precise immune system lesion which causes increased susceptibility to S. suis infection is also not known. The palatine tonsil has been suggested as the portal of entry for S. suis (33). Evidence suggests that the ability of macrophages to kill S. suis is critical for resistance to disease (32). Therefore, piglets may be more susceptible to S. suis type II infection from the environment if the activity of their tonsillar macrophages is inhibited by PRRSV infection. This hypothesis is supported by several studies which show a loss of bactericidal function in pulmonary intravascular macrophages and PAM following PRRSV infection (25, 30). Systemic infection and incapacitation of macrophages may also account for the rapid spread of S. suis throughout the body.
Vertical infection of piglets by PRRSV, as recreated within the present study, may play a significant role in the respiratory virus-bacterial disease complex seen in nursery-aged pigs during acute PRRS outbreaks or circulation of the virus among susceptible subpopulations of pregnant sows in infected herds. This hypothesis is supported by reports (8, 15) that total depopulation of the nursery was unable to eliminate the endemic respiratory disease complex in PRRSV-infected herds until after virus circulation had stopped among sows in the herd. Ending virus circulation among pregnant sows would likely prevent in utero PRRSV infection of piglets and their subsequent increased susceptibility to secondary bacterial infections during lactation or after weaning.
In summary, our observations suggest that in utero infection with PRRSV negatively affects the piglet immune system, increasing susceptibility to secondary bacterial infection. Further characterization of events that occur during in utero PRRSV infection of piglets will be necessary for us to understand the mechanisms causing enhanced susceptibility to S. suis under laboratory and natural conditions.
ACKNOWLEDGMENTS
We thank Jack Britt, Carlos Pijoan, Gail Brown, and Sandi Amass for their assistance in this study.
This study was funded by the North Carolina Pork Producers Association, the College of Veterinary Medicine, and the College of Agriculture and Life Sciences, North Carolina State University.
REFERENCES
- 1.Andrew J T, Ritter M A. Thymic involution with ageing: obsolescence or good housekeeping. Immunol Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 2.Benfield D A, Nelson E, Collins J E, Harris L, Hennings J C, Shaw D P, Goyal S M, McCullough S, Morrison R B, Joo H S, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J Vet Diagn Investig. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- 3.Benfield D A, Christopher-Hennings J, Nelson E A, Rowland R R, Nelson J K, Chase C L, Rossow K D, Collins J E. Proceedings of 28th Annual Meeting of American Association of Swine Practitioners 1997. 1997. Persistent fetal infection of porcine reproductive and respiratory syndrome (PRRS) virus; pp. 455–467. [Google Scholar]
- 4.Carvalho L F O S, Segales J, Pijoan C. Effect of porcine reproductive and respiratory syndrome virus on subsequent Pasteurella multocida challenge in pigs. Vet Microbiol. 1997;55:241–246. doi: 10.1016/S0378-1135(96)01324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanagh D. Nidovirales: a new order comprising coronaviridae and arterividae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 6.Christianson W T, Choi C S, Collins J E, Molitor T W, Morrison R B, Joo H S. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid-gestation sows and fetuses. Can J Vet Res. 1993;57:262–268. [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper V L, Doster A R, Hesse R A, Harris N B. Porcine reproductive and respiratory syndrome: NEB-1 PRRSV infection did not potentiate bacterial pathogens. J Vet Diagn Investig. 1995;7:313–320. doi: 10.1177/104063879500700303. [DOI] [PubMed] [Google Scholar]
- 8.Dee S A, Morrison R B, Joo H. Eradicating porcine reproductive and respiratory syndrome (PRRS) virus using two-site production and nursery depopulation. Swine Health Product. 1993;1:20–23. [Google Scholar]
- 9.Dial G D, Hull R D, Olson C L, Hill H T, Erickson G A. Proceedings of Mystery Swine Disease Committee Meeting. 1990. Executive summary, mystery swine disease: implications and needs of the North American swine industry; pp. 3–6. [Google Scholar]
- 10.Done S H, Paton D J. Porcine reproductive and respiratory syndrome: clinical disease, pathology and immunosuppression. Vet Rec. 1995;136:32–35. doi: 10.1136/vr.136.2.32. [DOI] [PubMed] [Google Scholar]
- 11.Galina L, Pijoan C, Sitjar M, Christianson W T, Rossow K, Collins J E. Interaction between Streptococcus suis serotype 2 and porcine reproductive and respiratory syndrome virus in specific pathogen-free piglets. Vet Rec. 1994;134:60–64. doi: 10.1136/vr.134.3.60. [DOI] [PubMed] [Google Scholar]
- 12.Gomez G G, Sandler R S, Seal E. High levels of inorganic sulfate cause diarrhea in neonatal piglets. J Nutr. 1995;125:2325–2332. doi: 10.1093/jn/125.9.2325. [DOI] [PubMed] [Google Scholar]
- 13.Halbur P G, Andrews J J, Huffman E L, Paul P S, Meng X J, Niyo Y. Development of a streptavidin-biotin immunoperoxidase procedure for the detection of porcine reproductive and respiratory syndrome virus antigen in porcine lung. J Vet Diagn Investig. 1994;6:254–257. doi: 10.1177/104063879400600219. [DOI] [PubMed] [Google Scholar]
- 14.Halbur P G, Miller L D, Paul P S, Meng X J, Huffman E L, Andrews J J. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of three-week-old colostrum-deprived pigs. Vet Pathol. 1995;32:200–204. doi: 10.1177/030098589503200218. [DOI] [PubMed] [Google Scholar]
- 15.Keffaber K, Stevenson G, Van Alstine W, Kanitz C, Harris L, Gorcyca D, Schlesinger K, Schults R, Chladek D, Morrison R. SIRS virus infection in nursery/grower pigs. Am Assoc Swine Pract Newslett. 1992;4:38–40. [Google Scholar]
- 16.Kim H S, Kwang J, Yoon I J. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in homogenous subpopulation of MA-104 cell line. Arch Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- 17.Meulenberg J J M, Hulst M M, De Meijer E J, Moonen P L J M, DenBesten A, De Kluyver E P, Wensvoort G, Moormana R J M. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molitor T W, Bautista E M, Choi C S. Immunity to PRRSV: double-edged sword. Vet Microbiol. 1997;55:265–276. doi: 10.1016/S0378-1135(96)01327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson E A, Christopher-Hennings J, Drew T, Wensvoort G, Collins J E, Benfield L T. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1995;34:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plagemann P G. Lactate dehydrogenase-elevating virus and related viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1150–1120. [Google Scholar]
- 21.Pol J A M, van Leengoed L A M G, Stockhofe N, Kok G, Wensvoort G. Dual infections of PRRSV/influenza or PRRSV/Actinobacillus pleuropneumoniae in the respiratory tract. Vet Microbiol. 1997;55:259–264. doi: 10.1016/s0378-1135(96)01323-5. [DOI] [PubMed] [Google Scholar]
- 22.Reeth K V, Nauwynck H, Pensaert M. Dual infection of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossow K D, Bautista E M, Goyal S M, Molitor T W, Murtaugh M P, Morrison R B, Benfield D A, Collins J E. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs. J Vet Diagn Investig. 1994;6:3–12. doi: 10.1177/104063879400600102. [DOI] [PubMed] [Google Scholar]
- 24.Rossow K D, Collins J E, Goyal S M, Nelson E A, Christopher-Hennings J, Benfield D A. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in gnotobiotic pigs. Vet Pathol. 1995;32:361–373. doi: 10.1177/030098589503200404. [DOI] [PubMed] [Google Scholar]
- 25.Solano G I, Bautista E, Molitor T W, Segales J, Pijoan C. Effect of porcine reproductive and respiratory syndrome virus infection on the clearance of Haemophilus parasuis by porcine alveolar macrophages. Can J Vet Res. 1998;62:251–256. [PMC free article] [PubMed] [Google Scholar]
- 26.Solano G I, Segales J, Colling J E, Molitor T W, Pijoan C. Porcine reproductive and respiratory syndrome virus (PRRSV) interaction with Haemophilus parasuis. Vet Microbiol. 1997;55:247–257. doi: 10.1016/S0378-1135(96)01325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson G W, VanAlstine W G, Kanitz C L, Keffaber K K. Endemic porcine reproductive and respiratory syndrome virus infection of nursery pigs in two swine herds without concurrent reproductive failure. J Vet Diagn Investig. 1993;5:432–434. doi: 10.1177/104063879300500322. [DOI] [PubMed] [Google Scholar]
- 28.Suarez P, Diaz-Guerra M, Prieto C, Esteban M, Castro J M, Nieto A, Ortin J. Open reading frame 5 of porcine reproductive and respiratory syndrome virus as a cause of virus-induced apoptosis. J Virol. 1996;70:2876–2882. doi: 10.1128/jvi.70.5.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpstra C, Wensvoort G, Pol J M A. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch's postulates fulfilled. Vet Q. 1991;13:131–136. doi: 10.1080/01652176.1991.9694297. [DOI] [PubMed] [Google Scholar]
- 30.Thanawongnuwech R, Thacker E L, Halbur P G. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) (isolate ATCC VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): in vitro comparisons with pulmonary alveolar macrophages (PAMs) Vet Immunol Immunopathol. 1997;59:323–335. doi: 10.1016/s0165-2427(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 31.Wensvoort G, Terpstra C, Pol J M A, Ter Lack E A, Bloemaraad M, de Kluyver E P, Kragten C, van Buiten L, den Besten A, Wagenaar F, Broekhuijsen J M, Moonen P L J M, Zetstra T, de Boer E A, Tibben H J, de Jong M F, van't Veld P, Groenland G J M, van Gennep J A, Voetv M T H, Verheijden J H M, Braamskamp J. Mystery swine disease in the Netherlands: isolation of Lelystad virus. Vet Q. 1991;3:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 32.Williams A E, Blakemore W F, Alexander T J L. Streptococcus suis type 2: virulence factors, macrophages and immunity. Proc Congr Int Pig Vet Soc. 1990;11:172. [Google Scholar]
- 33.Williams D M, Lawson G H K, Rowland A C. Streptococcal infection in piglets: the palatine tonsils as portals of entry for Streptococcus suis. Res Vet Sci. 1973;15:352–362. [PubMed] [Google Scholar]
- 34.Wills R W, Gray J T, Fedorka-Cray P J, Yoo K-J, Ladely S, Zimmerman J J. Synergism between porcine reproductive and respiratory syndrome virus (PRRSV) and Salmonella choleraesuis in swine. Vet Microbiol. 2000;71:177–192. doi: 10.1016/S0378-1135(99)00175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]