Abstract
Aims
We report the results of a real‐world study based on heart failure (HF) patients' continuous remote monitoring strategy using the CardioMEMS system to assess the impact of this device on healthcare outcomes, costs, and patients' management and quality of life.
Methods and results
We enrolled seven patients (69.00 ± 4.88 years; 71.43% men) with HF, implanted with CardioMEMS, and daily remote monitored to optimize both tailored adjustments of home therapy and/or hospital infusions of levosimendan. We recorded clinical, pharmacological, biochemical, and echocardiographic parameters and data on hospitalizations, emergency room access, visits, and costs. Following the implantation of CardioMEMS, we observed a 50% reduction in the total number of hospitalizations and a 68.7% reduction in the number of days in the hospital. Accordingly, improved patient quality of life was recorded with EQ‐5D (pre 58.57 ± 10.29 vs. 1 year post 84.29 ± 19.02, P = 0.008). Echocardiographic data show a statistically significant improvement in both systolic pulmonary artery pressure (47.86 ± 8.67 vs. 35.14 ± 9.34, P = 0.022) and E/e′ (19.33 ± 5.04 vs. 12.58 ± 3.53, P = 0.023). The Quantikine® HS High‐Sensitivity Kit determined elevated interleukin‐6 values at enrolment in all patients, with a statistically significant reduction after 6 months (P = 0.0211). From an economic point of view, the net savings, including the cost of CardioMEMS, were on average €1580 per patient during the entire period of observation, while the analysis performed 12 months after the implant vs. 12 months before showed a net saving of €860 per patient. The ad hoc analysis performed on the levosimendan infusions resulted in 315 days of hospital avoidance and a saving of €205 158 for the seven patients enrolled during the observation period.
Conclusions
This innovative strategy prevents unplanned access to the hospital and contributes to the efficient use of healthcare facilities, human resources, and costs.
Keywords: Heart failure, CardioMEMS, Telemonitoring, Levosimendan
Introduction
Heart failure (HF) is characterized by structural and/or functional cardiac abnormalities, resulting in reduced cardiac output and/or elevated intracardiac pressures at rest or during stress. 1 Specifically, the disease exhibits exacerbation phases and periods of clinical stability. 2 Precisely, worsening HF is considered a phase in the natural history of the disease that marks its progression, and it portends a worse prognosis. 3 The steady increase in HF prevalence worldwide and the aging population are associated with significant mortality, morbidity, and healthcare expenditures. The pressure on healthcare payers to reduce hospitalizations will continue unabated. 4 , 5 , 6 , 7 Exactly, HF affects more than 64 million people worldwide. The incidence of HF is estimated to be generally 1–20 cases per 1000 person‐years or 1000 population, and several studies report that HF incidence significantly increases with age. The prevalence of HF ranges between 1% and 3% in the general adult population in industrialized countries, with the annual healthcare costs per person with HF amounting to €25 000. 8
The cost of HF management is projected to increase markedly: a 2.5‐fold increase from US$20.9 billion in 2012 to US$53.1 billion by 2030. Of note, 80% of the costs are related to HF hospitalizations. The total cost, including indirect costs, is estimated to increase from US$31 billion in 2012 to US$70 billion by 2030. The estimated average cost for persons with HF during the final 2 years of life is more than US$156 000, and 75% of this cost is attributed to HF‐related hospital admissions during the last 6 months of life. 9 , 10
In 2012, the national cost of HF was estimated to have surpassed US$4.5 billion (approximately €3.5 billion) in Germany, France, and the UK, and more than US$1 billion (about €781 million) in Italy, Spain, and Belgium. 11
Maggioni et al. 12 analysed data from the ARNO Observatory, including inhabitants of five Local Health Units of the Italian National Health Service (INHS); precisely, patients were selected when discharged for HF (1 January 2008 to 31 December 2012) and prescribed at least one HF treatment. The results of this study showed that the direct cost per patient per year to the INHS was €11 867 (€7426 if the first hospitalization was excluded). Analysing the components of this expenditure during the 1 year follow‐up (FU), the main cost could be ascribed to hospitalizations (76%; €5621), followed by drug prescriptions (16%; €1177) and specialist examinations/diagnostic procedures (8.5%; €629).
During the last few years, the management of HF has made significant progress, focusing on pharmacological 13 , 14 , 15 and device‐based therapies 16 to meet the challenges of this complex syndrome. 17 In particular, the CardioMEMS (Abbott Medical, Inc., Abbott Park, IL, USA) is an implantable device positioned in the pulmonary artery (PA) able to detect higher cardiac filling pressures, an objective measure of ‘haemodynamic congestion’, estimated to rise more than 2 weeks before the onset of symptomatic clinical congestion, 18 , 19 as demonstrated by the CHAMPION trial, 20 which clearly showed a benefit of the pressure‐guided therapy, regardless of left ventricular ejection fraction (LVEF).
According to the European conformity (CE) mark, the device is indicated for patients with chronic HF in functional New York Heart Association (NYHA) class III with at least one admission for HF in the past 12 months. 20 Specifically, PA pressure (PAP) monitoring through the CardioMEMS system aims to prevent the worsening of HF by optimizing medical therapy, reducing HF hospitalizations, and improving health outcomes such as quality‐adjusted life years (QALYs). 5 , 21 , 22 Mainly, the haemodynamic data reduce hospitalizations by modulating the dose of diuretics 20 to identify the patient before the onset of symptoms and exacerbation of the disease.
In this article, we report the results of a real‐world study based on an HF patient's continuous remote monitoring strategy using the CardioMEMS system. Specifically, this study aims to assess the impact of the CardioMEMS system on healthcare outcomes, costs, and patients' management and quality of life. Ad hoc analysis was performed on optimizing pharmaceutical treatment, including levosimendan infusions.
Materials and methods
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee (protocol code SJM‐CIP‐10147 CRD_879 of 23 May 2018); moreover, informed consent was obtained from all participants, and all investigations were carried out according to the principles of the Helsinki Declaration.
Study design
The implants were performed in our department between December 2019 and December 2021. The last patient FU was on 31 January 2023.
Inclusion criteria were age > 18 years old; diagnosis of HF and HF therapy at the maximum tolerated dose, according to the indications of the European Society of Cardiology (ESC) 2021 guidelines 1 ; NYHA class III; at least one hospitalization for HF in the previous 12 months on optimal medical therapy; body mass index (BMI) < 35 kg/m2 or a measurement of the chest circumference (at the armpit level) of <65 cm; PA diameter > 7 mm; regular attendance for periodic outpatient FU and optimal compliance with the proposed pharmacological treatment; availability of baseline anamnestic, anthropometric, clinical, laboratory, and instrumental data; and possibility of their acquisition at FU.
Exclusion criteria were active infection; recurrent history of pulmonary embolism or deep vein thrombosis; inability to tolerate right heart catheterization; history of major cardiovascular events within 2 months before enrolment; cardiac resynchronization therapy device implanted within 3 months before the enrolment; estimated glomerular filtration rate (eGFR) < 25 mL/min (obtained within 2 weeks of the FU visit) and unresponsive to diuretic therapy or chronic dialysis; congenital heart disease or right heart mechanical valves; heart transplant or ventricular assist device (VAD) implant planned within 6 months of the FU visit; coagulation disorders; and hypersensitivity or allergy to aspirin and/or clopidogrel (not applicable to subjects who are on anticoagulant therapy).
Patients with the previous criteria underwent CardioMEMS implantation and were monitored daily to optimize home therapy and/or levosimendan infusions. After the CardioMEMS implantation, the treating cardiologists, with daily reports, monitored the patient. Specifically, if the cardiologist detected a tendency for diastolic PAP to rise (in three consecutive readings), patients were contacted for home therapeutic changes to lower diastolic PAP to the patient‐specific goal [guideline‐directed medical therapy (GDMT) optimization and/or increasing the diuretic dosage].
The typical daily initial furosemide dose ranges between 50 and 250 mg prior to the device. After CardioMEMS implantation, the patients restarted the previous diuretic dose, which was titrated based on the recorded PAP. Usually, furosemide was increased by 25 mg at a time if an increasing trend in pulmonary pressures was noted or reduced by 25 mg if a decreasing trend was noted.
If no further changes were possible, the suitable patients [only HF with reduced ejection fraction (HFrEF)] were hospitalized for a 24 h levosimendan infusion (0.1 μg/kg/min without a bolus), a calcium sensitizer that exerts its positive inotropic action by increasing the calcium sensitivity of myocytes without an increase in intracellular calcium by binding to cardiac troponin C. By precisely monitoring the parameters of each patient, it was possible to avoid the fixed periodic administration of levosimendan and modulate it based on the measured parameters. The cost of a vial of this drug at our hospital goes from €670.00 (until April 2021) to €298.00 (from May 2021); the infusion takes place during a hospitalization lasting an average of 3 days. The average hospital length of stay of 3 days per patient, resulting from retrospective data on patients under levosimendan treatment, was considered. Usually, the patient is hospitalized for blood testing on Day 1, then a levosimendan infusion is administered on Day 2, and on Day 3, the patient is discharged.
Clinical and economic data have been gathered for each enrolled patient to estimate the CardioMEMS system's clinical impact on chronic HF patients' management.
Clinical data
At the baseline visit, patients' medical histories were gathered using patient and medical documentation to identify the causal factors of HF. Cardiovascular risk factors were recorded, including family history, arterial hypertension, dyslipidaemia, smoking, and other comorbidities [diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic renal failure, dysthyroidism, etc.]. Additionally, the pharmacological history was gathered. The interview was then completed with pathological anamnesis (symptoms, functional capacity, and NYHA class). Subsequently, the fundamental anthropometric parameters (weight, height, and BMI) were evaluated, a 12‐lead electrocardiogram (ECG) was performed, blood pressure values were measured in a sitting position, and a complete physical examination was performed. The health‐related quality of life (HRQoL) was assessed using the Italian adaptation of the EuroQol Group. 23 Moreover, the MAGGIC (Meta‐analysis Global Group in Chronic Heart Failure) score was calculated for each patient; precisely, this score estimates 1 and 3 year all‐cause mortality in persons with HF. 24
Echocardiographic measurements
Echocardiographic examinations were performed with a 3.5 MHz monoplane ultrasound probe from Vivid E‐9 (GE Vingmed Ultrasound, Horten, Norway), according to international guidelines. 25 Specifically, the patients underwent serial echocardiographic examinations, even during hospitalizations; however, we report baseline and 1‐year‐FU data. All parameters were analysed offline by two expert operators blinded to clinical data. LVEF was calculated by the Simpson biplane method according to the following formula: LVEF = [left ventricular end‐diastolic volume (LVEDV) − left ventricular end‐systolic volume (LVESV)]/LVEDV × 100 as the mean of two measures in four and two apical chambers. Mitral E and A velocities, E/A ratio, tissue Doppler analysis of mitral annular E′ velocity, and mitral E/e′ ratio were recorded.
Moreover, the diameter of the inferior vena cava and its respiratory variation were measured in the subcostal view and used to estimate right atrial pressure; consequently, peak systolic PAP (sPAP) was estimated by adding right atrial pressure to the systolic tricuspid regurgitation gradient. Right ventricular (RV) systolic parameters were also evaluated and assessed by calculating the tricuspid annulus plane systolic excursion (TAPSE) and tricuspid annular S′ velocity (RVs′), measured using pulsed‐wave tissue Doppler. RV function was assessed using an off‐axis apical four‐chamber view for better visualization of RV.
Blood samples
Furthermore, at the time of enrolment, at the 1 year FU, and at each hospitalization, the same standard blood chemistry parameters were evaluated [glycaemia, aspartate aminotransferase (AST), alanine aminotransferase (ALT), low‐density lipoprotein (LDL), haemoglobin, uric acid, creatinine, urea nitrogen, and eGFR]. In cases of hospitalization, blood sampling was performed before and at the end of the levosimendan infusion. In addition to standard blood chemistry tubes, 5–8 mL of whole blood was collected from each patient (at implantation and after 6 months), placed into ethylenediaminetetraacetic acid (EDTA) tubes, and centrifuged at 1000 g for 20 min at 25°C to separate cells and plasma. After collection, plasma samples were aliquoted and stored at −80°C until analysis. Interleukin‐6 (IL‐6) levels were duplicated by an enzyme‐linked immunosorbent assay (microplates coated with a mouse monoclonal antibody against IL‐6; Quantikine® HS High‐Sensitivity Kit, R&D Systems, USA). Results are reported as the mean of duplicate samples in pg/mL ± SD, following manufacturer guidelines (within‐assay coefficient of variance = 6.9–7.4%; between‐assay coefficient of variance = 9.6–6.5%; sensitivity = 0.016–0.110 pg/mL; mean = 0.039 pg/mL).
Resource consumption and cost estimation
The healthcare resource consumptions (hospitalizations, emergency room access, visits, and pharmacological therapies) were collected, and a costing method was used to evaluate the economic and organizational impact of the remote monitoring strategy. To assess the benefit of CardioMEMS, we compared pre‐ and post‐CardioMEMS implantation data at the patient level. We included in the analysis the same number of months pre‐ and post‐implantation, meaning that the observation period can be different from patient to patient; precisely, patient data refer to a period of at least 12 months after the implant, apart from Patient 3, as the diagnosis of HF was made 5 months before the CardioMEMS implant without any data available before, consequently only 5 months after the CardioMEMS implant has been considered for this patient. Specifically, the following data were collected during the pre‐ and post‐CardioMEMS implantation: number of hospitalizations (including length of stay, diagnosis, and procedures and high‐cost drugs, e.g., levosimendan), number of emergency room access, number of in‐hospital visits, and number of therapy adjustments. The cost of the above healthcare resources was defined by using reimbursement tariffs for hospitalizations and outpatients for specialistic visits and examinations of our region's welfare (Decree No. 32/2013). The cost of emergency room access was not taken into consideration.
Considering the levosimendan, the actual number of vials used has been compared with a hypothetical scenario in which the levosimendan infusions have occurred every 21 days, assuming an average time between the LION‐HEART study strategy (infusions every 15 days) 26 and the Ortis et al. (infusions every 28 days). 27 The cost for the levosimendan infusion hospitalization was obtained by summing the price of the drug paid by the hospital to the hospital stay cost.
Statistical analysis
Data are expressed as frequencies and percentages for qualitative variables, as mean ± SD for quantitative ones, and paired t‐test calculated statistical significance between means. For all analyses, a P‐value < 0.05 was considered statistically significant.
SPSS software for Windows Version 22.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The graphs were processed with GraphPad Prism 6.
Results
Patient characteristics
A total of seven patients (69.00 ± 4.88 years; 71.43% men) underwent a CardioMEMS implantation at our departmental HF clinic. The baseline characteristics of the study population are reported in Tables 1 and 2 . Specifically, six patients had HFrEF, while one had preserved LVEF (HFpEF).
Table 1.
Baseline characteristics of the enrolled patients
| ID | Gender | CRT | H–H distance (km) | Cost analysis observation period (months pre‐ and post‐device) | Age (years) | BMI (kg/m2) | LVEF (%) | sPAP (mmHg) | BNP (pg/mL) | eGFR (mL/min) | EQ 5‐D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Yes | 70.1 | 56 (28 pre + 28 post) | 70 | 34.02 | 32 | 45 | 620 | 25.3 | 50 |
| 2 | F | No | 5.5 | 72 (36 pre + 36 post) | 78 | 25.64 | 23 | 45 | 271 | 27 | 60 |
| 3 | M | No | 2.2 | 10 (5 pre + 5 post) | 60 | 26.45 | 22 | 56 | 743 | 49.1 | 50 |
| 4 | F | No | 98.9 | 24 (12 pre + 12 post) | 84 | 25.78 | 55 | 35 | 473 | 45.6 | 60 |
| 5 | M | Yes | 14.7 | 44 (22 pre + 22 post) | 82 | 24.84 | 20 | 45 | 1531 | 52.8 | 55 |
| 6 | M | Yes | 278 | 36 (18 pre + 18 post) | 58 | 27.18 | 20 | 58 | 588 | 49.7 | 55 |
| 7 | M | No | 9.3 | 26 (13 pre + 13 post) | 51 | 33.63 | 33 | 32 | 278 | 113.4 | 80 |
BMI, body mass index; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; F, female; H–H, home–hospital; LVEF, left ventricular ejection fraction; M, male; sPAP, systolic pulmonary artery pressure.
Table 2.
Clinical parameters, medical history, and pharmacological therapy at enrolment
| Variables | Baseline | |
|---|---|---|
| N | % | |
| Men | 5 | 71.43 |
| CAD | 4 | 57.14 |
| Hypertension | 7 | 100.00 |
| Diabetes | 3 | 42.86 |
| Dyslipidaemia | 7 | 100.00 |
| PAD | 1 | 14.29 |
| Current smokers | 2 | 28.57 |
| COPD | 1 | 14.29 |
| Chronic kidney disease | 5 | 71.43 |
| Atrial fibrillation | 6 | 85.71 |
| Previous implantation of ICD/CRT | 6 | 85.71 |
| Primitive dilated cardiomyopathy | 2 | 28.58 |
| Valvular cardiomyopathy | 1 | 14.29 |
| SBP (mmHg) | 107.14 ± 9.51 | |
| DBP (mmHg) | 65.71 ± 7.87 | |
| HR (b.p.m.) | 74.43 ± 7.61 | |
| Medications | ||
| ACE inhibitors or ARBs | 3 | 42.86 |
| ARNI | 4 | 57.14 |
| Furosemide | 7 | 100.00 |
| Mineralocorticoid receptor antagonist | 6 | 85.71 |
| Metolazone | 0 | 0 |
| Beta‐blockers | 6 | 85.71 |
| Statins | 5 | 71.43 |
| Antiarrhythmics (amiodarone) | 3 | 42.86 |
| SGLT2 inhibitors | 7 | 100.00 |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; ARNI, angiotensin receptor‐neprilysin inhibitor; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; HR, heart rate; ICD, implantable cardioverter‐defibrillator; PAD, peripheral arterial disease; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2.
Moreover, from a therapeutic point of view, Table 2 shows that all patients at enrolment were already following anti‐compensation therapy when tolerated, per the ESC 2021 guidelines. 1
Laboratoristic and echocardiographic parameters
Table 3 shows laboratory parameters essentially stable at 1 year and a statistically significant reduction in uric acid [serum uric acid (sUA)] (P = 0.048). On the other hand, echocardiographic data show a statistically significant improvement in both sPAP (P = 0.022) and E/e′ (P = 0.023) (Table 4 ). Moreover, the Quantikine® HS High‐Sensitivity Kit determined elevated IL‐6 values at enrolment in all patients; however, after 6 months, there was a statistically significant reduction in these levels (P = 0.0211) (Figure 1 ).
Table 3.
Laboratoristic parameters (baseline vs. 1 year follow‐up)
| Variables | Baseline | 1 year FU | P |
|---|---|---|---|
| sGlucose (mg/dL) | 112.86 ± 48.14 | 119.57 ± 38.70 | 0.779 |
| AST (mg/dL) | 19.57 ± 5.26 | 19.86 ± 7.40 | 0.935 |
| ALT (mg/dL) | 16.71 ± 10.92 | 19.14 ± 14.30 | 0.727 |
| LDL (mg/dL) | 90.2 ± 37.80 | 82.8 ± 50.59 | 0.799 |
| Haemoglobin (g/dL) | 11.83 ± 1.53 | 12.58 ± 2.98 | 0.568 |
| sUA (mg/dL) | 10.15 ± 2.68 | 7.09 ± 2.29 | 0.048 |
| eGFR (mL/min/1.73 m2) | 51.41 ± 29.80 | 28.11 ± 15.64 | 0.083 |
| sUrea (mg/dL) | 89.57 ± 32.64 | 135.14 ± 75.61 | 0.169 |
| sCreatinine (mg/dL) | 1.31 ± 0.86 | 1.57 ± 0.79 | 0.568 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; FU, follow‐up; LDL, low‐density lipoprotein; sCreatinine, serum creatinine; sGlucose, serum glucose; sUA, serum uric acid; sUrea, serum urea.
We have reported in bold the statistically significant P‐value.
Table 4.
Echocardiographic data (baseline vs. 1 year follow‐up)
| Variables | Baseline | 1 year FU | P |
|---|---|---|---|
| LVEF (%) | 28.57 ± 10.89 | 29.57 ± 13.01 | 0.879 |
| E/e′ | 19.33 ± 5.05 | 12.58 ± 3.53 | 0.023 |
| TAPSE (mm) | 17.57 ± 2.64 | 18.14 ± 2.67 | 0.694 |
| RVs′ (cm/s) | 10.86 ± 2.54 | 9.71 ± 1.11 | 0.298 |
| sPAP (mmHg) | 47.86 ± 8.67 | 35.14 ± 9.34 | 0.022 |
| LVEDV (mL) | 196.00 ± 69.08 | 215.29 ± 96.97 | 0.676 |
| LVESV (mL) | 144.03 ± 61.65 | 153.14 ± 79.78 | 0.815 |
E, early‐wave transmitral diastolic velocity; e′, early‐diastolic velocity at tissue Doppler imaging; FU, follow‐up; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; RVs′, TDI‐derived tricuspid lateral annular systolic velocity; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
We have reported in bold the statistically significant P‐value.
Figure 1.
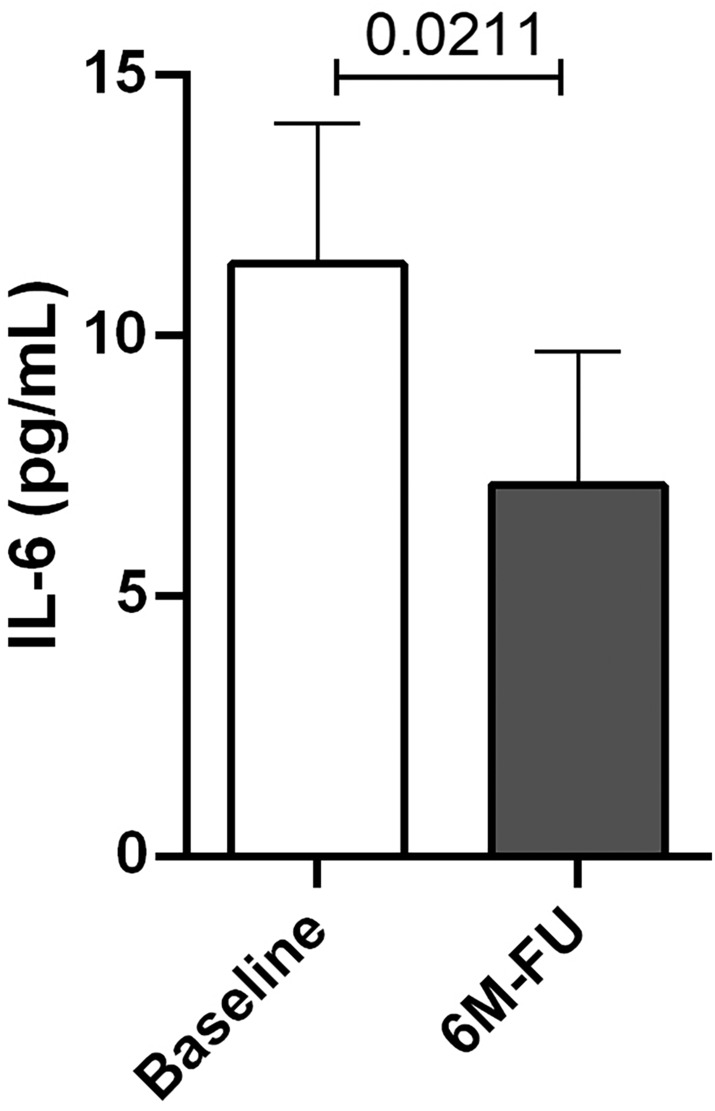
The figure shows reduced interleukin‐6 (IL‐6) levels 6 months after CardioMEMS implantation [baseline vs. 6 month FU (6M‐FU)]. The difference is statistically significant (P = 0.0211).
Health‐related quality of life
Data regarding HRQoL at baseline, 1 month, 6 months, and 1 year after implantation were based on the EQ‐5D questionnaire. Specifically, at baseline, the EQ‐5D was 58.57 ± 10.29 vs. 84.29 ± 19.02 at 1 year after the implantation (P = 0.008), with an improvement in quality of life for six out of seven patients (Figure 2 ).
Figure 2.
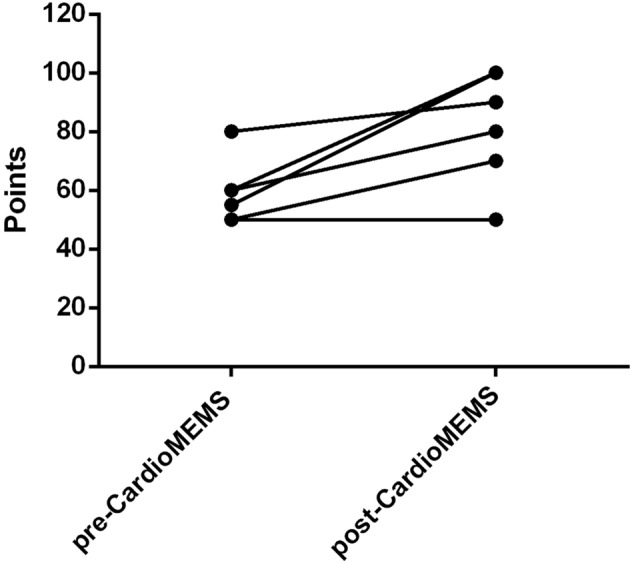
Quality of life (EQ‐5D) pre‐CardioMEMS HF System and post‐1 year. Specifically, the graph shows the improvement in quality of life for six of the seven patients.
Healthcare resource consumption
As described in the Methods section, for each patient, the analysis considered the same number of months before and after the CardioMEMS implant, although the FU period varied among patients (Table 1 ).
Hospitalization rate
The number of hospitalizations was 36 (0.46 events/patient‐year) before the implant and 18 (0.23 events/patient‐year) after the implant, resulting in a reduction of 50% (Table 5 ). Precisely, before CardioMEMS implantation, 23 hospitalizations out of 36 (64%) were not planned, with patients accessed through the emergency department (ED). In contrast, during the CardioMEMS monitoring, only 7 hospitalizations out of 18 were unplanned (39%) (Table 5 ). These seven not‐planned hospitalizations, which occurred after the CardioMEMS implant, might be considered scheduled because, during the COVID‐19 pandemic, patients were hospitalized only after access to the ED, as per hospital directives.
Table 5.
Detailed comparison of pre‐ and post‐implantation hospitalizations of the CardioMEMS heart failure system in all seven enrolled patients
| Years of observation | Total number of hospitalizations | Total number of days in the hospital | Number of days in the hospital per month | Length of stay in the hospital | Total number of hospitalizations from the ED | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐implant (A) | Post‐implant (B) | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | (B)–(A) in % | Pre‐implant (A) | Post implant (B) | (B)–(A) | (B)–(A) in % | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | (B)–(A) in % | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | (B)–(A) in % | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | (B)–(A) in % |
| 2.3 | 2.3 | 6 | 6 | ‐ | 120 | 75 | −45 | −38% | 4.29 | 2.68 | −1.61 | −161% | 20.0 | 12.5 | −7.5 | 1 | 1 | 0 | |||
| 3.0 | 3.0 | 11 | 8 | −3 | 135 | 60 | −75 | −56% | 3.75 | 1.67 | −2.08 | −208% | 12.3 | 7.5 | −4.8 | −39% | 11 | 6 | −5 | ||
| 0.4 | 0.4 | 5 | 3 | −2 | 126 | 14 | −112 | −89% | 25.20 | 2.80 | −22.40 | −2240% | 25.2 | 4.7 | −20.5 | 5 | 0 | −5 | |||
| 1.0 | 1.0 | 4 | ‐ | −4 | 33 | ‐ | −33 | −100% | 2.75 | 0.00 | −2.75 | −275% | 8.3 | 0 | −8.3 | 1 | 0 | −1 | |||
| 1.8 | 1.8 | 2 | ‐ | −2 | 19 | ‐ | −19 | −100% | 0.86 | 0.00 | −0.86 | −86% | 9.5 | 0 | −9.5 | 0 | 0 | 0 | |||
| 1.5 | 1.5 | 3 | ‐ | −3 | 12 | ‐ | −12 | −100% | 0.67 | 0.00 | −0.67 | −67% | 4.0 | 0 | −4.0 | 1 | 0 | −1 | |||
| 1.1 | 1.1 | 5 | 1 | −4 | 41 | 3 | −38 | −93% | 3.15 | 0.23 | −2.92 | −292% | 8.2 | 3 | −5.2 | 4 | 0 | −4 | |||
| 36 | 18 | −18 | −50.0% | 486 | 152 | −334 | −68.7% | 87.4 | 27.7 | −59.8 | −68.4% | 23 | 7 | −16 | −69.6% | ||||||
| Pre‐implant: hospitalizations/patient‐year | Post‐implant: hospitalizations/patient‐year | Diff | Annualized HF hospitalization rates | Total number of days in the hospital/patient‐year | Total number of days in the hospital/patient‐year | Diff | Annualized days in hospital rate | Pre‐implant: hospitalizations from ED/patient‐year | Post‐implant: hospitalizations from ED/patient‐year | Diff | Annualized HF hospitalization rates from ED | ||||||||||
| 0.46 | 0.23 | −0.23 | −50.0% | 6.22 | 1.94 | −4.27 | −68.7% | 0.29 | 0.09 | −0.20 | −69.6% | ||||||||||
ED, emergency department.
Considering the days of hospitalizations, patients stayed in a total of 486 days in hospital during the pre‐CardioMEMS period vs. 152 days in hospital during the post‐device implantation, with a reduction of hospital bed occupancy of 68.7% (Figure 3 ).
Figure 3.
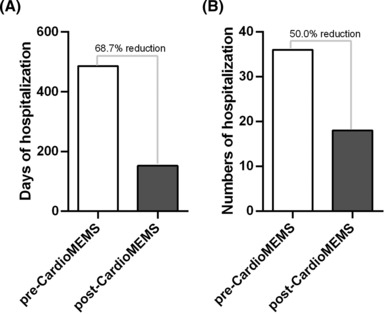
Days and numbers of hospitalization post‐CardioMEMS device. (A) The graph shows a 68.7% reduction in the days of hospitalization. (B) The graph shows a 50% reduction in the total number of hospitalizations.
Considering the length of stay, all patients have been discharged earlier after the CardioMEMS implantation. Specifically, during the pre‐CardioMEMS implant, the average length of stay ranged from 4 days for Patient 6 to 20 days for Patient 1, while during the post‐CardioMEMS implant, the average length of stay ranged from 0 days for Patients 4–6 to 12.5 days for Patient 1 (Table 5 ).
Additionally, we compared hospitalization rate, total number of days, and length of stay in the 12 months before the CardioMEMS implant vs. 12 months after the CardioMEMS implant. To perform this analysis, we excluded Patient 3 as the HF diagnosis had been made 5 months before the CardioMEMS implant. There were 24 hospitalizations in the 12 months before the device implant vs. 6 hospitalizations in the 12 months post‐device, resulting in a reduction of 75%. Patients stayed 231 days in the hospital 12 months before vs. 45 days post, resulting in a reduction of 81% (Supporting Information, Table S1 ).
Access to the emergency department without hospitalization
During the entire period of observation pre‐CardioMEMS, three patients out of seven accessed the ED without hospitalization (treat‐and‐release), for a total of 12 ED accesses, while during the post‐CardioMEMS, only one patient out of seven accessed the ED without hospitalization, for a total of 1 access.
Drug therapy management
Considering the medication changes, the results revealed an increase in the number of changes in the post‐CardioMEMS period vs. pre‐implantation phase by five times.
In the pre‐CardioMEMS implantation phase, an average of 0.56 therapy changes/month was performed, while we recorded 2.56 changes/month post‐device.
Considering the levosimendan infusions, the drug has been administered to four patients in the post‐CardioMEMS phase, and a total of 15 levosimendan vials have been used in the real setting vs. 120 levosimendan vials used in the hypothetical scenario (fixed periodic infusion every 21 days), resulting in 105 levosimendan vials saved using the CardioMEMS system.
Cardiological examinations, diagnostic, and instrumental exams
Considering outpatient visits, the results showed a reduction in the number of cardiological examinations by 59.4% on average (9 per patient in the pre‐implant vs. 3.7 per patient post‐implant), laboratory exams by 17% (8.1 per patient in the pre‐implant vs. 6.7 per patient in the post‐implant), and instrumental examinations by 40% (8.6 per patient in the pre‐implant vs. 5.2 per patient in the post‐implant). By applying the regional tariffs, a reduction of €294 on average per patient was estimated by comparing the period pre‐ and post‐CardioMEMS (total saving of €2059).
Economic results
In terms of costs—calculated by applying the reimbursement tariff recognized by our region over the observation period—the total savings for hospitalizations of the seven patients were estimated at around €95 000. Specifically, we considered hospitalizations for HF‐related reasons. The cost of CardioMEMS was €84 000, €12 000 for each of the seven patients. As the cost of CardioMEMS was not included in the Diagnosis Related Groups (DRG) code for implant hospitalizations, we calculated the net cost by subtracting the cost of the CardioMEMS (€12 000 per patient, a total of €84 000) from the cost of hospitalizations, resulting in a total savings of €11 000 (almost €1570 on average per patient).
Additionally, a total reduction, including CardioMEMS cost, of €6000 in hospitalization has been shown when considering a period of observation of 12 months before and 12 months after the CardioMEMS implant.
Furthermore, we performed an ad hoc analysis of the impact of the use of levosimendan. The hospitalization cost due to the levosimendan infusion was around €1500.00 per infusion (estimate of €500.00/day). As said in the Methods section, the CardioMEMS HF system allows the modulation of the levosimendan administration, avoiding the fixed periodic administration of 21 days (mean time between the infusion period of Ortis et al. 27 and the LION‐HEART trial 26 ). Our strategy leads to optimal management of the levosimendan. In terms of costs, we estimated a total cost savings for the drug of around €205 158 and 315 days of hospitalization avoided (Tables 6A and 6B ).
Table 6A.
Comparison of high‐cost therapy with 21 day infusions pre‐ and post‐implantation of the CardioMEMS heart failure system
| Years of observation | Real number of vials utilized | Number of vials utilized in case of levosimendan infusion every 21 days | Avoided number of levosimendan vials | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐implant (A) | Post‐implant (B) | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | Pre‐implant (A) | Post‐implant (B) | (B)–(A) |
| 2 | 2 | 3 | 6 | 3 | 3 | 40 | 37 | 0 | 34 | 34 |
| 3 | 3 | 0 | 5 | 5 | 0 | 53 | 53 | 0 | 48 | 48 |
| 0 | 0 | 0 | 3 | 3 | 0 | 8 | 8 | 0 | 5 | 5 |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 1 | 1 | 19 | 19 | 0 | 18 | 18 | |
| 3 | 15 | 12 | 3 | 120 | 117 | 0 | 105 | 105 | ||
The mean of the LION‐HEART study strategy (infusions every 15 days) and the Ortis et al. (infusions every 28 days).
Table 6B.
Comparison in monetary terms of high‐cost therapy with pre‐ and post‐implant 21 day infusions of the CardioMEMS system
| Potential avoided costs for levosimendan infusions (€) | Potential avoided costs for hospitalizations due to levosimendan infusions (€) | Potential avoided costs (vials + hospitalizations) (€) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐implant (A) | Post‐implant (B) | (B)–(A) | Pre‐implant (A) | Post‐implant (B) | (B)–(A) | Pre‐implant (A) | Post‐implant (B) | (B)–(A) |
| 0 | 17 572 | 17 572 | 0 | 51 000 | 51 000 | 0 | 68 572 | 68 572 |
| 0 | 21 372 | 21 372 | 0 | 72 000 | 72 000 | 0 | 93 372 | 93 372 |
| 0 | 3350 | 3350 | 0 | 7500 | 7500 | 0 | 10 850 | 10 850 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 5364 | 5364 | 0 | 27 000 | 27 000 | 0 | 32 364 | 32 364 |
| 0 | 47 658 | 47 658 | 0 | 157 500 | 157 500 | 0 | 205 158 | 205 158 |
The mean of the LION‐HEART study strategy (infusions every 15 days) and the Ortis et al. (infusions every 28 days).
To conclude, the total savings were approximately €216 223, and the days of hospitalization avoided were around 649 (Table 7 ).
Table 7.
Economic impact of the CardioMEMS system and guided infusions of levosimendan
| All seven patients | ||
|---|---|---|
| € | ||
| Avoided hospitalizations | Avoided costs | −95 065 |
| Pre‐implant hospitalizations | 163 522 | |
| Post‐implant hospitalizations | 68 457 | |
| CardioMEMS system | Emerging costs | 84 000 |
| Net savings, including the cost of CardioMEMS | −11 065 | |
| Avoided levosimendan infusions | Avoided costs | −205 158 |
| Potential avoided costs for levosimendan infusion | 47 658 | |
| Potential avoided hospitalization costs for levosimendan infusion | 157 500 | |
| Total savings | −216 223 | |
| Days | ||
| Avoided number of days at hospital | −334 | |
| Pre‐implant number of days at hospital | 486 | |
| Post‐implant number of days at hospital | 152 | |
| Avoided number of days at hospital for levosimendan infusions | −315 | |
| Avoided total number of days at hospital | −649 | |
Application of CardioMEMS in three different phenotypes
A patient with heart failure with reduced ejection fraction and a need for periodic levosimendan infusions (Patient 2)
In January 2020, a 78‐year‐old female with HFrEF and 14 HF hospitalizations in the last 4 years, despite the optimal guideline‐based pharmacological therapy, was implanted with a CardioMEMS device. In particular, the woman had post‐ischaemic dilated heart disease, an apex aneurysm, and severely reduced LVEF (23%). Her comorbidities included permanent atrial fibrillation, dyslipidaemia, diabetes mellitus, stage III chronic kidney disease, and dysthyroidism. Moreover, the patient had had an implantable cardioverter‐defibrillator implantation for primary prevention. At the time of CardioMEMS implantation, the MAGGIC score was 36 points (1 year mortality was 39.8% and 3 year mortality was 72.5%); however, after 36 months, the patient is still alive.
The cardiologist monitored the patient using daily reports. If he detected a trend towards increasing diastolic PAP, the patient was contacted, and his medications were adjusted, as described in the Methods section.
Explicitly, when the cardiologist noticed a steadily increased diastolic PAP despite the maximum tolerated dose therapy changes, he contacted the patient for hospitalization and a levosimendan infusion.
During the observation period (72 months: 36 pre‐ and 36 post‐device), the patient underwent 19 hospitalizations: 90% were hospitalizations following visits to the ED, while the remaining 10% were all planned in the post‐implantation period. Considering the hospitalizations of this patient, a total of 135 days in hospitals were recorded in the pre‐implantation phase vs. 60 days post‐device, resulting in a reduced number of days in hospital by 56%. Considering the length of stay in the hospital, there was a reduction of 39% (pre‐device 12.3 days vs. post‐device 7.5 days).
The cost of hospitalization—valued with the reimbursement tariffs approved by our region plus the cost of the implanted system—decreased by approximately €3200 in the post‐implantation period. As far as high‐cost drugs are concerned, this patient used five vials of levosimendan in the post‐implantation period for approximately €2000. Considering the standard levosimendan infusion every 21 days, the CardioMEMS avoided the potential consumption of 48 vials and 144 days of hospitalization, as we considered 3 days in the hospital for the infusion of one vial. We estimated a potential avoided cost for vials and hospitalizations of about €93 000.
As regards the observation of further services, we recorded that in the post‐implantation period, only one access to the ED without hospitalization was performed, compared with six in the previous period. Regarding examinations, a 23% decrease was observed in both cardiac echocolordoppler and cardiological visits (12 pre vs. 9 post); on the other hand, haematological tests have increased by 19% in the post‐phase (12 pre vs. 14 post). In monetary terms, this led to a saving of around €185 for all examinations.
Finally, regarding therapy optimization, we recorded 12 therapy changes pre‐device vs. 214 in the post‐implantation period, of which 98% related to CardioMEMS.
A patient with heart failure with reduced ejection fraction and no need for periodic levosimendan infusions (Patient 5)
In April 2021, an 82‐year‐old male with HFrEF (LVEF 20%) and a dilated cardiomyopathy with evidence of normal coronary was implanted with CardioMEMS. His comorbidities included permanent atrial fibrillation, dyslipidaemia, stage I chronic kidney disease, and dysthyroidism. Moreover, the patient had had a biventricular implantable cardioverter‐defibrillator implantation for primary prevention and a MitraClip placement for severe functional mitral regurgitation. At the time of CardioMEMS implantation, the MAGGIC score was 32 points (1 year mortality was 29.2% and 3 year mortality was 59%); however, after 27 months, the patient is still alive.
During the observation period (44 months: 22 pre‐ and 22 post‐device), the patient underwent two scheduled hospitalizations (both in the pre‐implantation period). Considering the number of days in the hospital, this patient experienced 19 days in the pre‐CardioMEMS phase vs. 0 days in the post‐CardioMEMS phase.
To date, the patient has not been treated with levosimendan infusions. As regards the observation of further services, no accesses to the ED were recorded, and a reduction of 45% of specialist cardiological visits (7 pre vs. 3 post), 9% of cardiac echocolordoppler (7 pre vs. 6 post), and an increase in the laboratory test by 12% (6 vs. 7) were observed. Finally, regarding therapy optimization, 6 therapy changes were observed in the pre‐implantation period against 22 in the post‐implantation period, of which 80% were related to CardioMEMS.
In monetary terms, this led to a saving of around €73 for all examinations.
The cost of hospitalization—valued with the reimbursement tariffs approved by our region plus the cost of the implanted system—was around €9920 in the pre‐CardioMEMS phase (22 months) vs. €12 000 in the post‐CardioMEMS phase (22 months) due to the cost of the device, increasing to €2080 (€94 per month).
A patient with heart failure with preserved ejection fraction (Patient 4)
An 84‐year‐old female presented to our ED with pulmonary oedema in February 2020. Her comorbidities included permanent atrial fibrillation, arterial hypertension, COPD, diabetes mellitus, dyslipidaemia, stage III chronic kidney disease, and carotid atheromasia. The echocardiogram showed an impaired LVEF (40%) with global hypokinesia, severe mitral regurgitation, dilated left atrium, and preserved RV function with an increased sPAP (65 mmHg). The pulmonary oedema was treated during the hospitalization, and coronary angiography revealed normal coronary; the patient was compensated, with a progressive LVEF improvement (LVEF 51%), and, at the end of hospitalization, the patient was enrolled in our HF clinic. Nevertheless, given the worsening of the patient's symptoms and recurrent decompensation events, in February 2021, during another HF hospitalization, the CardioMEMS was implanted.
At the time of implantation, the MAGGIC score was 25 points (1 year mortality was 16% and 3 year mortality was 36.9%); however, after 29 months, the patient is alive.
For this patient, we considered 24 months (12 months pre‐ and 12 months post‐device). Precisely, the patient had four hospitalizations (25% with access to the ED in the pre‐CardioMEMS phase); on the other hand, following device implantation, no further hospitalizations or accesses to the ED were recorded.
The cost of hospitalization, including the device cost, was approximately €4000 less than in the pre‐implantation phase. Moreover, a 33% reduction was observed for specialist cardiological visits and laboratory tests, and an increase of 25% was observed for cardiac doppler. In monetary terms, there was an increase of €66 in 12 months for all examinations. Finally, regarding therapy optimization, we recorded 3 therapy changes (on average <1 per month) pre‐device vs. 27 in the post‐implantation period (on average 2.5 per month) related to the CardioMEMS device.
Discussion
This study provides the first Italian experience assessing PAP‐guided HF therapy's clinical and economic benefits and the first real‐world experience of levosimendan infusions guided by CardioMEMS. Specifically, we considered not only the costs but also the improvement of clinical and echocardiographic parameters, in addition to IL‐6 levels. The study demonstrated a safe and feasible strategy to remotely manage patients with advanced HF and the maximum tolerated dose of HF drugs and to prevent worsening HF.
First, in terms of safety, the patients did not experience procedural complications or device malfunctioning, as previously evidenced in several studies. 20 , 28 , 29 , 30
Moreover, PAP‐guided HF management shows an improvement in echocardiographic data at 1 year FU, such as sPAP and E/e′ ratio; accordingly, during the first year of COAST‐UK trial 28 FU, PAP decreased significantly from baseline, with significant differences observed in sPAP (−4.2 ± 6.6 mmHg), diastolic PAP (−2.7 ± 3.7 mmHg), and mean PAP (−3.3 ± 4.5 mmHg) (P < 0.0001 for all).
The key pathways implicated in the evolution of HF are neurohormonal activation, oxidative stress, and immune activation. In support of the immune activation theory, several studies have shown increased levels of inflammatory cytokines in HF patients. 31 , 32 In this study, our patients showed a reduction in IL‐6 levels 6 months after implantation of the device (Figure 2 ); furthermore, even in the event of an increase in PAP on CardioMEMS monitoring, the IL‐6 levels in each patient never reached the pre‐implantation values.
Noteworthy, increased cardiac IL‐6 and IL‐6 receptor mRNA levels have been associated with worsening haemodynamics in advanced HF. 33 Indeed, the effects of IL‐6 on myocardial function are well documented, just as studies on the use of anti‐IL‐6 in HF have been conducted. 33 , 34
Improving the quality of life is an important therapeutic goal. Recently, the MEMS‐HF study demonstrated significant improvements across all patient‐reported quality of life outcomes [including the nine‐item Patient Health Questionnaire (PHQ‐9) for depression]. 29 MEMS‐HF captured the health status of enrolled patients using the Kansas City Cardiomyopathy Questionnaire (KCCQ) (from 47 to 60.5, P < 0.001); depressive symptoms were assessed using the PHQ‐9 depression module (from 8.7 to 6.3); and additionally, patients completed the EQ‐5D‐5L questionnaire (from 54.4 to 61.1). Similarly, our study, which used the same study design as MEMS‐HF, showed improved quality of life on the EQ‐5D questionnaire (Figure 1 ).
Regarding pharmaceutical therapy, this study showed continuous drug therapy optimization (average number of therapy changes per month/patient: 2.56 in the post‐device implant vs. 0.56 in the pre‐device implant); additionally, the levosimendan infusions were optimized, allowing a reduction in the number of infusions compared with the evidence from the literature. In particular, an excellent therapeutic alliance was found by the association of CardioMEMS with the calcium‐sensitizer levosimendan. 26 Levosimendan improves symptoms, quality of life, and LVEF of persons with HF; in particular, LION‐HEART shows that in patients with advanced chronic HF, the i.v. 6 h levosimendan dose (0.2 μg/kg/min, no bolus) every 2 weeks for 12 weeks (6 cycles) significantly reduced the rehospitalization rate and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels. 26 Furthermore, patients receiving infusions experienced a smaller decline in quality of life on the EQ‐5D Visual Analogue Scale (VAS), in accordance with our results. Similarly, Ortis et al. reported that intermittent infusions of levosimendan in patients with advanced HF improve LVEF and reduce hospitalizations; in particular, in this trial, infusions of levosimendan (0.1–0.2 μg/kg/min) were performed in 24–48 h at an interval of 28 days. 27
Unlike the LION‐HEART trial 26 and the experience of Ortis et al., 27 in our study, it was possible to practise levosimendan infusions over time, thanks to the monitoring of PAP by CardioMEMS, 35 which has therefore proved to be a valid tool for guiding drug infusions, leading to a reduction in patient hospitalizations and savings in healthcare costs.
The HF hospitalization rate was reduced, consistent with several studies (Champion, 20 COAST‐HF, 28 and MEMS‐HF 29 ). This result is fundamental because it is now widely proven that for each HF exacerbation hospitalization, there is a reduction in cardiac function and a worsening of the prognosis. 36
Specifically, our results showed a reduction in patients' HF exacerbation hospitalizations after device implantation, consistent with the 2011 CHAMPION study, the first clinical study confirming the decrease in HF hospitalizations with the CardioMEMS system. 37 In 6 months, in the CHAMPION study, there were 120 hospitalizations for HF in the control group (rate 0.44) and 84 in the CardioMEMS group (rate 0.32), with a significant reduction in HF hospitalizations of 28% [hazard ratio (HR) 0.72, 95% confidence interval (CI) 0.60–0.85; P < 0.0001]. During the entire 15 month FU, the device‐implanted group had a 37% reduction in HF hospitalizations compared with the control group.
Recently, MONITOR‐HF, 38 the first randomized clinical trial designed in Europe, showed a reduction in the rate of total HF hospitalizations by 44% (0.381 per patient‐year in the CardioMEMS‐HF group and 0·678 per patient‐year in the control group).
The European study MEMS‐HF 29 showed a 62% reduction in the annualized HF hospitalization from the pre‐implant to the post‐implant. In comparison, this analysis showed a reduction of 49%, though we have to consider that MEMS‐HF included n = 234 patients with a CardioMEMS sensor implanted vs. 7 patients included in this real‐world analysis.
The reduction in the hospitalization rate is lower than in the COAST‐UK study 28 (82%). However, the rate observed in the COAST‐UK can only be used as a point estimate of effectiveness with control of the confounding factors (such as the possibility that patients enrolled in the COAST did not have structured HF management in the 12 months before sensor implantation).
The analysis showed a reduction in the total number of days in the hospital and the average length of each hospitalization after the CardioMEMS implant, meaning patients are less complex to manage once hospitalized. Consequently, hospital facilities might be better utilized as bed occupancy has been reduced in favour of hospitalizations for other types of patients on the waiting list.
In several published cost‐effectiveness analyses based on the CHAMPION clinical trial, it has been demonstrated that the CardioMEMS strategy costs more than the standard of care, but it offers a better quality of life, resulting in an affordable cost‐effectiveness ratio in several healthcare systems, including Italy. 21 , 28 , 39 , 40
In our analysis, the cost reduction for HF hospitalization after a CardioMEMS implant was about €13 571 per patient, consistent with the Desai study. 39 Even when we include the cost of the CardioMEMS system, the results still show a reduction in the total cost sustained by the hospital by an average of €1571 per patient. Considering the savings calculated, including the optimized levosimendan infusion strategy, the average saving per patient was €31 000. Only for Patient 5 was an increase in costs recorded (€2080); however, this increase is related to 22 months of patient management (about €94 per month). Moreover, thanks to the device, the patient did not have hospitalizations or ED accesses in the post‐CardioMEMS period, despite the natural progression of his disease. Indeed, in terms of therapy optimization, 6 therapy changes were observed in the pre‐implantation period against 22 in the post‐implantation period, of which 80% related to CardioMEMS. Finally, although an increase in spending has been recorded, the improvement in the quality of life (EQ‐5D) must not be overlooked (pre‐ 55 vs. post‐1 year 100).
The real‐world reduction in hospitalizations after the CardioMEMS implant and the significant cost reductions for hospitalizations support the real‐world effectiveness of this approach to HF management. Specifically, this supports the idea that the savings due to the CardioMEMS system may be durable over time.
Finally, considering the number of diagnostic services, laboratory, and cardiological visits, a reduction in the services provided, in addition to guaranteeing savings, also generates effectiveness and efficiency in the management of waiting lists (a decrease of 59% for cardiological visits, 37 visits avoided; 40% of echocolordoppler, 24 exams avoided; and 17% of laboratory tests, 10 avoided).
In conclusion, this new strategy contributes to the organizational efficiency of the healthcare facility, the adequate use and allocation of financial and human resources as patients will experience less unplanned hospitalization, and easy management of their HF status in different clinical settings, as demonstrated by the three different HF phenotypes we have described.
Study limitation
First, the sample study included in this analysis is small: only seven patients have been enrolled; however, our experience provides a glimpse of how this innovative strategy can be applied at other institutions and countries.
As this study compares outcomes before and after device implantation, not a prospective randomized study, we cannot exclude bias; however, to reduce potential bias, we included only patients in NYHA class III and with optimal HF medical therapy at the maximum tolerated dose, and we made a comparison pre‐ and post‐CardioMEMS implantation for each patient; consequently, each patient becomes his own control.
Patients have different observation periods (minimum 10 months, maximum 72 months), and the benefits observed in this study may be different if the analysis was performed considering 12 months before the CardioMEMS implant and 12 months after the CardioMEMS implant.
The impact of CardioMEMS on the levosimendan infusion has been calculated assuming a hypothetical scenario in which levosimendan was administered every 21 days. Even if this period were more extended (e.g. 42 days), we would still save 52 vials and €102 000 in total hospitalization costs (hospital stay + drug cost).
Moreover, COVID‐19 significantly impacted the analysis 41 ; changes in the number of hospitalizations have been widely reported. In our study, hospitalizations during COVID‐19 have been considered unplanned, even if planned, as patients have been hospitalized after access to the ED.
Conclusions
The new concept of associating CardioMEMS with levosimendan infusions and its role as a ‘bridge’ device to ventricular assist or heart transplantation appears extremely promising but needs further studies on larger patient cohorts to better define its efficacy and tolerability.
Nevertheless, our preliminary results support the usefulness of this system in the remote management of persons with HF and the rehospitalization reduction for exacerbation and drug management. The parameters' monitoring through the CardioMEMS device allows personalized drug therapy management. Finally, our innovative strategy contributes to achieving organizational efficiency in healthcare facilities and the adequate use and allocation of financial and human resources, resulting in better outcomes for HF patients.
As informed by scientific evidence, national and regional healthcare systems should recognize the value of remote monitoring for challenging illnesses, such as HF. CardioMEMS should be considered the standard of care for persons with HF who are eligible for the device implant.
Payers, decision‐makers, and clinicians should collaborate to redesign patient pathways and define financial mechanisms based on value and outcome.
Conflict of interest
Valeria Visco, Cristina Esposito, Antonella Rispoli, Paola Di Pietro, Carmine Izzo, Francesco Loria, Daniele Di Napoli, Nicola Virtuoso, Alessia Bramanti, Michele Manzo, Carmine Vecchione, and Michele Ciccarelli declare that they have no conflict of interest.
Funding
This work was funded by the SOLOMAX SociaLNetwOrk of MedicAlEXperiences Prog. N. F/260007/05/X51—CUP: B49J21031700008 and Innovation Agreement D.M. 02.8.2019 Life Sciences—MISE.
Supporting information
Table S1. Detailed comparison pre and post implant hospitalization of the CardioMEMS System in all 7 enrolled patients (12 months pre vs. 12 months post).
Acknowledgements
The authors sincerely acknowledge Irene Colangelo, Health Economics and Reimbursement Manager; Valentina Caviglia, Health Economics and Reimbursement Specialist; and Melinda Bechini, Health Economics and Reimbursement Specialist of Abbott Medical Italia S.r.l., for their support in cost analysis.
Visco, V. , Esposito, C. , Rispoli, A. , Di Pietro, P. , Izzo, C. , Loria, F. , Di Napoli, D. , Virtuoso, N. , Bramanti, A. , Manzo, M. , Vecchione, C. , and Ciccarelli, M. (2024) The favourable alliance between CardioMEMS and levosimendan in patients with advanced heart failure. ESC Heart Failure, 11: 2835–2848. 10.1002/ehf2.14838.
Contributor Information
Valeria Visco, Email: vvisco@unisa.it.
Michele Ciccarelli, Email: mciccarelli@unisa.it.
References
- 1. Authors/Task Force Members , McDonagh T, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4‐131. doi: 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 2. Visco V, Esposito C, Manzo M, Fiorentino A, Galasso G, Vecchione C, et al. A multistep approach to deal with advanced heart failure: A case report on the positive effect of cardiac contractility modulation therapy on pulmonary pressure measured by CardioMEMS. Front Cardiovasc Med 2022;9:874433. doi: 10.3389/fcvm.2022.874433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;73:935‐944. doi: 10.1016/j.jacc.2018.11.049 [DOI] [PubMed] [Google Scholar]
- 4. Ciccarelli M, Giallauria F, Carrizzo A, Visco V, Silverio A, Cesaro A, et al. Artificial intelligence in cardiovascular prevention: New ways will open new doors. J Cardiovasc Med (Hagerstown) 2023;24:e106‐e115. doi: 10.2459/JCM.0000000000001431 [DOI] [PubMed] [Google Scholar]
- 5. Martinson M, Bharmi R, Dalal N, Abraham WT, Adamson PB. Pulmonary artery pressure‐guided heart failure management: US cost‐effectiveness analyses using the results of the CHAMPION clinical trial. Eur J Heart Fail 2017;19:652‐660. doi: 10.1002/ejhf.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guha K, McDonagh T. Heart failure epidemiology: European perspective. Curr Cardiol Rev 2013;9:123‐127. doi: 10.2174/1573403x11309020005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail 2013;6:606‐619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc Res 2023;118:3272‐3287. doi: 10.1093/cvr/cvac013 [DOI] [PubMed] [Google Scholar]
- 9. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med 2009;360:1418‐1428. doi: 10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 10. Ayyadurai P, Alkhawam H, Saad M, al‐Sadawi MA, Shah NN, Kosmas CE, et al. An update on the CardioMEMS pulmonary artery pressure sensor. Ther Adv Cardiovasc Dis 2019;13:1753944719826826. doi: 10.1177/1753944719826826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368‐376. doi: 10.1016/j.ijcard.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 12. Maggioni AP, Orso F, Calabria S, Rossi E, Cinconze E, Baldasseroni S, et al. The real‐world evidence of heart failure: Findings from 41 413 patients of the ARNO database. Eur J Heart Fail 2016;18:402‐410. doi: 10.1002/ejhf.471 [DOI] [PubMed] [Google Scholar]
- 13. Visco V, Radano I, Campanile A, Ravera A, Silverio A, Masarone D, et al. Predictors of sacubitril/valsartan high dose tolerability in a real world population with HFrEF. ESC Heart Fail 2022;9:2909‐2917. doi: 10.1002/ehf2.13982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campanile A, Visco V, de Carlo S, Ferruzzi GJ, Mancusi C, Izzo C, et al. Sacubitril/valsartan vs. standard medical therapy on exercise capacity in HFrEF patients. Life (Basel) 2023;13:1174. doi: 10.3390/life13051174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993‐1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 16. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539‐1549. doi: 10.1056/NEJMoa050496 [DOI] [PubMed] [Google Scholar]
- 17. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: The Better Effectiveness After Transition—Heart Failure (BEAT‐HF) randomized clinical trial. JAMA Intern Med 2016;176:310‐318. doi: 10.1001/jamainternmed.2015.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Visco V, Ferruzzi GJ, Nicastro F, Virtuoso N, Carrizzo A, Galasso G, et al. Artificial intelligence as a business partner in cardiovascular precision medicine: An emerging approach for disease detection and treatment optimization. Curr Med Chem 2021;28:6569‐6590. doi: 10.2174/0929867328666201218122633 [DOI] [PubMed] [Google Scholar]
- 19. Klein L. (Re)discovering the neurohormonal and hemodynamic duality of heart failure. J Am Coll Cardiol 2017;70:1887‐1889. doi: 10.1016/j.jacc.2017.08.058 [DOI] [PubMed] [Google Scholar]
- 20. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011;377:658‐666. doi: 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 21. Schmier JK, Ong KL, Fonarow GC. Cost‐effectiveness of remote cardiac monitoring with the CardioMEMS heart failure system. Clin Cardiol 2017;40:430‐436. doi: 10.1002/clc.22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brugts JJ, Veenis JF, Radhoe SP, Linssen GCM, van Gent M, Borleffs CJW, et al. A randomised comparison of the effect of haemodynamic monitoring with CardioMEMS in addition to standard care on quality of life and hospitalisations in patients with chronic heart failure: Design and rationale of the MONITOR HF multicentre randomised clinical trial. Neth Heart J 2020;28:16‐26. doi: 10.1007/s12471-019-01341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savoia E, Fantini MP, Pandolfi PP, Dallolio L, Collina N. Assessing the construct validity of the Italian version of the EQ‐5D: Preliminary results from a cross‐sectional study in North Italy. Health Qual Life Outcomes 2006;4:47. doi: 10.1186/1477-7525-4-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404‐1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233‐270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 26. Comín‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, García Pinilla JM, Almenar L, et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: The LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018;20:1128‐1136. doi: 10.1002/ejhf.1145 [DOI] [PubMed] [Google Scholar]
- 27. Ortis B, Villani A, Oldani M, Giglio A, Ciambellotti F, Facchini M, et al. Intermittent levosimendan infusions in advanced heart failure: A real world experience. J Int Med Res 2017;45:361‐371. doi: 10.1177/0300060516655244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowie MR, Flett A, Cowburn P, Foley P, Chandrasekaran B, Loke I, et al. Real‐world evidence in a national health service: Results of the UK CardioMEMS HF System Post‐Market Study. ESC Heart Fail 2022;9:48‐56. doi: 10.1002/ehf2.13748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, et al. Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF). Eur J Heart Fail 2020;22:1891‐1901. doi: 10.1002/ejhf.1943 [DOI] [PubMed] [Google Scholar]
- 30. Vaduganathan M, DeFilippis EM, Fonarow GC, Butler J, Mehra MR. Postmarketing adverse events related to the CardioMEMS HF system. JAMA Cardiol 2017;2:1277‐1279. doi: 10.1001/jamacardio.2017.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep 2017;19:27. doi: 10.1007/s11883-017-0660-3 [DOI] [PubMed] [Google Scholar]
- 32. Tamariz L, Hare JM. Inflammatory cytokines in heart failure: Roles in aetiology and utility as biomarkers. Eur Heart J 2010;31:768‐770. doi: 10.1093/eurheartj/ehq014 [DOI] [PubMed] [Google Scholar]
- 33. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin‐6 in heart failure: Results from the BIOSTAT‐CHF study. Eur J Heart Fail 2019;21:965‐973. doi: 10.1002/ejhf.1482 [DOI] [PubMed] [Google Scholar]
- 34. Ridker PM, Rane M. Interleukin‐6 signaling and anti‐interleukin‐6 therapeutics in cardiovascular disease. Circ Res 2021;128:1728‐1746. doi: 10.1161/CIRCRESAHA.121.319077 [DOI] [PubMed] [Google Scholar]
- 35. Visco V, Esposito C, Vitillo P, Vecchione C, Ciccarelli M. It is easy to see, but it is better to foresee: A case report on the favourable alliance between CardioMEMS and levosimendan. Eur Heart J Case Rep 2020;4:1‐5. doi: 10.1093/ehjcr/ytaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: Rates and long‐term mortality. J Card Fail 2004;10:374‐379. doi: 10.1016/j.cardfail.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 37. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137‐e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 38. Brugts JJ, Radhoe SP, Clephas PRD, Aydin D, van Gent MWF, Szymanski MK, et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR‐HF): A randomised clinical trial. Lancet 2023;401:2113‐2123. doi: 10.1016/S0140-6736(23)00923-6 [DOI] [PubMed] [Google Scholar]
- 39. Desai AS, Bhimaraj A, Bharmi R, Jermyn R, Bhatt K, Shavelle D, et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real‐world” clinical practice. J Am Coll Cardiol 2017;69:2357‐2365. doi: 10.1016/j.jacc.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 40. Sandhu AT, Goldhaber‐Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost‐effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail 2016;4:368‐375. doi: 10.1016/j.jchf.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zile MR, Desai AS, Costanzo MR, Ducharme A, Maisel A, Mehra MR, et al. The GUIDE‐HF trial of pulmonary artery pressure monitoring in heart failure: Impact of the COVID‐19 pandemic. Eur Heart J 2022;43:2603‐2618. doi: 10.1093/eurheartj/ehac114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed comparison pre and post implant hospitalization of the CardioMEMS System in all 7 enrolled patients (12 months pre vs. 12 months post).


