Abstract
Background
Cardiogenic shock (CS) is associated with high in‐hospital mortality. Objective assessment of its severity and prognosis is paramount for timely therapeutic interventions. This study aimed to evaluate the efficacy of the shock index (SI) and its variants as prognostic indicators for in‐hospital mortality.
Methods
A retrospective study involving 1282 CS patients were evaluated. Baseline patient characteristics, clinical trajectory, hospital outcomes, and shock indices were collected and analysed. Receiver operating characteristic (ROC) curves were employed to determine the predictive accuracy of shock indices in predicting in‐hospital mortality.
Results
Of those evaluated, 866 (67.6%) survived until discharge. Non‐survivors were older (66.0 ± 13.7 vs. 57.4 ± 16.2, P < 0.001), had a higher incidence of cardiac risk factors, and were more likely to present with acute coronary syndrome (33.4% vs. 16.1%, P < 0.001) and out‐of‐hospital cardiac arrest (11.3% vs. 5.3%, P < 0.001). All mean shock indices were significantly higher in non‐survivors compared with survivors. ROC curves demonstrated that adjusted shock index (ASI), age‐modified shock index (AMSI), and shock index‐C (SIC) had the highest predictive accuracy for in‐hospital mortality, with AUC values of 0.654, 0.667, and 0.659, respectively. Subgroup analysis revealed that SIC had good predictive ability in patients with STEMI (AUC: 0.714) and ACS (AUC: 0.696) while AMSI and ASI were notably predictive in the OHCA group (AUC: 0.707 and 0.701, respectively).
Conclusions
Shock index and its variants, especially ASI, AMSI, and SIC, may be helpful in predicting in‐hospital mortality in CS patients. Their application could guide clinicians in upfront risk stratification. SIC, ASI, and AMSI show potential in predicting in‐hospital mortality in specific CS subsets (STEMI and OHCA). This is the first study to evaluate SI and its variants in CS patients.
Keywords: Cardiogenic shock, Hospital mortality, Shock index, Adjusted shock index, Age‐modified shock index, Shock index‐C
Introduction
Cardiogenic shock (CS) is a clinical syndrome characterized by a primary cardiac disorder leading to hypotension and signs of organ hypoperfusion in the presence of euvolaemia or hypervolaemia. 1 CS remains challenging to manage given the high mortality rate of 40%. 2 Effective management of CS requires early identification, rapid intervention, and an understanding of early prognostic factors to guide clinical decision making. 3 , 4
Originally proposed in 1967 for the management of haemorrhagic and septic shock, the shock index (SI), defined as the ratio of heart rate (HR) to systolic blood pressure (SBP), has emerged as a promising tool for risk stratification and prognostication. Values of SI greater than 1.0 indicate imminent shock, underscoring its significance as an early warning indicator in critical care settings. 5 The simplicity of the SI makes it a valuable parameter for predicting mortality in various acute pathologies, including myocardial infarction, pulmonary thromboembolism, sepsis, hypovolaemia, and trauma, as it can be easily obtained upon admission. 6 , 7 , 8 , 9 , 10 , 11 Previous studies have found SI to be a more reliable measure of haemodynamic stability than SBP or HR alone. 12
In patients undergoing revascularization for ST‐segment elevation myocardial infarction (STEMI), an elevated SI is an independent predictor of microvascular damage, extent of myocardial injury, and short‐term and long‐term mortality. 11 SI may also reflect worsening stroke volume, cardiac index, 8 severity of cardiac dysfunction, which in turn, is associated with higher mortality rates. 13 Due to its practicality, SI has been validated for assessing the severity and prognosis of different cardiac conditions such as acute heart failure (AHF), acute coronary syndrome (ACS), out‐of‐hospital cardiac arrest (OHCA), and critical illness. 14 , 15 , 16 , 17 , 18
Other indices have since been developed. In early studies, the modified shock index (MSI), which includes mean arterial pressure (MAP) instead of SBP has been shown to predict mortality rates more accurately than SI. 19 As older patients may be moribund, the adjusted shock index (ASI), defined as age in years multiplied by SI, is another tool for mortality prediction. 20 Furthermore, the shock index‐C (SIC), including estimated creatinine clearance (CrCl), has demonstrated superior predictive value compared with SI in patients with STEMI. 21 Chiang et al. found that that a value of SIC >21.0 showed statistically significant association with in‐hospital mortality in patients with STEMI. 22 These indices have not been evaluated in patients with CS.
In this retrospective cohort study, we defined the utility of the SI and its variants in predicting in‐hospital mortality in patients presenting with CS. We provided insights into the prognostic potential of these indices, offering clinicians a simple tool for risk assessment early in the disease course of CS.
Methods
Study design and setting
A retrospective observational analysis was conducted in consecutive adult patients (>18 years) diagnosed with CS admitted to the Cardiac Intensive Care Unit (CICU) at Toronto General Hospital (University Health Network) between January 2014 and December 2021.
Patient population
CS was defined as: SBP < 90 mm Hg sustained for >30 min or the need for vasopressors to maintain a SBP > 90 mmHg, pulmonary congestion or elevated left ventricular filling pressures, and signs of organ perfusion impairment evident through at least one of the following: altered mental status, cold clammy skin, oliguria, or elevated serum lactate. 1 Patients were excluded if they had: incomplete data, prior CS admission during the same hospital stay, pre‐existing sepsis, or haemorrhagic shock. This study adhered to the ethical guidelines of Declaration of Helsinki 1975 and was approved by institutional research ethics board. Due to the retrospective nature of the study and the usage of de‐identified clinical data, informed consent was waived.
Data collection
Patient characteristics, vital signs, clinical presentation, laboratory values, comorbidities, management during hospital stay, and in‐hospital outcomes were included. Various shock indices were calculated using first record at the cardiac care unit (CCU) admission as follows: SI is the ratio of HR (beats per minute, bpm) to SBP (mmHg) 5 ; MSI being the ratio of HR (bpm) to MAP (mmHg) 23 ; ASI multiplies age (years) with SI 20 ; age‐modified shock index (AMSI) multiplies age (years) with MSI 20 ; and SIC is (SI × 100) minus CrCl. 21 CrCl was calculated using the first creatinine at CCU admission and the Cockcroft–Gault published equations. 24 The primary outcome evaluated was in‐hospital mortality.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are presented as numbers and percentages. T‐tests (Levene) were applied for continuous variables and Pearson χ 2 tests for discrete variables. A linear regression model was used to calibrate the data, and an ANOVA test was used to determine the statistical significance of the model. The diagnostic performance was assessed using ROC curves and sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), negative likelihood ratio (LR−), and Youden index (YI). 25 Area under the curve (AUC) was used to compare the discriminatory power of various SIs, with an AUC of 1.0 considered as perfect discrimination and 0.5 considered as equal to chance. AUC values were compared using the nonparametric approach described by DeLong et al. 26 Separate subgroup analyses were performed stratified by age, sex, ACS, and OHCA. All statistical tests were two‐sided, and P values of <0.05 were considered statistically significant. All analyses were performed using SPSS software (version 28; SPSS Inc, DE, USA).
Results
Patient characteristics
A total of 1282 patients diagnosed with CS were included in the analysis. The mean age of the participants was 60.2 ± 15.9 years, and 901 patients (70.3%) were male. In this study, 866 (67.6%) survived until time of hospital discharge. The baseline patient characteristics are summarized in Table 1 .
Table 1.
Baseline characteristics
| Characteristics | Total population | Non‐survivors | Survivors | P value |
|---|---|---|---|---|
| Patients, n (%) | 1282 | 416(32.4) | 866(67.6) | — |
| Age, years, mean [SD] | 60.2 [15.98] | 66.0(13.7) | 57.4(16.2) | <0.001 |
| Gender, n (%) | ||||
| Male | 901 (70.3) | 278(66.8) | 623(71.9) | — |
| Comorbidities, n (%) | ||||
| Hypertension | 555 (43.3) | 212(51.4) | 343(39.6) | 0.002 |
| Arrhythmia (VF/VT) | 114(8.9) | 28(6.7) | 86(6.7) | 0.06 |
| Atrial fibrillation/flutter | 372(29.0) | 116(27.9) | 256(29.6) | 0.54 |
| Diabetes mellitus | 423(33.0) | 156(37.5) | 267(30.8) | 0.01 |
| Dyslipidaemia | 457 (35.6) | 165(39.7) | 292(33.7) | 0.03 |
| COPD | 89(6.9) | 33(7.9) | 56(6.5) | 0.33 |
| CVA/TIA | 117(9.1) | 46(11.1) | 71(8.2) | 0.10 |
| Previous MI | 270(21.1) | 96(23.1) | 174(20.1) | 0.22 |
| Previous PCI | 206(16.1) | 74(17.8) | 132(15.2) | 0.24 |
| Previous CABG | 118(9.2) | 45(10.8) | 73(8.4) | 0.17 |
| CKD | 326(25.4) | 117(28.2) | 209(24.2) | 0.12 |
| PVD | 75(5.9) | 32(7.7) | 43(4.9) | 0.05 |
| CHF | 638(49.8) | 178(42.8) | 460(53.3) | <0.001 |
| PPM | 50(3.9) | 17(4.1) | 33(3.8) | 0.81 |
| ICD | 182(14.2) | 46(11.0) | 136(15.7) | 0.02 |
| Clinical characteristics, n (%) | ||||
| Smoking history | 521(40.6) | 150(36.0) | 371(42.8) | 0.05 |
| ACS (AMI‐CS) | 279(21.8) | 139(33.4) | 140(16.1) | <0.001 |
| STEMI at presentation | 200(15.6) | 102(27.3) | 98(13.4) | <0.001 |
| NSTE‐ACS at presentation | 79(6.2) | 37(10.1) | 42(5.8) | 0.009 |
| Transfer | 513(40.0) | 161(45.2) | 352(49.3) | 0.20 |
| OHCA | 93(7.3) | 47(11.3) | 46(5.3) | <0.001 |
| SCAI stage n (%) | <0.001 | |||
| SCAI‐B | 96(7.5) | 22(5.3) | 74(8.5) | |
| SCAI‐C | 56(4.4) | 9(2.1) | 47(5.4) | |
| SCAI‐D | 1,076(83.9) | 357(85.8) | 719(83.0) | |
| SCAI‐E | 54(4.2) | 28(6.7) | 26(3.0) | |
| Prior vasopressor/inotrope use | 520(40.6) | 185(44.7) | 335(38.9) | 0.05 |
| Vital signs, mean [SD] | ||||
| HR, bpm | 90.8 [22.4] | 91.1 [22.2] | 90.6 [22.5] | 0.7 |
| MAP, mmHg | 75.8 [14.1] | 72.8 [15.1] | 77.3 [13.4] | <0.001 |
| SBP, mmHg | 104.0 [20.0] | 101.0 [22.2] | 105.5 [18.8] | <0.001 |
| DBP, mmHg | 61.8 [13.5] | 58.9 [14.1] | 63.2 [12.9] | <0.001 |
| Laboratory tests, mean [SD] | ||||
| Hb, g/L | 120.3 [25.5] | 114.98 [25.8] | 122.9 [25.1] | <0.001 |
| WBC, ×109/L | 12.7 [7.3] | 14.1 [8.0] | 12.0 [6.9] | <0.001 |
| Sodium, mEq/L | 13.0 [6.9] | 135.0 [6.7] | 135.0 [7.0] | 0.94 |
| Lactate, mmol/L | 3.5 [3.8] | 4.9 [4.8] | 2.9 [2.9] | <0.001 |
| Creatinine, μmol/L | 192.8 [135.7] | 236.35 [155.0] | 172.1 [120.1] | <0.001 |
| Creatinine clearance, mL/min | 42.8 [28.0] | 32.6 [22.2] | 47.6 [29.1] | <0.001 |
| In‐hospital treatment, n (%) | ||||
| Vasopressor/inotrope use | 1150(89.7) | 387(93.0) | 763(88.1) | 0.007 |
| PAC | 600(46.8) | 174(41.8) | 426(49.5) | 0.010 |
| IMV | 436(34.0) | 204(49.1) | 232(26.8) | <0.001 |
| IABP | 212(16.5) | 99(23.8) | 113(13.0) | <0.001 |
| Impella | 47(3.7) | 19(4.6) | 28(3.2) | 0.171 |
| ECMO | 67(5.3) | 28(6.8) | 39(4.5) | 0.085 |
| dLVAD implantation | 138(10.8) | 29(6.9) | 109(12.6) | 0.004 |
| OHT | 88(8.1) | 4 (1.1) | 84 (11.5) | <0.001 |
| Hospital LOS (days) | 25.7 [36.5] | 21.3 [41.0] | 27.8 [34.2] | 0.004 |
| CCU LOS (days) | 6.3 [6.8] | 5.5 [8.5] | 6.7 [5.9] | 0.11 |
Abbreviations: ACS, acute coronary syndrome; AMI‐CS, acute myocardial infarction–cardiogenic shock; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DBP, diastolic blood pressure; dLVAD, durable left ventricular assist device; ECMO, extracorporeal membrane oxygenation; Hb, haemoglobin; HR, heart rate; IABP, intra‐aortic balloon pump; ICD, implantable cardioverter‐defibrillator; IMV, invasive mechanical ventilation; LOS, length of stay; MAP, mean arterial pressure; MI, myocardial infarction; NSTE‐ACS, non ST‐elevation acute coronary syndrome; OHCA, out‐of‐hospital cardiac arrest; OHT, orthotopic heart transplantation; PAC, pulmonary artery catheter; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; PVD, peripheral vascular disease; SBP, systolic blood pressure; SCAI, Society for Cardiovascular Angiography and Interventions; SD, standard deviation; STEMI, ST‐elevation myocardial infarction; TIA, transient ischaemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia; WBC, white blood cell count.
Non‐survivors were older than survivors (66.0 vs. 57.4 years, P < 0.001), were more likely to have hypertension (51.4% vs. 39.6%, P < 0.001), diabetes mellitus (37.5% vs. 30.8%, P = 0.01), and dyslipidaemia (39.7% vs. 33.7%, P = 0.03), and had lower rates of congestive heart failure (CHF) compared with survivors (42.8% vs. 53.3%, P < 0.001). Clinical presentation with ACS (33.4% vs. 16.1%, P < 0.001) and/or OHCA (11.3% vs. 5.3%, P < 0.001) were more common in the non‐survivors. Moreover, non survivors, more frequently presented with a Society for Cardiovascular Angiography and Interventions (SCAI) classification of D or E.
Table 1 also provides an overview of major in‐hospital treatments. Non‐survivors were more likely to receive inotrope/vasopressor therapy during their stay in the critical care unit compared with survivors (93% vs. 88%, P = 0.007). Additionally, non‐survivors had higher rates of mechanical ventilation (49% vs. 26.8%, P < 0.001) and intra‐aortic balloon pumps (IABP) (23.8% vs. 13%, P < 0.001). Conversely, there was no significant difference in survival between the two groups regarding the use of Impella or ECMO (P = 0.171 and P = 0.085, respectively). Among survivors, heart transplantation during admission was more frequent than among non‐survivors (84 vs. 4, P < 0.001), as were durable left ventricular assist devices (dLVAD) (12.6% vs. 6.9%, P = 0.004) and pulmonary artery catheters (PAC) (49.5% vs. 41.8%, P = 0.010). Survivors also had a longer average length of hospital stay (27.8 vs. 21.3, P = 0.004), with no significant difference in critical care unit length of stay.
Shock indices and outcomes
The mean SIs were all significantly higher in deceased patients compared with those who survived until hospital discharge (Table 2 ).
Table 2.
Shock indices
| Indices, mean [SD] | Total population | Non‐survivors | Survivors | P value |
|---|---|---|---|---|
| SI | 0.90 [0.29] | 0.95 [0.34] | 0.88 [0.26] | 0.001 |
| MSI | 1.24 [0.39] | 1.31 [0.47] | 1.20 [0.35] | <0.001 |
| ASI | 53.83 [21.77] | 61.9 [23.5] | 50.0 [19.8] | <0.001 |
| AMSI | 73.78 [29.79] | 85.4 [31.9] | 68.3 [27.1] | <0.001 |
| SIC | 47.30 [38.89] | 61.9 [39.3] | 40.4 [36.8] | <0.001 |
Abbreviations: AMSI, age‐modified shock index; ASI, adjusted shock index; MSI, modified shock index; SI, shock index; SIC, shock index‐C.
The ROC curves and analyses for the various SIs for predicting in‐hospital mortality can be found in Table 3 and Figure 1 . Optimal cut‐off values were identified by maximizing YI, which revealed the following threshold values for the total sample: SI: 0.96 (sensitivity 43.6%, specificity 66.7%, YI: 0.10); MSI: 1.23, (sensitivity 52.0%, specificity 59.6%, YI: 0.12); ASI: 58.10, ASI (sensitivity 50.1%, specificity 72.2%, YI: 0.22); AMSI: 67.59 (sensitivity of 68.4%, specificity of 57.9%, YI: 0.26); and lastly, SIC: 54.22 (sensitivity of 57.8%, specificity of 67.3%, YI: 0.25).
Table 3.
Receiver operating characteristic curve analysis and the optimal thresholds of the SI, MSI, ASI, AMSI, and SIC for predicting in‐hospital mortality
| Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden index | AUC | SE | Lower limit | Upper limit | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SI | 0.96 | 43.6 | 66.7 | 23 | 71 | 0.10 | 0.553 | 0.18 | 0.518 | 0.588 | 0.003 |
| MSI | 1.23 | 52.0 | 59.6 | 29 | 72 | 0.12 | 0.564 | 0.18 | 0.528 | 0.600 | <0.001 |
| ASI | 58.10 | 50.1 | 72.2 | 24 | 75 | 0.22 | 0.654 | 0.16 | 0.621 | 0.686 | <0.001 |
| AMSI | 67.59 | 68.4 | 57.9 | 35 | 79 | 0.26 | 0.667 | 0.17 | 0.634 | 0.700 | <0.001 |
| SIC | 54.22 | 57.8 | 67.3 | 28 | 77 | 0.25 | 0.659 | 0.17 | 0.627 | 0.692 | <0.001 |
Youden index = (sensitivity + specificity) − 1.
Abbreviations: MSI, modified shock index; ASI, adjusted shock index; AMSI, age‐modified shock index; SI, shock index; SIC, shock index‐C; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; SE, standard error.
Figure 1.
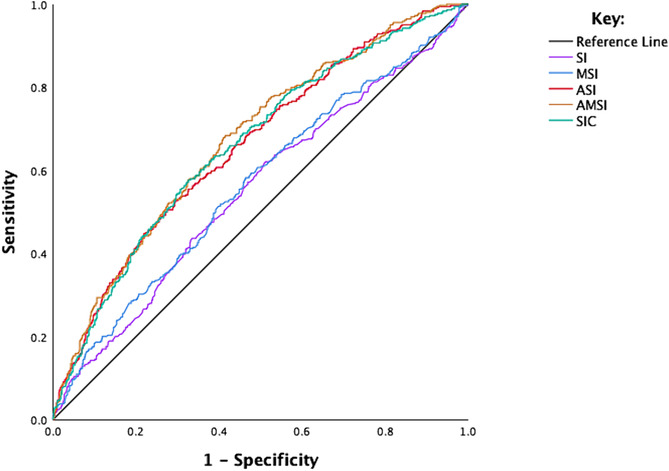
ROC analysis of the SI, MSI, ASI, AMSI and SIC for predicting in‐hospital mortality. AMSI, age‐modified shock index; ASI, adjusted shock index; MSI, modified shock index; ROC, receiver operating characteristic; SI, shock index; SIC, shock index‐C.
In the DeLong comparison of all tests together, SIs exhibited significant differences in their prognostic capabilities for in‐hospital mortality (P < 0.0001). The comparison of AUC values revealed that AMSI (AUC: 0.667, 95% CI: 0.634–0.700, P < 0.001) exhibited superior predictive power compared with all other indices. Specifically, the predictive ability of AMSI was relatively higher than SIC (AUC: 0.659, 95% CI: 0.627–0.692, P < 0.001) but the difference did not achieve statistical significance (P = 0.37). Moreover, SIC showed better predictive power when compared with ASI (AUC: 0.654, 95% CI: 0.621–0.686, P < 0.001) although this difference was not statistically significant (P = 0.92) (Supporting information, Table S1 ). In the individual linear regression analyses, all SI indices showed statistically significant positive associations with in‐hospital mortality (P < 0.001). Notably, the ASI and SIC demonstrated the strongest correlations (Table S2 and Figure S1 ).
Subgroup analysis
We conducted a comprehensive subgroup analysis to assess the discriminatory power of SI indices within specific patient populations defined by important clinical characteristics, including age, sex, acute MI (STEMI, non‐STEMI, and unstable angina), and OHCA. The AUC from ROC for these indices were used to evaluate their predictive performance for in‐hospital mortality within each subgroup (Table 4 , Figure 2 ). Significant AUC values were found for the ASI and AMSI indices for OHCA and SIC index for AMI‐CS and STEMI.
Table 4.
Overall analysis of AUC for shock indices in sub‐groups
| Subgroup | SI AUC (95% CI) | MSI AUC (95% CI) | ASI AUC (95% CI) | AMSI AUC (95% CI) | SIC AUC (95% CI) |
|---|---|---|---|---|---|
| Age (years) | |||||
| >60 | 0.573 [0.529–0.617] | 0.571 [0.526–0.616] | 0.592 [0.549–0.635] | 0.591 [0.546–0.636] | 0.628 [0.586–0.671] |
| <60 | 0.571 [0.512–0.630] | 0.601 [0.540–0.663] | 0.646 [0.590–0.702] | 0.669 [0.611–0.727] | 0.660 [0.601–0.719] |
| Gender | |||||
| Female | 0.502 [0.440–0.563] | 0.487 [0.423–0.551] | 0.636 [0.577–0.695] | 0.625 [0.564–0.686] | 0.663 [0.605–0.721] |
| Male | 0.573 [0.531–0.616] | 0.597 [0.554–0.640] | 0.660 [0.621–0.699] | 0.685 [0.646–0.723] | 0.654 [0.614–0.694] |
| STEMI | 0.601 [0.520–0.682] | 0.615 [0.530–0.700] | 0.652 [0.574–0.730] | 0.651 [0.569–0.734] | 0.714 [0.638–0.789] |
| OHCA | 0.577 [0.453–0.701] | 0.573 [0.447–0.700] | 0.701 [0.592–0.811] | 0.707 [0.594–0.819] | 0.666 [0.550–0.782] |
| AMI‐CS | 0.583 [0.514–0.651] | 0.599 [0.528–0.670] | 0.646 [0.581–0.712] | 0.655 [0.586–0.723] | 0.696 [0.632–0.760] |
Abbreviations: AMI‐CS, acute myocardial infarction–cardiogenic shock; AMSI, age‐modified shock index; ASI, adjusted shock index; AUC, area under the curve; CI, confidence interval; MSI, modified shock index; OHCA, out‐of‐hospital cardiac arrest; SI, shock index; SIC, shock index‐C; STEMI, ST‐ elevation myocardial infarction.
Figure 2.
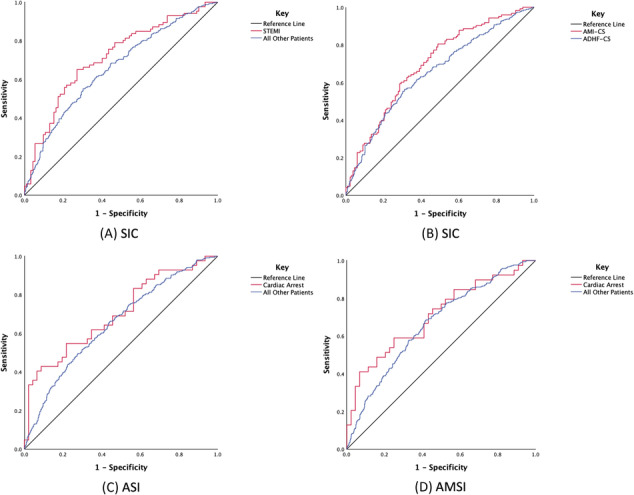
Shock indices for predicting in‐hospital mortality in different sub‐groups: ROC curves for SIC in STEMI (A), SIC in AMI‐CS (B), ASI in OHCA (C), and AMSI in OHCA (D). AMI‐CS: acute myocardial infarction–cardiogenic shock; AMSI, age‐modified shock index; ASI, adjusted shock index; OHCA, out‐of‐hospital cardiac arrest; ROC, receiver operating characteristic; SIC, shock index‐C; STEMI, ST‐ elevation myocardial infarction.
Subgroup analysis by age (<60 and >60 years) and sex displayed AUC values in the range of 0.5 to 0.7 for all indices, indicating moderate predictive performance for in‐hospital mortality. Notably, SIC demonstrated relatively higher AUC values in STEMI patients (0.714, 95% CI: 0.638–0.789, P < 0.001) and in patients with ACS 0.696, 95% CI: 0.632–0.760, P < 0.001), indicating good discriminative ability. Among patients with OHCA, AMSI exhibited a notable AUC of 0.708 (95% CI: 0.593–0.823, P < 0.001), and the ASI displayed a significant AUC of 0.701 (95% CI: 0.592–0.811, P < 0.001), indicating good predictive abilities for in‐hospital mortality. Other indices in the STEMI and OHCA categories predominantly had AUC values within the 0.5 to 0.7 range (Tables S 3–S7 ).
Table 5 summarizes the threshold values for the subgroups of STEMI, AMI‐CS, and OHCA where particular indices show good discriminative ability.
Table 5.
Prognostic performance of shock indices in different sub‐groups
| SIC in STEMI | SIC in AMI‐CS | ASI in OHCA | AMSI in OHCA | |
|---|---|---|---|---|
| Threshold | 53.57 | 35.36 | 74.12 | 97.98 |
| Sensitivity (%) | 65.1 | 80.5 | 42.9 | 41 |
| Specificity (%) | 72.8 | 51.1 | 91.3 | 93.2 |
| PPV (%) | 45 | 59 | 30 | 28 |
| NPV (%) | 52 | 52 | 52 | 53 |
| Youden index | 0.38 | 0.32 | 0.34 | 0.34 |
| LR+ | 2.39 | 1.65 | 4.95 | 6.08 |
| LR− | 0.48 | 0.38 | 0.63 | 0.63 |
Youden index = (sensitivity + specificity) – 1.
Abbreviations: AMI‐CS, acute myocardial infarction—cardiogenic shock; AMSI, age‐modified shock index; ASI, adjusted shock index; LR, likelihood ratio; NPV, negative predictive value; OHCA, out‐of‐hospital cardiac arrest; PPV, positive predictive value; SIC, shock index‐C; STEMI, ST‐elevation myocardial infarction.
Discussion
Clinical characteristics
Identifying higher risk patients presenting with CS is instrumental in managing this population given its significant morbidity. This analysis demonstrates the importance of the SI and its variants in predicting in‐hospital mortality in CS patients. Our population included consecutive CS patients and was device agnostic, representing a ‘real‐world population’. Our findings corroborate the literature indicating that older patients with chronic comorbidities do worse when admitted with CS. 27 Interestingly, non‐survivors were more likely to have de novo presentations of CS—an observation not described previously. 28 Given our institution is a quaternary referral centre for CS management, 40% of patients were transferred in. These patients possibly had higher SCAI stage upon arrival and were ineligible for short term mechanical circulatory support (MCS) or advanced HF therapies after assessment. Furthermore, given the large number of patients with non‐AMI‐CS in our cohort, earlier recognition and therapies for stabilization may have contributed to this observed trend.
Unsurprisingly, patients presenting with ACS and/or OHCA were less likely to survive their hospital stay, which is consistent with the literature. 29 Non‐survivors exhibited a more pronounced manifestation of shock and multi‐organ dysfunction compared with survivors, given the profound haemodynamic and metabolic sequelae resulting from these events as reflected by lower Hb and CrCl levels and elevated WBC and lactates levels. Non‐survivors received more aggressive interventions during their hospitalization, including higher use of vasopressors/inotropes (P = 0.007), mechanical ventilation (P < 0.001) and IABP (P < 0.001). These patients may lack the compensatory mechanisms of low output seen in patients with chronic HF, highlighting the need for a tailored approach in management for varying aetiologies of CS. In contrast, survivors were more likely to receive PAC (P = 0.010) and dLVAD (P < 0.001). Further studies could identify how timing of early MCS, varying CS management strategies based on aetiology, and rapid referral to a CS centre of excellence contribute to outcomes of this population.
Shock indices and mortality
Although the SI and its variants have been used in various acute clinical scenarios to predict outcomes, 6 , 8 , 9 , 30 its utility in CS has not been explored. Our findings show that non‐survivors had statistically significant higher indices across all scores, including calculations incorporating age and renal function, both known to increase mortality in CS. 31 Although all indices were statistically significant between survivors and non‐survivors, their discriminative abilities may be limited. The AUC values for SI were predominantly in the range of 0.5 to 0.7, with the highest value observed being 0.667 for AMSI. Generally, AUC values in this range suggest moderate predictive performance. Based on the DeLong analysis, the SI and MSI exhibited more modest AUCs than the ASI, AMSI, and SIC, suggesting that haemodynamics should not be used in isolation. 21 Prior atrial fibrillation or vasoactive medicine use prior to CICU admission had no effect on AUC of any of the SIs (Tables S10 and S11 ).
Several studies have explored the predictive value of SI derivatives in AHF patients, shedding light on their potential applicability in CS. El‐Menyar et al. conducted a retrospective study with 4818 AHF subjects and found that SI demonstrated the highest AUC for predicting in‐hospital mortality (AUC: 0.70), with a suggested cutoff of 0.9. 16 Pourafkari et al. studied 554 AHF patients, reporting significantly different AUC values for ASI, SI, and MSI, favouring ASI (AUC: 0.68). 32 Bondariyan et al. enrolled 3652 patients in a retrospective study, reporting AUC values for SI (0.668), ASI (0.684), MSI (0.640), and AMSI (0.659), suggesting their utility in assessing in‐hospital mortality. 33 These results align with ours and demonstrate that the application of these indices may not be useful for all CS patients and cannot be used as prognostic tools in isolation for in‐hospital mortality, being one‐time assessments, which do not reflect changes in patient status during their admission. From a clinical perspective, calculated indices can integrate existing tools to intensify and individualize the management of patients at higher risk. These include informed discussions with families, early access to palliative care, and timely utilization of invasive haemodynamics/MCS. 14
If we consider recent CS prognostic scores, it is evident that all approaches share a common challenge in achieving definitive prognostic accuracy. In a recent investigation, Ortega‐Hernández et al. assessed the efficacy of seven CS scores, including SCAI, CARDSHOCK, CSS, and IABP‐SHOCK II score, in predicting in‐hospital mortality among 872 patients. The findings revealed a generally moderate to good performance across all scores in the overall cohort, with CARDSHOCK exhibiting the highest AUC at 0.666. 34 SIs offer simplicity and real‐time assessment, making them accessible and cost‐effective. Complex scoring systems offer a detailed, disease‐specific evaluation but may be resource intensive. The observed limitations emphasize the need for a more comprehensive and detailed phenotyping of CS patients. It becomes crucial to delve deeper into haemodynamic parameters and differentiate between aetiologies to better capture the full spectrum of factors influencing outcomes.
Subgroup analysis
Subgroup analyses revealed moderate performance across age groups (<60 or >60 years and sex) and superior predictive capacity for patients presenting with STEMI, AMI‐CS, and OHCA of particular scores (SIC, ASI, and AMSI). Our findings demonstrated a superior predictive ability of SIC in STEMI and AMI‐CS patients who presented with cardiogenic shock, where it achieved an AUC of 0.714 and 0.696, respectively. This observation is similar to the results of other studies that enrolled patients with a STEMI diagnosis alone. Ran et al. identified the SIC as the best predictor in a broader STEMI patient population (AUC of 0.877). 21 Similarly, Chiang et al. endorsed the SIC's predictive superiority among other SIs in all STEMI patients (AUC of 0.818). 22 Our findings suggest that the SIC's predictability varies depending on the patient subset and severity of the condition resulting in a lower AUC in those with STEMI‐CS than those not in CS.
Our analysis revealed that AMSI and ASI stand out in their predictive ability for in‐hospital mortality in those presenting with OHCA. Van Bergen et al. assessed the utility of SI and MSI predictors of mortality in all patients with OHCA regardless of aetiology. They found an AUC which aligns with our data presented in Table S 7 . 17 The refined predictive prowess of ASI and AMSI may arise from factoring age in its calculation. The thresholds for OHCA patients reflect high specificity, albeit with a lower sensitivity. Consequently, caution is warranted before extrapolating the efficacy of these indices to a broader OHCA demographic given our focus on patients with acute cardiac disease.
Although SI presents an appealing, readily computed tool for clinical guidance, our results show that its application is most useful in distinct CS subgroups including STEMI or OHCA patients. Furthermore, the study underlines the principle that ‘one size fits all’ is not applicable in predicting mortality in CS patients. Prognostic indicators must be interpreted with discretion and should be complementary instruments in a patient's comprehensive clinical analyses. A comprehensive tool incorporating clinical, laboratory, and haemodynamic indicators for different aetiologies is needed. 35 Future studies should focus on creating and validating such integrated models, ensuring they are robust and generalizable across diverse patient populations.
Limitations
The observational nature of our research allows us to establish associations and not causation. It is crucial to highlight that 40% of our CS patients were transferred from another hospital. This could introduce a significant bias, given that recorded SI might not reflect the true initial presentation of the patient in the CCU. Instead, these indices were potentially captured at a later stage. To address this concern, we calculated the AUCs for all indices in the subgroup of patients transferred from another hospital and compared the values with those non‐transferred. The analysis revealed no significant difference between the two groups (Tables S 8 and S9 ).
Furthermore, while our cohort is sizable, it is derived from a single centre. Future multicentre, prospective studies are crucial to validate our findings and to compare SI metrics directly with established risk scores for CS.
Conclusions
While SI was independently associated with in‐hospital mortality in CS patients, the clinical application is limited. Notably, SIC, ASI, and AMSI showed enhanced predictive capacity in subgroups, like STEMI‐CS, ACS‐CS, and in shock patients presenting with OHCA, suggesting that context‐specific utilization might be beneficial. To maximize the utility of these indices, integration into a broader prognostic model that encompasses clinical, laboratory, and haemodynamic parameters could be more effective.
Funding
Darshan H. Brahmbhatt is supported by TRANSFORM HF. Adriana Luk is supported by the Heart and Stroke Foundation/University of Toronto Polo Chair in Cardiology Young Investigator Award.
Conflict of interest
None of the authors declare that they have a conflict of interest.
Supporting information
Table S1. DeLong pairwise comparison tests between different shock indices using the AUC values.
Table S2. Individual linear regression results for each index *.
Figure S1. Calibration for in‐hospital mortality of SI (a), MSI (b), ASI (c), AMSI (d) and SIC (e).
Table S3. Age Subgroup analysis.
Table S4. Sex Subgroup analysis.
Table S5. STEMI Subgroup analysis.
Table S6. AMI‐CS Subgroup analysis.
Table S7. OHCA Subgroup analysis.
Table S8. Transferred patients Subgroup analysis.
Table S9. Comparison of ROC Curve Area Differences in Independent Shock Indices.
Table S10. Comparison of ROC Curve Area Differences for Patients with Atrial Arrhythmia.
Table S11. Comparison of ROC Curve Area Differences for Patients with Prior Vasopressor/Inotrope use.
Colarusso, L. , Brahmbhatt, D. H. , Scolari, F. L. , Keon, K. A. , Shin, E. , De Pellegrin Overgaard, A. I. , Nisar, M. , Fung, N. , Ibrahimova, N. , Billia, F. , Overgaard, C. B. , and Luk, A. C. (2024) Decoding cardiogenic shock: assessing shock index and its variants as prognostic indicators for in‐hospital mortality. ESC Heart Failure, 11: 3023–3032. 10.1002/ehf2.14853.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017;136:e232‐e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2. Thiele H, Zeymer U, Neumann F‐J, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287‐1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895‐e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 4. Sciaccaluga C, Mandoli GE, Ghionzoli N, Anselmi F, Dini CS, Righini F, et al. Risk stratification in cardiogenic shock: a focus on the available evidence. Heart Fail Rev 2022;27:1105‐1117. doi: 10.1007/s10741-021-10140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allgöwer M, Burri C. Schockindex. DMW ‐ Deutsche Medizinische Wochenschrift 1967;92:1947‐1950. doi: 10.1055/s-0028-1106070 [DOI] [PubMed] [Google Scholar]
- 6. el‐Menyar A, Goyal P, Tilley E, Latifi R. The clinical utility of shock index to predict the need for blood transfusion and outcomes in trauma. J Surg Res 2018;227:52‐59. doi: 10.1016/j.jss.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 7. Ozsu S, Erbay M, Durmuş ZG, Ozlu T. Classification of high‐risk with cardiac troponin and shock index in normotensive patients with pulmonary embolism. J Thromb Thrombolysis 2017;43:179‐183. doi: 10.1007/s11239-016-1443-3 [DOI] [PubMed] [Google Scholar]
- 8. Rady MY, Nightingale P, Little RA, Edwards JD. Shock index: a re‐evaluation in acute circulatory failure. Resuscitation 1992;23:227‐234. doi: 10.1016/0300-9572(92)90006-X [DOI] [PubMed] [Google Scholar]
- 9. Berger T, Green J, Horeczko T, Hagar Y, Garg N, Suarez A, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168‐174. doi: 10.5811/westjem.2012.8.11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cannon CM, Braxton CC, Kling‐Smith M, Mahnken JD, Carlton E, Moncure M. Utility of the shock index in predicting mortality in traumatically injured patients. J Trauma: Injury Infect Crit Care 2009;67:1426‐1430. doi: 10.1097/TA.0b013e3181bbf728 [DOI] [PubMed] [Google Scholar]
- 11. el‐Menyar A, Al Habib KF, Zubaid M, Alsheikh‐Ali AA, Sulaiman K, Almahmeed W, et al. Utility of shock index in 24,636 patients presenting with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2020;9:546‐556. doi: 10.1177/2048872619886307 [DOI] [PubMed] [Google Scholar]
- 12. Zarzaur BL, Croce MA, Fischer PE, Magnotti LJ, Fabian TC. New vitals after injury: shock index for the young and age × shock index for the old. J Surg Res 2008;147:229‐236. doi: 10.1016/j.jss.2008.03.025 [DOI] [PubMed] [Google Scholar]
- 13. Mudd JO, Kass DA. Tackling heart failure in the twenty‐first century. Nature 2008;451:919‐928. doi: 10.1038/nature06798 [DOI] [PubMed] [Google Scholar]
- 14. Oh GC, An S, Lee H, Cho HJ, Jeon ES, Lee SE, et al. Modified reverse shock index predicts early outcomes of heart failure with reduced ejection fraction. ESC Heart Fail 2022;9:3232‐3240. doi: 10.1002/ehf2.14031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Wang Z, Wang Z, Fang M, Shu Z. The prognostic value of shock index for the outcomes of acute myocardial infarction patients. Medicine 2017;96:e8014. doi: 10.1097/MD.0000000000009313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. el‐Menyar A, Sulaiman K, Almahmeed W, al‐Motarreb A, Asaad N, AlHabib KF, et al. Shock index in patients presenting with acute heart failure: a multicenter multinational observational study. Angiology 2019;70:938‐946. doi: 10.1177/0003319719857560 [DOI] [PubMed] [Google Scholar]
- 17. van Bergen KMG, van Kooten L, Eurlings CGMJ, Foudraine NA, Lameijer H, Meeder JG, et al. Prognostic value of the shock index and modified shock index in survivors of out‐of‐hospital cardiac arrest: a retrospective cohort study. Am J Emerg Med 2022;58:175‐185. doi: 10.1016/j.ajem.2022.05.039 [DOI] [PubMed] [Google Scholar]
- 18. Rady MY, Smithline HA, Blake H, Nowak R, Rivers E. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med 1994;24:685‐690. doi: 10.1016/S0196-0644(94)70279-9 [DOI] [PubMed] [Google Scholar]
- 19. Yu T, Tian C, Song J, He D, Sun Z, Sun Z. Age shock index is superior to shock index and modified shock index for predicting long‐term prognosis in acute myocardial infarction. Shock 2017;48:545‐550. doi: 10.1097/SHK.0000000000000892 [DOI] [PubMed] [Google Scholar]
- 20. Zhou J, Shan P‐R, Xie Q‐L, Zhou XD, Cai MX, Xu TC, et al. Age shock index and age‐modified shock index are strong predictors of outcomes in ST‐segment elevation myocardial infarction patients undergoing emergency percutaneous coronary intervention. Coron Artery Dis 2019;30:398‐405. doi: 10.1097/MCA.0000000000000759 [DOI] [PubMed] [Google Scholar]
- 21. Ran P, Wei X, Lin Y, Li G, Huang JL, He XY, et al. Shock index‐C: an updated and simple risk‐stratifying tool in ST‐segment elevation myocardial infarction. Front Cardiovasc Med 2021;8:657817. doi: 10.3389/fcvm.2021.657817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiang C‐Y, Lin C‐F, Liu P‐H, Chen FC, Chiu IM, Cheng FJ. Clinical validation of the shock index, modified shock index, delta shock index, and shock index‐C for emergency department ST‐segment elevation myocardial infarction. J Clin Med 2022;11:5839. doi: 10.3390/jcm11195839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Liu J, Fang ZA, Shan GL, Xu J, Qi ZW, et al. Modified shock index and mortality rate of emergency patients. World J Emerg Med 2012;3:114‐117. doi: 10.5847/wjem.j.issn.1920-8642.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Booysen HL, Woodiwiss AJ, Raymond A, Sareli P, Hsu HC, Dessein PH, et al. Chronic kidney disease epidemiology collaboration‐derived glomerular filtration rate performs better at detecting preclinical end‐organ changes than alternative equations in black Africans. J Hypertens 2016;34:1178‐1185. doi: 10.1097/HJH.0000000000000924 [DOI] [PubMed] [Google Scholar]
- 25. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32‐35. doi: [DOI] [PubMed] [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 27. Kalra S, Ranard LS, Memon S, Rao P, Garan AR, Masoumi A, et al. Risk prediction in cardiogenic shock: current state of knowledge, challenges and opportunities. J Card Fail 2021;27:1099‐1110. doi: 10.1016/j.cardfail.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 28. Olarte N, Rivera NT, Grazette L. Evolving presentation of cardiogenic shock: a review of the medical literature and current practices. Cardiol Ther 2022;11:369‐384. doi: 10.1007/s40119-022-00274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Obling L, Frydland M, Hansen R, Møller‐Helgestad OK, Lindholm MG, Holmvang L, et al. Risk factors of late cardiogenic shock and mortality in ST‐segment elevation myocardial infarction patients. Eur Heart J Acute Cardiovasc Care 2018;7:7‐15. doi: 10.1177/2048872617706503 [DOI] [PubMed] [Google Scholar]
- 30. Shariefuddin WWA, Pramudyo M, Martha JW. Shock index creatinine: a new predictor of mortality in acute coronary syndrome patients. BMC Cardiovasc Disord 2024;24:87. doi: 10.1186/s12872-024-03730-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cywinski JB, Mascha EJ, Kurz A, Sessler DI. Estimated glomerular filtration rate better predicts 30‐day mortality after non‐cardiac surgery than serum creatinine: a retrospective analysis of 92,888 patients. Can J Anesthesia/J Canadien d'anesthésie 2015;62:745‐752. doi: 10.1007/s12630-015-0398-8 [DOI] [PubMed] [Google Scholar]
- 32. Pourafkari L, Wang CK, Schwartz M, Nader ND. Does shock index provide prognostic information in acute heart failure? Int J Cardiol 2016;215:140‐142. doi: 10.1016/j.ijcard.2016.04.083 [DOI] [PubMed] [Google Scholar]
- 33. Bondariyan N, Vakhshoori M, Sadeghpour N, Shafie D. Prognostic value of shock index, modified shock index, and age‐adjusted derivatives in prediction of in‐hospital mortality in patients with acute decompensated heart failure: Persian registry of cardiovascular disease/heart failure study. Anatolian J Cardiol 2022;26:210‐217. doi: 10.5152/AnatolJCardiol.2021.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ortega‐Hernández J, González‐Pacheco H, Gopar‐Nieto R, Araiza‐Garaygordobil D, Sierra Lara‐Martínez D, Briseño de la Cruz JL, et al. Comparison of the predictive performance of cardiogenic shock scores in a real‐world LATIN AMERICA country. Shock 2023;59:576‐582. doi: 10.1097/SHK.0000000000002091 [DOI] [PubMed] [Google Scholar]
- 35. Saku K, Nakata J. How should we develop new risk scores for cardiogenic shock? Circ J 2022;86:695‐698. doi: 10.1253/circj.CJ-21-0953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. DeLong pairwise comparison tests between different shock indices using the AUC values.
Table S2. Individual linear regression results for each index *.
Figure S1. Calibration for in‐hospital mortality of SI (a), MSI (b), ASI (c), AMSI (d) and SIC (e).
Table S3. Age Subgroup analysis.
Table S4. Sex Subgroup analysis.
Table S5. STEMI Subgroup analysis.
Table S6. AMI‐CS Subgroup analysis.
Table S7. OHCA Subgroup analysis.
Table S8. Transferred patients Subgroup analysis.
Table S9. Comparison of ROC Curve Area Differences in Independent Shock Indices.
Table S10. Comparison of ROC Curve Area Differences for Patients with Atrial Arrhythmia.
Table S11. Comparison of ROC Curve Area Differences for Patients with Prior Vasopressor/Inotrope use.


