Abstract
Aims
A set of indicators to assess the quality of care for patients hospitalized for heart failure was developed by an expert working group of the Italian Health Ministry. Because a better performance profile measured using these indicators does not necessarily translate to better outcomes, a study to validate these indicators through their relationship with measurable clinical outcomes and healthcare costs supported by the Italian National Health System was carried out.
Methods and results
Residents of four Italian regions (Lombardy, Marche, Lazio, and Sicily) who were newly hospitalized for heart failure (irrespective of stage and New York Heart Association class) during 2014–2015 entered in the cohort and followed up until 2019. Adherence to evidence‐based recommendations [i.e. renin–angiotensin–aldosterone system (RAS) inhibitors, beta‐blockers, mineralocorticoid receptor antagonists (MRAs), and echocardiograms (ECCs)] experienced during the first year after index discharge was assessed. Composite clinical outcomes (cardiovascular hospital admissions and all‐cause mortality) and healthcare costs (hospitalizations, drugs, and outpatient services) were assessed during the follow‐up. The restricted mean survival time at 5 years (denoted as the number of months free from clinical outcomes), the hazard of clinical outcomes (according to the Cox model), and average annual healthcare cost (expressed in euros per person‐year) were compared between adherent and non‐adherent patients. A non‐parametric bootstrap method based on 1000 resamples was used to account for uncertainty in cost‐effectiveness estimates. A total of 41 406 patients were included in this study (46.3% males, mean age 76.9 ± 9.4 years). Adherence to RAS inhibitors, beta‐blockers, MRAs, and ECCs were 64%, 57%, 62%, and 20% among the cohort members, respectively. Compared with non‐adherent patients, those who adhered to ECCs, RAS inhibitors, beta‐blockers, and MRAs experienced (i) a delay in the composite outcome of 1.6, 1.9, 1.6, and 0.6 months and reduced risks of 9% (95% confidence interval, 2–14%), 11% (7–14%), 8% (5–11%), and 4% (−1–8%), respectively; and (ii) lower (€262, €92, and €571 per year for RAS inhibitors, beta‐blockers, and MRAs, respectively) and higher costs (€511 per year for ECC). Adherence to RAS inhibitors, beta‐blockers, and MRAs showed a delay in the composite outcome and a saving of costs in 98%, 84%, and 93% of the 1000 bootstrap replications, respectively.
Conclusions
Strict monitoring of patients with heart failure through regular clinical examinations and drug therapies should be considered the cornerstone of national guidelines and audits.
Keywords: Heart failure; Healthcare utilization database, population‐based cohort study; Care pathways; Guideline‐driven recommendations
Introduction
Heart failure (HF) is a common chronic condition characterized by a decline in the heart's ability to function as a pump. 1 An overall lifetime risk of developing HF of 33% in men and 28% in women has been reported. 2 The hospitalization rate for HF, particularly in developed countries with aging populations, has steadily increased over the last decade, 3 , 4 making HF the leading cause of hospital admission among the elderly. 5 In addition, HF imposes a huge economic burden, estimated at USD 108 billion annually in 2008, and this value is projected to continue to rise with the rapid increase in aging populations. 6 Taken together, these data explain why HF has become a major public health concern and has been described as one of the ‘emerging’ pandemics of the 21st century. 7 , 8
In‐hospital mortality from HF has substantially decreased in most developed countries, 9 probably because of the widespread adoption of good practices for acute management of the disease. 1 , 10 , 11 This suggests that the healthcare of patients with HF after hospital discharge presents a major challenge for reducing rehospitalization, lengthening survival, and enhancing quality of life. 11 Accordingly, the current guidelines include several evidence‐based recommendations for out‐of‐hospital healthcare. 1 , 10 , 11 Among them, drug therapy [mainly using renin–angiotensin–aldosterone system (RAS) inhibitors, beta‐blockers, and mineralocorticoid receptor antagonists (MRAs)] has been shown to delay disease progression and improve survival, 1 including the sodium–glucose transport protein 2 inhibitors, which are the most important update in the last European guidelines. 1 In addition, monitoring with echocardiogram (ECC) is recommended for identifying cardiac dysfunction and for early initiation of suitable treatment. 12 , 13 Unfortunately, adherence to guidelines for patients with chronic HF in primary healthcare settings has been systematically reported to be suboptimal. 12 , 13 , 14 , 15 , 16 , 17 Several factors are involved in this phenomenon, including patients' (e.g. socio‐demographic and clinical determinants 16 ), physicians' (e.g. years of experience and medical specialty 18 ), and healthcare system's characteristics (e.g. the presence of chronic care models 19 ). However, some strategies that involve patients in the transition from the hospital to the home represent a promising opportunity to improve out‐of‐hospital care for HF patients. 20
A panel of experts operating under the auspices of the Italian Health Ministry, the so‐called MAP (monitoring and assessing diagnostic‐therapeutic paths) working group, focuses on measuring and comparing integrated care pathways for patients with specific clinical conditions, including hospitalized HF, through a system of performance indicators. Because a better performance profile measured using these indicators does not necessarily translate to better outcomes, at least one study was designed to validate the set of indicators by examining their association with measurable clinical and economic outcomes for each considered condition. The performance indicators developed for women operated for breast cancer, 21 pregnant women, 22 and patients with severe mental disorders 23 were associated with better clinical outcomes whereas the corresponding indicators for diabetes also showed a reduction in healthcare costs. 24 , 25 This study aimed to assess the impact of adherence to evidence‐based recommendations for the out‐of‐hospital management of patients with HF on measurable clinical outcomes and healthcare costs supported by the Italian National Health System (NHS).
Methods
Data source
All Italian citizens have equal access to healthcare services as part of the NHS. An automated system of healthcare utilization (HCU) databases allows each Italian region to manage the NHS locally. HCU data collect a variety of information, including (i) an archive of residents who receive NHS assistance (NHS beneficiaries); (ii) a database on hospital discharge records, including information about diagnoses and procedures for inpatients hospitalized in public or private hospitals (coded according to ICD‐CM‐9 codes); (iii) an outpatient drug prescription database (coded according to ATC codes); and (iv) a database on outpatient services, including specialist visits and diagnostic exams reimbursable by the NHS (e.g. ECC). The cost of each service provided to an NHS beneficiary and reimbursed to a healthcare provider is also recorded regularly. As a unique identification code is systematically used across all HCU databases within each region, their record linkage allows searching for the complete care pathway for NHS beneficiaries. Additional details regarding HCU databases in the context of cardiovascular diseases in general, and more specifically for HF, are available in existing literature. 6 , 26 , 27
In this study, we established links between the aforementioned four databases and each participating region in the study. The analyses were conducted separately within each participating region, and summarized estimates were derived.
Harmonization and data processing
This study is based on computerized HCU databases from four regions located in the north (Lombardy), centre (Marche and Lazio), and south (Sicily) of Italy. Approximately 22 million beneficiaries of the Italian NHS were recorded in the corresponding databases, accounting for 37% of the Italian population.
Although databases did not substantially differ across regions in several aspects, some information were differently recorded. For example, the patient's sex was encoded as ‘M’ or ‘F’ in some regions, and as ‘1’ or ‘0’ in others. Thus, a between‐region data harmonization was performed, which allowed data extraction processes to be targeted to the same semantic concepts (e.g. information was uniformly encoded using the same names, values, and formats). This allowed to extract and process locally anonymized data using a common script, which reduces the chance of heterogeneity in data analysis between regions (and therefore increases the consistency of results across regions). The script was developed by one of the authors (F. R.) in accordance with the protocol previously approved by the MAP working group, and all analyses were conducted using Statistical Analysis System Software (version 9.4; SAS Institute, Cary, NC, USA).
The specific diagnostic and therapeutic codes used in this study are provided in Supporting information, Table S1 .
Privacy‐by‐design issues
Privacy‐by‐design (or data protection‐by‐design) principles were adopted in this study. 28 In general, a safe environment was ensured by the regional infrastructure, which prevented the unauthorized import/export of individual records. Data security was ensured by the pseudo‐anonymization of identification codes and by the condition that outputs were exported only when more than three individual records typified each category with which the beneficiaries were stratified. Secure individuals were guaranteed that only a few of the authors (F. R., M. I., A. L., and G. F.) were authorized for data analysis. Finally, the safety of the project was ensured through the assessment of Regional Health Authorities regarding potential public benefits. Because data of this nature (i.e. HCU data collected primarily for reimbursing healthcare providers) should be used for secondary purposes admitted that data owners (i.e. the regions) recognize their implications, the authorization granted by Regional Health Authorities for this project reflects their acknowledgment that the study serves a functional purpose in generating knowledge aimed at improving the quality of care. A comprehensive overview of ‘privacy research environments by design’ can be found in existing literature. 29
Cohort selection and follow‐up
Just over 10 million NHS beneficiaries aged ≥50 years who were residents of one of the four participating Italian regions formed the target population. Among these, those who were hospitalized at least once for HF during 2014–2015 were identified from the hospital discharge database, and the first hospitalization that occurred during this period was denoted as the index one (i.e. index hospital admission, index hospital discharge, and index hospital stay, where applicable). To ensure that the data were relevant to the aim of the study, several categories of patients were excluded, including (i) patients who died during the index hospital stay (because the aim was to investigate healthcare provided), (ii) those who had been beneficiaries of the NHS for <3 years before the index admission (because we were interested in characterizing each cohort member according to the NHS services previously received), (iii) those who had been hospitalized with a primary or secondary diagnosis of HF within 3 years before the index admission (because we aimed to include patients with a common onset of the event that initiated the observation), and (iv) those who died, were hospitalized for cardiovascular events, moved to another region, or emigrated to another country during the first year after the index discharge (to allow cohort members to accumulate at least 1 year of exposure to recommendations). The remaining patients were included as the final cohort.
Adherence to recommendations
During the first year after the index discharge (i.e. during the period that we called ‘exposure time‐span’), four indicators for measuring the quality of HF care were assessed, inspired by the recommendations for adherence to drug therapy and ECC. 1 For adherence to drug therapy (i.e. RAS inhibitors, beta‐blockers, and MRAs), we proceeded as follows: following the user‐only paradigm, 30 patients who did not dispense the prescribed drug within 3 months after the index discharge were excluded from the drug adherence evaluation to minimize potential confounding by indication. For each patient, drugs dispensed during the year after the date of the first drug prescription were identified. The period covered by a prescription was calculated by dividing the total amount of the drug prescribed by a defined daily dose. To consider the lower maintenance dosage used among patients with HF rather than that for their main indication (i.e. hypertension), 31 the coverage for beta‐blockers and MRAs was adjusted based on specific weights (Table S2 ). In case of overlapping prescriptions, the patient was assumed to have completed the former before starting the latter. Adherence to therapy was determined by dividing the cumulative number of days covered by the drug by 365 days, with the ratio defined as the proportion of days covered by treatment or proportion of days covered (PDC). 32 Patients with a PDC of ≥75% were defined as adherent to the drug therapy, while those below this threshold were considered non‐adherent. The 75% threshold was selected because in previous studies using the Lombardy database, this adherence level was associated with a marked reduction in clinical outcomes for patients hospitalized due to cardiovascular events. 33
Regarding adherence to ECC, patients were considered adherent if they underwent at least one control during the exposure time‐span; otherwise, they were classified as non‐adherent.
Data on dispensed drugs and ECC were retrieved from outpatient drug prescription and outpatient service databases, respectively.
Additional information
Baseline characteristics of the cohort members were collected, which included sex, age, non‐pharmacological treatments received during the index hospitalization, drug therapies, and comorbidities. Drug therapies included blood pressure‐lowering and lipid‐lowering agents, digitalis glycosides, organic nitrates, antiarrhythmics, antithrombotics, antidiabetics, non‐steroidal anti‐inflammatory and anti‐gout agents, drugs for pulmonary diseases, and antidepressants. Comorbidities included ischemic heart disease, cerebrovascular disease, diabetes, kidney disease, respiratory disease, and cancer. Comorbidities were identified from in‐hospital diagnoses and drug treatments recorded in the outpatient prescription database during the previous 3 years. The Multisource Comorbidity Score (MCS), a simple score developed and validated in Italy, 34 was used to assess the clinical profile of each cohort member. Three categories of clinical profiles were considered: good (MCS = 0), intermediate (1 ≤ MCS ≤ 2), and poor (3 ≤ MCS ≤ 4). Finally, the number of contacts that each cohort member had with the NHS during the 3‐year period before the index hospital admission was recorded. This measure was considered a proxy for the patient's behaviour in searching for health services.
Propensity score matching
To enhance the comparability of patients classified as adherent or non‐adherent to each indicator, a propensity score (PS) matching design was used. 35 With this aim, logistic regression was employed to model the probability of being classified as adherent, taking into account the previously mentioned covariates (i.e. sex, age, non‐pharmacological treatments received during the index hospitalization, drug therapies, and comorbidities). For each cohort member classified as adherent to a given healthcare recommendation, one non‐adherent patient was randomly selected to establish a 1:1 match for the PS using the nearest neighbour‐matching algorithm. 36 To assess between‐group differences in baseline characteristics, the standardized difference was calculated for each measured covariate both before and after PS matching. 37
Health‐related outcomes
The composite outcomes of all‐cause mortality and hospital admission for cardiovascular disease (primary endpoint) and all‐cause mortality (secondary endpoint) were considered. Follow‐up started from the date corresponding to 1 year after the index hospital discharge when analysing adherence to ECC or 1 year after the date of the first drug prescription until the first occurring outcome or censoring (emigration, 5 years after cohort entry, or endpoint of follow‐up, i.e. 31 December 2019). A proportional hazard Cox model was fitted to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the association between adherence to the considered out‐of‐hospital healthcare and the occurrence of health‐related outcomes. In addition, the average time free from health‐related outcomes experienced by each cohort member during follow‐up was considered. The restricted mean survival times, 38 calculated using the area under the Kaplan–Meier curves, were denoted as the number of months free from health‐related outcomes at 5 years experienced by each cohort member. 39
Healthcare costs
Costs from the NHS perspective were assessed as the amount reimbursed by the Regional Health Authority to healthcare providers, and were available in our databases. These costs included costs related to hospital admissions, drugs, and outpatient services (e.g. specialist visits, laboratory examinations, and imaging). Healthcare costs accumulated by each cohort member started from the date of the index hospital discharge until death, migration, 5 years after cohort entry, or the end of follow‐up (i.e. 31 December 2019). Healthcare costs accumulated by a given matched cohort were divided by the number of person‐years accumulated in that cohort and expressed as a rate. The corresponding measure was denoted as the average annual healthcare cost and expressed in euros per person‐year.
Because retrospective data were analysed without forecasting outcomes in the future, no adjustment were performed on cost data.
Cost‐effectiveness profiles
The cost‐effectiveness profile of adherence to each of the aforementioned recommendations at 5 years was investigated using the incremental cost‐effectiveness ratio (ICER), which represents the additional cost required to gain a month free from health‐related outcomes due to enhanced adherence to recommendations. 40 This measure was calculated by dividing the difference in healthcare costs and restricted mean survival times between groups (adherent and non‐adherent patients). A non‐parametric bootstrap method based on 1000 resamples was used to account for uncertainty in cost‐effectiveness estimates. 41
Sensitivity analysis
As an intention‐to‐treat approach was adopted in the main analysis (i.e. exposure experienced in the first year after discharge was linked to subsequent outcomes), an as‐treated approach was used to assess the robustness of the health‐related findings. To account for the dynamic nature of adherence over time, dummy factors for adherence categories were introduced into the Cox models as time‐dependent covariates. Adherence to recommendations was assessed across all years preceding the onset of outcomes using the following definition: regarding adherence to drug therapy, patients were defined as ‘full adherents’ if they spent at least 75% of the time span of each year (previous to the outcome onset) with the drug available, ‘partial adherents’ if their PDC was ≥75% for at least half of the years of follow‐up, and ‘non‐adherents’ otherwise; regarding adherence to ECC, patients were considered ‘fully adherents’ if they underwent at least a control during each year (previous to the outcome onset), ‘partial adherents’ if they underwent control in at least half of the years of follow‐up, and ‘non‐adherents’ otherwise.
Summarizing between‐region estimates
Given the constraint that the data could not leave the regional platform, the described procedure was independently applied within each of the four participating regions. Subsequently, estimates were summarized using meta‐analytic procedures, specifically employing a weighted average of the estimates by using the reciprocal of the variance (HRs and restricted mean survival times) or by the number of patients (costs and ICERs). 42
Results
Patients
Among the 120 023 eligible NHS beneficiaries hospitalized for HF, 41 406 met the inclusion criteria and were included in this study (Figure S1 ). The characteristics of the cohort members are presented in Table 1 . The mean patient age was 77 years, and less than half of the patients were men. Approximately one in ten patients had ischemic heart disease, cerebrovascular disease, and diabetes. Four in five patients were co‐treated with antihypertensive drugs; four in ten with lipid‐lowering drugs; and three in ten with antidiabetic drugs. Almost one in six patients had a poor clinical profile.
Table 1.
Baseline characteristics of cohort members
| Characteristics | N (%) |
|---|---|
| Male sex | 19 185 (46.3%) |
| Age (years): mean [SD] | 76.9 [9.4] |
| Region | |
| Lombardy | 17 306 (41.8%) |
| Marche | 3354 (8.1%) |
| Lazio | 10 105 (24.4%) |
| Sicily | 10 641 (25.7%) |
| Procedures during the index hospitalization | |
| CRT implantation | 598 (1.4%) |
| ICD | 708 (1.7%) |
| PTCA | 725 (1.8%) |
| CABG | 642 (1.6%) |
| Comorbidities a | |
| Ischemic heart disease | 5718 (13.8%) |
| Cerebrovascular disease | 3683 (8.9%) |
| Diabetes | 4565 (11.0%) |
| Kidney disease | 2730 (6.6%) |
| Respiratory disease | 5803 (14.0%) |
| Cancer | 3133 (7.6%) |
| Medications a | |
| RAS inhibitors /beta‐blockers/MRAs | 34 383 (83.0%) |
| Other antihypertensive drugs | 28 449 (68.7%) |
| Digitalis glycosides | 4088 (9.9%) |
| Organic nitrates | 6932 (16.7%) |
| Antiarrhythmics | 5630 (13.6%) |
| Lipid‐lowering agents | 18 136 (43.8%) |
| Antithrombotic agents | 30 455 (73.6%) |
| Antidiabetic drugs | 12 131 (29.3%) |
| NSAIDs | 18 519 (44.7%) |
| Anti‐gout drugs | 7960 (19.2%) |
| Drugs for pulmonary diseases | 12 978 (31.3%) |
| Antidepressants | 6896 (16.7%) |
| Clinical profile b | |
| Good | 10 787 (26.1%) |
| Intermediate | 24 170 (58.4%) |
| Poor | 6449 (15.6%) |
| Echocardiogram a | 12 201 (29.5%) |
| Number of services received by the NHS a : mean [SD] | 288.1 [200.4] |
CABG, coronary artery bypass graft; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter‐defibrillator; MRAs, mineralocorticoid receptor antagonists; NSAIDs, non‐steroidal anti‐inflammatory drugs; PTCA, percutaneous transluminal coronary angioplasty; RAS, renin–angiotensin system; SD, standard deviation.
Within 3 years from index hospitalization.
The clinical profile was assessed by the Multisource Comorbidity Score (MCS) according to the hospital admission and the drugs prescribed in the 3‐year period before the index date. Three categories of clinical profile were considered: good (MCS = 0), intermediate (1 ≤ MCS ≤ 2), and poor (3 ≤ MCS ≤ 4).
Adherence to recommendations
Table 2 shows that almost 20% of patients adhered to the ECC (18.9%), approximately 60% received prescriptions for RAS inhibitors (64.1%) and beta‐blockers (60.5%), and just over a third received prescriptions for MRAs (35.3%). Among drug users, adherence to therapy was achieved by 64.0%, 57.3%, and 61.6% of RAS inhibitor, beta‐blocker, and MRA users, respectively.
Table 2.
Adherence to recommendations in the first year after discharge from the index hospital admission for heart failure
| Recommendations | N (%) |
|---|---|
| Echocardiogram a | 7846 (18.9%) |
| RAS inhibitors | |
| Users b | 26 558 (64.1%) |
| Adherents c | 16 996 (64.0%) |
| Beta‐blockers | |
| Users b | 25 056 (60.5%) |
| Adherents c | 14 350 (57.3%) |
| MRAs | |
| Users b | 14 599 (35.3%) |
| Adherents c | 8991 (61.6%) |
MRAs, mineralocorticoid receptor antagonists; RAS, renin–angiotensin system.
At least a control.
At least a prescription within 3 months after the index discharge.
Proportion of days covered by drug prescription ≥75%. Adherence was assessed among drug users.
Baseline characteristics of cohort members according to adherence to recommendations are shown in Table S3–S6 . With a few exceptions, there were no substantial differences in covariate distributions between adherent and non‐adherent patients. Conversely, compared with patients who did not undergo ECC, those who experienced ECC were younger men who received more extensive non‐pharmacological therapy during the index admission and fewer previous medications and had a good clinical profile. After the PS matching procedure, there was no evidence of between‐group differences in covariate distributions.
Adherence → health‐related outcomes
The event rates of health‐related outcomes are reported in Table S7 . As shown in Table 3 , compared with non‐adherent patients, those who adhered to ECC, RAS inhibitors, beta‐blockers, and MRAs experienced a delay in the composite outcome of 1.6, 1.9, 1.6, and 0.6 months, respectively. Additionally, they exhibited reduced risks of 9% (95% CI, 2–14%), 11% (95% CI, 7–14%), 8% (95% CI, 5–11%), and 4% (95% CI, −1% to 8%). Similar benefits associated with adherence to recommendations were observed for all‐cause mortality when the outcomes were considered separately.
Table 3.
Health‐related outcome estimates among matched cohort members
| Outcome | Adherence | Echocardiogram | RAS inhibitors | Beta‐blockers | MRAs | ||||
|---|---|---|---|---|---|---|---|---|---|
| RMST (months) | HR (95% CI) | RMST (months) | HR (95% CI) | RMST (months) | HR (95% CI) | RMST (months) | HR (95% CI) | ||
| Composite outcome a | No adherent | 36.9 | 1.00 (Ref.) | 35.7 | 1.00 (Ref.) | 35.2 | 1.00 (Ref.) | 34.8 | 1.00 (Ref.) |
| Adherent | 38.5 | 0.91 (0.86–0.98) | 37.6 | 0.89 (0.86–0.93) | 36.8 | 0.92 (0.89–0.95) | 35.4 | 0.96 (0.92–1.01) | |
| All‐cause death | No adherent | 44.9 | 1.00 (Ref.) | 42.9 | 1.00 (Ref.) | 42.3 | 1.00 (Ref.) | 42.3 | 1.00 (Ref.) |
| Adherent | 46.5 | 0.88 (0.83–0.93) | 45.3 | 0.84 (0.80–0.88) | 44.3 | 0.89 (0.85–0.93) | 42.1 | 1.01 (0.96–1.07) | |
CI, confidence interval; HR, hazard ratio; MRAs: mineralocorticoid receptor antagonists; RAS, renin–angiotensin system; RMST, restricted mean survival time.
Cardiovascular hospitalization or all‐cause death.
Adherence → healthcare costs
Table 4 presents the average annual healthcare costs for each patient. Patients who adhered to ECC incurred higher healthcare costs during follow‐up than non‐adherent patients (€5936 vs. €5425 per year). Conversely, adherence to drug therapy was associated with lower costs (€4610 vs. €4872 for RAS inhibitors, €4883 vs. €4975 for beta‐blockers, and €4859 vs. €5430 for MRAs).
Table 4.
Average annual healthcare cost (Euro) per patient according to adherence with recommendations
| Echocardiogram | RAS inhibitors | Beta‐blockers | MRAs | |||||
|---|---|---|---|---|---|---|---|---|
| Non‐adherent | Adherent | Non‐adherent | Adherent | Non‐adherent | Adherent | Non‐adherent | Adherent | |
| Hospitalizations | ||||||||
| Heart failure | 332 | 417 | 300 | 304 | 306 | 309 | 334 | 316 |
| Other cardiovascular diseases | 462 | 549 | 376 | 362 | 358 | 358 | 356 | 336 |
| All other causes | 1852 | 1849 | 1911 | 1611 | 1811 | 1704 | 2012 | 1698 |
| Medications | ||||||||
| RAS inhibitors or beta‐blockers or MRAs | 136 | 165 | 123 | 167 | 126 | 173 | 142 | 166 |
| All other drugs | 1548 | 1773 | 1440 | 1505 | 1528 | 1591 | 1883 | 1815 |
| Outpatient services | ||||||||
| Echocardiogram | 10 | 43 | 14 | 14 | 13 | 15 | 14 | 15 |
| Other outpatient services | 1085 | 1140 | 708 | 646 | 834 | 732 | 689 | 514 |
| Total | 5425 | 5936 | 4872 | 4610 | 4975 | 4883 | 5430 | 4859 |
MRAs, mineralocorticoid receptor antagonists; RAS, renin–angiotensin system.
Cost‐effectiveness profiles
As shown in Figure 1 , patients who adhered to ECC experienced a gain in time free from health‐related outcomes (positive differential effectiveness) at the expense of increased costs (positive healthcare costs differential), resulting in an ICER of 225.6 (95% CI, 153.4–300.7). In addition, patients who adhered to RAS inhibitors, beta‐blockers, and MRAs exhibited positive differential effectiveness compared with non‐adherent patients; however, in this case, a cost saving (negative healthcare costs differential) was observed in 98%, 84%, and 93% of the 1000 bootstrap replications, respectively.
Figure 1.
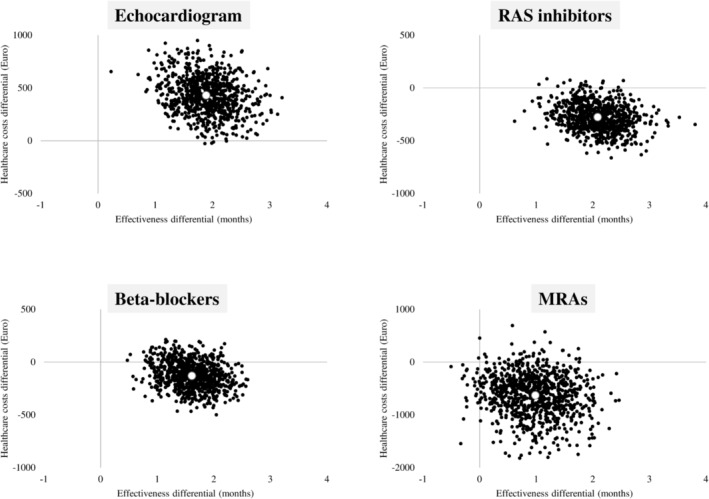
Incremental cost‐effectiveness ratio scatterplots representing cost‐effectiveness profiles obtained by comparing matched cohorts differentiated according to whether they adhered or did not adhere to recommendations. MRAs, mineralocorticoid receptor antagonists; RAS, renin–angiotensin system.
Sensitivity analysis
The association between adherence to recommendations and health‐related outcomes did not change substantially when using an as‐treated (time‐dependent) approach (Table S8 ). Compared with non‐adherent patients, full adherents had a risk reduction for the composite outcome, ranging from 8% (MRAs) to 17% (RAS inhibitors).
Discussion
This study provides real‐world evidence of the clinical outcomes and healthcare costs associated with adherence to out‐of‐hospital healthcare recommendations for patients with HF. Our findings showed that health‐related outcomes (i.e. cardiovascular hospitalizations and all‐cause deaths) occurred at reduced rates, ranging from 8% to 17% for patients fully adherent to MRAs and RAS inhibitors, respectively. Adherence to ECC was associated with higher healthcare costs (on average, €511 per year per patient) while adherence to drug therapies resulted in cost savings (ranging from €92 for beta‐blockers to €571 for MRAs). Therefore, although efforts to increase adherence to drug therapies should be pursued because of the expected benefits for patients and the healthcare system, decision makers should carefully consider the balance between improved prognosis and higher ECC‐related costs. These data could hence be the starting point for planning resource allocation and other healthcare policies.
In our cohort, approximately 60% of patients received prescriptions for RAS inhibitors and beta‐blockers, and 35% received prescriptions for MRAs within 3 months of hospital discharge. In addition, 36%, 43%, and 38% of RAS inhibitor, beta‐blocker, and MRA users, respectively, exhibited suboptimal adherence to treatment during the first year of follow‐up. The lower use of MRAs than that of RAS inhibitors and beta‐blockers was likely due to the restricted indication of this drug class by the guidelines at the time of recruitment (2014–2015), that is MRAs were not indicated for patients with any one of the following criteria: New York Heart Association (NYHA) class 1, left ventricular ejection fraction >35%, and not being treated with RAS inhibitors and beta‐blockers. 43 Our study confirms previous observations that adherence to drug therapies is low in real‐life settings and is associated with better outcomes. 14 , 15 , 16 , 44 However, the novelty of our findings lies in our assessment of the cost‐effectiveness profile, which showed that not only patients but also the healthcare system could benefit from improving adherence to drug therapies.
In line with previous observations, 45 only a few patients (<20%) underwent ECC within the first year after hospital discharge in this study. The effectiveness of ECC during hospital stay has already been demonstrated previously. 46 One of the most novel results of this study is that we extended this finding by assessing the impact of outpatient management of HF through cardiac imaging on long‐term outcomes. Our study showed that although adherence to ECC was associated with a delay in the onset of outcomes, it involved additional costs. As the unit cost of reimbursing an ECC was low (approximately €60), our results suggest that this examination serves as a surrogate for the overall quality of care, that is patients adhering to this recommendation may also be more likely to follow healthy lifestyle advice and treatment indications regularly. Finally, the additional healthcare cost associated with ECC required to gain a year free from health‐related outcomes was €2707, which was much lower than the frequently adopted willingness‐to‐pay threshold of €50 000–€100 000 in western countries for HF‐related interventions. 47
Our study has several strengths. First, the study was based on a very large and unselected population, which was made possible because in Italy, the healthcare system is free or almost free for virtually all citizens. Second, the data included in the HCU database are accurate because all services claimed by health providers to obtain reimbursement from the Regional Health Authority are checked, with potential legal consequences for incorrect reports. 26 Third, the user‐only design adopted for assessing the effectiveness of drug therapies reduces the potential for confounding effects. 30 Finally, the robustness of our main findings was confirmed using a sensitivity analysis.
While our study provides valuable insights, it has certain limitations. First, the strict inclusion criteria may compromise the external validity of the study. As we excluded patients aged <50 years and who were hospitalized for cardiovascular events or those who died within the first year after hospital discharge, the generalizability of our findings to younger and high‐risk patients requires extreme caution. Second, owing to privacy rules, hospital records were unavailable for scrutiny, with a consequent lack of validation studies performed in the Italian healthcare system. 48 , 49 Third, misclassification of exposure may have affected our findings in several ways. For example, adherence to recommendations observed in the first year after hospital discharge was implicitly considered a proxy for adherence during follow‐up, which may not invariably be the case. However, the as‐treated approach analysis confirmed our main findings. In addition, adherence to drug therapies relies on drug dispensing and we have no data on actual drug assumption. 26 Moreover, out‐of‐pocket payments for healthcare services (e.g. over‐the‐counter drugs and ECC in private facilities) are not recorded in HCU databases. 26 Fourth, clinical characterization was probably imperfect because diseases not requiring hospitalization may have been underestimated in our study. Fifth, because the prescriptions for ECC were not recorded in our databases, we could not assess whether the lack of ECC was related to the patient or to the physician. Sixth, we have no data on the quality of the ECC assessments. Finally, our results may be affected by confounding factors, that is because patients who adhered to recommendations may have different clinical features from those who did not adhere to them, the observed reduction in clinical and economic outcomes might have been generated by factors other than adherence to recommendations. To minimize the potential for residual confounding, we adopted a PS matching design. Although PS is a widely used method in observational studies, it does not entirely avoid confounding, especially when important clinical data are lacking, as in our HCU databases (e.g. left ventricular ejection fraction, NYHA class, blood pressure, and drug doses prescribed). Therefore, our findings could not entirely exclude confounding factors.
In summary, adherence to HF recommendations improves patient prognosis, and adherence to evidence‐based drug therapies reduces healthcare costs in patients with HF after hospital discharge. Therefore, strict monitoring of patients with HF through regular clinical examinations and drug therapies should be considered the cornerstone of national guidelines and audits. Our results support the application of performance indicators based on adherence to evidence‐based recommendations for the out‐of‐hospital management of patients with HF in a national monitoring and evaluation system for healthcare provision, similar to the one activated by the Italian Ministry of Health.
Conflict of interest
Giovanni Corrao received research support from the European Community (EC), the Italian Medicines Agency (AIFA), Italian Ministry of Health, and the Italian Ministry of Education, University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (i.e. Novartis, GSK, Roche, AMGEN, BMS, and Servier). He also received honoraria from Roche as a member of its Advisory Board. Other authors have no disclosures.
Funding
This study was funded by grant from the Italian Ministry of Health (‘Metodi per il monitoraggio e la valutazione dell'assistenza sanitaria nell'ambito del Nuovo Sistema di Garanzia, con particolare riferimento alle cure integrate e al confronto dei risultati pre e post pandemia da CoViD‐19’ project, grant number J59H06000160001). The Italian Ministry of Health had no role in the design of the study, the collection, the analysis, the interpretation of the data, or the decision to approve publication of the finished manuscript.
Supporting information
Figure S1. Flow‐chart of inclusion and exclusion criteria that were used to select the final cohort.
Table S1. Clinical diagnoses, drugs and outpatient services codes used for the study purpose.
Table S2. Weights used to adjust the drug coverage of prescriptions for beta‐blockers and mineralocorticoid receptor antagonists.
Table S3. Baseline characteristics of cohort members who adhered and no adhered with echocardiogram in the first year after index discharge.
Table S4. Baseline characteristics of cohort members who adhered and no adhered with renin‐angiotensin system inhibitors in the first year after index discharge.
Table S5. Baseline characteristics of cohort members who adhered and no adhered with beta‐blockers in the first year after index discharge.
Table S6. Baseline characteristics of cohort members who adhered and no adhered with mineralocorticoid receptor antagonists in the first year after index discharge.
Table S7. Event rates of health‐related outcomes.
Table S8. Hazard ratios, and 95% confidence intervals, for health‐related outcomes and time‐dependent adherence to recommendations.
Acknowledgements
We thank the ‘Monitoring and assessing diagnostic‐therapeutic paths (MAP)’ working groups of the Italian Ministry of Health.
Italian Ministry of Health, Department of Health Planning: Office Director, Cristina Giordani, Maria Donata Bellentani, Rosanna Mariniello, Modesta Visca, Carla Ceccolini; Department of Health Prevention: Natalia Magliocchetti, Giovanna Romano; External Expert: Andrea Di Lenarda, Antonio Lora, Paola Pisanti, Rinaldo Zanini.
Polytechnic University of Marche: Flavia Carle (scientific coordinator), Marica Iommi, Edlira Skrami.
University of Milano‐Bicocca, Laboratory of Healthcare Research and Pharmacoepidemiology: Giovanni Corrao, Federico Rea, Anna Cantarutti, Matteo Monzio Compagnoni, Pietro Pugni.
Department of Epidemiology Lazio Region: Marina Davoli, Mirko Di Martino, Adele Lallo.
Aosta Valley Region: Guido Giardini, Patrizia Vittori.
Campania Region: Alfonso Bernardo, Anna Frusciante.
Emilia‐Romagna Region: Rossana De Palma.
Friuli‐Venezia Giulia Region: Marisa Prezza, Alfredo Perulli.
Lazio Region: Danilo Fusco, Chiara Marinacci.
Lombardy Region: Francesco Cideni, Olivia Leoni.
Marche Region: Marco Pompili, Simone Pizzi.
Molise Region: Lolita Gallo.
Puglia Region: Ettore Attolini, Vito Lepore.
Sicily Region: Salvatore Scondotto, Giovanni De Luca.
Tuscany Region: Paolo Francesconi, Carla Rizzuti.
Veneto Region: Francesco Avossa, Silvia Vigna.
Research and Health Foundation (Fondazione ReS ‐Ricerca e Salute‐): Nello Martini, Antonella Pedrini, Carlo Piccinni, Letizia Dondi.
National Agency for Regional Health Services: Mimma Cosentino, Maria Grazia Marvulli.
ANMCO (National Association of Hospital Cardiologists) Study Center: Aldo Maggioni.
Corrao, G. , Rea, F. , Iommi, M. , Lallo, A. , Fantaci, G. , Di Martino, M. , Davoli, M. , Leoni, O. , Pompili, M. , Scondotto, S. , De Luca, G. , Carle, F. , Lorusso, S. , Giordani, C. , Di Lenarda, A. , Maggioni, A. P. , and Monitoring and Assessing care Pathways (MAP)' working group of the Italian Ministry of Health (2024) Cost‐effectiveness of outpatient adherence to recommendations for monitoring of patients hospitalized for heart failure. ESC Heart Failure, 11: 2719–2729. 10.1002/ehf2.14779.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure the Rotterdam study. Eur Heart J 2004;25:1614‐1619. doi: 10.1016/j.ehj.2004.06.038 [DOI] [PubMed] [Google Scholar]
- 3. Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc 1997;45:968‐974. doi: 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 4. Westert GP, Lagoe RJ, Keskimaki I, Leyland A, Murphy M. An international study of hospital readmissions and related utilization in Europe and the USA. Health Policy 2002;61:269‐278. doi: 10.1016/s0168-8510(01)00236-6 [DOI] [PubMed] [Google Scholar]
- 5. Kozak LJ, DeFrances CJ, Hall MJ. National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat 2006;13:1‐209. [PubMed] [Google Scholar]
- 6. Corrao G, Ghirardi A, Ibrahim B, Merlino L, Maggioni AP. Short‐ and long‐term mortality and hospital readmissions among patients with new hospitalization for heart failure: a population‐based investigation from Italy. Int J Cardiol 2015;181:81‐87. doi: 10.1016/j.ijcard.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 7. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368‐376. doi: 10.1016/j.ijcard.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 8. Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997;337:1360‐1369. doi: 10.1056/NEJM199711063371906 [DOI] [PubMed] [Google Scholar]
- 9. Akintoye E, Briasoulis A, Egbe A, Dunlay SM, Kushwaha S, Levine D, et al. National trends in admission and in‐hospital mortality of patients with heart failure in the United States (2001–2014). J Am Heart Assoc 2017;6:e006955. doi: 10.1161/JAHA.117.006955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776‐803. doi: 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 11. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure—digest version. Circ J 2019:2084‐2184. doi: 10.1253/circj.CJ-19-0342 [DOI] [PubMed] [Google Scholar]
- 12. Avaldi VM, Lenzi J, Urbinati S, Molinazzi D, Descovich C, Campagna A, et al. Effect of cardiologist care on 6‐month outcomes in patients discharged with heart failure: results from an observational study based on administrative data. BMJ Open 2017;7:e018243. doi: 10.1136/bmjopen-2017-018243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonagh TA, Blue L, Clark AL, Dahlström U, Ekman I, Lainscak M, et al. European Society of Cardiology Heart Failure Association Standards for delivering heart failure care. Eur J Heart Fail 2011;13:235‐241. doi: 10.1093/eurjhf/hfq221 [DOI] [PubMed] [Google Scholar]
- 14. Giezeman M, Arne M, Theander K. Adherence to guidelines in patients with chronic heart failure in primary health care. Scand J Prim Health Care 2017;35:336‐343. doi: 10.1080/02813432.2017.1397253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirt MN, Muttardi A, Helms TM, van den Bussche H, Eschenhagen T. General practitioners' adherence to chronic heart failure guidelines regarding medication: the GP‐HF study. Clin Res Cardiol 2016;105:441‐450. doi: 10.1007/s00392-015-0939-8 [DOI] [PubMed] [Google Scholar]
- 16. Buja A, Solinas G, Visca M, Federico B, Gini R, Baldo V, et al. Prevalence of heart failure and adherence to process indicators: which socio‐demographic determinants are involved? Int J Environ Res Public Health 2016;13:238. doi: 10.3390/ijerph13020238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail 2011;17:664‐669. doi: 10.1016/j.cardfail.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 18. Hoorn CJGM, Crijns HJGM, Dierick‐van Daele ATM, Dekker LRC. Review on factors influencing physician guideline adherence in cardiology. Cardiol Rev 2019;27:80‐86. doi: 10.1097/CRD.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 19. Yeoh EK, Wong MCS, Wong ELY, Yam C, Poon CM, Chung RY, et al. Benefits and limitations of implementing chronic care model (CCM) in primary care programs: a systematic review. Int J Cardiol 2018;258:279‐288. doi: 10.1016/j.ijcard.2017.11.057 [DOI] [PubMed] [Google Scholar]
- 20. Butler J, Petrie MC, Bains M, Bawtinheimer T, Code J, Levitch T, et al. Challenges and opportunities for increasing patient involvement in heart failure self‐care programs and self‐care in the post‐hospital discharge period. Res Involv Engagem 2023;9:23. doi: 10.1186/s40900-023-00412-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corrao G, Rea F, Di Felice E, Di Martino M, Davoli M, Merlino L, et al. Influence of adherence with guideline‐driven recommendations on survival in women operated for breast cancer: real‐life evidence from Italy. Breast 2020;53:51‐58. doi: 10.1016/j.breast.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corrao G, Cantarutti A, Locatelli A, Porcu G, Merlino L, Carbone S, et al. Association between adherence with recommended antenatal care in low‐risk, uncomplicated pregnancy, and maternal and neonatal adverse outcomes: evidence from Italy. Int J Environ Res Public Health 2020;18:173. doi: 10.3390/ijerph18010173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corrao G, Monzio Compagnoni M, Barbato A, D'Avanzo B, Di Fiandra T, Ferrara L, et al. From contact coverage to effective coverage of community care for patients with severe mental disorders: a real‐world investigation from Italy. Front Psych 2022;13:1014193. doi: 10.3389/fpsyt.2022.1014193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corrao G, Rea F, Di Martino M, Lallo A, Davoli M, De Palma R, et al. Effectiveness of adherence to recommended clinical examinations of diabetic patients in preventing diabetes‐related hospitalizations. International J Qual Health Care 2019;31:464‐472. doi: 10.1093/intqhc/mzy186 [DOI] [PubMed] [Google Scholar]
- 25. Corrao G, Rea F, Mancia G, Perseghin G, Merlino L, Martini N, et al. Cost‐effectiveness of the adherence with recommendations for clinical monitoring of patients with diabetes. Nutr Metab Cardiovasc Dis 2021;31:3111‐3121. doi: 10.1016/j.numecd.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 26. Corrao G, Mancia G. Generating evidence from computerized healthcare utilization databases. Hypertension 2015;65:490‐498. doi: 10.1161/HYPERTENSIONAHA.114.04858 [DOI] [PubMed] [Google Scholar]
- 27. Corrao G, Ghirardi A, Ibrahim B, Merlino L, Maggioni AP. Burden of new hospitalization for heart failure: a population‐based investigation from Italy. Eur J Heart Fail 2014;16:729‐736. doi: 10.1002/ejhf.105 [DOI] [PubMed] [Google Scholar]
- 28. Arbuckle L, Ritchie F. The five safes of risk‐based anonymization. IEEE Secur Priv 2019;17:84‐89. doi: 10.1109/MSEC.2019.2929282 [DOI] [Google Scholar]
- 29. Zhang P, Kamel Boulos MN. Privacy‐by‐design environments for large‐scale health research and federated learning from data. Int J Environ Res Public Health 2022;19:11876. doi: 10.3390/ijerph191911876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corrao G, Ghirardi A, Segafredo G, Zambon A, Della Vedova G, Lapi F, et al. User‐only design to assess drug effectiveness in clinical practice: application to bisphosphonates and secondary prevention of fractures. Pharmacoepidemiol Drug Saf 2014;23:859‐867. doi: 10.1002/pds.3650 [DOI] [PubMed] [Google Scholar]
- 31. Goldberger JJ, Bonow RO, Cuffe M, Dyer A, Rosenberg Y, O'Rourke R, et al. Beta‐blocker use following myocardial infarction: low prevalence of evidence‐based dosing. Am Heart J 2010;160:435‐442.e1. doi: 10.1016/j.ahj.2010.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565‐574. doi: 10.1002/pds.1230 [DOI] [PubMed] [Google Scholar]
- 33. Rea F, Ronco R, Pedretti RFE, Merlino L, Corrao G. Better adherence with out‐of‐hospital healthcare improved long‐term prognosis of acute coronary syndromes: evidence from an Italian real‐world investigation. Int J Cardiol 2020;318:14‐20. doi: 10.1016/j.ijcard.2020.06.017 [DOI] [PubMed] [Google Scholar]
- 34. Corrao G, Rea F, Di Martino M, De Palma R, Scondotto S, Fusco D, et al. Developing and validating a novel multisource comorbidity score from administrative data: a large population‐based cohort study from Italy. BMJ Open 2017;7:e019503. doi: 10.1136/bmjopen-2017-019503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang SV, Jin Y, Fireman B, Gruber S, He M, Wyss R, et al. Relative performance of propensity score matching strategies for subgroup analyses. Am J Epidemiol 2018;187:1799‐1807. doi: 10.1093/aje/kwy049 [DOI] [PubMed] [Google Scholar]
- 36. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057‐1069. doi: 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009;28:3083‐3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao L, Claggett B, Tian L, Uno H, Pfeffer MA, Solomon SD, et al. On the restricted mean survival time curve in survival analysis. Biometrics 2016;72:215‐221. doi: 10.1111/biom.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol 2017;2:1179‐1180. doi: 10.1001/jamacardio.2017.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen DJ, Reynolds MR. Interpreting the results of cost‐effectiveness studies. J Am Coll Cardiol 2008;52:2119‐2126. doi: 10.1016/j.jacc.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang H, Zhao H. A study on confidence intervals for incremental cost‐effectiveness ratios. Biom J 2008;50:505‐514. doi: 10.1002/bimj.200810439 [DOI] [PubMed] [Google Scholar]
- 42. Scotti L, Rea F, Corrao G. One‐stage and two‐stage meta‐analysis of individual participant data led to consistent summarized evidence: lessons learned from combining multiple databases. J Clin Epidemiol 2018;95:19‐27. doi: 10.1016/j.jclinepi.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 43. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787‐1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 44. Rea F, Iorio A, Barbati G, Bessi R, Castrichini M, Nuzzi V, et al. Patient adherence to drug treatment in a community based‐sample of patients with chronic heart failure. Int J Cardiol 2022;349:144‐149. doi: 10.1016/j.ijcard.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 45. Braga JR, Leong‐Poi H, Rac VE, Austin PC, Ross HJ, Lee DS. Trends in the use of cardiac imaging for patients with heart failure in Canada. JAMA Netw Open 2019;2:e198766. doi: 10.1001/jamanetworkopen.2019.8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanaka H, Nabeshima Y, Kitano T, Nagumo S, Tsujiuchi M, Ebato M, et al. Optimal timing of echocardiography for heart failure inpatients in Japanese institutions: OPTIMAL study. ESC. Heart Fail 2020;7:4213‐4221. doi: 10.1002/ehf2.13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Di Tanna GL, Bychenkova A, O'Neill F, Wirtz HS, Miller P, Ó Hartaigh B, et al. Evaluating cost‐effectiveness models for pharmacologic interventions in adults with heart failure: a systematic literature review. Pharmacoeconomics 2019;37:359‐389. doi: 10.1007/s40273-018-0755-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hyeraci G, Spini A, Roberto G, Gini R, Bartolini C, Lucenteforte E, et al. A systematic review of case‐identification algorithms based on Italian healthcare administrative databases for three relevant diseases of the cardiovascular system: acute myocardial infarction, ischemic heart disease, and stroke. Epidemiol Prev 2019;43:37‐50. doi: 10.19191/EP19.4.S2.P037.091 [DOI] [PubMed] [Google Scholar]
- 49. Lorenzoni G, Baldi I, Soattin M, Gregori D, Buja A. A systematic review of case‐identification algorithms based on Italian healthcare administrative databases for three relevant diseases of the cardiovascular system: hypertension, heart failure, and congenital heart diseases. Epidemiol Prev 2019;43:51‐61. doi: 10.19191/EP19.4.S2.P051.092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow‐chart of inclusion and exclusion criteria that were used to select the final cohort.
Table S1. Clinical diagnoses, drugs and outpatient services codes used for the study purpose.
Table S2. Weights used to adjust the drug coverage of prescriptions for beta‐blockers and mineralocorticoid receptor antagonists.
Table S3. Baseline characteristics of cohort members who adhered and no adhered with echocardiogram in the first year after index discharge.
Table S4. Baseline characteristics of cohort members who adhered and no adhered with renin‐angiotensin system inhibitors in the first year after index discharge.
Table S5. Baseline characteristics of cohort members who adhered and no adhered with beta‐blockers in the first year after index discharge.
Table S6. Baseline characteristics of cohort members who adhered and no adhered with mineralocorticoid receptor antagonists in the first year after index discharge.
Table S7. Event rates of health‐related outcomes.
Table S8. Hazard ratios, and 95% confidence intervals, for health‐related outcomes and time‐dependent adherence to recommendations.


