Abstract
Insulin-regulated aminopeptidase (IRAP) is an abundant cargo protein of Glut4 storage vesicles (GSVs) that traffics to and from the plasma membrane in response to insulin. We used the amino terminus cytoplasmic domain of IRAP, residues 1–109, as an affinity reagent to identify cytosolic proteins that might be involved in GSV trafficking. In this way, we identified p115, a peripheral membrane protein known to be involved in membrane trafficking. In murine adipocytes, we determined that p115 was localized to the perinuclear region by immunofluorescence and throughout the cell by fractionation. By immunofluorescence, p115 partially colocalizes with GLUT4 and IRAP in the perinuclear region of cultured fat cells. The amino terminus of p115 binds to IRAP and overexpression of a N-terminal construct results in its colocalization with GLUT4 throughout the cell. Insulin-stimulated GLUT4 translocation is completely inhibited under these conditions. Overexpression of p115 C-terminus has no significant effect on GLUT4 distribution and translocation. Finally, expression of the p115 N-terminus construct has no effect on the distribution and trafficking of GLUT1. These data suggest that p115 has an important and specific role in insulin-stimulated Glut4 translocation, probably by way of tethering insulin-sensitive Glut4 vesicles at an as yet unknown intracellular site.
INTRODUCTION
Insulin normalizes blood glucose levels by mobilizing the muscle and adipocyte glucose transporter isoform, GLUT4, from intracellular storage vesicles and moving it to the plasma membrane (Simpson et al., 2001; Bryant et al., 2002). Various models of GLUT4 trafficking suggest that GLUT4 must exist in more than one intracellular compartment and the major insulin sensitive pool is localized to a compartment that is distinct from endosomal markers and is commonly referred to as glucose transporter storage vesicles (GSVs), or insulin responsive vesicle (IRVs). Despite the critical function of glucose transport in glucose homeostasis, many of the details by which adipocytes and muscle form a pathway of insulin-sensitive GLUT4 trafficking remain unknown. However, it is virtually certain that the major cargo proteins of GSVs must interact with a number of cytosolic and membrane proteins, such as adaptors and tethers, in order to be properly sorted and regulated by insulin.
Insulin-responsive aminopeptidase (IRAP) was identified as an abundant cargo protein associated with GLUT4 vesicles that translocates in response to insulin in a manner seemingly identical to GLUT4 (Kandror and Pilch, 1994; Kandror et al., 1994; Mastick et al., 1994; Keller et al., 1995; Malide et al., 1997; Martin et al., 1997; Ross et al., 1997). In fact, it is more abundantly expressed in vesicles than the transporter (Kupriyanova et al., 2002). When the cytoplasmic N-terminus of IRAP was microinjected into 3T3-L1 adipocytes, GLUT4 was localized on the plasma membrane even in the basal state (Waters et al., 1997), suggesting IRAP can play a role in GSV movement/targeting. A chimeric protein containing the intracellular domain of IRAP and the extracellular and transmembrane domains of the transferrin receptor displays IRAP- and GLUT4-like trafficking in 3T3-L1 adipocytes (Subtil et al., 2000). IRAP displays similar trafficking kinetics to GLUT4 in 3T3-L1 adipocytes (Garza and Birnbaum, 2000), although there may be some differences in the internalization rate of these two proteins in rat adipocytes (Kandror, 1999). In any case, these citations document considerable evidence that IRAP is a marker for insulin-dependent GLUT4/GSV trafficking.
IRAP undergoes increased intracellular sequestration upon differentiation of 3T3-L1 adipocytes in correlation with the development of an insulin responsive compartment whose formation precedes GLUT4 expression during the differentiation process (Ross et al., 1998; El-Jack et al., 1999). Recently, we reported that GLUT4 is not absolutely necessary for the formation of GSVs in engineered adipocytes and that it is targeted to a preexisting vesicle, which can form independently of GLUT4 as adipocytes differentiate (Gross et al., 2004). Similarly, IRAP fractionates and responds normally to insulin in denervated muscle under conditions where GLUT4 expression is decreased by 90% (Zhou et al., 2000). All this strongly suggests the involvement of IRAP with the retention and sorting machinery of GSVs and its potential association with the targeting and tethering proteins involved in this process.
IRAP has a single transmembrane domain with the N-terminus projecting into the cytosol (Keller et al., 1995), whereas GLUT4 has potential sorting and targeting motifs in three cytosolic domains corresponding to N- and C-termini as well as the central loop that connects helices 6 and 7 (Fukumoto et al., 1989). The N-terminus of IRAP has dileucine motifs and an acidic cluster domain similar to that found in the C-terminus of GLUT4, which is thought to be important region for its trafficking (Verhey et al., 1993, 1995; Corvera et al., 1994; Verhey and Birnbaum, 1994; Haney et al., 1995; Marsh et al., 1995). In this study, we used the N-terminal cytoplasmic domain of IRAP, residues 1–109, conjugated to a chitin-binding protein in order to find cytosolic proteins involved in GSV trafficking, and we identified p115 as one such protein. p115 is known to be involved in vesicular traffic, for example, by tethering vesicle in the process of docking and fusing in the Golgi apparatus and in in vitro membrane trafficking protocols (Waters et al., 1992; Barroso et al., 1995; Sapperstein et al., 1995). Here, we document that p115 binds GLUT4 storage vesicles via IRAP and plays an important role in GLUT4 trafficking.
MATERIALS AND METHODS
Dexamethasone, 3-isobutyl-methylxanthine, insulin, sodium fluoride, sodium orthovanadate, fetal bovine serum (FBS; Australian origin), benzamidine, and mouse immunoglobulin G (IgG) antibody were purchased from Sigma (St. Louis, MO). LB broth, ampicillin, kanamycin, aprotinin, leupeptin, and pepstatin A were obtained from American Bioanalytical (Natick, MA). Calf serum was purchased from Life Technologies (Gaithersburg, MD), and DMEM was from Mediatech (Herndon, VA). Lipofectamine 2000 reagent and the pcDNA 3.1 expression vector were purchased from Invitrogen (Carlsbad, CA). BCA protein assay kit was from Pierce (Rockford, IL). Protein A-agarose was from Santa Cruz Biotechnology (Santa Cruz, CA). Penicillin, streptomycin, and trypsin were purchased from Life Technologies (Carlsbad, CA). pGEX-3X and pGEX-5X-1 vectors were purchased from Amersham Biosciences (Piscataway, NJ), and the IMPACT fusion protein system was from New England Biolabs (Beverly, MA). Collagenase B was purchased from Roche Applied Sciences (Indianapolis, IN). pEGFP-N3 and pEGFP-C3 vector were purchased from BD Bioscience Clontech (Palo Alto, CA).
Cell Culture
3T3-L1 fibroblasts were maintained in DMEM containing 4.5 g/liter glucose and l-glutamine supplemented with 10% calf serum and 100 U/ml penicillin, and 100 μg/ml streptomycin. Two days after confluence, cells were induced to differentiate by changing media to DMEM with 10% FBS, 0.5 mM 3-isobutyl-methylxanthine, 1 μM dexamethasone, and 1.7 μM insulin. After 48 h, the induction medium was removed and cells were maintained in DMEM with 10% FBS as described (Stephens et al., 1997). COS7 cells were maintained in DMEM with 10% FBS in 5% CO2. When they reached 90% confluence, they were transfected with 2 μg of the cDNA of interest by means of the Lipofectamine 2000 reagent.
Expression Vectors
The pTYB4 IRAP 1–109 construct (IMPACT) was made by PCR and ligated via NcoI and SmaI sites. The pcDNA3.1 rat p115 cDNA construct was a kind gift from Dr. E. Sztul (University of Alabama, Birmingham, AL). The pcDNA3.1 N-terminal p115 construct (1–766 amino acid) was ligated with a BamHI site. The pcDNA3.1 C-terminal p115 construct (325–959 amino acid) was ligated with EcoRI and NotI sites. pEGFP-C3 C-terminal p115 was made (EcoRI and ApaI site) by PCR from pcDNA3.1 C-terminal p115 and pEGFP-N3 N-terminal p115 was made (ApaI and KpnI site) by PCR from pcDNA3.1 N-terminal p115.
Antibodies
In this study we used the monoclonal anti-GLUT4 antibody 1F8 (James et al., 1988) for Western blotting, and a polyclonal anti-GLUT1 antibody (a kind gift from Dr. C. Carter-Su, University of Michigan, Ann Arbor, MI). Monoclonal anti-p115 antibody 7D1 (a kind gift from Dr. G. Waters, Princeton University, Princeton, NJ) was used for immunofluorescence, and polyclonal anti-p115 antibodies made by immunizing rabbits with synthetic peptides corresponding to amino acids 40–57 and 888–905 of murine p115 and recognizing N- and C-terminal residues, respectively, were used for Western blots (see Figure 4). Polyclonal anti-Syntaxin 6 antibody was a kind gift from Dr. E. M. van Dam (Garvan Institute of Medical Research, Darlinghurst, Australia), antimonoclonal anti-IRAP antibody (a kind gift from Dr. M. Birnbaum, University of Pennsylvania, Philadelphia, PA), antipolyclonal IRAP antibody obtained from Dr. S. Waters (Metabolex, Hayward, CA) was used for immunoprecipitation (see Figure 3) and anti-goat polyclonal GLUT4 antibody (C20) for immunofluorescence purchased from Santa Cruz Biotechnology. The GM130 antibody was made as described in Alvarez et al. (2001).
Figure 4.
The N-terminus of p115 binds to the N-terminus of IRAP. COS 7 cells were grown and transfected with pCDNA3.1 vector alone or ligated to wild-type p115, C-terminal p115 and N-terminal p115 as described in Materials and Methods. After 48 h, total cell lysates were obtained and 200 μg protein were subjected to affinity purification with an IRAP1–109-CBD column. Bound material was solubilized with Laemmli sample buffer and equal proportions from each transfection were separated by SDS-PAGE and analyzed by Western blotting using (A) anti-p115 mAb (7D1) recognizing the C-terminus and (B) anti-p115 polyclonal antibody recognizing the N terminus. Detection was by ECL.
Figure 3.
p115 coimmunoprecipitates with IRAP. 3T3-L1 adipocytes were starved for 2 h and then treated with or without 100 nM insulin for 15 min. Total cell lysates were prepared (see Materials and Methods) and antipolyclonal IRAP antibody or nonspecific IgG (each 2 μg) was incubated with 200 μg of lysate over night. Protein A (60 μl) was added to the lysate and incubated for 60 min and then washed three times with 0.1% Triton X-100 in PBS. Bound samples were solubilized in Laemmli sample buffer and equal portions were subjected to SDS-PAGE and analyzed by Western blotting using anti-IRAP monoclonal and anti-p115 monoclonal antibodies as in Figures 1 and 2.
Bacterial Expression and Purification of IRAP1–109 Peptide
LB media containing ampicillin (100 μg/ml) was inoculated with Escherichia coli ER2566 cells harboring pTYB1 IRAP 1–109 or CBD only at 37°C until the OD600 was 0.5–0.6. Isopropyl thiogalactoside was added to the culture at a final concentration of 0.5 mM to induce expression of the fusion protein, and then the culture was grown for 6 h at 30°C. Bacteria were concentrated by centrifugation, and the cell pellet was resuspended in 1/20 culture volume of column buffer (phosphate-buffered saline [PBS], 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride [PMSF]) and sonicated with a probe sonicator. A 10% solution of Triton X-100 was added at a 1:10 dilution and the extract was incubated 30 min with shaking. The bacterial extract was spun at 16,000 × g at 4°C for 30 min to remove debris, and the supernatant was loaded onto a column of chitin beads. The column was washed with at least 15 volumes of column buffer and was used for affinity chromatography as described below. For peptide cleavage, the column was quickly flushed with 3 column volumes of PBS containing 50 mM dithiothreitol, and the flow was stopped and kept at 4°C overnight. The IRAP 1–109 peptide was eluted with PBS and passed through a Microcon column (10-kDA cutoff) to remove the reducing agent. The purity of IRAP peptide was confirmed by SDS-PAGE.
Affinity Purification of p115 with the IRAP-CBD Column
The IRAP-CBD column was equilibrated with 10 bed volumes of HES buffer (20 mM HEPES, pH 7.4, 250 mM sucrose, 1 mM EDTA) containing the protease inhibitors, aprotinin and pepstatin (each 1 μM), leupeptin 10 μM, and 2 mM PMSF. Nine 10-cm2 dishes of differentiated 3T3-L1 adipocytes (8–10 d) were washed two times in ice-cold PBS and one time in HES buffer. The cells were scraped from the dishes in ice-cold HES (1 ml/dish) and lysed by Potter-Elverhjem homogenization. The homogenate was spun at 16,000 × g for 10 min at 0°C, the fat layer was carefully removed, and the supernatant was transferred and centrifuged at 220,000 × g for 60 min at 4°C to yield a membrane pellet and supernatant (cytosol). The cytosol was passed over CBD beads to remove nonspecific CBD binders and applied to the IRAP-CBD column. The IRAP-CBD column was washed with 10 volumes of HES, and adherent proteins were eluted with three column volumes of HES containing 5 μM NT-IRAP 1–109 peptide. The eluates were concentrated using a Centricon-3 device followed by a Microcon-3 device and solubilized in Laemmli sample buffer, separate by SDS-PAGE, and visualized with Bio-Safe Coomassie Blue-G250 or Western blotting.
Total cell lysates of COS7 cells overexpressing p115 proteins were subjected to pulldown assays with IRAP-CBD beads in protocols similar to the above.
Subcellular Fractionation of Adipocytes
This was performed on differentiated 3T3-L1 adipocytes (Kandror et al., 1995) essentially as described for rat adipocytes (Simpson et al., 1983). Cells were treated with insulin (100 nM) or carrier for 15 min at 37°C. Hormonal action was stopped with 2 mM KCN and cells were transferred to HES and homogenized with a Potter-Elverhjem Teflon-glass tissue grinder. Subcellular fractions (plasma membrane [PM], heavy microsomes [HM], and light microsomes [LM]) were obtained by differential centrifugation and resuspended in HES. Buffers used with subcellular fractionation contained a mix of protease inhibitors consisting of 1 μM aprotinin, 10 μM leupeptin, 1 μM pepstatin, and 5 mM benzamidine.
Western Blotting
Adipocytes or COS7 cells were rinsed three times with ice-cold PBS and lysed in buffer of 25 mM Tris, pH 7.4, 50 mM NaCl, 0.5% sodium deoxycholate, 2% Nonidet P-40, 0.2% SDS, 1 μM aprotinin, 10 μM leupeptin, 1 μM pepstatin, 0.1 μM PMSF, 50 mM sodium fluoride, and 1 mM sodium orthovanadate. Cell lysates were incubated on ice for 15 min and then soluble proteins were obtained by centrifugation at 12,000 × g for 20 min at 4°C and separated by SDS-PAGE (Laemmli, 1970). After electrophoresis, the proteins were transferred to a 0.2 μ PVDF membrane and incubated in PBS-T (PBS with 0.1% Tween20) containing 10% nonfat evaporated milk for 1 h at room temperature. The membranes were then incubated with the primary antibodies described above. Horseradish peroxidase–conjugated secondary antibodies (Sigma Chemical) and either an enhanced chemiluminescent substrate kit (NEN) or Super signal West Femto Maximum Sensitivity Substrate kit (Pierce) was used for detection.
Immunofluorescence
3T3-L1 fibroblasts were split onto glass coverslips, grown, and differentiated as described above. The cells were washed twice with warm DMEM, serum-starved for 4 h, and then exposed to 100 nM insulin or carrier for 15 min at 37°C. They were then washed once with ice-cold PBS and fixed with 3.7% formaldehyde in PBS for 20 min at room temperature. They were blocked and permeabilized for 10 min at room temperature in buffer P (PBS with 5% FBS and 1% Triton X-100). The cells were then incubated for 2 h with primary antibody at a dilution of 1/100 in the buffer P solution at room temperature. Then the cells were washed four times in buffer P and incubated with Cy3 or Cy2-conjugated second antibody at a dilution of 1/250 for 30 min at room temperature. Then the cells were washed four times in buffer P solution. The stained cells were mounted with Vectashield and fixed with nail polish. The cells were observed using a Zeiss LSM510 confocal fluorescence microscope (Carl Zeiss, Thornwood, NY). Images were imported into an ISM5 image browser (Carl Zeiss) for processing and analysis.
Electroporation of 3T3-L1 Adipocytes
This protocol was performed as described by Okada et al. (2003). Briefly, differentiated 3T3-L1 adipocytes from one 15-cm dish were dislodged by incubation with trypsin-EDTA and transferred to conical tubes. The cells were washed twice with PBS by centrifugation at 900 rpm at room temperature. The cells were resuspended in PBS to a final concentration of about 1.0 × 107 cells/500 μl. EGFP DNA plasmid (50 μg) was transferred to a 0.4-mm cuvette and then the cell suspension (500 μl) was added to the cuvette. The cuvette was placed in a Gene Pulser II apparatus and charged with 975 μF capacitance at 0.16 kV. The cells were immediately diluted with 1 ml of DMEM and transferred to conical tube. The cell suspensions were adjusted to 6 ml with DMEM and incubated for 10 min at room temperature. After a 10-min incubation, the cells were plated onto glass coverslips in a six-well dish. After a 48-h incubation, the cells were visualized by confocal microscopy.
RESULTS
Identification of p115 as an IRAP-binding Protein
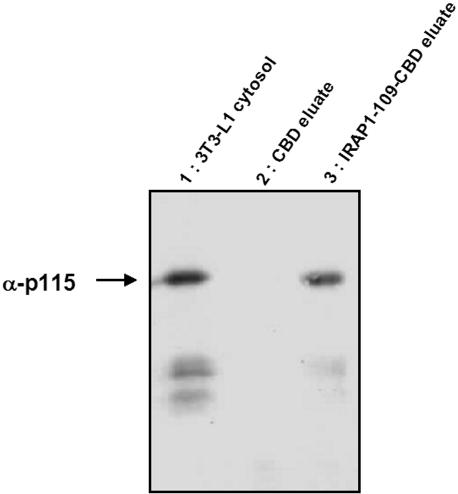
To orient the 109 residue cytoplasmic amino terminus of IRAP in the same way as it would be in the cell cytoplasm, we used the IMPACT fusion protein system to create an IRAP-CBD chimera. We then passed cytosol from rat adipocytes and 3T3-L1 cultured adipocytes over an IRAP-CBD affinity column and found a number of Coomassie staining bands that could be specifically eluted with IRAP 1–109 peptide (unpublished data). The slowest migrating band from both rat and mouse cytosols was subjected to proteomic analysis by mass spectrometry (Dr. W. Lane, Harvard Microchemistry and Proteomics Facility, Boston, MA), and nine individual peptides were identified corresponding to p115 (Waters et al., 1992; Barroso et al., 1995; Sapperstein et al., 1995). We confirmed this identification by Western blotting as shown in Figure 1 (see also Figure 4) where we passed cytosol from 3T3-L1 adipocytes over the affinity column and see nearly complete retention of p115 on an IRAP-CBD column and specific elution with IRAP 1–109 peptide. The p115 protein is a bona fide vesicular transport protein; therefore, we focused our attention on the characterization of its possible functional role in adipocytes.
Figure 1.
Affinity purification of p115 by CBD-IRAP 1–109 fusion protein. 3T3-L1 fibroblasts were differentiated and cytosol obtained as described in Materials and Methods. Cytosol was passed over columns of CBD alone (lane 2) or CBD-IRAP 1–109 (lane 3) and the columns were washed extensively. The adherent proteins were eluted from beads with Laemmli sample buffer, separated by SDS-PAGE, and analyzed by Western blotting using anti-p115 monoclonal antibody (mAb) and enhanced chemiluminescence (ECL) detection. Lane 1 depicts the results from an amount of 3T3-L1 cytosol proportional to that eluted from the CBD beads.
Adipocyte p115 Localizes Primarily to Cytosol but Also to Membrane Fractions
We examined the subcellular localization of p115 in adipocytes by a widely used, standard fractionation protocol that results in a PM preparation of >90% purity and membrane fractions enriched in Golgi and GSVs (LM) and ER (HM; Simpson et al., 1983). The level of p115 is highest in the cytosol, but is present at detectable levels in all membrane fractions from 3T3-L1 adipocytes, consistent with its peripheral membrane protein nature (Figure 2). Similar results were obtained with rat fat cells (unpublished data). There was no effect of insulin on the subcellular distribution of p115 as assessed by fractionation where the redistribution/translocation of GLUT4 and IRAP from the LM to the PM can be clearly demonstrated (Figure 2). The apparent insulin-dependent migration shift of cytosolic p115 (Figure 2) is a gel edge effect.
Figure 2.
Subcellular distribution of p115 in 3T3-L1 adipocytes. Subcellular fractionation (PM, plasma membrane; HM, heavy microsomes; LM, light microsomes) of fat cells treated with insulin or not was performed as described in Materials and Methods. Equal protein amounts (10 μg, Figure 2) of the fractions were separated by SDS-PAGE and analyzed by Western blot using the antibodies indicated. Detection was by ECL.
Biochemical Verification of p115-IRAP Interaction in Cells
To show direct interaction between IRAP and p115, we performed coimmunoprecipitation (IP) assays with anti-IRAP and anti-p115 antibodies in 3T3-L1 adipocytes. As shown in Figure 3, we can specifically coimmunoprecipitation p115 with anti-IRAP antibody, but we did not succeed in the converse experiment for unknown reasons. Moreover and interestingly, the amount of IRAP immunoprecipitated was less from cells exposed to insulin, whereas the amount of p115 is enhanced. The IP was performed on total cell lysates containing the same amount of IRAP, therefore it appears that insulin is having some effect on the interaction between these two proteins.
To determine which domain of p115 binds IRAP, we created truncated forms of p115. N-terminal p115 (residues 1–776) was made lacking the C-terminus coiled coil domain, which can bind to some Golgi-localized proteins, and C-terminal p115 (residues 325–959) was made lacking the N-terminal site, which may bind to vesicles. These constructs were transiently expressed in COS7 cells, and after 48 h, cell lysates were subjected to a pulldown assay with IRAP-CBD beads. As shown in Figure 4, A and B, we confirmed that the N-terminus of p115, the expected vesicle-binding locus, actually bound to IRAP. Note that we also pulldown with the IRAP-CBD beads the endogenous COS7 cell p115 that is more easily detected (Figure 4A) with the antibodies to its C-terminus than with the N-terminus specific reagent (Figure 4B).
Characterization of p115 Localization and Function by Immunofluorescence
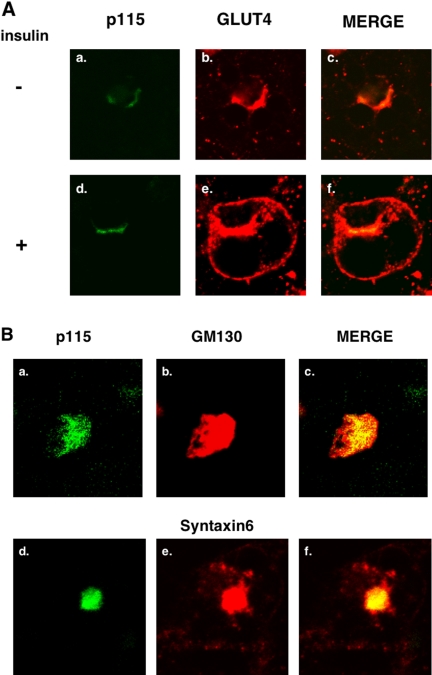
As shown in Figure 5A, p115 colocalizes with GLUT4 in the perinuclear region of 3T3-L1 adipocytes under basal conditions (panels a–c). Unlike GLUT4, the distribution of p115 does not change after insulin stimulation (panels d–f). Similarly, p115 also colocalizes to a considerable extent with IRAP under basal and insulin-stimulated conditions but only IRAP translocates to the cell surface (unpublished data). Thus, p115 shows significant colocalization with the two major GSV cargo proteins. To further determine the relationship of GLUT4 and p115, we compared the localization of the latter to the Golgi proteins, syntaxin6 and GM130, as shown in Figure 5B. These three proteins overlap to a much greater extent than do p115 and Glut4, consistent with previous data (Shewan et al., 2003).
Figure 5.
Colocalization of p115 with GLUT4 in 3T3-L1 adipocytes. 3T3-L1 fibroblasts were grown and differentiated as described in Materials and Methods. The cells were serum starved for 4 h and then treated with 100 nM insulin or carrier for 15 min and then immunostained as described in Materials and Methods. (A) The cells were incubated with anti-mouse monoclonal p115 and anti-goat polyclonal GLUT4 antibody, followed by incubation with anti-mouse Cy2 and anti-goat Cy3 antibody. (B) The cells were incubated with anti-mouse monoclonal P115 and rabbit polyclonal GM130 and syntaxin6 antibody followed by incubating with anti-mouse Cy2 and anti-rabbit Cy3 antibody. Figures are representative of four independent experiments.
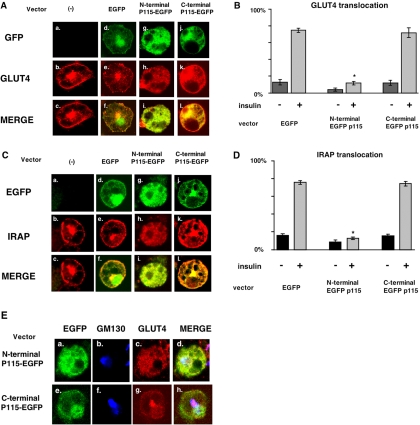
To determine if this p115-GSV colocalization has functional significance, we used electroporation to overexpress the N- and C-terminal p115 constructs described in Figure 4 that we fused to EGFP for reporting their presence by immunofluorescence. We show that the electroporation procedure itself, using EGFP expression alone, does not affect endogenous GLUT4 trafficking (Figure 6, A and B). When N-terminal p115 is overexpressed, the GLUT4 signal disperses throughout the cytosol and decreases in the perinuclear region in basal state (unpublished data). Insulin-stimulated GLUT4 translocation is completely blocked under these conditions (Figure 6A, g–i, and 6B). However, there was minimal, if any, effect of the C-terminal p115 expression on GLUT4 trafficking in response to insulin (Figure 6, j–l). We also examined the effect of electroporating wild-type p115 in adipocytes. This had the same effect as N-terminal p115 in that it dispersed GLUT4 and blocked its ability to translocate (unpublished data). We confirmed that N-terminal p115 has the same effects on IRAP as it does on GLUT4 (Figure 6, C and D), namely dispersion of the IRAP signal throughout the cytosol and a loss of insulin-mediated translocation. To determine if this construct also disrupted the Golgi, we determined the localization of GM130 by immunofluorescence after electroporation of p115 N- and C-terminal constructs and found it to be unchanged (Figure 6E), thus supporting the specificity of the p115 effect for GLUT4 trafficking and localization.
Figure 6.
N-terminus p115 expression blocks insulin-stimulated GLUT4 and IRAP translocation. (A) Constructs consisting of 100 μg of pCDNA3.1 EGFP, N-terminal p115 EGFP, and C-terminal p115 EGFP were electroporated into 3T3-L1 adipocytes as described in Materials and Methods. After 48 h, cells were serum-starved for 4 h and stimulated without or with 100 nM insulin for 15 min. The cells were fixed with 3.7% formaldehyde and stained with antipolyclonal goat GLUT4 antibody followed by Cy3 antibody as described in Materials and Methods. (B) Electroporated cells (100 each from 3 cover slips) were scored for translocation and analyzed with Excel software. Statistical significance was determined by a Student's t test; *p > 0.01. (C) After insulin stimulation, the cells were stained antipolyclonal rabbit IRAP antibody followed by Cy3 antibody, and the data were analyzed (D) as in B. (E) Electroporation was performed as in A, and the cells were stained with the indicated antibodies followed by appropriate secondary antibodies labeled with Cy2 (GLUT4) and Cy5 (GM 130). The figure is representative of four independent experiments.
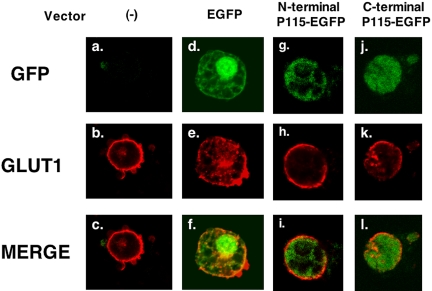
To further determine if the effects of N-terminal p115 expression were specific for GLUT4, we examined its effects on GLUT1 trafficking. Interestingly, p115 has no significant effect on insulin-dependent GLUT1 trafficking (Figure 7). Moreover, we examined p115 effects on endocytosis of the transferrin receptor. We incubated transferrin receptor-conjugated with Texas Red with adipocytes for 30 min, after which time the receptor internalized and became concentrated in the perinuclear region in 3T3-L1 adipocytes. There was no difference in this process regardless of whether N-terminal p115 or EGFP alone was expressed (unpublished data).
Figure 7.
p115 N-terminus has no effect on GLUT1 trafficking. Constructs consisting of 100 μg N-terminal p115 EGFP, and C-terminal p115 EGFP were electroporated into 3T3-L1 adipocytes as described in Materials and Methods. Two days after electroporation, cells were serum-starved for 4 h and stimulated with 100 nM insulin for 15 min. The cells were then fixed with 3.7% formaldehyde and stained antipolyclonal rabbit GLUT1 antibody followed by Cy3 antibody as described in Materials and Methods. Pictures are representative of three independent experiments.
DISCUSSION
Skeletal muscle and adipocytes are specifically able to elaborate an intracellular pool of GLUT4 glucose transporters that move to the cell surface in response to insulin and thus modulate blood glucose homeostasis. Considerable morphological and biochemical evidence supports a model for GLUT4 trafficking, whereby approximately half the intracellular GLUT4 is targeted to a specific storage vesicle pool called GSVs or IRVs that is relatively deficient in other endosomal trafficking markers such as the transferrin receptor (TfR) and undergoes a marked increase in its rate of exocytosis in response to insulin (Simpson et al., 2001; Bryant et al., 2002). The molecular bases for this increased exocytic rate, the formation of GSVs, and the segregation of GLUT4 from endosomal markers such as the TfR remain incompletely understood.
To address this issue, we and others have used purification of GLUT4-rich vesicles to identify their protein content by immunological and biochemical (proteomics) means. These protocols have resulted in the identification of numerous proteins such as VAMP2 (Cain et al., 1992) and sorting receptors such as M6PR (Kandror and Pilch, 1996) and sortillin (Lin et al., 1997; Morris et al., 1998) that one might expect to be involved in vesicular traffic. Importantly and as cited in the introduction, IRAP was also identified in this way as marker for GSV trafficking that is apparently more abundant than GLUT4 itself. Indeed, we have shown that IRAP is directed to GSVs during the course of 3T3-L1 adipocyte differentiation before significant GLUT4 expression occurs (El-Jack et al., 1999). Moreover, in engineered adipocytes, IRAP can become essentially the only major cargo constituent of GSVs and it responds to insulin in a manner similar or identical to GSVs from 3T3-L1 cells (Gross et al., 2004). Thus, it can be expected that IRAP must interact with additional cellular proteins, adaptors, and tethers, to undergo proper targeting and trafficking. In particular the cytoplasmic sequences of IRAP likely interact with cytosolic proteins for steps in vesicle formation and targeting.
In this study, we used chitin binding domain (CBD) protein fused to the cytosolic sequence of IRAP, residues 1–109, to identify possible IRAP-binding proteins that might function in GSV trafficking. This fusion protein has the same orientation as does full-length IRAP in vesicles, and thus, we used it to identify p115 as an IRAP-interacting protein by a variety of criteria. Immobilized CBD-IRAP specifically binds p115 from the cytosol of 3T3-L1 cells (Figure 1) and rat adipocytes (not shown). Anti-IRAP antibody coimmunoprecipitates p115 from 3T3-L1 adipocytes (Figure 3), and pulldown assays demonstrate that CBD-IRAP binds to the N-terminus of p115 as well as full-length protein (Figure 4). Finally, immunofluorescence shows partial colocalization of p115 with GLUT4 (Figure 5) and IRAP (unpublished data). Importantly, expression of the N-terminal, membrane-binding sequence of p115 completely disrupts insulin-dependent GLUT4 and IRAP translocation (Figure 6) with no effect on GLUT1 or the Golgi marker GM130 (Figures 7 and 6E). Thus we conclude that p115 plays a specific role in insulin-dependent GSV trafficking.
The question remains as to where in the cell this IRAP-p115 interaction occurs and if there is any insulin-dependent regulation of this interaction. With regard to the latter issue, phosphorylation of p115 on serine 942 by casein kinase (Brunati et al., 2001) has been reported to enhance its binding to GM130 on Golgi membranes (Brandon et al., 2003), but there are no reports that insulin or other hormones can effect the phosphorylation of p115. In our experiments, we do not see a significant insulin-dependent mobility shift of bulk p115 (Figure 2) that might reflect this phosphorylation, but insulin does seem to effect the p115-IRAP interaction and decrease the efficiency of the IRAP coimmunoprecipitation, while simultaneously increasing the amount of p115 pulled down (Figure 3). Efforts to further understand this phenomenon are underway. The development of GSVs occurs during the differentiation of adipocytes, presumably as a result of the expression of an as yet unknown protein or proteins that initially results in the sequestration of IRAP, then GLUT4 (El-Jack et al., 1999). It is unlikely that p115 is one of these putative proteins as its expression is unchanged over the 8–10 d required for adipocytes to fully differentiate (unpublished data)
As for the cellular locus of this interaction, p115 has been described to play a variety of roles in vesicular transport at different cellular loci. In vitro vesicle fusion assays suggest p115 to be a general factor required for transport vesicle fusion to target membranes (Barroso et al., 1995; Sapperstein et al., 1995), in the present example, presumably the plasma membrane. And although the intensity of p115 fluorescence shows it to be mainly perinuclear and similar to syntaxin 6 and GM130 in adipocytes (Figure 5), subcellular fractionation reveals it to be ubiquitous in adipocytes membrane compartments, including the PM, as well as in the cytosol (Figure 2). As additional support for a possible functional role for p115 localization at the plasma membrane, we recently detected this protein in detergent-resistant PM lipid rafts isolated from insulin-treated adipocytes (unpublished data). We did not observe a significant signal for p115 in Western blots of immunoadsorbed GLUT4 vesicles (not shown).
A possible site of p115 function relevant to GSV trafficking is the trans-Golgi apparatus, which has been suggested to play a part in the trafficking of GSVs/GLUT4 based, in part, on colocalization of GLUT4 with the trans-Golgi marker, syntaxin6 (Shewan et al., 2003). However, whereas the expression of the p115-N-terminus disperses the GLUT4 signal throughout the cytosol and blocks its translocation, this construct is without effect on GM130 distribution (Figure 6E). Moreover, p115 function has usually been localized to the cis and medial Golgi where it attaches COP1-coated transport vesicles to the Golgi membrane and assists in vesicle fusion by assembling SNARE pin complexes (Shorter et al., 2002). However, p115 in adipocytes is clearly distributed throughout the cell (Figure 2).
How does the N-terminal domain of p115 disrupt GLUT4 distribution and translocation? We postulate that overexpressing the membrane/vesicle-binding domain of p115 allows it to displace endogenous full-length protein and release/disperse the GSVs so that they are no longer in position to interact with other cellular proteins necessary for their translocation and proper trafficking. Such proteins could be the actin filament–interacting protein, FHOS, which was cloned recently in our lab by the yeast two-hybrid system using IRAP as bait (Tojo et al., 2003). Other putative GSV-binding proteins include Tankyrase, a Golgi-associated MAPK substrate, which was also shown to interact with the cytoplasmic domain of IRAP by yeast two-hybrid (Chi and Lodish, 2000) and TUG (tether containing UBX domain for GLUT4), which was shown to bind GSVs by a translocation assay (Bogan et al., 2003). It remains to be determined at what step these proteins act in insulin-regulated GSV trafficking.
What is the role of GLUT4 cytoplasmic sequences in GSV trafficking? IRAP null mice exhibit apparently normal GLUT4 translocation (Keller et al., 2002), indicating, as expected, that the cytoplasmic domains of GLUT4 possess the information requisite for proper GSV trafficking. Indeed and as noted above, dileucine and acidic cluster sequences in IRAP's N-terminus are very similar to those in the GLUT4 C-terminus. We were unable to show direct p115-GLUT4 interactions with C-terminus-GST fusion proteins (unpublished data) either because the orientation of the fusion protein was such to prevent this interaction, or because GLUT4 has putative trafficking information in three noncontiguous cytoplasmic domains, all of which might be required for interaction with p115 (Khan et al., 2004). Still, it seems highly likely that the cytosolic sequences of both IRAP and GLUT4 play important roles in the cellular itinerary of GSVs. Finally, it also seems quite likely that p115 is not the only cytoplasmic protein that interacts with GSV components and we are continuing our efforts to identify more such molecules.
Acknowledgments
We thank Dr. Tsugumichi Saito for help and advice with the electroporation protocol and Dr. Libin Liu for help with the immunofluorescence figures. The study was supported by research grants from the National Institutes of Diabetes and Digestive and Kidney Diseases (DK-30425 and DK-49147 to P.F.P.)
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-01-0072) on March 30, 2005.
Abbreviations used: GSV, Glut4 storage vesicle; Glut, glucose transporter; CBD, chitin binding domain; IRAP, insulin-responsive aminopeptidase; EGFP, enhanced green fluorescent protein; ECL, enhanced chemiluminescence.
References
- Alvarez, C., Garcia-Mata, R., Hauri, H. P., and Sztul, E. (2001). The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 276, 2693-2700. [DOI] [PubMed] [Google Scholar]
- Barroso, M., Nelson, D. S., and Sztul, E. (1995). Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc. Natl. Acad. Sci. USA 92, 527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan, J. S., Hendon, N., McKee, A. E., Tsao, T. S., and Lodish, H. F. (2003). Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 425, 727-733. [DOI] [PubMed] [Google Scholar]
- Brandon, E., Gao, Y., Garcia-Mata, R., Alvarez, C., and Sztul, E. (2003). Membrane targeting of p115 phosphorylation mutants and their effects on Golgi integrity and secretory traffic. Eur. J. Cell Biol. 82, 411-420. [DOI] [PubMed] [Google Scholar]
- Brunati, A. M., Marin, O., Folda, A., Meggio, F., and Pinna, L. A. (2001). Possible implication of the Golgi apparatus casein kinase in the phosphorylation of vesicle docking protein p115 Ser-940, a study with peptide substrates. Biochem. Biophys. Res. Commun. 284, 817-822. [DOI] [PubMed] [Google Scholar]
- Bryant, N. J., Govers, R., and James, D. E. (2002). Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 3, 267-277. [DOI] [PubMed] [Google Scholar]
- Cain, C. C., Trimble, W. S., and Lienhard, G. E. (1992). Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J. Biol. Chem. 267, 11681-11684. [PubMed] [Google Scholar]
- Chi, N. W., and Lodish, H. F. (2000). Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 275, 38437-38444. [DOI] [PubMed] [Google Scholar]
- Corvera, S., Chawla, A., Chakrabarti, R., Joly, M., Buxton, J., and Czech, M. P. (1994). A double leucine within the GLUT4 glucose transporter COOH-terminal domain functions as an endocytosis signal. J. Cell Biol. 126, 979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jack, A. K., Kandror, K. V., and Pilch, P. F. (1999). The formation of an insulin-responsive vesicular cargo compartment is an early event in 3T3–L1 adipocyte differentiation. Mol. Biol. Cell 10, 1581-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto, H., Kayano, T., Buse, J. B., Edwards, Y., Pilch, P. F., Bell, G. I., and Seino, S. (1989). Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J. Biol. Chem. 264, 7776-7779. [PubMed] [Google Scholar]
- Garza, L. A., and Birnbaum, M. J. (2000). Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J. Biol. Chem. 275, 2560-2567. [DOI] [PubMed] [Google Scholar]
- Gross, D. N., Farmer, S. R., and Pilch, P. F. (2004). Glut4 storage vesicles without Glut4, transcriptional regulation of insulin-dependent vesicular traffic. Mol. Cell. Biol. 24, 7151-7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney, P. M., Levy, M. A., Strube, M. S., and Mueckler, M. (1995). Insulin-sensitive targeting of the GLUT4 glucose transporter in L6 myoblasts is conferred by its COOH-terminal cytoplasmic tail. J. Cell Biol. 129, 641-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, D. E., Brown, R., Navarro, J., and Pilch, P. F. (1988). Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333, 183-185. [DOI] [PubMed] [Google Scholar]
- Kandror, K., and Pilch, P. F. (1994). Identification and isolation of glycoproteins that translocate to the cell surface from GLUT4-enriched vesicles in an insulin-dependent fashion. J. Biol. Chem. 269, 138-142. [PubMed] [Google Scholar]
- Kandror, K. V. (1999). Insulin regulation of protein traffic in rat adipose cells. J. Biol. Chem. 274, 25210-25217. [DOI] [PubMed] [Google Scholar]
- Kandror, K. V., and Pilch, P. F. (1996). The insulin-like growth factor II/mannose 6-phosphate receptor utilizes the same membrane compartments as GLUT4 for insulin-dependent trafficking to and from the rat adipocyte cell surface. J. Biol. Chem. 271, 21703-21708. [DOI] [PubMed] [Google Scholar]
- Kandror, K. V., Stephens, J. M., and Pilch, P. F. (1995). Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J. Cell Biol. 129, 999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror, K. V., Yu, L., and Pilch, P. F. (1994). The major protein of GLUT4-containing vesicles, gp160, has aminopeptidase activity. J. Biol. Chem. 269, 30777-30780. [PubMed] [Google Scholar]
- Keller, S. R., Davis, A. C., and Clairmont, K. B. (2002). Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J. Biol. Chem. 277, 17677-17686. [DOI] [PubMed] [Google Scholar]
- Keller, S. R., Scott, H. M., Mastick, C. C., Aebersold, R., and Lienhard, G. E. (1995). Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J. Biol. Chem. 270, 23612-23618. [DOI] [PubMed] [Google Scholar]
- Khan, A. H., Capilla, E., Hou, J. C., Watson, R. T., Smith, J. R., and Pessin, J. E. (2004). Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is dependent upon both the amino terminus and the large cytoplasmic loop. J. Biol. Chem. 279, 37505-37511. [DOI] [PubMed] [Google Scholar]
- Kupriyanova, T. A., Kandror, V., and Kandror, K. V. (2002). Isolation and characterization of the two major intracellular Glut4 storage compartments. J. Biol. Chem. 277, 9133-9138. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Lin, B. Z., Pilch, P. F., and Kandror, K. V. (1997). Sortilin is a major protein component of Glut4-containing vesicles. J. Biol. Chem. 272, 24145-24147. [DOI] [PubMed] [Google Scholar]
- Malide, D., St-Denis, J. F., Keller, S. R., and Cushman, S. W. (1997). Vp165 and GLUT4 share similar vesicle pools along their trafficking pathways in rat adipose cells. FEBS Lett. 409, 461-468. [DOI] [PubMed] [Google Scholar]
- Marsh, B. J., Alm, R. A., McIntosh, S. R., and James, D. E. (1995). Molecular regulation of GLUT-4 targeting in 3T3–L1 adipocytes. J. Cell Biol. 130, 1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S., Rice, J. E., Gould, G. W., Keller, S. R., Slot, J. W., and James, D. E. (1997). The glucose transporter GLUT4 and the aminopeptidase vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J. Cell Sci. 110(Pt 18), 2281-2291. [DOI] [PubMed] [Google Scholar]
- Mastick, C. C., Aebersold, R., and Lienhard, G. E. (1994). Characterization of a major protein in GLUT4 vesicles. Concentration in the vesicles and insulin-stimulated translocation to the plasma membrane. J. Biol. Chem. 269, 6089-6092. [PubMed] [Google Scholar]
- Morris, N. J., Ross, S. A., Lane, W. S., Moestrup, S. K., Petersen, C. M., Keller, S. R., and Lienhard, G. E. (1998). Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J. Biol. Chem. 273, 3582-3587. [DOI] [PubMed] [Google Scholar]
- Okada, S., Mori, M., and Pessin, J. E. (2003). Introduction of DNA into 3T3–L1 adipocytes by electroporation. Methods Mol. Med. 83, 93-96. [DOI] [PubMed] [Google Scholar]
- Ross, S. A., Herbst, J. J., Keller, S. R., and Lienhard, G. E. (1997). Trafficking kinetics of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. Biochem. Biophys. Res. Commun. 239, 247-251. [DOI] [PubMed] [Google Scholar]
- Ross, S. A., Keller, S. R., and Lienhard, G. E. (1998). Increased intracellular sequestration of the insulin-regulated aminopeptidase upon differentiation of 3T3–L1 cells. Biochem. J. 330(Pt 2), 1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein, S. K., Walter, D. M., Grosvenor, A. R., Heuser, J. E., and Waters, M. G. (1995). p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc. Natl. Acad. Sci. USA 92, 522-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan, A. M., van Dam, E. M., Martin, S., Luen, T. B., Hong, W., Bryant, N. J., and James, D. E. (2003). GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38, involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., Beard, M. B., Seemann, J., Dirac-Svejstrup, A. B., and Warren, G. (2002). Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, F., Whitehead, J. P., and James, D. E. (2001). GLUT4 —at the cross roads between membrane trafficking and signal transduction. Traffic 2, 2-11. [DOI] [PubMed] [Google Scholar]
- Simpson, I. A., Yver, D. R., Hissin, P. J., Wardzala, L. J., Karnieli, E., Salans, L. B., and Cushman, S. W. (1983). Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim. Biophys. Acta 763, 393-407. [DOI] [PubMed] [Google Scholar]
- Stephens, J. M., Lee, J., and Pilch, P. F. (1997). Tumor necrosis factor-alpha-induced insulin resistance in 3T3–L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 272, 971-976. [DOI] [PubMed] [Google Scholar]
- Subtil, A., Lampson, M. A., Keller, S. R., and McGraw, T. E. (2000). Characterization of the insulin-regulated endocytic recycling mechanism in 3T3–L1 adipocytes using a novel reporter molecule. J. Biol. Chem. 275, 4787-4795. [DOI] [PubMed] [Google Scholar]
- Tojo, H., Kaieda, I., Hattori, H., Katayama, N., Yoshimura, K., Kakimoto, S., Fujisawa, Y., Presman, E., Brooks, C. C., and Pilch, P. F. (2003). The Formin family protein, formin homolog overexpressed in spleen, interacts with the insulin-responsive aminopeptidase and profilin IIa. Mol. Endocrinol. 17, 1216-1229. [DOI] [PubMed] [Google Scholar]
- Verhey, K. J., and Birnbaum, M. J. (1994). A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. J. Biol. Chem. 269, 2353-2356. [PubMed] [Google Scholar]
- Verhey, K. J., Hausdorff, S. F., and Birnbaum, M. J. (1993). Identification of the carboxy terminus as important for the isoform-specific subcellular targeting of glucose transporter proteins. J. Cell Biol. 123, 137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey, K. J., Yeh, J. I., and Birnbaum, M. J. (1995). Distinct signals in the GLUT4 glucose transporter for internalization and for targeting to an insulin-responsive compartment. J. Cell Biol. 130, 1071-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, M. G., Clary, D. O., and Rothman, J. E. (1992). A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol. 118, 1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, S. B., D'Auria, M., Martin, S. S., Nguyen, C., Kozma, L. M., and Luskey, K. L. (1997). The amino terminus of insulin-responsive aminopeptidase causes Glut4 translocation in 3T3–L1 adipocytes. J. Biol. Chem. 272, 23323-23327. [DOI] [PubMed] [Google Scholar]
- Zhou, M., Vallega, G., Kandror, K. V., and Pilch, P. F. (2000). Insulin-mediated translocation of GLUT-4-containing vesicles is preserved in denervated muscles. Am. J. Physiol. Endocrinol. Metab. 278, E1019-E1026. [DOI] [PubMed] [Google Scholar]