Abstract
Aims
In low‐risk patients with severe aortic stenosis (AS), sutureless surgical aortic valve replacement (SU‐SAVR) may be an alternative to transcatheter aortic valve implantation (TAVI). The risk of heart failure hospitalization (HFH) after aortic valve replacement (AVR) in this population is incompletely characterized. This study aims to investigate the incidence, predictors, and outcomes of HFH in patients undergoing SU‐SAVR versus TAVI.
Methods and results
Patients referred for AVR between 2013 and 2020 at two centres were consecutively included. The decision for SU‐SAVR or TAVI was determined by a multidisciplinary Heart Team. Cox regression and competing risk analysis were conducted to assess adverse events. Of 594 patients (mean age 77.5 ± 6.4, 59.8% male), 424 underwent SU‐SAVR, while 170 underwent TAVI. Following a mean follow‐up of 34.1 ± 23.1 months, HFH occurred in 112 (27.8%) SU‐SAVR patients and in 8 (4.8%) TAVI patients (P < 0.001). The SU‐SAVR cohort exhibited higher all‐cause mortality (138 [32.5%] patients compared with 30 [17.6%] in the TAVI cohort [P < 0.001]). These differences remained significant after sensitivity analyses with 1:1 propensity score matching for baseline variables. SU‐SAVR with HFH was associated with increased all‐cause mortality (61.6% vs. 23.1%, P < 0.001). Independent associates of HFH in SU‐SAVR patients included diabetes, atrial fibrillation, chronic obstructive pulmonary disease, lower glomerular filtration rate and lower left ventricular ejection fraction. SU‐SAVR patients with HFH had a 12‐month LVEF of 59.4 ± 12.7.
Conclusions
In low‐risk AS, SU‐SAVR is associated with a higher risk of HFH and all‐cause mortality compared to TAVI. In patients with severe AS candidate to SU‐SAVR or TAVI, TAVI may be the preferred intervention.
Keywords: Aortic stenosis cardiomyopathy, Aortic valve stenosis, Heart failure, Mortality, Prostheses and implants
This study compares SU‐SAVR and TAVI in low‐risk patients with severe aortic stenosis (AS), focusing on heart failure hospitalization (HFH). Among 594 patients from 2013 to 2020, 424 underwent SU‐SAVR and 170 TAVI. HFH was significantly higher in SU‐SAVR patients (27.8% vs. 4.8%), who also had higher all‐cause mortality. Factors like diabetes, atrial fibrillation, and lower LVEF increased HFH risk in SU‐SAVR patients. TAVI shows lower HFH and mortality, suggesting it as the preferred intervention for low‐risk AS patients.

Introduction
Randomized controlled trials have consolidated transcatheter aortic valve implantation (TAVI) as a good therapeutic option over surgical aortic valve replacement (SAVR) in patients with symptomatic severe aortic stenosis (AS), spanning from low surgical risk 1 , 2 , 3 , 4 to inoperable patients, 5 , 6 , 7 , 8 , 9 particularly when transfemoral access is feasible.
Sutureless aortic valves were developed to allow a minimally invasive SAVR through a rapid‐deployment implantation technique. Compared to conventional aortic valve prosthesis, SAVR with a sutureless aortic valve (SU‐SAVR) has been associated with reduced procedural, aortic cross‐clamping (ACC) and cardiopulmonary bypass (CPB) times along with lower postoperative complications and non‐inferior mid‐term outcomes, although with higher rates of pacemaker implantation. 10 , 11 , 12 Trials and metanalyses comparing SU‐SAVR and TAVI have shown similar prosthetic valve haemodynamics, particularly among patients with high‐ or intermediate‐surgical risk. 13 , 14 , 15 , 16 , 17
Despite the advantages of SU‐SAVR, recent data show an increased risk of heart failure hospitalization (HFH) in patients with sutureless aortic valves. 17 Furthermore, the incidence and impact of HFH among low‐risk patients undergoing AVR with sutureless valves is still uncertain. The present study aimed to evaluate the incidence of HFH in low‐risk patients with symptomatic severe AS treated with SU‐SAVR and TAVI and to explore the clinical implications of HFH among patients treated with SU‐SAVR.
Methods
This is a retrospective, observational study. Data, methods, and materials used to conduct the research will be provided to any researcher for purposes of reproducing the results upon reasonable request. The study was performed according to the Ethics Committee of participating centres, and the patients provided informed consent for the procedures.
Study population
The flowchart of the study population is shown in Figure 1 . A total of 594 consecutive low‐risk (EuroSCORE II < 4%) patients diagnosed with symptomatic severe AS who underwent either SU‐SAVR with the Perceval valve or TAVI between 2013 and 2020 in two centres were included. Patients' suitability for each procedure was evaluated by a multidisciplinary Heart Team in each participating centre. All procedures were performed following standard institutional clinical protocols. The choice of the transcatheter aortic bioprosthesis was left to the operator's discretion. Combined procedures (concomitant revascularization with a coronary artery bypass graft or another valvular intervention) were excluded from the analysis.
Figure 1.
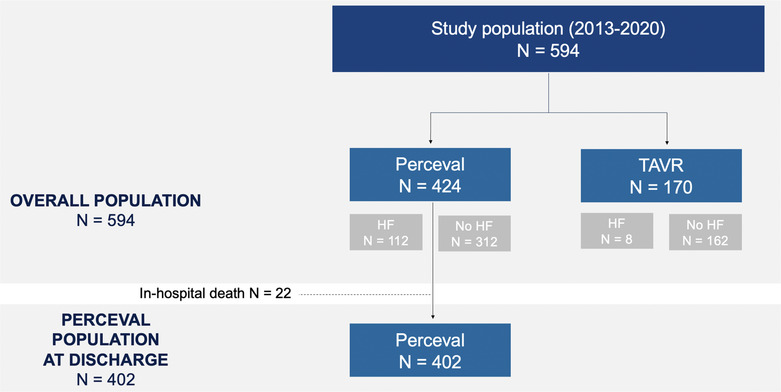
Study flowchart of included patients.
Follow‐up
All baseline, procedural, and follow‐up (FU) data were retrospectively collected in a dedicated database at each participating centre. All clinical events were recorded and defined according to criteria established by the Valve Academic Research Consortium‐3. 18 Transthoracic echocardiographic data of the overall cohort at 3‐month, 1‐year, 2‐year, and 3‐year follow‐up was available in 273 (48.9%), 429 (79.8%), 362 (73.2%), and 307 (64%) patients, respectively. HF hospitalizations were defined as admissions to a hospital, intensive care unit or the emergency department that lasted >6 h and required intravenous diuretic treatment. Day‐stay hospital admissions and admissions that only required oral treatment were excluded from the current analysis.
Statistical analysis
Categorical variables were expressed as absolute number and percentages. Continuous variables were expressed as mean ± standard deviation or medians and interquartile range for data with normal and non‐normal distributions, respectively. Data distribution was assessed with normal Q–Q plots. Differences between groups were assessed using the χ 2 test, Fisher exact test, Student's t‐test, or Mann–Whitney U test, as appropriate. 1:1 propensity score matching without replacement using the nearest neighbour method was performed as a sensitivity analysis when comparing HF hospitalization between the SU‐SAVR and TAVI cohorts. The following variables were included for calculation of the propensity score: Age, sex, body mass index, hypertension, dyslipidaemia, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, prior stroke, cancer, coronary artery disease, history of myocardial infarction, previous percutaneous coronary intervention, previous cardiac surgery, prior coronary artery bypass graft, atrial fibrillation, prior left bundle branch block, previous pacemaker, New York Heart Association functional Class III or IV, basal left ventricle ejection fraction, basal left ventricle diastolic diameter, basal peak aortic velocity and mean aortic gradient, basal haemoglobin, basal estimated glomerular filtration rate, Euroscore II, STS score and follow‐up time. A calliper width of 0.2 of the pooled standard deviation of logit propensity scores was used for matching. When analysing the SU‐SAVR cohort for predictors of HF hospitalization, multivariable logistic regression analyses included all clinical variables that exhibited a P‐value of <0.05 in the univariable analysis and that were considered clinically significant. The risk of all‐cause mortality was evaluated with the use of a Cox proportional‐hazard model, while the risk of hospitalization due to HF was assessed using a competing risk analysis adopting the Fine and Grey method, considering all‐cause death as competing event. A two‐sided P‐value <0.05 was considered significant. Stata v.15.1 (College Station, TX) was used for descriptive and inferential analytics, while graphical representations were performed with R v.4.3.1 (R Core Team, 2023).
Results
Overall study population
Of 594 consecutive low‐risk (median EuroSCORE II 2.87%) patients who underwent AVR, 424 patients were treated with SU‐SAVR and 170 patients with TAVI (Figure 1 ). The baseline characteristics of the overall cohort are presented in Table 1 . Information regarding prosthesis type and size can be found in the supplementary material (Table S1 ).
Table 1.
Demographic, clinical and perioperative characteristics of the study population
| SU‐SAVR | TAVI | P value | |
|---|---|---|---|
| (n = 424) | (n = 170) | ||
| Demographics | |||
| Age, years | 77.6 ± 6.4 | 77.3 ± 8.4 | 0.701 |
| Male sex | 177 (41.7) | 62 (36.5) | 0.236 |
| BMI, kg/m2 | 29 ± 5.0 | 29 ± 7.2 | 0.679 |
| Arterial hypertension | 364 (85.8) | 143 (84.1) | 0.59 |
| Dyslipidaemia | 321 (75.7) | 130 (76.5) | 0.844 |
| Diabetes mellitus | 158 (37.3) | 58 (34.1) | 0.471 |
| COPD | 63 (14.9) | 21 (12.4) | 0.428 |
| CAD | 169 (39.9) | 34 (20.0) | <0.001 |
| Prior MI | 38 (8.9) | 14 (8.2) | 0.777 |
| Prior PCI | 33 (7.8) | 30 (17.6) | <0.001 |
| Prior cardiac surgery | 39 (9.2) | 10 (5.9) | 0.184 |
| Prior CABG | 5 (1.2) | 0 (0) | 0.155 |
| Atrial fibrillation | 112 (26.4) | 42 (24.7) | 0.667 |
| Prior LBBB | 27 (6.4) | 11 (6.5) | 0.963 |
| Prior PM | 25 (5.9) | 13 (7.6) | 0.431 |
| NYHA III/IV | 185 (53.8) | 83 (48.8) | 0.29 |
| eGFR, (mL/min/1.73 m2) | 67.4 ± 25.8 | 71.9 ± 23.2 | 0.046 |
| Haemoglobin, g/dL | 12.4 ± 1.5 | 12.5 ± 1.6 | 0.291 |
| Baseline echocardiography | |||
| LVEF, % | 61.7 ± 12.9 | 60.4 ± 5.6 | 0.203 |
| LVEDD, mm | 47.8 ± 7.3 | 44.1 ± 7.3 | <0.001 |
| Peak Ao vel, m/seg | 4.3 ± 0.7 | 4.4 ± 0.8 | 0.164 |
| Mean Ao Grad, mmHg | 49.4 ± 16.0 | 48.4 ± 18.5 | 0.523 |
| Indexed AVA, cm2/m2 | 0.41 ± 0.1 | 0.39 ± 0.2 | 0.64 |
| Risk scores | |||
| EuroSCORE II, % | 3.2 ± 3.9 | 1.9 ± 0.9 | <0.001 |
| STS PROM, % | 7.9 ± 93.3 | 2.6 ± 1.1 | 0.463 |
| Periprocedural biomarkers | |||
| Troponin peak, pg/mL | 2,930 (3524) | 1,361 (1933) | 0.005 |
| CK peak, IU/L | 193 (297) | 145.5 (125) | 0.351 |
| CRP peak, mg/L | 105.6 (100) | 48 (43) | <0.001 |
| Fibrinogen peak, mg/dL | 762 (168) | 394 (142) | <0.001 |
| In‐hospital death | 22 (5.2) | 3 (1.8) | 0.06 |
| Cardiac | 6 (27.3) | 1 (33.3) | 0.003 |
| Systemic | 16 (72.7) | 0 (0) | |
| Stroke | 0 (0) | 2 (66.7) | |
| Echocardiography at discharge | |||
| LVEF, % | 64 (15) | 60.0 (5) | <0.001 |
| LVEDD, mm | 44.9 (6.2) | 42.9 (7.2) | 0.04 |
| Mean Ao Grad, mmHg | 16.8 (6.4) | 11.6 (5.8) | <0.001 |
| Aortic regurgitation | |||
| None | 334 (78.8) | 80 (47.1) | <0.001 |
| Grade 1 | 68 (16) | 53 (31.2) | |
| Grade 2 | 13 (3.1) | 24 (14.1) | |
| Grade 3 | 2 (0.5) | 2 (1.2) | |
| Paravalvular leak | 33 (7.8) | 28 (16.5) | 0.522 |
| Indexed AVA, cm2/m2 | 0.94 (0.3) | 0.99 (0.3) | 0.608 |
| Treatment at discharge | |||
| VKA | 109 (26.9) | 22 (13.0) | <0.001 |
| DOAC | 18 (4.5) | 24 (14.2) | <0.001 |
| Beta‐blocker | 192 (47.4) | 65 (38.9) | 0.064 |
| Calcium antagonist | 71 (17.6) | 76 (45.5) | <0.001 |
| Diuretics | 333 (82.4) | 71 (42.5) | <0.001 |
| ACEI | 150 (37.0) | 34 (20.4) | <0.001 |
| ARB | 45 (11.1) | 43 (25.8) | <0.001 |
| Follow‐up HF hospitalization | 112 (27.8) | 8 (4.8) | <0.001 |
| Follow‐up death | 138 (32.5) | 30 (17.6) | <0.001 |
Results expressed as mean ± SD or median (interquartile range) for quantitative variables and n (%) for categorical.
ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin 2 receptor blockers; AVA, aortic valve area; AVR, aortic valve replacement; BMI: body mass index; CABG, Coronary artery bypass grafting; CAD, coronary artery disease; CK, creatine kinase; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HF, heart failure; LBBB, left bundle branch block; LVEDD, left ventricular end‐diastolic diameter; LVEF left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association class; PCI, percutaneous coronary intervention; PM, permanent pacemaker; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI, transcatheter aortic valve implantation; VKA, vitamin K antagonist.
Both groups were comparable in terms of age, sex, cardiovascular risk factors, chronic obstructive pulmonary disease (COPD), atrial fibrillation (AF) history, cardiac conduction disturbances, and baseline New York Heart Association class (NYHA). Patients treated with SU‐SAVR had worse renal function (eGFR 67 ± 25.8 mL/min/1.73 m2 vs. 71.9 ± 23.2 mL/min/1.73 m2, P = 0.046) and larger left ventricles (left ventricular end‐diastolic diameter 47.8 ± 7.3 mm vs. 44.1 ± 7.3 mm, P < 0.001) with a comparable left ventricular ejection fraction (LVEF, 61.7 ± 12.9 vs. 60.4 ± 5.6 P = 0.203), as compared with their counterparts.
While still classified as low‐risk patients, SU‐SAVR patients exhibited a higher surgical risk (EuroSCORE II 3.2% vs. 1.9%; P < 0.001) and a higher elevation of cardiac biomarkers and acute phase reactants during the perioperative period. Postoperatively, patients treated with SU‐SAVR showed higher LVEF (64 ± 15 vs. 60 ± 5, P < 0.001) and mean aortic gradients (16.8 ± 6.4 vs. 11.6 ± 5.8, P < 0.001) and larger left ventricles (LVEDD 44.9 ± 6.2 vs. 42.9 ± 7.2, P = 0.04), while TAVI patients presented more frequently any degree of aortic regurgitation, mostly grades 1 and 2. Paravalvular leak rates were comparable between groups. There were 22 (5.2%) in‐hospital deaths among patients treated with SU‐SAVR and 3 (1.8%) among patients treated with TAVI. The proportion of cardiac death was comparable between groups [6 (27.2%) and 1 (33%) for SU‐SAVR and TAVI patients, respectively], but the origin of the rest of deceases differs: 16 (72.7%) SU‐SAVR patients died due to systemic affections (six respiratory insufficiency or non‐cardiac origin, four abdominal complications, and four haemorrhagic events), while in the remaining 2 (67%) TAVI patients, the death cause was a fatal cerebrovascular accident.
At discharge, SU‐SAVR patients received more often diuretics (82.4% vs. 42.5%, P < 0.001) and angiotensin‐converting enzyme inhibitors (37% vs. 20.4%, P < 0.001) while patients treated with TAVI received more often angiotensin 2 receptor blockers (25.8% vs. 11.1%, P < 0.001).
Mid‐term outcomes of the overall cohort
After a mean follow‐up of 34.1 ± 23.1 months, hospitalization for HF occurred in 112 (27.8%) patients in the SU‐SAVR cohort and in 8 (4.8%) patients in the TAVI cohort (P < 0.001). Heart failure‐free survival curves are depicted in Figure 2 A . SU‐SAVR patients exhibited higher LVEF values at 3 months (65 ± 13.8 vs. 60 ± 5, P = 0.029) and 12 months (64 ± 11 vs. 60 ± 5, P = 0.005) and higher mean aortic gradients at 3 months (14 ± 7 mmHg vs. 12 ± 6 mmHg, P = 0.003) compared with TAVI patients to become comparable thereafter (Figure S1 ). After 1:1 propensity score‐matching for baseline variables, differences in HF hospitalization between groups remained statistically significant. Notably, significant differences regarding cardiac and inflammatory biomarkers as well as postoperative mean aortic gradients were also maintained (Table S2 ).
Figure 2.
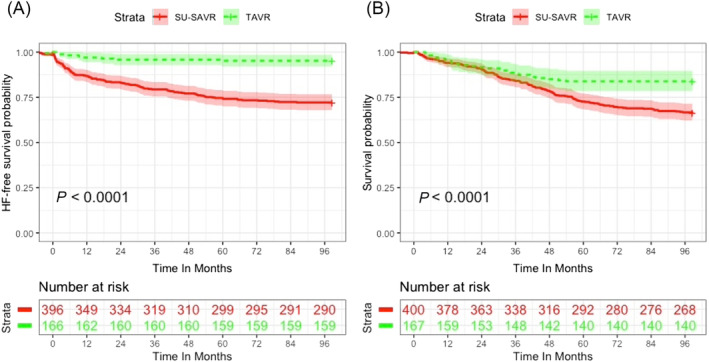
Cumulative incidence of HF readmission (A) and death (B) for SU‐SAVR and TAVI patients.
The SU‐SAVR cohort also showed a higher mid‐term all‐cause mortality [138 (32.5%) SU‐SAVR patients vs. 30 (17.6%) TAVI patients, P < 0.001]. Survival curves are represented in Figure 2 B . After 1:1 propensity score‐matching for baseline variables, differences in follow‐up death between groups remained statistically significant (Table S2 ).
Characterization of the sutureless surgical aortic valve replacement population according to heart failure hospitalization
Of the 402 SU‐SAVR patients included in the follow‐up analysis, 112 (27.8%) were hospitalized for HF after a mean follow up of 1,330 ± 604 days. The baseline characteristics and in‐hospital outcomes of the SU‐SAVR cohort according to HF hospitalization are presented in Table 2 .
Table 2.
Baseline characteristics and in‐hospital outcomes of SU‐SAVR patients according to HF readmission
| Sutureless SAVR cohort (n = 402) | |||
|---|---|---|---|
| HF (n = 112) | No HF (n = 290) | P value | |
| Demographics | |||
| Age, years | 76.9 ± 6.2 | 77.8 ± 6.4 | 0.242 |
| Arterial hypertension | 104 (92.9) | 238 (82.1) | 0.007 |
| Diabetes mellitus | 55 (49.1) | 90 (31.0) | 0.001 |
| COPD | 25 (22.3) | 34 (11.7) | 0.007 |
| NYHA III/IV | 59 (52.7) | 112 (38.6) | 0.024 |
| Permanent atrial fibrillation | 27 (24.1) | 24 (8.3) | <0.001 |
| Paroxysmal atrial fibrillation | 16 (14.3) | 36 (12.4) | 0.616 |
| Prior LBBB | 12 (10.7) | 14 (4.8) | 0.031 |
| Prior MI | 16 (14.3) | 19 (6.6) | 0.014 |
| Prior cardiac surgery | 10 (8.9) | 27 (9.3) | 0.906 |
| eGFR (mL/min/1.73 m2) | 60.4 ± 24.7 | 70.8 ± 24.9 | <0.001 |
| Baseline echocardiography | |||
| LVEF, % | 58.8 ± 13.8 | 63.2 ± 12.0 | 0.002 |
| LVEDD, mm | 50.0 ± 7.3 | 47.0 ± 7.2 | <0.001 |
| Peak Ao vel, m/seg | 4.1 ± 0.7 | 4.4 ± 0.7 | <0.001 |
| Mean Ao Grad, mmHg | 44.4 ± 13.2 | 51.4 ± 16.4 | <0.001 |
| Indexed AVA, cm2/m2 | 0.42 ± 0.1 | 0.39 ± 0.1 | 0.145 |
| Risk scores | |||
| EuroSCORE II, % | 3.67 ± 4 | 2.99 ± 3.7 | 0.109 |
| STS PROM, % | 3.84 ± 2.6 | 9.7 ± 112.8 | 0.586 |
| Periprocedural biomarkers | |||
| Troponin peak, pg/mL | 3,521 (3380) | 2,220 (2921) | <0.001 |
| CK peak, IU/L | 138 (230) | 175 (210) | 0.098 |
| CRP peak, mg/L | 106 (96) | 95 (95) | 0.604 |
| Fibrinogen peak, mg/dL | 162 (120) | 162 (136) | 0.831 |
| In‐hospital outcomes | |||
| Median sternotomy | 81 (72.3) | 190 (65.5) | 0.192 |
| CPB time, min | 77.6 ± 27.2 | 71.6 ± 22.8 | 0.025 |
| Clamping time, min | 54.2 ± 21.5 | 50.3 ± 17.8 | 0.06 |
| In‐hospital HF | 17 (15.2) | 33 (11.4) | 0.301 |
| Acute kidney injury | 61 (54.5) | 100 (34.5) | <0.001 |
| Hospitalization days | 20 ± 10.3 | 17.1 ± 12.5 | 0.029 |
| Echocardiography at discharge | |||
| LVEF, % | 59.1 ± 14.1 | 62.5 ± 10.7 | 0.009 |
| LVED diameter, mm | 47.1 ± 6.3 | 44.3 ± 6.1 | 0.028 |
| Mean Ao Grad, mmHg | 16.7 ± 6.4 | 16.9 ± 6.4 | 0.791 |
| Aortic regurgitation | |||
| None | 83 (74.1) | 226 (77.9) | 0.619 |
| Grade 1 | 23 (20.5) | 55 (19) | |
| Grade 2 | 5 (4.5) | 7 (2.4) | |
| Grade 3 | 0 (0) | 1 (0.3) | |
| Mitral regurgitation | |||
| None | 37 (33) | 125 (43.1) | 0.11 |
| Grade 1 | 56 (50) | 132 (45.5) | |
| Grade 2 | 16 (14.3) | 30 (10.3) | |
| Grade 3 | 3 (2.7) | 2 (0.7) | |
| PASP, mmHg | 44.6 ± 9.5 | 39.7 ± 8.6 | <0.001 |
| Treatment at discharge | |||
| VKA | 41 (36.6) | 68 (23.4) | 0.012 |
| DOAC | 8 (6.7) | 10 (7.5) | 0.177 |
| Beta‐blocker | 52 (46.4) | 139 (47.9) | 0.764 |
| Calcium antagonist | 30 (26.8) | 41 (14.1) | 0.003 |
| Diuretic | 96 (85.7) | 235 (81) | 0.281 |
| ACEI | 41 (36.6) | 108 (37.2) | 0.979 |
| ARB | 11 (9.8) | 33 (11.4) | 0.646 |
| Mid‐term outcomes | |||
| Need for redo procedure (ViV) | 4 (3.6) | 3 (1) | 0.083 |
| QRS length | 129 ± 32 | 120 ± 29 | 0.01 |
| AF prevalence | 43 (38.3) | 55 (18.9) | <0.001 |
| LBBB prevalence | 34 (30.3) | 60 (20.7) | 0.073 |
| PM prevalence | 13 (11.6) | 21 (7.2) | 0.007 |
| MI | 5 (4.5) | 8 (2.8) | 0.097 |
Results expressed as mean ± SD or median (interquartile range) for quantitative variables and n (%) for categorical after excluding in‐hospital deaths (n = 22).
ACEI, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin 2 receptor blockers; AVA, aortic valve area; AVR, aortic valve replacement; CK, creatine kinase; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HF, heart failure; LBBB, left bundle branch block; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association class; PASP, pulmonary arterial systolic pressure; PM, permanent pacemaker; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement; ViV, valve‐in‐valve; VKA, vitamin K antagonist.
Patients with at least one HF hospitalization were more likely to have hypertension (92.9% vs. 82.1%, P = 0.007) and diabetes (49.1% vs. 31%, P = 0.001), were most commonly diagnosed with COPD (22.3% vs. 11.7%, P = 0.007), permanent atrial fibrillation (24.1% vs. 8.3%, P < 0.001), left bundle branch block (10.7% vs. 4.8%, P = 0.031), and myocardial infarction (14.3% vs. 6.6%, P = 0.014), and presented worse renal function (eGFR 60.4 ± 24.7 mL/min/1.73 m2 vs. 70.8 ± 24.9 mL/min/1.73 m2, P < 0.001). These patients also showed larger left ventricles (left ventricular end‐diastolic diameter 50 ± 7.3 mm vs. 47 ± 7.2 mm, P < 0.001), lower LVEF (58.8 ± 13.8% vs. 63.2 ± 12.0%, P = 0.002), and lower basal mean transvalvular aortic pressure gradients (44.4 ± 13.2 mmHg vs. 51.4 ± 16.4 mmHg, P < 0.001).
Admitted patients experienced longer cardiopulmonary bypass times (CPB) (77.6 min vs. 71.6 min, P = 0.025) and, although borderline for statistical significance, longer aortic cross‐clamp (ACC) times (54.2 min vs. 50.3 min, P = 0.06). Perioperatively, they presented a higher rise in cardiac biomarkers (troponin peak 3,521 pg/mL vs. 2,220 pg/mL, P < 0.001), higher rates of acute kidney injury (54.5% vs. 34.5%, P < 0.001), and longer hospital stays (20 days vs. 17 days, P = 0.029). Postoperatively, they exhibited lower LVEF (59.1% vs. 62.5%, P = 0.009), larger left ventricles (47.1 mm vs. 44.3 mm, P = 0.028), and higher pulmonary arterial systolic pressure (44.6 mmHg vs. 39.7 mmHg, P < 0.001), with aortic gradients and regurgitation comparable with those of non‐admitted patients.
Mid‐term outcomes of the sutureless surgical aortic valve replacement cohort according to heart failure hospitalization
The midterm outcomes and echocardiographic monitoring of SU‐SAVR patients according to HF hospitalization are presented in Table 2 and Figure S2 , respectively.
Patients admitted for HF showed lower (while always preserved) LVEF at 3‐month (60 ± 14.5 vs. 66 ± 12, P < 0.001), 1‐year (61.5 ± 12 vs. 65 ± 10, P < 0.001), and 2‐year (60 ± 13.8 vs. 64.5 ± 9.8, P = 0.006) follow‐up to become comparable thereafter, while mean aortic gradients remained comparable throughout the whole follow‐up period. These patients were more likely to present interventricular conduction disturbances (QRS of 129 ms vs. 120 ms, P = 0.010; left bundle branch block 31% vs. 22%, P = 0.073), AF (40% vs. 20%, P < 0.001), and permanent pacemaker (PM, 10.8% vs. 4.4%, P = 0.007) during follow‐up and showed a significantly higher all‐cause mortality rate (61.6% vs. 23.1%, P < 0.001) (Figure 3 ).
Figure 3.
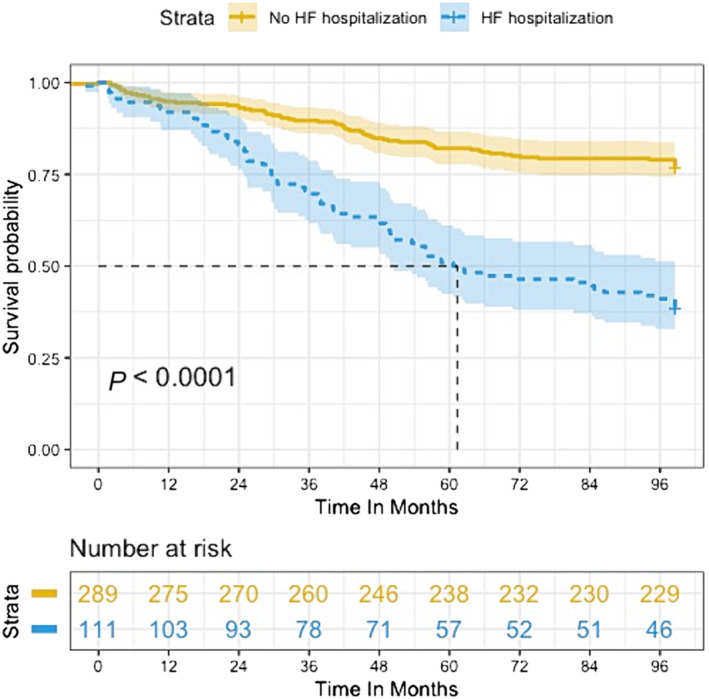
Kaplan–Meier survival curve for SU‐SAVR patients according to HF readmission.
Independent associates of heart failure in sutureless surgical aortic valve replacement patients
In the multivariate analysis, the independent associates of HF hospitalization in the SU‐SAVR cohort were diabetes (OR 1.76, 95% CI 1.07 to 2.87, P = 0.024), chronic AF (OR 3.32, 95% CI 1.73 to 6.41, P < 0.001), COPD (OR 1.96, 95% CI: 1.01 to 3.76, P = 0.045), a lower basal eGFR (OR 0.99, 95% CI 0.98 to 0.99, P = 0.049), and a lower basal LVEF (OR 0.98, 95% CI 0.96 to 0.99, P = 0.025) (Table 3 ).
Table 3.
Multivariate analysis for HF readmission in SU‐SAVR patients
| OR | 95% CI | P value | |
|---|---|---|---|
| Hypertension | 2.24 | 1.02–5.54 | 0.05 |
| Diabetes | 1.76 | 1.07–2.87 | 0.024 |
| Chronic AF | 3.32 | 1.73–6.41 | <0.001 |
| COPD | 1.96 | 1.01–3.76 | 0.045 |
| Basal eGFR (mL/min/1.73 m2) | 0.99 | 0.98–1 | 0.049 |
| In‐hospital AKI | 1.47 | 0.87–2.47 | 0.148 |
| In‐hospital HF | 1.08 | 0.52–2.18 | 0.832 |
| CBP time, min | 1.01 | 0.99–1.02 | 0.219 |
| Troponin peak, pg/mL | 1 | 0.99–1 | 0.542 |
| Basal LVEF, % | 0.98 | 0.96–0.99 | 0.025 |
| Permanent pacemaker | 2.16 | 0.89–5.11 | 0.081 |
AF, atrial fibrillation; AKI, acute kidney injury; CBP, cardiopulmonary bypass; CI, confidence interval; COPD, chronic obstructive pulmonar disease; eGFR, estimated glomerular filtrate rate; HF, heart failure; LVEF, left ventricular ejection fraction.
Discussion
Our main findings can be summarized as follows: (1) SU‐SAVR patients presented a higher rise in perioperative cardiac and inflammatory biomarkers compared to TAVI patients. (2) Hospitalizations due to HF occurred more frequently (and early after discharge) in the SU‐SAVR group. (3) Patients admitted for HF within the SU‐SAVR cohort presented preserved LVEF, larger left ventricles, longer CPB times, higher rise in perioperative cardiac biomarkers, higher rates of AF, and more conduction disturbances compared with the non‐HF group. (4) Patients admitted for HF within the SU‐SAVR cohort presented a higher mid‐term overall mortality (Graphical abstract).
Risk of heart failure hospitalization in sutureless surgical aortic valve replacement compared with transcatheter aortic valve implantation patients
The SU‐SAVR and TAVI cohorts were mostly comparable in terms of baseline characteristics, aside from non‐clinically significant differences in left ventricular diameters and renal function and the rate of HF admission was higher in the SU‐SAVR group, even when these patients presented, postprocedurally, higher LVEF, and, consistent with previous data, 19 , 20 , 21 lower rates of aortic regurgitation.
Perioperative cardiac and inflammatory biomarkers were non‐surprisingly higher in the SU‐SAVR cohort. It has been reported that cardiac procedures using extracorporeal circulation are a source of cardiovascular stress and damage due to a systemic inflammatory response and surgical trauma, 22 , 23 and head‐to‐head comparisons have proven those to be greater after SAVR compared with TAVI. 22 , 23
Importantly, these biological responses have been linked to a higher risk of HF 24 alongside an increase in short/mid‐term mortality risk. 22 Consequently, while surgical times are considerably shortened with SU‐SAVR, we hypothesize that the systemic inflammation occurring after surgery could be a contributing factor to the increased incidence of heart failure hospitalization, along with in‐hospital and mid‐term mortality. This observation gains support from the fact that HF hospitalization‐free curves start to separate soon after discharge despite SU‐SAVR patients received more frequently diuretics and ACE inhibitors at discharge and exhibited higher left ventricular ejection fraction (LVEF) values during the first 2 years of follow‐up. Moreover, even when, consistent with previous studies, 25 transprosthetic pressure gradients were slightly higher postprocedurally in SU‐SAVR patients, comparable postinterventional indexed AVA and transprosthetic gradients at mid‐term follow‐up suggest similar valve haemodynamics in both groups.
Risk factors and clinical impact of heart failure hospitalization in sutureless surgical aortic valve replacement patients
Similar to diabetes mellitus, 26 , 27 COPD, 26 , 27 , 28 chronic renal insufficiency 26 , 28 (particularly in end‐stage 26 , 27 but also in mild cases 26 ), atrial fibrillation, 26 , 27 and a lower baseline LVEF 28 being identified as factors elevating the likelihood of HF hospitalization following conventional SAVR, they emerged as independent predictors of HF in SU‐SAVR patients. This association may be linked to a reduced tolerance to systemic volume shifts and/or an existing proinflammatory state.
The numerical increase in in‐hospital HF rates among patients admitted for HF, although without a statistical difference, might be partially attributed to the multifactorial nature of postoperative congestive symptoms. Notably, the considerable weight of addressing fluid overload to manage vasoplegia could contribute to this phenomenon. Nevertheless, admitted patients experienced longer hospital stays, 26 , 27 , 28 potentially reflecting the intricacies of the postoperative course in individuals with the mentioned co‐morbidities.
When evaluating surgical variables, we found that peak troponin levels and mean CPB and ACC times, which were comparable with usually reported times for this kind of valve, 29 showed a directly proportional relationship with the risk of HF hospitalization. Several studies have explored the role of these variables in postoperative outcomes, leading to the same inferences: the longer the ACC 12 and CPB times 12 , 22 , 24 and the higher the troponin peak levels, 30 the higher the risk of readmission, in many cases due to HF, and mid‐term mortality. This phenomenon could be a reflection of the intricacy of the intervention or, in line with the previous argument, indicate perioperative cardiovascular damage. In this context, extracorporeal circulation times and cardiac biomarkers might serve as surrogate measures for systemic inflammation and myocardial damage.
Despite having significantly lower LVEF values (potentially associated with higher rates of intraventricular conduction delay and paced rhythm), SU‐SAVR patients maintain values within the normal range and exhibit adequate, downward, and comparable transvalvular pressure gradients after discharge and throughout the 3‐year follow‐up period. In other words, these individuals present a profile consistent with heart failure with preserved ejection fraction (HFpEF), where haemodynamic valve performance does not seem to be the differentiating factor between admitted patients and their counterparts. Consistently with existing literature, 31 , 32 HF hospitalization was associated with a significantly higher risk of short‐term and mid‐term all‐cause mortality, which was maintained during the follow‐up period.
Potential mechanisms of heart failure with preserved ejection fraction in sutureless surgical aortic valve replacement patients
Altogether, we are identifying patients who, despite presenting normal systolic function, having undergone successful AVR and maintaining a stable LVEF and favourable prosthetic valve haemodynamics over time, present a significant incidence of HF and all‐cause mortality, both in absolute and relative (compared with TAVI) terms. While several risk factors for HF differentiated admitted from non‐admitted patients within the SU‐SAVR group, not many differed in a clinically significant manner between the TAVI and the SU‐SAVR cohorts.
At this stage, it is pertinent to introduce the concept of AS cardiomyopathy—a HFpEF phenotype recently elucidated elsewhere. 33 This condition is characterized by adaptive but adverse left ventricular (LV) remodelling, 31 , 32 , 33 resulting from years of excessive afterload. AS cardiomyopathy has been linked to an increased risk of HF hospitalization and mortality following successful AVR. 33 This heightened risk may be attributed to a multihit pathogenesis involving concentric LV hypertrophy, myocyte, and extracellular matrix remodelling, and capillary rarefaction. Various insults, including perioperative damage secondary to cardioplegia, cardiopulmonary bypass, ischemia–reperfusion, inflammation, and direct myocardial injury, 33 have been implicated in precipitating HF decompensation.
Further exploration of this aspect is warranted, considering that while SU‐SAVR reduces surgical times and minimizes cardiac trauma compared to conventional SAVR, these factors cannot be entirely eliminated due to their intrinsic connection to the surgical procedure. Therefore, we must regard global surgical damage as a non‐modifiable risk factor for HF hospitalization, consistently differentiating SU‐SAVR from TAVI patients. This distinction holds particular significance when dealing with relatively young, low‐risk patients, especially considering the increased odds of surplus mortality from systemic complications in surgical patients.
Results of dedicated trials are awaited, as in‐depth knowledge of this entity is required to properly understand the factors driving HF after AVR, to help improve therapy selection, to define the best moment for intervention and to identify therapeutic targets aimed not only at the diseased valve but also to the impaired myocardium. It remains to be elucidated if previous effective treatments for reducing HF rehospitalizations in HFpEF patients, such as sodium–glucose cotransporter 2 inhibitors (SGLT2i), 34 maintain their impact on this new entity.
Study limitations
Given the observational and retrospective nature of the present study, our findings are subject to selection bias, for which they may not be applicable to the general population. Also, the lack of some echocardiographic data at follow‐up such as LV mass and hypertrophy, left atrial volume, diastolic function and indexed AVA is another limitation of the present study, as these variables could have helped to better define the profile of patient more prone to HF hospitalization and would allow to properly rule out other predisposing factors such as patient‐prosthesis mismatch or early prosthesis degeneration. Finally, the final sample size was relatively small, for which a type II error cannot be excluded regarding the comparison between groups for some clinical events.
Conclusions
In summary, among low‐risk patients with severe symptomatic AS, SU‐SAVR exhibited a more pronounced myocardial damage and postoperative inflammatory response that might be related to a heightened risk of HF hospitalization and mid‐term all‐cause death when compared to TAVI. These data would support TAVI over SU‐SAVR in most of such patients. Readmitted SU‐SAVR patients displayed a HFpEF profile possibly resultant of a previous adverse but adaptive LV remodelling (AS cardiomyopathy), where perioperative damage and co‐morbidities conditioning a proinflammatory state or a poor volume handling capacity (diabetes, AF, COPD, renal dysfunction, and lower LVEF) would act as precipitants of HF decompensation, in turn correlated with a higher mortality risk.
Funding
This paper was not funded.
Conflict of interest
Dr. Bayés has lectured and/or participated in advisory boards for Abbott, AstraZeneca, Boehringer Ingelheim, Novartis, Bayer, Roche Diagnostics, Vifor. Dr. Rodés‐Cabau is consultant for and has received institutional research grants from Edwards Lifesciences and Medtronic. Dr. Victoria Delgado received speaker fees from Edwards Lifesciences, GE Healthcare, Novartis, Philips, and MSD; consulting fees from Novo Nordisk, Edwards Lifesciences, and Abbott Vascular. The rest of authors do not report any conflict of interest with respect to the content of this article.
Supporting information
Table S1. Bioprosthesis type and size by study group.
Table S2. Demographic, clinical and perioperative characteristics of the study population after 1:1 propensity score matching.
Figure S1. Follow‐up LVEF (A) and mean aortic gradient (B) evolution by study group. LVEF, left ventricle ejection fraction; F3M, 3 months after discharge; F12M, 12 months after discharge; F24M, 24 months after discharge, F3Y, 3 years after discharge.
Figure S2. Follow‐up LVEF (A) and mean aortic gradient (B) evolution in SU‐SAVR patients according to HF readmission. LVEF, left ventricle ejection fraction; F3M, 3 months after discharge; F12M, 12 months after discharge; F24M, 24 months after discharge, F3Y, 3 years after discharge.
Lopez‐Martinez, H. , Vilalta, V. , Farjat‐Pasos, J. , Ferrer‐Sistach, E. , Mohammadi, S. , Escabia, C. , Kalavrouziotis, D. , Resta, H. , Borrellas, A. , Dumont, E. , Carrillo, X. , Paradis, J.‐M. , Fernández‐Nofrerías, E. , Delgado, V. , Rodés‐Cabau, J. , and Bayes‐Genis, A. (2024) Heart failure hospitalization following surgical or transcatheter aortic valve implantation in low‐risk aortic stenosis. ESC Heart Failure, 11: 2531–2541. 10.1002/ehf2.14887.
[Correction added on 15 July 2024, after first online publication: Helena Lopez‐Martinez's family name has been corrected in this version.]
References
- 1. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med 2019;380:1706‐1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 2. Thyregod HGH, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis. J Am Coll Cardiol 2015;65:2184‐2194. doi: 10.1016/j.jacc.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol 2021;77:1149‐1161. doi: 10.1016/j.jacc.2020.12.052 [DOI] [PubMed] [Google Scholar]
- 4. Baron SJ, Magnuson EA, Lu M, Wang K, Chinnakondepalli K, Mack M, et al. Health status after transcatheter versus surgical aortic valve replacement in low‐risk patients with aortic stenosis. J Am Coll Cardiol 2019;74:2833‐2842. doi: 10.1016/j.jacc.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 5. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two‐year outcomes after transcatheter or surgical aortic‐valve replacement. N Engl J Med 2012;366:1686‐1695. doi: 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 6. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med 2014;370:1790‐1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 7. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med 2016;374:1609‐1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 8. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet 2016;387:2218‐2225. doi: 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 9. Siontis GCM, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta‐analysis of randomized trials. Eur Heart J 2016;37:3503‐3512. [DOI] [PubMed] [Google Scholar]
- 10. Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S, et al. Sutureless perceval aortic valve versus conventional stented bioprostheses: meta‐analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Assoc 2018;7:e006091. doi: 10.1161/JAHA.117.006091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischlein T, Caporali E, Asch FM, Vogt F, Pollari F, Folliguet T, et al. Hemodynamic performance of sutureless vs. conventional bioprostheses for aortic valve replacement: the 1‐year core‐lab results of the randomized PERSIST‐AVR trial. Front Cardiovasc Med 2022;9:844876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jolliffe J, Moten S, Tripathy A, Skillington P, Tatoulis J, Muneretto C, et al. Perceval valve intermediate outcomes: a systematic review and meta‐analysis at 5‐year follow‐up. J Cardiothorac Surg 2023;18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santarpino G, Pfeiffer S, Jessl J, Dell'Aquila AM, Pollari F, Pauschinger M, et al. Sutureless replacement versus transcatheter valve implantation in aortic valve stenosis: a propensity‐matched analysis of 2 strategies in high‐risk patients. J Thorac Cardiovasc Surg 2014;147:561‐567. doi: 10.1016/j.jtcvs.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 14. Santarpino G, Pfeiffer S, Jessl J, Dell'Aquila A, Vogt F, von Wardenburg C, et al. Clinical outcome and cost analysis of sutureless versus transcatheter aortic valve implantation with propensity score matching analysis. Am J Cardiol 2015;116:1737‐1743. doi: 10.1016/j.amjcard.2015.08.043 [DOI] [PubMed] [Google Scholar]
- 15. Kamperidis V, van Rosendael PJ, de Weger A, Katsanos S, Regeer M, van der Kley F, et al. Surgical sutureless and transcatheter aortic valves: hemodynamic performance and clinical outcomes in propensity score‐matched high‐risk populations with severe aortic stenosis. JACC Cardiovasc Interv 2015;8:670‐677. doi: 10.1016/j.jcin.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 16. Repossini A, Di Bacco L, Passaretti B, Grubitzsch H, Schäfer C, Claus B, et al. Early hemodynamics and clinical outcomes of isolated aortic valve replacement with stentless or transcatheter valve in intermediate‐risk patients. J Thorac Cardiovasc Surg 2017;153:549‐558.e3. doi: 10.1016/j.jtcvs.2016.10.086 [DOI] [PubMed] [Google Scholar]
- 17. Vilalta V, Alperi A, Cediel G, Mohammadi S, Fernández‐Nofrerias E, Kalvrouziotis D, et al. Midterm outcomes following sutureless and transcatheter aortic valve replacement in low‐risk patients with aortic stenosis. Circ Cardiovasc Interv 2021;14:e011120. doi: 10.1161/CIRCINTERVENTIONS.121.011120 [DOI] [PubMed] [Google Scholar]
- 18. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol 2021;77:2717‐2746. doi: 10.1016/j.jacc.2021.02.038 [DOI] [PubMed] [Google Scholar]
- 19. Muneretto C, Alfieri O, Cesana BM, Bisleri G, De Bonis M, Di Bartolomeo R, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in “real‐world” patients with aortic stenosis and intermediate‐ to high risk profile. J Thorac Cardiovasc Surg 2015;150:1570‐1577. doi: 10.1016/j.jtcvs.2015.08.052 [DOI] [PubMed] [Google Scholar]
- 20. Shinn SH, Altarabsheh SE, Deo SV, Sabik JH, Markowitz AH, Park SJ. A systemic review and meta‐analysis of sutureless aortic valve replacement versus transcatheter aortic valve implantation. Ann Thorac Surg 2018;106:924‐929. doi: 10.1016/j.athoracsur.2018.03.059 [DOI] [PubMed] [Google Scholar]
- 21. Muneretto C, Solinas M, Folliguet T, Di Bartolomeo R, Repossini A, Laborde F, et al. Sutureless versus transcatheter aortic valves in elderly patients with aortic stenosis at intermediate risk: a multi‐institutional study. J Thorac Cardiovasc Surg 2022;163:925‐935. doi: 10.1016/j.jtcvs.2020.04.179 [DOI] [PubMed] [Google Scholar]
- 22. Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart 2015;101:537‐545. doi: 10.1136/heartjnl-2014-307057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimura N, Nomura Y, Aomatsu A, Matsuda A, Imamura Y, Taniguchi Y, et al. Effect of transcatheter aortic valve implantation on the immune response associated with surgical aortic valve replacement. Am J Cardiol 2020;128:35‐44. doi: 10.1016/j.amjcard.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 24. Guerrero‐Orriach JL, Carmona‐Luque MD, Gonzalez‐Alvarez L. Heart failure after cardiac surgery: the role of halogenated agents, myocardial conditioning and oxidative stress. Int J Mol Sci 2022;23:1360. doi: 10.3390/ijms23031360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdel‐Wahab M, Fujita B, Frerker C, Bauer T, Beckmann A, Bekeredjian R, et al. Transcatheter versus rapid‐deployment aortic valve replacement: a propensity‐matched analysis from the german aortic valve registry. JACC Cardiovasc Interv 2020;13:2642‐2654. doi: 10.1016/j.jcin.2020.09.018 [DOI] [PubMed] [Google Scholar]
- 26. Khoury H, Ragalie W, Sanaiha Y, Boutros H, Rudasill S, Shemin RJ, et al. Readmission after surgical aortic valve replacement in the United States. Ann Thorac Surg 2020;110:849‐855. doi: 10.1016/j.athoracsur.2019.11.058 [DOI] [PubMed] [Google Scholar]
- 27. Trooboff SW, Magnus PC, Ross CS, Chaisson K, Kramer RS, Helm RE, et al. A multi‐center analysis of readmission after cardiac surgery: experience of the northern New England cardiovascular disease study group. J Card Surg 2019;34:655‐662. doi: 10.1111/jocs.14086 [DOI] [PubMed] [Google Scholar]
- 28. Maniar HS, Bell JM, Moon MR, Meyers BF, Marsala J, Lawton JS, et al. Prospective evaluation of patients readmitted after cardiac surgery: analysis of outcomes and identification of risk factors. J Thorac Cardiovasc Surg 2014;147:1013‐1020. doi: 10.1016/j.jtcvs.2013.10.066 [DOI] [PubMed] [Google Scholar]
- 29. Mujtaba SS, Ledingham S, Shah AR, Clark S, Pillay T, Schueler S. Early clinical results of perceval sutureless aortic valve in 139 patients: Freeman experience. Braz J Cardiovasc Surg 2018;33:8‐14. doi: 10.21470/1678-9741-2017-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auensen A, Hussain AI, Falk RS, Walle‐Hansen MM, Bye J, Pettersen KI, et al. Associations of brain‐natriuretic peptide, high‐sensitive troponin T, and high‐sensitive C‐reactive protein with outcomes in severe aortic stenosis. PLoS ONE 2017;12:e0179304. doi: 10.1371/journal.pone.0179304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Durand E, Doutriaux M, Bettinger N, Tron C, Fauvel C, Bauer F, et al. Incidence, prognostic impact, and predictive factors of readmission for heart failure after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2017;10:2426‐2436. doi: 10.1016/j.jcin.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 32. Auffret V, Bakhti A, Leurent G, Bedossa M, Tomasi J, Belhaj Soulami R, et al. Determinants and impact of heart failure readmission following transcatheter aortic valve replacement. Circ Cardiovasc Interv 2020;13:e008959. doi: 10.1161/CIRCINTERVENTIONS.120.008959 [DOI] [PubMed] [Google Scholar]
- 33. Aziminia N, Nitsche C, Mravljak R, Bennett J, Thornton GD, Treibel TA. Heart failure and excess mortality after aortic valve replacement in aortic stenosis. Expert Rev Cardiovasc Ther 2023;21:193‐210. doi: 10.1080/14779072.2023.2186853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451‐1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Bioprosthesis type and size by study group.
Table S2. Demographic, clinical and perioperative characteristics of the study population after 1:1 propensity score matching.
Figure S1. Follow‐up LVEF (A) and mean aortic gradient (B) evolution by study group. LVEF, left ventricle ejection fraction; F3M, 3 months after discharge; F12M, 12 months after discharge; F24M, 24 months after discharge, F3Y, 3 years after discharge.
Figure S2. Follow‐up LVEF (A) and mean aortic gradient (B) evolution in SU‐SAVR patients according to HF readmission. LVEF, left ventricle ejection fraction; F3M, 3 months after discharge; F12M, 12 months after discharge; F24M, 24 months after discharge, F3Y, 3 years after discharge.


