Abstract
The study aims to evaluate whether rhythm control by catheter ablation is superior to medical therapy for the patients with atrial fibrillation (AF) and heart failure (HF). The literatures were searched by using PubMed, Cochrane Library, Embase, and Web of Science databases up to 12 October 2023. The randomized controlled trials (RCTs) comparing rhythm control using catheter ablation vs. medical therapy in AF patients with HF were pooled. The primary outcomes included all‐cause mortality, HF re‐hospitalization, and stroke, and the secondary outcomes included left ventricular ejection fraction (LVEF), atrial tachyarrythmia recurrence, quality of life (Minnesota Living with Heart Failure Questionnaire score, MLHFQ score), 6 min walking distance (6MWD), the level of N‐terminal B‐type natriuretic peptide precursor (NT‐proBNP), and adverse events. Nine RCTs involving in 2293 patients met the inclusion criteria. Compared with medical therapy, catheter ablation reduced all‐cause mortality [10.07% (121/1201) vs. 15.26% (175/1147), risk ratio (RR):0.60, 95% confidence interval (CI): 0.48–0.74, P < 0.00001, I 2 = 0%] and the rate of HF re‐hospitalization (RR: 0.65, P = 0.02, 95% CI: 0.45 to 0.94, I 2 = 74%), but had no obvious difference in incidence of stroke (RR: 0.67, P = 0.27, 95% CI: 0.32 to 1.38, I 2 = 0%). Catheter ablation enhanced LVEF [mean difference (MD), 6.26%, P < 0.00001, I 2 = 89%], reduced AT recurrence (RR: 0.37, P < 0.00001, 95% CI: 0.26 to 0.52, I 2 = 89%), improved the quality of life (MLHFQ score) (MD: −6.83, P = 0.003, I 2 = 67%), elevated 6MWD (MD: 15.92, P = 0.006, I 2 = 76%), and diminished the level NT‐proBNP (MD: −44.19, P < 0.00001, I 2 = 75%), but had no significant difference in adverse events [25.81% (310/1201) vs. 30.25% (347/1147), RR: 0.81, 95% CI: 0.65–1.01, P = 0.06, I 2 = 55%]. Catheter ablation as rhythm control strategy substantially enhances the survival rate, reduces HF re‐hospitalization, increases the rate of sinus rhythm maintenance, improves the left ventricular function and the quality of life for AF patients with HF, and has similar safety, compared with medical therapy. The rhythm control by catheter ablation may be a better strategy for the AF patients with HF.
Keywords: Atrial fibrillation, Heart failure, Rhythm control, Catheter ablation, Medical therapy, Meta‐analysis
Introduction
Atrial fibrillation (AF) and heart failure (HF) are common cardiovascular diseases. AF is the most common arrhythmia among patients with HF, and HF is the most common cause of death for the patients presenting with clinical AF. 1 They often coexist and promote each other and have great adverse effects on cardiovascular health. 2 HF and AF together increase the risks of all‐cause mortality, stroke, and re‐hospitalization.
Whether the rhythm control strategy is superior to ventricular rate control has always been controversial for AF patients with HF. Early studies have shown that rhythm control strategies based on antiarrhythmic drug (AAD) therapy are not superior to ventricular rate control, as the low rate of sinus rhythm maintenance and the side effects of the AADs for rhythm control. 3 The catheter ablation for AF has robustly shown a significant improvement in sinus rhythm maintenance and reduces the burden of AF. 4 In AF patients with HF, catheter ablation improves cardiovascular outcomes (death, disabling stroke, severe bleeding, or cardiac arrest) and prognosis. 5 , 6
According to 2023 ACC/AHA/ACCP/HRS Guidelines for the diagnosis and management of AF, catheter ablation has also been upgraded to class I recommendation to improve cardiovascular outcomes, ventricular function, symptoms, and quality of life for the AF patients with HF. 7 However, the RAFT‐AF trial, 8 currently the largest study comparing clinical outcomes of the AF patients with HF between rhythm control via catheter ablation and rate control by medical therapy, recently disclosed that rhythm control by catheter ablation does not significantly improve clinical outcomes in AF patients with HF, compared with medical therapy. This finding adds a critical dimension to our understanding of treatment efficacy in this patient cohort.
Therefore, the present systematic review and meta‐analysis aims to compare long outcomes of catheter ablation with medical therapy in AF patients with HF basing on the randomized controlled trials (RCTs), focusing on the impacts on all‐cause mortality, the rate of HF re‐hospitalization, the rate of stroke, left ventricular function, the recurrence of atrial tachyarrhythmia (AT), and the quality of life.
Methods
This study was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement and Cochrane Handbook for Systematic Reviews of Interventions. 9
Search strategy
Two investigators independently searched the literatures in PubMed, Cochrane Library, Embase, and Web of Science databases up to 12 October 2023. We searched the literatures of RCTs evaluating the efficacy of medical therapy and catheter ablation‐based rhythm control on reducing cardiovascular outcomes in patients with AF and HF. The keywords ‘atrial fibrillation’ and ‘heart failure’ and ‘rate control’, and ‘rhythm control’ were used to search the literatures, and a list of relevant literatures were further evaluated for inclusion in the study (The details of search strategy were showed in Supp orting information, Data S1 ).
Selection criteria and quality assessment
The inclusion criteria of this study, structured according to the Population Intervention Comparison Outcome Study Design framework, encompass the following: (i) population, referring to patients diagnosed with AF and HF; (ii) intervention, involving rhythm control via catheter ablation in RCTs; (iii) control, including standard medical therapy; and (iv) outcomes, focusing on the evaluation of major cardiovascular endpoints that must include all‐cause mortality.
Two investigators used Cochrane collaboration's tool for assessing risk of bias to assess the quality of included studies independently. The items included in this tool were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.
Data extraction and outcomes measurement
Two reviewers independently extracted data from included studies. Data extracted from studies included study characteristics, patient characteristics, details regarding catheter ablation and control groups, and outcomes. The primary outcomes were all‐cause mortality, re‐hospitalization, and stroke. The secondary outcomes included left ventricular ejection fraction (LVEF), the recurrence of AT (defined as AF, atrial flutter, or atrial tachycardia lasting ≥30 s), Minnesota Living with Heart Failure Questionnaire (MLHFQ) score, 6 min walking distance (6MWD), the level of N‐terminal B‐type natriuretic peptide precursor (NT‐proBNP), and adverse events.
Statistical analysis
Data from the included RCTs will be pooled using Review Manager (RevMan) software. Continuous outcomes will be analysed using mean difference (MD). Binary outcomes will be presented as risk ratios (RR) with 95% confidence intervals (CIs). Random‐effect models were used for all outcomes due to the clinical heterogeneity of the included studies. The Cochran's Q test and I 2 test were performed to assess the heterogeneity of the summary effects. If the P value of Cochran's Q test was <0.10 and I 2 was >50%, there was a heterogeneity in the study. All P values were two‐tailed, and a P value <0.05 was considered significant. All the statistical analyses were performed using the RevMan software package (Review Manager, Version 5.4).
Results
Overall summary of included studies
The process of study selection was shown in Figure 1 . The search produced 988 articles. According to the inclusion and exclusion criteria, 9 RCTs were included in this meta‐analysis finally. 5 , 6 , 8 , 10 , 11 , 12 , 13 , 14 , 15 A total of 2293 patients (mean age 64.8 ± 10.1 years) with AF and HF were included in the present meta‐analysis. Four trials enrolled paroxysmal and persistent AF patients, 5 , 6 , 8 , 14 but only persistent AF patients were included in another five trials. 10 , 11 , 12 , 13 , 15 All the patients with catheter ablation underwent pulmonary vein isolation (PVI). Most of the patients with catheter ablation underwent additional linear and complex fractionated electrogram ablation. In patients with medical therapy, rate control was a strategy in four RCTs, 8 , 12 , 13 , 15 rhythm control was a strategy in the AATAC trial, 11 and rhythm control or rate control was a strategy in the CASTLE‐AF trial, 6 AMICA trial, 10 CABANA HF subgroup trials, 5 and CASTLE‐HTx trial. 14 The characteristics of patients in the included studies were summarized in Table 1 .
Figure 1.
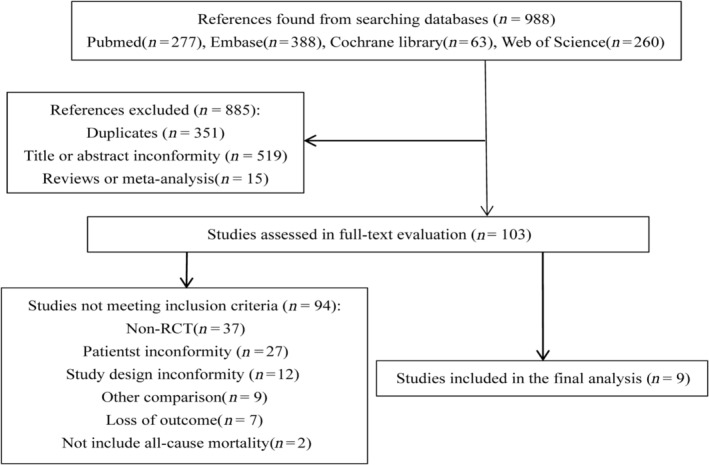
Search criteria and flow chart of the studies screened and included in the systematic review.
Table 1.
Clinical features of patients included in the selected studies
| Trials (RCT) | ARC‐HF 2013 13 | CAMTAF 2014 12 | AATAC 2016 11 | CASTLE‐AF 2018 6 | AMICA 2019 10 | CABANA HF subgroup 2019 5 | RAFT‐AF 2022 8 | Zakeri 2022 15 | CASTLE‐HTx 2023 14 |
|---|---|---|---|---|---|---|---|---|---|
| Sample size | 52 | 50 | 203 | 363 | 140 | 778 | 411 | 102 | 194 |
| Age, years, mean ± SD | 63 ± 9 | 63 ± 10 | 61 ± 11 | 64 ± 12 | 65 ± 8 | 68 ± 8 | 67 ± 8 | 61 ± 11 | 63 ± 11 |
| Male, n (%) | 45 (87) | 48 (96) | 151 (74) | 311 (86) | 126 (90) | 433 (56) | 353 (86) | 93 (91) | 157 (87) |
| AF type | Persistent AF | Persistent AF | Persistent AF | Paroxysmal, 118 (33); persistent AF, 245 (67) | Persistent AF | Paroxysmal, 246 (32); persistent AF, 532 (68) | Paroxysmal, 30 (7); persistent AF, 381 (93) | Persistent AF | Paroxysmal, 59 (30); persistent AF, 135 (70) |
| AF duration, months | 23 ± 25 | 24 ± 19 | 102 ± 44 | — | — | 13 ± 11 | — | — | 42 ± 54 |
| Cardiac function | NYHA II–III | NYHA II–III | — | NYHA I–IV | NYHA II–III | NYHA II–IV | NYHA II–III | NYHA II–III | NYHA II–IV |
| Hypertension, n (%) | — | 16 (32) | 94 (46) | 265 (73) | 111 (79) | 665 (85.5) | 272 (66) | 33 (32) | — |
| Diabetes, n (%) | — | — | 46 (23) | 110 (30) | 44 (33) | 195 (25.1) | 125 (30) | — | 56 (29) |
| CHD, n (%) | 24 (46) | 13 (26) | 129 (64) | 168 (46) | 70 (50) | 170 (21.9) | 129 (31) | 38 (37) | — |
| Cerebrovascular disease, n (%) | — | — | — | — | — | 79 (10.2) | 39 (9) | — | — |
| COPD, n (%) | — | — | — | — | — | — | 33 (8) | — | — |
| LVEF, %, mean ± SD | 23.5 ± 7.6 | 32.7 ± 10.0 | 29.5 ± 6.7 | 32.0 ± 8.5 | 26.3 ± 9.2 | 55 ± 8.1 | 40.6 ± 14.8 | 30.0 ± 10.1 | 27.0 ± 6.3 |
| LA diameter, mm, mean ± SD | 48 ± 7 | 51 ± 9 | 47 ± 5 | 49 ± 5 | 51 ± 6 | — | 46 ± 6 | 50 ± 7 | 49 ± 7 |
| Intervention group | Ablation | Ablation | Ablation | Ablation | Ablation | Ablation | Ablation | Ablation | Ablation |
| Control group | Medical rate control | Medical rate control | AMIO | Medical rate or rhythm control | Medical rate or rhythm control | Medical rhythm control | Medical rate control, AVN ablation with BIV pacing | Medical rate control | Medical rate or rhythm control |
| Follow‐up, years | 1 | 0.5 | 2 | 3.2 | 1 | 4.0 | 3.1 | 7.8 | 1.5 |
| Primary outcomes | Peak VO2 | LVEF | AF recurrence | All‐cause mortality, HF hospitalization | LVEF | Composite of death, disabling stroke, serious bleeding, or cardiac arrest | All‐cause mortality and HF events | All‐cause mortality | Composite of death from any cause, implantation of a left ventricular assist device, or urgent heart transplantation |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; AMIO, amiodarone; AVN, atrioventricular node; BIV, biventricular; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; LA, left atrium; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association Classification; VO2, oxygen consumption.
All‐cause mortality, HF re‐hospitalization, and stroke
Compared with the medical therapy, catheter ablation was associated with a significantly decreased all‐cause mortality [10.07% (121/1201) vs. 15.26% (175/1147), RR: 0.60, 95%CI: 0.48–0.74, P < 0.0001, I 2 = 0%] (Fig. 2 A ), and HF re‐hospitalization [17.65% (168/952) vs. 26.85% (243/905), RR: 0.65, 95% CI: 0.45–0.94, P = 0.02, I 2 = 74%] (Fig. 2 B ). However, the rate of stroke was no difference between two groups [1.37% (12/876) vs. 2.05% (17/828), RR: 0.67, 95% CI: 0.32–1.38, P = 0.27, I 2 = 0%] (Fig. 2 C ).
Figure 2.
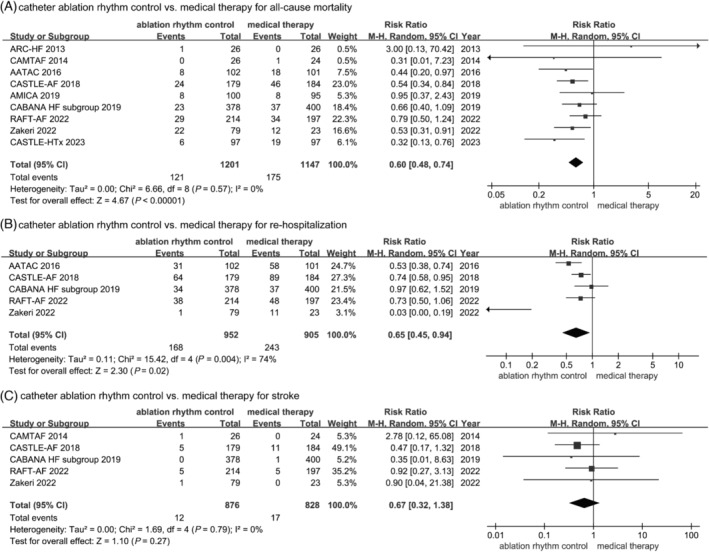
Forest plots of the included studies concerning on the primary outcomes. (A) All‐cause mortality; (B) re‐hospitalization; (C) stroke.
Because composite endpoints were available in the CABANA HF subgroup 5 and CASTLE‐HTx, 14 we performed additional sensitivity analysis to test the robustness of the outcome on all‐cause mortality by using the ‘One Study removing technique’, by which a study was removed to observe the change of all‐cause mortality. After a total of nine removals, the sensitivity analysis consistently demonstrated that catheter ablation significantly reduced all‐cause mortality compared with medical therapy (RR: 0.60, 95% CI: 0.48–0.74, P < 0.01, I 2 = 0%) (Fig. 3 ). These findings ensured the robustness and reliability of the results.
Figure 3.
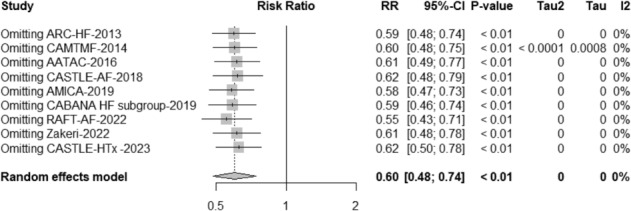
Sensitivity analysis for all‐cause mortality by using the ‘One Study removing technique’.
Left ventricular ejection fraction, recurrence of AT, quality of life, 6 min walk distance, and the level of NT‐proBNP
As shown in Figure 4 , compared with medical therapy, catheter ablation had a greater improvement in LVEF (MD: 6.26%, 95% CI: 4.18% to 8.34%, P < 0.00001, I 2 = 89%), significantly less recurrence of AT [30.27% (329/1087) vs. 69.85% (725/1038), RR: 0.37, 95% CI: 0.26–0.52, P < 0.00001, I 2 = 89%], remarkable improvement in quality of life based on MLHFQ score (MD: −6.83, 95% CI: −11.27 to −2.40, P = 0.003, I 2 = 67%), significantly reduced the level of NT‐proBNP (MD: −44.19, 95% CI: −58.07 to −30.32, P < 0.00001, I 2 = 75%), and apparently enhanced 6MWD (MD: 15.92, 95% CI: 4.59–27.24, P = 0.006, I 2 = 76%).
Figure 4.
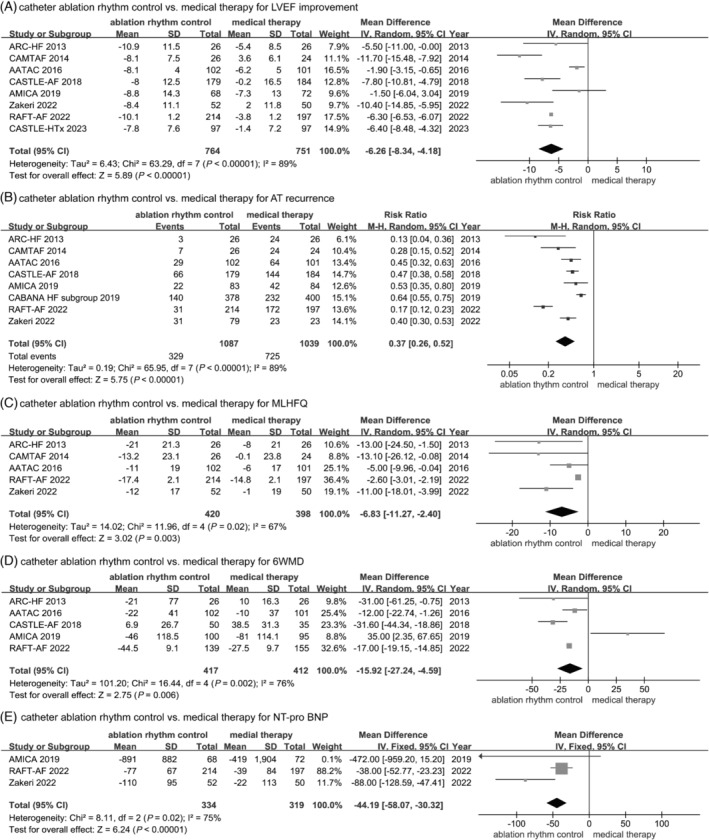
Forest plots of the included studies concerning on the secondary outcomes. (A) Left ventricular ejection fraction (LVEF). (B) Atrial tachyarrhythmia (AT) recurrence. (C) Minnesota Living with Heart Failure Questionnaire score (MLHFQ) score. (D) Six minute walking distance (6WMD). (E) N‐terminal B‐type natriuretic peptide precursor (NT‐proBNP).
Adverse events
We summarized common adverse events, including death, stroke, cardiac tamponade, groin haematoma, worsening HF, pulmonary vein stenosis, and major bleeding (Table S1 ). Adverse events were similar between the population with catheter ablation and population with medical therapy [25.81% (310/1201) vs. 30.25% (347/1147), RR: 0.81, 95% CI: 0.65 to 1.01, P = 0.06, I 2 = 55%] (Figure 5 ). All procedure‐related complications were treated appropriately in these trials.
Figure 5.
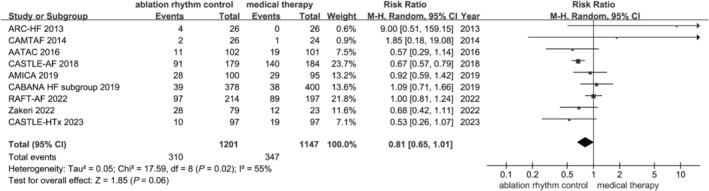
Forest plot of the included studies concerning on the composite adverse events. Composite adverse events include death, stroke, transient ischaemic attack, cardiac tamponade, groin complications, worsening heart failure, pulmonary vein stenosis, atrial oesophageal fistula, cardiogenic shock, oesophageal ulcer, pericardial effusion, myocardial infarction, pulmonary oedema, vascular access complications, haematoma, and major bleeding.
Discussion
The present meta‐analysis uncovers that rhythm control based on catheter ablation significantly decreases the all‐cause mortality and re‐hospitalization of HF in AF patients with HF, compared with rate control or rhythm control based on medical therapy. In addition, an obvious improvement of LVEF and quality of life, remarkably decreased recurrence of AT, significantly increased 6MWD, and markedly down‐regulated level of NT‐proBNP were observed in the patients with catheter ablation, compared with medical therapy. However, there is no significant difference in the rates of stroke and adverse events between catheter ablation and medical therapy. To the best of our knowledge, the present study first reported that rhythm control by catheter ablation remarkably improves clinical outcomes in AF patients with HF compared with medical therapy after including the latest RAFT‐AF trial 8 and CASTLE‐HTx trial. 14
AF is the most frequently arrhythmia in the patients with HF. In recent years, tachycardia‐induced cardiomyopathy (TCMP) caused by AF has been increasingly recognized and differentiated from dilated cardiomyopathy. TCMP is a particular form of reversible cardiomyopathy secondary to incessant tachyarrhythmia. Once heart rhythm is normalized, the left ventricular function is usually recovered, and clinical outcomes of the patients are improved. 16 Meanwhile, the clinical complexity should be considered in these patients. The co‐existing diseases such as diabetes, hypertension, and coronary artery disease will affect the benefits of AF catheter ablation. Our results suggested that rhythm control based on catheter ablation was associated with an almost 40% reduction in all‐cause mortality and 35% reduction in the rate of HF re‐hospitalization, compared with medical therapy. The early ARC‐HF 13 and CAMTAF 12 trials included in the present study showed that catheter ablation of AF was not beneficial for HF patients during short‐term follow‐up (0.5 to 1 year), but the long‐term follow‐up results (average 7.8 years) of the study published in 2022 revealed that AF catheter ablation was associated with a significantly decreased all‐cause mortality and cardiovascular hospitalization, compared with medical therapy alone. 15 The subsequent ATTAC trial unexpectedly suggested that AF catheter ablation reduced unplanned hospitalization and mortality in persistent AF patients with HF, 11 which was reconfirmed by the CASTLE‐AF 6 and CABANA HF subgroup trials. 5 More recently, CASTLE‐HTx trial, enrolling the patients with symptomatic AF and end‐stage HF, revealed that catheter ablation significantly reduced the primary endpoint with a composite of death from any cause, implantation of a left ventricular assist device, or urgent heart transplantation, compared with guideline‐directed medical therapy. 14 These evidences consistently suggest that rhythm control by catheter ablation improves clinical outcomes in AF patients with HF.
However, the latest RAFT‐AF trial failed to show a significant benefit on all‐cause mortality and HF events from rhythm control by catheter ablation verse rate control via medical therapy in paroxysmal or persistent AF patients with HF. 8 These findings are inconsistent with previous studies and confused us again. The result of RAFT‐AF trial showed a modest, non‐significant benefit (50 vs. 64 primary events of mortality or HF events) with ablation, representing a 29% relative risk reduction that did not reach statistical significance (P = 0.066). 8
We speculate that the following causes may accounts for it. First, the patients in RAFT‐AF trial were under stricter ventricular rate control that required resting heart rate <80 beats per minute, and <110 beats per minute during a 6 min walk. Once heart rate was not controlled with medical therapy, atrioventricular node ablation with bi‐ventricular pacing was recommended. There were 60 patients in the rate‐control group who underwent atrioventricular node ablation and permanent pacing in the RAFT‐AF trial, and in the rate‐control group, the mean resting heart rate in beats per minute was 74.3 ± 11.8 at 12 months and 74.7 ± 11.8 at 24 months. During the 6 min walk, the heart rates were 88.7 ± 15.2 and 87.4 ± 14.4 at 12 and 24 months. 8 In another 8 RCTs, resting heart rate <80 beats per minute, and <110 beats per minute during moderate exercise were required in the patients with medical therapy in the ARC‐HF trial, 13 CAMTAF trial, 12 and study of Zakeri et al. 15 Beta‐blockers or/and digitalis or/and calcium channel blockers (if not contraindicated) were used to control heart rate, but atrioventricular node ablation with pacemaker was not recommended in these three trials. Rhythm control or rate control was a strategy in the patients with medical therapy in the CASTLE‐AF trial, 10 AMICA trial, 11 CABANA HF subgroup trials, 5 and CASTLE‐HTx trial. 14 If rate control failed, or the patient was feasible for rhythm control according to guideline, rhythm control was performed by using AADs or/and cardioversion in these four trials. Rhythm control was a strategy in the patients with medical therapy in the AATAC trial. 11 Amiodarone was used to maintain sinus rhythm, and cardioversion was preformed, if AT was recurrent. Although a post‐hoc analysis of the RACE II study revealed that the strict rate control have no effect on cardiovascular morbidity and mortality, symptoms, and quality of life in AF patients with HF, the majority of the patients had preserved LVEF at baseline, 17 and the consistence in AF patients with HF with reduced EF(HFrEF) needs to be verified. Of note, in the strict rate control group, 72 patients (24.8%) did not achieve resting heart‐rate target, and 83 patients (27.4%) did not achieve exercise heart‐rate target in the RACE II trial, 18 which may affect the results of RACE II trial. In addition, a study disclosed that high heart rate is a risk factor in HF. 19 Theoretical concern when using a lenient control strategy is that patients may develop HF if the heart rate is too fast. 20 Recently, the APAF‐CRT mortality trial reveals that atrio‐ventricular (AV) junction ablation and cardiac resynchronization therapy (CRT) were superior to pharmacological therapy in reducing mortality in permanent AF patients with HF and narrow QRS wave, irrespective of their baseline EF. Of note, the mean heart rate was 70 beats per minute in population with AV junction ablation + CRT, and 82 beats per minute in population with pharmacological therapy. 21 These evidences indicate that strict rate control may be better than lenient rate control in AF patients with HF. Therefore, the strategy of stricter rate control may attenuate relatively benefit from catheter ablation, compared with medical therapy in RAFT‐AF trial. The ongoing DanAF randomized clinical trial may provide us partial answer in future. 20 Second, it is unfortunate that this trial was not completed as planned to the original sample size of 1000 patients. 22 The more obvious benefit from catheter ablation may be present as amplified sample size. Noteworthily, the LVEF and 6MWD were significantly improved, and the level of NT‐proBNP was markedly decreased in the patients with rhythm control by catheter ablation, compared with medical therapy. Therefore, we think that the result of this study is prone to catheter ablation for AF patients with HF. Except RAFT‐AF trial, there were another eight RCTs included in the present meta‐analysis.. The result showed significantly decreased all‐cause mortality by catheter ablation, compared with medical therapy. The heterogeneity of this meta‐analysis on all‐cause mortality is very low based on the value of I 2. Furthermore, we verify the validation using the ‘One Study removing technique’. Therefore, this result is reliable.
Meanwhile, a meta‐analysis including RAFT‐AF trial and another 4 RCTs was performed. The result suggested remarkably decreased rate of HF re‐hospitalization by catheter ablation, compared with medical therapy. In addition, the present meta‐analysis shows obvious improvement of LVEF, life quality and 6MWD, and less recurrence of AT in the patients with catheter ablation, compared with medical therapy. These results are similar with previous studies. 23 , 24 However, there was no difference in the rate of stroke, adverse events between catheter ablation, and medical therapy. The lack of difference in stroke rates could potentially be attributed to the standardized anticoagulant usage across both populations. Our study shows a very low incidence of stroke in two groups [1.37% (12/876) vs. 2.05% (17/828)].
Of course, there are some limits in our study. First, we should note that some trials included in the present meta‐analysis were performed before 2018, which still has some defects, such as the lack of relatively new strategies including the adjunctive ethanol infusion into the vein of Marshall (EI‐VOM), 25 , 26 high‐power short‐duration ablation guided by ablation index (AI‐HPSD), 27 , 28 and the low efficiency of ablation catheter. On the other hand, the majority of trials included our study had insufficient guideline‐directed medical treatment according to contemporary concepts. Only angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, beta‐blockers, and aldosterone receptor antagonists were applied, except CASTLE‐HTx trial, in which angiotensin receptor–neprilysin inhibitor, and sodium–glucose cotransporter‐2 inhibitor (SGLT2i) were used. These defects may attenuate the benefits from medical therapy. Moreover, recent studies disclosed that omecamtiv mecarbil and vericiguat may improve clinical outcomes in the patients with HFrEF. 29 , 30 These novel drugs for intervention of HF may lead to significant effect on the strategy of management for AF patients with HF in the future. These defects have an established impact on results of our study. Second, the heterogeneity of present meta‐analysis on LVEF, recurrence of AT, quality of life, the level of NT‐proBNP, and 6MWD is very high. We think the following factors related to heterogeneity: (i) the sample size was very small in trials before 2016; (ii) the LVEF was markedly elevated in the CABANA HF subgroup trial and RAFT‐AF trial, compared with another trials; (iii) the strategy of AF catheter ablation had subtle differences among the three RCTs included our study; (iv) the proportion of implantation of implantable cardioverter‐defibrillator (ICD)/CRT‐D/CRT‐P was different among the nine RCTs; (v) the follow‐up duration was various among the nine RCTs; (vi) AV junction ablation and CRT were only recommended as a strategy of rate control in the RAFT‐AF trial, when the heart rate was not be controlled by medical therapy. Although random‐effect models were used, the effect of heterogeneity on our results could not be avoided completely. Third, meta‐regression analysis does not allow clinicians to drive causative inferences, but only speculative. Lastly, a stricter rate control was performed in the RAFT‐AF trial, compared with other RCTs. This strategy has an established effect on clinical outcomes of AF patients with HF. Therefore, larger prospective multicenter clinical trials where a stricter rate control is performed are needed to define the efficacy and safety of AF catheter ablation in the AF patients with HF.
Conclusions
In conclusion, the present meta‐analysis suggests that catheter ablation as rhythm control strategy substantially decreases all‐cause mortality, rate of HF re‐hospitalization, recurrence of AT, and the level of NT‐proBNP, and significantly improves LVEF, quality of life, and 6MWD in AF patients with HF, compared with those with medical therapy. Moreover, incidence of adverse events is similar between catheter ablation and medical therapy. Therefore, catheter ablation as rhythm control strategy is effective and safe for AF patients with HF, rhythm control based on catheter ablation should be a preferred option for this population.
Conflict of interest
All the authors declare that there is no conflict of interests.
Funding
This study is supported by the Natural Science Foundation of China (NSFC) Grant (No. 82160066 and No. 81760060).
Supporting information
Data S1. Supporting Information.
Table S1. Adverse events between rhythm control by catheter ablation and medical therapy.
Acknowledgements
We acknowledge that English language was edited by professor Jiang‐Zhang from Guangxi Medical University, who is jointly awarded PhD degree by University of Florence, University of Pisa, and University of Siena in Italy.
Zhang, Z. , Zheng, Y. , He, W. , Wei, J. , Li, P. , Zhong, G. , and Jiang, Z. (2024) Efficacy of catheter ablation for atrial fibrillation in heart failure: a meta‐analysis of randomized controlled trials. ESC Heart Failure, 11: 2684–2693. 10.1002/ehf2.14814.
Zhongyin Zhang and Yan Zheng contributed equally to this work.
Contributor Information
Guoqiang Zhong, Email: gq_zhong@126.com.
Zhiyuan Jiang, Email: mr_jzy@163.com.
References
- 1. Reddy YNV, Borlaug BA, Gersh BJ. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation 2022;146:339‐357. doi: 10.1161/circulationaha.122.057444 [DOI] [PubMed] [Google Scholar]
- 2. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484‐492. doi: 10.1161/circulationaha.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow‐up investigation of rhythm management (AFFIRM) study. Circulation 2004;109:1509‐1513. doi: 10.1161/01.Cir.0000121736.16643.11 [DOI] [PubMed] [Google Scholar]
- 4. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HT, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. Bmj‐British Med J 2021;373:14. doi: 10.1136/bmj.n991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Packer DL, Piccini JP, Monahan KH, al‐Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure results from the CABANA trial. Circulation 2021;143:1377‐1390. doi: 10.1161/circulationaha.120.050991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. New Engl J Med 2018;378:417‐427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 7. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical practice guidelines. J Am Coll Cardiol 2024;83:109‐279. doi: 10.1016/j.jacc.2023.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes A, et al. Randomized ablation‐based rhythm‐control versus rate‐control trial in patients with heart failure and atrial fibrillation: results from the RAFT‐AF trial. Circulation 2022;145:1693‐1704. doi: 10.1161/circulationaha.121.057095 [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj‐British Med J 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure the randomized AMICA trial. Circ‐Arrhythm Electrophysiol 2019;12:12. doi: 10.1161/circep.119.007731 [DOI] [PubMed] [Google Scholar]
- 11. di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637‐1644. doi: 10.1161/circulationaha.115.019406 [DOI] [PubMed] [Google Scholar]
- 12. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circulation‐Arrhythmia and Electrophysiology 2014;7:31‐38. doi: 10.1161/circep.113.000806 [DOI] [PubMed] [Google Scholar]
- 13. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman‐Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894‐1903. doi: 10.1016/j.jacc.2013.01.069 [DOI] [PubMed] [Google Scholar]
- 14. Sohns C, Fox H, Marrouche NF, Crijns HJGM, Costard‐Jaeckle A, Bergau L, et al. Catheter ablation in end‐stage heart failure with atrial fibrillation. New England Journal of Medicine 2023;389:1380‐1389. doi: 10.1056/NEJMoa2306037 [DOI] [PubMed] [Google Scholar]
- 15. Zakeri R, Ahluwalia N, Tindale A, Omar F, Packer M, Khan H, et al. Long‐term outcomes following catheter ablation versus medical therapy in patients with persistent atrial fibrillation and heart failure with reduced ejection fraction. Eur J Heart Fail 2023;25:77‐86. doi: 10.1002/ejhf.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grandi E, Maleckar MM. Anti‐arrhythmic strategies for atrial fibrillation the role of computational modeling in discovery, development, and optimization. Pharmacol Ther 2016;168:126‐142. doi: 10.1016/j.pharmthera.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulder BA, van Veldhuisen D, Crijns H, Tijssen JG, Hillege HL, Alings M, et al. Lenient vs. strict rate control in patients with atrial fibrillation and heart failure: a post‐hoc analysis of the RACE II study. Eur J Heart Fail 2013;15:1311‐1318. doi: 10.1093/eurjhf/hft093 [DOI] [PubMed] [Google Scholar]
- 18. van Gelder I, Groenveld HF, Crijns H, Tuininga YS, Tijssen JG, Alings AM, et al. Lenient versus strict rate control in patients with atrial fibrillation. New Engl J Med 2010;362:1363‐1373. doi: 10.1056/NEJMoa1001337 [DOI] [PubMed] [Google Scholar]
- 19. Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet 2010;376:886‐894. doi: 10.1016/s0140-6736(10)61259-7 [DOI] [PubMed] [Google Scholar]
- 20. Feinberg JB, Olsen MH, Brandes A, Raymond, Nielsen WB, Nielsen EE, et al. Lenient rate control versus strict rate control for atrial fibrillation: a protocol for the Danish atrial fibrillation (DanAF) randomised clinical trial. BMJ Open 2021;11:e044744. doi: 10.1136/bmjopen-2020-044744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF‐CRT mortality trial. Eur Heart J 2021;42:4731‐4739. doi: 10.1093/eurheartj/ehab569 [DOI] [PubMed] [Google Scholar]
- 22. Parkash R, Wells G, Rouleau J, Talajic M, Essebag V, Skanes A, et al. A randomized ablation‐based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation: the RAFT‐AF trial rationale and design. Am Heart J 2021;234:90‐100. doi: 10.1016/j.ahj.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 23. Blomström‐Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation the CAPTAF randomized clinical trial. Jama‐J Am Med Assoc 2019;321:1059‐1068. doi: 10.1001/jama.2019.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu G, Huang H, Cai L, Yang Y, Liu X, Yu B, et al. Long‐term observation of catheter ablation vs. pharmacotherapy in the management of persistent and long‐standing persistent atrial fibrillation (CAPA study). Europace 2021;23:731‐739. doi: 10.1093/europace/euaa356 [DOI] [PubMed] [Google Scholar]
- 25. Lai YW, Liu XX, Sang CH, Long D, Li M, Ge W, et al. Effectiveness of ethanol infusion into the vein of Marshall combined with a fixed anatomical ablation strategy (the “upgraded 2C3L” approach) for catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:1849‐1856. doi: 10.1111/jce.15108 [DOI] [PubMed] [Google Scholar]
- 26. Valderrábano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN, et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation the VENUS randomized clinical trial. Jama‐J Am Med Assoc 2020;324:1620‐1628. doi: 10.1001/jama.2020.16195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wielandts JY, Kyriakopoulou M, Almorad A, Hilfiker G, Strisciuglio T, Phlips T, et al. Prospective randomized evaluation of high power during CLOSE‐guided pulmonary vein isolation the POWER‐AF study. Circ‐Arrhythm Electrophysiol 2021;14:7. doi: 10.1161/circep.120.009112 [DOI] [PubMed] [Google Scholar]
- 28. Shin DG, Ahn J, Han SJ, Lim HE. Efficacy of high‐power and short‐duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace 2020;22:1495‐1501. doi: 10.1093/europace/euaa144 [DOI] [PubMed] [Google Scholar]
- 29. Docherty KF, McMurray JJV, Claggett BL, Miao ZM, Adams KF, Arias‐Mendoza A, et al. Efficacy of omecamtiv mecarbil in heart failure with reduced ejection fraction according to N‐terminal pro‐B‐type natriuretic peptide level: insights from the GALACTIC‐HF trial. Eur J Heart Fail 2023;25:248‐259. doi: 10.1002/ejhf.2763 [DOI] [PubMed] [Google Scholar]
- 30. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. New Engl J Med 2020;382:1883‐1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Table S1. Adverse events between rhythm control by catheter ablation and medical therapy.


