Abstract
Aims
Diagnosing acute heart failure (AHF) remains particularly challenging in older patients. Natriuretic peptides are recommended as valuable diagnostic tools in this context. This study aims to establish the diagnostic thresholds of B‐type natriuretic peptide (BNP) and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) for AHF in patients aged over 75 years, both with and without co‐morbidities.
Methods and results
In this retrospective longitudinal multicentre cohort study, data were gathered from 12 071 hospitalized patients aged 75 years or older, presenting with acute dyspnoea and undergoing BNP or NT‐proBNP measurement within 48 h of admission across 10 Assistance Publique‐Hôpitaux de Paris facilities between 2011 and 2022, encompassing geriatrics, cardiology, and pulmonology departments. Final diagnoses were categorized using ICD‐10 criteria as either AHF or other acute respiratory conditions such as COPD exacerbation, pulmonary embolism, and pneumonia. The mean (SD) age of the population was 84.0 (80.0, 89.0) years, with 52.7% being female. Out of these, 7946 (65.8%) were diagnosed with AHF upon discharge. For NT‐proBNP, the identified ‘optimal’ threshold for diagnosing AHF was 1748 ng/L, with a positive predictive value (PPV) of 84%. Among patients aged over 85 years, a threshold of 2235 pg/mL for NT‐proBNP was associated with an 84% PPV. In patients with atrial fibrillation (AF), a threshold of 2332 pg/mL for NT‐proBNP demonstrated a PPV of 90% for AHF diagnosis. Additionally, in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min, a threshold of 3474 pg/mL for NT‐proBNP yielded a 90% PPV for AHF diagnosis. In male patients, a threshold of 1800 pg/mL showed an 85% PPV for AHF diagnosis, while in patients with obesity, a threshold of 1375 pg/mL demonstrated an 85% PPV for AHF diagnosis.
Conclusions
In older patients, we found significant effects of co‐morbidities on natriuretic peptides results, particularly in patients over 85 years old, older patients with abnormal renal function, obesity, and atrial fibrillation. Despite the consideration of those co‐morbid conditions, NT‐proBNP and BNP level continue to demonstrate utility in the diagnosis of AHF in older patients.
Keywords: Acute heart failure, Diagnosis, NT‐proBNP, Older
Introduction
Accurate diagnostic tools are pivotal in managing heart failure (HF), facilitating timely interventions and optimizing patient care. Presently, the diagnosis of acute heart failure (AHF) relies on clinical assessment, natriuretic peptide measurements, and echocardiography. 1 B‐type natriuretic peptide (BNP) and N‐terminal proBNP (NT‐proBNP) have emerged as integral components in HF diagnosis due to their high sensitivity and specificity, especially in screening patients with acute dyspnoea. 2 For younger and middle‐aged patients, an NT‐proBNP cut‐off value of 125 pg/mL effectively excludes cardiac dysfunction as the cause of symptoms. 3
However, determining precise cut‐off values for BNP and NT‐proBNP is contingent upon various factors including age, renal function, atrial fibrillation, obesity, and HF type. 4 , 5 Evolving demographics in HF, particularly the increasing prevalence of older patients with higher rates of co‐morbidities such as renal disease and atrial fibrillation, necessitate a re‐evaluation of current diagnostic cut‐offs for natriuretic peptides 6 , 7 , 8 , 9 ). This demographic shift, coupled with a scarcity of cardiologists in the French medical system, underscores the importance of establishing accurate HF diagnoses before echocardiography, especially with the advent of novel treatments like gliflozins. 10
Despite the efficacy of natriuretic peptides in discerning the aetiology of acute dyspnoea, there remains a paucity of data on specific cut‐off values for patients over 75 years old, particularly in the context of co‐morbidities. 11 , 12 , 13 Data from the PRIDE (N‐Terminal Pro‐BNP Investigation of Dyspnoea in the Emergency Department) and ICON (International Collaborative of NT‐proBNP) studies suggested optimal diagnostic cut‐offs NT‐proBNP of 450, 900, and 1800 pg/mL for age categories of <50, 50 to 75, and >75 years, respectively, for the identification of acute HF. 14 These cut‐offs have been widely endorsed. 1 , 8 , 15 It is hypothesized that older patients, especially those with co‐morbidities such as atrial fibrillation, renal failure, and obesity, may require different cut‐off values for BNP and NT‐proBNP to enhance diagnostic accuracy. This study aims to identify age‐specific BNP and NT‐proBNP cut‐offs in patients over 75 years old, considering various co‐morbidities such as atrial fibrillation, renal failure, and obesity, thereby addressing a crucial gap in current diagnostic strategies for AHF.
Methods
Population
Our study was a retrospective, observational, longitudinal multicentre cohort study conducted in patients aged 75 years or older, hospitalized in 48 geriatrics, cardiology, and pulmonology departments for acute respiratory failure in all the Assistance Publique des Hôpitaux de Paris (APHP) hospitals.
Between January 2011 and December 2022, patients aged 75 or older were included if admitted to APHP for a final principal diagnosis of acute heart failure, pulmonary embolism, pneumonia, or COPD exacerbation. These patients also had an NT‐proBNP or a BNP measurement 48 h following admission. Patients could have been admitted to the intensive care unit before the hospital wards. Exclusion criteria were ambulatory day hospitalization, BNP or NT‐proBNP assay performed >48 h after admission, and having several principal diagnoses. The regional ethics committee approved the protocol (EDS CSE 180032).
Baseline data collection
All diagnoses were recorded in the APHP data warehouse, ‘entrepot de données de santé’ (EDS), with one principal diagnosis and secondary diagnoses for each patient.
EDS collects data on more than 11 million patients treated in the 39 AP‐HP establishments. In addition to demographic data, EDS contains medico‐administrative data relating to the MPIS (Medicalization Programme for Information Systems), diagnoses, procedures, biological and imaging results, drug prescriptions, and medical reports associated with hospital visits. This includes emergency department data, which are part of the latest integrated information flows. In the EDS, all diagnostic are coded with ICD‐10.
All patients hospitalized in AP‐HP have a ICD‐10 exhaustive code of principal diagnoses and co‐morbidities.
The data collection was obtained by EDS available via ORBIS‐JUPYTER software and coded with ICD‐10. We recorded the principal diagnosis (AHF, COPD exacerbation pulmonary embolism and pneumonia) and medical history was coded with the secondary diagnoses (diabetes mellitus, arterial hypertension, obesity, ischaemic cardiopathy, atrial fibrillation, kidney failure, dyslipidaemia, and depression were selected as coded co‐morbidities). Socio‐demographic characteristics (sex, age, and BMI) were obtained as well as weight at admission that is a data item filled in the reports. Laboratory data (such as the blood haemoglobin level and the serum BNP or NT‐proBNP and creatinine levels) were available in the ORBIS‐JUPYTER software window. The samples were taken at different times of the day, which correspond to the time of the diagnostic stage when the patient arrives at the emergency department. Samples were taken from venous blood upon the patient's arrival, at any time of the day or night. BNP and NT‐proBNP exhibit a diurnal rhythm that results in even higher diagnostic accuracy among evening/night‐time presenters versus daytime presenters. 16
eGFR was calculated with CKD‐EPI formula. The measurements were done before treatment; 80% of biological assays were completed within 448 min of admission (Médiane: 83 min, q1 = 38 min, q3 = 238 min).
Dyspnoea cause of principal diagnosis
The principal and the secondary diagnoses were determined by a physician at discharge and coded in compliance with the ICD‐10 system, which is mandatory for each hospital stay. We included all ICD‐10 codes included for acute dyspnoea which were ‘acute heart failure’, ‘pulmonary embolism’, ‘COPD exacerbation’, ‘asthma exacerbation’, ‘acute pneumonia’, and ‘acute bronchitis’. Patients who were classified as having patients having both AHF and PE or any other pulmonary pathology, as the principal diagnosis were excluded of the study.
Statistical analysis
Continuous variables were reported as mean (standard deviation) or median [interquartile range (IQR)], while categorical variables were presented as numbers (percentage). For statistical analysis, quantitative variables were compared using Student, Wilcoxon, or Kruskal–Wallis rank‐based test as required. Categorical variables were compared using either a chi‐squared test or Fisher's exact test as appropriate. All baseline characteristics were included in the univariate analysis.
Diagnosis of AHF was based on the ICD‐10 code. BNP and NT‐proBNP levels were depicted regarding diagnosis in box plot figures (Figure 2 ). Receiver operating characteristic (ROC) curves were used to determine the best BNP or NT‐proBNP cut‐off levels and rounded to the nearest clinically relevant integer when applicable. The diagnostic performance of the prediction score was then evaluated using the area under the ROC curve (AUC).
Figure 2.
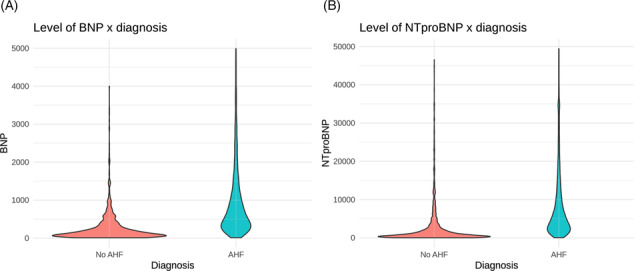
BNP and NT‐proBNP values between diagnostic groups.
The selection of cut‐offs was designed based on the patient distribution in each sample, ensuring homogeneity across samples. We tested different thresholds to find out which were the most interesting: between the number of patients, and the diagnostic relevance. Finally we choose ‘age = 85 years’, ‘eGFR = 30, 45 and 60 mL/min’, and ‘BMI = 30’. The optimal cut‐off was determined using the Youden Index, which measures the diagnostic test's ability to balance sensitivity and specificity. The threshold for BNP or NT‐proBNP is set by maximizing the sum of the sensitivity and the specificity. The Youden Index is considered a measure of a diagnostic test's performance, with a value of greater than 50% considered to be acceptable.
Other potential predictors for AHF were analysed through descriptive statistics to identify significant variables associated with disease status. The logistic regression was performed taking into account the principal factors influencing diagnosis in the population, and the result of baselines characteristics. We identify the dependent variable and independent variables in this binary classification problem. The ROC curves were calculated for each group of significant variables and the difference in the AUC of the ROC curves was tested. A P‐value of <0.05 was considered statistically significant. The statistical analysis was performed using the R project language within ORBIS‐JUPYTER software.
Results
Between January 2011 and December 2022, 15 211 patients aged over 75 years with an initial emergency department admission for acute dyspnoea and subsequent hospitalization within 48 h in the geriatrics, cardiology, or pulmonology department within the enlarged APHP hospitals were included in this study. These patients also had natriuretic peptide levels available. Among them, 15 037 patients had a complete stay, 12 730 patients had a BNP or NT‐proBNP dosage within 48 h after emergency department admission, and 12 220 had only one principal diagnosis. In an acceptable range of 5–5000 ng/L for BNP or 10–50 000 ng/L for NT‐proBNP, 12 071 patients were identified. The primary diagnosis of these patients was classified as ‘AHF’ or ‘other causes of dyspnoea’, including pulmonary embolism. The diagnosis of AHF was observed in 66% (n = 7946) of patients in this study, and respiratory dyspnoea was the diagnosis in the remaining 35% (n = 4125) (Figure 1 ).
Figure 1.
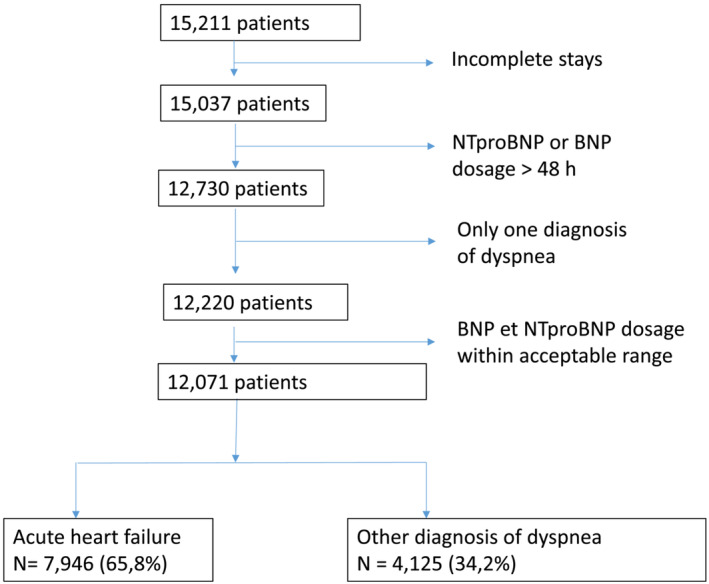
Flow chart of the study population.
Patients' general characteristics
Compared with other causes of dyspnoea, AHF patients were older (85 vs. 83, P < 0.01), more often women (53.5% vs. 51.2%, P < 0.01), had more frequent diabetes mellitus (27.2 vs. 19.1%, P < 0.01), hypertension (53.5 vs. 42.3%, P < 0.01), and dyslipidaemia (27.5% vs. 19.9%, P < 0.01). AHF patients had more frequent co‐morbidities such as atrial fibrillation (57.3 vs. 23.9%, P < 0.01), coronary artery disease (34.2 vs. 14.5%, P < 0.01), and chronic kidney disease (15.5 vs. 6.7%, P < 0.01).
At admission, blood analysis of patients with AHF compared to patients without AHF showed lower levels of CRP (14 vs. 84 mg/L, P < 0.01), leukocytes (8.2 vs. 9.9 ×10*9/L, P < 0.01), and haemoglobin (11.9 vs. 12.5 g/dL, P < 0.01). Conversely, AHF patients had higher blood levels of T troponin (45 vs. 31 ng/L, P < 0.01) and creatinine (110 vs. 84 μmol/L, P < 0.01) (Table 1 ); 9509 patients have treatments at admission; 70% had loop diuretics; 6% had thiazide diuretic; 3% had SGLT2i, and 2% had ARNi.
Table 1.
| AHF (N = 7946) | No AHF (N = 4125) | Total (N = 12071) | P value | |
|---|---|---|---|---|
| Age | 85.0 (80.0, 89.0) | 83.0 (79.0, 88.0) | 84.0 (80.0, 89.0) | <0.01 |
| Age (>85), n (%) | 3500 (44.0%) | 1566 (38.0%) | 5066 (42.0%) | <0.01 |
| Sex (W), n (%) | 4254 (53.5%) | 2113 (51.2%) | 6367 (52.7%) | 0.02 |
| Hypertension, n (%) | 4299 (54.1%) | 1754 (42.5%) | 6053 (50.1%) | <0.01 |
| Ischaemic heart disease, n (%) | 2721 (34.2%) | 597 (14.5%) | 3318 (27.5%) | <0.01 |
| COPD, n (%) | 949 (11.9%) | 1272 (30.8%) | 2221 (18.4%) | <0.01 |
| Atrial fibrillation, n (%) | 4555 (57.3%) | 986 (23.9%) | 5541 (45.9%) | <0.01 |
| Dyslipidaemia, n (%) | 2327 (29.3%) | 820 (19.9%) | 3147 (26.1%) | <0.01 |
| Diabetes, n (%) | 2182 (27.5%) | 785 (19.0%) | 2967 (24.6%) | <0.01 |
| Depression, n (%) | 490 (6.2%) | 324 (7.9%) | 814 (6.7%) | <0.01 |
| Chronic kidney disease, n (%) | 1232 (15.5%) | 276 (6.7%) | 1508 (12.5%) | <0.01 |
| Acute ischaemic stroke, n (%) | 291 (3.7%) | 170 (4.1%) | 461 (3.8%) | 0.21 |
| Glycated haemoglobin (%) | 6.1 (5.7, 7.0) | 6.2 (5.7, 7.1) | 6.1 (5.7, 7.0) | 0.02 |
| ALT (U/L) | 23.0 (16.0, 35.0) | 20.0 (14.0, 30.0) | 22.0 (15.0, 33.0) | <0.01 |
| AST (U/L) | 28.0 (21.0, 40.0) | 25.0 (20.0, 35.0) | 27.0 (21.0, 38.0) | <0.01 |
| Bilirubin (μmol/L) | 12.0 (8.0, 18.0) | 9.0 (6.0, 13.0) | 11.0 (7.0, 16.0) | <0.01 |
| Creatinine clearance (mL/min or mL/min/1.73 m2) | 45.0 (32.0, 62.0) | 64.0 (45.0, 81.0) | 51.0 (35.0, 70.2) | <0.01 |
| Creatinine (μmol/L) | 110.0 (83.0, 152.0) | 84.0 (65.0, 112.0) | 101.0 (75.0, 140.0) | <0.01 |
| CRP (mg/L) | 14.0 (5.0, 40.0) | 37.0 (9.2, 106.7) | 18.3 (6.0, 59.0) | <0.01 |
| Haematocrit (%) | 36.2 (32.4, 40.0) | 38.2 (34.7, 41.5) | 37.0 (33.3, 40.6) | <0.01 |
| Haemoglobin (g/dL) | 11.9 (10.6, 13.2) | 12.6 (11.4, 13.8) | 12.1 (10.9, 13.4) | <0.01 |
| Leukocytes (×10*9/L) | 8.2 (6.5, 10.7) | 9.9 (7.5, 13.4) | 8.8 (6.8, 11.7) | <0.01 |
| Platelets (×10*9/L) | 224.0 (176.0, 281.0) | 244.0 (193.0, 309.0) | 231.0 (182.0, 292.0) | <0.01 |
| Potassium (mmol/L) | 4.3 (3.9, 4.7) | 4.2 (3.9, 4.6) | 4.3 (3.9, 4.7) | <0.01 |
| Sodium (mmol/L) | 139.0 (136.0, 141.0) | 139.0 (136.0, 141.0) | 139.0 (136.0, 141.0) | <0.01 |
| Troponin I (μg/L) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.10 |
| Troponin T (ng/L) | 45.0 (29.0, 77.0) | 31.5 (19.0, 57.0) | 42.0 (25.7, 72.0) | <0.01 |
| TSH (mUI/L) | 1.9 (1.1, 3.2) | 1.5 (0.9, 2.5) | 1.8 (1.0, 3.0) | <0.01 |
| NT‐proBNP (ng/L) log | 8.5 (7.8, 9.3) | 6.7 (5.7, 7.8) | 8.1 (7.0, 9.0) | <0.01 |
| BNP (ng/L) log | 6.4 (5.7, 7.1) | 4.9 (4.1, 5.8) | 5.9 (5.0, 6.8) | <0.01 |
| NT‐proBNP (ng/L) | 5021.5 (2412.8, 10642.2) | 832.0 (285.5, 2405.5) | 3308.0 (1094.5, 8092.5) | <0.01 |
| BNP (ng/L) | 607.0 (312.0, 1178.8) | 134.0 (60.2, 318.0) | 383.0 (153.0, 858.2) | <0.01 |
| In‐hospital death, n (%) | 588 (7.4%) | 325 (7.9%) | 913 (7.6%) | 0.35 |
The data are quoted as the number (%), or mean ± SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (mL/min); NT‐proBNP, N terminal pro brain natriuretic peptide.
Natriuretic peptide concentrations and diagnostic performance for AHF
Compared with patients with other causes of dyspnoea, AHF patients had higher BNP (607 vs. 134, P < 0.01) and NT‐proBNP levels (5021 vs. 832, P < 0.01) (Figure 2 A,B ).
For BNP, the ‘optimal’ threshold for the AHF diagnosis was 256 [234–276] ng/L, while sensitivity was 82% and specificity was 70%, with a maximal accuracy of 77%.
It was found that BNP alone was effective in identifying patients with AHF (AUC = 0.83; 95% CI [0.81–0.84]).
For NT‐proBNP, the ‘optimal’ threshold for the AHF diagnosis was 1748 [1670–2015] ng/L, while sensitivity was 84% and specificity was 67%, with maximal accuracy of 79% (Figure 3).
Figure 3.
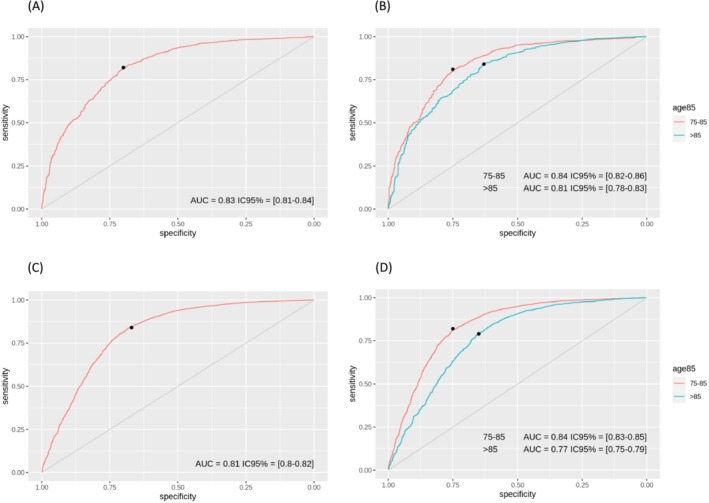
BNP and NT‐proBNP cutpoint diagnosis of Acute HF. (A) BNP in the overall population, (B) BNP in age subgroups. (C) NT‐proBNP in the overall population. (D) NT‐proBNP in age subgroups.
In logistic regression, a BNP cutpoint of 250 pg/mL and NT‐proBNP cutpoint of 1750 pg/mL had a high OR of all variables retained (OR: 10.4 and 10.94; 95% CI: 8.82–12.43 and 9.87–12.13). Other variables significantly predictive of acute HF included AF (OR: 4.27; 95% CI: 3.93–4.65; P < 0.01), ischaemic cardiopathy (OR: 3.07; 95% CI: 2.79–3.39; P < 0.01), and kidney failure (OR: 2.55; 95% CI: 2.23–2.93) (Table 2 ).
Table 2.
| Variable | OR, 95% CI | Estimate | P value |
|---|---|---|---|
| Age | 1.024 [1.018; 1.031] | 0.02 | <0.001 |
| Sex | 1.097 [1.018; 1.183] | 0.09 | 0.016 |
| Kidney failure | 2.559 [2.236; 2.938] | 0.94 | <0.001 |
| HTA | 1.593 [1.477; 1.719] | 0.47 | <0.001 |
| Ischaemic cardiopathy | 3.077 [2.791; 3.398] | 1.12 | <0.001 |
| COPD | 0.304 [0.277; 0.334] | −1.19 | <0.001 |
| Atrial fibrillation | 4.276 [3.932; 4.654] | 1.45 | <0.001 |
| Dyslipidaemia | 1.669 [1.525; 1.828] | 0.51 | <0.001 |
| Diabetes | 1.611 [1.47; 1.767] | 0.48 | <0.001 |
| Depression | 0.771 [0.667; 0.892] | −0.26 | <0.001 |
| Stroke | 0.884 [0.73; 1.074] | −0.12 | 0.212 |
| HbA1c | 0.935 [0.882; 0.992] | −0.07 | 0.025 |
| eGRF | 0.97 [0.968; 0.971] | −0.03 | <0.001 |
| Creatininemia | 1.01 [1.009; 1.011] | 0.01 | <0.001 |
| CRP | 0.992 [0.991; 0.993] | −0.01 | <0.001 |
| Haematocrit | 0.941 [0.933; 0.949] | −0.06 | <0.001 |
| Haemoglobin | 0.832 [0.814; 0.85] | −0.18 | <0.001 |
| Leucocytes | 0.935 [0.927; 0.943] | −0.07 | <0.001 |
| Potassium | 1.231 [1.159; 1.306] | 0.21 | <0.001 |
| Sodium | 0.984 [0.977; 0.992] | −0.02 | <0.001 |
| Tropo I | 1.04 [0.997; 1.099] | 0.04 | 0.111 |
| Tropo T | 1.001 [1; 1.001] | 0.00 | 0.002 |
| TSH | 1.054 [1.037; 1.073] | 0.05 | <0.001 |
| BNP100 (BNP/100) | 1.31 [1.275; 1.347] | 0.27 | <0.001 |
| NT‐proBNP100 (NT‐proBNP/100) | 1.019 [1.018; 1.02] | 0.02 | <0.001 |
| BNP (cut off 250 ng/mL) | 10.463 [8.826; 12.434] | 2.35 | <0.001 |
| NT‐proBNP (cut off 1750 ng/mL) | 10.943 [9.879; 12.131] | 2.39 | <0.001 |
| Intra hospital death | 0.934 [0.812; 1.077] | −0.07 | 0.345 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimate glomerular filtration rate; NT‐proBNP, N terminal pro brain natriuretic peptide.
Cutpoint analysis according to age
For the age‐dependent cut‐offs of 85 years old (75–85 or >85 years old), the cutpoint strategy for BNP was associated with a predictive positive value (PPV) of 83% (95% CI: 0.8 to 0.85) and 81% (95% CI: 0.8 to 0.87) with a threshold of 236 and 262 pg/mL, respectively. The corresponding sensitivities for each cut‐off were 81% (95% CI: 0.76–0.87) and 84% (95% CI: 0.67–0.87), respectively (Table 3).
Table 3.
| (a) Mesures of diagnostic accuracy according to subgroup comorbidities | ||||||||
|---|---|---|---|---|---|---|---|---|
| BNP | ||||||||
| Threshold | Sens | Spec | PPV | NPV | Accuracy | AUC | P value | |
| BNP | 256 [234; 276] | 0.82 [0.79–0.85] | 0.7 [0.67–0.74] | 0.82 [0.81–0.84] | 0.7 [0.67–0.73] | 0.77 [0.76–0.79] | 0.83 [0.81–0.84] | |
| 75–85 | 236 [176; 280] | 0.81 [0.76–0.87] | 0.75 [0.67–0.8] | 0.83 [0.8–0.85] | 0.72 [0.69–0.79] | 0.78 [0.77–0.81] | 0.84 [0.82–0.86] | 0.015 |
| >85 | 262 [236; 442] | 0.84 [0.67–0.87] | 0.63 [0.59–0.8] | 0.81 [0.8–0.87] | 0.68 [0.56–0.71] | 0.77 [0.71–0.79] | 0.81 [0.78–0.83] | |
| eGFR < 30 | 446 [341; 568] | 0.76 [0.69–0.86] | 0.66 [0.55–0.77] | 0.87 [0.84–0.9] | 0.48 [0.43–0.58] | 0.74 [0.69–0.79] | 0.76 [0.71–0.81] | <0.01 |
| eGFR ≥ 30 | 254 [230; 270] | 0.81 [0.78–0.83] | 0.72 [0.7–0.77] | 0.82 [0.8–0.84] | 0.72 [0.68–0.74] | 0.78 [0.76–0.79] | 0.83 [0.82–0.85] | |
| eGFR < 45 | 358 [222; 468] | 0.7 [0.68–0.91] | 0.71 [0.49–0.76] | 0.88 [0.84–0.9] | 0.45 [0.43–0.66] | 0.7 [0.69–0.81] | 0.78 [0.75–0.81] | <0.01 |
| eGFR ≥ 45 | 236 [215; 262] | 0.78 [0.75–0.81] | 0.75 [0.72–0.79] | 0.8 [0.78–0.82] | 0.74 [0.71–0.77] | 0.77 [0.75–0.79] | 0.83 [0.81–0.85] | |
| eGFR < 60 | 270 [248; 455] | 0.84 [0.67–0.88] | 0.62 [0.57–0.79] | 0.84 [0.83–0.89] | 0.62 [0.5–0.67] | 0.78 [0.7–0.8] | 0.8 [0.78–0.83] | 0.155 |
| eGFR ≥ 60 | 236 [152; 274] | 0.75 [0.71–0.88] | 0.78 [0.65–0.82] | 0.78 [0.72–0.81] | 0.75 [0.73–0.83] | 0.77 [0.75–0.79] | 0.83 [0.8–0.85] | |
| BMI < 30 | 310 [224; 380] | 0.81 [0.76–0.9] | 0.79 [0.68–0.84] | 0.87 [0.83–0.89] | 0.7 [0.67–0.81] | 0.8 [0.78–0.84] | 0.87 [0.84–0.9] | 0.036 |
| BMI ≥ 30 | 184 [172; 206] | 0.79 [0.74–0.88] | 0.77 [0.64–0.89] | 0.9 [0.86–0.95] | 0.59 [0.51–0.7] | 0.79 [0.74–0.85] | 0.78 [0.7–0.86] | |
| No AF | 254 [222; 272] | 0.82 [0.79–0.86] | 0.76 [0.72–0.79] | 0.78 [0.75–0.8] | 0.8 [0.78–0.83] | 0.79 [0.77–0.81] | 0.85 [0.83–0.87] | <0.01 |
| AF | 302 [247; 438] | 0.76 [0.62–0.83] | 0.62 [0.54–0.75] | 0.87 [0.86–0.9] | 0.44 [0.37–0.5] | 0.73 [0.65–0.77] | 0.75 [0.72–0.78] | |
| W | 236 [198; 268] | 0.82 [0.78–0.85] | 0.68 [0.63–0.73] | 0.82 [0.81–0.84] | 0.67 [0.63–0.71] | 0.77 [0.75–0.79] | 0.81 [0.78–0.83] | <0.01 |
| M | 274 [234; 358] | 0.84 [0.74–0.87] | 0.72 [0.68–0.82] | 0.82 [0.8–0.86] | 0.74 [0.67–0.79] | 0.79 [0.76–0.81] | 0.86 [0.84–0.88] | |
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| NT‐proBNP | ||||||||
| Threshold | Sens | Spec | PPV | NPV | Accuracy | AUC | P value | |
| NT‐proBNP | 1748 [1670; 2015] | 0.84 [0.8–0.85] | 0.67 [0.66–0.73] | 0.84 [0.84–0.86] | 0.68 [0.63–0.69] | 0.79 [0.77–0.8] | 0.81 [0.8–0.82] | |
| 75–85 | 1680 [1387; 1760] | 0.82 [0.8–0.85] | 0.75 [0.71–0.77] | 0.86 [0.84–0.87] | 0.69 [0.67–0.72] | 0.79 [0.78–0.8] | 0.84 [0.83–0.85] | <0.01 |
| >85 | 2235 [1751; 2772] | 0.79 [0.77–0.87] | 0.65 [0.57–0.68] | 0.84 [0.83–0.86] | 0.56 [0.55–0.65] | 0.75 [0.74–0.79] | 0.77 [0.75–0.79] | |
| eGFR < 30 | 3474 [1840; 5286] | 0.79 [0.68–0.92] | 0.51 [0.36–0.62] | 0.9 [0.89–0.92] | 0.3 [0.25–0.47] | 0.75 [0.68–0.84] | 0.68 [0.64–0.72] | <0.01 |
| eGFR ≥ 30 | 1682 [1552; 1980] | 0.82 [0.78–0.84] | 0.7 [0.68–0.74] | 0.83 [0.82–0.84] | 0.69 [0.66–0.71] | 0.78 [0.77–0.79] | 0.82 [0.81–0.83] | |
| eGFR < 45 | 2437 [1972; 2798] | 0.85 [0.78–0.88] | 0.51 [0.47–0.59] | 0.88 [0.87–0.89] | 0.44 [0.38–0.49] | 0.79 [0.74–0.81] | 0.72 [0.7–0.74] | <0.01 |
| eGFR ≥ 45 | 1593 [1292; 1757] | 0.78 [0.77–0.84] | 0.75 [0.69–0.77] | 0.81 [0.78–0.82] | 0.72 [0.71–0.77] | 0.77 [0.76–0.78] | 0.83 [0.82–0.84] | |
| eGFR < 60 | 2010 [1708; 2411] | 0.85 [0.8–0.88] | 0.56 [0.52–0.62] | 0.87 [0.86–0.88] | 0.51 [0.46–0.56] | 0.78 [0.75–0.81] | 0.75 [0.73–0.76] | <0.01 |
| eGFR ≥ 60 | 1553 [1183; 1698] | 0.76 [0.74–0.83] | 0.78 [0.71–0.8] | 0.76 [0.73–0.78] | 0.77 [0.76–0.82] | 0.77 [0.76–0.78] | 0.83 [0.82–0.85] | |
| BMI < 30 | 1995 [1410; 2260] | 0.85 [0.81–0.9] | 0.72 [0.66–0.77] | 0.83 [0.81–0.85] | 0.74 [0.7–0.81] | 0.8 [0.78–0.82] | 0.83 [0.81–0.85] | 0.227 |
| BMI ≥ 30 | 1375 [777; 2178] | 0.79 [0.66–0.91] | 0.69 [0.56–0.84] | 0.85 [0.82–0.91] | 0.61 [0.52–0.77] | 0.76 [0.7–0.82] | 0.8 [0.76–0.84] | |
| No AF | 1738 [1664; 1984] | 0.8 [0.76–0.81] | 0.74 [0.72–0.78] | 0.77 [0.76–0.79] | 0.77 [0.74–0.78] | 0.77 [0.76–0.78] | 0.83 [0.82–0.84] | <0.01 |
| AF | 2332 [2008; 2743] | 0.78 [0.74–0.85] | 0.57 [0.5–0.62] | 0.9 [0.9–0.91] | 0.33 [0.31–0.38] | 0.75 [0.72–0.79] | 0.71 [0.69–0.73] | |
| W | 1698 [1565; 2100] | 0.84 [0.77–0.86] | 0.66 [0.63–0.72] | 0.84 [0.83–0.86] | 0.66 [0.6–0.69] | 0.78 [0.75–0.79] | 0.8 [0.78–0.81] | <0.01 |
| M | 1800 [1669; 2157] | 0.84 [0.8–0.86] | 0.7 [0.68–0.76] | 0.85 [0.84–0.87] | 0.7 [0.65–0.72] | 0.8 [0.78–0.81] | 0.83 [0.82–0.84] | |
AF, atrial fibrillation; BMI, body mass index; eGFR, estimate glomerular filtration rate, W, women; M, Men.
For the age‐dependent cut‐offs of 85 years old (75–85 or >85 years old), the cutpoint strategy for NT‐proBNP was associated with a PPV of 86% (95% CI: 0.84 to 0.87) and 84% (95% CI: 0.83 to 0.86) with a threshold of 1680 and 2235 pg/mL, respectively. The corresponding sensitivities for each cut‐off were 82% (95% CI: 0.8–0.85) and 79% (95% CI: 0.77–0.87), respectively.
For BNP, when testing a PPV of 95%, we found a cut off of 2727 pg/mL for the diagnosis. For the age‐dependent cut‐offs of 85 years old (75–85 or >85 years old), the cutpoint strategy for BNP was associated with a PPV of 95% with a threshold of 1480 and 3532 pg/mL, respectively.
For NT‐proBNP, when testing a PPV of 95%, we found a cut off of 45925 pg/mL for the diagnosis. For the age‐dependent cut‐offs of 85 years old (75–85 or >85 years old), the cutpoint strategy for BNP was associated with a PPV of 95% with a threshold of 33 135 and 38 990 pg/mL, respectively.
Cutpoint analysis: Exclusion of acute heart failure
The age‐independent approach for ruling out acute HF using the single BNP cutpoint of 256 pg/mL exhibited an NPV of 70% (95% CI: 0.67–0.73) and a specificity of 70% (95% CI: 0.67–0.74). In our cohort, to reach a NPV of 95%, the BNP cutpoint was 12 pg/mL.
For the age‐dependent cut‐offs of 85 years, with a threshold BNP of 236 and 258 pg/mL, the corresponding specificity for each cut‐off was 74% (95% CI: 67–0.8) and 62% (95% CI: 0.59–0.81) respectively with NPV of 72% and 68%.
The age‐independent approach for ruling out AHF using the single NT‐proBNP cutpoint of 1748 pg/mL exhibited an NPV of 68% (95% CI: 0.63–0.69) and a specificity of 67% (95% CI: 0.66–0.73). In our cohort, to reach a NPV of 95%, the NT‐proBNP cutpoint was 45 925 pg/mL.
For the age‐dependent cut‐offs of 85 years, with a threshold of 1680 and 2235 pg/mL, the corresponding specificity for each cut‐off was 75% (95% CI: 0.71–0.77) and 65% (95% CI: 0.57–0.68) respectively with NPV of 69% and 56%.
Subgroup findings
Subgroup analyses assessing the AUC of the optimal BNP and NT‐proBNP threshold for the diagnosis or exclusion of AHF are presented in Table 3. Regarding conditions and co‐morbidities, the threshold of BNP and NT‐proBNP in older patients increased with age, the presence of AF, renal failure, in males. The threshold was reduced with higher BMI. We found good AUC for the different subgroups.
For BNP in older patients with AF, a threshold of 302 pg/mL had a good sensitivity and specificity for the diagnosis of AHF and was higher than in patients without AF. In older patients with eGFR < 30 mL/min, a threshold of 446 pg/mL had a good sensitivity and specificity for the diagnosis of AHF, and this threshold was higher than in patients without kidney failure. In older male patients, a threshold of 274 pg/mL, had a good sensitivity and specificity for the diagnosis of AHF, and this threshold was higher than in older women.
In patients with obesity, a threshold of 184 pg/mL had a good sensitivity and specificity for the diagnosis of AHF, and this threshold was lower than in patients without obesity.
For NT‐proBNP, in patients with AF, a threshold of 2332 pg/mL had a good sensitivity and specificity for the diagnosis of AHF and was higher than in patients without AF. In patients with eGFR < 30 mL/min, a threshold of 3474 pg/mL had a good sensitivity and specificity for the diagnosis of AHF, and this threshold was higher than in patients without kidney failure. In male patients, a threshold of 1800 pg/mL, had a good sensitivity and specificity for the diagnosis of AHF, and this threshold was higher than in women.
In patients with obesity, a threshold of 1375 pg/mL had had a good sensitivity and specificity for the diagnosis of AHF, and this threshold was lower than in patients without obesity.
Discussion
Although widely used in clinical practice, the age‐stratified BNP and NT‐proBNP rule‐in cutpoint strategy and the impact of co‐morbidities for diagnosis of AHF in patients over 75 year‐old has not been validated in a large, real life cohort. Our results provide contemporary information about BNP and NT‐proBNP cutpoints to help in the diagnosis and exclusion of AHF in a cohort of old patients hospitalized in geriatrics, cardiology and pulmonology departments for acute respiratory failure. The novelty of our study is that we analysed retrospectively the diagnostic performances of NP thresholds when accounting for age‐related subgroups, gender and patients with kidney failure, AF and obesity.
Our study encompasses a diverse cohort of older patients, with nearly equal representation across genders. The findings underscore the robust performance of BNP and NT‐proBNP in identifying acute HF, affirming the efficacy of an age‐stratified diagnostic approach. 12 , 13
The demographic profile of participants aligns with evolving trends in patients with acute HF and those with measurable BNP or NT‐proBNP levels, indicating a shift from previous evaluations conducted over a decade ago. 7 , 8 Notably, the prevalence of acute HF in our study, encompassing all‐comers with acute dyspnoea (2/3), surpasses recent reports, possibly due to the inclusion of patients with available natriuretic peptide data. 7 , 8
BNP and NT‐proBNP demonstrate excellent sensitivity and a favourable area under the ROC curve for diagnosing AHF, particularly when using age‐adjusted cut‐offs. Sensitivity and positive predictive value in our study outperform those reported in the ICON‐RELOADED Study, 13 reflecting the substantial prevalence of acute HF in our sample. However, our study highlights lower specificity and negative predictive value, prompting a preference for maximizing sensitivity, especially in older patients with frequent co‐morbidities. We adopt a rule‐in cut‐point strategy for BNP and NT‐proBNP, balancing accuracy and availability while prioritizing the best positive predictive value for our cut‐offs. Our results affirm the utility of age‐stratified cut‐offs, even in patients aged over 75, with concentrations exceeding 260 pg/mL for BNP or 1750 pg/mL for NT‐proBNP, providing significant diagnostic value.
The incorporation of age‐stratified approaches into clinical guidelines and textbooks globally underscores their relevance in contemporary practice. Emphasizing sensitivity in our diagnostic approach, particularly for rapid exclusion of AHF, aligns with the ease of diuretic treatment and the availability of reliable diagnostic tests. 1 The incorporation of age‐stratified approaches into clinical guidelines and textbooks globally underscores their relevance in contemporary practice. Emphasizing sensitivity in our diagnostic approach, particularly for rapid exclusion of AHF, aligns with the ease of diuretic treatment and the availability of reliable diagnostic tests.
Consistent cut‐offs and adherence to clinical guidelines are crucial to mitigate confusion surrounding diagnostic thresholds. Notably, the performance of BNP and NT‐proBNP in our study compares favourably with previous large‐scale trials, such as the BED Study, affirming their efficacy in diagnosing AHF. 11
Furthermore, our study reveals that age‐stratified cut‐offs for BNP and NT‐proBNP increase with age, demonstrating good sensitivity and positive predictive value even in patients over 85. This study marks a significant contribution by testing diagnostic cut‐offs in this demographic with robust statistical power, filling a crucial gap in current literature. 3 , 13
Analysis of pre‐specified subgroups with demographics or co‐morbidities that could potentially confound the performance of BNP or NT‐proBNP demonstrated that the diagnostic and performance of the test remained accurate in these patient subgroups, including abnormal renal function, obesity, and atrial fibrillation as previously showed. 13 , 17 , 18 , 19 , 20 , 21
In our study focusing on older patients, we observed adjustments to the BNP and NT‐proBNP cut‐off values based on individual co‐morbidities such as an eGFR < 30 mL/min, presence of atrial fibrillation, and male sex, while higher BMI corresponded to lower cut‐off values. This observation is noteworthy as no prior studies have specifically examined AHF diagnosis in older patients with atrial fibrillation or renal failure, aligning with existing research findings in younger cohorts.
Interestingly, we found that BNP and NT‐proBNP levels were higher in men compared to women, despite women being older and exhibiting a higher prevalence of HF. Further investigations are warranted to elucidate this discrepancy. 22
At the specified cut‐off points, BNP and NT‐proBNP demonstrated an odds ratio exceeding 10 for diagnosing HF, significantly outperforming traditional diagnostic variables derived from history, physical examination, or other laboratory tests, consistent with previous research. 12 , 13 This underscores the potency of BNP and NT‐proBNP as diagnostic biomarkers in older individuals burdened with multiple co‐morbidities.
Timely and accurate diagnosis of AHF in patients presenting with acute dyspnoea in the emergency department is crucial, given the association between delayed treatment and increased mortality. Swift and precise diagnosis not only reduces uncertainty but also holds the potential to enhance patient outcomes and curtail healthcare expenditures. Therefore, validating our study's diagnostic cut‐offs across diverse patient cohorts could facilitate early AHF diagnosis in emergency settings. 3 , 23 , 24 Additionally, our study revealed elevated CRP levels upon admission in AHF patients, potentially correlating with heightened short‐term cardiac and non‐cardiac mortalities. 25
This study contributes novel insights into the diagnostic performance of BNP/NT‐proBNP across various age groups and subgroups characterized by co‐morbidities known to influence NP levels, including atrial fibrillation, obesity, and renal disease. These findings provide compelling evidence for integrating NP cut‐offs into clinical practice.
Limitations
The major limitation of our retrospective study is that the diagnosis of AHF was done by ICD‐10 code by a physician that takes natriuretic peptide into account for the diagnosis of AHF, without the independent judgement of two or more clinicians at discharge. This study reliance on elevated natriuretic levels could inadvertently lead to misclassification as AHF. This potential bias poses a constraint on the diagnostic accuracy of natriuretic peptides. AHF is rarely the only diagnosis in older patients and the admitting physicians could have made a mistake when coding the principal ICD‐10 diagnosis. It is important to note that in our study, only a few clinical parameters of congestion were accessible in the software. With the ICD‐10 code, we only have the access at the information « atrial fibrillation », but we cannot precise if it was chronic or acute AF.
Conclusions
In conclusion, our study of over 12 000 older patients admitted for acute dyspnoea reveals distinct clinical profiles for Acute Heart Failure (AHF). Patients with AHF exhibit advanced age, higher prevalence of co‐morbidity, and distinct laboratory findings. Natriuretic peptide levels, particularly BNP and NT‐proBNP, are effective in diagnosing AHF, with optimal thresholds identified. Age‐dependent cut‐off analysis enhances diagnostic precision. Subgroup analyses highlight nuanced associations between biomarker thresholds and patient characteristics. Clinicians can utilize BNP and NT‐proBNP alongside clinical evaluation for improved AHF diagnosis. Tailored diagnostic approaches based on individual patient profiles are essential. Our findings contribute to enhancing diagnostic accuracy and optimizing treatment strategies for older patients with acute dyspnoea.
Funding
No specific funding was received for this work.
Conflict of interest
None declared.
Berthelot, E. , Bailly, M. T. , Lehova, X. C. , Rahmani, M. E. B. , Bounab, R. , Mewton, N. , Dobbs, J. E. , Mas, R. , Frank, M. , Lellouche, N. , Paclot, M. , and Jourdain, P. (2024) Setting the optimal threshold of NT‐proBNP and BNP for the diagnosis of heart failure in patients over 75 years. ESC Heart Failure, 11: 3232–3241. 10.1002/ehf2.14894.
Impact statement: We certify that this work is novel.
Data availability statement
Please contact Marion Paclot at marion.paclot@gmail.com if you need the raw data.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Mueller C, McDonald K, De Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart failure Association of the European Society of cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715‐731. doi: 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 3. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161‐167. doi: 10.1056/NEJMoa020233 [DOI] [PubMed] [Google Scholar]
- 4. Wong LL, Zou R, Zhou L, Lim JY, Phua DCY, Liu C, et al. Combining circulating MicroRNA and NT‐proBNP to detect and categorize heart failure subtypes. J Am Coll Cardiol 2019;73:1300‐1313. doi: 10.1016/j.jacc.2018.11.060 [DOI] [PubMed] [Google Scholar]
- 5. Krauser DG, Lloyd‐Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a proBNP investigation of dyspnea in the emergency department (PRIDE) substudy. Am Heart J 2005;149:744‐750. doi: 10.1016/j.ahj.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 6. Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Sørensen HT. Thirty‐year trends in heart failure hospitalization and mortality rates and the prognostic impact of co‐morbidity: a Danish nationwide cohort study: heart failure hospitalization, outcome, and co‐morbidity. Eur J Heart Fail 2016;18:490‐499. doi: 10.1002/ejhf.486 [DOI] [PubMed] [Google Scholar]
- 7. Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, et al. Age‐specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation 2017;135:1214‐1223. doi: 10.1161/CIRCULATIONAHA.116.02594 [DOI] [PubMed] [Google Scholar]
- 8. Baggish AL, van Kimmenade RRJ, Januzzi JL. The differential diagnosis of an elevated amino‐terminal pro–B‐type natriuretic peptide level. Am J Cardiol 2008;101:S43‐S48. doi: 10.1016/j.amjcard.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 9. Tsutsui H, Albert NM, Coats AJS, Anker SD, Bayes‐Genis A, Butler J, et al. Natriuretic peptides: role in the diagnosis and management of heart failure: a scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur J Heart Fail 2023;ejhf.2848. doi: 10.1002/ejhf.2848 [DOI] [PubMed] [Google Scholar]
- 10. Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient‐level, pooled meta‐analysis of DAPA‐HF and DELIVER. Nat Med 2022;28:1956‐1964. doi: 10.1038/s41591-022-01971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plichart M, Orvoën G, Jourdain P, Quinquis L, Coste J, Escande M, et al. Brain natriuretic peptide usefulness in very elderly dyspnoeic patients: the BED study: BNP in very elderly dyspnoeic patients. Eur J Heart Fail 2017;19:540‐548. doi: 10.1002/ejhf.699 [DOI] [PubMed] [Google Scholar]
- 12. Booth RA, Hill SA, Don‐Wauchope A, Santaguida PL, Oremus M, McKelvie R, et al. Performance of BNP and NT‐proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev 2014;19:439‐451. doi: 10.1007/s10741-014-9445-8 [DOI] [PubMed] [Google Scholar]
- 13. Januzzi JL, Chen‐Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, et al. N‐terminal pro–B‐type natriuretic peptide in the emergency department. J Am Coll Cardiol 2018;71:1191‐1200. doi: 10.1016/j.jacc.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 14. Januzzi JL, Van Kimmenade R, Lainchbury J, Bayes‐Genis A, Ordonez‐Llanos J, Santalo‐Bel M, et al. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients. Eur Heart J 2006;27:330‐337. doi: 10.1093/eurheartj/ehi631 [DOI] [PubMed] [Google Scholar]
- 15. Januzzi JL, Rehman SU, Mohammed AA, Bhardwaj A, Barajas L, Barajas J, et al. Use of amino‐terminal pro–B‐type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011;58:1881‐1889. doi: 10.1016/j.jacc.2011.03.072 [DOI] [PubMed] [Google Scholar]
- 16. Breidthardt T, Van Doorn WPTM, Van Der Linden N, Diebold M, Wussler D, Danier I, et al. Diurnal variations in natriuretic peptide levels: clinical implications for the diagnosis of acute heart failure. Circ Heart Fail 2022. [cité 25 mars 2024];15(6):e009165. Disponible sur: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.121.009165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rørth R, Jhund PS, Yilmaz MB, Kristensen SL, Welsh P, Desai AS, et al. Comparison of BNP and NT‐proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail 2020;13:e006541. doi: 10.1161/CIRCHEARTFAILURE.119.006541 [DOI] [PubMed] [Google Scholar]
- 18. Morello A, Lloyd‐Jones DM, Chae CU, van Kimmenade RRJ, Chen AC, Baggish AL, et al. Association of atrial fibrillation and amino‐terminal pro–brain natriuretic peptide concentrations in dyspneic subjects with and without acute heart failure: results from the proBNP investigation of dyspnea in the emergency department (PRIDE) study. Am Heart J 2007;153:90‐97. doi: 10.1016/j.ahj.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 19. Anwaruddin S, Lloyd‐Jones DM, Baggish A, Chen A, Krauser D, Tung R, et al. Renal function, congestive heart failure, and amino‐terminal pro‐brain natriuretic peptide measurement. J Am Coll Cardiol 2006;47:91‐97. doi: 10.1016/j.jacc.2005.08.051 [DOI] [PubMed] [Google Scholar]
- 20. Bayes‐Genis A. Effect of body mass index on diagnostic and prognostic usefulness of amino‐terminal pro–brain natriuretic peptide in patients with acute dyspnea. Arch Intern Med 2007;167:400. doi: 10.1001/archinte.167.4.400 [DOI] [PubMed] [Google Scholar]
- 21. Letsas KP, Filippatos GS, Pappas LK, Mihas CC, Markou V, Alexanian IP, et al. Determinants of plasma NT‐pro‐BNP levels in patients with atrial fibrillation and preserved left ventricular ejection fraction. Clin Res Cardiol 2009;98:101‐106. doi: 10.1007/s00392-008-0728-8 [DOI] [PubMed] [Google Scholar]
- 22. Hildebrandt P, Collinson PO, Doughty RN, Fuat A, Gaze DC, Gustafsson F, et al. Age‐dependent values of N‐terminal pro‐B‐type natriuretic peptide are superior to a single cut‐point for ruling out suspected systolic dysfunction in primary care†. Eur Heart J 2010;31:1881‐1889. doi: 10.1093/eurheartj/ehq163 [DOI] [PubMed] [Google Scholar]
- 23. Meijers WC, Bayes‐Genis A, Mebazaa A, Bauersachs J, Cleland JGF, Coats AJS, et al. Circulating heart failure biomarkers beyond natriuretic peptides: review from the biomarker study Group of the Heart Failure Association (hfa), European Society of Cardiology (esc). Eur J Heart Fail 2021;23:1610‐1632. doi: 10.1002/ejhf.2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCullough PA, Nowak RM, McCord J, Hollander JE, Herrmann HC, Steg PG, et al. B‐type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from breathing not properly (BNP) multinational study. Circulation 2002;106:416‐422. doi: 10.1161/01.cir.0000025242.79963.4c [DOI] [PubMed] [Google Scholar]
- 25. Minami Y, Kajimoto K, Sato N, Hagiwara N, Takano T, on behalf of the ATTEND Study Investigators . C‐reactive protein level on admission and time to and cause of death in patients hospitalized for acute heart failure. Eur Heart J ‐ Qual Care Clin Outcomes 2016;qcw054. doi: 10.1093/ehjqcco/qcw054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact Marion Paclot at marion.paclot@gmail.com if you need the raw data.


