Abstract
Aims
Anthracycline chemotherapy (AC) for breast cancer can cause cancer therapy‐related cardiac dysfunction (CTRCD) with resultant heart failure, traditionally defined as a reduction in left ventricular (LV) ejection fraction on echocardiography. In recent years, global longitudinal systolic strain (GLS) has been used to identify subclinical cardiac dysfunction prior to development of overt CTRCD. Recent harmonized guidelines have incorporated GLS into definitions for CTRCD to identify cardiac dysfunction and inform decisions regarding cardioprotective strategies.
Methods and results
We evaluated subclinical dysfunction in breast cancer patients treated with AC and determined the echocardiographic and patient factors associated with significant GLS changes. One hundred fourteen HER2 negative patients treated with AC were prospectively recruited and underwent serial echocardiograms (LVEF and LVGLS) at three time points (prior to AC, 3 months, and 1 year). CTRCD was defined as an asymptomatic reduction in LVEF of 10% or symptomatic drop of 5% to LVEF <53%. Subclinical LV dysfunction was defined as a reduction of ≥10% in GLS compared with baseline, recognizing that this cut off identified an ‘at risk cohort’ rather than patients with established CTRCD. No participant demonstrated CTRCD by reduction in LVEF. Forty‐three patients (38%) demonstrated a ≥10% relative reduction in GLS at 12 months; 20/43 (47%) had a reduced absolute GLS to <16%, and were older, had hypertension, increased LV mass, lower baseline e′ velocity and GLS. GLS ≥20.5% at baseline yielded a sensitivity of 79% and specificity of 87% for a normal GLS (i.e., ≥16%) at 1 year despite a ≥10% reduction from baseline.
Conclusions
We present a stepwise evaluation for subclinical LV dysfunction using both a relative reduction in GLS combined with an absolute reduction in GLS. We believe our findings may re‐stratify patients with a high baseline GLS into a lower risk group despite transient relative GLS decrements ≥10%.
Keywords: Anthtracycline chemotherapy, Breast cancer, Global longitudinal systolic strain, Transthoracic echocardiography
Introduction
With early cancer detection and the development of newer therapies, cancer survivorship has significantly improved over the last decade. 1 , 2 Cardiotoxicity has demonstrated increased prevalence with improved survivorship in patients with cancer. 3 Anthracycline chemotherapy (AC) is an essential therapy to treat various cancers including breast cancer and has contributed to improved long‐term patient survival. 4 , 5 The effectiveness of AC is balanced against a significant risk of cancer therapy‐related cardiac dysfunction (CTRCD)and future development of heart failure. 6 There have been varying definitions for CTRCD with previous expert consensus statement from the European association of cardiovascular imaging (EACVI) defining CTRCD as any reduction in left ventricular ejection fraction (LVEF) to <50% or a reduction of 10% to <53%. 7 The American Society of Echocardiography (ASE) expert consensus statement defined cardiotoxicity as a symptomatic reduction in LVEF >5% or an asymptomatic reduction in LVEF >10% to <53%. 8 The most contemporary guidelines have described mild asymptomatic CTRCD as a new relative decline in global longitudinal systolic strain (GLS) of ≥15% from baseline. 9 The CTRCD threshold for GLS was chosen in an effort to optimize specificity for interpreting relative GLS changes, with prior data reinforcing that a reduction in GLS between −8% and −15% may still identify patients at risk for CTRCD. 8 , 10
Transthoracic echocardiography (TTE) is widely used to assess cardiac function in breast cancer patients, although it has been historically under‐utilized. 11 TTE is recommended at baseline prior to AC 12 and during therapy, based on expert opinion. 4 , 10 TTE derived LVEF remains an important metric in assessing cardiac function, although an overt reduction in LVEF represents a late pathophysiological process whereby cardiac dysfunction may be permanent with resultant heart failure. 13 Speckle tracking strain imaging has utilized GLS to identify subclinical cardiac dysfunction prior to development of clinically meaningful cardiac injury, expressed as a reduction in LVEF. 7 , 13 Identifying subclinical cardiac dysfunction can inform decisions regarding cancer treatment including whether to avoid, interrupt or cease therapies and has demonstrated improved cardiac outcomes. 11
The incidence of cardiac dysfunction and consequent heart failure with AC is well recognized, and is often increased with other concomitant cardiotoxic therapies (eg trastuzumab). 12 , 14 A meta‐analysis of previous studies using GLS in the setting of chemotherapy has suggested the need for prospective studies with more clearly defined patient groups. 10 This prospective study characterizes echocardiographic changes in LVEF and GLS in a clearly defined group of HER2 negative breast cancer patients who received only AC therapy. We evaluated patient demographic, clinical and echocardiographic characteristics at baseline prior to AC therapy that could be associated with subclinical LV dysfunction over 12 months.
Methods
Study population
This is prospective cohort study that recruited patients from multiple sites within the Western Sydney Local Health District. Serial transthoracic echocardiograms were performed at a single centre, at baseline, and at 3 and 12 months after AC. A total of 134 patients with histologically confirmed HER2 negative breast cancer were prospectively recruited. Anthracycline chemotherapy (doxorubicin or epirubicin) was administered (4 to 6 cycles) as per current protocols, determined by the treating oncologist. All patients underwent detailed baseline clinical evaluation; patients were not offered enrolment if there was significant valvular disease, a history of arrhythmia, prior cardiomyopathy, a history of coronary artery disease or revascularization, previous radiation or chemotherapy, previous cardiac surgery or implanted cardiac devices. The study ethics approval was obtained from the Human Research Ethics Committee of Western Sydney Local Health District.
Recruited participants underwent serial comprehensive transthoracic echocardiogram (TTE) with 2‐dimensional, colour and Doppler images obtained from parasternal, apical, and subcostal views. Figure 1 outlines the patient longitudinal follow up timeline during the study. Baseline echocardiograms (T1) were performed at least 1 week prior to the first cycle of AC chemotherapy. Fourteen patients were excluded at T1 due to inadequate image quality for offline strain analysis, often related to recent breast surgery. Serial echocardiograms were performed within a fortnight of anthracycline treatment completion (~3 months from baseline) (T2) and 12 months from baseline (T3). Four patients were excluded due to inadequate image quality at T2, with a further 2 excluded at T3. The remaining 114 patients with interpretable TTEs at all 3 time points were included in the analysis.
Figure 1.
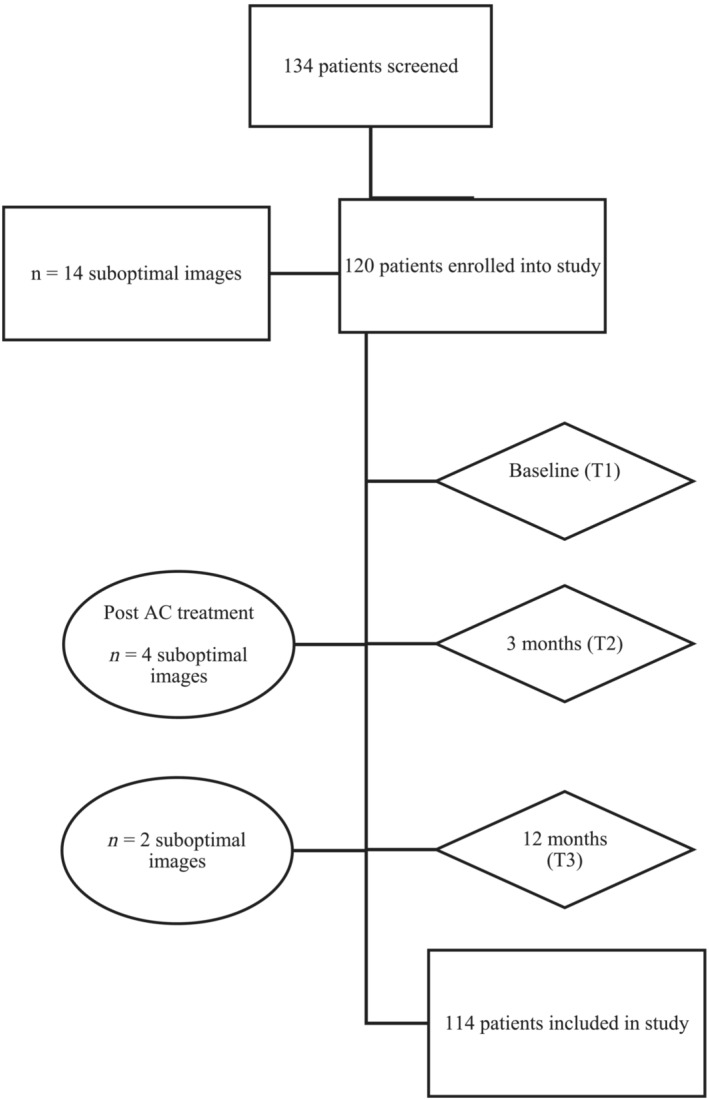
Timeline for longitudinal follow up. Flow chart of timepoints of serial echocardiograms, prior to (T1), at 3 (T2), and 12 months (T3). Patients were excluded if suboptimal image quality. AC, anthracycline.
Two‐dimensional echocardiography
Echocardiographic examinations were performed using commercially available ultrasound machines (Vivid E9 and Vivid 7, General Electric Healthcare, Horton, Norway). LV volumes were measured from apical 4‐ and 2‐chamber views utilizing the Simpson's modified biplane method of disks and LVEF was calculated. 15 Mitral inflow velocities to assess LV diastolic filling, were obtained with the pulsed‐wave Doppler sample volume placed at the mitral leaflet tips. Pulsed wave Doppler tissue imaging was used to measure the septal and lateral peak velocities in systole (s′) and early diastole (e′) with sample volume placed at the septal and lateral mitral annulus, and mean annular velocities were calculated. GLS was measured offline (EchoPac version 203, General Electric‐Vingmed) from the three apical LV focused views acquired at high frame rate (>60 fps). The endocardial border of the left ventricle was traced at end‐systole and the region of interest was set to include the LV myocardium, with the software automatically subdividing each LV wall into three regions (basal, mid, and apical). Peak systolic strain was measured as the peak negative strain during systole. Longitudinal strain rate in systole (S‐Sr), early diastole (E‐Sr), and late diastole (A‐Sr) were also measured. GLS was calculated as the average of the 18 segments from the 4‐, 2‐, and 3‐chamber views. If two or more segments had uninterpretable longitudinal strain, it was excluded from analysis. GLS was collected from all three layers (endocardial, mid‐myocardial, and epicardial). If not otherwise stated, GLS measurements represent mid‐myocardial strain. Although LV GLS is a ‘negative’ value to represent myocardial shortening, for simplicity, the absolute values of GLS are reported in the results.
We defined CTRCD as a symptomatic reduction in LVEF of >5% or an asymptomatic reduction in LVEF of >10% from baseline to a value of ≤53%. 8 We evaluated GLS to identify subclinical cardiac dysfunction (rather than GLS CTRCD) and defined this as a relative reduction in GLS ≥10% from baseline, as previously reported. 16 , 17 There were 43 patients with ≥10% reduction in GLS at T3. We further stratified this group with subclinical dysfunction based on patients with an absolute value of GLS <16% or GLS ≥16% at T3 (Figure 2 ). 18 Hence, three patient subgroups were identified at T3: Group 1 (n = 15): ≥10% reduction in GLS compared with baseline, with GLS <16% at T3, Group 2 (n = 28): ≥10% reduction in GLS compared with baseline, with GLS ≥16% at T3, Group 3 (n = 71): <10% reduction in GLS compared with baseline.
Figure 2.
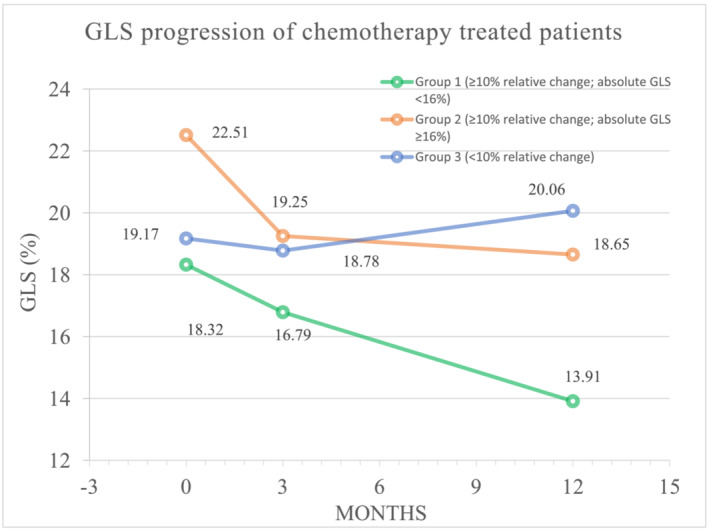
Timeline of GLS alteration in the three groups. Line graph of alterations in LV GLS between groups prior to and at 3 and 12 months post‐AC treatment. AC, anthracycline; GLS, global longitudinal strain; LV, left ventricular.
Inter‐observer variability for LVEF and LV GLS was performed by 2 independent operators blinded to measurements in 20 randomly selected patients. Inter‐observer variability was performed by the same operator in the same 20 patients at least 4 weeks after the initial measurements.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 26 (SPSS, Chicago, IL, USA), and differences were considered significant if P < 0.05. Continuous data are expressed as mean ± standard deviation and categorical variables as a number (percentage). Baseline and follow‐up data were compared by repeated measures analysis of variance (ANOVA) with least significant difference (LSD) post‐hoc testing. Independent t‐test was used to compare continuous variables in subgroup analysis. Chi‐square test was used for analysis of categorical variables.
Logistic regression analysis was performed to identify univariate predictors for patients in Group 1 (i.e., individuals that would have a ≥10% relative reduction to a GLS <16% at T3). Significant univariate predictors were entered into a multivariable logistic regression model and examined by backwards binary multivariate regression analysis to determine independent predictors specifically for patients in group 1.
Inter‐observer and intra‐observer variability were assessed for LV GLS. Measurements were done using 20 randomly selected subjects. Intra‐class correlation coefficients (ICC) using a two‐way random effects model was used to assess variability. Values between 0.75 and 0.9 represented good reliability and values ≥0.90 excellent reliability. 19
Results
A 114 prospectively recruited breast cancer patients underwent serial echocardiograms over 12 months. Cardiovascular risk factors and cardioactive medications are listed in Table 1 and Table S1 . Eighty‐six patients received radiation with 44 receiving left sided radiation. Thirty‐eight patients (33%) had more than one cardiovascular risk factor. No participant reported any symptoms or had any signs of cardiac failure during the entirety of the 12 month follow up. Eighty‐eight (77%) patients received doxorubicin (cumulative dose 343.6 ± 107.8 mg/m2), and 26 (23%) patients received epirubicin (cumulative dose 461.7 ± 142.2 mg/m2).
Table 1.
Echo parameters of cohort T1–T3
| T1 | T2 | T3 | ANOVA (P value) | |
|---|---|---|---|---|
| Time to follow‐up (days) | N/A | 97 ± 23 | 372 ± 33 | N/A |
| Height (cm) | 160.1 ± 6.1 | N/A | N/A | N/A |
| Weight (kg) | 74.7 ± 15.8 | 74.4 ± 15.7 | 73.7 ± 15.4 | 0.177 |
| BSA (m2) | 1.77 ± 0.17 | 1.77 ± 0.17 | 1.77 ± 0.18 | 0.501 |
| BMI (kg/m2) | 29.17 ± 6.06 | 29.00 ± 5.99 | 28.58 ± 5.80 | 0.082 |
| Heart rate (b.p.m.) | 71.7 ± 11.5 | 77.5 ± 11.7 a | 69.3 ± 9.9 a , b | <0.001 |
| Systolic BP (mmHg) | 118.0 ± 11.3 | 119.1 ± 12.9 | 120.9 ± 20.0 | 0.606 |
| Diastolic BP (mmHg) | 73.9 ± 8.7 | 74.7 ± 8.0 | 72.8 ± 8.6 | 0.478 |
| LV mass (g/m2) | 75.73 ± 15.74 | 76.57 ± 15.06 | 75.36 ± 13.99 | 0.470 |
| LVEDV (mL/m2) | 56.74 ± 10.31 | 57.21 ± 11.05 | 62.97 ± 11.48 a , b | <0.001 |
| LVESV (mL/m2) | 22.31 ± 4.03 | 22.82 ± 4.27 | 24.81 ± 5.27 a , b | <0.001 |
| Stroke volume (mL/m2) | 34.43 ± 6.61 | 34.39 ± 7.11 | 38.15 ± 6.62 a , b | <0.001 |
| LVEF (%) | 60.71 ± 2.39 | 60.01 ± 2.28 a | 60.61 ± 2.08 b | 0.008 |
| Mean s′ (cm/s) | 7.98 ± 1.69 | 7.82 ± 1.39 | 7.20 ± 1.16 a , b | <0.001 |
| E velocity (cm/s) | 69.55 ± 13.24 | 63.56 ± 12.54 a | 64.06 ± 12.79 a | <0.001 |
| A velocity (cm/s) | 67.75 ± 16.16 | 68.01 ± 16.07 | 65.65 ± 14.56 a | 0.057 |
| Mean e′ (cm/s) | 9.04 ± 3.98 | 8.06 ± 1.95 a | 7.55 ± 1.89 a , b | <0.001 |
| E/e′ | 8.44 ± 2.28 | 8.09 ± 2.46 | 8.92 ± 2.27 a , b | <0.001 |
| GLSendo (%) | 22.19 ± 3.31 | 20.81 ± 3.55 a | 21.08 ± 3.52 a | <0.001 |
| GLSmyo (%) | 19.88 ± 2.92 | 18.63 ± 3.20 a | 18.91 ± 3.14 a | <0.001 |
| GLSepi (%) | 17.94 ± 2.60 | 16.83 ± 2.89 a | 17.09 ± 2.77 a | <0.001 |
| S‐Sr (1/s) | 1.11 ± 0.21 | 1.08 ± 0.20 a | 1.02 ± 0.18 a , b | <0.001 |
| E‐Sr (1/s) | 1.33 ± 0.37 | 1.16 ± 0.34 a | 1.18 ± 0.32 a | <0.001 |
| A‐Sr (1/s) | 0.84 ± 0.23 | 0.86 ± 0.24 | 0.86 ± 0.23 | 0.232 |
Mean ± standard deviation for continuous variables.
BMI, body mass index; BP, blood pressure; BSA, body surface area; GLS, global longitudinal strain; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; Sr, strain rate.
P < 0.05 when compared with T1.
P < 0.05 when compared with T2.
Clinical and echocardiographic parameters at each of the three visits are outlined in Table 2 . The mean follow‐up time at T2 and T3 was 92 and 372 days, respectively. Heart rate was significantly higher at T2, and then decreased at T3. Left ventricular end diastolic and end systolic volumes were significantly increased at T3 compared with T1 and T2. LVEF at T2 was significantly reduced, although within the normal clinical range. No patient developed CTRCD throughout the entirety of the study period (defined by alteration in LVEF). 8 Average s′ was significantly reduced at T3 compared with T1 and T2. Similarly, GLS at T2 and T3 were significantly reduced compared with T1, with this being reflected in all three myocardial layers. S‐Sr was significantly decreased at T2, and persisted at T3. Peak E, average e′, and E‐Sr were significantly reduced at T2 and T3 compared with T1, while E/e′ only showed a significant difference between T2 and T3 (Table 2 ).
Table 2.
Baseline parameter comparisons between groups
| Echo parameters at T1 | ||||
|---|---|---|---|---|
| Group 1 (n = 15) | Group 2 (n = 28) | Group 3 (n = 71) | P value | |
| Age (years) | 60.22 ± 9.72 | 50.65 ± 9.61 a | 55.71 ± 8.68 | 0.005 |
| Height (cm) | 158.73 ± 5.66 | 160.07 ± 6.57 | 160.30 ± 6.10 | 0.663 |
| Weight (kg) | 76.97 ± 17.48 | 72.48 ± 17.48 | 74.88 ± 14.70 | 0.716 |
| BSA (m2) | 1.78 ± 0.17 | 1.75 ± 0.21 | 1.78 ± 0.16 | 0.782 |
| BMI (kg/m2) | 30.64 ± 7.00 | 28.22 ± 6.40 | 29.19 ± 5.73 | 0.495 |
| Heart rate (b.p.m.) | 71.00 ± 15.03 | 75.18 ± 10.80 | 70.44 ± 10.70 | 0.153 |
| Systolic BP (mmHg) | 129.53 ± 10.29 | 119.41 ± 12.47 a | 122.12 ± 11.21 | 0.021 |
| Diastolic BP (mmHg) | 81.53 ± 4.26 | 74.85 ± 7.09 a | 76.76 ± 8.94 | 0.031 |
| Hypertension | 9 (60.0) | 5 (17.9) a | 20 (28.2) a | 0.014 |
| Diabetes | 2 (13.3) | 1 (3.5) | 3 (4.2) | 0.321 |
| Hypercholesterolaemia | 7 (46.7) | 4 (14.3) a | 13 (18.3) a | 0.046 |
| LV mass (g/m2) | 83.84 ± 20.36 | 69.48 ± 13.71 a | 76.17 ± 14.66 | 0.019 |
| LVEDV (mL/m2) | 59.90 ± 10.25 | 55.23 ± 8.40 | 56.60 ± 11.04 | 0.393 |
| LVESV (mL/m2) | 23.69 ± 3.84 | 21.64 ± 3.35 | 22.27 ± 4.29 | 0.289 |
| LV stroke volume (mL/m2) | 36.21 ± 6.81 | 33.59 ± 5.50 | 34.33 ± 7.05 | 0.502 |
| LVEF (%) | 60.33 ± 2.53 | 60.93 ± 2.48 | 60.63 ± 2.33 | 0.549 |
| Mean s′ (cm/s) | 6.99 ± 2.39 | 8.46 ± 1.68 a | 8.10 ± 1.4 | 0.039 |
| E velocity (cm/s) | 66.22 ± 8.64 | 72.91 ± 12.60 | 6.88 ± 1.41 | 0.203 |
| A velocity (cm/s) | 74.82 ± 17.20 | 65.79 ± 14.93 | 6.63 ± 1.60 | 0.180 |
| Mean e′ (cm/s) | 7.48 ± 1.96 | 9.46 ± 2.50 | 9.20 ± 4.60 | 0.288 |
| E/e′ | 9.57 ± 2.85 | 8.07 ± 1.89 | 8.25 ± 2.13 | 0.126 |
| GLSendo (%) | 20.48 ± 1.91 | 25.15 ± 2.26 a | 21.33 ± 3.16 b | <0.001 |
| GLSmyo (%) | 18.32 ± 1.72 | 22.51 ± 1.97 a | 19.13 ± 2.78 b | <0.001 |
| GLSepi (%) | 16.54 ± 1.57 | 20.27 ± 1.80 a | 17.29 ± 2.47 b | <0.001 |
| S‐Sr (1/s) | 1.01 ± 0.19 | 1.24 ± 0.19 a | 1.07 ± 0.21 b | <0.001 |
| E‐Sr (1/s) | 1.04 ± 0.30 | 1.62 ± 0.37 a | 1.28 ± 0.33 a , b | <0.001 |
| A‐Sr (1/s) | 0.81 ± 0.27 | 0.91 ± 0.22 | 0.81 ± 0.22 | 0.082 |
Mean ± standard deviation for continuous variables.
BMI, body mass index; BP, blood pressure; BSA, body surface area; GLS, global longitudinal strain; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; Sr, strain rate.
P < 0.05 when versus group 1.
P < 0.05 when versus group 2.
We identified patients with subclinical CTRCD defined as a ≥10% decrease in GLS at T3 compared with baseline (n = 43), and further stratified this group into those with an absolute reduction in GLS <16% (Group 1; n = 15) versus those with a preserved absolute GLS ≥16% (Group 2; n = 28). Group 3 comprised patients who experienced <10% decrease in GLS over the course of the study (n = 71). Figure 2 presents the GLS at the three time points for each of these groups. Table S1 demonstrates the number of patients with GLS <16% versus GLS ≥16% in all 114 patients (with and without a relative change ≥10%) at T3. Of the patients with GLS <16% at 12 months, 79% had a ≥10% relative reduction in GLS compared with baseline. A total of 43 (37.7%) patients exhibited a ≥10% relative reduction in GLS between T1 and T3, with only 15 (34.9%) of this group having a GLS <16% at T3. Group 3 (n = 71) had a normal GLS throughout the census period without a ≥10% relative change but did appear to have a trend towards a GLS recovery between 3 and 12 months.
Figure 3 presents box plots of the GLS measurements between groups 1 and 2 at 3 time points. GLS for group 1 patients was significantly lower at all time points compared with group 2 (Figure 3 ). Group 1 exhibited significant reductions at T3 compared with T1 (P < 0.001) and T2 compared with T3 (P = 0.002), with no reduction between T1 and T2 (P = 0.106). Group 2 exhibited significant reductions at T2 and T3 compared with T1 (P < 0.001 for both), with no reduction in GLS between T2 and T3.
Figure 3.
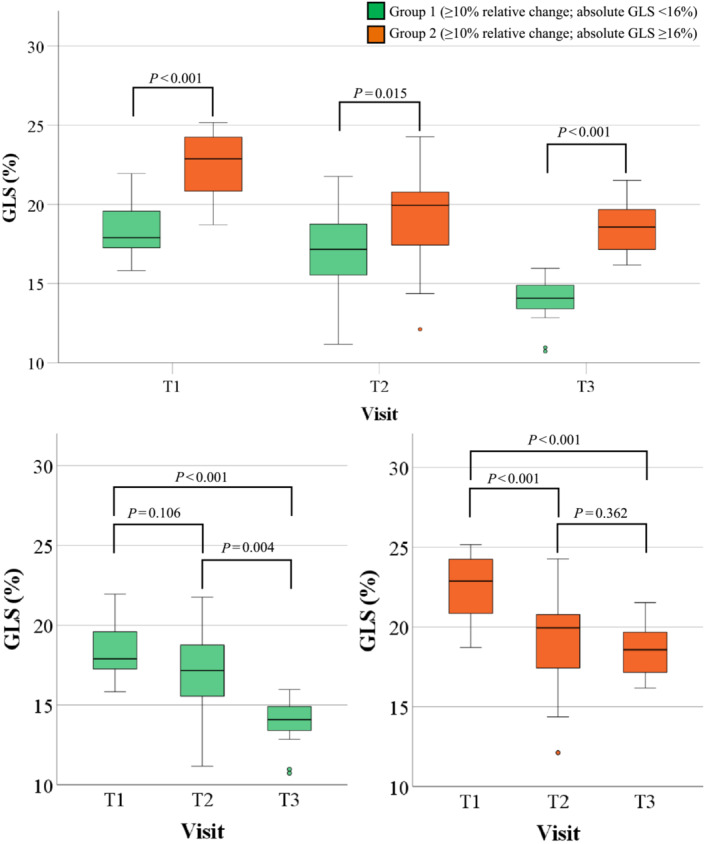
Comparison between the 2 groups at risk of LV subclinical dysfunction. Boxplots of groups with ≥10% relative decrease in GLS at 12 months compared with baseline, stratified by an absolute GLS value of 16%. AC, anthracycline; GLS, global longitudinal strain; LV, left ventricular; T1, prior to AC treatment; T2, post‐AC; T3, 12 months post‐T1.
Table 2 compares baseline characteristics and echocardiographic parameters between groups 1 and 2. Group 1 patients were older (P = 0.024), with a higher prevalence of hypertension (P = 0.004), higher diastolic blood pressure and greater LV mass (P = 0.009 and P = 0.012, respectively). LV volumes were similar at T1; however, LVEF was significantly higher in group 2 at baseline (P = 0.012), albeit still within normal clinical range. GLS in all myocardial layers was significantly lower in group 1 compared with group 2 (P < 0.001).
Univariate clinical and echocardiographic predictors for patients in group 1 were age, presence of hypertension, hypercholesterolaemia, LV mass, average e′ velocity and GLS. Backwards stepwise binary multivariate regression analysis (Table 3 ) was performed using nested analysis for demographic and clinical parameters (model 1), and for echocardiographic parameters (model 2), given the relatively small number of patients in group 1. In model 1, age, hypertension, and hypercholesterolaemia all showed modest univariate correlation to group 1 (P = 0.003, P = 0.004 and P = 0.020, respectively). Backwards binary regression analysis identified age as the only significant parameter, with an odds ratio of 0.905 (P = 0.009). In model 2, LV mass, average e′ and LV GLS all demonstrated univariate correlation to group 1 (P = 0.009, P = 0.013 and P < 0.001, respectively). Backwards binary regression analysis identified only GLS as being significant, with an odds ratio of 0.354 (P = 0.001). A final analysis (model 3) was performed using the independent predictors for group 1 from models 1 and 2 (i.e., age and GLS). This demonstrated that only baseline GLS was a significant predictor of patients in group 1, with an odds ratio of 0.897 (P = 0.002).
Table 3.
Univariant and multivariant backward binary regression analysis of baseline parameters against designation to group 1 in at‐risk groups
| Univariant | Multivariant | |||
|---|---|---|---|---|
| Parameters | R | P value | OR (CI) | P value |
| Nested model 1: clinical variables | ||||
| Age | −0.436 | 0.003 | 0.905 (0.840–0.975 | 0.009 |
| Hypertension | −0.429 | 0.004 | N/A | N/A |
| Hypercholesterolaemia | −0.354 | 0.020 | N/A | N/A |
| Nested model 2: echocardiographic variables | ||||
| LV mass | −0.395 | 0.009 | N/A | N/A |
| Average e′ | −0.380 | 0.013 | N/A | N/A |
| GLS | −0.734 | <0.001 | 0.354 (0.190–0.660 | 0.001 |
| Nested model 3: combined variables | ||||
| Age | 0.335 (0.167–0.671) | 0.091 | ||
| GLS | 0.897 (0.790–1.018) | 0.002 | ||
BP, blood pressure; CI, confidence interval; GLS, global longitudinal strain; LV, left ventricular; OR, odds ratio.
We further examined the baseline GLS value that would identify patients in group 1 versus group 2. A cut‐off value for GLS was established by calculating the mean minus 1 standard deviation of the GLS at T1 for group 2 patients (GLS of 20.5%). Using this cut‐off GLS of 20.5%, the sensitivity and specificity to identify patients in group 2 (≥10% reduction in GLS to ≥16%) from group 1 at timepoint T3 was 79% and 87%, respectively (Table S2 ).
Regarding intra‐observer variability, the ICC for LV GLS was 0.989 (0.973–0.996). The inter‐observer variability for LV GLS was 0.990 (0.976–0.996). The results here demonstrate excellent reproducibility for LV GLS.
Discussion
We present one of the largest prospective ‘anthracycline only’ breast cancer patient groups with comprehensive TTE assessment before, immediately after, and at 1 year following chemotherapy.
The key findings of our study are as follows (Figure 4 ):
43/114 (38%) patients demonstrated subclinical cardiac dysfunction with a ≥10% reduction in LV GLS at 12 months after AC therapy.
Of the 43 patients with ≥10% relative reduction in GLS compared with baseline, 15/43 (34.9%) had a reduction with an absolute GLS of <16%.
Baseline clinical and echocardiographic characteristics associated with ≥10% GLS reduction with absolute GLS <16% (group 1), were older age, hypertension, increased LV mass, lower e′ velocity and GLS prior to AC therapy.
A baseline GLS ≥20.5% had a sensitivity and specificity of 79% and 87% respectively, to maintain LV GLS at ‘normal’ levels (≥16%), despite ≥10% relative reduction from baseline GLS.
Figure 4.
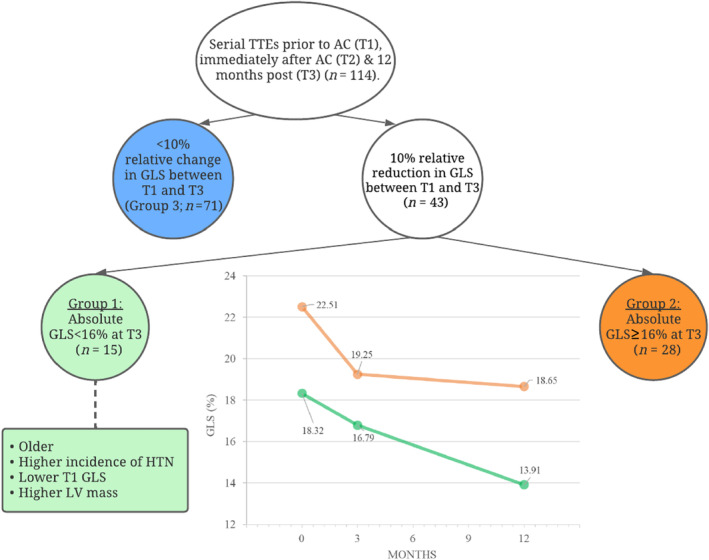
Subclinical LV dysfunction in breast cancer patients 12 months post‐AC. Relative and absolute LV GLS were used to identify patients developing subclinical LV dysfunction at 12 months. The stepwise process included (1) relative decrease in LV GLS ≥10%. (2) Absolute LV GLS <16%. In this cohort, patients who exhibited ≥10% relative decrease in GLS to an absolute GLS <16% were older, had a higher incidence of hypertension, a lower T1 LV GLS and higher LV mass compared with those with a GLS ≥16%. AC, anthracycline; GLS, global longitudinal strain; LV, Left ventricular; TTE, transthoracic echocardiogram.
The study design included a strict enrolment protocol ensuring that recruited patients had no known cardiac pathology and with only one third of patients having more than one cardiac risk factor. Based on a recently developed cardiac risk assessment tool applicable to patients on AC, there was only 1 high risk patient in group 1 (6.7%) and group 2 (3.6%), while group 3 comprised 3 high risk patients (4.2%). There were 8 medium risk patients in group 1 (53.3%) and 6 medium risk patients in group 2 (21.4%) while group 3 included 22 medium risk patients (31%)—the remainder of the patients were all low risk (Table S3 ). 20 While the percentage of medium risk patients varies among the groups a Pearson Chi‐square analysis was not significant. Our study was free of patients developing CTRCD defined by LVEF, which may reflect the small percentage of high risk patients in the study, improved contemporary cancer regimens as well as a medium term follow up of 12 months. Group 3 (n = 71) did not experience a relative GLS change of 10% and remained within the normal GLS range throughout therapy although there was a subtle downward trend of GLS between baseline and 3 months and a recovery of GLS between months 3 and 12 (Figure 2 ) suggesting that small initial relative GLS changes during AC are unlikely to correspond with significant change at 1 year. While no patients experienced overt CTRCD, 38% of our cohort experienced subclinical cardiac dysfunction with a reduction in GLS ≥10% over 1 year. Among patients who experienced a ≥10% drop in GLS, a pre‐treatment GLS ≥20.5% was protective against an absolute reduction in GLS (i.e., <16%) at 1 year. Our findings suggest the potential for a stepwise GLS guided model, whereby patients with a relative reduction in GLS of >10% could be re‐stratified as lower risk individuals based on a pre‐treatment absolute GLS >20.5%. Such an approach once validated may enable fidelity to chemotherapeutic regimens and inform decisions regarding cardioprotective strategies. Relative changes in GLS changes can also provoke anxiety among patients and indecision among clinicians and once validated a high baseline GLS cut‐off may serve to alleviate concerns.
TTE is the primary imaging modality used to determine CTRCD with AC therapy where even a mildly abnormal pre‐treatment LVEF (i.e., 50–54%) has been identified as a significant risk factor for developing heart failure. 7 While AC‐related cardiotoxicity can occur at variable periods after chemotherapy, previous research has demonstrated that most cases of AC cardiotoxicity occur within the first year after treatment, underscoring the importance of serial echocardiography. 7 A reduction in LVEF is now appreciated as a late change reflecting a degree of irreversible myocardial damage 6 , 7 , 13 with translation to poor patient outcomes. 21 Previous studies of AC in breast cancer patients have yielded a 10–20% rate of CTRCD, 10 although contemporary analyses suggest modern AC regimens are less likely to decrease LVEF, 12 , 22 with a recent meta‐analysis estimating the effect of AC in contemporary settings only decreases LVEF by ~5.4%. 22 The LVEF in our cohort was within normal limits throughout the census period; however, a statistically significant reduction in LVEF between baseline and 3 months was noted with no persistence of this negative trend beyond 3 months. Peak systolic annular s′ velocity was decreased at 3 months and demonstrated a small but significant reduction which persisted at 1 year, indicative of AC selectively affecting longitudinal function prior to a change in LVEF. Both end diastolic and end systolic LV volumes were also increased from baseline at 1 year, underscoring the development of LV remodelling with AC even when LVEF is preserved. Similar changes have been reported in studies using cardiac magnetic resonance imaging in breast cancer patients. 23
In recent years, GLS has been utilized to identify subclinical cardiac dysfunction in cardiovascular disease in general, and also related to cancer therapies. GLS is technically superior to LVEF, with relative angle independence of 2D speckle tracking strain and fewer geometric assumptions. 12 , 24 , 25 The most recent ESC guidelines define GLS‐CTRCD as a >15% relative change and this threshold was chosen to yield a high specificity to minimize overdiagnosis of CTRCD. 9 While a 15% cut‐off to define GLS‐CTRCD is reasonable, there can be no doubt that relative GLS changes below this threshold may still be relevant and indicate subclinical dysfunction. Previous studies have suggested a relative drop in GLS of ≥10% during chemotherapy represents subclinical dysfunction, 16 , 26 , 27 and prior guidelines have supported cardiac specialist referral for such patients irrespective of symptoms to reduce development of heart failure. 28 Our study demonstrated a significant drop in relative GLS of ≥10% in 43 patients (37.7%), underscoring the fact that subclinical myocardial changes occur even in a relatively healthy patient group following AC therapy. Moreover, changes in LV GLS independently predicts cardiac mortality and major adverse cardiac events, with a prognostic value superior to LVEF in non‐cancer populations. 29
Prior data has suggested improved cardiac outcomes when GLS is monitored throughout cancer therapy. 11 Recently the SUCCOUR study sought to determine whether a GLS guided approach to cardioprotective therapies was superior to a traditional approach guided by changes in LVEF. 13 The authors selected a relative GLS change of ≥12% to guide the administration of cardioprotective therapies. 13 However, no difference in final LVEF among the two approaches was observed, although the GLS guided group received more cardioprotective therapies and had less of a drop in LVEF overall. 13 Our data support the notion that absolute GLS value combined with relative GLS change may have a role in risk stratifying patients and therefore allow for a more nuanced GLS assessment, and potentially allow for better identification of patients with GLS CTRCD.
A recent classification of LV GLS has suggested that normal LV GLS is 18–20%, borderline LV GLS is 16–18% 25 while LV GLS <16% is considered reduced GLS 25 and has clinical relevance in the context of CTRCD. AC specific data has demonstrated a 4.7 fold increase in major adverse cardiac events in patients with a GLS of ≤16%. 30
In our study, patient characteristics associated with a relative reduction in GLS of >10% to an absolute value <16% included older age, presence of hypertension and a higher diastolic blood pressure, while baseline echocardiographic parameters included increased LV mass, reduced e′ velocity and LV GLS. However, baseline LV GLS was the only independent determinant of subclinical LV dysfunction at 12 months, as GLS may represent the composite additive effects of increased age, hypertension, and increased LV mass, with a resultant lower GLS.
Although guidelines recommend a pre‐chemotherapy baseline echocardiogram in breast cancer patients, associations between absolute pre‐treatment GLS thresholds and CTRCD have been studied infrequently. 14 , 31 , 32 A pretreatment GLS below 19.95% predicted CTRCD in a study of patients with haematological malignancies. 31 Similarly, a retrospective analysis of breast cancer patients demonstrated a lower pre‐treatment mean GLS of 19.4% among those who developed CTRCD while CTRCD‐free patients demonstrated a higher baseline GLS of 23.1%. 14 Our current study has added further merit to the concept of a ‘protective’ pre‐treatment GLS (i.e., >20.5%) among breast cancer patients receiving AC.
AC is beneficial in the treatment of breast cancer although it nonetheless poses a risk of permanent irreversible myocardial dysfunction with development of heart failure. 13 , 28 Conversely, interrupting essential cancer therapy may worsen outcomes or result in patient distress. 28 Our study suggests the consideration of a simple stepwise model where patients treated with AC who experience a drop in GLS of ≥10% may be re‐categorized as low risk if their baseline GLS is ≥20.5%. To our knowledge, this is the first study to suggest a simple and intuitive stepwise approach, which could potentially help alleviate patient anxiety, ensure completion of chemotherapy regimens and determine when to employ cardioprotective medications. However, further validation is required with a longer duration of follow up for adverse cardiovascular events. Our findings may not necessarily be applicable to other chemotherapeutic regimens but needs to be considered.
Study limitations and future directions
By virtue of recruitment of a low risk cohort, the patient subset did not experience significant CTRCD or adverse cardiac events within the follow up period. The persistence of GLS perturbation (as both a relative change and absolute GLS value), at 1 year suggests a future risk of cardiovascular morbidity. However, longer‐term follow up for adverse cardiovascular events is not available in the current study cohort.
Cardiac biomarker abnormalities have been noted in prior AC studies 6 , 16 but were not routinely performed in our study.
3D echocardiography was novel at the inception of our study but is now accepted as superior to standard 2D LVEF assessment reducing both inter‐observer and test to test variability. 7 In the current study, LVEF was well above the normal threshold with an average of 60%, making the probability of significant clinical reassignment (e.g., from normal range to abnormal LVEF) unlikely.
A high pre‐treatment GLS may be protective against a subsequent reduced absolute GLS; however, the specific GLS cut off is unclear and a validation study is required to determine whether the proposed GLS ≥20.5% may predict ‘protection’ even in patients with significant relative change in GLS (i.se., >10%) with AC therapy.
Conclusion
This HER2 negative breast cancer cohort demonstrated no CTRCD by LVEF with AC therapy at 1 year, although 38% (n = 43) dropped GLS by ≥10% with 15/43 patients demonstrating an absolute LV GLS <16% at 1 year. GLS at baseline was the single determinant of absolute GLS <16% at 1 year and was more commonly seen in older women with hypertension. Patients with GLS of ≥20.5% before AC treatment who experienced a ≥10% relative drop in GLS, were more likely to have a normal absolute GLS at 1 year, suggesting that this group may be erroneously classified as subclinical CTRCD or in instances even commenced on cardioprotective therapy. Using absolute pre‐treatment LV GLS along with relative LV GLS change may help to re‐stratify a patient's risk for subclinical AC cardiotoxicity. Future validation studies with longer‐term follow up are required to further confirm these preliminary findings.
Conflict of interest
None declared.
Supporting information
Table S1. Patient characteristics and cardiovascular risk factors.
Table S2. Sensitivity and Specificity for Group 2 in the at‐risk groups.
Table S3. Patient cardiotoxicity risk utilising the AC risk tool as proposed by Lyon AR et al. Eur J Heart Fail. 2020 Nov;22 (11):1945‐60.
Acknowledgements
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Terluk, A. , Stefani, L. , Boyd, A. , Vo, K. , Byth, K. , Hui, R. , Richards, D. , and Thomas, L. (2024) Redefining anthracycline‐related subclinical cardiotoxicity: ‘Absolute’ and ‘relative’ change in longitudinal strain. ESC Heart Failure, 11: 3210–3221. 10.1002/ehf2.14884.
Andrew Terluk and Luke Stefani are co‐first authors.
References
- 1. Rana JS, Khan SS, Lloyd‐Jones DM, Sidney S. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med 2021;36:2517‐2518. PubMed PMID: 32705476. Pubmed Central PMCID: PMC7377530. Epub 2020/07/25. doi: 10.1007/s11606-020-06070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363‐385. PubMed PMID: 31184787. Epub 2019/06/12. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 3. Bansal N, Adams MJ, Ganatra S, Colan SD, Aggarwal S, Steiner R, et al. Strategies to prevent anthracycline‐induced cardiotoxicity in cancer survivors. Cardiooncology 2019;5:18. PubMed PMID: 32154024. Pubmed Central PMCID: PMC7048046. Epub 2020/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171‐190. PubMed PMID: 31959335. Pubmed Central PMCID: PMC8019325. Epub 2020/01/22. doi: 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014;63:2751‐2768. PubMed PMID: 24703918. Epub 2014/04/08. doi: 10.1016/j.jacc.2014.01.073 [DOI] [PubMed] [Google Scholar]
- 6. Inoue K, Iida N, Tajiri K, Bando H, Chiba S, Tasaka N, et al. Rationale, design, and feasibility of a prospective multicenter registry study of anthracycline‐induced cardiotoxicity (AIC registry). J Clin Med 2021;10. PubMed PMID: 33801734. Pubmed Central PMCID: PMC8036590. Epub 2021/04/04:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Čelutkienė J, Pudil R, López‐Fernández T, Grapsa J, Nihoyannopoulos P, Bergler‐Klein J, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio‐Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:1504‐1524. PubMed PMID: 32621569. Epub 2020/07/06. doi: 10.1002/ejhf.1957 [DOI] [PubMed] [Google Scholar]
- 8. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911‐939. PubMed PMID: 25172399. Epub 2014/08/31. doi: 10.1016/j.echo.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 9. Lyon AR, López‐Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler‐Klein J, et al. 2022 ESC guidelines on cardio‐oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio‐Oncology Society (IC‐OS). Eur Heart J Cardiovasc Imaging 2022;23:e333‐e465. PubMed PMID: 36017575. Epub 2022/08/27. doi: 10.1093/ehjci/jeac106 [DOI] [PubMed] [Google Scholar]
- 10. Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy‐induced cardiotoxicity: a systematic review and meta‐analysis. JAMA Cardiol 2019;4:1007‐1018. PubMed PMID: 31433450. Pubmed Central PMCID: PMC6705141. Epub 2019/08/23. doi: 10.1001/jamacardio.2019.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ammon M, Arenja N, Leibundgut G, Buechel RR, Kuster GM, Kaufmann BA, et al. Cardiovascular management of cancer patients with chemotherapy‐associated left ventricular systolic dysfunction in real‐world clinical practice. J Card Fail 2013;19:629‐634. PubMed PMID: 24054339. Epub 2013/09/24. doi: 10.1016/j.cardfail.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 12. Yu C, Pathan F, Tan TC, Negishi K. The utility of advanced cardiovascular imaging in cancer patients‐when, why, how, and the latest developments. Front Cardiovasc Med 2021;8:728215. PubMed PMID: 34540922. Pubmed Central PMCID: PMC8446374. Epub 2021/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thavendiranathan P, Negishi T, Somerset E, Negishi K, Penicka M, Lemieux J, et al. Strain‐guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol 2021;77:392‐401. PubMed PMID: 33220426. Epub 2020/11/22. doi: 10.1016/j.jacc.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 14. Milks MW, Velez MR, Mehta N, Ishola A, van Houten T, Yildiz VO, et al. Usefulness of integrating heart failure risk factors into impairment of global longitudinal strain to predict anthracycline‐related cardiac dysfunction. Am J Cardiol 2018;121:867‐873. PubMed PMID: 29454478. Epub 2018/02/20. doi: 10.1016/j.amjcard.2017.12.022 [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233‐270. PubMed PMID: 25712077. Epub 2015/02/26. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 16. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596‐603. PubMed PMID: 22744937. Pubmed Central PMCID: PMC3703313. Epub 2012/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laufer‐Perl M, Derakhshesh M, Milwidsky A, Mor L, Ravid D, Amrami N, et al. Usefulness of global longitudinal strain for early identification of subclinical left ventricular dysfunction in patients with active cancer. Am J Cardiol 2018;122:1784‐1789. PubMed PMID: 30217373. Epub 2018/09/16. doi: 10.1016/j.amjcard.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 18. Negishi T, Negishi K. How to standardize measurement of global longitudinal strain. J Med Ultrason 2001;49:45‐52. PubMed PMID: 34787744. Epub 2021/11/18. [DOI] [PubMed] [Google Scholar]
- 19. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155‐163. PubMed PMID: 27330520. Pubmed Central PMCID: PMC4913118. Epub 2016/06/23. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyon AR, Dent S, Stanway S, Earl H, Brezden‐Masley C, Cohen‐Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio‐Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio‐Oncology Society. Eur J Heart Fail 2020;22:1945‐1960. PubMed PMID: 32463967. PMCID: PMC8019326. Epub 2020/08/06. doi: 10.1002/ejhf.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, de Giacomi G, et al. Anthracycline‐induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213‐220. PubMed PMID: 20117401. Epub 2010/02/02. doi: 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 22. Jeyaprakash P, Sangha S, Ellenberger K, Sivapathan S, Pathan F, Negishi K. Cardiotoxic effect of modern anthracycline dosing on left ventricular ejection fraction: a systematic review and meta‐analysis of placebo arms from randomized controlled trials. J Am Heart Assoc 2021;10:e018802. PubMed PMID: 33660514. Pubmed Central PMCID: PMC8174208. Epub 2021/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jordan JH, D'Agostino RB Jr, Hamilton CA, Vasu S, Hall ME, Kitzman DW, et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1‐weighted and T2‐weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2014;7:872‐879. PubMed PMID: 25273568. Pubmed Central PMCID: PMC4241241. Epub 2014/10/03. doi: 10.1161/CIRCIMAGING.114.002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, et al. Two‐dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr 2011;12:945‐952. PubMed PMID: 21965152. Epub 2011/10/04. doi: 10.1093/ejechocard/jer187 [DOI] [PubMed] [Google Scholar]
- 25. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 2018;11():260–274. PubMed PMID: 29413646. Epub 2018/02/08. [DOI] [PubMed] [Google Scholar]
- 26. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab‐induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493‐498. PubMed PMID: 23562088. Epub 2013/04/09. doi: 10.1016/j.echo.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 27. Florescu M, Magda LS, Enescu OA, Jinga D, Vinereanu D. Early detection of epirubicin‐induced cardiotoxicity in patients with breast cancer. J Am Soc Echocardiogr 2014;27:83‐92. PubMed PMID: 24268372. Epub 2013/11/26. doi: 10.1016/j.echo.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 28. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017;35:893‐911. PubMed PMID: 27918725. Epub 2016/12/06. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 29. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356‐364. PubMed PMID: 19808623. Epub 2009/10/08. doi: 10.1161/CIRCIMAGING.109.862334 [DOI] [PubMed] [Google Scholar]
- 30. Mousavi N, Tan TC, Ali M, Halpern EF, Wang L, Scherrer‐Crosbie M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50‐59% treated with anthracyclines. Eur Heart J Cardiovasc Imaging. 2015;16:977‐984. PubMed PMID: 25925220. Epub 2015/05/01. doi: 10.1093/ehjci/jev113 [DOI] [PubMed] [Google Scholar]
- 31. Charbonnel C, Convers‐Domart R, Rigaudeau S, Taksin AL, Baron N, Lambert J, Ghez S, Georges JL, Farhat H, Lambert J, Rousselot P, Livarek B Assessment of global longitudinal strain at low‐dose anthracycline‐based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging 2017;18():392–401. PubMed PMID: 28064155. Epub 2017/01/09. [DOI] [PubMed] [Google Scholar]
- 32. Narayan HK, Wei W, Feng Z, Lenihan D, Plappert T, Englefield V, et al. Cardiac mechanics and dysfunction with anthracyclines in the community: results from the PREDICT study. Open Heart 2017;4:e000524. PubMed PMID: 28123764. Pubmed Central PMCID: PMC5255565. Epub 2017/01/27. doi: 10.1136/openhrt-2016-000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics and cardiovascular risk factors.
Table S2. Sensitivity and Specificity for Group 2 in the at‐risk groups.
Table S3. Patient cardiotoxicity risk utilising the AC risk tool as proposed by Lyon AR et al. Eur J Heart Fail. 2020 Nov;22 (11):1945‐60.


