Abstract
Avian influenza A H9N2 viruses are widespread among domestic poultry and were recently isolated from humans with respiratory illness in China. Two antigenically and genetically distinct groups of H9N2 viruses (G1 and G9) are prevalent in China. To evaluate a strategy for vaccination, we compared G1 and G9 viruses for their relative immunogenicity and cross-protective efficacy. Infection of BALB/c mice with representative viruses of either group protected against subsequent challenge with the homologous or heterologous H9N2 virus in the absence of detectable cross-reactive serum hemagglutination inhibition antibody. Mice injected intramuscularly with inactivated G1 whole virus vaccine were completely protected from challenge with either H9N2 virus. In contrast, mice administered inactivated G9 vaccine were only partially protected against heterologous challenge with the G1 virus. These results have implications for the development of human vaccines against H9N2 viruses, a priority for pandemic preparedness.
Avian influenza A H9N2 viruses are circulating in domestic poultry worldwide (1, 13, 14, 15, 24, 32). Although this avian subtype is generally not highly pathogenic for avian species, these viruses have recently been transmitted to mammalian species, including humans (2, 15, 22, 27, 28). In Hong Kong, H9N2 viruses were isolated from domestic pigs in 1998 and 1 year later were isolated from two children with uncomplicated febrile respiratory illnesses (2, 22, 27, 28). An additional five human cases of H9N2 influenza infection in southern China have been reported (15). Three genetically and antigenically distinct (≥4-fold differences in titers in serologic assays) Eurasian H9 sublineages, represented by A/Quail/Hong Kong/G1/97 (Qa/G1; G1 group), A/Chicken/Hong Kong/G9/97 (Ck/G9; G9 group), and A/Duck/Hong Kong/Y439/97 (Korean group) viruses have been identified in Asia (12). A surveillance study conducted in Hong Kong poultry markets in 1999 resulted in the isolation of G1 group viruses from 16% of quail (n = 101), while the majority of viruses isolated from 4.7% of chickens (n = 1,180) were antigenically similar to G9 viruses (13). Qa/G1 and Ck/G9 viruses differ by 8% in their hemagglutinin (HA) amino acid sequences (12). The H9N2 viruses isolated from humans in Hong Kong are G1 group viruses, sharing >99% nucleotide homology with the prototype Qa/G1 virus (22). In contrast, the viruses isolated from swine belong to the G9 group (22). Furthermore, seroprevalence studies suggest that G9 viruses may also have been transmitted to humans exposed to infected poultry in Hong Kong (Jacqueline M. Katz, unpublished data). All six internal genes of the G1 group viruses and the PB1 and PB2 genes of the Ck/G9 virus share a high degree of nucleotide homology with those of the highly pathogenic H5N1 viruses isolated from humans in 1997 (12, 14, 22), suggesting that these viruses may share molecular determinants that facilitate their replication in mammalian species.
Both Eurasian H5N1 and H9N2 viruses have proven their ability to directly infect humans (4, 6, 15, 20, 22, 28, 34, 35). However, unlike the H5N1 viruses, H9N2 viruses are currently widespread in domestic poultry in southern China (13, 15, 32) and thus remain a potential source of further human infections and, possibly, a new pandemic strain. A virus with the ability to be efficiently transmitted among humans may arise by mutation of the avian H9N2 virus genome and/or by reassortment between this avian and a human influenza A virus. Humans, as well as swine, must now be considered a likely “mixing vessel” for a reassortment event with pandemic consequences. Therefore, the development of a human influenza vaccine for H9N2 viruses is considered a high priority for pandemic preparedness. To provide a rational basis for vaccine development, we compare here the relative immunogenicity of G1 and G9 viruses in mice and evaluate each as a candidate strain for the development of an inactivated vaccine against H9N2 viruses.
Replication, immunogenicity, and cross-protective efficacy of live H9N2 viruses.
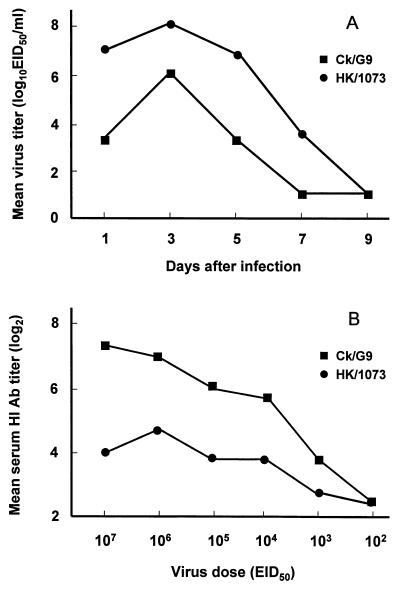
Qa/G1 and Ck/G9 viruses have been shown to replicate in BALB/c mice without prior adaptation (14). In this study, we have used an H9N2 virus isolated in a human, A/Hong Kong/1073/99 (HK/1073), as a prototype G1 virus together with Ck/G9, the prototype G9 virus. To compare the infectivity of these viruses, 6- to 8-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were infected intranasally (i.n.) under light CO2 anesthesia with 106 50% egg infectious doses (EID50) of HK/1073 or Ck/G9 virus. At various times postinfection (p.i.), mice were euthanized and lungs were collected and titrated for virus as previously described (18). As shown in Fig. 1A, the lung virus titers of mice infected with the HK/1073 virus were consistently ≥15-fold higher than those of mice infected with the Ck/G9 virus at each time point tested (P < 0.05). Neither H9N2 virus replicated in extrapulmonary organs, caused significant weight loss, or caused fatal disease in mice following i.n. inoculation. The 50% lethal doses for HK/1073 and Ck/G9 viruses were >108.0 EID50. Similar results were obtained with other G1 group (Qa/G1 and another human isolate, A/Hong Kong/1074/99) and G9 group (A/Swine/Hong Kong/10/98) viruses (data not shown). Thus, all of the H9N2 viruses studied failed to replicate outside the respiratory tract and caused no morbidity or mortality in mice. In contrast, Guo et al. (14) reported that Qa/G1 virus caused 37% mortality and replicated in mouse brains. Sequence analysis of the HA and neuraminidase (NA) genes of the viruses used in the present study failed to identify any consistent molecular basis for this difference between studies.
FIG. 1.
Replication and serum HI antibody production in mice infected with H9N2 viruses. (A) Kinetics of replication of H9N2 viruses in mouse lungs. Mice were infected i.n. with 106 EID50 of each virus. Lungs from three mice per group were harvested at the indicated times and titrated in embryonated eggs. The limit of virus detection was 101.2 EID50/ml. (B) Serum HI antibody responses of mice infected with H9N2 viruses. HI titers are the reciprocal of the highest dilution of serum that completely inhibited agglutination of 0.5% turkey red blood cells by 4 hemagglutinating units of virus. Antibody titers are expressed as the mean log2 titer of five mice per group.
To evaluate the relative immunogenicity of G1 and G9 group viruses, serum antibodies were measured in mice that had been infected i.n. 1 month previously with 107 to 102 EID50 of either HK/1073 or Ck/G9 virus (Fig. 1B). Sera were collected from five mice per group, treated with receptor-destroying enzyme, and tested by the hemagglutination inhibition (HI) assay as previously described (21). Homologous HI antibody titers induced by Ck/G9 virus were ≥4-fold higher than those induced by the HK/1073 virus. Similar differences in serum HI and neutralizing antibody titers were obtained when the other G1 and G9 group viruses listed above were compared (data not shown). These results indicated that G9 group viruses were more immunogenic than G1 group viruses, despite replicating to lower titers in mouse lungs.
To better understand the extent of cross-protective immunity between G1 and G9 group viruses, mice infected with either H9N2 virus 1 month previously were tested for the presence of cross-reactive serum HI and neutralizing antibody (31) and were then challenged i.n. with 106 EID50 of either HK/1073 or Ck/G9 virus. As shown in Table 1, mice infected with the HK/1073 virus had low HI and neutralizing antibodies to HK/1073 virus but no detectable antibody to Ck/G9 virus. Similarly, sera from mice infected with Ck/G9 virus had substantial HI and neutralizing antibody titers to Ck/G9 virus but failed to react with heterologous HK/1073 virus. Despite the lack of cross-reactive antibody, mice infected with HK/1073 virus were completely protected from lung infection with either HK/1073 or Ck/G9 virus on day 4 p.i. Likewise, mice previously infected with a Ck/G9 virus were essentially protected from challenge with virus of either group. Only one of five mice infected with Ck/G9 virus and subsequently challenged with HK/1073 virus had a detectable lung virus titer (103.9 EID50/ml). Similar cross-protection was achieved between other G1 and G9 group viruses (data not shown). These results indicated that infection of mice with live G1 or G9 group virus resulted in protection from challenge with the heterologous H9N2 virus in the absence of detectable cross-reactive serum HI and neutralizing antibody responses.
TABLE 1.
Cross-protection in mice following primary infection with influenza A H9N2 viruses
| Primary virus used to infecta | Antibody titerb
|
Protection against challengec
|
||||||
|---|---|---|---|---|---|---|---|---|
| HK/1073
|
Ck/G9
|
|||||||
| HK/1073
|
Ck/G9
|
Mean virus titer | No. protectedd/ total no. | Mean virus titer | No. protectedd/ total no. | |||
| HI | Neut | HI | Neut | |||||
| HK/1073 (G1 group) | 20 | 106 | 5 | 20 | ≤1.2 | 5/5 | ≤1.2 | 5/5 |
| Ck/G9 (G9 group) | 5 | 20 | 105 | 485 | 1.7 ± 0.1 | 4/5 | ≤1.2 | 5/5 |
| None | 5 | 20 | 5 | 20 | 8.8 ± 0.3 | 0/5 | 6.1 ± 0.3 | 0/5 |
Groups of mice were infected i.n. with 106 EID50 of influenza A H9N2 virus.
Sera collected 1 month after infection were tested by HI and neutralization (Neut) assays against HK/1073 and Ck/G9 viruses. Antibody titers are expressed as the geometric mean titer of five mice per group.
Two months after primary infection, mice were challenged i.n. with 106 EID50 of HK/1073 or Ck/G9 virus and euthanized 4 days later. The lungs were homogenized in 1 ml of phosphate-buffered saline and were titrated for virus infectivity in eggs. Titers are expressed as the mean log10 EID50/ml ± the standard deviation of five mice per group. All mice that were previously infected with an H9N2 virus showed significant reduction in virus titers (P < 0.01) compared with the previously uninfected group of mice upon challenge with homologous or heterologous H9N2 virus.
Mice were considered protected if no virus was detectable in 0.2 ml of a 1:10 dilution of lung homogenate (virus titer, ≤101.2).
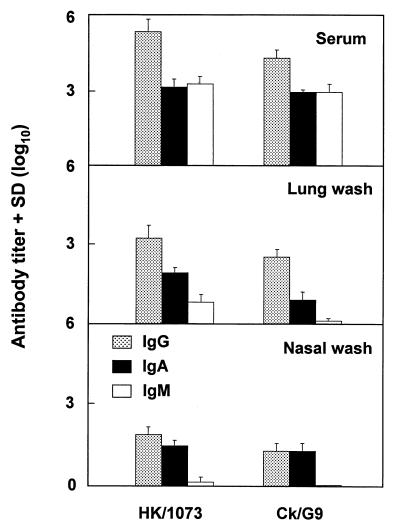
To further investigate the anti-H9 HA antibody response following infection, sera and mucosal washes were collected and tested for anti-HA-specific immunoglobulin G (IgG), IgA, and IgM antibodies by enzyme-linked immunosorbent assay (ELISA) as previously described (18, 19) but using a purified baculovirus-expressed recombinant HK/1073 HA protein (Protein Sciences Corporation, Meriden, Conn.; 1 μg/ml). Purified Ck/G9 HA was not available for this study. No significant differences in serum and mucosal wash antibodies were observed among mice infected i.n. with either HK/1073 or Ck/G9 virus, despite the fact that antibody was detected with HK/1073 HA (Fig. 2). These results demonstrated that serum IgG, IgA, and IgM antibodies and mucosal wash IgG and IgA antibodies that cross-react with the HK/1073 G1 group HA are induced in mice infected with Ck/G9 virus.
FIG. 2.
Anti-HK/1073 HA antibody responses after infection with H9N2 viruses. Mice were infected i.n. with 107 EID50 of HK/1073 or Ck/G9 virus. Two months later, antibody samples were collected from 10 animals per group and were tested by ELISA for the presence of IgG, IgM, and IgA antibody using a purified HK/1073 virus HA recombinant protein. Antibody responses are expressed as reciprocal endpoint titers + standard deviations (SD).
Immunogenicity and cross-protective efficacy of inactivated H9N2 virus vaccines.
We next compared inactivated whole virus vaccines prepared from HK/1073 (G1 group) or Ck/G9 (G9 group) virus for their relative immunogenicity and cross-protective efficacy in mice. The viruses were concentrated from allantoic fluid and purified on a linear sucrose gradient as described previously (5) and were inactivated by treatment with 0.025% formalin at 4°C for 3 days. The HA content of the purified viruses was estimated to be 35 to 39% of the total viral proteins by densitometric analysis of viral protein bands separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (26). Mice were immunized once intramuscularly (i.m.) with 10 μg of purified inactivated vaccine, in the presence or absence of 1% alum (Alhydrogel; Superfos Biosectors, Kvistgaard, Denmark), as previously described (23). As shown in Table 2, the administration of vaccine alone resulted in 100% of mice achieving an HI titer of ≥40 to the homologous virus. The addition of alum resulted in homologous HI titers that were 6- to 12-fold higher than titers achieved without adjuvant. HK/1073 vaccine also induced modest titers of antibody to the heterologous Ck/G9 virus, which were slightly enhanced by alum. In contrast, the Ck/G9 vaccine induced only G9 strain-specific HI antibody, even following coadministration of Ck/G9 vaccine with alum. A microneutralization assay also failed to detect any cross-reactive antibody in sera from animals vaccinated with Ck/G9 virus, in either the presence or absence of alum (data not shown). Next, the protective efficacy of the H9N2 vaccines was investigated 3 days p.i. (Table 2). The level of viral replication in the lower and upper respiratory tract of mice was determined. Lung and anterior nasal (nose) tissue were collected, homogenized, and titrated for virus infectivity in eggs as previously described (19). Mice administered HK/1073 vaccine were completely protected from infection of the lung with homologous HK/1073 virus and only one of seven mice had a low titer of virus in the nose. The HK/1073 vaccine also substantially protected mice from challenge with heterologous Ck/G9 virus, and when formulated with alum, the vaccine was 100% cross-protective. Mean virus titers were reduced by >104-fold in the lung and by ≥6-fold in the nose. While mice immunized with Ck/G9 vaccine were essentially protected from homologous virus challenge in both the lungs and noses, only partial protection from heterologous challenge with HK/1073 virus was demonstrated. Following HK/1073 virus challenge, virus was detected in the lungs and noses of six of seven mice that received the Ck/G9 vaccine alone. Nevertheless, the mean lung virus titers were significantly lower than for mice that received alum alone but were significantly higher than for those that received the HK/1073 vaccine (P < 0.01). Although the addition of alum to the Ck/G9 vaccine resulted in a further reduction of virus titers, protection from HK/1073 virus infection was again incomplete. These results suggest that inactivated vaccines made from HK/1073 viruses offered more complete protection from heterologous H9 virus challenge than did the Ck/G9 vaccine. Nevertheless, the Ck/G9 vaccine provided partial protection from HK/1073 virus challenge in the absence of a cross-reactive serum HI and neutralizing antibody responses (data not shown), suggesting a role for cellular immunity or antibody that is not detected by HI and microneutralization assays.
TABLE 2.
Protective efficacy of influenza H9N2 inactivated vaccines against infection with HK/1073 and Ck/G9 viruses
| Vaccine groupa | HI antibody titerb
|
Protection against challengec
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HK/1073
|
Ck/G9
|
|||||||||
| Mean virus titerd
|
No. protectede/total no.
|
Mean virus titer
|
No. protectede/total no.
|
|||||||
| HK/1073 | Ck/G9 | Lung | Nose | Lung | Nose | Lung | Nose | Lung | Nose | |
| Vaccinated mice | ||||||||||
| HK/1073 | 91 | 40 | ≤1.2 | 0.9 ± 0.2 | 7/7 | 6/7 | 1.8 ± 1.6 | 1.1 ± 0.7 | 7/8 | 5/8 |
| HK/1073 + alum | 564 | 62 | ≤1.2 | ≤0.8 | 7/7 | 7/7 | ≤1.2 | ≤0.8 | 8/8 | 8/8 |
| Ck/G9 | 10 | 219 | 3.5 ± 1.5f | 2.4 ± 1.2g | 1/7 | 1/7 | ≤1.2 | 0.9 ± 0.2 | 8/8 | 7/8 |
| Ck/G9 + alum | 12 | 2,560 | 1.5 ± 0.5 | 1.2 ± 0.4 | 5/7 | 3/7 | ≤1.2 | ≤0.8 | 8/8 | 8/8 |
| Alum | 10 | 10 | 7.5 ± 0.7 | 2.9 ± 0.8 | 0/7 | 0/7 | 5.9 ± 1.1 | 1.9 ± 0.4 | 0/8 | 0/8 |
| Infected mice | ||||||||||
| HK/1073 | 103 | 13 | ≤1.2 | ≤0.8 | 7/7 | 7/7 | ≤1.2 | ≤0.8 | 8/8 | 8/8 |
Groups of 6-week-old female BALB/c mice (n = 20) were injected i.m. with 1 dose of 10 μg of formalin-inactivated vaccine alone, vaccine with 1% alum, or alum alone or were infected i.n. with 106 EID50 of HK/1073 virus as a positive control.
Serum samples were collected 3 months after vaccination or infection and tested by HI assay against HK/1073 and Ck/G9 viruses. HI titers are expressed as the geometric mean titer of 10 mice per group.
Mice were challenged i.n. 3 months later with 100 50% mouse infectious doses of HK/1073 (105.5 EID50) or Ck/G9 (106.3 EID50) virus. Virus titers and protection from infection were determined on day 3 p.i. Virus titers were calculated by the method of Reed and Muench (29) and are expressed as the mean log10 EID50/ml ± the standard deviation of seven or eight mice per group.
With one exception (footnote g), mice administrated H9N2 vaccine either with or without alum had significant reductions in lung and nasal virus titers compared with mice that received alum alone following challenge with either HK/1073 or Ck/G9 virus (P < 0.05).
Mice were considered protected if no virus was detectable in 0.2 ml of a 1:10 dilution of lung homogenate (virus titer, ≤101.2) or a 1:2 dilution of nasal passage tissue homogenate (virus titer, ≤100.8).
Viral titer was significantly higher than lung titer for mice that received HK/1073 vaccine alone (P < 0.01).
Viral titer was not significantly different from that for mice administered alum alone.
To determine whether vaccination with Ck/G9 vaccine induced any serum antibody response to HK/1073 virus HA, IgG, IgM, and IgA serum antibodies were measured by ELISA using the HK/1073 recombinant HA protein. Mucosal antibody responses were not measured because systemic vaccination with inactivated influenza vaccine typically does not induce mucosal antibodies in mice (18). As shown in Fig. 3, vaccination with either of the H9N2 vaccines without alum induced substantial serum anti-HK/1073 HA IgG and lower levels of IgM and IgA antibody responses. In particular, mice immunized with Ck/G9 vaccine mounted a substantial anti-HK/1073 HA IgG antibody response but the response was significantly lower than that of mice receiving HK/1073 vaccine alone (P < 0.01). Coadministration of alum with the vaccines enhanced IgG responses four- to eightfold (P < 0.01) and enhanced IgM responses two- to fourfold but did not augment IgA responses. Formulation of the Ck/G9 vaccine with alum enhanced the IgG response to HK/1073 HA eightfold. These results suggest that the partial protection against HK/1073 virus challenge observed in mice vaccinated with Ck/G9 virus may, in part, be associated with the induction of non-HI antibody that is cross-reactive for HK/1073 HA.
FIG. 3.
Comparison of serum antibody responses following vaccination of mice with inactivated H9N2 vaccines. Twenty mice per group were vaccinated i.m. with 10 μg of purified formalin-inactivated H9N2 virus vaccine alone, vaccine with alum, or alum alone. Three months later, serum from 10 mice per group were collected and anti-HK/1073 HA IgG, IgA, and IgM antibodies were detected by ELISA. Antibody responses are expressed as reciprocal endpoint titers + standard deviations (SD).
Since H9N2 viruses that possess HA antigenically similar to G1 and G9 viruses have continued to circulate in Asia, it was important to determine the cross-protective efficacy that a vaccine of one H9 sublineage afforded against a virus of the other sublineage. Infection of mice with either live G1 or G9 group viruses resulted in essentially complete cross-protection against subsequent reinfection with the heterologous virus, despite a lack of detectable HI or neutralizing cross-reactive serum anti-H9 antibody. However, mice infected with the Ck/G9 virus had substantial titers of anti-HK/1073 HA (G1 group) serum IgG, IgA, and IgM and mucosal IgG and IgA antibody, suggesting that these antibody responses may play a role in cross-protection. Although primary infection with one H9N2 virus may have also induced anti-NA antibody and a memory cytotoxic T lymphocyte response that may contribute to enhanced viral clearance, it is unlikely that either of these immune mechanisms alone would result in a complete lack of virus 4 days after reinfection with the heterologous virus (3, 17). O'Neill et al. (25) have demonstrated that infection with the H9N2 virus protected C57BL/6 mice against lethal challenge with an H5N1 virus. Such heterosubtypic immunity is typically the result of only a modest reduction in lung virus titers measured on or after day 5 p.i. (11).
Although the protective immunity induced by the HK/1073 inactivated vaccine was more cross-reactive, the Ck/G9 vaccine induced higher titers of homologous antibody, particularly when coadministered with alum (Table 2). Ck/G9 virus also induced substantially higher HI antibody titers in infected mice compared with HK/1073, despite the fact that Ck/G9 replicated less efficiently in the lungs of mice (Fig. 1). One reason for the observed differences in immunogenicity and antigenicity between the G1 and G9 group viruses may be the differential glycosylation of their HA molecules. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the viruses used in the present study revealed a higher molecular weight for the HA1 polypeptides of G1 group viruses that was consistent with the presence of additional glycosylation sites predicted by nucleotide sequence analysis (12) (data not shown). Oligosaccharide side chains are known to affect both B- and T-cell recognition of the HA (9, 16, 33). In addition, oligosaccharides at the distal tip of the HA may reduce receptor binding (7, 8), which may, in turn, affect the uptake of virus by antigen-presenting cells (10).
The optimal strategy for control of pandemic influenza is early intervention with a vaccine produced, ideally, from the actual pandemic strain, or at least from a related strain that is a close antigenic match. Our results suggest that the HK/1073 vaccine induced antibody that was more broadly cross-reactive as well as more cross-protective against the heterologous H9 virus than that induced by the Ck/G9 vaccine. The HK/1073 virus has the further advantage of growing to high titers in embryonated eggs and may eliminate the need for preparation of a high-growth reassortant for human vaccine preparation. Furthermore, unlike the poor immunogenicity of H5 vaccine in animals (23, 30), a single dose of inactivated H9 vaccine was sufficient to induce homologous serum HI antibody titers that were comparable to the HI antibody response in infected mice (Table 2) and provided essentially complete homologous protection. Nevertheless, the cross-protective efficacy of both H9N2 vaccines was improved by the coadministration of alum. It will be important to determine whether H9 vaccine candidates are similarly immunogenic in humans and whether inactivated vaccines prepared from an HK/1073 virus can induce titers of cross-reactive HI antibody that may be considered adequate for protection in humans.
Acknowledgments
We thank Wilina Lim, Government Virus Unit, Department of Health, Hong Kong, for providing the human influenza A H9N2 virus isolates; Robert Webster, Department of Virology and Molecular Biology, St. Jude Children's Research Hospital, Memphis, Tenn., and Alan Hay, Division of Virology, National Institute for Medical Research, Mill Hill, London, for providing avian and swine H9N2 influenza virus isolates, respectively; Yumiko Matsuoka and Jing Huang for assistance in sequence analyses; Thomas Rowe for preparation of figures and technical support; and Nancy Cox and Kanta Subbarao for critical reviews of the manuscript.
REFERENCES
- 1.Alexander D J. A review of avian influenza in different bird species. Vet Microbiol. 2000;74:3–13. doi: 10.1016/s0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Influenza: Hong Kong Special Administrative Region of China. WHO Wkly Epidemiol Rec. 1999;14:111. [Google Scholar]
- 3.Chen Z, Matsuo K, Asanuma H, Takahashi H, Iwasaki T, Suzuki Y, Aizawa C, Kurata T, Tamura S. Enhanced protection against a lethal influenza virus challenge by immunization with both hemagglutinin- and neuraminidase-expressing DNAs. Vaccine. 1999;17:653–659. doi: 10.1016/s0264-410x(98)00247-3. [DOI] [PubMed] [Google Scholar]
- 4.Claas E C J, Osterhaus A D M E, Van Beek R, de Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 5.Cox N J, Kendal A P. Genetic stability of A/Ann Arbor/6/60 cold-mutant (temperature-sensitive) live influenza virus genes: analysis by oligonucleotide mapping of recombinant vaccine strains before and after replication in volunteers. J Infect Dis. 1984;149:194–200. doi: 10.1093/infdis/149.2.194. [DOI] [PubMed] [Google Scholar]
- 6.De Jong J C, Claas E C J, Osterhaus A D M E, Webster R G, Lim W L. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deom C M, Caton A J, Schulze I T. Host cell-mediated selection of a mutant influenza A virus that has lost a complex oligosaccharide from the tip of the hemagglutinin. Proc Natl Acad Sci USA. 1986;83:3771–3775. doi: 10.1073/pnas.83.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deom C M, Schulze I T. Oligosaccharide composition of an influenza virus hemagglutinin with host-determined binding properties. J Biol Chem. 1985;260:14771–14774. [PubMed] [Google Scholar]
- 9.Drummer H E, Jackson D C, Brown L E. Modulation of CD4+ T-cell recognition of influenza hemagglutinin by carbohydrate side chains located outside a T-cell determinant. Virology. 1993;192:282–289. doi: 10.1006/viro.1993.1031. [DOI] [PubMed] [Google Scholar]
- 10.Eisenlohr L C, Gerhard W, Hackett C J. Role of receptor-binding activity of the viral hemagglutinin molecule in the presentation of influenza virus antigens to helper T cells. J Virol. 1987;61:1375–1383. doi: 10.1128/jvi.61.5.1375-1383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein S L, Chia Y L, Misplon J A, Lawson C M, Hendrickson B A, Max E E, Subbarao K. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, β2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158:1222–1230. [PubMed] [Google Scholar]
- 12.Guan Y, Shortridge K F, Krauss S, Webster R G. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Shortridge K F, Krauss S, Chin P S, Dyrting K C, Ellis T M, Webster R G, Peiris M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol. 2000;74:9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y J, Krauss S, Senne D A, Mo P, Lo K S, Xiong X P, Norwood M, Shortridge K F, Webster R G, Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y J, Li J G, Cheng X W, Wang M, Zhou Y, Li X H, Cai F, Miao H L, Zhang H, Guo F. Discovery of men infected by avian influenza A (H9N2) virus. Chin J Exp Clin Virol. 1999;13:105–108. [PubMed] [Google Scholar]
- 16.Jackson D C, Drummer H E, Urge L, Otvos L, Jr, Brown L E. Glycosylation of a synthetic peptide representing a T-cell determinant of influenza virus hemagglutinin results in loss of recognition by CD4+ T-cell clones. Virology. 1994;199:422–430. doi: 10.1006/viro.1994.1140. [DOI] [PubMed] [Google Scholar]
- 17.Johansson B E, Grajower B, Kilbourne E D. Infection-permissive immunization with influenza virus neuraminidase prevents weight loss in infected mice. Vaccine. 1993;11:1037–1039. doi: 10.1016/0264-410x(93)90130-p. [DOI] [PubMed] [Google Scholar]
- 18.Katz J M, Lu X, Young S A, Galphin J C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 19.Katz J M, Lu X, Todd C W, Newman M J. A nonionic block co-polymer adjuvant (CRL1005) enhances the immunogenicity and protective efficacy of inactivated influenza vaccine in young and aged mice. Vaccine. 2000;18:2177–2187. doi: 10.1016/s0264-410x(00)00022-0. [DOI] [PubMed] [Google Scholar]
- 20.Katz J M, Lim W, Bridges C B, Rowe T, Hu-Primmer J, Lu X, Abernathy R A, Clarke M, Conn L, Kwong H, Lee M, Au G, Ho Y Y, Mak K H, Cox N J, Fukuda K. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180:1763–1770. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 21.Kendal A P, Skehel J J, Pereira M S. Concepts and procedures for laboratory-based influenza surveillance. Publication no. B17–35. Atlanta, Ga: Centers for Disease Control; 1982. [Google Scholar]
- 22.Lin Y P, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, Subbarao K, Guan Y, Krauss S, Shortridge K, Webster R, Cox N, Hay A. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci USA. 2000;97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu X H, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naeem K, Ullah A, Manvell R J, Alexander D J. Avian influenza A subtype H9N2 in poultry in Pakistan. Vet Rec. 1999;145:560. doi: 10.1136/vr.145.19.560. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill E, Krauss S L, Riberdy J M, Webster R G, Woodland D L. Heterologous protection against lethal A/Hong Kong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J Gen Virol. 2000;81:2689–2696. doi: 10.1099/0022-1317-81-11-2689. [DOI] [PubMed] [Google Scholar]
- 26.Oxford J S, Corcoran T, Hugentobler A L. Quantitative analysis of the protein composition of influenza A and B viruses using high resolution SDS polyacrylamide gels. J Biol Stand. 1981;9:483–491. doi: 10.1016/s0092-1157(81)80041-8. [DOI] [PubMed] [Google Scholar]
- 27.Peiris M, Yam W C, Chan K H, Ghose P, Shortridge K F. Influenza A H9N2: aspects of laboratory diagnosis. J Clin Microbiol. 1999;37:3426–3427. doi: 10.1128/jcm.37.10.3426-3427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peiris M, Yuen K Y, Leung C W, Chan K H, Ip P L S, Lai R W M, Orr W K, Shortridge K F. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 29.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 30.Rimmelzwaan G F, Class E C J, van Amerongen G, de Jong J C, Osterhaus A D M E. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999;17:1355–1358. doi: 10.1016/s0264-410x(98)00390-9. [DOI] [PubMed] [Google Scholar]
- 31.Rowe T, Abernathy R A, Hu-Primmer J, Thompson W W, Lu X H, Lim W, Fukuda K, Cox N J, Katz J M. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shortridge K F. Poultry and the influenza H5N1 outbreak in Hong Kong, 1997: abridged chronology and virus isolation. Vaccine. 1999;17:26–29. doi: 10.1016/s0264-410x(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 33.Skehel J J, Stevens D J, Daniels R S, Douglas A R, Knossow M, Wilson I A, Wiley D C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci USA. 1984;81:1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 35.Yuen K Y, Chan P K S, Peiris M, Tsang D N C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T F, Sung R, Cheng A F B Members of the H5N1 Study Group. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]